Abstract
Abbreviated pathogenesis and clinical course of the acute liver failure syndrome. The pathogenesis and clinical course of the syndrome of acute liver failure (ALF) differs depending upon the etiology of the primary liver injury. In turn, the severity of the liver injury and resulting synthetic failure is often the primary determinant of whether a patient is referred for emergency liver transplantation. Injuries by viral etiologies trigger the innate immune system via pathogen-associated molecular patterns (PAMPs), while toxin-induced (and presumably ischemia-induced) injuries do so via damage-associated molecular patterns (DAMPs). The course of the clinical syndrome further depends upon the relative intensity and composition of cytokine release, resulting in an early proinflammatory phenotype (SIRS) and later compensatory anti-inflammatory response phenotype (CARS). The outcomes of overwhelming immune activation are the systemic (extrahepatic) features of ALF (cardiovascular collapse, cerebral edema, acute kidney injury, respiratory failure, sepsis) which ultimately determine the likelihood of death.
Acute liver failure (ALF) continues to carry a high risk of mortality or the need for transplantation despite recent improvements in overall outcomes over the past two decades. Optimal management begins with identifying that liver failure is indeed present and its etiology, since outcomes and the need for transplantation vary widely across the different etiologies. Most causes of ALF can be divided into hyperacute (ischemia and acetaminophen) and subacute types (other etiologies), based on time of evolution of signs and symptoms of liver failure; the former evolve in 3 to 4 days and the latter typically in 2 to 4 weeks. Both involve intense release of cytokines and hepatocellular contents into the circulation with multiorgan effects/consequences.
Management involves optimizing fluid balance and cardiovascular support, including the use of continuous renal replacement therapy, vasopressors, and pulmonary ventilation. Early evaluation for liver transplantation is advised particularly for acetaminophen toxicity, which evolves so rapidly that delay is likely to lead to death.
Vasopressor support, high-grade hepatic encephalopathy, and unfavorable (subacute) etiologies heighten the need for urgent listing for liver transplantation. Prognostic scores such as Kings Criteria, Model for End-Stage Liver Disease, and the Acute Liver Failure Group prognostic index take these features into account and provide reasonable but imperfect predictive accuracy. Future treatments may include liver support devices and/or agents that improve hepatocyte regeneration.
Keywords: encephalopathy, coagulopathy, hepatocyte injury, renal replacement therapy, prognostic score
Graphical Abstract
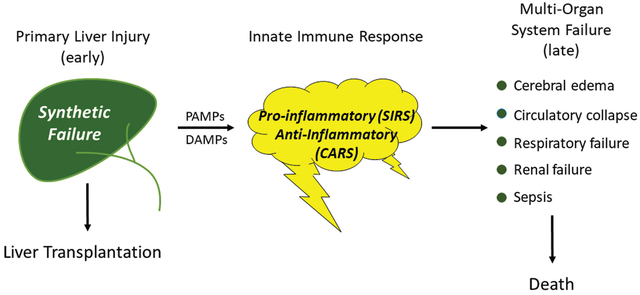
The past 40 years has seen remarkable changes in all of medicine and in particular the management of patients with acute liver failure (ALF). ALF is the term applied to a cluster of diseases that all cause rapid evolution of liver injury to liver failure with similar signs and symptoms. Despite encompassing several etiologies, ALF remains an orphan or perhaps a super-orphan disease, affecting only approximately 2 to 3,000 per year in North America. Hallmark features include coagulopathy with increased prothrombin time/international normalized ratio (INR) as well as altered mentation (hepatic encephalopathy [HE], graded for severity from 0 to 4). Interest in ALF remains high due to these dramatic presentation features as well as its high overall mortality. Prior to the 1980s and 1990s, more than 90% of patients with ALF died within weeks of disease onset.1 With the advent of liver transplantation, outcomes began to improve but mortality still hovers around 30% in Europe and North America and is considerably higher in underdeveloped countries.2,3 ALF is a distinct clinical entity which should not be confused with acute-on-chronic-liver-failure,4 since the latter condition is characterized by the presence of underlying cirrhosis and complications thereof, while the definition of ALF excludes patients with cirrhosis except in specific conditions which present as ALF in a patient with unrecognized sub-clinical chronic liver disease (acute Wilson disease, acute autoimmune hepatitis [AIH], and reactivation of chronic hepatitis B). The current update will begin with a brief review of the overall spectrum (and evolution) of etiologies and their pathogenesis, before focusing on current management practices, and an assessment of current prognostic modeling and the role of liver transplantation for ALF.
Section I: Etiologies and Diagnosis
Case series beginning in the 1990s have highlighted the central role of acetaminophen (APAP; also called paracetamol in Europe) as the dominant etiology in Western countries, including North America, and much of Northern Europe.5 Next most common is usually idiosyncratic drug-induced liver injuries (DILIs),6 followed by viral hepatitis, and a raft of smaller numbers of cases of AIH, Wilson disease, ALF during pregnancy, and a host of even rarer agents.7 By contrast, the Middle East, Asia, South America, and Africa (where records are available) vary from country-to-country with hepatitis B and E represented prominently, with many fewer DILI or APAP cases.8 Herbal and dietary supplements (HDSs) have become a much larger problem in North America and in Asia in recent decades.9
Over the 20+ years of the Acute Liver Failure Study Group (ALFSG) registry, 1998 to 2018, there has been little change in the overall pattern of etiologies (Fig. 1). The incidence of APAP-induced ALF has remained steady over the years at around 46% of all adult cases. That said, there is some evidence from the United Kingdom that APAP has declined due to better public information concerning its toxicity and legal limitations on package size, neither of which have been implemented in North America. Over the two decades, the percentages in the ALFSG registry showed DILI remaining roughly steady at approximately 11%, hepatitis B 8%, and hepatitis A 3%. Over the 20-year period, however, hepatitis A and B initially began to decline somewhat, thought due to vaccination for the overall population, followed by a recent increase in the annual number of cases of infection of both A and B, apparently linked to the opioid crisis. An additional part of the increase in hepatitis B-related ALF is due to cases of reactivation in the setting of immunosuppressive medications, including cancer chemotherapy or other biologics.10 Hepatitis A requiring hospitalization has increased in recent years in association with injection drug use and homelessness,11 but this has not appeared to translate to more cases of full-blown hepatitis A-related ALF, at least in our experience: no hepatitis A ALF cases were registered between 2015 and 2019 by the ALFSG while nine acute liver injury (ALI) cases (INR ≥ 2.0, no encephalopathy) were recorded in the same period.12 Within the overall DILI group, the mix of drugs implicated has evolved; antibiotics remain the most common overall group, but the incidence of HDSs has doubled in the past decade from 10 to 20%.13 Overall, ALF due to HDS are associated with worse overall outcomes than other forms of DILI and will be discussed in more detail below. Certain agents are less commonly used and thus less frequently implicated. Medications that are declining in use/incidence include phenytoin, isoniazid, nitrofurantoin, and the early and toxic highly active antiretroviral agents.13
Fig. 1.
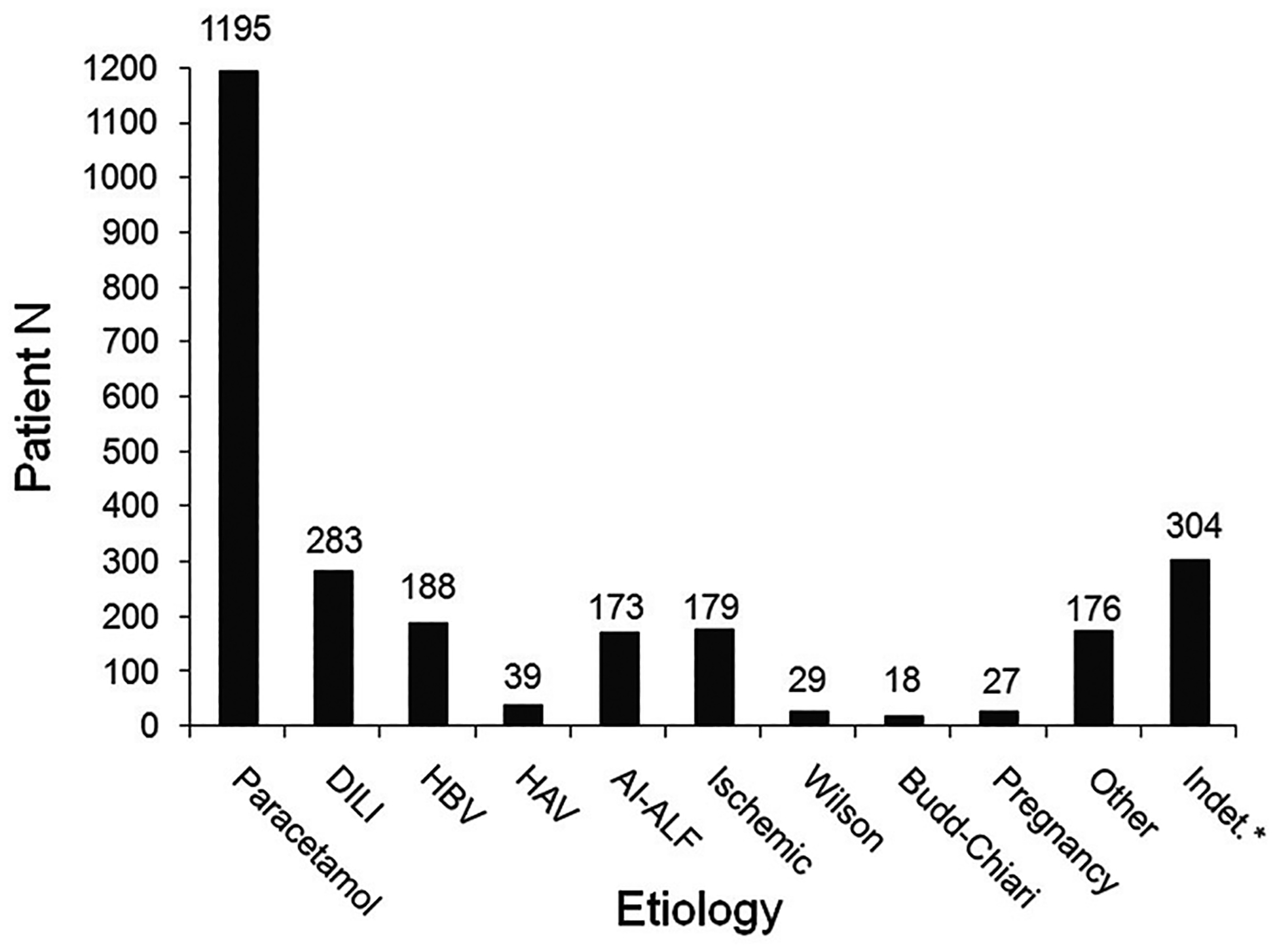
Incidence of specific etiologies of acute liver failure (ALF) as determined by the site principal investigator (PI) in the Adult Acute Liver Failure Study Group Registry: January 1998–March 2019.
In summary, evolution of different etiologies over the past two decades has been relatively minimal for overall groups (APAP, DILI, AIH) while changes within the groups have been seen. We are not aware of differing trends in etiologies worldwide over this same time period.
Making the Correct Etiologic Diagnosis
It is vitally important to discern the correct etiology since antidotes and prognosis are both tied to the cause of the liver injury from first principles. N-acetylcysteine (NAC) is the recognized antidote for APAP poisoning but ideally must be given within 12 hours of ingestion of a single dose with no clear timing requirement (early is best) for unintentional cases.14 Other therapies such as corticosteroids (CS) for AIH and nucleoside analogs for hepatitis B are of uncertain value (see below), and do not preclude listing and ultimately transplantation.
