Abstract
Reptilia exploit a large diversity of food resources from plant materials to living mobile prey. They are among the first tetrapods that needed to drink to maintain their water homeostasis. Here were compare the feeding and drinking mechanisms in Reptilia through an empirical approach based on the available data to open perspectives in our understanding of the evolution of the various mechanisms determined in these Tetrapoda for exploiting solid and liquid food resources.
This article is part of the theme issue ‘Food processing and nutritional assimilation in animals’.
Keywords: feeding, transport, evolution, Reptilia, Aves
1. Introduction
All Reptilia are characterized by a series of characters related to their survival at different times of their life (e.g. eggshell properties). Regarding the phylogenetic relationship between non-avian and avian Reptilia, it is now accepted that modern birds are theropod dinosaurs with crown groups including modern species generally dating from the Eocene Epoch (55–55 Myr) [1,2]. Non-avian living Reptilia involve two groups (i) Pan-Lepidosauria, which includes Rhyncocephalia and Squamata and (ii) Archosauria, including modern Crocodilia and Testudines. The evolution of food transport in these tetrapods has attracted attention for the past four decades on the basis of integrating morphological and functional studies in many lineages. Regardless of their phylogenetic relationships, these clades show two main kinds of feeding behaviour: terrestrial and aquatic feeding. The ancestral Pan-Reptilia that gave rise to Reptilian clades probably lived in swamps of the late Carboniferous period [3–6]. Although the origin of the different Reptilian clades is still discussed based on molecular studies and novel discoveries of fossils, it seems that their ancestors exploited food resources regardless of their properties and transported their food in the aerial environment. In Reptilia, liquid and solid food/prey transport is considered as a phase of the feeding and drinking sequences. Both types of sequences primarily involve ingestion, transport and processing in feeding where it occurs (e.g. mastication), and swallowing.
These tetrapods were probably able to catch food in water, in air and at their interface, but the use of water as a vehicle to transport their food remains unknown. During their history, various lineages of Reptilia secondarily returned to the aquatic habitat with a complete aquatic feeding behaviour from food catching to swallowing (e.g. turtles; the Galapagos marine iguana; penguins) [7,8]. Aquatic transport is probably the most common mode of food transport in Testudines, with wholly terrestrial feeding being unusual (i.e. limited to Testudinidae) [9]. In Crocodilia, derived from truly terrestrial ancestors, prey capture occurs either in water or at its surface [10]. Food transport could occur in both fluids, but has been studied in animals with their heads out of water [10,11]. In Aves, some specialized lineages using various types of food are able to complete the entire feeding sequence from capture to swallowing in water (e.g. penguins, gannets, ducks), while a large number of species are able to catch their food items in water (e.g. shorebirds, gulls, cormorants, grebes), but remain in the air for transporting items to the pharyngeal cavity and subsequent swallowing [12]. Species with aquatic lifestyles consume food resources at various depths of the water column and on the substrata of marine and fresh water habitats. Some Aves are adapted to catch and transport prey (mainly arthropods) living on the water surface by virtue of surface tension [13,14]. It is salient to note that some shorebirds are able to feed on and therefore to transport superficial biofilms of mudflats at some stages of their migratory history [15]. All other Reptilia (e.g. the majority of Lepidosauria, and many bird species) catch and transport their food/prey solely in air. Here we highlight the collected knowledge of the transport mechanism of liquid and solid foods to suggest some comparative approaches in non-avian and avian Reptilia. Because of the diversity of these transport modes, Reptilia, including Aves, can be viewed as key model organisms to study the evolutionary pathways that show how vertebrates use their feeding apparatus to exploit a great diversity of liquid and solid food resources in aquatic and terrestrial environments.
Regardless of their feeding ecology and morphological traits, food transport in all clades of Reptilia occurs in both fluids, water and air, and at their interfaces. In the first case, water can be used as a fluid to help food transport in association with each gape and hyobranchium cycle. Incompressible water can either be the only vehicle to transport the food, or used as a complementary vehicle to the tongue to move the food backwards towards the pharynx. In the aerial environment, food transport cannot be based on compressible air as a vehicle to move the food into the oro-pharyngeal cavity. Only elements of the feeding apparatus—whether aided by postcranial movements or not—can be used to move the food through the oro-pharyngeal cavity. The action of the feeding apparatus is strongly linked to the postcranial action in both environments (e.g. suction/ram feeding in aquatic habitats) [9,16].
The salient point is that reptilians were among the first tetrapods (including stem mammals in addition to pan-reptiles) that required use of the same feeding apparatus designs for transporting both liquid and solid food with highly variable diverse properties (e.g. size, volume, texture, behaviour in case of living prey), regardless of the morphological specializations of this apparatus. They also have to feed and transport solid food and water either immediately after hatching or after yolk sac exhaustion [17,18]. In the majority of Reptilia, neonates have to feed like adults, without any period of transition. In Aves, altricial hatchling birds have a transitional nesting phase when food is delivered by the parents, often deep into the pharynx. However, precocial species, in which hatchlings are immediately mobile after leaving the egg, must feed independently like adults; many of these are aquatic birds (e.g. ducks, geese).
The results of functional studies of the feeding apparatus in solid food transport were used to propose two general models for the motor pattern of food transport, regardless of historical (morphological) properties and feeding ecology: Bramble and Wake's (BW) and Reilly and Lauder's (RL) models [19–21]. These models combine data from analyses of images (by high-speed photography, electromyography (at least for a small number of species) and more recently from data recorded with new techniques such as XROMM, combined with electromyography, fluoromicrometry and contrast-enhanced CT-scanning) and finite element analysis (FEA) [22,23]. These models, initially emerging in the 1980s and 1990s, show that rhythmic coordinated elements of the feeding apparatus ensure solid food and liquid transport from the environment to the pharyngeal cavity. Both models depict the spatial and temporal relationships between the skull, the upper and lower jaws, and the hyo-lingual apparatus. The opening phase of the BW model has been discussed for many Reptilia, including squamates, on the basis of methodological approach [20]. In birds, food transport is included in the story of the evolution of the pecking behaviour [24,25]. The BW and RL models were primarily established from comparative studies in non-mammalian tetrapods (e.g. Lissamphibia and Reptilia) and have been tested in a large number of living Reptilia lineages with various behavioural feeding ecologies and diets. Functionally, each clade is often studied separately, thus emphasizing different evolutionary pathways to explain their feeding success. However, these models were not tested in drinking behaviour because gape follows a rather simple opening–closing cycle associated with lingual cycles [26,27]. Here we predominantly compare the food transport behaviour of Reptilia in terrestrial habitats (including the interface of water/air, such as on the sea shore) and not the fully aquatic transport, which has been recently reviewed [11].
2. Drinking
All liquids are characterized by similar physical properties, including molecular cohesion, incompressibility, viscosity and wettability. Reptilia were among the first tetrapods that absolutely had to gain water by drinking to maintain water content homeostasis (i.e. osmoregulation or fluid balance). This was mainly achieved by transporting water through their oro-pharyngeal cavity to the digestive tract. The hyo-lingual apparatus employ a completely different actions in two modes of drinking: (i) suction, used by the majority of Testudines (although the role of the tongue remains to be clarified in various species) and some Squamata (e.g. snakes) and (ii) lingual-based drinking or licking [28]. Various species living in habitats with little free water (e.g. xeric habitats, brackish and seawater) have even developed specialized morphological devices and/or behavioural patterns to gain and transport water to the buccal cavity. In xeric lizards, the water always flows from the body surface to the edge of the mouth, to be transported toward the pharynx by the tongue as in non-xeric squamates, regardless of their lingual structures [29–33].
A buccal pump mechanism is used by snakes and turtles [27,34–38]. Water enters the oro-pharyngeal cavity by depression cycles of the anterior tips of the mandibles and the enlargement of the oro-pharyngeal cavity produced by depression of the buccal floor (with or without lingual action in snakes). In turtles, cyclic action of the hyo-lingual apparatus acts to modify the buccal pressure and induce water entrance during the immersion phase. It is interesting to note that some turtles can reject water from their cavity, probably when too much water has accumulated in the oro-pharyngeal cavity during the phase before swallowing.
