Abstract
Background:
Identifying risk factors for an infection after anterior cruciate ligament reconstruction (ACLR) and following targeted preventive strategies can effectively reduce this potentially serious complication.
Purpose:
To perform a systematic review and meta-analysis to identify the risk factors for an infection after ACLR.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
The PubMed, Embase, and Web of Science databases were searched from inception to September 1, 2022, for prospective and retrospective studies investigating risk factors for any type of infection after ACLR. Odds ratios (ORs) or mean differences were calculated for potential risk factors if ≥2 studies assessed the same risk factor. A qualitative analysis of variables was performed if a meta-analysis could not be conducted.
Results:
A total of 17 studies with 141,991 patients were included in this review. The overall pooled infection rate was 0.86% (range, 0.24%-5.50%). There were 20 risk factors identified for analysis. Of these, 7 variables independently increased the odds of an infection after ACLR: (1) male sex (OR, 1.90 [95% CI, 1.33-2.73]), (2) diabetes (OR, 2.69 [95% CI, 1.66-4.35]), (3) hamstring tendon autograft (OR, 2.51 [95% CI, 2.03-3.10]), (4) revision ACLR (OR, 2.31 [95% CI, 1.22-4.37]), (5) professional athlete status (OR, 6.21 [95% CI, 1.03-37.38]), (6) lateral tenodesis (OR, 3.45 [95% CI, 1.63-7.28]), and (7) corticosteroid use (OR, 7.83 [95% CI, 3.68-16.63]). No significant associations were found between postoperative infections and age, body mass index, smoking, meniscal repair, or outpatient surgery.
Conclusion:
This review revealed that an increased risk of infections after ACLR was associated with male sex, diabetes, hamstring tendon autograft, revision surgery, professional athlete status, lateral tenodesis, and steroid use. Knowledge of the risk factors associated with an infection after ACLR may facilitate the identification of high-risk cases and the implementation of preventive measures to mitigate the serious consequences of this complication.
Keywords: anterior cruciate ligament, ACL, infection, septic arthritis, risk factors
An infection (including superficial wound infections and septic arthritis) after anterior cruciate ligament reconstruction (ACLR) is a rare but devastating complication, with a reported incidence of 0.28% to 1%.7,9,51 It can result in prolonged rehabilitation, functional deficits, arthrofibrosis, and often the need for repeated surgery.37,42,59 The treatment of joint infections after ACLR usually requires arthroscopic debridement combined with intravenous antibiotics and on occasion requires graft removal.11,63 Infection-induced prolongation of an illness and escalation of treatment costs not only impose a significant burden on patients and their families but also exert additional pressure on the health care system. Therefore, it is critical to determine which factors put patients at a greater risk of a postoperative infection, especially those that can be modified to minimize the hazard of such serious events.
Numerous previous studies have investigated the risk factors associated with an infection after ACLR, such as sex,44,51 high body mass index (BMI), 29 diabetes, 9 tobacco use,12,51 being a professional athlete, 57 and hamstring tendon (HT) autografts.6,31 However, some studies have also found that smoking, diabetes, and being a professional athlete do not increase the risk of infections in patients after ACLR.8,9,64 Given the range of risk factors, inconsistent findings, limited sample sizes, and single institution–based cohorts in numerous studies, further research is warranted.
The purpose of the current study was to perform a systematic review and meta-analysis of risk factors associated with an infection after ACLR and to help develop effective preventive management strategies for this complication.
Methods
We performed and conducted this review with a meta-analysis according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. 41 The study protocol was preregistered at PROSPERO before starting this review (registration No. CRD42022360506).
Literature Search
PubMed, Embase, and Web of Science were searched for any relevant studies published before September 1, 2022. The following terms were used for the search: “risk,”“associated with,”“anterior cruciate ligament,”“infection,” and “septic arthritis.”Appendix Table A1 shows the search strategy for each database. In addition, the gray literature and databases of unpublished studies were also examined, and the reference lists of all included studies were hand searched for potentially eligible studies. There was no restriction on the publication date. Overall, 2 reviewers (L.Z. and R.Y.) independently screened the studies identified in the search. Any discrepancies were resolved by a consensus between the reviewers.
Inclusion criteria were as follows: (1) cohort studies, case-control studies, or cross-sectional studies; (2) cases and controls defined according to the presence or absence of infections after ACLR, respectively; (3) studies that reported at least 1 risk factor for any type of infection (superficial wound infection, septic arthritis, etc) after ACLR; and (4) valid data presented to estimate odds ratios (ORs) or mean differences (MDs) with 95% confidence intervals (CIs). Exclusion criteria were as follows: (1) case reports, conferences, commentaries, and reviews; (2) articles written in a language other than English; (3) nonclinical studies (eg, cadaveric studies, animal studies, and basic science articles); and (4) patients undergoing ACLR with concomitant open surgery or an additional ligament reconstruction procedure (eg, medial collateral ligament, lateral collateral ligament). There was no minimum follow-up time limit because an infection could theoretically occur at any time after ACLR. If several studies focused on the same group of patients, the most recent data containing more samples were used for analysis. Risk factors were defined as any variables potentially associated with an infection after ACLR, including sociodemographic factors, intraoperative factors, and activity level.
Quality Assessment
The Methodological Index for Non-Randomized Studies (MINORS) 23 checklist was used to evaluate the quality of all included studies. The 12 items of the MINORS questionnaire were scored as 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate), with a maximum score of 16 for noncomparative studies and 24 for comparative studies. The methodological quality was assessed according to the MINORS score for noncomparative studies as very low (0-5), low (6-10), fair (11-15), and good (16). Again, 2 reviewers (L.Z. and R.Y.) independently evaluated the included studies, and any discrepancies between the reviewers were resolved by a consensus.
Data Extraction and Synthesis
After identifying all eligible studies, the same 2 reviewers independently evaluated each study, extracted data, and cross-checked the data. The country, number of cases and controls, and risk factors evaluated were extracted from the studies. Any disagreements were resolved by a discussion between the 2 reviewers. We synthesized the risk factors associated with an infection after ACLR as examined in the enrolled studies, conducting a meta-analysis when ≥2 studies reported on a given risk factor and performing a qualitative analysis when only 1 study addressed a particular risk factor. If risk factors were used to match cohorts in a 1-to-1 ratio by exact values, they were not included in the meta-analysis. In addition, considering the many risk factors that may affect the incidence of infections after ACLR, adjusted effect estimates in multivariate analyses were preferred over unadjusted effect estimates because they were closer to the actual effects. In multivariate analyses, adjusting for other variables can help to reduce bias and confounding in estimating the effect of a particular variable on the outcome of interest.
Statistical Analysis
Dichotomous outcomes were reported as ORs with 95% CIs, while continuous outcomes were reported as MDs with 95% CIs. The I2 statistic was used to estimate heterogeneity. I2 > 50% indicated high heterogeneity; a random-effects model was then used. Otherwise, a fixed-effects model was applied. P < .05 was considered statistically significant. If there was high heterogeneity between studies, we conducted a sensitivity analysis by removing individual studies one by one to explore the source of heterogeneity. Review Manager (Version 5.3; Cochrane Collaboration) was used to analyze all extracted data.