There are two consistent clinical syndromes that account for all etiologies: “hyperacute” which includes APAP and ischemic liver injury, and “subacute” which includes nearly everything else: AIH, viral hepatitis, and DILI (Table 1).7 The table highlights the differences between the hyperacute and subacute groups, in terms of onset, laboratory values, and outcomes, which are vastly different. The hyperacute group evolves over 2 to 4 days and resolution occurs by day 5 or 6.7 In the case of APAP and most ischemic injuries, by day 5, either death or transplantation has occurred, or the patient is ready to be discharged! APAP cases are never subacute, and recovery rarely is prolonged to 2 weeks or more. By contrast, the subacute group typically has been ill for at least 2 weeks before seeking medical care or evolving tosigns of ALF. History-taking is vital to discern exposure to possible drugs, toxins, or viruses; however, since patients have altered sensorium, it may be difficult or impossible to obtain a reliable history. The availability of serologic testing for most viruses is paramount if clarity of diagnosis is to be obtained. A useful but not yet fully available test to determine exposure to APAP is the APAP-CYS adduct assay, which is currently “research only,” while a point of care assay is under development.15,16 After an overdose, blood levels of APAP disappear quite rapidly due to its short half-life (~ 6 hours), and levels are often undetectable upon arrival in the emergency department, raising uncertainty about the etiology of liver injury.17 In contrast, APAP-CYS adducts, hepatocyte proteins covalently bound to the reactive metabolite of APAP (N-aminoparaquinoneimine), remain in the circulation as long as aminotransferases are elevated, up to 9 days.18 Comparable specific diagnostic tests to determine definitively the causative agent in idiosyncratic DILI are not yet available but are under study.
Table 1.
Clinical and laboratory features of the different etiologies of acute liver failure
| Clinical feature | Etiology of ALF, N = 2,614 | |||||||
|---|---|---|---|---|---|---|---|---|
| Paracetamol, N = 1,195 | Ischemia, N = 181 | DILI, N = 283 | AI-ALF, N = 173 | HBV, N = 188 | HAV, N = 39 | Pregnant, N = 27 | All others, N = 528 | |
| Age (median years) | 37 | 53 | 47 | 46 | 45 | 50 | 31 | 40 |
| Gender (% female) | 75 | 58 | 67 | 81 | 45 | 44 | 100 | 64 |
| Jaundice-coma (median days) | 1 | 2 | 12 | 16 | 8 | 4 | 7 | 7 |
| HE grade ≥ 3 (%) | 54 | 56 | 36 | 27 | 51 | 54 | 54 | 44 |
| ALT (median IU/L) | 3,780 | 2,311 | 654 | 404 | 1,410 | 2,229 | 43 | 758 |
| Bilirubin (median mg/dL) | 4.3 | 3.8 | 19.6 | 22.8 | 19.2 | 12.0 | 9.0 | 16.2 |
| Transplanted* (%) | 9 | 2 | 39 | 57 | 40 | 33 | 4 | 36 |
| Transplant-free survival* (%) | 65 | 57 | 24 | 14 | 19 | 51 | 78 | 22 |
| Overall survival* (%) | 72 | 58 | 58 | 63 | 53 | 77 | 82 | 55 |
Abbreviations: AI, autoimmune; ALF, acute liver failure; ALT, alanine aminotransferase; DILI, drug-induced liver injury; HAV, hepatitis A virus; HBV, hepatitis B virus; HE, hepatic encephalopathy.
Note: Striking differences are seen between the two hyperacute etiologies (paracetamol and ischemia) and all other etiologies in terms of time from onset to failure, liver laboratory values, and outcomes.
indicates outcome 21 days after admission for ALF to the study site hospital
Completing the Diagnostic Workup
The basic sequence of events in triaging patients with presumed ALF is as follows:
Determine that ALF criteria are met (INR ≥ 1.5, any degree of encephalopathy.
Search for etiology.
Determine severity and need for liver transplant listing; if poor prognostic indicators are present, transfer to a liver transplant center.
Start etiology-specific treatments and consider NAC.
Set up general support measures and alerts for changes in status.
Determining an etiology requires extensive initial testing. A smartphone app provides a checklist for initial and daily laboratory testing to sort out etiology of each case (access as “ALF Checklist” in the App Store). The checklist process was summarized in 2016; a modified version of the admission and daily checklist is included in Tables 2 and 3.19 A list of basic common etiologies and their appropriate tests precedes searching for more obscure conditions that are very rare.
Table 2.
Items to be considered on admission and daily in all cases of ALF
| Neuro checks every 1–2 hours |
| Head of the bed at 30° |
| Head in neutral position |
| Minimize stimulation (tracheal suctioning, chest physiotherapy, sternal rubbing) |
| Consider N-acetylcysteine (NAC) IV |
| CXR and surveillance cultures (blood, urine, sputum) on admission and every 24–48 hours |
| Monitor blood glucose every 1–2 hours |
| Avoid nephrotoxic drugs (aminoglycosides, NSAIDs, neomycin, etc.) and IV contrast |
| DVT prophylaxis (sequential compression device) despite coagulopathy |
| PPI for stress ulcer prophylaxis |
| Communication: (1) intensivist and/or transplant hepatologist, (2) nurse, (3) patient’s family |
Abbreviations: ALF, acute liver failure; CXR, chest X-ray; DVT, deep vein thrombosis; IV, intravenous; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton-pump inhibitor.
Table 3.
Diagnostic testing based on possible etiologies of ALF
| Possible etiology | Diagnostic items to do in all cases of ALF | Diagnostic items to consider | Specific therapiesa |
|---|---|---|---|
| Drug/toxin |
|
Consider liver biopsy | Acetaminophen toxicity: NAC Mushroom poisoning: charcoal, NAC, penicillin G, and/or silibininb |
| Viral |
|
Anti-HEV HSV DNA EBV DNA CMV DNA Anti-HDV/HDV RNA |
HBV: Nucleos(t)ide analog HSV: Acyclovir |
| Autoimmune |
|
Anti-liver/kidney microsomal antibody Liver biopsy |
Consider corticosteroids |
| Vascular Budd–Chiari ischemia |
|
CT/MRI Assess for hypercoagulable state including search for malignancy Interventional radiology consultation Echocardiography/ECG |
Budd–Chiari: Anticoagulation, TIPS |
| Wilson |
|
Ceruloplasmin 24-hour urine for copper Serum copper Ophthalmology consultation to look for Kayser–Fleischer rings |
Consider early CRRT |
| AFLP/HELLP | β-HCG Obstetrical consultation Ultrasound for fat |
Early delivery | |
| Malignancy | CT/MRI Liver biopsy |
||
| Indeterminate | Liver biopsy |
Abbreviations: AFLP, acute fatty liver of pregnancy; ALF, acute liver failure; β-HCG, beta-human chorionic gonadotropins; CMV, cytomegalovirus; CRRT, continuous renal replacement therapy; CT, computed tomography; DNA, deoxyribonucleic acid; EBV, Epstein-Barr virus; ECG, electrocardiogram; HAV, hepatitis A virus; HBc, hepatitis B core; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; HELLP, hemolysis, elevated liver enzymes and low platelets; HEV, hepatitis E virus; HSV, herpes simplex virus; IgM, immunoglobulin M; MRI, magnetic resonance imaging; NAC, N-acetylcysteine; OTC, over-the-counter; RNA, ribonucleic acid.
NAC should be administered to all patients with ALF and encephalopathy grade I/II regardless of etiology, and for all cases of suspected acetaminophen toxicity.
Not United States Food and Drug Administration (FDA) approved.
What Constitutes Indeterminate ALF?
When no diagnosis is apparent, this “indeterminate” group, sometimes referred to as seronegative ALF, comprises a mélange of essentially undiagnosed etiologies. In rural communities in parts of Asia, indeterminate ALF may comprise more than 60% of cases20 since serological testing is quite limited. In North America, indeterminate ALF was diagnosed by the ALFSG in 12% of registrants. However, upon further analysis of each case, the percent was diminished to around 5%, through thorough adjudication by a panel of expert hepatologists, additional serological testing, the use of the APAP-CYS adduct assay, and screening sera for viral genomes.21 In many cases, the site investigator was uncertain between two diagnoses and chose “indeterminate ALF” to reflect this uncertainty. In the future, it is likely that nonbiased genomic sequencing with one sample will probe for most known viruses rather than the hit-or-miss testing we currently perform.21 Despite the commonly held suspicion that occult viral infections were responsible for some indeterminate cases, searches for undiagnosed hepatitis viruses capable of causing ALF so far failed to disclose novel agents.22
Challenges to immediate diagnosis are real: patients have altered mentation (by definition), may not recall medications even if they are somewhat alert, and may not admit to overdosing as well. Thus, the APAP patient represents an ongoing diagnostic challenge. Use of the new APAP-CYS adduct assay will increase certainty of diagnosis once a point-of-care assay becomes available.15,16
As we look to the future, it is clear that the overall number of cases of ALF has not been increasing in most parts of the world and may actually be decreasing. Better education regarding the risks associated with APAP toxicity may be making some impact in the U.S. in terms of severity if not overall number of cases.23 Outcomes have continued to improve in the past two decades with a decline in listing and liver transplantation accompanied by an increase in transplant-free survival.3 These trends have occurred without a diminution of the severity of liver injury on presentation. Thus, improvements in intensive care as well as posttransplant management have evolved with greater experience with these difficult and challenging patients.
Section II: Management
Management Practices in ALF: A Developing Consensus Despite Weak Empirical Data
Although the management of patients with ALF remains unstandardized, general principles are emerging by consensus of experts in the field. Unfortunately, many of these principles are supported by weak empirical data. In 2007, the U.S. ALFSG comprehensively reviewed practices within the consortium,24 many of which differed significantly. In 2016, the European ALF Consortium subsequently surveyed 22 liver transplant centers from 11 nations.25 An overall approach to intensive care unit (ICU) management is outlined in Table 4. The etiologies of ALF in this report resembled those in the U.S., with approximately half of cases due to APAP overdose, 8% non-APAP idiosyncratic DILI, 5% viral, and 21% indeterminate etiologies. Although some management decisions were center-specific, many practices were quite similar; for example, intracranial pressure (ICP) monitoring was selectively performed in most centers, usually for clinical evidence of intracranial hypertension (ICH). The ICP goal parameters were also similar, and treatment triggered by ICP greater than 25 mm Hg, with a goal cerebral perfusion pressure of 50 to 60 mm Hg. Mannitol was universally the first-line treatment. Interestingly, second-line therapy after failure of mannitol varied by center (increased sedation, followed by hypertonic saline [HTS] boluses), although there was a clear tendency for high-transplant volume centers to use HTS compared with low-volume centers (86 vs. 31%, respectively).
Table 4.
ICU management
| 1. Neurologic |
| Abrupt deterioration in mental status? |
| Yes: Head CT to look for intracranial hemorrhage |
| Serum sodium < 145 mMol/L? |
| Yes → Consider using hypertonic saline for prophylaxis of intracranial hypertension to maintain serum Na between 145–155 mMol/L; carefully monitor rate of Na rise; discuss serum Na goal with health care team if patient on CRRT |
| Intubated, agitated or in pain? |
| No → Avoid sedating medications (benzodiazepines, narcotics, central-acting anti-emetics) |
| Yes → Use propofol and/or fentanyl |
| Spontaneous hypothermia (34–37°C)? |
| Yes → Do not warm patient |
| Encephalopathy grade III/IV? |
| Yes → Consider mannitol 0.25–1.0 g/kg IV q6 hours if serum osmolality < 320 mOsm/L or hypertonic saline boluses for treatment of suspected intracranial hypertension |
| Yes → Consider intracranial pressure monitoring |
| ■ Goal intracranial pressure < 25 mm Hg |
| ■ Goal cerebral perfusion pressure 50–80 mm Hg |
| 2. Pulmonary |
| Encephalopathy grade III/IV? |
| Yes → Intubate; prefer low tidal volume ventilation to avoid acute lung injury |
| Intubated and spontaneously hyperventilating? |
| Yes → Do not correct ventilation |
| 3. Infectious disease |
| (1) Progression of encephalopathy or grade III/IV or (2) SIRS or (3) clinical deterioration or (4) patient listed for transplant? Surveillance cultures daily |
| Yes/positive surveillance culture → Consider broad-spectrum antibiotics |
| 4. Cardiovascular |
| Mean arterial pressure (MAP) < 75 despite volume repletion AND encephalopathy grade III/IV? |
| Yes → Begin vasopressors (prefer norepinephrine over epinephrine or vasopressin) |
| Yes → Consider trial of hydrocortisone |
| 5. Renal |
| 1) Oliguria or (2) rise in creatinine > 0.3 mg/dL or (3) ammonia > 150 μM or (4) volume overload or (5) established/suspected intracranial hypertension? |
| No → Consider renal consultation/early hemodialysis |
| Yes → Initiate CRRT (CRRT preferred over intermittent HD even if hemodynamically stable) |
| 6. Hematology |
| Clinically significant bleeding? |
| No → Do not correct INR |
| Yes → Correct thrombocytopenia, hypofibrinogenemia and coagulopathy |
| Planned invasive procedure? |
| No → Do not correct INR, if possible upon consultation with intensivists/radiologists/neurosurgeons |
| Yes → Correct thrombocytopenia and hypofibrinogenemia (INR does not predict bleeding risk in patients with ALF) |
| 7. Endocrine |
| Glucose < 80 mg/dL? |
| Yes → Dextrose |
| Glucose > 180 mg/dL? |
| Yes → Insulin |
| 8. Gastrointestinal |
| Enteral feeding possible (PO or NG)? |
| Yes → Begin as early as possible if no evidence of gastroparesis |
| 9. Early transplant evaluation |
| Encephalopathy? |
| Yes → Consult transplant center/transplant hepatologist early |
| Potential liver transplant candidate? |
| Yes → Begin transplant evaluation per center protocol |
| All criteria for Status IA listing met? |
| All 3 of following criteria must be met: |
|
| Yes→ Consider listing, in consultation with transplant team |
Abbreviations: ALF, acute liver failure; CRRT, continuous renal replacement therapy; CT, computed tomography; HD, hemodialysis; ICU, intensive care unit; INR, international normalized ratio; IV, intravenous; Na, sodium; NG, nasogastric; PO, per oral; SIRS, systemic inflammatory response syndrome.