In lingual-based drinking, the liquid uptake and transport are a continuous process produced by rhythmic lingual protrusion–retraction cycles in a phase called the immersion phase [26,28,31,32,39]. In all Reptilia, even in the highly specialized nectarivorous birds, the liquid must be offloaded and intraorally transported to the pharynx, where liquids are swallowed [40]. Water swallowing either occurs in a phase called emersion, or in continuance of the intraoral transport (e.g. hummingbirds). The liquid is gathered by the tongue through various mechanisms including (but not exclusively) (i) capillarity on the dorsal lingual surface in contact with water (e.g. lizards) [41], (ii) lingual suction [31], (iii) scooping (e.g. finches) [32], (iv) fluid trapping and elastic filling (e.g. nectarivorous birds) [12,42,43], (v) holding action of the immersed bill tips accompanied by the surface tension along the mouth epidermis (e.g. mallard) [44,45], (vi) hydraulic piston of the tongue to move the water at the entrance of the oral cavity (e.g. Tegu lizards, V Bels 2023, personal observation) and (vii) generation of water columns by inertia produced by lapping action of the adhesive curled tongue again gravity (e.g. lorikeets, V Bels 2017, personal observation), as in cats [46] and dogs [47]. In all of the mechanisms used by the tongue to gather the liquid, the tongue acts to produce a continuous water/nectar movement from uptake through the buccal cavity to the pharynx, regardless of the jaw (and rhamphotheca) plus lingual morphologies. All of these mechanisms are related to lingual movements and tongues with highly specialized keratinous surface structures (e.g. lamellae in hummingbird), or with papillary structures covering the fore tongue that contacts water (e.g. tongues of pigeons and lizards).
An overview of drinking in Reptilia shows that tongue contact between its surface loaded with water and the surfaces of internal buccal cavities is the main mechanism for liquid delivering corresponding to intrabuccal transport. In lizards, water is delivered to a first compartment and then moved to a second compartment bounded between the tongue, the side and the roof of the buccal cavity. Finally, water is accumulated in the pharyngeal cavity corresponding to a final compartment and variously swallowed in separate phases (e.g. emersion phase; pharyngeal scope) [26,48]. In hummingbirds, special internal structures are able to move the nectar from the tongue to the buccal cavity by wringing (i.e. squeezing their tongues between the upper and lower bills) [49]. In addition to drinking, extremely specialized nectivorous birds also have to capture and swallow solid food (particularly flying insects) to take in enough protein for survival. These birds catch their prey in flight and the prey directly enters the pharynx to be swallowed without any mode of transport within the buccal cavity [50].
The emerging message from all drinking studies is that the relationship between lingual structures and the internal shape and structures of the oro-pharyngeal cavity plays a key role in the immersion phase by (i) gathering liquid on the tongue to draw the liquid into the buccal cavity and (ii) transporting the liquid into the spaces between the internal epidermal surfaces of the skull, regardless of the mechanism of gaining the liquid (e.g. water and nectar). A main finding is that only the structural organization of the oro-pharyngeal cavity has an effect on the amount of water delivered during the lingual cycles before the emersion phase (swallowing). The transport of liquid to the pharynx is similar in all Reptilia, with a key role played by the contact between the lingual surface and the internal surface of the buccal cavity regardless of the related specialized lingual keratinous features (e.g. papillae or lamellae).
3. Feeding
Gaining solid food requires its initial capture from the environment. Regardless of the lineages and their feeding ecologies, classical Darwinian theory supports the relationship between diet specialization and some characteristics of anatomical design such as the skull and its properties (e.g. shape, kinetism, biting force) [51–57], although dietary preference does not always have a major effect on some of its morphological traits [58]. It is well recognized that several structural characteristics of feeding apparatus designs (e.g. skull, tongue) [10,59–62], as well as the head–neck articulation that can play a key role in transport, are influenced by integrated historical (phylogenetic) and ecological (feeding ecology) factors, with the relative weight of each factor remaining variable among the studied species [11,63,64]. Furthermore, the feeding apparatus is often used in many behaviours independent of feeding ecology; these include sexual and agonistic displaying (including biting), plus vocalization and nest building in Aves [65,66]. The constraints imposed by these other functions can probably modulate the use of the feeding apparatus in feeding (e.g. such as chemoreception in squamates). In such Reptilia, the characteristics (kinematic and neuro-motor control) of food transport are mainly discussed in relation to a trade-off between lingual functional and morphological properties in any phase of feeding and chemoreception [67,68]. In all other Reptilia, the mode of transport is discussed in relation to the habitat where feeding occurs and its impact on food properties (e.g. terrestrial/aquatic evolution and diet in various Testudines species [9,69]).
Feeding on solid food poses completely different problems from dealing with liquid food. Transporting solids relies on their variable properties (e.g. volume, size, toughness, texture, anti-predator behaviour) and the medium in which they are transported. Food transport is the feeding phase where the selected food/prey is moved toward the pharynx to be swallowed, regardless of its properties. It also depends on the mode of food/prey catching (figure 1). In aquatic Reptilia, food/prey catching in water is achieved by suction and/or biting, and the tongue and water movement in the oral cavity act for food transport (as in Testudines) [9] Some birds [71] display the highly specialized mode of filter feeding [45,72,73]. In terrestrial habitats, Reptilia use two modes of food/prey catching: lingual prehension and jaw prehension (biting). Food can be transported with or without the action of the tongue (figure 2). Avian–Reptilia predation at the water surface uses surface tension (e.g. shorebirds eating various insects or larvae), and Crocodilia eating at this interface clearly use biting to catch their food. One point emerging from studies of food transport in water is that both tongue and water play combined roles in moving the food/prey intraorally [16]. In air, food transport occurs in two ways: (i) use of the tongue and (ii) use of the jaw to give inertia to resist any motion of the food that moves freely in air, as discussed below.
Figure 1.
Schematic representation of the phylogenetic distribution of the modes of food transport determined for Reptilia. The phylogeny was built by using TimeTree 5 (http://timetree.org/; [70]) and ITOL (iTOL: https://itol.embl.de/). Each type of food transport was added to the branches of the phylogenetic tree on the basis of the literature. Straight-drawn transport is a mode of ballistic transport (see §3 for explanation).
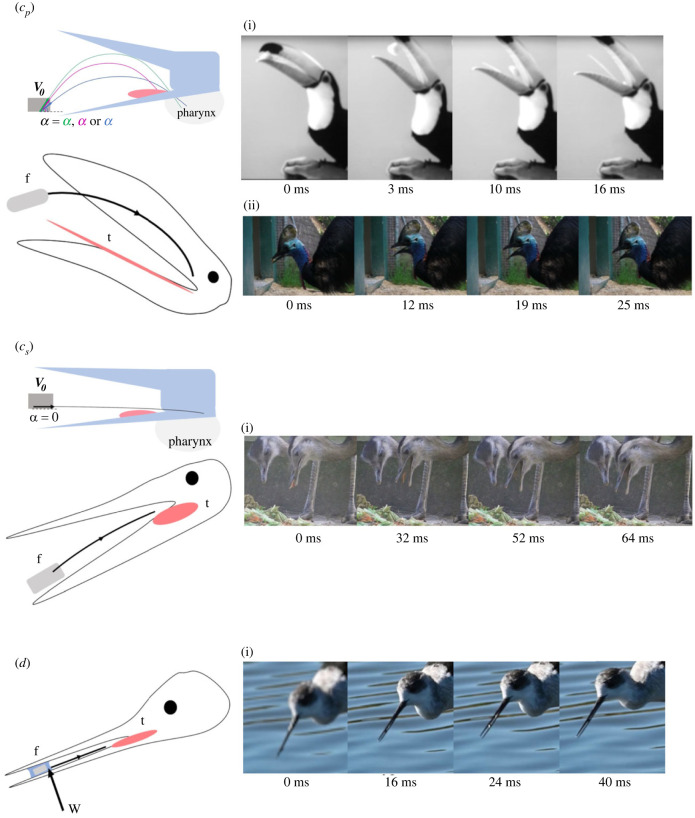
Figure 2.
(a–d) Schematic representation of the food trajectories in the five modes of solid food transport in Reptilia illustrated with typical sequences in various species eating solid food/prey. The tongue movements are only illustrated in case of their action on food during its transport through the oral cavity. (a) Lingual-based transport; (b) inertial feeding following by lingual-based transport. (c) Upper: cp, ballistic transport with parabolic (p) trajectory. Lower: cs, straight-drawn transport (quasi-horizontal trajectory). (d) Transport using surface tension (and capillarity). The food schematic trajectories are represented by the black lines and arrows. Each case is illustrated by various species. (a)(i) Eublepharis macularius; (a)(ii) Kinixys homeana; (a)(iii) Ara ararauna; (b)(i) Alligator mississipiensis; (e) Rhamphastos toco; (f) Casuarius casuarius; (g) Leptoptilos crumenifer; (h) Himantopus himantopus. f, food; t, tongue; w, water.
The jaw apparatus can also be covered by teeth or a keratinous rhamphotheca (i.e. beak; birds and turtles). These last designs mainly rely on food processing (i.e. reduction, mastication) and probably facilitate food transformation. Food processing and transport during its trajectory in the buccal cavity are not always easy to distinguish because both phases occur as parts of a continuous process. For example, food/prey can be moved between the jaws (and the teeth) and processed during typical cycles (e.g. inertial crushing bite in Crocodilia) before the transport cycles or during the transport cycles. In this case, the food is reduced and moved backward to the pharynx. This phase of processing occurs variably in the feeding sequence (see electronic supplementary material, S1 and S2), with various associations with backward movements of food to the pharynx (e.g. puncture crushing in lizards, crushing cycle in Crocodilia and seed processing in granivorous birds) [74–76]. Some aquatic turtles (e.g. Carettochelys) also seem able to move large food items between the jaws before finally transporting them toward the pharynx for swallowing.
