Abstract
Escherichia coli TUM1083, which is resistant to ampicillin, carbenicillin, cephaloridine, cephalothin, piperacillin, cefuzonam, and aztreonam while being sensitive to cefoxitin, moxalactam, cefmetazole, ceftazidime, and imipenem, was isolated from the urine of a patient treated with β-lactam antibiotics. The β-lactamase (Toho-2) purified from the bacteria hydrolyzed β-lactam antibiotics such as penicillin G, carbenicillin, cephaloridine, cefoxitin, cefotaxime, ceftazidime, and aztreonam and especially had increased relative hydrolysis rates for cephalothin, cephaloridine, cefotaxime, and ceftizoxime. Different from other extended-spectrum β-lactamases, Toho-2 was inhibited 16-fold better by the β-lactamase inhibitor tazobactam than by clavulanic acid. Resistance to β-lactams was transferred by conjugation from E. coli TUM1083 to E. coli ML4909, and the transferred plasmid was about 54.4 kbp, belonging to the incompatibility group IncFII. The cefotaxime resistance gene for Toho-2 was subcloned from the 54.4-kbp plasmid. The sequence of the gene was determined, and the open reading frame of the gene was found to consist of 981 bases. The nucleotide sequence of the gene (DDBJ accession no. D89862) designated as blatoho was found to have 76.3% identity to class A β-lactamase CTX-M-2 and 76.2% identity to Toho-1. It has 55.9% identity to SHV-1 β-lactamase and 47.5% identity to TEM-1 β-lactamase. Therefore, the newly isolated β-lactamase designated as Toho-2 produced by E. coli TUM1083 is categorized as an enzyme similar to Toho-1 group β-lactamases rather than to mutants of TEM or SHV enzymes. According to the amino acid sequence deduced from the DNA sequence, the precursor consisted of 327 amino acid residues. Comparison of Toho-2 with other β-lactamase (non-Toho-1 group) suggests that the substitutions of threonine for Arg-244 and arginine for Asn-276 are important for the extension of the substrate specificity.
β-Lactam antibiotics are widely used as front line agents in the clinical field. In the early 1980s, expanded-spectrum β-lactams, with stability for β-lactamase and good activity against gram-negative bacteria, were first used in the clinical setting. Not long after the beginning of wide use of the expanded-spectrum β-lactams, extended-spectrum β-lactamases were isolated in Europe and the United States and now have become a serious problem in the clinical field (29). In the late 1980s and early 1990s, those enzymes hydrolyzing the expanded-spectrum β-lactams were generally derived from TEM- or SHV-type β-lactamases through several mutations (6, 24). The mutations of Glu-104, Arg-164, and Glu-240 have been suggested to be important for the spectrum expansion (16, 24). In more recent years, non-TEM- or non-SHV-type β-lactamases such as Toho-1 (13), CTX-M-2 (5), and MEN-1 (3) have been identified. Those β-lactamases have high homology to the chromosomally encoded β-lactamase of Proteus vulgaris or Klebsiella oxytoca (2, 9, 23). In most cases, the β-lactamase-producing organisms show resistance to expanded-spectrum β-lactams such as cefotaxime and ceftazidime (6, 24). On the other hand, they are susceptible to carbapenems such as imipenem (6, 24). The main characteristic of those class A β-lactamases, except TEM-30 to TEM-40, is that they are sensitive to β-lactamase inhibitors such as clavulanic acid, sulbactam, and tazobactam (6). The reaction mechanism and the amino acid residues associated with the spectrum expansion of β-lactamases are still under investigation. Ishii et al. (13) proposed that mutations at positions 244 and 276 are important for the substrate extension after performing sequence alignment of Toho-1 and other β-lactamases.
The expanded-spectrum β-lactam-resistant strains isolated from several hospitals were surveyed and collected. We investigated those strains by enzymological and molecular biological methods and focused upon Escherichia coli TUM1083, a cefotaxime-resistant clinical isolate.
In this report, we discuss a correlation between the mutation and the substrate specificity of the β-lactamase from E. coli TUM1083 based on the sequence alignment and a three-dimensional structure of a related β-lactamase of Bacillus licheniformis (17).
MATERIALS AND METHODS
Bacterial strains and plasmids.
