Abstract
The evolution of the mother/infant dyad providing a source of nutrition for infants is essential for the origin and subsequent diversification of mammals. Despite the importance of this dyad, research on maternal and infant function is often treated independently. Our goal is to synthesize the work on maternal and infant function, discuss our own studies of suckling, and compare the origins of lactation and suckling with their ensuing diversification. Our central premise is that while extensive work has demonstrated variation across mammals in the maternal aspect of this system, very little has been done to address how this relates to infant function. We start with a discussion of the fundamental anatomy and physiology of both mother and infant. We next discuss the origin of mammary glands and milk, and infant suckling, which is distinct from their subsequent diversification. We then discuss the diversification of maternal and infant function, highlighting the evolutionary diversity present in maternal function (both anatomically and physiologically), before arguing that the diversity of infant function is unexplored, and needs to be better studied in the future. We end by discussing some of the holes in our understanding, and suggestions for future work that can address these lacunae.
This article is part of the theme issue ‘Food processing and nutritional assimilation in animals’.
Keywords: suckling, mammalian evolution, oropharyngeal anatomy
1. Introduction
Maternal mammary glands, including both milk production and the delivery of milk to an infant, and the ability of infants to obtain that sustenance, have fascinated biologists since Darwin, who discussed the mammary gland in his book On the Origin of Species [1]. He, and others since then, have questioned how these processes have evolved, and what their evolution means for the diversification and success of mammals as a lineage [2,3]. When we look at the differences between mammals and their nearest living relatives, as well as the differences that exist among extant mammals, there are two distinct evolutionary events that have occurred. The first, the origin of lactation and the origin of suckling behaviour probably evolved prior to or near the origin of mammals. The second, the diversification of the two parts of this dyad (maternal and infant function), happened after the mammalian features were established in the earliest-known fossil mammals and their extant mammalian descendants. These are two distinct evolutionary stories, although the second is dependent on and a consequence of the first. A great deal of insight and analysis exists for both stories, over a number of different disciplines.
The goal of this paper is to synthesize the sometimes disparate work on both maternal and infant function, discuss our own studies of suckling, and compare the origins of lactation and suckling with their subsequent diversification to gain insight into both of these stories. We will start with the fundamentals of both components of this dyad. While broken into sections, we acknowledge that this is a continuous system of a mammary gland that produces milk which is consumed by an infant. The information and research on mammary glands and milk is far more detailed and pervasive than on infant function. The first two sections (mammary gland and milk) are shorter because excellent review papers of current understanding exist. We focus more on infant function, and the evolution of infant function as being our contribution to the understanding of the evolution and diversification of this system. Next, we will discuss the current knowledge of the evolutionary origins of these three components of the mother/infant system. This is followed by a section detailing the subsequent evolution of these characters in the context of diversification of mammals. We end by discussing some of the holes in our understanding, and suggestions for future work that can address these lacuna (figure 1).
Figure 1.
Scheme of paper for understanding the evolution of the mother-infant dyad for feeding in mammals. Joint consideration of maternal and infant interactions, the dyad, are critical for both origin and subsequent diversification. The evolutionary forces that originated these interactions are distinct from, but the basis of, diversificatin within mammals.
Despite the recognition that infants are an equal partner in the evolutionary processes that have shaped maternal lactation/infant feeding, the impact of evolution on both the origin and subsequent diversification of this dyad has largely focused on the maternal side of this partnership. Our central premise is that selection has acted strongly on both mother and infant, and that acquiring data on infant feeding is a critical part of this story. By bringing together our current understanding, new palaeontological discoveries and interpretations, and comparative studies of extant species, we hope to move the focus of this field to a more synthetic interpretation that can be the building blocks for further study.
2. Fundamentals
(a) . Maternal anatomy and physiology
At its core, the function of the maternal mammary gland is to provide an avenue for nutrients to be provided to an infant [4]. Specifically, the mammary gland is responsible for synthesizing, secreting and providing a means for delivering milk to an infant [5]. This can occur through various means and varies across mammalian species (reviewed later). The mammary gland differs from most other organs in the body, in that the mammary gland only reaches a functional state during the pregnancy-lactation cycle in adult females [5]. In this state, the generalized mammary gland has a complex, hierarchical organization, of lobes and ducts that emerge at the nipple [6,7]. The production of milk by the mammary gland is also extremely metabolically expensive and can require up to 25% of the daily maternal energy intake during lactation [8]. The production of milk is by female mammals is the source of sizable amounts of work, for evolutionary, agricultural and clinical reasons [9].
Milk, the product of the mammary gland, is regarded as a complete food source for infants. Milk is composed primarily of lipids, carbohydrates (such as oligosaccharides and lactose) and proteins, in addition to several minerals and vitamins [10]. One of the primary proteins within milk are caseins, which are unique to milk, and are a primary transport vehicle for calcium, phosphorus and amino acids that are required by offspring [11]. Caseins found in milk are thus essential for neonatal nutrition and have been posited to be one of the most important evolutionary novelties associated with lactation [11,12]. Similarly, milk lipids possess unique features, in that they are packaged into membrane-enclosed structures known as milk fat globules [11]. Milk also possesses several unique carbohydrates, such as lactose [13]. Beyond its specific nutritional composition, milk also provides several key components to offspring function, as detailed in several reviews [9,10]. For example, milk provides antimicrobial properties, as well as functioning as a microbial ecosystem engineer, in addition to providing a hormonal signal for infants, a source of stem cells, hydration for infants, and may enhance neurodevelopment [14–16].
(b) . Infant anatomy and physiology
Infant suckling is biomechanically and neurologically distinct from adult feeding [17–19]. Suckling is primarily a tongue-based mechanism, consisting of several distinct actions. The first is acquisition of liquid (milk) from a nipple. Next, the milk is transported through the oral cavity into the valleculae at the base of the tongue in the oropharynx. Finally, the milk is moved out of the oropharynx, across the laryngeal opening, through the upper oesophageal sphincter, and into the oesophagus. Acquisition requires two seals, an anterior and a posterior. Anteriorly, the tongue wraps around the nipple, and in some species the lips also close. The posterior seal is formed by the back of the tongue pressing against the soft palate. This isolates the oral cavity so that dorsal/ventral contraction of the tongue functions as a pump to reduce intraoral pressure. The pressure change pulls milk from the nipple into the oral cavity [20,21]. Transport and movement of the milk from the oral cavity to the point of swallowing requires breaking of the posterior seal, and a subsequent pumping movement of the more posterior tongue [22,23].