Figure 2 summarizes the food transport mechanisms identified in numerous species of living Reptilia in air. A survey of solid food transport in all reptilian lineages (except fossorial and completely aquatic species) shows that there are three major modes of food transport: lingual-base transport, ballistic transport and inertial feeding. Each mode can be roughly associated with some traits of the hard (skull and mandible) and soft (buccal floor and tongue) feeding apparatus. A first emerging message from the comparison of food transport in Reptilia is that the complexity of this feeding phase is highly variable in the studied species, mainly depending on the hyo-lingual morphology and on feeding ecology. For example, lingual-based transport is used by many Pan-Lepidosauria regardless of the morphological specializations of the feeding apparatus. Even in Varanids and Teeids, which have a highly modified tongue associated with vomerolfaction, lingual feeding still depends on the properties of the food and its position within the buccal cavity, together with the feeding sequence. Except in ballistic transport [77–79] and prey transport based on surface tension [14,80–82], all of the food transport categories are primarily based on rhythmic neuro-motor cyclic movements of the jaws and the hyo-lingual apparatus, even in inertial feeding [75]. Basically, all gape cycles in any sort of food transport involve motor cycles ensured by the mouth opening and closing [19,21].
(a) . Lingual-based transport mode
The most widespread mode of transport in Reptilia is lingual-based, regardless of the lingual characteristics (electronic supplementary material, S1 and S3), their phylogeny or their feeding ecology in aquatic and terrestrial habitats. In lingual transport, the head remains relatively motionless during each jaw cycle, which is divided into slow opening (SO) and fast opening (FO) phases, followed by a closing phase (e.g. Testudines). In various Reptilia (e.g. Testudines), the gape remains variably at its maximum during lingual and food movements. The only required conditions are (i) anterior–posterior movements of the tongue (as in drinking) and (ii) potential deformation of the tongue permitting movements under the food item. These lingual performances have been demonstrated on the basis of muscular activities in squamates [83,84] and Aves [12,85]. This mode is used by reptilian species to process, transport and swallow any types of solid food with various textures in both water and air. When the tongue is used for food/prey prehension and therefore used as a gripper [86], the tongue must lose its contact with the food just after the capture cycle to be able to move it under the food item and then continue movements under the food to ensure its role in backwards food displacement within the buccal cavity, regardless of the division of the SO (slow opening) phase into SOI (slow opening I) and SOII (slow opening II). Mouth closing plays a major role in the spatial and temporal characteristics of the transport cycles. Processing and transporting of the food are also associated with lingual deformations that remain to be investigated. It is not always clear that processing and transport cycles are separated, for example, when large prey items being transported toward the pharynx are crushed between the jaws. Food processing that occurs in a separate feeding phase (e.g. puncture crushing, mastication) is also strongly dependent on the lingual–gape coordinated cycles. In terrestrial Reptilia, several studies demonstrate that lingual performances related to the deformation of the tongue are different in processing and transport cycles [87,88]. In water, food transport is more complex because this feeding phase can be based on the physical contact between captured food and the tongue or based on using water as the primary vehicle to move the food toward the entrance of the oesophagus. For example, Cuora sp. (Testudines) can use both modes of food transport before swallowing per se [9].
(b) . Non-lingual based transport modes
In the following sections, we present and sort different modes of non-lingual transport according to their functionality in air. Such non-lingual based transport modes include behaviours that have been termed ‘catch and throw’ [45,89], ‘cranio-inertial transport’ [54,90], ‘ballistic transport’ [77–79] and ‘inertial feeding’ [67,68,91]. Transport based on surface tension only occurs in some specialized avian Reptilia that exploit seashore prey or prey at the interface between water and air [85].
(i) . Ballistic transport
In avian Reptilia such as mallard ducks and chickens, ‘catch-and-throw’ cycles have been described as cycles that can be intermittent in the transport cycles between lingual-based cycles. In young chickens, the frequency of ‘catch-and-throw’ was modulated with respect to the size of the food, being greater with greater diameters of food items (over 1.2 mm). Lingual transport was primarily used for smaller food items (diameters between 0.5 and 0.7 mm) [89]. Regardless of the terminology used, the tongue never contacts the food in these modes of transport and the initial velocity of the food at launch angle is provided either by (i) a sudden backwards linear movement of the head (always in the case of straight-draw transport with quasi-horizontal trajectory; electronic supplementary material, S4) or (ii) a backward rotation of the head (always in the case of visible parabolic trajectories). Therefore, both modes of head movements under different motor controls, including no tongue action on the food, produce various trajectories along a continuum with a launch angle varying from of 0° (zero launch angle, which corresponds to a quasi-horizontal trajectory here called straight-draw transport) to 90° (all other parabolic trajectories). Therefore, straight-draw transport is physically a case of ballistic transport with a trajectory that gives the impression of being horizontal for the short distance between the beak tips and the pharynx. Indeed, in moving in the field of gravity, the food is submitted to a gravity force (weight) while moving that prevents the trajectory from being straight (food curves downward according to a portion of its parabolic trajectory).
In this mode of transport (quasi-horizontal trajectory), the head follows a stereotyped cycle involving a sudden backward movement to provide the required inertial velocity to the food, followed by a forward movement to variously bring the tongue and/or pharynx to the moving food (electronic supplementary material, S4). During the food trajectory, the animal can keep the beak variably orientated toward the soil or water surface (electronic supplementary material, S5). In the case of food parabolic trajectories with launch angles greater than 0°, the animal moves the head backwards and forwards (e.g. Rhea sp.). In the other cases, movement of the beak is produced by the rotation of the skull (e.g. cassowaries, toucans, hornbills) to provide the initial velocity needed to launch the food toward the pharynx as the beak opens (electronic supplementary material, S6). The head either stops rotating and the gape increases to avoid contact between the roof of the upper beak (e.g. toucans) and the food during its parabolic trajectory, or the head moves variably during gape increase to optimize the food's entrance into the pharynx (e.g. hornbills) [78,79].
Empirically, ballistic transport depends on (i) the relative size of the jaw apparatus covered with the rhamphotheca and the tongue and (ii) the potential contact of the food and the tongue. The comparison of ballistic transport in toucans, which have a long thin tongue, with aerial and terrestrial hornbills that have a short tongue, supports this relationship between modes of transport with parabolic curves of the food between the upper and lower bill, regardless of their lingual morphology [77,79]. In rhea using horizontal food trajectories and cassowaries employing parabolic food trajectories, the tongue is short and leaves a rather large space in the beak that is simply used to clamp the food [90]. Rheas follow stereotypical backwards and forwards head movements regardless of the type of food item (e.g. artificial food pellets, plant materials.) By contrast to rheas, cassowaries do not always show forward movements of the head when the food follows its trajectory and this is therefore not included as a stereotyped motor action of the transport cycles [78]. Many birds show great plasticity in their food trajectories by controlling initial velocities and launch angles (e.g. toucans, [79]).
(ii) . Inertial feeding: a mixture of non-lingual and lingual transport
Inertial feeding has been recorded in various Reptilia and has been suggested to be present in some Lissamphibia as well [92]. This mode of food transport is considered to be a transport mechanism associated (or not) with tongue action (e.g. some Lepidosauria [19,67,68,93]) or as an obligatory mode of transport (e.g. Crocodilia [75]). The role of the tongue in inertial feeding has been clearly demonstrated in Crocodilia, showing the role of the coordinated head, jaw and hyo-lingual apparatus in efficient displacement of the prey toward the pharynx. The tongue pushes the prey toward the roof of the skull as the head is accelerated under the effects of neck musculature, and it then releases the prey to inflate the throat and increase the open space between skull and the tongue (electronic supplementary material, S2). Because the prey is variably placed between the jaws, movements of the tongue during killing/crushing bites may facilitate repositioning of the prey [74,75]. When the food is placed at the rear of the oro-pharyngeal cavity, the tongue is used to transport it (electronic supplementary material, S2). Swallowing the food does not involve the tongue, which passively follows the movements of the hyoid apparatus (which plays the key role). In two families of Lepidosauria (Teiidae and Varanidae), the food is inertially transported with a rapid movement of the cranio-cervical system, providing acceleration to the food that moves freely between the upper and lower jaws. In lizards, individual differences in jaw and tongue movements (e.g. angle and acceleration) have been detected in relationship with prey characteristics (e.g. mass and size), while the head–neck movements are not modulated by prey types [93].