Table 1 shows the bacterial strains and plasmids used in this study. E. coli TUM1083 was isolated in March 1995 from the urine of a 69-year-old male who suffered from colon cancer. Before strain isolation, the patient had received an empiric antibiotic treatment consisting of a combination of piperacillin, cefuzonam, cefotiam, imipenem, levofloxacin, and tosulfloxacin. The strain did not ferment lactose but was identified as E. coli by tests with API 20E (Asuka, Tokyo, Japan) and the Vitek system (bioMerieux Vitek, Inc., Hazelwood, Mo.). E. coli ML4909, used for plasmid conjugation, was provided by Matsuhisa Inoue of Kitasato University. E. coli AS226 (13) was used for β-lactamase purification, and E. coli MV1184 (31) was used for transformation.
TABLE 1.
Bacterial strains and plasmids used
| E. coli strain or plasmid | Characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| TUM1083 | Clinical isolate, cefotaxime-resistant strain | This study |
| TUM1103 | Transformant, E. coli AS226 harboring pMTY036 | This study |
| ML4909 | F−galK2 galT22 hsdR metB1 relA supE44 Rifr | M. Inoue |
| C600 | F−thi-1 thr-2 leuB6 lacY1 tonA21 supE44 λ−Rifr | 27 |
| AS226 | F−thr-1 leuB6 thi-1 hsdS1 lacY1 tonA21 supE44 ampCΔ λ− | 13 |
| MV1184 | ara Δ (lac-proAB) Δ (srl-racA) 306::Tn10 φ80 dlacZ ΔM15 rpsL thi [F′ lacIqlacZ ΔM15proAB traD36] | 31 |
| Plasmids | ||
| pHSG397 | lac cat | 30 |
| pMTY002 | Cefotaxime resistant | This study |
| pMTY036 | 2.1-kb Sau3AI fragment from pMTY002 cloned into pHSG397 | This study |
| R388 | IncW | 33 |
| R386 | IncFI | 27 |
| R100-1 | IncFII | 27 |
| R124 | IncFIV | 27 |
| R64-11 | IncIα | 27 |
| R621α | IncIγ | 10 |
| R27 | IncHI | 27 |
| N3 | IncN | 27 |
| R751 | IncP | 15 |
| R446B | IncM | 27 |
| R401 | IncT | 27 |
| R6K | IncX | 27 |
Conjugation.
Conjugation was performed by the broth method (7). E. coli TUM1083 and E. coli ML4909 (recipient) were incubated for 30 min at 35°C before selection of transconjugant.
Antibacterial agents.
Penicillin G and ampicillin (Meiji Seika, Ltd., Tokyo, Japan); oxacillin and imipenem (Banyu Pharmaceutical Co., Ltd., Tokyo, Japan); carbenicillin and ceftizoxime (Fujisawa Pharmaceutical Co., Ltd., Tokyo, Japan); piperacillin (Toyama Chemical Co., Ltd., Tokyo, Japan); cefuzonam (Lederle Japan Ltd., Tokyo, Japan); cephalothin, cephaloridine, and moxalactam (Shionogi & Co., Ltd., Osaka, Japan); ceftazidime (Nippon Glaxo Ltd., Tokyo, Japan); cefotaxime (Hoechst Marion Roussel, Ltd., Tokyo, Japan); aztreonam (Sankyo Co., Ltd., Tokyo, Japan); and tazobactam and YP-14, a combination of tazobactam and piperacillin at a ratio of 1 to 4, respectively (Taiho Pharmaceutical Co., Ltd., Tokyo, Japan), all with known potencies, were used.
Drug sensitivity tests.
MICs were determined by the broth microdilution method with Mueller-Hinton broth (Difco, Detroit, Mich.). The organisms were inoculated at about 5 × 105 cells/well with MIC2000 (Dynatech, McLean, Va.).
The MIC was defined as the lowest concentration preventing visible growth after incubation for 18 h at 35°C.
Incompatibility tests and β-lactamase assay.
Incompatibility tests were carried out according to the method described in a previous report (7). The plasmids and strains used in this study were described previously (13).
Purification of β-lactamase.