While the tongue, including both intrinsic and extrinsic muscles, is clearly the major structure responsible for these movements, other structures play a critical role. In acquisition, the lips and tongue sealing around the nipple anteriorly, and the tongue sealing against the hard and soft palates posteriorly is necessary for being able to change the pressure within the oral cavity. The tensor veli palatini, which tenses the soft palate, as well as the pillars of the fauces, composed by the palatoglossal muscle (anterior pillar) and the palatopharyngeus (posterior pillar) are critical structures in suckling [21]. These structures are largely muscle, covered with oral mucosa, but they are attached to bones. The soft palate is attached to the hard palate, but the pterygoid bones, including the hamulus, are critical for the suction generated [24]. The subsequent movement of the milk also depends on the contraction of the palatal, pharyngeal, and hyoid muscles, as well as tongue movements. Finally, the hyoid bone must be anchored by its inferior muscles, as it is the base of the tongue, an attachment for the pharyngeal muscles, and important for the pillars of the fauces [21,24–26].
3. The evolutionary origins of the infant/mother dyad in lactation and suckling
It is generally thought that mammalian milk evolved before or with mammalian diphyodont dentition that consists of two generations of teeth: the ‘milk teeth’ (deciduous teeth) erupting during neonatal to juvenile growth, and their replacement by permanent teeth during later growth [27–30]. The diphyodont tooth replacement, which is used as a proxy for lactation, first appeared in mammaliaforms [28,31], a phylogenetic group including extant mammals and their extinct relatives of the Mesozoic, collectively diagnosed by the dentary and squamosal craniomandibular joint [28,31]. Based on this fossil evidence, the lactation occurred in mammaliaforms, well before the common ancestor of crown Mammalia [25,26]. Various other aspects of mammalian biology, including endothermy [27], may also predate the origin of crown mammals [32], if defined by a tempro-mandibular jaw joint. Yet, the determination of the specific origin of both lactation and suckling is scientifically difficult. Neither mammary glands nor tongues and oral cavities fossilize well and determination of a primitive-derived character axis for these traits is challenging [33]. Nonetheless, we have made substantial progress in understanding the origins of lactation and suckling in the past 50 years, which we highlight briefly here.
(a) . The origins of lactation and milk
Mammalian mammary glands are largely considered to be built from apocrine glands [11,34], although some evidence suggests they may have originated as part of the immune system as mucous skin secretion [35]. Because there is no fossil record, only comparative studies of modern taxa are possible. The origin of milk is closely tied to the origin of mammary glands [11,12]. Both earlier work [2,4] and more recent biochemical studies [11] suggest that the ability to feed infants would confirm a significant selective advantage to either true mammals or the immediate ancestors.
(b) . The origin of infant suckling
Determining the origin of suckling is difficult, given that this behaviour does not rely on hard structures, unlike extrapolating locomotor mechanics from bones, or from teeth and jaw joint to ecology and biomechanics of adult feeding. However, one intriguing source of palaeontological data is the evolution of the hyoid bone. A recent discovery, the mammaliaform fossil Microdocodon by Zhou, Luo and colleagues, shows that a mammal-like hyoid appeared well before the common ancestor of extant mammals [28]. The hyoid bone forms the base for most oropharyngeal functions in mammals, and many of the muscles that control suckling, as well as adult feeding are associated with this bone [36]. The presence of the modern mammal-like hyoid in this mammaliaform precursor to mammals suggests that the muscles that are required to produce suction would be able to attach to this hyoid apparatus, and support a functional tongue capable of suckling.
Other recent data and interpretations support this position [27]. A study of inner ear morphology integrating modern physiology with detailed measurements of fossils suggests that endothermy evolved in the Mammaliamorpha [31]. The evolution of milk and suckling would need to evolve at a similar point in time to support infants. These palaeontological results provide the foundation for much of the argument that, as with the mammary gland and milk, the ability of an infant to suckle evolved prior to the evolution of mammals. The evolution of suckling prior to the origin of mammals may have played a key role in the development of the temporomandibular joint and thus their ecological success. Unlike infants that must feed as adults from birth (such as most reptiles), suckling would allow for a delay in the development of the musculoskeletal system needed to support rotary jaw motions and adult mastication.
4. The evolutionary diversification among mammalia of the infant/mother dyad in lactation and suckling
The diversification of mammals, which has roots in the Mesozoic, but was strongest in the Cenozoic, is evolutionarily distinct from the origin of mammals. While the origin of mammals established the particular traits that characterize mammals, including endothermy, the temporomandibular joint, complex multi-cuspid teeth and mammary glands/lactation, this is a separate story from the evolution of the consequent variation in these traits that characterizes modern mammals. These traits, plus the exigencies of history made it possible for the subsequent diversification of mammals [37,38].