(iii) . Surface tension transport
Surface tension and capillarity underlie a mode of prey transport recognized in many avian Reptilia [81,82]. Surface tension can be influential when prey are seized on the surface or at any depth, regardless of the shape and size of beak, or the prey capture strategies (e.g. pecking and probing prey under water surface, or predating on the water surface). In shorebirds, the adaptive radiation of the beak size and shape in relation to foraging behaviour is, therefore, free of any constraints associated with food transport toward the pharynx. This mode of transport has been suggested for some shorebirds, with droplets including small prey items being transported by surface tension (electronic supplementary material, S7) and capillarity [14]. The drop motion is induced by the opening and closing beak cycles, enhanced by beak rhynchokinesis (the ability to flex the upper mandible) [54]. In a model comparing various beak shapes, the droplet containing the prey advances through a ratcheting motion, with asynchronous movements of the contact lines. During the opening phase, the leading edge of the droplet remains pinned, while the trailing edge retreats. During closing, the leading edge moves forwards while the trailing edge remains pinned. As demonstrated in almost all descriptions of the foraging behaviour in shorebirds [94], the animals remain with the beak variably vertically orientated toward the substratum during feeding as soon as the prey is caught in water or at its surface. Our current analyses of filmed sequences of feeding shorebird species show that, in all cases when surface tension is used to move the prey toward the tongue or the pharynx directly, the bird provides an inertial velocity to the water drop and the ensconced prey by an initial backward head movement immediately followed by forward movement of the head (as in straight-draw ballistic transport).
The effect of a highly variable morphological beak taper or beak curvature (e.g. Recuvirostra avosetta) on the association between the movement of droplets (and included prey [14]) and the head's backward–forward cycle remains to be investigated. The prey is transported in the droplet from the beak's tip to the buccal cavity with a variable number of opening–closing beak cycles (2–3 in Phalaropus lobatus; 7 in Phalaropus tricolor), or moved directly on the tongue or into the pharynx in one cycle (e.g. Limosa limosa—electronic supplementary material, S7).
(c) . Plasticity
Furthermore, some shorebirds are able to completely adapt their feeding behaviour and even use lingual transport only in relation to the size of the prey (e.g. P. lobatus—electronic supplementary material, S8) or ballistic transport for the extracted flesh from the shell (e.g. oystercatcher). This plasticity in the mechanisms, and subsequent modulation of the motor patterns used in various species with highly different beak shapes and modes of food catching (e.g. pecking, probing) remains to be investigated. As soon as the food contacts the tongue, the last transport cycle(s) before swallowing are always lingual-based.
4. Discussion
Food transport can be compared on the basis of studies in Reptilia. Briefly, food transport is the feeding phase during which food that is gathered from the environment either by using the jaws (e.g. jaw prehension or pecking) or caught by the foretongue (e.g. lingual prehension) is moved to the pharynx for delivery to the digestive tract (via swallowing). Reptilia use various modes of food transport in aquatic and terrestrial habitats [9,10,12,83]. Four modes of food transport are used in air: (i) lingual-based transport, (ii) ballistic transport, (iii) inertial feeding (with and without tongue use—electronic supplementary material, S2) and (iv) surface tension. The last mode is unique to birds with various feeding ecologies, but is always associated with water as an aid to moving food from the tips of the beak toward the pharynx (e.g. in shorebirds).
In reptiles inhabiting aquatic environments, both water (e.g. suction) and tongue can act in transporting food (with or without processing that changes the food properties) to facilitate its entrance into the oesophagus and the initiation of digestion. This has been particularly demonstrated for all Testudines [11]. In birds, food transport has mainly been studied in terrestrial habitats and concentrated upon comprehension of the pecking mechanism and its evolution [55]. However, clearly both modes of food transport can be extracted from the available data: ((i) lingual-based and (ii) ballistic transport). In Crocodilia, study of the transport mechanism has mainly concentrated upon the role of head movements in inertial feeding, associated (or not) with action of the tongue on the food material (e.g. inertial bite and transport) [10,75]. In Lepidosauria, the question of the effects of properties of the tongue on transport mechanisms and their use in delivering molecules to the Jacobson's organ have been much investigated [41]. It has been suggested that vomerolfaction strongly constrained the mode of capture (lingual versus jaw prehension), but did not constrain the use of the tongue for lingual-based transport (regardless of the tongue's biomechanical properties). It is also recognized that lizards with specialized tongues mainly use them for gathering chemicals with the dorsal surface, but they are often still able to catch the prey with their tongue. All lizards, including the species with highly specialized tongues related to vomerolfaction, are able to use their tongues in food transport (even if in a different manner). In Testudines in either aquatic or terrestrial habitats, the evolution of morphological and functional properties of the tongue used in moving food through the buccal cavity, or in producing buccal depression to admit water to move the food toward the oesophageal entrance, has been thoroughly investigated in several species, particularly in those that have aquatic or terrestrial feeding ecologies [9].
In lingual-based transport, the motor pattern (and CPG—central pattern generators) involves coordinated movements of the tongue (and hyobranchium) together with the jaws. However, the movements of the head remain rather variable with, for example, a difference between the head movements associated with transport in Testudines (electronic supplementary material) and Lepidosauria (electronic supplementary material). Head movements under the control of head–neck musculature and the characteristics of the neck–head articulation remain to be investigated in many species (e.g. by use of XROMM) [95]. With the exception of two squamate families (Teeidae and Varanidae), the lingual-based intraoral transport cycles are similar throughout the Lepidosauria, despite the differences in the hyo-lingual morphological properties. A review of available data suggests that tongue properties (e.g. length and width relative to the space between the lower and upper jaws, plus potential deformations due to their muscular characteristics) can modulate their performance in any food transport, but not the motor pattern as suggested in the general BW and RL models [19,21]. Only the performance of the skull related to neck–head articulation and potential neck movements, the jaw apparatus and the hyo-lingual system can be modulated in food transport by integrating properties of the food (e.g. size, volume, texture, resistance to breaking/tearing) and characteristics of the feeding apparatus.
Birds can use both lingual-based transport or ballistic transport regardless of the morphology of the feeding apparatus and their feeding ecology. Indeed, ballistic transport occurs in birds that are not able to touch the food with their short tongue or act on the food with their tongue to move it toward the pharynx after its capture between the tips of the beak. Thus, the relative length between tongue and tip of the ramphotheca is one of the main constraints upon use of ballistic transport, but not exclusively. Indeed this mode of transport is present in Rhamphastidae (e.g. toucans with long and thin tongue) and Bucerotidae [77,79] but not as a result of convergent evolution since it is widespread in Paleognathous (e.g. Rhea and Casuarius [54,78,90]) and Neognathous species (figure 2) with short tongues compared with the length of the ramphotheca.
Shorebirds pose another question because they are able to use lingual-based transport, ballistic transport and surface tension for moving food in droplets. At one period of their life cycle, many shorebird species are unable to gain access to water or to eat large prey (e.g. reproductive periods in tundra without access to aquatic prey compared with periods of feeding during their migrations when they have access to sea shore or freshwater surfaces). In this case, they probably use ballistic transport as they do with large food items during migration and on their wintering grounds (e.g. large worms for Limosa sp. or crabs in Numenius sp.). However, the relationship between size of the prey and use of surface tension associated with head–neck movements and ballistic trajectory per se remains to be investigated (e.g. in the absence of water) not only in adults but also in nestlings. In the case of those shorebird species that have tongues of a variety of lengths compared with beak length, the adaptive evolution of the beak morphology (e.g. in decreasing viscous forces) and motor control facilitating the use of surface tension (e.g. using a decreased gape to ensure contact between the film of water and the beak edges) could have been favoured in those species that exploit small food resources in environments with water nearby (e.g. foraging in water or on/in substratum left at low tide), regardless of beak characteristics (e.g. length, curvature, rhynchokinesis). The motor pattern remains similar in all birds using ballistic transport and surface tension; simply modulating the gape angle permits prey to be transported in droplets of water over a variable length of the beak in one or several cycles until tongue contact can be reached to initiate lingual transport. The question about the use of lingual-based transport, ballistic transport and surface tension in relation to the evolution of their foraging behaviour and food selection (e.g. prey size and volume) in these birds remains open [96].