We classified the risk factors according to the OR as having strong, moderate, minimal, or marginal to no evidence. Risk factors with strong evidence doubled the risk for an infection after ACLR compared with baseline (OR > 2.0), or had a strong protective effect (OR < 0.8), and were significant. Risk factors with moderate evidence had an OR between 1.5 and 2.0, or between 0.8 and 0.9 if protective, and were significant (P < .05). Risk factors with minimal evidence had an OR between 1.0 and 1.5, or between 0.9 and 1.0 if protective, and were significant. Risk factors with marginal to no evidence had a nonsignificant OR (P > .05) or presented no plausible explanation for being a risk factor for infections after ACLR. These criteria have been used in previously published studies.33,55
Results
The initial search strategy generated a total of 834 studies across PubMed, Embase, and Web of Science. Another study 32 was found through a manual search of the reference lists of the included studies. After removing 249 duplicate studies, the remaining studies were screened according to their titles and abstracts. The full text of 55 studies was considered for screening, 38 studies that did not meet the inclusion criteria were excluded, and 17 studies ‡ were ultimately included in the meta-analysis (Figure 1).
Figure 1.

Flowchart of the study inclusion process.
Characteristics of Included Studies
The 17 eligible studies involved a total of 141,991 patients, consisting of 1223 cases and 140,768 controls (overall incidence of 0.86%). Table 1 shows the characteristics of all included studies. There were 8 case-control studies (47.1%), 1 case series (5.9%), and 8 retrospective cohort studies (47.1%). There were 11 studies that were conducted in the United States, 5 in Europe, and 1 in Japan. The infection rate after ACLR in this review was similar to rates previously reported in the literature.54,58,62 Additionally, 4 studies27,29,39,64 reported all types of infections but with deep surgical site infections (SSIs) and superficial SSIs described separately, 10 studies § only included septic arthritis, and 3 studies9,12,16 reported all types of infections together. The publication year of the included articles ranged from 2005 to 2022.
Table 1.
Characteristics of Included Studies a
| Lead Author (Year) | Country | Study Design (LOE) | Study Period | No. of Cases/ Controls | Infection Type | Risk Factors |
|---|---|---|---|---|---|---|
| Crawford 16 (2005) | USA | Retrospective cohort (3) | 2000-2002 | 11/320 | All SSIs | Male sex |
| Judd 27 (2006) | USA | Case series (4) | 1994-2001 | 23/395 | All SSIs | Previous knee surgery, tibial ACL graft with post-and-washer fixation, HT autograft |
| Katz 28 (2008) | USA | Retrospective cohort (3) | 2001-2005 | 6/795 | Septic arthritis | Preoperative use of antibiotic clindamycin (vs cefazolin) |
| Barker 6 (2010) | USA | Retrospective cohort (3) | 2002-2006 | 18/3108 | Septic arthritis | HT autograft |
| Sonnery-Cottet 56 (2011) | France | Case-control (3) | 2003-2008 | 12/1945 | Septic arthritis | Professional athlete, lateral tenodesis |
| Maletis 39 (2013) | USA | Retrospective cohort (3) | 2005-2010 | 51/10,575 | All SSIs | HT autograft |
| Brophy 9 (2015) | USA | Retrospective cohort (3) | 2002-2005 | 17/2181 | All SSIs | Diabetes, HT autograft |
| Cancienne 12 (2016) | USA | Retrospective cohort (3) | 2007-2011 | 135/13,223 | All SSIs | Smoking |
| Murphy 44 (2016) | USA | Retrospective cohort (3) | 2000-2008 | 121/11,651 | Septic arthritis | HT autograft, connective tissue disorder, male sex, age, immunosuppressive medications |
| Krutsch 32 (2017) | Germany | Case-control (3) | 2008-2019 | 17/1792 | Septic arthritis | Outdoor summer sports |
| Westermann 64 (2017) | USA | Case-control (3) | 2007-2013 | 39/6359 | All SSIs | Postoperative hospital admission |
| Kawata 29 (2018) | Japan | Case-control (3) | 2010-2015 | 374/30,162 | All SSIs | Atopic dermatitis, preoperative steroid use, age ≤19 y, BMI ≥30 kg/m2, male sex, diabetes |
| Bohu 8 (2019) | France | Case-control (3) | 2012-2016 | 7/1802 | Septic arthritis | Prior knee surgery, hemarthrosis during immediate postoperative period |
| Sonnery-Cottet 57 (2019) | France | Case-control (3) | 2009-2017 | 15/4406 | Septic arthritis | Professional athlete |
| Hurvitz 24 (2020) | USA | Retrospective cohort (3) | 2008-2016 | 38/15,633 | Septic arthritis | Screw-and-sheath tibial fixation |
| Kraus Schmitz 31 (2021) | Sweden | Case-control (3) | 2006-2013 | 291/25,018 | Septic arthritis | Male sex, HT autograft, longer operating time |
| Marom 40 (2022) | USA | Case-control (3) | 2010-2018 | 48/11,403 | Septic arthritis | Revision surgery, younger age |
ACL, anterior cruciate ligament; BMI, body mass index; HT, hamstring tendon; LOE, level of evidence; SSI, surgical site infection.
Quality Assessment
The results of the methodological quality assessment using the MINORS score are summarized in Appendix Table A2. The mean MINORS score was 17.29 ± 2.11 among all studies. The mean MINORS score of the 16 comparative studies was 17.75 ± 1.10 of 24, and the mean MINORS score for the 1 noncomparative study 27 was 10 of 16.
Risk Factors for Infection After ACLR
Our analysis ultimately comprised 20 risk factors, with 12 subjected to a meta-analysis: sex, age, BMI, diabetes, smoking, meniscal repair, HT autograft, revision surgery, professional athlete status, lateral tenodesis, steroid use, and outpatient surgery (Table 2). The remaining 8 risk factors (connective tissue disorder, immunosuppressive medications, postoperative hospital admission, atopic dermatitis, prior knee surgery, hemarthrosis during the immediate postoperative period, screw-and-sheath tibial fixation, longer operating time) underwent a qualitative analysis (Appendix Table A3).
Table 2.
Meta-analysis of Risk Factors for Postoperative Infection a
| Risk Factor | Study Citations | OR (95% CI) b | P | I2, % |
|---|---|---|---|---|
| Male sex | 8, 16, 29, 31, 40, 44, 56, 57, 64 | 1.90 (1.33 to 2.73) | .0005 | 70 |
| Age | 9, 28, 40, 44, 64 | MD: –0.79 (–2.21 to 0.63) | .27 | 49 |
| BMI | 9, 40, 64 | MD: –0.57 (–1.58 to 0.44) | .27 | 0 |
| Diabetes | 9, 29, 31, 40, 44, 64 | 2.69 (1.66 to 4.35) | <.0001 | 31 |
| Smoking | 9, 12, 29, 40, 64 | 1.47 (0.99 to 2.18) | .06 | 55 |
| Meniscal repair | 31, 64 | 0.92 (0.60 to 1.41) | .69 | 7 |
| HT autograft | 6, 8, 9, 24, 27, 31, 39, 40, 44, 56, 57 | 2.51 (2.03 to 3.10) | <.00001 | 44 |
| Revision surgery | 8, 27, 31, 40, 56, 57 | 2.31 (1.22 to 4.37) | .01 | 62 |
| Professional athlete | 8, 32, 56, 57 | 6.21 (1.03 to 37.38) | .05 | 83 |
| Lateral tenodesis | 8, 56, 57 | 3.45 (1.63 to 7.28) | .001 | 0 |
| Steroid use | 29, 64 | 7.83 (3.68 to 16.63) | <.00001 | 0 |
| Outpatient surgery | 8, 31, 64 | 1.18 (0.91 to 1.54) | .22 | 0 |
Boldface P values indicate statistical significance (P < .05). BMI, body mass index; HT, hamstring tendon; MD, mean difference; OR, odds ratio.