(a) . Variation and comparative anatomy/physiology of the mammary gland and milk
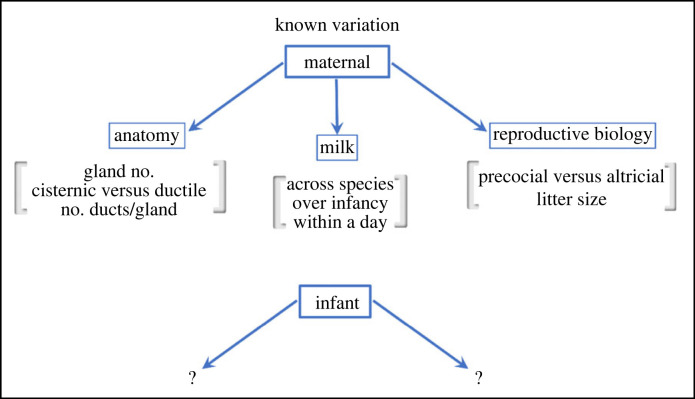
As the structure that provides the foundation for the name of the clade of Mammals (meaning ‘of the breast’), the structure and evolution of mammary glands have received significant study [39]. There is vast variation in breast (and mammary gland) development and anatomy, both across all mammals [12,40,41], as well as within eutherians [12,42]. For example, the number of mammary glands varies across mammals, and is closely linked to the number of offspring born at a time [42]. Similarly, the number of ducts per gland varies across mammals, although the function of this remains relatively unexplored [12,42]. Furthermore, ruminants possess a cisternic breast anatomy, where milk is collected from glandular lobes and stored, whereas in other eutherian mammals ducts empty into the infant mouth directly [43–46]. Finally, there is variation within and across species in the strength of milk letdown that would impact flow rates, driven by variation in myoepithelial cell function that drives letdown [46,47]. Critically, despite the major variation in both the embryology and gross morphology of the mammalian breast, we have very little insight into how this variation is reflected by infant feeding function across mammals.
Milk composition in mammals has also received comprehensive study over the past 50 years. There is large variation in the composition of milk across species, even within eutherians. In most species, caseins are the primary protein found in milk and have been posited to have evolved prior to the evolution of mammals [48]. However, their concentration within milk varies by more than an order of magnitude [10,49–51]. There is further variation across mammals in the type of casein present in different species, as well as variability in phosphorylation patterns across mammals [10,52–54]. Similar patterns are also present in other proteins found in milk, as well as in lipid content across mammals, with some species having lipid contents in milk of less than 1%, and others up to more than 60% [11]. Overall, milk composition across mammals appears to be primarily a function of a combination of phylogenetic relationships, maternal nutrient intake, and the duration of milk production [55,56]. Any variation in milk composition will also impact its viscosity, and thus its flow rate, which in turn may impact infant function [57]. This high level of variation emphasizes the fact that milk composition is evolutionarily tuned to the demands of different species and that this composition has bearing on infant function (figure 2). For example, although bears consume less milk per body mass compared to other mammals, their milk is more concentrated, which allows them to obtain their nutritional requirements, just with less volume [58,59].
Figure 2.
Known variation in aspects of maternal and infant anatomy, physiology, and function. Several maternal aspects have well documented variation. However, almost nothing is known about variation within infant feeding. Because this variation is the basis of natural selection, and evolution, this deficit represents a significant roadblock in understanding the evolution of the maternal/infant dyad.
Milk concentration also varies through development in a nursing infant. One drastic example of this in eutherians occurs through the production of colostrum (secretion with high levels of antibodies) for the first few days of life before milk is secreted [60,61]. Colostrum also has numerous bioactive components, and its content and function varies across mammals. For example, ungulates, with exclusive postpartal care, have higher concentrations of immunoglobulin G in colostrum than species with prepartal transfer of immunoglobulins such as humans and rabbits [62]. Beyond colostrum, there is also variation in the composition of milk through the time spent nursing. This is especially true in monotremes and marsupials, which have extended periods of nursing [13,63–66], and also occurs in most eutherian species, including humans, albeit less dramatically [67–69]. The concentration of milk also differs within a single feeding session, and varies depending on the time of the day, and is thought to provide infants with cues for self-satiation [70,71] (figure 2). Thus, the composition of milk probably has been a target of selection to match the nutritional demands of infants, balanced with maternal (adult) diets.
Taken together, these data demonstrate that selection has acted powerfully upon mothers physiology and morphology in all aspects of delivering milk to an infant: from the structure of the mammary gland, through the composition of milk, to the anatomical structure that delivers that milk. However, mothers only represent half of the infant–mother dyad, and very little attention has been paid to how infant anatomy and physiology in relation to feeding function have evolved across mammals in concert with their mothers.
(b) . Variation in suckling anatomy and physiology across mammals
In contrast to the sweeping work done on the maternal half of the mother/infant dyad, very little comparative work has been done to understand how infants are acquiring milk (figure 2). Across all mammals, data exists on relatively few species, including widespread data on humans [72–74] and pigs [25,75–77], and sparse data on marsupials [17,78], non-human primates [20], rodents [79] and carnivores [80,81], making phylogenetic comparisons nearly impossible.
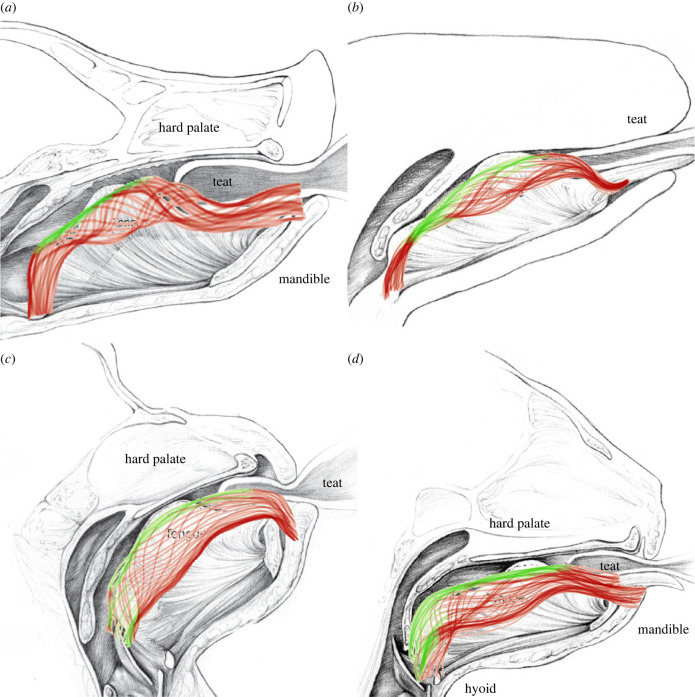
In the few species with detailed biomechanical studies, the general mechanisms of suckling are consistent in that a seal is made to generate suction and acquire milk, although there is variation in the specific mechanisms used. Within this variation, it appears as though similarities and differences are driven more by oropharyngeal anatomy than by evolutionary relationships. For example, felids and primates appear to suckle more similarly to each other than to possums, which suckle more similarly to pigs [80,81]. Instead, differences in suckling appear to relate strongly to the relative length of the oral cavity (figure 3), which differs across infant mammals, although not as significantly as it does in adult members of these groups [82–84]. For example, species with longer faces (possums and suids) appear to use two tongue–palate contact points during suckling (one at the hard palate anteriorly, and one posteriorly at the soft palate), whereas the tongue in species with shorter faces (primates and felids) only contacts the hard palate. This probably impacts suction generation, milk transport, and suck–swallow–breathe dynamics across mammalian infants. For example, within pigs, infants that latch onto only the tip of the nipple (sippers) are able to generate higher suction pressures, acquire more milk, and feed more efficiently than individuals that tend to latch onto a larger portion of the nipple [22]. This is clinically relevant, as premature infants typically latch onto a nipple with the tip deeper into their mouth, further compounding their abilities to generate suction and acquire milk [22,85,86]. Thus, interspecific differences in the available space within the oral cavity could drive variation in performance across species, and suggests a functional coupling between infant oropharyngeal anatomy, and maternal breast morphology. In addition, even within species that have been studied, we have very little insight into how the mechanisms of milk extraction by the infant are related to performance and survival.