Empirical comparative analysis of ballistic food transport in birds shows that the food always follows a trajectory with variable maximum distance and height. The bird controls this trajectory with only two physical parameters: (i) the initial velocity provided by the head–jaw acceleration (neglecting air resistance) and (ii) the angle of launch. The initial velocity is given by different motor movements of the neck–skull morphological system to the launched food: (i) the backward rotation of the head (e.g. toucans and hornbills) or (ii) sudden backward movements of the head (e.g. Rhea sp., and Ciconia sp.; electronic supplementary material, S4). In these cases, the head always follows backwards–forwards cycles (with various amplitudes). The trajectory of the food allows it to contact the tongue, which can then play an active role in moving the food into the pharynx toward the oesophagus (e.g. hornbill), or directly entering the pharynx with the tongue and hyobranchium, ensuring swallowing (e.g. toucans). The trajectory is controlled by each animal through the launch angle (zero angle in case of straight draw trajectory) and the initial velocity (at the moment when the food leaves its contact with the jaw tips), with a maximal height allowing avoidance of any contact with the internal upper jaw ramphotheca. Cranio-inertial feeding and ‘throw and catch’ are only different terminologies used to functionally define the same mechanism of ballistic transport in various Aves.
The question of inertial feeding can also be addressed in terms of food trajectory. In both Squamate families using inertial feeding, the role of the tongue remains somewhat unclear because these lizards are variously reported to use/not use their tongues [67,68,93,97]. The role of the tongue could be associated with selected food/prey because these lizards catch prey items of highly differing sizes and masses, including pieces of meat and bones torn from carrion [98,99]. Furthermore, the use of the tongue in Squamates is reported to be kinematically different from its use by Crocodilia, with lingual anterodorsal movements being associated with the SO phase of the gape cycle in lizards, and the FO phase of Crocodilia [93]. The most salient point used to characterize inertial feeding in Crocodilia is that its trajectory depends both on the initial velocity provided to the food to move it freely through the buccal cavity, and on the launch angle, the tongue being used to enlarge the buccal cavity by inflating the throat to facilitate the passage of the food (electronic supplementary material, S2). We suggest that inertial feeding without any contact between the tongue and the food in both Squamates with highly modified tongue characteristics (e.g. Teeidae and Varanidae) and Crocodilia (with simpler tongue characteristics) corresponds to ballistic transport in birds. In the case of processing (e.g. repositioning, killing, crushing), the food makes contact with the jaws depending on its volume and/or size. Since salamanders (Lissamphibia) also seem to use inertial feeding (but with tongue contact) [94], it currently remains unclear whether inertial feeding associating food trajectory and tongue contact is phylogenetically or convergently selected in Tetrapoda. We also suggest that inertial feeding is only a sort of ballistic transport.
In conclusion, we hypothesize that these tetrapods use two main modes for transporting their food, regardless of the diet regime and the selected food: (i) lingual-based transport and (ii) ballistic transport. These modes follow jaw prehension (including pecking in Aves [100]) or lingual prehension. The selection of one or other mode of transport depends on lingual functional properties (e.g. various mechanisms of protrusion/projection, and adhesion on food/prey) in relation to tongue morphology, and also to feeding ecology (e.g. prey size, volume, habitats) [101–105]. Both modes can be used alternately or in succession during a single feeding sequence and can be associated (or not) with food processing. In birds exploiting aquatic resources either in water or in wet sediments, these modes have evolved to employ surface tension to help to move small prey items to the pharynx [12,85]. For drinking, the tongue plays a key role in moving the liquid in the buccal cavity (e.g. lingual-based drinking and scooping and suction) and from outside to inside the buccal cavity (e.g. drinking in hummingbirds, lorikeets and some lizards) regardless of its morphological features, with highly specialized morphological and functional specializations in Aves exploiting nectar as their main source of food [40]. Drinking in aquatic tetrapods (e.g. Testudines and Crocodilia) relies on various strategies, one of them being to have sufficient access to water through food [106]. This brief empirical survey of food transport opens up perspectives on (i) the coevolution of solid and liquid food transport in Reptilia, (ii) understanding how the various transport mechanisms evolved within the taxon and (iii) how the motor pattern of modes of transport of solid and liquid resources is controlled on the basis of the general BW and RL models. It is not yet clear whether both modes of transport were present in early tetrapods or whether one mode derives from the other.
In ballistic transport, the tongue movements and deformations can also be coordinated with the jaw cycle. For example, in toucans, the movement of the hyolingual apparatus is coordinated with gape cycle, as demonstrated by the tongue and throat movements. The hyolingual movements open the pharynx, despite the tongue never touching the food [79]. However, this coordination remains to be investigated in many more species because functionally, tongue movements can be stopped by simply silencing the lingual motor neurons of the CPG. This modification of the motor pattern does not affect the success of the transport cycles. As soon as food touches the tongue, these motor neurons can be activated by sensory information and the animals can use lingual-based transport if needed. Such activation is probably required to move from transport cycles to swallowing cycles that implicate the tongue in all Reptilia.
Acknowledgements
M.B. is part of the Laboratoire Excellence TULIP (ANR-10-LABX-41, France). We thank the Zoos de La Bourbansais (Pleugueneuc, France) and the Ménagerie du Jardin des Plantes (Muséum National d'Histoire Naturelle, Paris, France) for providing the opportunity and helping to film the animals. We particularly thank Chris Gilette, who allowed us to use his films on Alligators (https://www.crocodilechris.com/).
Data accessibility
All data are simply video films to support the text of the paper, and they can be found in the electronic supplementary material [107]. These data were recorded as simple bird watching without any need for permission.
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
V.B.: conceptualization, writing—original draft; G.L.F.: methodology; F.K.: investigation, writing—original draft; G.G.: methodology; J.D.: writing—review and editing; M.B.: writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Agnolin FL, Motta MJ, Brissón Egli F, Lo Coco G, Novas FE. 2019. Paravian phylogeny and the dinosaur-bird transition: an overview. Front. Earth Sci. 6, 252. ( 10.3389/feart.2018.00252) [DOI] [Google Scholar]
- 2.Hu H, et al. 2023. Cranial osteology and palaeobiology of the Early Cretaceous bird Jeholornis prima (Aves: Jeholornithiformes). Zool. J. Linn. Soc. 198, 93-112. ( 10.1093/zoolinnean/zlac089) [DOI] [Google Scholar]
- 3.Prum RO, Berv JS, Dornburg A, Field DJ, Townsend JP, Lemmon EM, Lemmon AR. 2015. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 526, 569-573. ( 10.1038/nature15697) [DOI] [PubMed] [Google Scholar]