Data are shown as ORs unless otherwise indicated. Risk factors with strong evidence doubled the risk for an infection (OR > 2.0), or had a strong protective effect (OR < 0.8), and were significant. Risk factors with moderate evidence had an OR of 1.5-2.0 (0.8-0.9 if protective) and were significant. Risk factors with minimal evidence had an OR of 1.0-1.5 (0.9-1.0 if protective) and were significant.
Male Sex
A total of 9 studies investigated the association between male sex and infections after ACLR, including 918 patients in the case group and 93,066 patients in the control group. The pooled results showed that male patients were more likely to develop infections after ACLR than female patients (OR, 1.90 [95% CI, 1.33-2.73]; P = .0005; I2 = 70%) (Figure 2A). Based on the statistical results, there was moderate evidence to suggest that male sex was a risk factor for infections after ACLR. High heterogeneity was also observed. Sensitivity analysis found that the study by Kawata et al 29 was the main source of heterogeneity. After removing this study, I2 was reduced to 48%, and pooled analysis of the remaining studies still showed significant differences and no significant change from the original result (OR, 1.73 [95% CI, 1.44-2.08]; P < .00001), indicating the robustness of the final finding. Based on the study characteristics, we speculated that the main source of heterogeneity is likely to be population differences. The patients in the Kawata et al 29 study were all from Japan, whereas the remaining studies included populations mainly from the United States or France.
Figure 2.
Forest plots showing the association of infections after anterior cruciate ligament reconstruction with patient characteristics: (A) sex, (B) age, (C) body mass index, (D) diabetes, and (E) smoking. IV, inverse variance; M-H, Mantel-Haenszel.
Age
A total of 5 studies investigated the association between age and infections after ACLR, including 231 patients in the case group and 32,389 patients in the control group. The pooled results showed that age was not significantly associated with infections after ACLR (MD, –0.79 [95% CI, –2.21 to 0.63]; P = .27; I2 = 49%) (Figure 2B). Based on the statistical results, age was not a risk factor for infections after ACLR.
Body Mass Index
A total of 3 studies investigated the association between higher BMI and infections after ACLR, including 104 patients in the case group and 19,943 patients in the control group. The pooled results demonstrated that BMI was not significantly associated with infections after ACLR (MD, –0.57 [95% CI, –1.58 to 0.44]; P = .27; I2 = 0%) (Figure 2C). Based on the statistical results, BMI was not a risk factor for infections after ACLR.
Diabetes
A total of 6 studies investigated the association between diabetes and infections after ACLR, including 890 patients in the case group and 86,774 patients in the control group. The pooled results showed that diabetes was significantly associated with infections after ACLR (OR, 2.69 [95% CI, 1.66-4.35]; P < .0001; I2 = 31%) (Figure 2D). Based on the statistical results, there was strong evidence to suggest that diabetes was a risk factor for infections after ACLR.
Smoking
A total of 5 studies investigated the association between smoking and infections after ACLR, including 613 patients in the case group and 63,328 patients in the control group. The pooled results showed that smoking was not significantly associated with infections after ACLR (OR, 1.47 [95% CI, 0.99-2.18]; P = .06; I2 = 55%) (Figure 2E). Based on the statistical results, smoking was not a risk factor for infections after ACLR. High heterogeneity was also observed. Sensitivity analysis found that the study of Cancienne et al 12 was the main source of heterogeneity. After this study was removed, I2 was reduced to 0%, and pooled analysis of the remaining studies still demonstrated no significant differences and no significant change from the original result (OR, 1.23 [95% CI, 1.00-1.52]; P = .05), indicating the robustness of the final finding.
Meniscal Repair
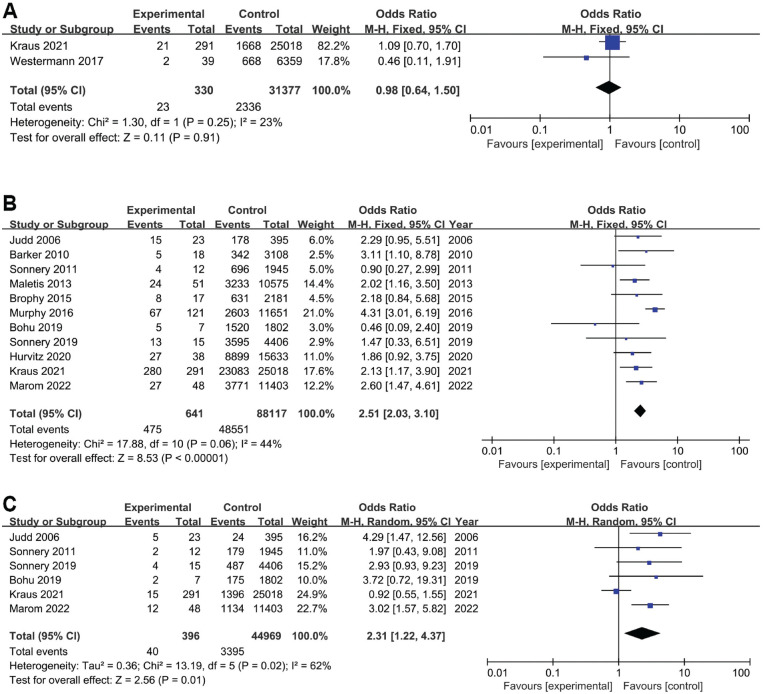
A total of 2 studies investigated the association between meniscal repair and infections after ACLR, including 330 patients in the case group and 31,377 patients in the control group. The pooled results showed that meniscal repair was not significantly associated with infections after ACLR (OR, 0.98 [95% CI, 0.64-1.50]; P = .91; I2 = 23%) (Figure 3A). Based on the statistical results, meniscal repair was not a risk factor for infections after ACLR.
Figure 3.
Forest plots showing the association of infections after anterior cruciate ligament reconstruction (ACLR) with (A) meniscal repair, (B) hamstring tendon autograft, and (C) revision ACLR. M-H, Mantel-Haenszel.
HT Autograft
A total of 11 studies investigated the association between HT autografts and infections after ACLR, including 641 patients in the case group and 88,117 patients in the control group. The pooled results showed that ACLR with HT autografts was more likely to lead to postoperative infections than ACLR with other types of grafts (OR, 2.51 [95% CI, 2.03-3.10]; P < .00001; I2 = 44%) (Figure 3B). Based on the statistical results, there was strong evidence to suggest that an HT autograft was a risk factor for infections after ACLR.
Revision Surgery
A total of 6 studies investigated the association between revision surgery and infections after ACLR, including 396 patients in the case group and 44,969 patients in the control group. The pooled results showed that patients undergoing revision were more likely to develop postoperative infections than those undergoing primary ACLR (OR, 2.31 [95% CI, 1.22-4.37]; P = .01; I2 = 62%) (Figure 3C). Based on the statistical results, there was strong evidence to suggest that revision surgery was a risk factor for infections after ACLR. High heterogeneity was also observed. Sensitivity analysis found that the study by Kraus Schmitz et al 31 was the main source of heterogeneity. After removing this study, I2 was reduced to 0%, and pooled analysis of the remaining studies still showed a significant difference and no significant change from the original result (OR, 3.13 [95% CI, 1.98-4.96]; P < .00001), indicating the robustness of the final finding.