The β-lactamase was purified from E. coli TUM1103, in which pMTY036 was transformed. The organisms were incubated for 5 h in 2 liters of Luria-Bertani broth and centrifuged at 7,000 × g for 10 min at 4°C. The supernatant was discarded, and MES (morpholineethanesulfonic acid)-NaOH buffer (20 mM, pH 6.5) was added to the tube to suspend the sediments. The suspended solution was disrupted by sonication (100 W, 30 min) in a volume of 15 ml of MES-NaOH buffer (20 mM, pH 6.5). The lysates were centrifuged again at 45,000 × g for 30 min. The supernatant was dialyzed for 24 h against 10 mM MES-NaOH buffer (pH 6.5), was then applied to carboxymethyl–Bio-Gel A (column size, 2.5 by 10 cm; Bio-Rad, Richmond, Calif.), and washed overnight with 10 mM MES-NaOH buffer (pH 6.5). Elution was performed with 10 mM MES-NaOH buffer (pH 6.5) containing 0.05 M NaCl. The activities of the eluted fractions were checked with nitrocefin (Oxoid, Basingstoke, England), and the active fractions were pooled and concentrated with a Centriprep-10 concentrator (Amicon, Beverly, Mass.) and purified again by fast protein liquid chromatography (column; Mono S 5/5 [Pharmacia Biotech, Uppsala, Sweden]; binding buffer, 10 mM MES-NaOH [pH 6.5]; elution buffer, 10 mM MES-NaOH [pH 6.5]–25 mM NaCl; flow rate, 0.5 ml/min; detector; UV, 280 nm). The purified β-lactamase was concentrated with a Centriprep-10 concentrator (Amicon). Isoelectric focusing was carried out with a Multiphor II electrophoresis system (Pharmacia Biotech) and a gel plate containing 2% Ampholine (pH 6.0 to 8.0). The enzyme protein on the gel plate was detected by staining with Coomassie brilliant blue R-250. The molecular weight was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (18).
Assay of β-lactamase activity.
The activity of the highly purified β-lactamase was measured by spectrophotometric assay (32) with a spectrophotometer (DU640; Beckman, Fullerton, Calif.) with a thermoregulator. The peak wavelength for each antibiotic used for the measurement was set according to that described in previous reports (13). Km and kcat values were derived by the linear regression analysis of Lineweaver-Burk plots (19) of initial velocity data that were obtained at different substrate concentrations ranging from 10 to 100 μM. The apparent Ki value was determined with nitrocefin as substrate at concentrations from 10 to 100 μM, after preincubation for 1 min with β-lactamase inhibitor concentrations from 1 to 25 μM, and data were analyzed by a Dixon plot (8).
Cloning and analysis of recombinant plasmids.
Plasmid DNA was purified by extracting plasmid DNA by the large-scale alkaline method and the ethidium bromide-CsCl linear gradient method (26) at 80,000 rpm for 16 h with a Beckman TLA centrifuge (Beckman). Restriction enzymes and T4 DNA ligase were purchased from Takara Shuzo Co., Ltd. (Shiga, Japan). The plasmid size was calculated from the size of the fragments obtained by cleaving the plasmid with restriction enzymes with cleaving λ phage DNA cleaved with StyI as a molecular marker. The cefotaxime resistance gene was cloned as follows. After plasmid DNA was cleaved partially by Sau3AI, the resultant fragments were ligated into the BamHI site of pHSG397 (30). E. coli MV1184 (31) was transformed with the ligated DNA, and cefotaxime-resistant colonies were selected on an L agar plate (26) supplemented with 10 μg of cefotaxime per ml.
DNA sequencing analysis.
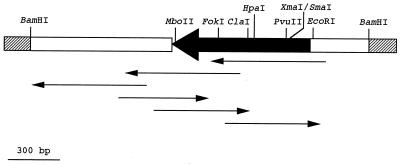
After pMTY036 was double digested with KpnI and XhoI, SphI and SalI deletion mutants were prepared by using a Takara deletion kit (Takara Shuzo). From these deletion mutants, five subclones were sequenced with the universal primer M13 pUC sequencing primer (Takara Shuzo), the Takara Bca BEST Dideoxy sequencing kit (Takara Shuzo), and a DSQ-1000 DNA sequencer (Shimadzu, Tokyo, Japan). Then, a 17-mer oligonucleotide reversed primer was prepared on the basis of the results obtained with the universal primer. The sequence was determined according to the scheme shown in Fig. 1.