- 4.Sues H-D. 2019. The rise of reptiles: 320 million years of evolution. Baltimore, MD: JHU Press. [Google Scholar]
- 5.Bever GS, Lyson TR, Field DJ, Bhullar B-AS. 2015. Evolutionary origin of the turtle skull. Nature 525, 239-242. ( 10.1038/nature14900) [DOI] [PubMed] [Google Scholar]
- 6.Evans SE, Jones ME. 2010. The origin, early history and diversification of lepidosauromorph reptiles. In New aspects of mesozoic biodiversity. Lecture Notes in Earth Sciences, vol. 132, pp. 27-44. Berlin, Germany: Springer. [Google Scholar]
- 7.Houssaye A. 2013. Bone histology of aquatic reptiles: what does it tell us about secondary adaptation to an aquatic life? Biol. J. Linn. Soc. 108, 3-21. ( 10.1111/j.1095-8312.2012.02002.x) [DOI] [Google Scholar]
- 8.Klein N, Canoville A, Houssaye A. 2019. Microstructure of vertebrae, ribs, and gastralia of Triassic sauropterygians—new insights into the microanatomical processes involved in aquatic adaptations of marine reptiles. Anat. Rec. 302, 1770-1791. ( 10.1002/ar.24140) [DOI] [PubMed] [Google Scholar]
- 9.Lemell P, Natchev N, Beisser CJ, Heiss E. 2019. Feeding in turtles: understanding terrestrial and aquatic feeding in a diverse but monophyletic group. In Feeding in Vertebrates. Fascinating Life Sciences (eds Bels V, Whishaw I). Cham, Switzerland: Springer. ( 10.1007/978-3-030-13739-7_16) [DOI] [Google Scholar]
- 10.Gignac PM, O'Brien HD, Turner AH, Erickson GM. 2019. Feeding in crocodylians and their relatives: functional insights from ontogeny and evolution. In Feeding in Vertebrates. Fascinating Life Sciences (eds Bels BL, Wishaw IQ), pp. 575-610. Cham, Switzerland: Springer. ( 10.1007/978-3-030-13739-7_15) [DOI] [Google Scholar]
- 11.Heiss E, Gignac PM, Porro LB, Lemell P. 2023. Convergence of aquatic feeding modes in the Sauropsida (crocodiles, birds, lizards, snakes and, turtles). In Convergent evolution: animal form and function (eds Bels VL, Russel AP), pp. 141-181. Berlin, Germany: Springer. [Google Scholar]
- 12.Rico-Guevara A, Sustaita D, Gussekloo S, Olsen A, Bright J, Corbin C, Dudley R. 2019. Feeding in birds: thriving in terrestrial, aquatic, and aerial niches. In Feeding in vertebrates: evolution, morphology, behavior, biomechanics (eds Bels VL, Wishaw IQ), pp. 643-693. Berlin, Germany: Springer. ( 10.1007/978-3-030-13739-7_17) [DOI] [Google Scholar]
- 13.van der Kolk H-J, et al. 2020. Shorebird feeding specialists differ in how environmental conditions alter their foraging time. Behav. Ecol. 31, 371-382. ( 10.1093/beheco/arz189) [DOI] [Google Scholar]
- 14.Prakash M, Quéré D, Bush JW. 2008. Surface tension transport of prey by feeding shorebirds: the capillary ratchet. Science 320, 931-934. ( 10.1126/science.1156023) [DOI] [PubMed] [Google Scholar]
- 15.Beninger PG, Elner RW. 2020. On the tip of the tongue: natural history observations that transformed shorebird ecology. Ecosphere 11, e03133. ( 10.1002/ecs2.3133) [DOI] [Google Scholar]
- 16.Lemell P, Lemell C, Snelderwaard P, Gumpenberger M, Wochesländer R, Weisgram J. 2002. Feeding patterns of Chelus fimbriatus (Pleurodira: Chelidae). J. Exp. Biol. 205, 1495-1506. ( 10.1242/jeb.205.10.1495) [DOI] [PubMed] [Google Scholar]
- 17.Rusli MU, Booth DT, Joseph J. 2016. Synchronous activity lowers the energetic cost of nest escape for sea turtle hatchlings. J. Exp. Biol. 219, 1505-1513. ( 10.1242/jeb.134742) [DOI] [PubMed] [Google Scholar]
- 18.Lee TN, Plummer MV, Mills NE. 2007. Use of posthatching yolk and external forage to maximize early growth in Apalone mutica hatchlings. J. Herpetol. 41, 492-500. ( 10.1670/0022-1511(2007)41[492:UOPYAE]2.0.CO;2) [DOI] [Google Scholar]
- 19.Bramble DM, Wake DB. 2013. Feeding mechanisms of lower tetrapods. In Functional vertebrate morphology (eds Hildebrand M, Bramble DM, Liem KF, Wake DB), pp. 230-261. Cambridge, MA: Harvard University Press. [Google Scholar]
- 20.McBrayer LD, Reilly SM. 2002. Testing amniote models of prey transport kinematics: a quantitative analysis of mouth opening patterns in lizards. Zoology 105, 71-81. ( 10.1078/0944-2006-00047) [DOI] [PubMed] [Google Scholar]
- 21.Reilly SM, Lauder GV. 1990. The evolution of tetrapod feeding behavior: kinematic homologies in prey transport. Evolution 44, 1542-1557. ( 10.2307/2409336) [DOI] [PubMed] [Google Scholar]
- 22.Brainerd EL, Camp AL. 2019. Functional morphology of vertebrate feeding systems: new insights from XROMM and fluoromicrometry In Feeding in vertebrates: evolution, morphology, behavior, biomechanics (eds Bels VL, Wishaw IQ), pp. 21-44. Berlin, Germany: Springer. ( 10.1007/978-3-030-13739-7_2) [DOI] [Google Scholar]
- 23.Rayfield EJ. 2019. What does musculoskeletal mechanics tell us about evolution of form and function in vertebrates? In Feeding in vertebrates: evolution, morphology, behavior, biomechanics (eds Bels VL, Wishaw IQ), pp. 45-70. Berlin, Germany: Springer.. ( 10.1007/978-3-030-13739-7_3) [DOI] [Google Scholar]
- 24.Zweers G. 1991. Transformation of avian feeding mechanisms: a deductive method. Acta Biotheor. 39, 15-36. ( 10.1007/BF00046405) [DOI] [Google Scholar]
- 25.Zweers GA, Berkhoudt H, Berge JV. 1994. Behavioral mechanisms of avian feeding. In Biomechanics of feeding in vertebrates (eds Bels VL, Chardon M, Vandewaelle P), pp. 241-279. Berlin, Germany: Springer. ( 10.1007/978-3-642-57906-6_9) [DOI] [Google Scholar]
- 26.Bels VL, Goosse V, Kardong KV. 1993. Kinematic analysis of drinking by the lacertid lizard, Lacerta viridis (Squamates, Scleroglossa). J. Zool. 229, 659-682. ( 10.1111/j.1469-7998.1993.tb02663.x) [DOI] [Google Scholar]
- 27.Bels VL, Davenport J, Renous S. 1995. Drinking and water expulsion in the diamondback turtle Malaclemys terrapin. J. Zool. 236, 483-497. ( 10.1111/j.1469-7998.1995.tb02726.x) [DOI] [Google Scholar]
- 28.Kim W, Bush JW. 2012. Natural drinking strategies. J. Fluid Mech. 705, 7-25. ( 10.1017/jfm.2012.122) [DOI] [Google Scholar]
- 29.Comanns P, Withers PC, Esser FJ, Baumgartner W. 2016. Cutaneous water collection by a moisture-harvesting lizard, the thorny devil (Moloch horridus). J. Exp. Biol. 219, 3473-3479. ( 10.1242/jeb.148791) [DOI] [PubMed] [Google Scholar]
- 30.Sherbrooke WC. 1990. Rain-harvesting in the lizard, Phrynosoma cornutum: behavior and integumental morphology. J. Herpetol. 24, 302-308. ( 10.2307/1564398) [DOI] [Google Scholar]
- 31.Zweers GA. 1982. Drinking of the pigeon (Columba livia L.). Behaviour 80, 274-316. ( 10.1163/156853982X00391) [DOI] [Google Scholar]
- 32.Heidweiller J, Zweers GA. 1990. Drinking mechanisms in the zebra finch and the bengalese finch. Condor 92, 1-28. ( 10.2307/1368379) [DOI] [Google Scholar]
- 33.Sherbrooke WC, Scardino AJ, de Nys R, Schwarzkopf L. 2007. Functional morphology of scale hinges used to transport water: convergent drinking adaptations in desert lizards (Moloch horridus and Phrynosoma cornutum). Zoomorphology 126, 89-102. ( 10.1007/s00435-007-0031-7) [DOI] [Google Scholar]
- 34.Cundall D. 2000. Drinking in snakes: kinematic cycling and water transport. J. Exp. Biol. 203, 2171-2185. ( 10.1242/jeb.203.14.2171) [DOI] [PubMed] [Google Scholar]
- 35.Cundall D, Brainerd EL, Constantino J, Deufel A, Grapski D, Kley NJ. 2012. Drinking in snakes: resolving a biomechanical puzzle. J. Exp. Zool. A Ecol. Genet. Physiol. 317, 152-172. ( 10.1002/jez.1710) [DOI] [PubMed] [Google Scholar]
- 36.Berkhoudt G, Kardong KV, Zweers GA. 1995. Mechanics of drinking in the brown tree snake, Boiga irregularis. Zoology 98, 92-92. [Google Scholar]
- 37.Moon BR, Penning DA, Segall M, Herrel A. 2019. Feeding in snakes: form, function, and evolution of the feeding system. In Feeding in vertebrates: evolution, morphology, behavior, biomechanics (eds Bels V, Whishaw I), pp. 527-574. ( 10.1007/978-3-030-13739-7_14) [DOI] [Google Scholar]