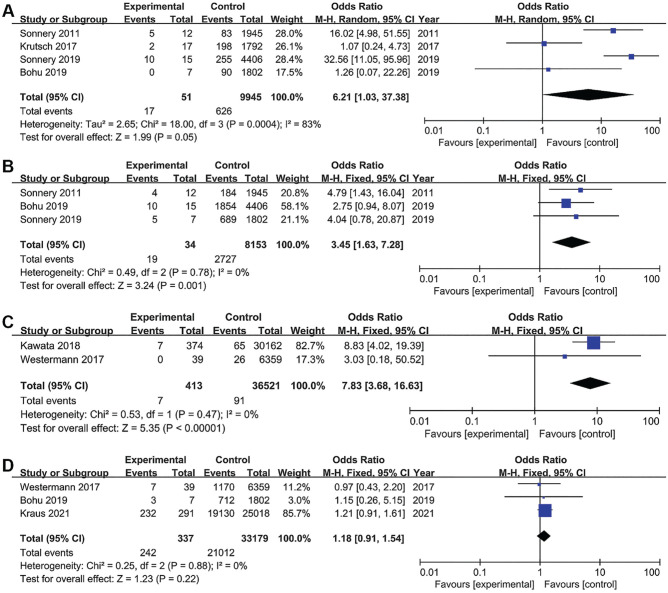
Professional Athlete
A total of 4 studies investigated the association between professional athlete status and infections after ACLR, including 51 patients in the case group and 9945 patients in the control group. The pooled results showed that professional athletes were significantly associated with infections after ACLR (OR, 6.21 [95% CI, 1.03-37.38]; P = .05; I2 = 83%) (Figure 4A). Based on the statistical results, there was strong evidence to suggest that professional athlete status was a risk factor for infections after ACLR. High heterogeneity was also observed. Sensitivity analysis found that the studies of Krutsch et al 32 and Bohu et al 8 were the main sources of heterogeneity. I2 was reduced to 0% after these studies were removed; however, pooled analysis of the remaining studies still showed significant differences and no significant change from the original result (OR, 23.48 [95% CI, 10.62-51.91]; P < .00001), indicating the robustness of the final finding. Based on the study characteristics, we found that the type of sports in which athletes participated varied across studies, which may be the source of heterogeneity.
Figure 4.
Forest plots showing the association of infections after anterior cruciate ligament reconstruction with (A) professional athlete status, (B) lateral tenodesis, (C) corticosteroid use, and (D) outpatient surgery. M-H, Mantel-Haenszel.
Lateral Tenodesis
A total of 3 studies investigated the association between lateral tenodesis and infections after ACLR, including 34 patients in the case group and 8153 patients in the control group. The pooled results showed that lateral tenodesis was significantly associated with infections after ACLR (OR, 3.45 [95% CI, 1.63-7.28]; P = .001; I2 = 0%) (Figure 4B). Based on the statistical results, there was strong evidence to suggest that lateral tenodesis was a risk factor for infections after ACLR.
Corticosteroid Use
A total of 2 studies investigated the association between steroid use and infections after ACLR, including 413 patients in the case group and 36,521 patients in the control group. The pooled results showed that steroid use was significantly associated with infections after ACLR (OR, 7.83 [95% CI, 3.68-16.63]; P < .00001; I2 = 0%) (Figure 4C). Based on the statistical results, there was strong evidence to suggest that steroid use was a risk factor for infections after ACLR.
Outpatient Surgery
A total of 3 studies investigated the association between outpatient surgery and infections after ACLR, including 337 patients in the case group and 33,179 patients in the control group. The pooled results showed that outpatient surgery was not significantly associated with infections after ACLR (OR, 1.18 [95% CI, 0.91-1.54]; P = .22; I2 = 0%) (Figure 4D). Based on the statistical results, outpatient surgery was not a risk factor for infections after ACLR.
Subgroup Analysis of Infection Type
Overall, 10 of the 17 included studies reported only septic arthritis, while the remaining 7 studies included cases of all SSIs. As mixing them together for pooled analysis may lead to confounding bias, we performed a further meta-analysis of studies that reported only septic arthritis. The new pooled results analyzing 11 risk factors showed that the statistical results did not change significantly, except for the factor of diabetes. Subgroup analysis results suggested that diabetes was not a risk factor for septic arthritis (OR, 1.56 [95% CI, 0.61-3.98]; P = .35; I2 = 19%). The results are shown in Table 3.
Table 3.
Meta-analysis of Risk Factors for Septic Arthritis a
| Risk Factor | Study Citations | OR (95% CI) b | P | I2, % |
|---|---|---|---|---|
| Male sex | 8, 29, 31, 40, 44, 56, 57 | 1.86 (1.53 to 2.25) | <.00001 | 14 |
| Age | 28, 40, 44 | MD: –1.78 (–5.81 to 2.25) | .39 | 72 |
| BMI | 40 | MD: –0.50 (–1.78 to 0.78) | .44 | NA |
| Diabetes | 31, 40, 44 | 1.56 (0.61 to 3.98) | .35 | 19 |
| Smoking | 40 | 0.80 (0.19 to 3.31) | .76 | NA |
| Meniscal repair | 31 | 1.01 (0.65 to 1.58) | .96 | NA |
| HT autograft | 6, 8, 24, 31, 40, 44, 56, 57 | 2.19 (1.43 to 3.35) | .0003 | 58 |
| Revision surgery | 8, 31, 40, 56, 57 | 2.04 (1.03 to 4.04) | .04 | 61 |
| Professional athlete | 8, 32, 56, 57 | 6.21 (1.03 to 37.38) | .05 | 83 |
| Lateral tenodesis | 8, 56, 57 | 3.45 (1.63 to 7.28) | .001 | 0 |
| Outpatient surgery | 8, 31 | 1.21 (0.91 to 1.60) | .19 | 0 |
Boldface P values indicate statistical significance (P < .05). BMI, body mass index; HT, hamstring tendon; MD, mean difference; NA, not applicable; OR, odds ratio.
Data are shown as ORs unless otherwise indicated. Risk factors with strong evidence doubled the risk for an infection (OR > 2.0), or had a strong protective effect (OR < 0.8), and were significant. Risk factors with moderate evidence had an OR of 1.5-2.0 (0.8-0.9 if protective) and were significant. Risk factors with minimal evidence had an OR of 1.0-1.5 (0.9-1.0 if protective) and were significant.
Discussion
In this systematic review and meta-analysis, the main findings were that diabetes, revision ACLR, HT autograft, professional athlete status, lateral tenodesis, and steroid use provided strong evidence for the occurrence of infections after ACLR. There was moderate evidence to suggest that male sex was an important risk factor for infections after ACLR, while age, BMI, smoking, meniscal repair, and outpatient surgery were not associated with an increased risk of infections after ACLR. The results of the subgroup analysis suggested that diabetes was a risk factor for overall infections but not specifically for septic arthritis.
Our study demonstrated that male patients exhibited 90% higher odds (OR, 1.90) of experiencing an infection after ACLR compared to their female counterparts. A couple of investigations conducted by Roecker et al 51 and Kraus Schmitz et al 31 on 217,541 and 25,309 patients, respectively, further bolster our findings, with male patients displaying a greater susceptibility to infections (OR, 1.58 and 1.65, respectively). Sex-related differences in daily life and sports intensity likely account for our findings. 60 This is also similar to the findings on risk factors for a periprosthetic infection after total knee arthroplasty14,25,26,46 in which male sex has been demonstrated to be an independent risk factor for infections. However, based on the present data, we cannot explain whether this difference is because of the sex itself or as a proxy for some risk factors. Although we cannot speculate on causation from systematic reviews, our conclusions highlight that orthopaedic surgeons should recognize that male patients are an at-risk population for infections after ACLR.