Note: Items to be checked on admission and daily.
Other management practices were also identified consistently across centers. Roughly 80% administered NAC to patients with non-APAP ALF despite limited data supporting the practice.26,27 Prophylactic antibiotics were uncommonly applied. Blood products (plasma and platelets) were generally not used without an indication, in only 5 and 16%, respectively, unless prior to ICP monitor placement (92 and 75%, respectively). Renal replacement therapy (RRT), usually continuous, was used by all centers. Interestingly, 55% of centers reported instituting RRT solely for treatment of hyperammonemia, as opposed to more traditional indications (acidosis, oliguria, volume overload).
Etiology-Specific Treatment
Steroids for suspected acute AIH:
AIH is the most difficult etiology of ALF to identify accurately and carries a particularly poor prognosis (14% transplant-free survival in the ALFSG registry).7 The international criteria for the diagnosis of chronic AIH were not developed for, and have not been validated in, patients with an acute presentation, and therefore are not readily applicable to patients with ALF. A simplification of the diagnostic criteria for chronic AIH has been adapted to the acute presentation, and include the presence of autoantibodies, hypergammaglobulinemia, a compatible liver histology, and the absence of viral markers.28,29 Although useful, these criteria lack sensitivity and specificity, as the prevalence of autoantibodies in the acute presentation is only approximately 50 to 70%, and autoantibodies are frequently found in patients with other etiologies of ALF.29–31 Liver biopsy features, which include those distinctive to the acute presentation, also lack sensitivity.29
Response to corticosteroids (CS) constitutes an important diagnostic feature of chronic AIH. Patients with severe acute presentations (coagulopathy without encephalopathy) may also respond to CS but have a poor prognosis overall.32,33 Many clinicians have extrapolated these observations to patients with ALF and autoimmune features, a practice that has been referred to as “therapeutic brinksmanship.”32 Unfortunately, no randomized, placebo-controlled studies of CS in AIH-ALF have been performed, and less rigorous studies are not particularly supportive of the practice.33,34 Consequently, guidelines regarding the use of CS in AIH-ALF have not advanced greatly. A study of CS therapy is currently underway for treatment of pediatric ALF, a disease that may be distinct from AIH-ALF in adults.
Without data-driven guidelines, an informal consensus has emerged regarding CS administration in suspected AIH-ALF. First, patients who present with, or evolve to, high-grade HE should be urgently evaluated for liver transplantation, and CS avoided, as CS rarely salvage such patients.25 Second, the course of CS should be limited to roughly a week or two,33 since longer application has been consistently shown to be futile and increases the risk of infection. Third, despite the acute presentation, cirrhosis underlies many cases of AIH-ALF,28 in which case liver transplantation may be the only viable long-term solution. De Martin et al34 have identified a pattern of nonresponse to CS which predicts the need for urgent liver transplantation within 3 days of CS initiation, including the evolution of the INR and total bilirubin. In general, higher Model for End-Stage Liver Disease (MELD) score, INR, bilirubin, and encephalopathy grade at diagnosis predict CS failure.32–35 Thus, the decision to list a patient for liver transplantation should be finalized within 3 days, and not more than 7 days, of CS administration. Evolution of encephalopathy may be the strongest indicator of the need for urgent transplantation, in which case CS should be forgone.
Antiviral agents for viral ALF:
Nucleos(t)ide analogs for hepatitis B virus (HBV)-induced ALF have been assessed in several studies. The most scientifically rigorous study randomized 71 patients to receive lamivudine or placebo36 but included patients with severe acute HBV (no encephalopathy). After 4 weeks, HBV deoxyribonucleic acid (DNA) titers were significantly lower in lamivudine-treated patients than placebo-treated controls, but otherwise, there were no clinically meaningful differences in the course of the two treatment groups. Other studies of DNA polymerase inhibitors in severe acute or fulminant HBV used historical controls, and some claim improvement in the poor transplant-free survival.37–39 A Cochrane Database Review of pharmacologic interventions for acute HBV attempted to synthesize results from 7 randomized trials (lamivudine vs. placebo, 4 trials; lamivudine vs. entecavir, 1 trial; entecavir vs. no treatment, 1 trial; and interferon vs. no treatment, 1 trial), but concluded the data were highly biased, of low quality, and patient populations were excessively dissimilar.40 Consequently, few data support the efficacy of any antiviral intervention in acute HBV; in patients with ALF, however, there is an emerging consensus that nucleos(t)ide analogs should be started on presentation to lower the risk of HBV infection of a transplanted liver. Herpes simplex virus causes ALF rarely but often with devastating severity. Acyclovir has been recommended based upon experience in small cases series.41 Rescue of affected patients by acyclovir, with or without liver transplantation, is unusual.
Management of Systemic Complications of ALF
Overall clinical management of the ALF syndrome is similar for different etiologies. The primary difference pertains to the special needs of patients with certain hyperacute versus subacute etiologies, specifically the critical mandate for rapid transplant listing of severely ill patients with APAP-induced ALF (hyperacute), and the more certain need for transplantation in all forms of sub-ALF, all of which have a dismal prognosis without liver transplantation. An overall management algorithm is shown in Fig. 2.
Fig. 2.
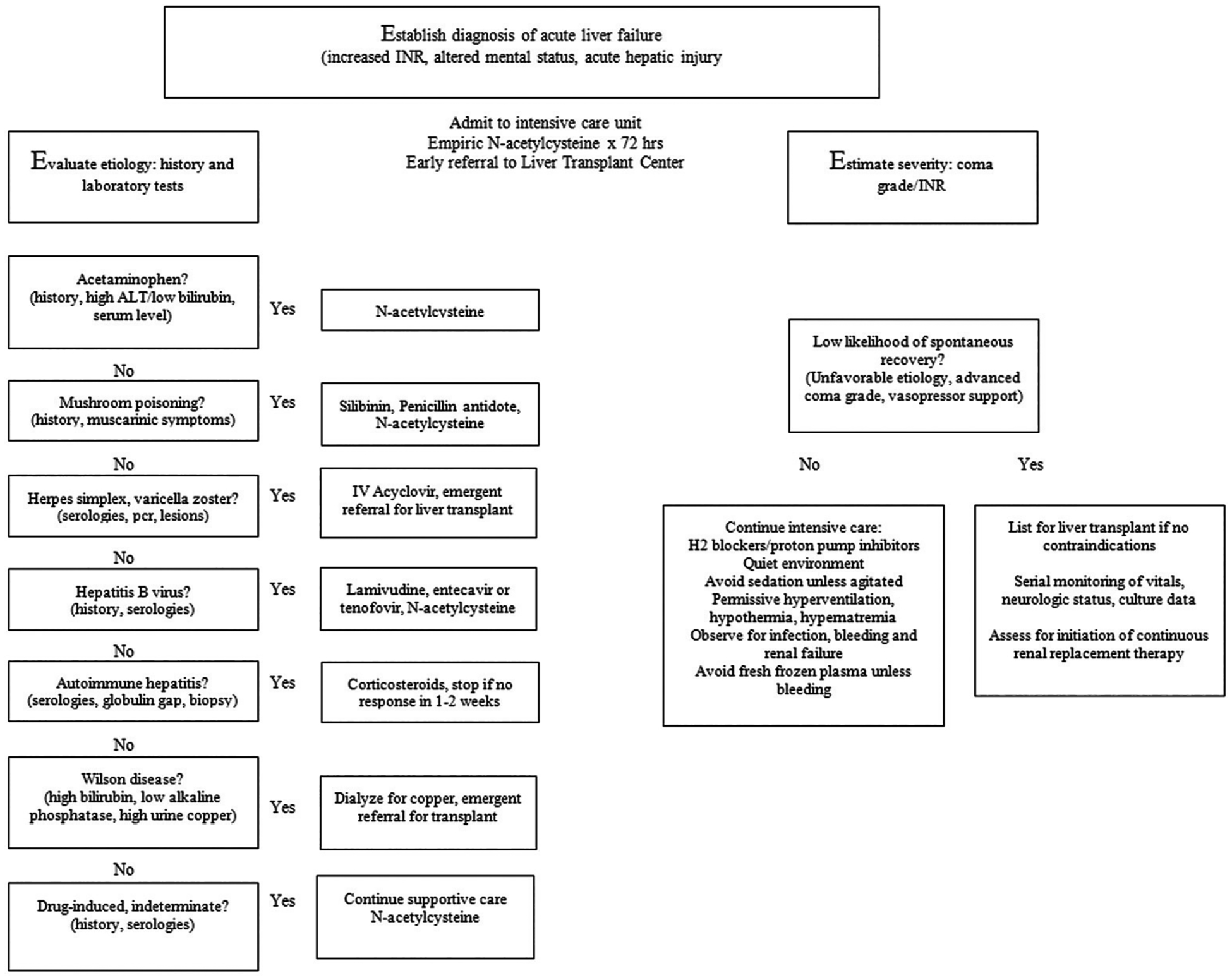
Algorithm for managing patients in the intensive care unit with acute liver failure.
Fluids and hemodynamics:
Upon initial admission to the hospital, patients with ALF are typically hypovolemic, particularly if the patient has been unconscious for any length of time. A hyperdynamic state with peripheral vasodilation ensues, the result of systemic inflammatory response syndrome (SIRS) and cytokine storm, and results in end-organ hypoperfusion including lactic acidosis and renal failure.42 Intravenous normal saline should be administered promptly to restore blood volume and pressure to a mean arterial pressure (MAP) of greater than 65 mm Hg.43 Assessment of end-organ perfusion should also guide the MAP in an individual patient. Persistent hypotension/hypoperfusion should prompt a search for infection, and broad-spectrum antibiotics and vasopressors initiated; norepinephrine is preferred.25 An inadequate response should prompt the addition of vasopressin. Relative adrenal insufficiency by abnormal short synacthen test has been shown in a majority of patients with ALF,44 and hydrocortisone (300 mg intravenously) should be added in persistently hypotensive, euvolemic patients on maximal doses of vasopressors. Fluid-responsiveness (cardiac index increase of ≥ 15% after a colloid challenge) was identified in only 29% of critically ill patients with ALF,45 suggesting that most patients have profound systemic vasodilation and an ineffective increase in cardiac preload after a volume challenge.