- 38.Kardong KV, Haverly JE. 1993. Drinking by the common boa, Boa constrictor. Copeia 1993, 808-818. ( 10.2307/1447246) [DOI] [Google Scholar]
- 39.Wagemans F, Chardon M, Gasc J-P, Renous S, Bels VL. 1999. Drinking behaviour in Anolis carolinensis (voigt, 1837) and Oplurus cuvieri (gray, 1831) (reptilia: Iguania: Iguanidae). Can. J. Zool. 77, 1136-1146. [Google Scholar]
- 40.Cuban D, Hewes AE, Sargent AJ, Groom DJ, Rico-Guevara A. 2022. On the feeding biomechanics of nectarivorous birds. J. Exp. Biol. 225, jeb243096. ( 10.1242/jeb.243096) [DOI] [PubMed] [Google Scholar]
- 41.Bels V, Baussart S, Davenport J, Shorten M, O'Riordan RM, Renous S, Davenport JL. 2007. Functional evolution of feeding behavior in turtles. In Biology of turtles: from structure to strategy of life (eds Wyneken J, Godfrey M, Bels V), pp. 201-226. Boca Raton, FL: CRC Press. [Google Scholar]
- 42.Rico-Guevara A. 2014. Functional morphology of hummingbird bill tips: their function as tongue wringers. Zoology 123, 1-10. ( 10.1016/j.zool.2017.06.001) [DOI] [PubMed] [Google Scholar]
- 43.Rico-Guevara A, Rubega MA, Hurme KJ, Dudley R. 2019. Shifting paradigms in the mechanics of nectar extraction and hummingbird bill morphology. Integr. Org. Biol. 1, oby006. ( 10.1093/iob/oby006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kooloos JGM, Zweers GA. 1989. Mechanics of drinking in the mallard (Anas platyrhynchos, Anatidae). J. Morphol. 199, 327-347. ( 10.1002/jmor.1051990308) [DOI] [PubMed] [Google Scholar]
- 45.Kooloos JGM, Zweers GA. 1991. Integration of pecking, filter feeding and drinking mechanisms in waterfowl. Acta Biotheor. 39, 107-140. ( 10.1007/BF00046595) [DOI] [PubMed] [Google Scholar]
- 46.Reis PM, Jung S, Aristoff JM, Stocker R. 2010. How cats lap: water uptake by Felis catus. Science 330, 1231-1234. ( 10.1126/science.1195421) [DOI] [PubMed] [Google Scholar]
- 47.Gart S, Socha JJ, Vlachos PP, Jung S. 2015. Dogs lap using acceleration-driven open pumping. Proc. Natl Acad. Sci. USA 112, 15 798-15 802. ( 10.1073/pnas.1514842112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bels VL, Jamniczky HA, Montuelle S, Pallandre J-P, Kardong KV, Russell AP. 2020. Mechanics and kinematics of fluid uptake and intraoral transport in the leopard gecko. J. Zool. 311, 33-44. ( 10.1111/jzo.12763) [DOI] [Google Scholar]
- 49.Rico-Guevara A, Rubega MA. 2017. Functional morphology of hummingbird bill tips: their function as tongue wringers. Zoology 123, 1-10. ( 10.1016/j.zool.2017.06.001) [DOI] [PubMed] [Google Scholar]
- 50.Yanega GM, Rubega MA. 2004. Hummingbird jaw bends to aid insect capture. Nature 428, 615. ( 10.1038/428615a) [DOI] [PubMed] [Google Scholar]
- 51.Stayton CT. 2006. Testing hypotheses of convergence with multivariate data: morphological and functional convergence among herbivorous lizards. Evolution 60, 824-841. ( 10.1111/j.0014-3820.2006.tb01160.x) [DOI] [PubMed] [Google Scholar]
- 52.McCurry MR, Mahony M, Clausen PD, Quayle MR, Walmsley CW, Jessop TS, Wroe S, Richards H, McHenry CR. 2015. The relationship between cranial structure, biomechanical performance and ecological diversity in varanoid lizards. PLoS ONE 10, e0130625. ( 10.1371/journal.pone.0130625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bout RG, Zweers GA. 2001. The role of cranial kinesis in birds. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 131, 197-205. ( 10.1016/S1095-6433(01)00470-6) [DOI] [PubMed] [Google Scholar]
- 54.Gussekloo SW, Bout RG. 2005. The kinematics of feeding and drinking in palaeognathous birds in relation to cranial morphology. J. Exp. Biol. 208, 3395-3407. ( 10.1242/jeb.01769) [DOI] [PubMed] [Google Scholar]
- 55.Zweers GA, Van Der Leeuw AH, Bout RG. 2001. Evolutionary morphology of the neck system in ratites, fowl and waterfowl. Neth. J. Zool. 51, 243-262. ( 10.1163/156854201X00297) [DOI] [Google Scholar]
- 56.Soons J, Genbrugge A, Podos J, Adriaens D, Aerts P, Dirckx J, Herrel A. 2015. Is beak morphology in Darwin's finches tuned to loading demands? PLoS ONE 10, e0129479. ( 10.1371/journal.pone.0129479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olsen AM. 2017. Feeding ecology is the primary driver of beak shape diversification in waterfowl. Funct. Ecol. 31, 1985-1995. ( 10.1111/1365-2435.12890) [DOI] [Google Scholar]
- 58.Bright JA, Marugán-Lobón J, Rayfield EJ, Cobb SN. 2019. The multifactorial nature of beak and skull shape evolution in parrots and cockatoos (Psittaciformes). BMC Evol. Biol. 19, 1-9. ( 10.1186/s12862-019-1432-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bestwick J, Jones AS, Purnell MA, Butler RJ. 2021. Dietary constraints of phytosaurian reptiles revealed by dental microwear textural analysis. Palaeontology 64, 119-136. ( 10.1111/pala.12515) [DOI] [Google Scholar]
- 60.Pigot AL, et al. 2020. Macroevolutionary convergence connects morphological form to ecological function in birds. Nat. Ecol. Evol. 4, 230-239. ( 10.1038/s41559-019-1070-4) [DOI] [PubMed] [Google Scholar]
- 61.Puga y Colmenares MC, Ramírez-Bautista A, Cruz-Elizalde R, García-Rosales A, Hernández-Salinas U. 2019. Feeding ecology and its relationship with head structures in two populations of the lizard Sceloporus minor (Squamata: Phrynosomatidae) from Northern Mexico. Copeia 107, 542-549. ( 10.1643/CH-19-182) [DOI] [Google Scholar]
- 62.Ferreira GS, Lautenschlager S, Evers SW, Pfaff C, Kriwet J, Raselli I, Werneburg I. 2020. Feeding biomechanics suggests progressive correlation of skull architecture and neck evolution in turtles. Sci. Rep. 10, 5505. ( 10.1038/s41598-020-62179-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terray L, Plateau O, Abourachid A, Böhmer C, Delapré A, de la Bernardie X, Cornette R. 2020. Modularity of the neck in birds (Aves). Evol. Biol. 47, 97-110. ( 10.1007/s11692-020-09495-w) [DOI] [Google Scholar]
- 64.Li Z, Clarke JA. 2016. The craniolingual morphology of waterfowl (Aves, Anseriformes) and its relationship with feeding mode revealed through contrast-enhanced X-ray computed tomography and 2D morphometrics. Evol. Biol. 43, 12-25. ( 10.1007/s11692-015-9345-4) [DOI] [Google Scholar]
- 65.Podos J, Nowicki S. 2004. Beaks, adaptation, and vocal evolution in Darwin's finches. Bioscience 54, 501-510. ( 10.1641/0006-3568(2004)054[0501:BAAVEI]2.0.CO;2) [DOI] [Google Scholar]
- 66.Podos J, Sung H-C. 2020. Vocal performance in songbirds: from mechanisms to evolution. In The neuroethology of birdsong (eds Sakata J, Wooley SC, Fay RR, Popper AN), pp. 245-268. Berlin Germany: Springer. ( 10.1007/978-3-030-34683-6_9) [DOI] [Google Scholar]
- 67.Elias JA, McBrayer LD, Reilly SM. 2000. Prey transport kinematics in Tupinambis teguixin and Varanus exanthematicus: conservation of feeding behavior in ‘chemosensory-tongued'lizards. J. Exp. Biol. 203, 791-801. ( 10.1242/jeb.203.4.791) [DOI] [PubMed] [Google Scholar]
- 68.Montuelle SJ, Herrel A, Schaerlaeken V, Metzger KA, Mutuyeyezu A, Bels VL. 2009. Inertial feeding in the teiid lizard Tupinambis merianae: the effect of prey size on the movements of hyolingual apparatus and the cranio-cervical system. J. Exp. Biol. 212, 2501-2510. ( 10.1242/jeb.026336) [DOI] [PubMed] [Google Scholar]
- 69.Natchev N, Tzankov N, Werneburg I, Heiss E. 2015. Feeding behaviour in a ‘basal'tortoise provides insights on the transitional feeding mode at the dawn of modern land turtle evolution. PeerJ 3, e1172. ( 10.7717/peerj.1172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar S, et al. 2022. TimeTree 5: an expanded resource for species divergence times. Mol. Biol. Evol. 39, msac174. ( 10.1093/molbev/msac174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Enstipp MR, Descamps S, Fort J, Grémillet D. 2018. Almost like a whale–first evidence of suction feeding in a seabird. J. Exp. Biol. 221, jeb182170. ( 10.1242/jeb.182170) [DOI] [PubMed] [Google Scholar]