The outcomes of our meta-analysis indicated a 2.69-fold elevated risk of infections after ACLR among patients with diabetes. Patients with diabetes, especially those with suboptimal glycemic control, have been well established to be associated with an increased risk of SSIs after multiple orthopaedic procedures, including total joint arthroplasty,2,30 spinal surgery,18,35 and ankle surgery. 47 Diabetes-increased susceptibility to infections is related to impaired immune responses within the hyperglycemic environment and microvascular complications. In addition, microvascular lesions leading to local tissue ischemia and factors associated with diabetes, including hypertension, increased oxidative stress, and inflammatory responses, also impede wound healing.17,49 It is intriguing that when only cases of septic arthritis were included, diabetes appeared not to be significantly associated with septic arthritis after ACLR. This may be because of the lack of sufficient data, with only 3 studies included in this comparison. Alternatively, diabetes may primarily impair the healing of superficial surgical incisions, and its effect on infections within the joint is not significant. However, the lack of descriptions for the type of diabetes in the included studies limited further discussions.
The use of HT autografts compared with other grafts (bone–patellar tendon–bone autografts or allografts) increased the risk of infections after ACLR (OR, 2.51). Because a possible source of septic arthritis after ACLR is contaminated grafts, several studies have investigated the infection rate depending on the choice of grafts. The contamination rate during autograft preparation was found to be higher for HT autografts compared to bone–patellar tendon–bone autografts (13% vs 10%, respectively). 22 The sources of graft contamination, either during the harvest process or the preparation phase on the back table, remain indeterminate. It is worth noting that the preparation time for HT autografts exceeds that of other grafts, consequently augmenting the window for potential contamination during graft preparation.19,21 The utilization of multifilament suture during the preparation of HT grafts is commonplace, despite its proclivity to serve as a vector for bacterial colonization. Additionally, bacterial contamination may ensue during the graft implantation phase, particularly if the graft or hardware is in proximity with the skin. 39 Bacterial cultures were performed in 8 of the 17 included studies, and the most common pathogen was Staphylococcus epidermidis8,27,56 or S. aureus.6,24,39,57 This finding also confirms the conclusions of the previous literature.1,5 Given recent evidence supporting a significant reduction in the incidence of infections after ACLR by soaking grafts in vancomycin,13,45 surgeons may consider using this prophylactic measure, especially for high-risk grafts. We acknowledge the fact that the use of specific grafts increases the risk of infections, but given the very low overall infection rate, the choice of grafts still requires a comprehensive evaluation.
Revision ACLR was found to be associated with an increased risk of infections after ACLR. Specifically, the OR was 2.31, suggesting that revision ACLR was 131% more likely to result in infections compared with primary ACLR. This is consistent with the results previously reported by Schuster et al 54 in that the ratio of revision to primary reconstruction for postoperative septic arthritis was 2.5. An analysis of 16,192 ACLR procedures by Maletis et al 38 found that a deep SSI developed in 0.3% of primary ACLR cases versus 0.9% of revision cases. This may be caused by the longer operative time and more complex procedures for revision surgery compared with primary ACLR.15,20 Recently, some strategies to prevent infections after revision ACLR have been explored, including the use of quadriceps tendon 53 and vancomycin-soaked grafts. 52
Our results showed that patients who were professional athletes were at a higher risk of infections after ACLR (OR, 6.21). This finding corroborates data reported in the previous literature that explored whether professional athletes are susceptible to infections. Sonnery-Cottet et al 57 found that being a professional player was associated with a significantly increased risk of infections after ACLR (OR, 21.0). Ristić et al 50 also reported a higher rate of infections in professional athletes (1.9%) than in nonprofessional athletes (0.8%). Conversely, Bohu et al 8 reported no significant difference in infection rates between the 2 groups, and no special precautions were required in professional athletes. However, this conclusion must be interpreted with caution, as the post hoc calculation suggested that the power of their study was close to zero. Krutsch et al 32 also found that the difference in postoperative infection rates did not depend on whether the athletes were professional but rather on the type of sports. Athletes who participate in summer outdoor sports (eg, football) have a significantly higher risk of infections after ACLR than athletes in winter sports. Possible reasons include external risk factors such as protective clothing at the time of the injury 10 or higher infection rates in athletes injured by frequent skin-to-skin contact. 60 Another explanation could be differences in skin bacterial colonization due to variations in temperature and sweat excretion depending on participation in different sports. 32
Patients who underwent combined lateral tenodesis at the time of ACLR were 3.45 times more likely to develop infections after ACLR than patients without lateral tenodesis. Potential explanations for the higher infection rate include increased operating time, the size of skin incisions, and the number of implants. However, several studies have reported opposite results, indicating that the infection rate for combined intra- and extra-articular reconstruction is not higher than that for isolated intra-articular ACLR.36,48 Our finding should be interpreted with caution because in the study by Sonnery-Cottet et al, 56 only 9% of nonprofessional athletes underwent lateral tenodesis, whereas 23% of professional athletes did. In the nonprofessional group, no patients had an infection after undergoing ACLR with combined lateral tenodesis. The strong correlation between lateral tenodesis and professional athletes suggests that one of the variables is a confounding factor. Sonnery-Cottet et al 56 speculated that it is this variable in professional athletes that plays a major role, conferring a higher infection rate. This is in accordance with the results of the current meta-analysis.
Our results showed that patients with corticosteroid use were more likely to develop infections after ACLR (OR, 7.83). However, only 2 studies29,64 were included in this meta-analysis, with a relatively small number of patients with postoperative infections. Considerable caution is needed when interpreting the results for steroid use. Kawata et al 29 focused on patients with regular preoperative steroid use, while Westermann et al 64 did not elucidate the administration of steroids. Neither mentioned the dose of steroid use. Steroids are frequently used to prevent postoperative nausea and vomiting. 43 They also confer other benefits, including postoperative pain relief 34 and reduced postoperative postural hypotension. 3 Previous studies have shown that intraoperative steroid injections increase the risk of SSIs after arthroscopic surgery, 4 but there is a paucity of literature on the systemic administration of steroids in the perioperative period of ACLR. However, the lack of specification on the mode of steroid administration in the included studies limited further discussions.
Limitations
The current study has some limitations that need to be noted. First, different study designs, included populations, and types of infections contributed to the heterogeneity of the review, but we conducted sensitivity and subgroup analyses to explore the sources of heterogeneity as much as possible. Second, most of the included studies identified cases of infections after ACLR based on disease diagnosis codes rather than clinical assessments. Possible coding errors, in addition to the fact that patients may not return to their original institution for treatment of the infection, can lead us to underestimate the incidence of infections. Third, the different techniques used to perform the procedure, the various surgeons, and the antibiotic regimen to treat infections have not been discussed, creating confounding bias and limiting the generalizability of this study. In addition, many studies have shown a significant reduction in infection rates with vancomycin-soaked grafts,13,45,65 but this factor was not analyzed in the studies that we included. Because the earliest study using vancomycin-soaked grafts was conducted in approximately 2012 61 and most of our included studies were performed earlier than this time point, none of them routinely applied this new technique. Future studies with more recent data will hopefully shed light on this issue. Fourth, some of the included studies provided only univariate rather than multivariate statistics, which may lead to some bias in our analysis. An infection after ACLR is caused by multiple factors that are interrelated and interdependent, and it cannot be explained by every single factor.