Figure 3.
Examples of suckling across mammals in the pig (a), opossum (b), macaque (c), and cat (d). Black and white illustrations demonstrate general suckling anatomy, whereas coloured traces indicate movement of the tongue across multiple cycles in each group. The pig and opossum generally use more similar movements of the tongue to each other, whereas the macaque and cat are more similar, probably owing to differences in infant anatomy rather than phylogenetic relatedness. Red lines: times when the tongue is not in contact with the hard or soft palate; green lines: points in time in which the tongue is in contact with the hard or soft palate. Modified and reproduced with permission from Devon Stuart (https://devonmedicalart.com/).
Outside of these few lineages, there are only intermittent details on the physiology of suckling in other species. For example, Cetaceans have evolved several maternal and infant synapomorphies associated with their aquatic habitat [87], including a high degree of maternal care and investment. Female cetaceans are known to eject milk, but infant sperm whales and Tursiops dolphins have both been observed to use tongue movements and generate suction similar to terrestrial infant mammals [88–90]. Thus, even in marine mammals, infant physiology remains a critical component of the mother–infant dyad. The only work that exists on the biomechanics of suckling in rodents is from Westneat & Hall [79], which demonstrated that electromyography (EMG) activity of many of the muscles associated with feeding is similar to those observed in infant pigs, the other lineage with robust EMG data of suckling [23,77,91]. However, there are no kinematic data in this work, so we lack a clear consensus of how the tongue functions during feeding in infant rodents. Finally, the small amount of data on infant feeding in monotremes is inconclusive. Monotremes have a mosaic of oropharyngeal features associated with suckling, and sucking has been hypothesized to occur based on visual observation and sonograms of sucking sounds [92]. However, while they have a mammal-like hyoid bone, they appear to lack pterygoid hamuli and muscles of the soft palate that are hypothesized to be necessary to generate a seal at the posterior of the mouth [24]. This morphology is probably derived, as they have several specialized oropharyngeal structures, including a long palatine bone, and keratinized pads on the palatine and posterior tongue that are thought to be used by adults [24] to feed.
These interspecific data, though scarce, demonstrate that there is variation in both anatomy and physiology of infants across these mammal species, that this variation is not necessarily solely owing to phylogenetic differences, and provides a potential substrate for selection to act on infants. Suckling, a critical aspect for surviving through the perilous time that is mammalian infancy, is likely as much a target for selection within mammals as the mechanism by which milk, and the content of that milk, is delivered to the infant that is consuming it. Further, while the variation in infants is more subtle than that in the anatomy and physiology of adult mammalian feeding, the developmental map of evolution in infants to the prevalent variation remains understudied.
5. Discussion
(a) . What we have learned, including problems with data and unexplored possibilities
The origin and subsequent diversification of the mammalian mother/infant dyad has provided a foundation for the success and diversification of mammals throughout the Cenozoic (although, to acknowledge the perspective of the late Karel Liem, that the Cenozoic is truly the age of teleosts). The evolution of suckling is a fundamental trait to support an endothermic infant, which has high metabolic and nutritional needs immediately after birth. However, suckling may additionally allow for a delay in the development of the anatomy and physiology needed for adult feeding, which requires a functional temporomandibular joint and teeth (both bony structures that must resist high forces). The consumption of milk via suckling may delay the need for these structures to function in the adult capacity, essentially buying offspring time to postnatally develop the jaw joint and teeth that are so characteristic of adult mammals [1,3,31]. This suggests that the evolution of suckling may have provided the essential foundation for mammals to have evolved into the diverse feeding ecologies and strategies that exist today.
While we have good evidence for how selection operated to establish the origins of the mammary gland and lactation, as well as how these have diversified across mammals, data on the comparative biomechanics of infant feeding is scarce and limited to a few species. The lack of data on suckling physiology and performance of infants across mammals inhibits our ability to understand the potential for selection to have acted on infant physiology, despite the voluminous evidence suggesting that selection has acted on maternal function during lactation.
Examination of existing comparative data on mammary glands suggests significant selection among mammalian taxa. For example, there are differences not just in the shape and size of the maternal teat, but also in how it delivers milk. Furthermore, variation in mammalian reproductive strategies within and between monotremes, marsupials, and eutherians is remarkable. Marsupials are born at an extremely altricial state and spend an extended period of time after birth latched on to the maternal teat prior to achieving an independent locomotor state, whereas many eutherians are mobile within minutes of being born [93]. Beyond litter size, much of infant mammalian physiology has involved classifying different taxa along an axis of altricial to precocial [94]. However, this perspective is grounded in locomotor function, and we have little understanding of the maturation of suckling physiology across mammals. How variation in the reproductive system across mammals is reflected in infant function is unexplored, although probably significant.