- 72.Crome FHJ. 1985. An experimental investigation of filter-feeding on zooplankton by some specialized waterfowl. Aust. J. Zool. 33, 849-862. ( 10.1071/ZO9850849) [DOI] [Google Scholar]
- 73.Zweers G, De Jong F, Berkhoudt H, Berge JV. 1995. Filter feeding in flamingos (Phoenicopterus ruber). Condor 97, 297-324. ( 10.2307/1369017) [DOI] [Google Scholar]
- 74.Cleuren J, de Vree F. 1992. Kinematics of the jaw and hyolingual apparatus during feeding in Caiman crocodilus. J. Morphol. 212, 141-154. ( 10.1002/jmor.1052120205) [DOI] [PubMed] [Google Scholar]
- 75.Cleuren J, De Vree F, Schwenk K. 2000. Feeding in crocodilians. In Feeding: form, function, and evolution in tetrapod vertebrates (ed. Schwenk K), pp. 337-358. Cambridge, MA: Academic. ( 10.1016/B978-012632590-4/50011-3) [DOI] [Google Scholar]
- 76.Mielke M, Van Wassenbergh S. 2022. Three-dimensional movement of the beak during seed processing in domestic canaries. J. Exp. Biol. 225, jeb244360. ( 10.1242/jeb.244360) [DOI] [PubMed] [Google Scholar]
- 77.Baussart S, Bels V. 2011. Tropical hornbills (Aceros cassidix, Aceros undulatus, and Buceros hydrocorax) use ballistic transport to feed with their large beaks. J. Exp. Zool. A Ecol. Genet. Physiol. 315, 72-83. ( 10.1002/jez.590) [DOI] [PubMed] [Google Scholar]
- 78.Harte M, Legreneur P, Pelle E, Placide MA, Bels V. 2012. Ballistic food transport in birds: the example of Casuarius casuarius. Comput. Methods Biomech. Biomed. Eng. 15, 137-139. ( 10.1080/10255842.2012.713665) [DOI] [PubMed] [Google Scholar]
- 79.Baussart S, Korsoun L, Libourel P-A, Bels V. 2009. Ballistic food transport in toucans. J. Exp. Zool. A Ecol. Genet. Physiol. 311, 465-474. ( 10.1002/jez.542) [DOI] [PubMed] [Google Scholar]
- 80.Rubega MA. 1997. Surface tension prey transport in shorebirds: how widespread is it? Ibis 139, 488-493. ( 10.1111/j.1474-919X.1997.tb04663.x) [DOI] [Google Scholar]
- 81.Rubega MA, Obst BS. 1993. Surface-tension feeding in phalaropes: discovery of a novel feeding mechanism. Auk 110, 169-178. [Google Scholar]
- 82.Estrella SM, Masero JA, Pérez-Hurtado A. 2007. Small-prey profitability: field analysis of shorebirds' use of surface tension of water to transport prey. Auk 124, 1244-1253. ( 10.1093/auk/124.4.1244) [DOI] [Google Scholar]
- 83.Bels V, Paindavoine A-S, Zghikh L-N, Paulet E, Pallandre J-P, Montuelle SJ. 2019. Feeding in lizards: form–function and complex multifunctional system. In Feeding in vertebrates: evolution, morphology, behavior, biomechanics (eds Bels VL, Wishaw IQ), pp. 469-525. Berlin, Germany: Springer. [Google Scholar]
- 84.Ross CF, Eckhardt A, Herrel A, Hylander WL, Metzger KA, Schaerlaeken V, Washington RL, Williams SH. 2007. Modulation of intra-oral processing in mammals and lepidosaurs. Integr. Comp. Biol. 47, 118-136. ( 10.1093/icb/icm044) [DOI] [PubMed] [Google Scholar]
- 85.Zweers GA, Berkhoudt H, Vanden Berge JC. 1994. Behavioral mechanisms of avian feeding. In Biomechanics of feeding in vertebrates (eds Bels VL, Chardon M, Vandewaelle P), pp. 241-279. ( 10.1007/978-3-642-57906-6_9) [DOI] [Google Scholar]
- 86.Noel AC, Hu DL. 2018. The tongue as a gripper. J. Exp. Biol. 221, jeb176289. ( 10.1242/jeb.176289) [DOI] [PubMed] [Google Scholar]
- 87.Bels V, Whishaw IQ. 2019. Feeding in vertebrates. Berlin, Germany: Springer. [Google Scholar]
- 88.Schwenk K. 2000. Feeding: form, function and evolution in tetrapod vertebrates. Baltimore, MD: Elsevier. [Google Scholar]
- 89.Neves DP, Mehdizadeh SA, Santana MR, Amadori MS, Banhazi TM, de Alencar Nääs I. 2019. Young broiler feeding kinematic analysis as a function of the feed type. Animals 9, 1149. ( 10.3390/ani9121149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tomlinson CB. 2000. Feeding in paleognathous birds. In Feeding: form, function, and evolution in tetrapod vertebrates (ed. Schwenk K), pp. 359-394. Cambridge, MA: Academic. ( 10.1016/B978-012632590-4/50012-5) [DOI] [Google Scholar]
- 91.Gans C. 1969. Comments on inertial feeding. Copeia 1969, 855-857. ( 10.2307/1441816) [DOI] [Google Scholar]
- 92.Schwarz D, Heiss E, Pierson T, Konow N, Schoch R. 2023. Using salamanders as model taxa to understand vertebrate feeding constraints during the late Devonian water-to-land transition. Phil. Trans. R. Soc. B 378, 20220541. ( 10.1098/rstb.2022.0541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schaerlaeken V, Montuelle SJ, Aerts P, Herrel A. 2011. Jaw and hyolingual movements during prey transport in varanid lizards: effects of prey type. Zoology 114, 165-170. ( 10.1016/j.zool.2010.11.008) [DOI] [PubMed] [Google Scholar]
- 94.Shealer DA. 2002. Foraging behavior and food of seabirds. Biol. Mar. Birds 14, 137-177. ( 10.1201/9781420036305) [DOI] [Google Scholar]
- 95.Brainerd EL, Camp AL. 2019. Functional morphology of vertebrate feeding systems: new insights from XROMM and fluoromicrometry. In Feeding in vertebrates: evolution, morphology, behavior, biomechanics (eds Bels V, Whishaw I), pp. 21-44. Cham, Switzerland: Springer. [Google Scholar]
- 96.Hicklin PW. 1979. The diets of five species of migrant shorebirds in the Bay of Fundy. Proc. N. S. Inst. Sci. 29, 483-488. [Google Scholar]
- 97.Smith KK. 1982. An electromyographic study of the function of the jaw adducting muscles in Varanus exanthematicus (Varanidae). J. Morphol. 173, 137-158. ( 10.1002/jmor.1051730203) [DOI] [PubMed] [Google Scholar]
- 98.Jessop TS, Ariefiandy A, Purwandana D, Benu YJ, Hyatt M, Letnic M. 2019. Little to fear: largest lizard predator induces weak defense responses in ungulate prey. Behav. Ecol. 30, 624-636. ( 10.1093/beheco/ary200) [DOI] [Google Scholar]
- 99.Maho T. 2022. Diet-Related Tooth Development and Replacement in Early Amniotes, with Comparison to a Stem Amniote and Extant Reptiles. PhD Thesis, University of Toronto (Canada). [Google Scholar]
- 100.Bhullar B-AS, Marugán-Lobón J, Racimo F, Bever GS, Rowe TB, Norell MA, Abzhanov A. 2012. Birds have paedomorphic dinosaur skulls. Nature 487, 223-226. ( 10.1038/nature11146) [DOI] [PubMed] [Google Scholar]
- 101.Noel AC, Guo H-Y, Mandica M, Hu DL. 2017. Frogs use a viscoelastic tongue and non-Newtonian saliva to catch prey. J. R. Soc. Interface 14, 20160764. ( 10.1098/rsif.2016.0764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brau F, Lanterbecq D, Zghikh L-N, Bels V, Damman P. 2014. Dynamics of the prey prehension by chameleons through viscous adhesion. Nature Physics 12, 931-935. ( 10.1038/nphys3795) [DOI] [Google Scholar]
- 103.Brau F, Lanterbecq D, Zghikh L-N, Bels V, Damman P. 2016. Dynamics of prey prehension by chameleons through viscous adhesion. Nat. Phys. 12, 931-935. ( 10.1038/nphys3795) [DOI] [Google Scholar]
- 104.de Groot JH, van Leeuwen JL. 2004. Evidence for an elastic projection mechanism in the chameleon tongue. Proc. R. Soc. Lond. B 271, 761-770. ( 10.1098/rspb.2003.2637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deban SM, Wake DB, Roth G. 1997. Salamander with a ballistic tongue. Nature 389, 27-28. ( 10.1038/37898)9288962 [DOI] [Google Scholar]
- 106.Rash R, Lillywhite HB. 2019. Drinking behaviors and water balance in marine vertebrates. Mar. Biol. 166, 122. ( 10.1007/s00227-019-3567-4) [DOI] [Google Scholar]
- 107.Bels V, Le Floch G, Kirchhoff F, Gastebois G, Davenport J, Baguette M. 2023. Food transport in reptilia: a comparative viewpoint. Figshare. ( 10.6084/m9.figshare.c.6834884) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bels V, Le Floch G, Kirchhoff F, Gastebois G, Davenport J, Baguette M. 2023. Food transport in reptilia: a comparative viewpoint. Figshare. ( 10.6084/m9.figshare.c.6834884) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data are simply video films to support the text of the paper, and they can be found in the electronic supplementary material [107]. These data were recorded as simple bird watching without any need for permission.