Conclusion
Our study revealed an increased risk of infections after ACLR associated with male sex, diabetes, HT autograft, revision surgery, professional athlete status, lateral tenodesis, and corticosteroid use. There was no significant association between age, BMI, smoking, meniscal repair, and outpatient surgery with infections after ACLR. Knowledge of the risk factors associated with an infection after ACLR may facilitate the identification of high-risk cases and the implementation of preventive measures to mitigate the serious consequences of this complication.
Appendix
Appendix Table A1.
Search Strategy According to Database
|
PubMed (324 results):
((((((“Risk Factors”[Mesh]) OR (risk[Title/Abstract])) OR (associated with[Title/Abstract])) OR (correlated with[Title/Abstract])) OR (predictor[Title/Abstract])) AND (((“Anterior Cruciate Ligament”[Mesh]) OR (“Anterior Cruciate Ligament Reconstruction”[Mesh])) OR (((anterior cruciate ligament[Title/Abstract]) OR (anterior cruciate ligament reconstruction[Title/Abstract])) OR (acl[Title/Abstract])))) AND ((“Arthritis, Infectious”[Mesh]) OR ((infection[Title/Abstract]) OR (septic arthritis[Title/Abstract]))) |
|
Embase (185 results):
1. ‘risk factor’/exp 2. ‘risk factors’:ab,ti 3. ‘associated with’:ab,ti 4. ‘correlated with’:ab,ti 5. predictors:ab,ti 6. #1 OR #2 OR #3 OR #4 OR #5 7. ‘anterior cruciate ligament’/exp 8. ‘anterior cruciate ligament reconstruction’/exp 9. ‘anterior cruciate ligament’:ab,ti 10. ‘anterior cruciate ligament reconstruction’:ab,ti 11. #7 OR #8 OR #9 OR #10 12. infection:ab,ti 13. ‘septic arthritis’:ab,ti 14. #12 OR #13 15. #6 AND #11 AND #14 |
|
Web of Science (325 results):
1. AB=(Risk Factors OR risk OR associated with OR correlated with OR predictor) 2. AB=(anterior cruciate ligament OR anterior cruciate ligament reconstruction OR acl) 3. AB=(infection OR septic arthritis) 4. #3 AND #2 AND #1 |
Appendix Table A2.
MINORS Scores a
| Study | MINORS Item b | Total Score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | ||
| Crawford 16 (2005) | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 1 | 2 | 2 | 1 | 1 | 16/24 |
| Judd 27 (2006) | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 0 | NA | NA | NA | NA | 10/16 |
| Katz 28 (2008) | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 0 | 2 | 2 | 1 | 1 | 18/24 |
| Barker 6 (2010) | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 0 | 2 | 2 | 2 | 1 | 19/24 |
| Sonnery-Cottet 56 (2011) | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 2 | 1 | 18/24 |
| Maletis 39 (2013) | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 19/24 |
| Brophy 9 (2015) | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 1 | 2 | 18/24 |
| Cancienne 12 (2016) | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 18/24 |
| Murphy 44 (2016) | 2 | 2 | 2 | 2 | 1 | 0 | 1 | 0 | 2 | 2 | 2 | 1 | 17/24 |
| Krutsch 32 (2017) | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 0 | 1 | 2 | 2 | 1 | 16/24 |
| Westermann 64 (2017) | 2 | 2 | 2 | 2 | 1 | 0 | 1 | 0 | 2 | 2 | 1 | 2 | 17/24 |
| Kawata 29 (2018) | 2 | 2 | 2 | 2 | 1 | 0 | 1 | 0 | 1 | 2 | 2 | 2 | 17/24 |
| Bohu 8 (2019) | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 2 | 2 | 2 | 0 | 17/24 |
| Sonnery-Cottet 57 (2019) | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 0 | 1 | 2 | 1 | 2 | 18/24 |
| Hurvitz 24 (2020) | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 0 | 2 | 2 | 1 | 2 | 19/24 |
| Kraus Schmitz 31 (2021) | 2 | 2 | 2 | 2 | 1 | 0 | 1 | 0 | 2 | 2 | 2 | 1 | 17/24 |
| Marom 40 (2022) | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 0 | 2 | 2 | 2 | 2 | 20/24 |
The items are scored as 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). MINORS, Methodological Index for Non-Randomized Studies; NA, not applicable.
MINORS items: (1) clearly stated aim, (2) inclusion of consecutive patients, (3) prospective collection of data, (4) endpoints appropriate to the aim of the study, (5) unbiased assessment of the study endpoint, (6) follow-up period appropriate to the aim of the study, (7) loss to follow-up <5%, and (8) prospective calculation of the study size. Additional criteria for comparative studies: (9) adequate control group, (10) contemporary groups, (11) baseline equivalence of groups, and (12) adequate statistical analyses.
Appendix Table A3.
Qualitative Analysis of 11 Risk Factors a
| Study | Risk Factor | Findings |
|---|---|---|
| Murphy 44 (2016) | • Connective tissue disorder • Immunosuppressive medications |
• Connective tissue disorder was a significant independent risk factor for infections after ACLR (OR, 21.7 [95% CI, 3.7-126.3]). • The use of immunosuppressive medications was a significant independent risk factor for infections after ACLR (OR, 6.7 [95% CI, 1.3-34.8]). |
| Westermann 64 (2017) | Postoperative hospital admission | On multivariate analysis, the only independent predictor of postoperative infections was hospital admission after surgery (OR, 2.67 [95% CI, 1.02-6.96]; P < .04). |
| Kawata 29 (2018) | Atopic dermatitis | Atopic dermatitis was a novel independent risk factor for SSIs after ACLR (OR, 7.19 [95% CI, 2.94-17.57]). |
| Bohu 8 (2019) | • Prior knee surgery • Hemarthrosis during immediate postoperative period |
Only prior knee surgery (OR, 15.0 [95% CI, 2.7-84.1]; P = .002) and the occurrence of immediate postoperative hemarthrosis (OR, 127.2 [95% CI, 16.0-1011.3]) were significantly associated with the development of septic arthritis on multivariate analysis. |
| Hurvitz 24 (2020) | Screw-and-sheath tibial fixation | HT autografts with a screw and sheath had a higher likelihood of 90-day deep infections compared with BPTB grafts (OR, 2.87 [95% CI, 1.29-6.38]). There was no difference between HT autografts without a screw and sheath and BPTB grafts (OR, 1.23 [95% CI, 0.54-2.77]). |
| Kraus Schmitz 31 (2021) | Longer operating time | An independent risk factor for septic arthritis was an operating time of ≥70 minutes (OR, 1.83 [95% CI, 1.42-2.36]). |
ACLR, anterior cruciate ligament reconstruction; BPTB, bone–patellar tendon–bone; HT, hamstring tendon; OR, odds ratio; SSI, surgical site infection.
Footnotes
Final revision submitted May 9, 2023; accepted May 19, 2023.