FIG. 1.
Sequencing strategies for the blatoho gene from pMTY036. The insert is shown as a dark, thick arrow, and lacZ is shown by open boxes. Sequence strategy is indicated by the arrows, which represent overlapping deletion mutants for sequencing of the blatoho gene.
Computer analysis.
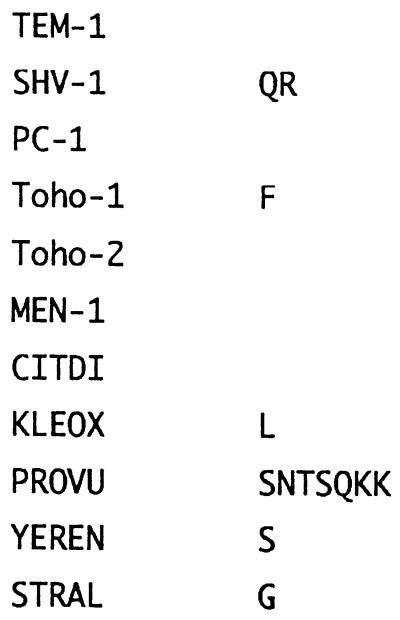
The DNA sequence data were analyzed primarily by using a UNIX computer and software from the DNA Data Bank of Japan (National Institute of Genetics, Mishima, Japan). The alignments of the DNA and peptide sequences were examined by using the Fasta mail server (22), and the multiple sequence alignment was examined by using the ODEN and Karashi programs (12). The sequences extracted from the database and used for examination of the multiple alignment were Toho-1 from E. coli TUH12191 (13), MEN-1 from E. coli MEN-1 (3), KLEOX from K. oxytoca E23004 (2), CITDI from Citrobacter diversus ULA 27 (25), STRAL from Streptomyces albus G (20), TEM-1 from E. coli TEM-1 (14), SHV-1 from E. coli SHV-1 (4), PC-1 from Staphylococcus aureus PC-1 (11), PROVU from Proteus vulgaris RO104 (23), and YEREN from Yersinia enterocolitica (28).
Nucleotide sequence accession number.
The nucleotide sequence data for the Toho-2 gene appear in the EMBL-GenBank-DDBJ data libraries under accession no. D89862.
RESULTS
One hundred fifty strains of E. coli or Klebsiella pneumoniae which show resistance to expanded-spectrum β-lactams were examined by PCR with the specific primer sets of the TEM (14), SHV (4), KOXY (2), Sme-1 (21), and Toho-1 (13) types of β-lactamases. No positive strain was detected by PCR with all the primer sets. The cefotaxime-resistant E. coli TUM1083 strain, which produces β-lactamase Toho-2, was identified from those strains. E. coli TUM1083 was isolated in March 1995 from the urine of a 69-year-old male patient. The patient suffered from colon cancer and was administered piperacillin, cefuzonam, cefotiam, imipenem, levofloxacin, and tosulfloxacin. The strain produced a new β-lactamase with an isoelectric point of 7.7 named Toho-2.
Plasmid profile.
Transconjugants which acquired cefotaxime resistance by conjugation appeared at a frequency of 10−4 to 10−6. A plasmid profile of 20 transconjugants revealed the presence in each of a single 54.4-kbp plasmid. The plasmid, which is called pMTY002 (pMTY; registered with the Plasmid Reference Center), was cleaved into seven segments by EcoRI or HindIII. From the size of the fragments obtained, the size of pMTY002 was estimated to be about 54.4 kbp. The incompatibility of pMTY002 was examined by conjugation with E. coli C600 strains containing various different Inc plasmids listed in Table 1. In this way, pMTY002 was shown to be within incompatibility group FII.
Cloning of the β-lactamase gene.
The fragments of pMTY002 generated by partial digestion with Sau3AI were inserted into pHSG397 and were transformed to E. coli MV1184. One plasmid, pMTY036, of about 4.3 kbp was isolated from a transformant selected on an L agar plate containing 10 μg of cefotaxime per ml. A restriction map and the position of the β-lactamase structural gene blatoho are shown in Fig. 1.
Susceptibility to antibiotics.