Finally, another unexplored axis of potential selection on infant function lies in the transition from drinking milk from a maternal nipple to feeding on solid food, also known as weaning [95]. Some work has been done on this from an evolutionary perspective, highlighting that extensive milk-only feeding is disadvantageous to mothers, and that having an elongated ‘weaning’ period provides an opportunity for infants to subsidize maternal investment [96]. However, very little work has been conducted on the physiology of weaning, and the possibility that variation in physiology may or may not covary with variation in how it has evolved across mammals.
Mammals are defined by suckling, yet exhibit extreme variation in their adult anatomy and physiology. For example, the hard and soft secondary palates involved in infant suckling are quite different between monotremes on one hand and the marsupials and placentals on the other [24,97]. Within eutherians, beyond tooth morphology, the length of the oral cavity and associated structures, especially the tongue, vary with trophic type and adult body size [26,84]. As such we might expect to see that the pathway to move from infancy to adulthood from the perspective of feeding physiology could be explained by phylogeny, diet, anatomy, or some other axis of variation. Understanding how this is achieved across mammals could provide insights into how the physiology of infant feeding might either constrain or facilitate the ability of mammals to evolve into feeding on novel diets.
(b) . The future
Examination of the correlations, let alone determination of causal relationships, between maternal anatomy and physiology with infant anatomy and physiology will be fruitful. Up until this point, despite discussion of the mother/infant dyad, evolutionary work has focused solely on the maternal aspect of this dyad. Understanding how variation in maternal anatomy (e.g. lactiferous versus cisternic), reproductive patterns (i.e. precocial versus altricial, single offspring versus litters) is reflected in variation in infant anatomy and physiology is critical in any discussion on the mechanisms and patterns driving evolution across mammals more broadly.
Acknowledgements
We thank decades of collaborators, but especially our mentors: Richard Blob, Stephen Jay Gould, Karen Hiiemae, A. W. Crompton and Allan Thexton. We are grateful to Zhe-Xi Luo for sharing information and insights about Microdocodon gracilis with us, and to Devon Stuart for an earlier version of figure 3. We also thank all three reviewers who made fabulous suggestions that improved this paper.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
This article has no additional data.
Authors' contributions
R.Z.G.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, writing—review and editing; C.J.M.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, writing—review and editing.
Both authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare no competing interests.
Funding
Funding for this work was provided by NIH NICHD to R.Z.G. (grant no. R01HD088561) and C.J.M. (grant no. R00HD105922).
References
- 1.Darwin C. 1964. On the origin of species: a facsimile of the first edition. Cambridge, MA: Harvard University Press. [Google Scholar]
- 2.Blackburn DG. 1991. Evolutionary origins of the mammary gland. Mamm. Rev. 21, 81-96. ( 10.1111/j.1365-2907.1991.tb00290.x) [DOI] [Google Scholar]
- 3.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: Penguin Classics. See http://www.penguin.co.uk/nf/Book/BookDisplay/0,,9780140436310,00.html. [Google Scholar]
- 4.Pond CM. 1977. The significance of lactation in the evolution of mammals. Evolution 31, 177-199. ( 10.1111/j.1558-5646.1977.tb00995.x) [DOI] [PubMed] [Google Scholar]
- 5.Hassiotou F, Geddes D. 2013. Anatomy of the human mammary gland: current status of knowledge. Clin. Anat. 26, 29-48. ( 10.1002/ca.22165) [DOI] [PubMed] [Google Scholar]
- 6.Negin Mortazavi S, Hassiotou F, Geddes D, Hassanipour F. 2015. Mathematical modeling of mammary ducts in lactating human females. J. Biomech. Eng. 137, 071009. ( 10.1115/1.4028967) [DOI] [PubMed] [Google Scholar]
- 7.Azarnoosh J, Hassanipour F. 2020. Fluid-structure interaction modeling of lactating breast. J. Biomech. 103, 109640. ( 10.1016/j.jbiomech.2020.109640) [DOI] [PubMed] [Google Scholar]
- 8.Hartmann PE. 2007. The lactating breast: an overview from down under. Breastfeed. Med. 2, 3-9. ( 10.1089/bfm.2006.0034) [DOI] [PubMed] [Google Scholar]
- 9.Hinde K, German JB. 2012. Food in an evolutionary context: insights from mother's milk. J. Sci. Food Agric. 92, 2219-2223. ( 10.1002/jsfa.5720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin P, Cebo C, Miranda G. 2013. Milk proteins: Introduction and historical aspects. In Advanced dairy chemistry: volume 1A: proteins: basic aspects, 4th edition (eds McSweeney PLH, Fox PF), Boston, MA: Springer US. ( 10.1007/978-1-4614-4714-6) [DOI] [Google Scholar]
- 11.Oftedal OT. 2020. The evolution of lactation in mammalian species. In Milk, mucosal immunity and the microbiome: impact on the neonate: 94th Nestlé nutrition institute workshop, Lausanne, September 2019 (eds Ogra PL, Walker WA, Lönnerdal B). Basel, Switzerland: S. Karger AG. ( 10.1159/isbn.978-3-318-06685-2) [DOI] [Google Scholar]