A relatively new strategy to manage vasopressor treatment failures has emerged: therapeutic plasma exchange (TPE). A multicenter, randomized, controlled study of “high-volume” TPE in 182 patients with ALF showed a higher cumulative proportion surviving compared with standard medical therapy (SMT).46 In the cohort receiving TPE, MAP increased promptly after institution, remained significantly higher than baseline (p < 0.0001), and required lower doses of vasopressors, while MAP in the SMT cohort remained unchanged. End-organ dysfunction such as the need for RRT was significantly lower in the TPE cohort (p < 0.0045). A nonrandomized trial of “low volume” plasma exchange,47 and a randomized study of “standard-volume” plasma exchange48 have documented similar benefits. The mechanism(s) by which TPE improves hemodynamic instability in ALF are likely multiple, including removal of vasoactive inflammatory cytokines and repletion of beneficial factors found in plasma.44 The former seems more likely, as treatment with the molecular adsorbent recirculating system (MARS), which uses an albumin dialysate, also improves MAP and end-organ function compared with matched controls.49
Cerebral edema (Table 5):
Table 5.
Management of cerebral edema
| Prevention of cerebral edema |
|---|
|
|
|
|
| Management of established cerebral edema |
|
|
|
|
|
|
|
|
Abbreviations: AKI, acute kidney injury; ALF, acute liver failure; CPP, cerebral perfusion pressure; CRRT, continuous renal replacement therapy; CVVH, continuous venovenous hemofiltration; HTS, hypertonic saline; ICP, intracranial pressure; IV, intravenous; MAP, mean arterial pressure;
OPA, L-ornithine phenylacetate; SIRS, systemic inflammatory response syndrome.
Note: The optimal management plan for cerebral edema in patients with ALF is undoubtedly prevention. In addition to the usual positional maneuvers (elevation of the head-of-bed; neutral neck rotation), patients should be allowed to hyperventilate such that their PCO2 decreases to 30–40 mm Hg and allowed to be hypothermic (usual in patients on CVVH). CRRT should be initiated early in patients with risk factors for developing cerebral edema. Serum sodium should be raised to high-normal.
In patients with established cerebral edema, mannitol or HTS boluses should be administered promptly, and CVVH continued. If an ICP monitor is placed, the CPP should be maintained > 60 mm Hg by increasing the MAP with norepinephrine. Therapeutic plasma exchange should be considered, as this will not only improve the MAP, but will remove ammonia from the circulation. As desperate measures, deeper sedation with propofol and/or therapeutic hypothermia may be considered. Although not studied in patients with ALF, sodium phenylacetate/sodium benzoate (Ammonul) might be considered as an ammonia scavenging agent. The compound has been studied in children with urea cycle defects and acute hyperammonemic crises and is commercially available; OPA is not.
In older series, complications of cerebral edema were a primary cause of death in patients with ALF. The two largest contemporary Western series of ALF, however, both documented dramatic decreases in the incidence of cerebral edema.2,50,51 In the U.S. ALFSG registry, ICH decreased from 52 to 30%, and death from cerebral edema from 12 to 4.5%, in early (1998–2007) and recent (2008–2018) cohorts, respectively. Undoubtedly, many improvements in the intensive care of patients with ALF contributed to these decreases, primary among them, the early and widespread use of continuous RRT (CRRT), and maintenance of serum sodium at high-normal levels.
Monitoring ICP:
The use of invasive ICP monitors also appears to be declining,3 due to the waning incidence of cerebral edema, often-fatal bleeding complications after insertion of the device, and the fact that no study has documented improvement in outcome or neurologic function in ICP-monitored patients. Invasive ICP monitoring has thus been relegated to the exceptional liver transplant candidate with high-risk features predictive of cerebral edema (presence of SIRS, high-grade HE, serum ammonia > 100 μM, renal failure) and clinical evidence of ICH. An argument may be made that ICP monitoring serves to identify which patient with ALF should not undergo liver transplantation because of long-standing ICH, and high risk of intraoperative herniation or postoperative poor neurologic recovery (Fig. 3).
Fig. 3.
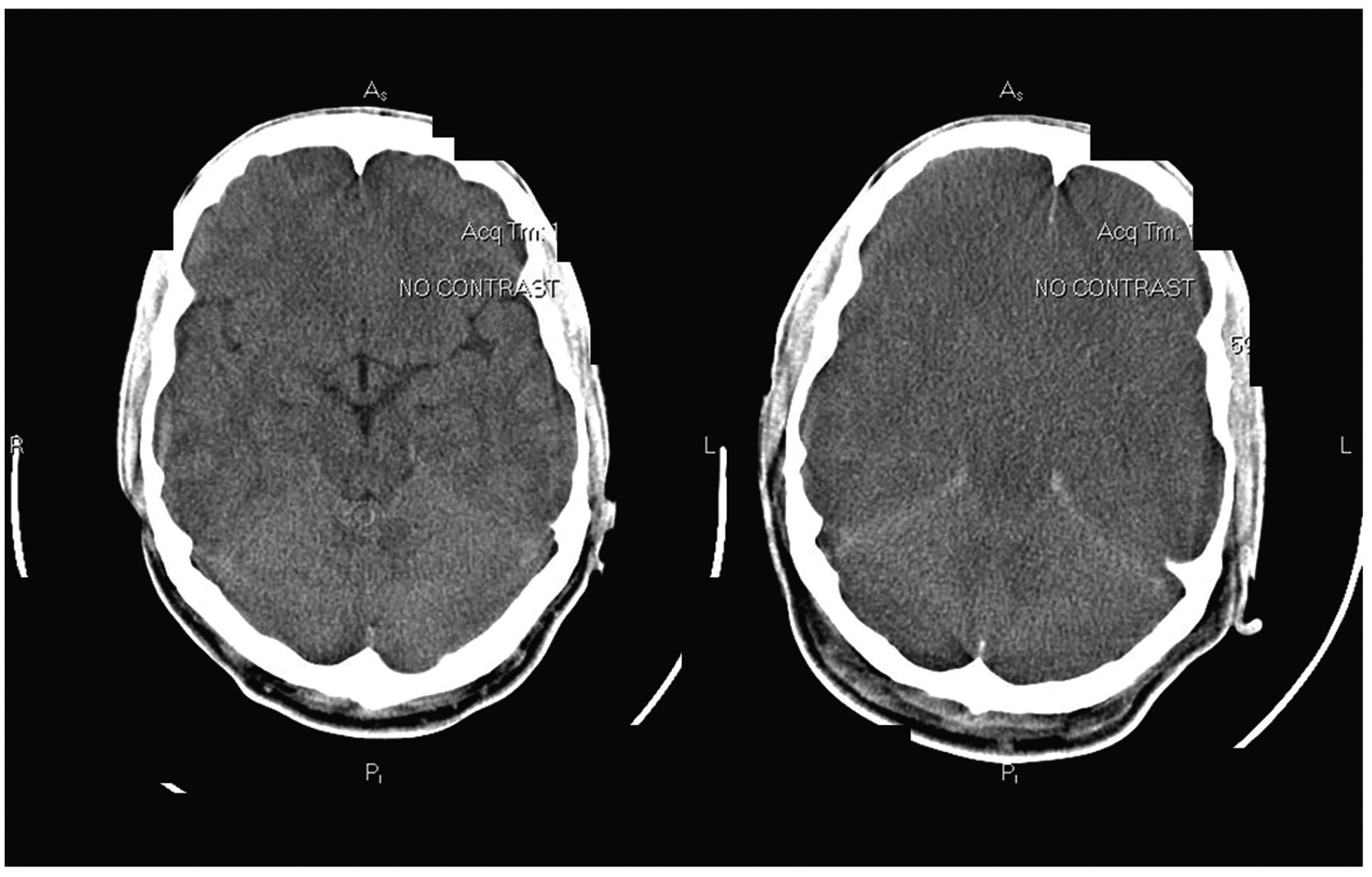
Head computed tomography (CT) scans of a 32-year-old man with acute liver failure of indeterminate etiology who died of progressive cerebral edema. At admission (left), the brain appeared normal; 48 hours later (right), there was diffuse loss of gray-white differentiation, effacement of sulci, and obliteration of the basal cisterns, consistent with severe diffuse edema and transtentorial herniation with brainstem compression. This patient died during attempted liver transplantation.
Unfortunately, noninvasive ICP monitoring remains an elusive goal in patients with ALF. For the reasons outlined above, studies of noninvasive ICP tests have not routinely used the gold standard of invasive pressure monitoring. In one study comparing noninvasive with invasive pressure monitoring, ultrasound-based tests were administered to a cohort of patients with ALF and grade 4 HE.52 Neurointensivists blinded to the ICP by invasive monitor concluded that only transcranial Doppler flow velocities accurately detected an ICP greater than 20 mm Hg (area under the curve [AUC] 0.90), while optic nerve sheath diameter (AUC 0.59) and middle cerebral artery pulsatility index (AUC 0.55), did not. While the optic nerve sheath diameter technique has been shown to accurately reflect ICP in other disorders of ICH, the pathogenesis of cerebral edema in ALF is unique (cytotoxic edema and cerebrovascular vasodilation) and may not be amenable to detection by this technique.52
Management of serum sodium:
The feasibility of prophylaxis against cerebral edema in patients with ALF was first shown by Murphy et al, in a prospective, randomized, placebo-controlled study of HTS to maintain mild hypernatremia (serum sodium 145–155 mM).53 Patients who received HTS maintained lower ICPs by invasive monitoring, were less likely to develop ICP of 25 mm Hg or higher, and required lower doses of norepinephrine, than those who were managed at normal serum sodium levels. In patients with established cerebral edema, HTS (3% as a constant infusion to maintain serum sodium < 160 mM) was recently compared with 20% mannitol (1 g/kg bolus[es]).54 The decline in ICP was approximately the same between groups but was measured by optic nerve sheath diameter and middle cerebral artery pulsatility index, results of which are questionable versus invasive ICP monitoring.52 However, rebound in ICP and renal dysfunction were more common in mannitol-compared with HTS-treated patients, suggesting a possible ancillary rationale for choosing the latter agent. The unpredictable titration of serum sodium during HTS boluses or infusion may be more closely controlled using modifications of CRRT, as recently proposed.55
RRT for hyperammonemia:
Another modification of standard ICU care in ALF patients has become the early application of RRT, usually CRRT, in moderately severely hyperammonemic patients. Although the association of serum ammonia greater than 200 μM with the development of ICH and cerebral herniation,56,57 and greater than 100 μM with high-grade HE58 has been recognized for decades, it has become more common to use RRT for the sole purpose of removing ammonia in ALF relatively recently. Blood and dialysate flow rates are the primary determinants of ammonia clearance.59 CRRT with hemofiltration clears ammonia in proportion to the ultrafiltration rate (r = 0.86) and can decrease median arterial ammonia over 24 hours by more than 20%.60 The cumulative duration of CRRT rather than hourly treatment intensity correlates with ammonia reduction most closely, suggesting that therapy should be started early and continued until ammonia levels are persistently less than 100 μM.60 Continuous may be superior to intermittent RRT in lowering serum ammonia and is associated with improved transplant-free survival.61
Novel treatments for refractory cerebral edema:
Based upon experience in children with hereditary forms of hyperammonemia, ammonia scavengers have been developed and tested in patients with ALF. L-ornithine L-aspartate (LOLA), a drug which provides a substrate for glutamine synthesis from glutamate and ammonia, was studied in a randomized, placebo-controlled population with ALF.62 Unfortunately, LOLA failed to lower serum ammonia more quickly than placebo and did not improve encephalopathy grade. These negative results were ascribed to gut glutaminases, which deamidate glutamine, returning ammonia back into the circulation.63 A similar drug, L-ornithine phenylacetate (OPA), provides a substrate for glutamate synthesis (ornithine), and also binds ammonia through the action of glutamine synthetase.64 In contrast to LOLA, the phenylacetate component of OPA binds glutamine and promotes its renal excretion before deamidation by glutaminases. In an open-label of patients with ALF and severe ALI, OPA was shown to promote renal excretion of ammonia in a dose-dependent manner and was deemed safe and well-tolerated.64 Further randomized, placebo-controlled studies in ALF have yet to be performed.