Table 2 shows MICs of β-lactam antibiotics against E. coli TUM1083 and E. coli ML4909 with and without pMTY002. Susceptibility tests were conducted with 10 clones of transconjugants. MICs of all penicillins, cephalothin, cefotaxime, and cefuzonam against E. coli TUM1083 and E. coli ML4909 (pMTY002) were ≥256 μg/ml. The MICs of inhibitors against E. coli TUM1083 and E. coli ML4909(pMTY002) were 512 μg/ml or more. However, MICs of piperacillin to these strains were markedly decreased to 16 and 2μg/ml in the presence of 4 and 0.5 μg/ml of tazobactam per ml. The MICs of aminoglycoside antibiotics against E. coli ML4909 were not changed in the presence of pMTY002.
TABLE 2.
MICs of various drugs for strains producing Toho-2 β-lactamase
| Drug | MIC (μg/ml) for E. coli strain:
|
||
|---|---|---|---|
| TUM1083 | ML4909(pMTY002) | ML4909 | |
| Ampicillin | >512 | >512 | 1.0 |
| Carbenicillin | >512 | >512 | 2.0 |
| Piperacillin | >512 | 512 | 0.5 |
| Cephalothin | >512 | 512 | 4.0 |
| Cefoxitin | 64 | 0.5 | 1.0 |
| Ceftizoxime | 128 | 1.0 | ≤0.3 |
| Cefotaxime | >512 | 256 | ≤0.3 |
| Ceftazidime | 4.0 | 0.5 | ≤0.3 |
| Cefuzonam | >512 | 512 | ≤0.3 |
| Moxalactam | 1.0 | ≤0.3 | ≤0.3 |
| Aztreonam | 256 | 4.0 | ≤0.3 |
| Imipenem | ≤0.3 | 0.5 | 0.5 |
| Piperacillin-tazobactama | 16 | 2.0 | 0.5 |
Tested at a ratio of 4:1, respectively.
Kinetic parameters.
β-Lactamase was purified from E. coli cells harboring pMTY036, as described under Materials and Methods. The purified enzyme gave a single band on isoelectric focusing (Coomassie blue staining) and on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, with an estimated molecular mass of 28,000 Da. As shown in Table 3, purified Toho-2 had high catalytic activity toward cephalothin, cephaloridine, cefotaxime, and piperacillin. the catalytic efficiency (kcat/Km) for those drugs was also much higher than for the other substrates tested. However, Toho-2 did not have high kcat values toward ampicillin, oxacillin, cefoxitin, ceftazidime, and imipenem. Relatively high Km values of these substrates reduced the catalytic efficiency (relative kcat/Km). The β-lactamase inhibitors, tazobactam and clavulanic acid, appeared to have the highest affinities of all the agents.
TABLE 3.
Kinetic parameters of Toho-2 β-lactamase
| Antibiotic | kcata (s−1) | Km or apparent Ki (μM) | kcat/Km (μM−1s−1) |
|---|---|---|---|
| Penicillin G | 3.6 | 6 | 0.6 |
| Ampicillin | 1.2 | 12 | 0.1 |
| Oxacillin | 9.1 | 91 | 0.1 |
| Carbenicillin | 14 | 18 | 0.8 |
| Piperacillin | 130 | 84 | 1.6 |
| Cephalothin | 12,000 | 470 | 25 |
| Cephaloridine | 4,500 | 180 | 25 |
| Cefoxitin | <0.1 | 4.8 | <0.1 |
| Ceftizoxime | 30 | 150 | 0.2 |
| Ceftazidime | 1.3 | 160 | <0.1 |
| Cefotaxime | 220 | 66 | 3.3 |
| Aztreonam | 0.1 | 140 | <0.1 |
| Imipenem | 4.8 | ||
| Clavulanic acid | 2.4 | ||
| Sulbactam | 4.5 | ||
| Tazobactam | 0.3 |
Absolute kcat value.
DNA sequencing.