- 12.Oftedal OT, Dhouailly D. 2013. Evo-devo of the mammary gland. J. Mammary Gland Biol. Neoplasia 18, 105-120. ( 10.1007/s10911-013-9290-8) [DOI] [PubMed] [Google Scholar]
- 13.Oftedal OT, Nicol SC, Davies NW, Sekii N, Taufik E, Fukuda K, Saito T, Urashima T. 2014. Can an ancestral condition for milk oligosaccharides be determined? Evidence from the Tasmanian echidna (Tachyglossus aculeatus setosus). Glycobiology 24, 826-839. ( 10.1093/glycob/cwu041) [DOI] [PubMed] [Google Scholar]
- 14.Hinde K, Lewis ZT. 2015. Mother's littlest helpers. Science 348, 1427-1428. ( 10.1126/science.aac7436) [DOI] [PubMed] [Google Scholar]
- 15.de Weerth C, et al. In press. Human milk: from complex tailored nutrition to bioactive impact on child cognition and behavior. Crit. Rev. Food Sci. Nutr. ( 10.1080/10408398.2022.2053058) [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Deng Q, Wang J, Wang H, Li Q, Zhu B, Ji C, Xu X, Johnston L. 2022. The impact of breast milk feeding on early brain development in preterm infants in China: an observational study. PLoS ONE 17, e0272125. ( 10.1371/journal.pone.0272125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.German RZ, Crompton AW. 1996. Ontogeny of suckling mechanisms in opossums (Didelphis virginiana). Brain Behav. Evol. 48, 157-164. ( 10.1159/000113194) [DOI] [PubMed] [Google Scholar]
- 18.Thexton AJ, Crompton AW, German RZ. 1998. Transition from suckling to drinking at weaning: a kinematic and electromyographic study in miniature pigs. J. Exp. Zool. 280, 327-343. () [DOI] [PubMed] [Google Scholar]
- 19.Bond LE, Mayerl CJ, Stricklen BM, German RZ, Gould FDH. 2020. Changes in the coordination between respiration and swallowing from suckling through weaning. Biol. Lett. 16, 20190942. ( 10.1098/rsbl.2019.0942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.German RZ, Crompton AW, Levitch LC, Thexton AJ. 1992. The mechanism of suckling in two species of infant mammal: miniature pigs and long-tailed macaques. J. Exp. Zool. 261, 322-330. ( 10.1002/jez.1402610311) [DOI] [PubMed] [Google Scholar]
- 21.Thexton AJ, Crompton AW, Owerkowicz T, German RZ. 2004. Correlation between intraoral pressures and tongue movements in the suckling pig. Arch. Oral Biol. 49, 567-575. ( 10.1016/j.archoralbio.2004.02.002) [DOI] [PubMed] [Google Scholar]
- 22.Mayerl CJ, Edmonds CE, Catchpole EA, Myrla AM, Gould FDH, Bond LE, Stricklen BM, German RZ. 2020. Sucking versus swallowing coordination, integration, and performance in preterm and term infants. J. Appl. Physiol. 129, 1383-1392. ( 10.1152/japplphysiol.00668.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayerl CJ, Steer KE, Chava AM, Bond LE, Edmonds CE, Gould FDH, Stricklen BM, Hieronymous TL, German RZ. 2021. The contractile patterns, anatomy and physiology of the hyoid musculature change longitudinally through infancy. Proc. R. Soc. B 288, 20210052. ( 10.1098/rspb.2021.0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crompton AW, Musinsky C, Bonaparte J, Bhullar B, Owerkowicz T. 2018. Evolution of the mammalian fauces region and the origin of suckling. Harvard Dash Repository. See https://nrs.harvard.edu/URN-3:HUL.INSTREPOS:37364482.
- 25.Mayerl CJ, Steer KE, Chava AM, Bond LE, Edmonds CE, Gould FDH, Hieronymous TL, Vinyard CJ, German RZ. 2021. Anatomical and physiological variation of the hyoid musculature during swallowing in infant pigs. J. Exp. Biol. 224, jeb243075. ( 10.1242/jeb.243075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, Ross CF, Luo Z-X. 2022. Morphological disparity and evolutionary transformations in the primate hyoid apparatus. J. Hum. Evol. 162, 103094. ( 10.1016/j.jhevol.2021.103094) [DOI] [PubMed] [Google Scholar]
- 27.Araújo R, et al. 2022. Inner ear biomechanics reveals a Late Triassic origin for mammalian endothermy. Nature 607, 726-731. ( 10.1038/s41586-022-04963-z) [DOI] [PubMed] [Google Scholar]
- 28.Luo Z-X. 2007. Transformation and diversification in early mammal evolution. Nature 450, 1011-1019. ( 10.1038/nature06277) [DOI] [PubMed] [Google Scholar]
- 29.Luo Z-X, Kielan-Jaworowska Z, Cifelli RL. 2004. Evolution of dental replacement in mammals. carb 2004, 159-175. ( 10.2992/0145-9058(2004)36[159:EODRIM]2.0.CO;2) [DOI] [Google Scholar]
- 30.O'Meara RN, Asher RJ. 2016. The evolution of growth patterns in mammalian versus nonmammalian cynodonts. Paleobiology 42, 439-464. ( 10.1017/pab.2015.51) [DOI] [Google Scholar]
- 31.Rowe T. 1988. Definition, diagnosis, and origin of Mammalia. J. Vertebr. Paleontol. 8, 241-264. ( 10.1080/02724634.1988.10011708) [DOI] [Google Scholar]
- 32.Newham E, et al. 2020. Reptile-like physiology in Early Jurassic stem-mammals. Nat. Commun. 11, 5121. ( 10.1038/s41467-020-18898-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith KK. 1992. The evolution of the mammalian pharynx. Zool. J. Linn. Soc. 104, 313-349. ( 10.1111/j.1096-3642.1992.tb00926.x) [DOI] [Google Scholar]
- 34.Oftedal OT. 2012. The evolution of milk secretion and its ancient origins. Animal 6, 355-368. ( 10.1017/S1751731111001935) [DOI] [PubMed] [Google Scholar]
- 35.Vorbach C, Capecchi MR, Penninger JM. 2006. Evolution of the mammary gland from the innate immune system? Bioessays 28, 606-616. ( 10.1002/bies.20423) [DOI] [PubMed] [Google Scholar]