The induction of therapeutic hypothermia in ALF was pioneered by Jalan et al, to treat established, refractory ICH.65,66 Candidates for hypothermia are usually awaiting liver transplantation, in grade 4 HE, and have an invasive ICP monitor in place. Although cooling patients to a core temperature of 32 to 34°C reliably decreases ICP, there are adverse effects of hypothermia particularly pertinent to patients with ALF to consider: increased risk of infection, exacerbation of coagulation abnormalities, and cardiac dysrhythmias. Therapeutic hypothermia has, therefore, been relegated to the most recalcitrant cases of ICH and is not a reliable bridge to liver transplantation in most cases. Prophylactic hypothermia has also received attention. In a randomized, multicenter study of management at low-normal (36°C) or hypothermic (33–34°C) conditions, patients with grade 4 encephalopathy and ICP monitors were followed until reaching a primary endpoint of ICP greater than 25 mm Hg or 72 hours of thermal management.67 There were no differences in outcomes in patients managed under hypothermic versus normothermic conditions (primary endpoint met in 35% vs. 27%, respectively; p = 0.56). Although this study was very likely underpowered, prophylactic hypothermia cannot be advocated based upon these data.
Hemostatic abnormalities (Table 6):
Table 6.
General recommendations for blood component transfusion prior to an invasive procedure in patients with ALF
| Hemostatic parameter | Transfusion/Infusion | Threshold to replete |
|---|---|---|
| INR | Plasma | Unknown |
| Platelet count | Platelets | < 60 × 109/L |
| Fibrinogen | Cryoprecipitate | < 100 mg/dL |
| Hemoglobin | RBCs | < 7 g/dL |
Abbreviations: ALF, acute liver failure; INR, international normalized ratio; RBC, red blood cell.
Note: Although the cause of much consternation to clinicians caring for patients with ALF, the implication of an elevated INR in estimating bleeding risk is unclear.68 Before an invasive procedure, we suggest considering the transfusion of 1–2 units of plasma shortly before the procedure, then proceeding with the procedure without repeating the INR.
Platelets should be transfused if peripheral counts are < 60 × 109, with consideration of a higher threshold for transfusion in the setting of renal failure. Fibrinogen should be repleted with cryoprecipitate or fibrinogen concentrate if blood concentration is < 100 mg/dL. Red blood cells (RBCs) should be transfused if the hemoglobin is < 7 g/dL. These recommendations are primarily made on the basis of studies in patients with cirrhosis rather than ALF.76 In a patient with ALF who is actively bleeding, all blood components should be repleted, including plasma.
Patients with ALF appear to have a bleeding diathesis based upon often-markedly elevated INR and thrombocytopenia. Bleeding complications, often fatal, were commonly reported in early series of ALF (20–30% mortality). However, bleeding complications in a more contemporary series occurred in only 11% of the ALFSG cohort, with bleeding as a proximal cause of death recorded in only approximately 2% (7/10 deaths attributable to intracranial bleeding including complications of invasive ICP monitoring).68 The explanation for the rarity of bleeding complications has been ascribed to “rebalanced hemostasis,” a concept first identified in cirrhosis but more recently described in ALF.69 Compensatory mechanisms for hemostatic deficiencies have been identified for each phase of coagulation, such that low procoagulant proteins are “rebalanced” by low anticoagulant proteins and increased levels of factor VIII.68–71 The development of thrombocytopenia, which is partly SIRS-driven,72 is “rebalanced” by SIRS-related hyperproduction of von Wille-brand factor,73 the binding protein of platelets to endothelium, and by production of prothrombotic platelet-derived microparticles.74
Despite the fact that transfusion of platelets and plasma in patients with ALF has decreased with time,3,68 bleeding complications have also decreased, suggesting that prophylactic transfusions may not be indicated. However, it must be emphasized that hemostatic abnormalities unquestionably exist and may contribute to bleeding complications in ALF.75 Therefore, the administration of plasma and platelets before an invasive procedure must be individualized by the clinician, with the following caveats. First, a platelet count of 60 × 109/L or higher is probably adequate for any invasive procedure except for ICP monitor placement, in which case individualized therapy discussed with the consulting neurosurgeon is mandatory. Considerations such as the presence of renal failure must be weighed. Second, there are no guidelines of plasma administration, or goal INR, to guide clinicians. Although an INR of 1.5 or lower is often stated as the goal of plasma infusion, there was no relationship between INR and bleeding complications in the ALFSG registry.68 Clinicians must weigh the loss of the most important single clinical indicator of severity and trajectory of liver injury (the INR) by plasma infusion versus the perception of decreasing bleeding risk. Global hemostasis as assessed by thromboelastography70,76 or rotational thromboelastometry75 may be a useful adjunct to guide transfusion of platelets and plasma in patients with ALF but require further study as their sensitivity to subtle hemostatic derangements has not been adequately studied.
Section III: Prognostic Scoring and Liver Transplantation for Acute Liver Failure
Prognostic Scoring
Determining who can survive with best supportive care and who will succumb without liver transplantation remains a contentious area. Prognostic scoring systems attempt to balance sensitivity identifying patients who will die without liver transplantation with specificity aimed at avoiding unnecessary transplantation. Although no model performs perfectly, the hallmark of understanding prognosis in ALF depends on the etiology, laboratory and clinical markers of disease severity, and the tempo of disease. While the hyper-ALF seen with APAP provides the most dramatic laboratory derangements and highest risk for cerebral edema, ALF due to APAP also has the most favorable prognosis with the antidote NAC and supportive medical care whereas those with sub-ALF often due to idiosyncratic DILI, AIH, or indeterminate causes have the poorest outcomes without transplantation.3 Historically, the King’s College Criteria (KCC) have been used to predict need for transplantation in both APAP and non-APAP ALF though many additional scoring systems have been developed (Table 7).77 Factor V was proposed early on as a more accurate measure than INR but is not used widely currently.78 More recent studies have demonstrated that while having high specificity (94% in APAP; 82% in non-APAP), KCC lack sensitivity potentially failing to identify patients that will not survive without transplant. When KCC are compared with the Sequential Organ Failure Assessment (SOFA) score and MELD score in APAP ALF, SOFA was significantly better with AUC of 0.72 compared with KCC and MELD, with AUC of 0.65 and 0.58, respectively.79 MELD score has been extensively studied in both APAP and non-APAP ALF cases with decreased spontaneous survival noted with MELD 33 or higher and 30 or higher, respectively.80,81 A meta-analysis of 23 studies comparing the accuracy of KCC and MELD determined KCC predicted the need for transplant better in APAP cases whereas MELD performed better in non-APAP cases.82 The first iteration of the ALFSG prognostic index used coma grade, INR, bilirubin, phosphorus, and levels of an apoptosis biomarker, M30, and correctly identified those who were transplanted or died with sensitivity of 86%. This score is limited in clinical practice by availability of the M30 assay and did not outperform SOFA score in further analysis.83,84
Table 7.
Parameters for predicting mortality and need for liver transplantation in patients with ALF
| Test or index | Etiology of ALF | Threshold for poor prognosis, need for liver transplantation | Reference |
|---|---|---|---|
| King’s College Criteria | Paracetamol | Arterial pH < 7.30 or all of the following:
|
O’Grady et al, 1989 |
| Non- paracetamol | PT > 100 s (INR > 6.5) or any three of the following:
|
||
| Factor V (Clichy criteria) | Viral | Age < 30 y: factor V < 20% or Any age: factor V < 30% and grade ¾ encephalopathy | Bernuau et al, 1986; 1991 |
| Liver biopsy | Mixed | Hepatocyte necrosis > 70% | Donaldson et al, 1993 |
| Arterial phosphate | Paracetamol | > 1.2 mmol/L; sensitivity 89%, specificity 100% | Schmidt and Dalhoff, 2002 |
| Serum lactate | Paracetamol | > 3.5 mmol/L postresuscitation | Bernal, 2002 114 |
| APACHE II score | Paracetamol | Score > 15; sensitivity 82%, specificity 98% Score > 20; sensitivity 68%, specificity 87% |
Mitchell et al, 1998 |
| MELD score | Paracetamol Non-paracetamol |
Score > 33 Score ≥ 30 |
Schmidt and Larsen, 2007
Yantorno et al, 2007 |
| SOFA score | Paracetamol | Score ≥ 12 | Cholongitas et al, 2012 |
| BiLE score | Mixed | Score > 6.9 | Hadem et al, 2008 |
| Volumetric CT | Non-paracetamol | Liver volume < 1,000cm3 | Zabron et al, 2018 |
| ALFSG prognostic indexa | Mixed | Continuous variables: Favorable/unfavorable, encephalopathy grade, use of pressors (Y/N), INR, bilirubin | Koch et al, 2016 |
Abbreviations: ALF, acute liver failure; ALFSG, Acute Liver Failure Study Group; APACHE, acute physiology, age and chronic health evaluation; BiLE, Bilirubin-Lactate-Etiology score; CT, computerized tomography; mixed, mixed etiologies; INR, international normalized ratio; MELD, Model for End-stage Liver Disease, PT, prothrombin time; SOFA, Sequential Organ Failure Assessment.
The ALFSG prognostic index, in contrast to the other indices listed, is designed to predict transplant-free survival rather than death/need for liver transplantation (see text).
Clinically, two readily available features on admission, coma grade and etiology of ALF, largely determine outcomes. Etiologies can be graded as favorable versus unfavorable (Fig. 4, Table 8) with advanced coma grade carrying a worse prognosis. The ALFSG subsequently developed a mathematical model available as a Web-based application (ALFSG Prognostic Index in the App Store) incorporating five clinical features: etiology (favorable vs. unfavorable), encephalopathy grade (low-grade 1–2, vs. high-grade 3–4), INR, bilirubin, and vasopressor use to successfully predict spontaneous survival with AUC of 0.843. This was superior to MELD (AUC 0.717) and KCC (AUC 0.560 for non-APAP, 0.655 for APAP) (Fig. 5).85 The addition of lactate to bilirubin and etiology to create the Bilirubin Lactate and Etiology score,86 as well as lactate when combined with creatinine may predict mortality better than MELD but has yet to be validated.87 The Acute Physiology and Chronic Health Evaluation score has also been applied.88
Fig. 4.
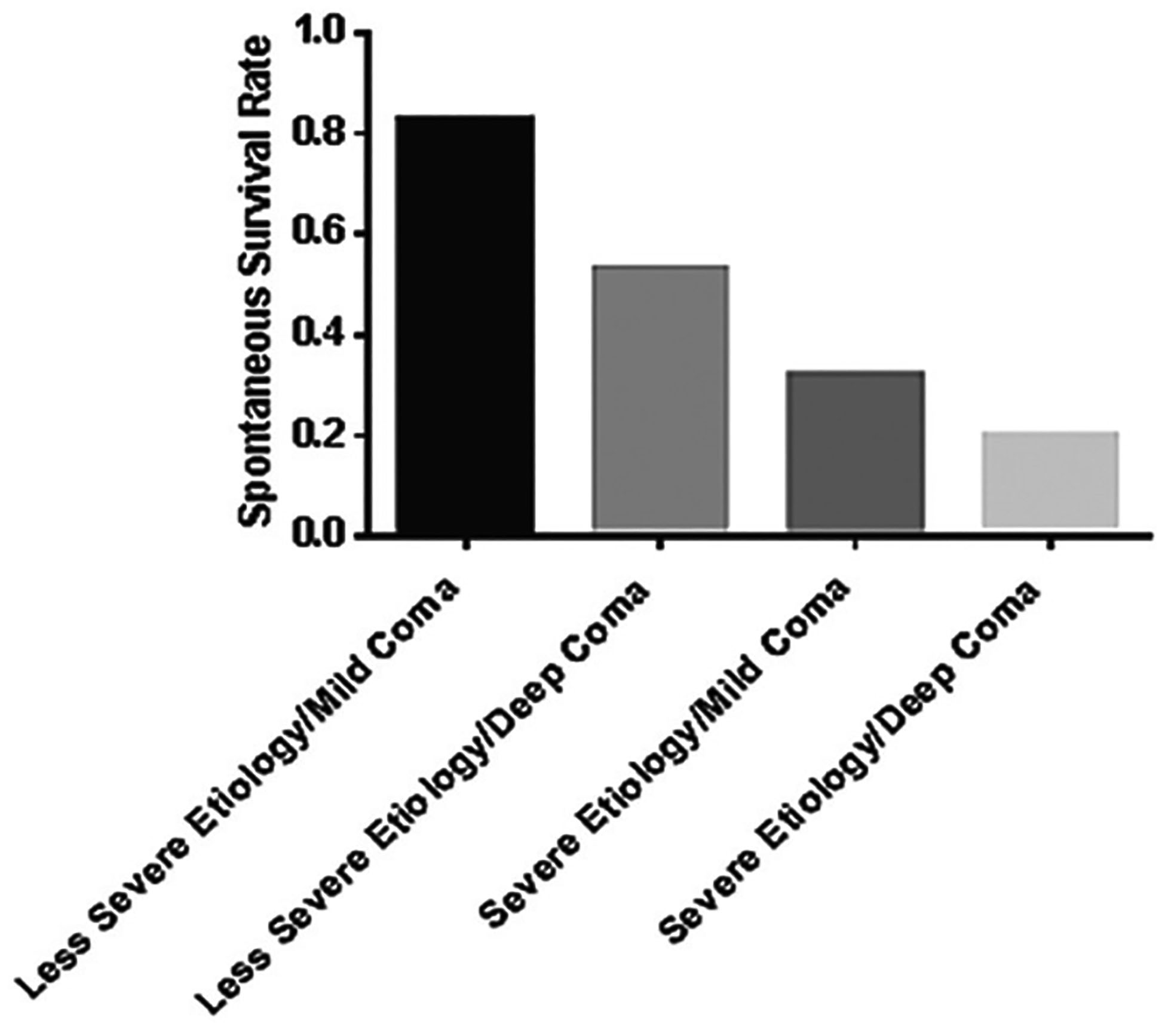
As noted in the table, prognosis depends on etiology but also on hepatic encephalopathy (HE) grade. Favorable etiologies and lower HE grades are associated with better transplant-free survival than unfavorable etiologies and advanced HE.