The nucleotide sequence of 981-bp blatoho was determined by the strategy shown in Fig. 1. An 870-nucleotide open reading frame with a GC content of 70.2% was present in this sequence (Fig. 2). The sequence initiation codon (ATG) was preceded by a possible −10 region (TGGAAT) and a −35 region (TTGAAG) of a putative promoter. The termination codon was TAA. From the putative open reading frame, the precursor form of Toho-2 seemed to consist of 289 amino acid residues with a molecular mass of 30,725 Da. The processing site was determined by the comparison of the hypothetical amino acid sequence predicted by the DNA sequence with the last 10 amino acids from the N-terminal sequence determined by a peptide sequencer. The mature form consisted of 261 amino acid residues with a molecular mass of 27,752 Da. The consensus sequences such as STSK, SDN, and KTG in class A β-lactamases were found in the amino acid sequence of Toho-2 β-lactamase. Thus, Toho-2 is a class A β-lactamase.
FIG. 2.
Nucleotide sequence of the blatoho gene. The conserved amino acid residues found in the active sites of class A β-lactamases are underlined. The deduced cleavage site of the signal peptide is shown by an arrow. Bold-face indicates −35 and −10 sequences.
Homology with other β-lactamases.
The DNA sequence of the gene for Toho-2 showed high homology (76% or higher) with those for plasmid-mediated β-lactamases of CTX-M-2 (5) and Toho-1. Amino acid sequence identities of Toho-2 with E. coli MEN-1 (3), K. oxytoca β-lactamase (2), and Toho-1 are 72, 72, and 70%, respectively, while Toho-2 showed less than 30% amino acid sequence identity with TEM and SHV. The multiple sequence alignment of Toho-2 and other β-lactamases is shown in Fig. 3. The consensus sequences S70XXK73, S130DN132, and K234TG236 (Ambler numbering) and the highly conserved E166 residue, which are essential for the catalysis of class A β-lactamases, were found in Toho-2.
FIG. 3.
Amino acid sequence alignment of 11 class A β-lactamases. Dashes indicate gaps inserted in the alignment; asterisks indicate identical residues. The unique sequence of Toho-2 is underlined (Ala-185 to Ala-219). ABL, standard numbering scheme for class A β-lactamases (1). References for the enzymes are as follows: Toho-1 (13), MEN-1 (3), KLEOX (2), CITDI (25), STRAL (20), TEM-1 (14), SHV-1 (4), PC-1 (11), PROVU (23), and YEREN (28).
DISCUSSION
Generally, most of the class A β-lactamases are strongly inhibited by β-lactamase inhibitors (6). Clavulanic acid is often the most potent inhibitor for these enzymes (6). For Toho-1, Ki values for sulbactam and tazobactam were 5.8 and 5.3 μM, respectively; on the other hand, the apparent Ki value of Toho-1 with clavulanic acid was 0.6 μM (13). However, the extended-spectrum β-lactamase Toho-2 was more strongly inhibited by tazobactam than by clavulanic acid or sulbactam. It is very different from the β-lactamase reported heretofore.
Toho-2 has three major sequences distinct from those of other class A β-lactamases. Two of those are found in the N- and C-terminal sequences, though Toho-1 has a sequence similar to that of Toho-2. The other distinct sequence is located at Ala-185 to Ala-219 (Fig. 3). This sequence has almost no sequence homology with the other class A β-lactamases. The sequence may constitute a loop structure at the substrate-binding site and has a deletion of 2 amino acid residues at the binding site. This deletion may well correlate with the observation that tazobactam, a β-lactamase inhibitor, showed a higher affinity for Toho-2 than for Toho-1, whereas sulbactam binds to both enzymes with similar affinities. Tazobactam has the 1,2,3-triazolylmethyl group at C-2 instead of methyl groups for sulbactam. Examination of the location of the triazolylmethyl moiety at the binding site through three-dimensional structure modeling of the acyl enzyme suggests that the triazole moiety interacts with the loop where the deletion of 2 amino acid residues occurred. Thus, the triazole moiety would find suitable room to reside in a larger substrate-binding pocket.
Ishii et al. (13) have pointed out that in Toho-1 the replacement of Asn-276 by Arg with the concomitant substitution of Thr for Arg-244 is important for the extension of the substrate specificity and that Arg-276 may function as Arg-244. The Arg-274 of Toho-1 is also a characteristic residue since this residue could also be located at a site similar to Arg-276. Toho-2 had the same substitutions as Toho-1 at positions 244 and 276. However, the Ser-274 residue was found in Toho-2, instead of the Arg-274 residue found in Toho-1. Since the Ser-274 residue is found also in the β-lactamases of E. coli MEN-1 (3) and K. oxytoca E23004 (2), the basic residue at position 274 in Toho-1 may not play an important role for the cefotaxime hydrolysis, while it would contribute to the higher affinity of aztreonam, which has the carboxylate in the oxyimino side chain.