- 36.German RZ, Campbell-Malone R, Crompton AW, Ding P, Holman S, Konow N, Thexton AJ. 2011. The concept of hyoid posture. Dysphagia 26, 97-98. ( 10.1007/s00455-011-9339-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gould SJ, Gilinsky NL, German RZ. 1987. Asymmetry of lineages and the direction of evolutionary time. Science 236, 1437-1441. ( 10.1126/science.236.4807.1437) [DOI] [PubMed] [Google Scholar]
- 38.Gould SJ. 2002. The structure of evolutionary theory. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 39.Linnaeus C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. Stockholm, SW: Holmia. See https://antcat.org/references/126902. [Google Scholar]
- 40.Propper AY, Howard BA, Veltmaat JM. 2013. Prenatal morphogenesis of mammary glands in mouse and rabbit. J. Mammary Gland Biol. Neoplasia 18, 93-104. ( 10.1007/s10911-013-9298-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spina E, Cowin P. 2021. Embryonic mammary gland development. Semin. Cell Dev. Biol. 114, 83-92. ( 10.1016/j.semcdb.2020.12.012) [DOI] [PubMed] [Google Scholar]
- 42.Ventrella D, et al. 2021. Animal models for in vivo lactation studies: anatomy, physiology and milk compositions in the most used non-clinical species: a contribution from the ConcePTION project. Animals 11, 714. ( 10.3390/ani11030714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira AM, Bislev SL, Bendixen E, Almeida AM. 2013. The mammary gland in domestic ruminants: a systems biology perspective. J. Proteomics 94, 110-123. ( 10.1016/j.jprot.2013.09.012) [DOI] [PubMed] [Google Scholar]
- 44.Hughes K. 2021. Comparative mammary gland postnatal development and tumourigenesis in the sheep, cow, cat and rabbit: exploring the menagerie. Semin. Cell Dev. Biol. 114, 186-195. ( 10.1016/j.semcdb.2020.09.010) [DOI] [PubMed] [Google Scholar]
- 45.Lérias JR, Hernández-Castellano LE, Suárez-Trujillo A, Castro N, Pourlis A, Almeida AM. 2014. The mammary gland in small ruminants: major morphological and functional events underlying milk production – a review. J. Dairy Res. 81, 304-318. ( 10.1017/S0022029914000235) [DOI] [PubMed] [Google Scholar]
- 46.Lincoln DW, Paisley AC. 1982. Neuroendocrine control of milk ejection. Reproduction 65, 571-586. ( 10.1530/jrf.0.0650571) [DOI] [PubMed] [Google Scholar]
- 47.Jonas W, Woodside B. 2016. Physiological mechanisms, behavioral and psychological factors influencing the transfer of milk from mothers to their young. Horm. Behav. 77, 167-181. ( 10.1016/j.yhbeh.2015.07.018) [DOI] [PubMed] [Google Scholar]
- 48.Brawand D, Wahli W, Kaessmann H. 2008. Loss of egg yolk genes in mammals and the origin of lactation and placentation. PLoS Biol. 6, e63. ( 10.1371/journal.pbio.0060063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kappeler S, Farah Z, Puhan Z. 1998. Sequence analysis of Camelus dromedarius milk caseins. J. Dairy Res. 65, 209-222. ( 10.1017/S0022029997002847) [DOI] [PubMed] [Google Scholar]
- 50.Ginger MR, Grigor MR. 1999. Comparative aspects of milk caseins. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 124, 133-145. ( 10.1016/S0305-0491(99)00110-8) [DOI] [PubMed] [Google Scholar]
- 51.Miranda G, Mahé M-F, Leroux C, Martin P. 2004. Proteomic tools to characterize the protein fraction of Equidae milk. Proteomics 4, 2496-2509. ( 10.1002/pmic.200300765) [DOI] [PubMed] [Google Scholar]
- 52.Chianese L, Garro G, Mauriello R, Laezza P, Ferranti P, Addeo F. 1996. Occurrence of five αs1-casein variants in ovine milk. J. Dairy Res. 63, 49-59. ( 10.1017/S0022029900031538) [DOI] [PubMed] [Google Scholar]
- 53.Poth AG, Deeth HC, Alewood PF, Holland JW. 2008. Analysis of the human casein phosphoproteome by 2-D electrophoresis and MALDI-TOF/TOF MS reveals new phosphoforms. J. Proteome Res. 7, 5017-5027. ( 10.1021/pr800387s) [DOI] [PubMed] [Google Scholar]
- 54.Matéos A, Miclo L, Mollé D, Dary A, Girardet J-M, Gaillard J-L. 2009. Equine αS1-casein: characterization of alternative splicing isoforms and determination of phosphorylation levels. J. Dairy Sci. 92, 3604-3615. ( 10.3168/jds.2009-2125) [DOI] [PubMed] [Google Scholar]
- 55.Skibiel AL, Downing LM, Orr TJ, Hood WR. 2013. The evolution of the nutrient composition of mammalian milks. J. Anim. Ecol. 82, 1254-1264. ( 10.1111/1365-2656.12095) [DOI] [PubMed] [Google Scholar]
- 56.Riek A. 2021. Comparative phylogenetic analysis of milk output at peak lactation. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 257, 110976. ( 10.1016/j.cbpa.2021.110976) [DOI] [PubMed] [Google Scholar]
- 57.Bakshi AS, Smith DE. 1984. Effect of fat content and temperature on viscosity in relation to pumping requirements of fluid milk products. J. Dairy Sci. 67, 1157-1160. ( 10.3168/jds.S0022-0302(84)81417-4) [DOI] [Google Scholar]
- 58.Riek A. 2011. Allometry of milk intake at peak lactation. Mamm. Biol. 76, 3-11. ( 10.1016/j.mambio.2010.03.004) [DOI] [Google Scholar]
- 59.Farley SD, Robbins CT. 1995. Lactation, hibernation, and mass dynamics of American black bears and grizzly bears. Can. J. Zool. 73, 2216-2222. ( 10.1139/z95-262) [DOI] [Google Scholar]
- 60.Uruakpa FO, Ismond MAH, Akobundu ENT. 2002. Colostrum and its benefits: a review. Nutr. Res. 22, 755-767. ( 10.1016/S0271-5317(02)00373-1) [DOI] [Google Scholar]