Table 8.
Etiologies divide nicely into those that are favorable (> 50% transplant-free survival) and those that are unfavorable (< 50% survival without transplantation)
| Favorable prognosis | |
|---|---|
|
66% |
|
66% |
|
55% |
|
56% |
| Unfavorable prognosis | |
|
27% |
|
27% |
|
26% |
|
26% |
|
0% |
Abbreviation: APAP, acetaminophen.
Fig. 5.
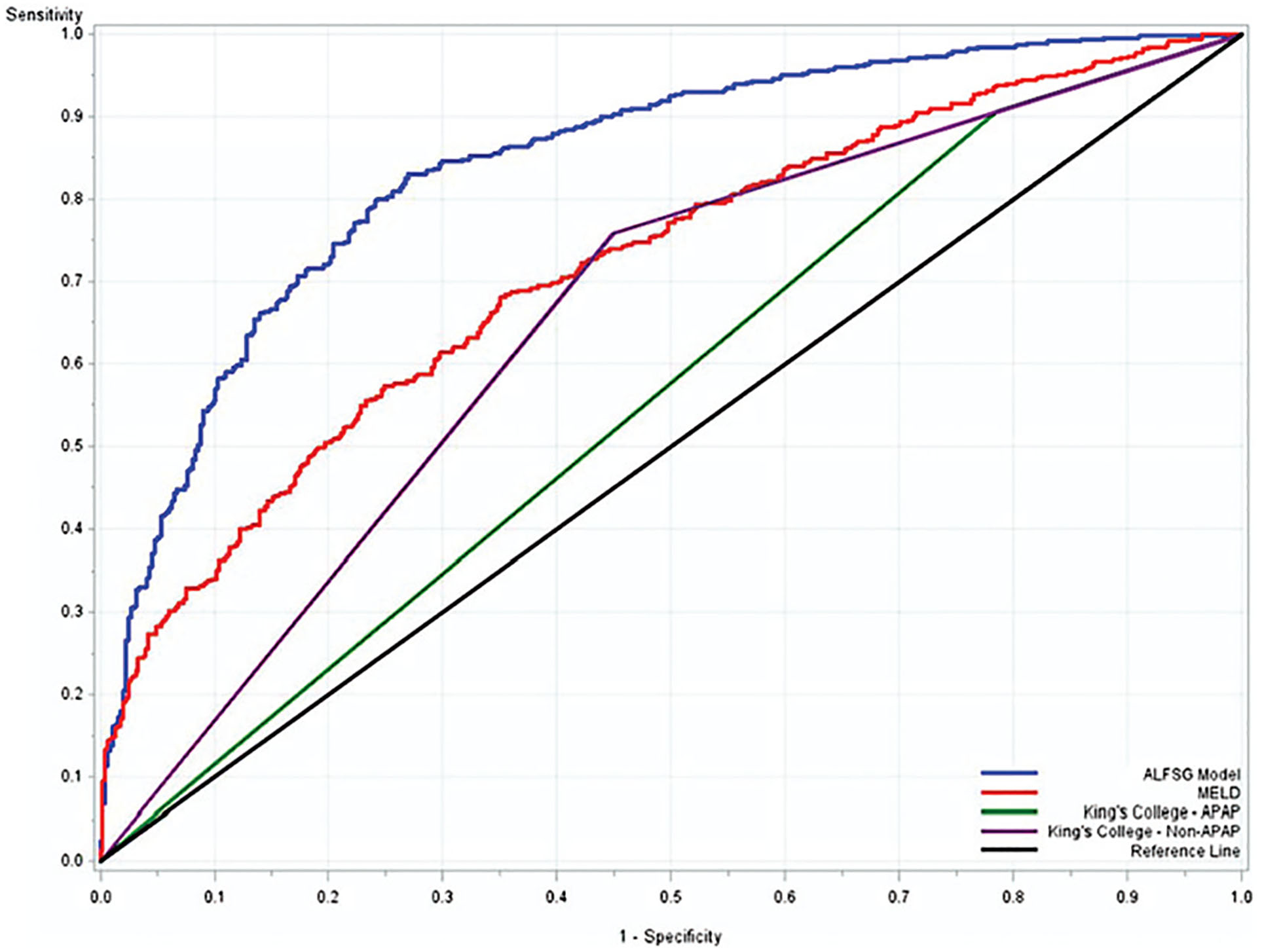
Area under the receiver operator curves (AUROC) comparing the Acute Liver Failure Study Group (ALFSG) prognostic index model to the King’s College Criteria (KCC) and Model for End-Stage Liver Disease (MELD) score, in predicting 80% survival. ALFSG model outperformed KCC and MELD in this analysis. Model c-statistics: ALFSG model = 0.843; MELD = 0.717; King’s College Criteria acetaminophen (APAP) (0.560), King’s College Criteria non-APAP (0.655).
Another more recent scoring system, the ALF early dynamic model, utilizes changes in ammonia, bilirubin, INR, and stage of encephalopathy over 3 days to predict outcomes more accurately.89 Sequential monitoring of readily available laboratory values INR and lactate also have been employed by the King’s College to discriminate between spontaneously recovering APAP ALF patients and those in need of liver transplant.90 Serial monitoring of hepatic metabolic function with the 13C-methacetin breath test predicted death or transplant with AUC of 0.88.91 Additional biomarkers such as α fetoprotein,92 phosphate,93 human leukocyte antigen-DR expression,94 Gc-globulin,95 fatty acid binding protein,96,97 and micro-ribonucleic acid levels98 have been reported to improve existing prognostic models but are limited by clinical availability and external validation.
Liver volume less than 1,000 cm3 determined by computed tomography may be a marker of irreversible massive necrosis predictive of development of high-grade encephalopathy and death in non-APAP ALF with 91% sensitivity and 63% specificity.99 While clinical status may preclude liver biopsy in these patients, specimens with greater than 75% necrosis is associated with need for transplant or death in ALF due to AIH, drug, or viral hepatitis.100–102
In summary, there is no consensus on best scoring system. Clinical judgment of experienced hepatologists and transplant surgeons seems the best guide, using the prognostic indices as aids in elucidating the proper timing and appropriateness of liver grafting.
Liver Support
Over the past three decades a variety of liver support devices have surfaced in early clinical trials only to falter and disappear from view. Many incorporated hepatocyte cartridges but either cell volumes were inadequate or the machines were too cumbersome to be feasible in the setting of a rare, rapidly evolving critical illness. Albumin-based dialysis systems, such as the MARS has been recommended for management of ALF patients particularly those with APAP injury. Equivocal results have led most centers to use these systems selectively, if at all.49 A recent meta-analysis of liver support devices discerned no difference in mortality between various support devices and standard medical treatment (SMT) in non-APAP ALF.103
Outcomes of Liver Transplantation
Mortality rate in ALF has improved greatly through the years, primarily with increased availability of liver transplantation. In the King’s College experience of over 3,300 patients between 1973 to 2008, the survival rate increased from 16.7 to 62.2%.2 Approximately half of non-APAP ALF patients will be transplanted, although ALF represents less than 10% of all liver transplants in Europe and the United States.104 One-year posttransplant survival of ALF is less than chronic liver disease but comparable to critically ill high MELD patients at over 80%.105 Risk factors for death after transplant for ALF in the European registry include male gender, donor age, recipient age, ABO incompatible graft, and small graft.104 Additional risk factors for poor outcomes in the United Network for Organ Sharing (UNOS) database include obesity, creatinine greater than 2 mg/dL, and history of life support. The highest risk of death is in the first 3 months related to infection including fungal, neurologic complications, and multisystem organ failure.106 In the European registry, noncompliance and repeated suicide resulted in graft loss and death 10 times more likely in APAP ALF than those transplanted for other etiologies.104 Among those in the large ALFSG registry (N = 2,264), only 22% of APAP ALF patients were listed for liver transplant and were twice as likely to die waiting for an organ compared with other etiologies.107 This finding was replicated in the UNOS database with APAP etiology associated with waitlist mortality but did not result in worse posttransplant outcomes after adjusting for disease severity. Most deaths occurred in the same hospitalization highlighting the need for timely and appropriate selection for transplant.108
Living donor liver transplant may be an option to combat organ availability though ethical concerns exist regarding expedited donor evaluation and undue coercion given the unique urgency of ALF patients. Such patients currently account for only 1% of the adult-to-adult living donor liver transplant evaluations in the United States with 1-year survival of 70%.109 In Asia where deceased donors are rare, living donor liver transplantations for ALF have a 1-year survival of 79%. Western transplant centers have more recently reported comparable complication rates to deceased donor transplantation.109,110 While more spontaneous recoveries of ALF are being achieved in recent years with supportive care, identifying those who will benefit from liver transplantation remains the ultimate task recognizing the limitations of current prognostic models.
Conclusion
Significant progress has been made over the past two decades in understanding and managing ALF. The incidence of ALF may be declining since several etiologies are less likely overall: DILI, hepatitis A and B, among others. The incidence of APAP ALF, however, does not appear to be declining. Spontaneous survival has improved in recent years owing to disease specific treatments such as NAC and improved critical care management including early initiation of CRRT. Despite numerous prognostic scoring systems, there is not one perfect prediction model and when to list and transplant for ALF remains a highly complex decision. The outcomes of patients transplanted for ALF are comparable to those transplanted for chronic liver disease. Management in the future is likely to include novel liver support devices,111 the possibility of stem cell mobilization to populate the damaged liver,112 and other agents which promote liver regeneration.113
Footnotes
Conflict of Interest
None declared.