The substitution of serine for Ala-237 is also a common mutation observed in substrate-extended class A β-lactamases. We assume that this mutation brings about an enlarged binding site for the bulky oxyimino moiety of the expanded-spectrum cephalosporins such as cefotaxime, and thus the mutation should be very important for the substrate extension.
In conclusion, Toho-2 has two characteristic mutation sites for the substrate extension. The mutations at 237, 244, and 276, which are aligned at a peripheral site of the substrate-binding site, may contribute to the specificity for oxyiminocephalosporins such as cefotaxime and ceftizoxime. The other mutation-deletion at 185 to 219, particularly at the triazole-binding loop, may be responsible for the higher affinity for tazobactam.
ACKNOWLEDGMENTS
We thank Hirosuke Matsuo for providing us with E. coli TUM1083. We express our thanks to Teru Ogura and Akira Inoue for their valuable advice.
This study was supported in part by a grant from the Ministry of Health and Welfare of Japan in 1996 (Scientific Research Foundation on Drug Resistant Bacteria) and by Project Research grant 8-25 from the Toho University School of Medicine. Ling Ma was supported by a grant from the Suntory Institute for Bioorganic Research.
REFERENCES
- 1.Ambler R P, Coulson A F W, Frére J-M, Ghuysen J-M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa Y, Ohta M, Kido N, Mori M, Ito H, Komatsu T, Fujii Y, Kato N. Chromosomal β-lactamase of Klebsiella oxytoca, a new class A enzyme that hydrolyzes broad-spectrum β-lactam antibiotics. Antimicrob Agents Chemother. 1989;33:63–70. doi: 10.1128/aac.33.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthelemy M, Peduzzi J, Bernard H, Tancrede C, Labia R. Close amino acid sequence relationship between the new plasmid-mediated extended-spectrum beta-lactamase MEN-1 and chromosomally encoded enzymes of Klebsiella oxytoca. Biochim Biophys Acta. 1992;1122:15–22. doi: 10.1016/0167-4838(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 4.Barthelemy M, Peduzzi J, Labia R. Complete amino acid sequence of p453-plasmid-mediated PIT-2 β-lactamase (SHV-1) Biochem J. 1988;251:73–79. doi: 10.1042/bj2510073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauernfeind A, Stemplinger I, Jungwirth R, Ernst S, Casellas J M. Sequences of beta-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other beta-lactamases. Antimicrob Agents Chemother. 1996;40:509–513. doi: 10.1128/aac.40.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush K, Jacoby G A, Medeiros A A. A function classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chabbert Y A, Scavizzi M R, Witchitz J L, Gerbaud G R, Bouanchaud D H. Incompatibility groups and the classification of fi− resistance factors. J Bacteriol. 1972;112:666–675. doi: 10.1128/jb.112.2.666-675.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon M. The determination of enzyme inhibitor constants. Biochem J. 1953;55:170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fournier B, Roy P H. Variability of chromosomally encoded β-lactamases from Klebsiella oxytoca. Antimicrob Agents Chemother. 1997;41:1641–1648. doi: 10.1128/aac.41.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedges R W, Datta N. Plasmids determining I pili constitute a compatibility group. J Gen Microbiol. 1973;77:19–25. doi: 10.1099/00221287-77-1-19. [DOI] [PubMed] [Google Scholar]
- 11.Herzberg O, Moult J. Bacterial resistance to β-lactam antibiotics: crystal structure of β-lactamase from Staphylococcus aureus PC1 at 2.5 Å resolution. Science. 1987;236:694–701. doi: 10.1126/science.3107125. [DOI] [PubMed] [Google Scholar]
- 12.Ina Y. ODEN: a program package for molecular evolutionary analysis and database search of DNA and amino acid sequences. Comput Appl Biosci. 1994;10:11–12. doi: 10.1093/bioinformatics/10.1.11. [DOI] [PubMed] [Google Scholar]