The authors have declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Alomar AZ, Alfayez SM, Somily AM. Hamstring autografts are associated with a high rate of contamination in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(5):1357-1361. [DOI] [PubMed] [Google Scholar]
- 2. Amusat N, Beaupre L, Jhangri GS, et al. Diabetes that impacts on routine activities predicts slower recovery after total knee arthroplasty: an observational study. J Physiother. 2014;60(4):217-223. [DOI] [PubMed] [Google Scholar]
- 3. Ashoor TM, Hussien NS, Anis SG, Esmat IM. Dexamethasone blunts postspinal hypotension in geriatric patients undergoing orthopedic surgery: a double blind, placebo-controlled study. BMC Anesthesiol. 2021;21(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Babcock HM, Carroll C, Matava M, L’Ecuyer P, Fraser V. Surgical site infections after arthroscopy: outbreak investigation and case control study. Arthroscopy. 2003;19(2):172-181. [DOI] [PubMed] [Google Scholar]
- 5. Badran MA, Moemen DM. Hamstring graft bacterial contamination during anterior cruciate ligament reconstruction: clinical and microbiological study. Int Orthop. 2016;40(9):1899-1903. [DOI] [PubMed] [Google Scholar]
- 6. Barker JU, Drakos MC, Maak TG, et al. Effect of graft selection on the incidence of postoperative infection in anterior cruciate ligament reconstruction. Am J Sports Med. 2010;38(2):281-286. [DOI] [PubMed] [Google Scholar]
- 7. Benner RW, Shelbourne KD, Freeman H. Infections and patellar tendon ruptures after anterior cruciate ligament reconstruction: a comparison of ipsilateral and contralateral patellar tendon autografts. Am J Sports Med. 2011;39(3):519-525. [DOI] [PubMed] [Google Scholar]
- 8. Bohu Y, Klouche S, Herman S, et al. Professional athletes are not at a higher risk of infections after anterior cruciate ligament reconstruction: incidence of septic arthritis, additional costs, and clinical outcomes from the French Prospective Anterior Cruciate Ligament Study (FAST) cohort. Am J Sports Med. 2019;47(1):104-111. [DOI] [PubMed] [Google Scholar]
- 9. Brophy RH, Wright RW, Huston LJ, Nwosu SK, Spindler KP. Factors associated with infection following anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2015;97(6):450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brucker PU, Katzmaier P, Olvermann M, et al. [Recreational and competitive alpine skiing: typical injury patterns and possibilities for prevention]. Article in German. Unfallchirurg. 2014;117(1):24-32. [DOI] [PubMed] [Google Scholar]
- 11. Cadet ER, Makhni EC, Mehran N, Schulz BM. Management of septic arthritis following anterior cruciate ligament reconstruction: a review of current practices and recommendations. J Am Acad Orthop Surg. 2013;21(11):647-656. [DOI] [PubMed] [Google Scholar]
- 12. Cancienne JM, Gwathmey FW, Miller MD, Werner BC. Tobacco use is associated with increased complications after anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(1):99-104. [DOI] [PubMed] [Google Scholar]
- 13. Carrozzo A, Saithna A, Ferreira A, et al. Presoaking ACL grafts in vancomycin decreases the frequency of postoperative septic arthritis: a cohort study of 29,659 patients, systematic review, and meta-analysis from the SANTI Study Group. Orthop J Sports Med. 2022;10(2):23259671211073928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen J, Cui Y, Li X, et al. Risk factors for deep infection after total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2013;133(5):675-687. [DOI] [PubMed] [Google Scholar]
- 15. Clement RC, Haddix KP, Creighton RA, et al. Risk factors for infection after knee arthroscopy: analysis of 595,083 cases from 3 United States databases. Arthroscopy. 2016;32(12):2556-2561. [DOI] [PubMed] [Google Scholar]
- 16. Crawford C, Kainer M, Jernigan D, et al. Investigation of postoperative allograft-associated infections in patients who underwent musculoskeletal allograft implantation. Clin Infect Dis. 2005;41(2):195-200. [DOI] [PubMed] [Google Scholar]
- 17. Evangelista V, Totani L, Rotondo S, et al. Prevention of cardiovascular disease in type-2 diabetes: how to improve the clinical efficacy of aspirin. Thromb Haemost. 2005;93(1):8-16. [DOI] [PubMed] [Google Scholar]
- 18. Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976). 2005;30(12):1460-1465. [DOI] [PubMed] [Google Scholar]
- 19. Gobbi A, Mahajan S, Zanazzo M, Tuy B. Patellar tendon versus quadrupled bone-semitendinosus anterior cruciate ligament reconstruction: a prospective clinical investigation in athletes. Arthroscopy. 2003;19(6):592-601. [DOI] [PubMed] [Google Scholar]
- 20. Gowd AK, Liu JN, Bohl DD, et al. Operative time as an independent and modifiable risk factor for short-term complications after knee arthroscopy. Arthroscopy. 2019;35(7):2089-2098. [DOI] [PubMed] [Google Scholar]
- 21. Greis PE, Koch BS, Adams B. Tibialis anterior or posterior allograft anterior cruciate ligament reconstruction versus hamstring autograft reconstruction: an economic analysis in a hospital-based outpatient setting. Arthroscopy. 2012;28(11):1695-1701. [DOI] [PubMed] [Google Scholar]
- 22. Hantes ME, Basdekis GK, Varitimidis SE, et al. Autograft contamination during preparation for anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2008;90(4):760-764. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hurvitz AP, Prentice HA, Funahashi TT, Maletis GB. Screw and sheath tibial fixation associated with a higher likelihood of deep infection after hamstring graft anterior cruciate ligament reconstruction. Am J Sports Med. 2020;48(4):806-811. [DOI] [PubMed] [Google Scholar]
- 25. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty: a register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47. [DOI] [PubMed] [Google Scholar]
- 26. Jämsen E, Varonen M, Huhtala H, et al. Incidence of prosthetic joint infections after primary knee arthroplasty. J Arthroplasty. 2010;25(1):87-92. [DOI] [PubMed] [Google Scholar]
- 27. Judd D, Bottoni C, Kim D, Burke M, Hooker S. Infections following arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2006;22(4):375-384. [DOI] [PubMed] [Google Scholar]
- 28. Katz LM, Battaglia TC, Patino P, et al. A retrospective comparison of the incidence of bacterial infection following anterior cruciate ligament reconstruction with autograft versus allograft. Arthroscopy. 2008;24(12):1330-1335. [DOI] [PubMed] [Google Scholar]
- 29. Kawata M, Sasabuchi Y, Taketomi S, et al. Atopic dermatitis is a novel demographic risk factor for surgical site infection after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(12):3699-3705. [DOI] [PubMed] [Google Scholar]
- 30. Kong L, Cao J, Zhang Y, Ding W, Shen Y. Risk factors for periprosthetic joint infection following primary total hip or knee arthroplasty: a meta-analysis. Int Wound J. 2017;14(3):529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kraus Schmitz J, Lindgren V, Edman G, et al. Risk factors for septic arthritis after anterior cruciate ligament reconstruction: a nationwide analysis of 26,014 ACL reconstructions. Am J Sports Med. 2021;49(7):1769-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krutsch W, Zellner J, Zeman F, et al. Sports-specific differences in postsurgical infections after arthroscopically assisted anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(12):3878-3883. [DOI] [PubMed] [Google Scholar]