References
- 1.Rakela J, Lange SM, Ludwig J, Baldus WP. Fulminant hepatitis: Mayo Clinic experience with 34 cases. Mayo Clin Proc 1985;60 (05):289–292 [DOI] [PubMed] [Google Scholar]
- 2.Bernal W, Hyyrylainen A, Gera A, et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol 2013;59(01):74–80 [DOI] [PubMed] [Google Scholar]
- 3.Reuben A, Tillman H, Fontana RJ, et al. Outcomes in adults with acute liver failure (ALF) from 1998–2013: an observational cohort study. Ann Intern Med 2016;164(11):724–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreau R, Jalan R, Gines P, et al. ; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144(07):1426–1437, 1437.e1–1437.e9 [DOI] [PubMed] [Google Scholar]
- 5.Lee WM. Acetaminophen (APAP) hepatotoxicity-isn’t it time for APAP to go away? J Hepatol 2017;67(06):1324–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoofnagle JH, Björnsson ES. Drug-induced liver injury—types and phenotypes. N Engl J Med 2019;381(03):264–273 [DOI] [PubMed] [Google Scholar]
- 7.Stravitz RT, Lee WM. Acute liver failure. Lancet 2019;394 (10201):869–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendizabal M, Marciano S, Videla MG, et al. Changing etiologies and outcomes of acute liver failure: perspectives from 6 transplant centers in Argentina. Liver Transpl 2014;20(04): 483–489 [DOI] [PubMed] [Google Scholar]
- 9.Chalasani N, Bonkovsky HL, Fontana R, et al. ; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology 2015;148(07):1340–52.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YTA-merican Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148 (01):215–219, quiz e16–e17 [DOI] [PubMed] [Google Scholar]
- 11.) Viral hepatitis surveillance—United States, 2017. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2019. Accessed on June 13, 2022, at: /hepatitis/statistics/2017surveillance/index.htm [Google Scholar]
- 12.Nelson NP, Weng MK, Hofmeister MG, et al. Prevention of hepatitis A virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR Recomm Rep 2020;69(05):1–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao A, Rule JA, Hameed B, Ganger D, Fontana RJ, Lee WM. Secular trends in severe idiosyncratic drug-induced liver injury in North America: an update from the ALFSG registry. Am J Gastroenterol 2022; In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heard K, Rumack BH, Green JL, et al. A single-arm clinical trial of a 48-hour intravenous N-acetylcysteine protocol for treatment of acetaminophen poisoning. Clin Toxicol (Phila) 2014;52(05): 512–518 [DOI] [PubMed] [Google Scholar]
- 15.Davern TJ II, James LP, Hinson JA, et al. ; Acute Liver Failure Study Group. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology 2006;130 (03):687–694 [DOI] [PubMed] [Google Scholar]
- 16.Khandelwal N, James LP, Sanders C, Larson AM, Lee WM Acute Liver Failure Study Group. Unrecognized acetaminophen toxicity as a cause of indeterminate acute liver failure. Hepatology 2011; 53(02):567–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leventhal TM, Gottfried M, Olson JC, Subramanian RM, Hameed B, Lee WM Acute Liver Failure Study Group. Acetaminophen is undetectable in plasma form more than half of patients believed to have acute liver failure due to overdose. Clin Gastroenterol Hepatol 2019;17(10):2110–2116 [DOI] [PubMed] [Google Scholar]
- 18.James LP, Letzig L, Simpson PM, et al. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos 2009;37 (08):1779–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fix OK, Liou I, Karvellas CJ, et al. ; Acute Liver Failure Study Group. Development and pilot of a checklist for management of acute liver failure in the intensive care unit. PLoS One 2016;11(05):e0155500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shalimar KS, Kedia S, Gunjan D, et al. Acute liver failure due to hepatitis E virus infection is associated with better survival than other etiologies in Indian patients. Dig Dis Sci 2017;62(04): 1058–1066 [DOI] [PubMed] [Google Scholar]
- 21.Ganger DR, Rule J, Rakela J, et al. ; Acute Liver Failure Study Group. Acute liver failure of indeterminate etiology: a comprehensive systematic approach by an expert committee to establish causality. Am J Gastroenterol 2018;113(09):1319–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somasekar S, Lee D, Rule J, et al. Viral surveillance in serum samples from patients with acute liver failure by metagenomic next-generation sequencing. Clin Infect Dis 2017;65(09): 1477–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long A, Magrath M, Mihalopoulos M, et al. Changes in epidemiology of overdoses in an urban county hospital after 20 years. Am J Gastro 2022; In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stravitz RT, Kramer AH, Davern T, et al. Acute Liver Failure Study Group. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med 2007;35(11):2498–2508 [DOI] [PubMed] [Google Scholar]
- 25.Rabinowich L, Wendon J, Bernal W, Shibolet O. Clinical management of acute liver failure: results of an international multicenter survey. World J Gastroenterol 2016;22(33):7595–7603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee WM, Hynan LS, Rossaro L, et al. ; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009;137(03):856–864, 864.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siu JT, Nguyen T, Turgeon RD. N-acetylcysteine for non-paracetamol (acetaminophen)-related acute liver failure. Cochrane Database Syst Rev 2020;12:CD012123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennes EM, Zeniya M, Czaja AJ, et al. ; International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48(01):169–176 [DOI] [PubMed] [Google Scholar]
- 29.Stravitz RT, Lefkowitch JH, Fontana RJ, et al. ; Acute Liver Failure Study Group. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology 2011;53(02):517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung PSC, Rossaro L, Davis PA, et al. ; Acute Liver Failure Study Group. Antimitochondrial antibodies in acute liver failure: implications for primary biliary cirrhosis. Hepatology 2007;46 (05):1436–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeoman AD, Westbrook RH, Zen Y, et al. Prognosis of acute severe autoimmune hepatitis (AS-AIH): the role of corticosteroids in modifying outcome. J Hepatol 2014;61(04):876–882 [DOI] [PubMed] [Google Scholar]
- 32.Czaja AJ. Corticosteroids or not in severe acute or fulminant autoimmune hepatitis: therapeutic brinksmanship and the point beyond salvation. Liver Transpl 2007;13(07):953–955 [DOI] [PubMed] [Google Scholar]
- 33.Rahim MN, Miquel R, Heneghan MA. Approach to the patient with acute severe autoimmune hepatitis. JHEP Rep 2020;2(06): 100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Martin E, Coilly A, Chazouillères O, et al. ; FILFOIE consortium – France. Early liver transplantation for corticosteroid non-responders with acute severe autoimmune hepatitis: the SURFASA score. J Hepatol 2021;74(06):1325–1334 [DOI] [PubMed] [Google Scholar]
- 35.Mendizabal M, Marciano S, Videla MG, et al. Fulminant presentation of autoimmune hepatitis: clinical features and early predictors of corticosteroid treatment failure. Eur J Gastroenterol Hepatol 2015;27(06):644–648 [DOI] [PubMed] [Google Scholar]
- 36.Kumar M, Satapathy S, Monga R, et al. A randomized controlled trial of lamivudine to treat acute hepatitis B. Hepatology 2007; 45(01):97–101 [DOI] [PubMed] [Google Scholar]
- 37.Yu J-W, Sun L-J, Yan B-Z, Kang P, Zhao Y-H. Lamivudine treatment is associated with improved survival in fulminant hepatitis B. Liver Int 2011;31(04):499–506 [DOI] [PubMed] [Google Scholar]
- 38.Tillmann HL, Hadem J, Leifeld L, et al. Safety and efficacy of lamivudine in patients with severe acute or fulminant hepatitis B, a multicenter experience. J Viral Hepat 2006;13(04):256–263 [DOI] [PubMed] [Google Scholar]
- 39.Dao DY, Seremba E, Ajmera V, Sanders C, Hynan LS, Lee WM Acute Liver Failure Study Group. Use of nucleoside (tide) analogues in patients with hepatitis B-related acute liver failure. Dig Dis Sci 2012;57(05):1349–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantzoukis K, Rodríguez-Perálvarez M, Buzzetti E, et al. Pharmacological interventions for acute hepatitis B infection: an attempted network meta-analysis. Cochrane Database Syst Rev 2017;3(03):CD011645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norvell JP, Blei AT, Jovanovic BD, Levitsky J. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl 2007;13(10):1428–1434 [DOI] [PubMed] [Google Scholar]
- 42.Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med 2020;48(03): e173–e191 [DOI] [PubMed] [Google Scholar]
- 43.Lee WM, Stravitz RT, Larson AM. Introduction to the AASLD Position Paper on acute liver failure. Hepatology 2012; 55:965–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harry R, Auzinger G, Wendon J. The clinical importance of adrenal insufficiency in acute hepatic dysfunction. Hepatology 2002;36(02):395–402 [DOI] [PubMed] [Google Scholar]
- 45.Audimoolam VK, McPhail MJ, Willars C, et al. Predicting fluid responsiveness in acute liver failure: a prospective study. Anesth Analg 2017;124(02):480–486 [DOI] [PubMed] [Google Scholar]
- 46.Larsen FS, Schmidt LE, Bernsmeier C, et al. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol 2016;64(01):69–78 [DOI] [PubMed] [Google Scholar]
- 47.Stahl K, Hadem J, Schneider A, et al. Therapeutic plasma exchange in acute liver failure. J Clin Apher 2019;34(05):589–597 [DOI] [PubMed] [Google Scholar]
- 48.Maiwall R, Bajpai M, Singh A, et al. Standard-volume plasma exchange improves outcomes in patients with acute liver failure: a randomized controlled trial. Clin Gastroenterol Hepatol 2022; 20(04):e831–e854 [DOI] [PubMed] [Google Scholar]
- 49.MacDonald AJ, Subramanian RM, Olson JC, et al. ; Use of the molecular adsorbent recirculating system in acute liver failure: results of a multicenter propensity score-matched study. Crit Care Med 2022;50(02):286–295 [DOI] [PubMed] [Google Scholar]
- 50.MacDonald AJ, Speiser JL, Ganger DR, et al. ; US Acute Liver Failure Study Group. Clinical and neurologic outcomes in acetaminophen-induced acute liver failure: A 21-year multicenter cohort study. Clin Gastroenterol Hepatol 2021;19(12):2615–2625.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karvellas CJ, Fix OK, Battenhouse H, Durkalski V, Sanders C, Lee WM U S Acute Liver Failure Study Group. Outcomes and complications of intracranial pressure monitoring in acute liver failure: a retrospective cohort study. Crit Care Med 2014;42(05): 1157–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rajajee V, Williamson CA, Fontana RJ, Courey AJ, Patil PG. Noninvasive intracranial pressure assessment in acute liver failure. Neurocrit Care 2018;29(02):280–290 [DOI] [PubMed] [Google Scholar]
- 53.Murphy N, Auzinger G, Bernel W, Wendon J. The effect of hypertonic sodium chloride on intracranial pressure in patients with acute liver failure. Hepatology 2004;39(02):464–470 [DOI] [PubMed] [Google Scholar]
- 54.Kalal CR, Maiwall R, Choudhary A, et al. Mannitol is comparable to hypertonic saline for raised intracranial pressure in Acute Liver Failure (MAHAL Study): a RCT. Dig Dis 2021 [DOI] [PubMed] [Google Scholar]
- 55.Hamdi T, Yessayan L, Yee J, Szamosfalvi B. High sodium continuous veno-venous hemodialysis with regional citrate anticoagulation and online dialysate generation in patients with acute liver failure and cerebral edema. Hemodial Int 2018;22(02): 184–191 [DOI] [PubMed] [Google Scholar]
- 56.Clemmesen JO, Larsen FS, Kondrup J, Hansen BA, Ott P. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology 1999;29(03): 648–653 [DOI] [PubMed] [Google Scholar]
- 57.Bernal W, Hall C, Karvellas CJ, Auzinger G, Sizer E, Wendon J. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology 2007;46(06):1844–1852 [DOI] [PubMed] [Google Scholar]
- 58.Cordoba J, Blei AT, Mujais S. Determinants of ammonia clearance by hemodialysis. Artif Organs 1996;20(07):800–803 [DOI] [PubMed] [Google Scholar]
- 59.Slack AJ, Auzinger G, Willars C, et al. Ammonia clearance with haemofiltration in adults with liver disease. Liver Int 2014;34 (01):42–48 [DOI] [PubMed] [Google Scholar]
- 60.Warrillow S, Fisher C, Bellomo R. Correction and control of hyperammonemia in acute liver failure: the impact of continuous renal replacement timing, intensity, and duration. Crit Care Med 2020;48(02):218–224 [DOI] [PubMed] [Google Scholar]