- 13.Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A beta-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jelsch C, Lenfant F, Masson J M, Samama J P. β-Lactamase TEM1 of E. coli. Crystal structure determination at 2.5 Å resolution. FEBS Microbiol Lett. 1992;299:135–142. doi: 10.1016/0014-5793(92)80232-6. [DOI] [PubMed] [Google Scholar]
- 15.Jobanputra R S, Datta N. Trimethoprim R factors in enterobacteria from clinical specimens. J Med Microbiol. 1974;7:169–177. doi: 10.1099/00222615-7-2-169. [DOI] [PubMed] [Google Scholar]
- 16.Knox J R. Extended-spectrum and inhibitor-resistant TEM-type beta-lactamases: mutations, specificity, and three-dimensional structure. Antimicrob Agents Chemother. 1995;39:2593–2601. doi: 10.1128/aac.39.12.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knox J R, Moews P C, Escobar W A, Fink A L. A catalytically-impaired class A β-lactamase: 2 Å crystal structure and kinetics of the Bacillus licheniformis E166A mutant. Protein Eng. 1993;6:11–18. doi: 10.1093/protein/6.1.11. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56:658–663. [Google Scholar]
- 20.Meester F D, Joris B, Lenzini M V, Dehottay P, Erpicium T, Dusart J, Klein D, Ghuysen J-M, Frére J-M, Beeumen V. The active sites of the β-lactamases of Streptomyces cacaoi and Streptomyces albus G. Biochem J. 1987;244:427–432. doi: 10.1042/bj2440427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naas T, Vandel L, Sougakoff W, Livermore D M, Nordmann P. Cloning and sequence analysis of the gene for a carbapenem-hydrolyzing class A beta-lactamase, Sme-1, from Serratia marcescens S6. Antimicrob Agents Chemother. 1994;38:1262–1270. doi: 10.1128/aac.38.6.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peduzzi J, Reynaud A, Baron P, Barthelemy M, Labia R. Chromosomally encoded cephalosporin-hydrolyzing β-lactamase of Proteus vulgaris RO104 belongs to Ambler’s class A. Biochim Biophys Acta. 1994;1207:31–39. doi: 10.1016/0167-4838(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 24.Raquet X, Lamotte-Brasseur J, Fonze E, Goussard S, Courvalin P, Frére J-M. TEM β-lactamase mutants hydrolysing third-generation cephalosporins. A kinetic and molecular modelling analysis. J Mol Biol. 1994;244:625–639. doi: 10.1006/jmbi.1994.1756. [DOI] [PubMed] [Google Scholar]
- 25.Rerilli M, Franceschini N, Segatore B, Amicosante G, Oratore A, Duez C, Joris B, Frére J-M. Cloning and nucleotide sequencing of the gene encoding the β-lactamase from Citrobacter diversus. FEMS Microbiol Lett. 1991;83:79–84. doi: 10.1016/0378-1097(91)90448-j. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Sasakawa C, Takamatsu N, Danbara H, Yoshikawa M. A method of plasmid classification by integrative incompatibility. Plasmid. 1980;3:116–127. doi: 10.1016/0147-619x(80)90103-1. [DOI] [PubMed] [Google Scholar]
- 28.Seoane A, Lobo J M G. Cloning of chromosomal β-lactamase genes from Yersinia enterocolitica. J Gen Microbiol. 1991;137:141–146. doi: 10.1099/00221287-137-1-141. [DOI] [PubMed] [Google Scholar]
- 29.Sirot D, De Champs C, Chanal C, Labia R, Darfeuille-Michaud A, Perroux R, Sirot J. Translocation of antibiotic resistance determinants including an extended-spectrum beta-lactamase between conjugative plasmids of Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother. 1991;35:1576–1581. doi: 10.1128/aac.35.8.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeshita S, Sato M, Toba M, Masahashi W, Gotoh T H. High-copy-number and low-copy-number plasmid vectors for lacZα-complementation and chloramphenicol- or kabanycin-resistance selection. Gene. 1987;61:63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- 31.Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 32.Waley S G. A spectrophotometric assay of β-lactamase action on penicillins. Biochem J. 1974;139:789–790. doi: 10.1042/bj1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward J M, Grinsted J. Physical and genetic analysis of the Inc-W group plasmids R388, Sa and R7K. Plasmid. 1982;7:239–250. doi: 10.1016/0147-619x(82)90005-1. [DOI] [PubMed] [Google Scholar]