- 33. Kunze KN, Wright-Chisem J, Polce EM, et al. Risk factors for ramp lesions of the medial meniscus: a systematic review and meta-analysis. Am J Sports Med. 2021;49(13):3749-3757. [DOI] [PubMed] [Google Scholar]
- 34. Li D, Wang C, Yang Z, Kang P. Effect of intravenous corticosteroids on pain management and early rehabilitation in patients undergoing total knee or hip arthroplasty: a meta-analysis of randomized controlled trials. Pain Pract. 2018;18(4):487-499. [DOI] [PubMed] [Google Scholar]
- 35. Liu JM, Deng HL, Chen XY, et al. Risk factors for surgical site infection after posterior lumbar spinal surgery. Spine (Phila Pa 1976). 2018;43(10):732-737. [DOI] [PubMed] [Google Scholar]
- 36. Lutz C. Role of anterolateral reconstruction in patients undergoing anterior cruciate ligament reconstruction. Orthop Traumatol Surg Res. 2018;104(1S):S47-S53. [DOI] [PubMed] [Google Scholar]
- 37. Makhni EC, Steinhaus ME, Mehran N, Schulz BS, Ahmad CS. Functional outcome and graft retention in patients with septic arthritis after anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2015;31(7):1392-1401. [DOI] [PubMed] [Google Scholar]
- 38. Maletis GB, Inacio MC, Funahashi TT. Analysis of 16,192 anterior cruciate ligament reconstructions from a community-based registry. Am J Sports Med. 2013;41(9):2090-2098. [DOI] [PubMed] [Google Scholar]
- 39. Maletis GB, Inacio MC, Reynolds S, et al. Incidence of postoperative anterior cruciate ligament reconstruction infections: graft choice makes a difference. Am J Sports Med. 2013;41(8):1780-1785. [DOI] [PubMed] [Google Scholar]
- 40. Marom N, Kapadia M, Nguyen JT, et al. Factors associated with an intra-articular infection after anterior cruciate ligament reconstruction: a large single-institution cohort study. Am J Sports Med. 2022;50(5):1229-1236. [DOI] [PubMed] [Google Scholar]
- 41. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mouzopoulos G, Fotopoulos VC, Tzurbakis M. Septic knee arthritis following ACL reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1033-1042. [DOI] [PubMed] [Google Scholar]
- 43. Movafegh A, Soroush AR, Navi A, et al. The effect of intravenous administration of dexamethasone on postoperative pain, nausea, and vomiting after intrathecal injection of meperidine. Anesth Analg. 2007;104(4):987-989. [DOI] [PubMed] [Google Scholar]
- 44. Murphy MV, Du DT, Hua W, et al. Risk factors for surgical site infections following anterior cruciate ligament reconstruction. Infect Control Hosp Epidemiol. 2016;37(7):827-833. [DOI] [PubMed] [Google Scholar]
- 45. Naendrup JH, Marche B, de Sa D, et al. Vancomycin-soaking of the graft reduces the incidence of septic arthritis following ACL reconstruction: results of a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1005-1013. [DOI] [PubMed] [Google Scholar]
- 46. Namba RS, Inacio MC, Paxton EW. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am. 2013;95(9):775-782. [DOI] [PubMed] [Google Scholar]
- 47. Ovaska MT, Mäkinen TJ, Madanat R, et al. Risk factors for deep surgical site infection following operative treatment of ankle fractures. J Bone Joint Surg Am. 2013;95(4):348-353. [DOI] [PubMed] [Google Scholar]
- 48. Panisset JC, Pailhé R, Schlatterer B, et al. Short-term complications in intra- and extra-articular anterior cruciate ligament reconstruction: comparison with the literature on isolated intra-articular reconstruction. A multicenter study by the French Arthroscopy Society. Orthop Traumatol Surg Res. 2017;103(8S):S231-S236. [DOI] [PubMed] [Google Scholar]
- 49. Reusch JEB. Diabetes, microvascular complications, and cardiovascular complications: what is it about glucose? J Clin Invest. 2003;112(7):986-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ristić V, Maljanović M, Harhaji V, Milankov M. Infections after reconstructions of anterior cruciate ligament. Med Pregl. 2014;67(1-2):11-15. [PubMed] [Google Scholar]
- 51. Roecker Z, Kamalapathy P, Werner BC. Male sex, cartilage surgery, tobacco use, and opioid disorders are associated with an increased risk of infection after anterior cruciate ligament reconstruction. Arthroscopy. 2022;38(3):948-952.e1. [DOI] [PubMed] [Google Scholar]
- 52. Schuster P, Schlumberger M, Mayer P, et al. Lower incidence of post-operative septic arthritis following revision anterior cruciate ligament reconstruction with quadriceps tendon compared to hamstring tendons. Knee Surg Sports Traumatol Arthrosc. 2020;28(8):2572-2577. [DOI] [PubMed] [Google Scholar]
- 53. Schuster P, Schlumberger M, Mayer P, et al. Soaking of autografts in vancomycin is highly effective in preventing postoperative septic arthritis after revision anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1154-1158. [DOI] [PubMed] [Google Scholar]
- 54. Schuster P, Schulz M, Immendoerfer M, et al. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: evaluation of an arthroscopic graft-retaining treatment protocol. Am J Sports Med. 2015;43(12):3005-3012. [DOI] [PubMed] [Google Scholar]
- 55. Snoeker BA, Bakker EW, Kegel CA, Lucas C. Risk factors for meniscal tears: a systematic review including meta-analysis. J Orthop Sports Phys Ther. 2013;43(6):352-367. [DOI] [PubMed] [Google Scholar]
- 56. Sonnery-Cottet B, Archbold P, Zayni R, et al. Prevalence of septic arthritis after anterior cruciate ligament reconstruction among professional athletes. Am J Sports Med. 2011;39(11):2371-2376. [DOI] [PubMed] [Google Scholar]
- 57. Sonnery-Cottet B, Saithna A, Abreu FG, et al. Professional athletes are at higher risk of septic arthritis after anterior cruciate ligament reconstruction: an analysis of 4421 consecutive patients including 265 elite athletes from the SANTI Study Group. Am J Sports Med. 2019;47(12):2910-2918. [DOI] [PubMed] [Google Scholar]
- 58. Stucken C, Garras DN, Shaner JL, Cohen SB. Infections in anterior cruciate ligament reconstruction. Sports Health. 2013;5(6):553-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Torres-Claramunt R, Pelfort X, Erquicia J, et al. Knee joint infection after ACL reconstruction: prevalence, management and functional outcomes. Knee Surg Sports Traumatol Arthrosc. 2013;21(12):2844-2849. [DOI] [PubMed] [Google Scholar]
- 60. Turbeville SD, Cowan LD, Greenfield RA. Infectious disease outbreaks in competitive sports: a review of the literature. Am J Sports Med. 2006;34(11):1860-1865. [DOI] [PubMed] [Google Scholar]
- 61. Vertullo CJ, Quick M, Jones A, Grayson JE. A surgical technique using presoaked vancomycin hamstring grafts to decrease the risk of infection after anterior cruciate ligament reconstruction. Arthroscopy. 2012;28(3):337-342. [DOI] [PubMed] [Google Scholar]
- 62. Wang C, Ao Y, Wang J, et al. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: a retrospective analysis of incidence, presentation, treatment, and cause. Arthroscopy. 2009;25(3):243-249. [DOI] [PubMed] [Google Scholar]
- 63. Wang C, Lee YH, Siebold R. Recommendations for the management of septic arthritis after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2136-2144. [DOI] [PubMed] [Google Scholar]
- 64. Westermann R, Anthony CA, Duchman KR, et al. Infection following anterior cruciate ligament reconstruction: an analysis of 6,389 cases. J Knee Surg. 2017;30(6):535-543. [DOI] [PubMed] [Google Scholar]
- 65. Xiao M, Sherman SL, Safran MR, Abrams GD. Significantly lower infection risk for anterior cruciate ligament grafts presoaked in vancomycin compared with unsoaked grafts: a systematic review and meta-analysis. Arthroscopy. 2021;37(5):1683-1690. [DOI] [PubMed] [Google Scholar]