- 61.Cardoso FS, Gottfried M, Tujios S, Olson JC, Karvellas CJ US Acute Liver Failure Study Group. Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatology 2018;67(02):711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acharya SK, Bhatia V, Sreenivas V, Khanal S, Panda SK. Efficacy of L-ornithine L-aspartate in acute liver failure: a double-blind, randomized, placebo-controlled study. Gastroenterology 2009; 136(07):2159–2168 [DOI] [PubMed] [Google Scholar]
- 63.Jalan R, Lee WM. Treatment of hyperammonemia in liver failure: a tale of two enzymes. Gastroenterology 2009;136(07): 2048–2051 [DOI] [PubMed] [Google Scholar]
- 64.Stravitz RT, Gottfried M, Durkalski V, et al. ; Acute Liver Failure Study Group. Safety, tolerability, and pharmacokinetics of l-ornithine phenylacetate in patients with acute liver injury/failure and hyperammonemia. Hepatology 2018;67(03):1003–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jalan R, Olde Damink SW, Deutz NE, Hayes PC, Lee A. Moderate hypothermia in patients with acute liver failure and uncontrolled intracranial hypertension. Gastroenterology 2004;127 (05):1338–1346 [DOI] [PubMed] [Google Scholar]
- 66.Stravitz RT, Larsen FS. Therapeutic hypothermia for acute liver failure. Crit Care Med 2009;37(7, Suppl):S258–S264 [DOI] [PubMed] [Google Scholar]
- 67.Bernal W, Murphy N, Brown S, et al. A multicentre randomized controlled trial of moderate hypothermia to prevent intracranial hypertension in acute liver failure. J Hepatol 2016;65(02): 273–279 [DOI] [PubMed] [Google Scholar]
- 68.Stravitz RT, Ellerbe C, Durkalski V, et al. ; Acute Liver Failure Study Group. Bleeding complications in acute liver failure. Hepatology 2018;67(05):1931–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lisman T, Stravitz RT. Rebalanced hemostasis in patients with acute liver failure. Semin Thromb Hemost 2015;41(05):468–473 [DOI] [PubMed] [Google Scholar]
- 70.Stravitz RT, Lisman T, Luketic VA, et al. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol 2012;56(01):129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Habib M, Roberts LN, Patel RK, Wendon J, Bernal W, Arya R. Evidence of rebalanced coagulation in acute liver injury and acute liver failure as measured by thrombin generation. Liver Int 2014;34(05):672–678 [DOI] [PubMed] [Google Scholar]
- 72.Stravitz RT, Ellerbe C, Durkalski V, Reuben A, Lisman T, Lee WM Acute Liver Failure Study Group. Thrombocytopenia is associated with multi-organ system failure in patients with acute liver failure. Clin Gastroenterol Hepatol 2016;14(04):613–620. e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Driever EG, Stravitz RT, Zhang J, et al. VWF/ADAMTS13 imbalance, but not global coagulation or fibrinolysis, is associated with outcome and bleeding in acute liver failure. Hepatology 2021;73 (05):1882–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stravitz RT, Bowling R, Bradford RL, et al. Role of procoagulant microparticles in mediating complications and outcome of acute liver injury/acute liver failure. Hepatology 2013;58(01): 304–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stravitz RT, Fontana RJ, Meinzer C, et al. ; ALF Study Group. Coagulopathy, bleeding events, and outcome according to rotational thromboelastometry in patients with acute liver injury/failure. Hepatology 2021;74(02):937–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stravitz RT. Algorithms for managing coagulation disorders in patients with liver disease. Hepatol Int 2018;12(05):390–401 [DOI] [PubMed] [Google Scholar]
- 77.O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989;97(02):439–445 [DOI] [PubMed] [Google Scholar]
- 78.Bernuau J, Goudeau A, Poynard T, et al. Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology 1986;6 (04):648–651 [DOI] [PubMed] [Google Scholar]
- 79.Cholongitas E, Theocharidou E, Vasianopoulou P, et al. Comparison of the Sequential Organ Failure Assessment score with the King’s College Hospital criteria and the model for end-stage liver disease score for the prognosis of acetaminophen-induced acute liver failure. Liver Transpl 2012;18(04):405–412 [DOI] [PubMed] [Google Scholar]
- 80.Schmidt LE, Larsen FS. MELD score as a predictor of liver failure and death in patients with acetaminophen-induced liver injury. Hepatology 2007;45(03):789–796 [DOI] [PubMed] [Google Scholar]
- 81.Yantorno SE, Kremers WK, Ruf AE, Trentadue JJ, Podestá LG, Villamil FG. MELD is superior to King’s College and Clichy’s criteria to assess prognosis in fulminant hepatic failure. Liver Transpl 2007;13(06):822–828 [DOI] [PubMed] [Google Scholar]
- 82.McPhail MJ, Farne H, Senvar N, Wendon JA, Bernal W. Ability of King’s College Criteria and Model for End-Stage Liver Disease scores to predict mortality of patients with acute liver failure. A meta-analysis. Clin Gastroenterol Hepatol 2016;14(04): 516–525.e5, quiz e43–e45 [DOI] [PubMed] [Google Scholar]
- 83.Rutherford A, King LY, Hynan LS, et al. ; ALF Study Group. Development of an accurate index for predicting outcomes of patients with acute liver failure. Gastroenterology 2012;143(05): 1237–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Craig DG, Simpson KJ. Accuracy of the ALFSG index as a triage marker in acute liver failure. Gastroenterology 2013;144(01): e25. [DOI] [PubMed] [Google Scholar]
- 85.Koch DG, Tillman H, Durkalski V, Lee WM, Reuben A. Development of a model to predict transplant-free survival of patients with acute liver failure. Clin Gastroenterol Hepatol 2016;14(08): 1199–1206.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hadem J, Stiefel P, Bahr MJ, et al. Prognostic implications of lactate, bilirubin, and etiology in German patients with acute liver failure. Clin Gastroenterol Hepatol 2008;6(03):339–345 [DOI] [PubMed] [Google Scholar]
- 87.Figueira ERR, Rocha-Filho JA, Lanchotte C, et al. Creatininelactate score predicts mortality in non-acetaminophen-induced acute liver failure in patients listed for liver transplantation. BMC Gastroenterol 2021;21(01):252–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitchell I, Bihari D, Chang R, Wendon J, Williams R. Earlier identification of patients at risk from acetaminophen-induced acute liver failure. Crit Care Med 1998;26(02):279–284 [DOI] [PubMed] [Google Scholar]
- 89.Kumar R, Shalimar, Sharma H, et al. Prospective derivation and validation of early dynamic model for predicting outcome in patients with acute liver failure. Gut 2012;61(07):1068–1075 [DOI] [PubMed] [Google Scholar]
- 90.Bernal W, Wang Y, Maggs J, et al. Development and validation of a dynamic outcome prediction model for paracetamol-induced acute liver failure: a cohort study. Lancet Gastroenterol Hepatol 2016;1(03):217–225 [DOI] [PubMed] [Google Scholar]
- 91.Fontana RJ, Stravitz RT, Durkalski V, et al. Prognostic value of the 13 C-methacetin breath test in adults with acute liver failure and non-acetaminophen acute liver injury. Hepatology 2021;74(02): 961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidt LE, Dalhoff K. Alpha-fetoprotein is a predictor of outcome in acetaminophen-induced liver injury. Hepatology 2005; 41(01):26–31 [DOI] [PubMed] [Google Scholar]
- 93.Schmidt LE, Dalhoff K. Serum phosphate is an early predictor of outcome in severe acetaminophen-induced hepatotoxicity. Hepatology 2002;36(03):659–665 [DOI] [PubMed] [Google Scholar]
- 94.Antoniades CG, Berry PA, Davies ET, et al. Reduced monocyte HLA-DR expression: a novel biomarker of disease severity and outcome in acetaminophen-induced acute liver failure. Hepatology 2006;44(01):34–43 [DOI] [PubMed] [Google Scholar]
- 95.Schiødt FV, Rossaro L, Stravitz RT, Shakil AO, Chung RT, Lee WM Acute Liver Failure Study Group. Gc-globulin and prognosis in acute liver failure. Liver Transpl 2005;11(10):1223–1227 [DOI] [PubMed] [Google Scholar]
- 96.Karvellas CJ, Speiser JL, Tremblay M, Lee WM, Rose CFUS Acute Liver Failure Study Group. Elevated FABP1 serum levels are associated with poorer survival in acetaminophen-induced acute liver failure. Hepatology 2017;65(03):938–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karvellas CJ, Speiser JL, Tremblay M, Lee WM, Rose CFUS Acute Liver Failure Study Group. Elevated serum liver-type fatty acid binding protein levels in non-acetaminophen acute liver failure patients with organ dysfunction. Dig Dis Sci 2021;66(01): 273–283 [DOI] [PubMed] [Google Scholar]
- 98.Tavabie OD, Karvellas CJ, Salehi S, et al. ; United States Acute Liver Failure Study Group. A novel microRNA-based prognostic model outperforms standard prognostic models in patients with acetaminophen-induced acute liver failure. J Hepatol 2021;75(02): 424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zabron A, Quaglia A, Fatourou E, et al. Clinical and prognostic associations of liver volume determined by computed tomography in acute liver failure. Liver Int 2018;38(09): 1592–1601 [DOI] [PubMed] [Google Scholar]
- 100.Donaldson BW, Gopinath R, Wanless IR, et al. The role of transjugular liver biopsy in fulminant liver failure: relation to other prognostic indicators. Hepatology 1993;18(06):1370–1376 [PubMed] [Google Scholar]
- 101.Singhal A, Vadlamudi S, Stokes K, et al. Liver histology as predictor of outcome in patients with acute liver failure. Transpl Int 2012;25(06):658–662 [DOI] [PubMed] [Google Scholar]
- 102.Ndekwe P, Ghabril MS, Zang Y, Mann SA, Cummings OW, Lin J. Substantial hepatic necrosis is prognostic in fulminant liver failure. World J Gastroenterol 2017;23(23):4303–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kanjo A, Ocskay K, Gede N, et al. Efficacy and safety of liver support devices in acute and hyperacute liver failure: a systematic review and network meta-analysis. Sci Rep 2021;11(01): 4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Germani G, Theocharidou E, Adam R, et al. Liver transplantation for acute liver failure in Europe: outcomes over 20 years from the ELTR database. J Hepatol 2012;57(02):288–296 [DOI] [PubMed] [Google Scholar]
- 105.Freeman RB Jr, Steffick DE, Guidinger MK, Farmer DG, Berg CL, Merion RM. Liver and intestine transplantation in the United States, 1997–2006. Am J Transplant 2008;8(4 Pt 2):958–976 [DOI] [PubMed] [Google Scholar]
- 106.Barshes NR, Lee TC, Balkrishnan R, Karpen SJ, Carter BA, Goss JA. Risk stratification of adult patients undergoing orthotopic liver transplantation for fulminant hepatic failure. Transplantation 2006;81(02):195–201 [DOI] [PubMed] [Google Scholar]
- 107.Reddy KR, Ellerbe C, Schilsky M, et al. ; Acute Liver Failure Study Group. Determinants of outcome among patients with acute liver failure listed for liver transplantation in the United States. Liver Transpl 2016;22(04):505–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wong NZ, Reddy KR, Bittermann T. Acute liver failure etiology is an independent predictor of waitlist outcome but not posttrans-plantation survival in a national cohort. Liver Transpl 2022;28 (01):39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Campsen J, Blei AT, Emond JC, et al. ; Adult-to-Adult Living Donor Liver Transplantation Cohort Study Group. Outcomes of living donor liver transplantation for acute liver failure: the adult-to-adult living donor liver transplantation cohort study. Liver Transpl 2008;14(09):1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamashiki N, Sugawara Y, Tamura S, et al. Outcomes after living donor liver transplantation for acute liver failure in Japan: results of a nationwide survey. Liver Transpl 2012;18(09):1069–1077 [DOI] [PubMed] [Google Scholar]
- 111.Chen HS, Joo DJ, Shaheen M, et al. Randomized trial of spheroid reservoir bioartificial liver in porcine model of post-hepatectomy liver failure. Hepatology 2019;69(01):329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ahmadi AR, Chicco M, Wesson RN, et al. Stem cell mobilization is lifesaving in a large animal preclinical model of acute liver failure. Ann Surg 2018;268(04):620–631 [DOI] [PubMed] [Google Scholar]
- 113.Wuestefeld T, Pesic M, Rudalska R, et al. A direct in vivo RNAi screen identifies MKK4 as a key regulator of liver regeneration. Cell 2013;153(02):389–401 [DOI] [PubMed] [Google Scholar]
- 114.Bernal W, Donaldson N, Wyncoll D, Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet 2002;359(9306):558–563 [DOI] [PubMed] [Google Scholar]