- 61.Hurley WL, Theil PK. 2011. Perspectives on immunoglobulins in colostrum and milk. Nutrients 3, 442-474. ( 10.3390/nu3040442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bigler NA, Bruckmaier RM, Gross JJ. 2022. Implications of placentation type on species-specific colostrum properties in mammals. J. Anim. Sci. 100, skac287. ( 10.1093/jas/skac287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lemon M, Bailey L. 1966. A specific protein difference in the milk from two mammary glands of a red kangaroo. Aust. J. Exp. Biol. Med. Sci. 44, 705-708. ( 10.1038/icb.1966.68) [DOI] [PubMed] [Google Scholar]
- 64.Urashima T, Messer M, Bubb WA. 1992. Biosynthesis of marsupial milk oligosaccharides. II: Characterization of a beta 6–N-acetylglucosaminyltransferase in lactating mammary glands of the tammar wallaby, Macropus eugenii. Biochim. Biophys. Acta 1117, 223-231. ( 10.1016/0304-4165(92)90083-7) [DOI] [PubMed] [Google Scholar]
- 65.Lefèvre CM, Sharp JA, Nicholas KR. 2010. Evolution of lactation: ancient origin and extreme adaptations of the lactation system. Annu. Rev. Genom. Hum. Genet. 11, 219-238. ( 10.1146/annurev-genom-082509-141806) [DOI] [PubMed] [Google Scholar]
- 66.Stannard HJ, Miller RD, Old JM. 2020. Marsupial and monotreme milk—a review of its nutrient and immune properties. PeerJ 8, e9335. ( 10.7717/peerj.9335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riedman M, Ortiz CL. 1979. Changes in milk composition during lactation in the northern elephant seal. Physiol. Zool. 52, 240-249. ( 10.1086/physzool.52.2.30152567) [DOI] [Google Scholar]
- 68.Karra MV, Udipi SA, Kirksey A, Roepke JLB. 1986. Changes in specific nutrients in breast milk during extended lactation. Am. J. Clin. Nutr. 43, 495-503. ( 10.1093/ajcn/43.4.495) [DOI] [PubMed] [Google Scholar]
- 69.Le Huërou-Luron I, Blat S, Boudry G. 2010. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr. Res. Rev. 23, 23-36. ( 10.1017/S0954422410000065) [DOI] [PubMed] [Google Scholar]
- 70.Daly S, Di Rosso A, Owens R, Hartmann P. 1993. Degree of breast emptying explains changes in the fat content, but not fatty acid composition, of human milk. Exp. Physiol. 78, 741-755. ( 10.1113/expphysiol.1993.sp003722) [DOI] [PubMed] [Google Scholar]
- 71.Saarela T, Kokkonen J, Koivisto M. 2007. Macronutrient and energy contents of human milk fractions during the first six months of lactation: macronutrient composition in human milk fractions. Acta Paediatrica 94, 1176-1181. ( 10.1111/j.1651-2227.2005.tb02070.x) [DOI] [PubMed] [Google Scholar]
- 72.Geddes DT, Kent JC, Mitoulas LR, Hartmann PE. 2008. Tongue movement and intra-oral vacuum in breastfeeding infants. Early Hum. Dev. 84, 471-477. ( 10.1016/j.earlhumdev.2007.12.008) [DOI] [PubMed] [Google Scholar]
- 73.Geddes DT, Chooi K, Nancarrow K, Hepworth AR, Gardner H, Simmer K. 2017. Characterisation of sucking dynamics of breastfeeding preterm infants: a cross sectional study. BMC Pregnancy Childbirth 17, 386. ( 10.1186/s12884-017-1574-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elad D, Kozlovsky P, Blum O, Laine AF, Po MJ, Botzer E, Dollberg S, Zelicovich M, Ben Sira L. 2014. Biomechanics of milk extraction during breast-feeding. Proc. Natl Acad. Sci. USA 111, 5230-5235. ( 10.1073/pnas.1319798111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.German RZ, Crompton AW, Hertweck DW, Thexton AJ. 1997. Determinants of rhythm and rate in suckling. J. Exp. Zool. 278, 1-8. () [DOI] [PubMed] [Google Scholar]
- 76.Mayerl CJ, Myrla AM, Bond LE, Stricklen BM, German RZ, Gould FDH. 2020. Premature birth impacts bolus size and shape through nursing in infant pigs. Pediatr. Res. 87, 656-661. ( 10.1038/s41390-019-0624-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mayerl CJ, Adjerid KA, Edmonds CE, Gould FDH, Johnson ML, Steer KE, Bond LE, German RZ. 2022. Regional variation in contractile patterns and muscle activity in infant pig feeding. Integr. Org. Biol. 4, obac046. ( 10.1093/iob/obac046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson EN, Biknevicius AR, German RZ. 2003. Ontogeny of feeding function in the gray short-tailed opossum Monodelphis domestica: empirical support for the constrained model of jaw biomechanics. J. Exp. Biol. 206, 923-932. ( 10.1242/jeb.00181) [DOI] [PubMed] [Google Scholar]
- 79.Westneat MW, Hal WG. 1992. Ontogeny of feeding motor patterns in infant rats: an electromyographic analysis of suckling and chewing. Behav. Neurosci. 106, 539-554. ( 10.1037/0735-7044.106.3.539) [DOI] [PubMed] [Google Scholar]
- 80.German RZ. 2001. Understanding infant swallowing through the use of animal models. Perspect. Swallowing Swallowing Disorders (Dysphagia) 10, 3-5. ( 10.1044/sasd10.2.3) [DOI] [Google Scholar]
- 81.German RZ, Matsuo K, Stuart DN. 2008. Swallowing in animal models: airway protection. Jpn J. Dysphagia Rehab. 12, 3-10. ( 10.32136/jsdr.12.1_3) [DOI] [Google Scholar]
- 82.Zelditch ML, Bookstein FL, Lundrigan BL. 1992. Ontogeny of integrated skull growth in the cotton rat Sigmodon fulviventer. Evolution 46, 1164-1180. ( 10.1111/j.1558-5646.1992.tb00626.x) [DOI] [PubMed] [Google Scholar]
- 83.Helm JW, German RZ. 1996. The epigenetic impact of weaning on craniofacial morphology during growth. J. Exp. Zool. 276, 243-253. () [DOI] [PubMed] [Google Scholar]
- 84.Cardini A, Polly PD. 2013. Larger mammals have longer faces because of size-related constraints on skull form. Nat. Commun. 4, 2458. ( 10.1038/ncomms3458) [DOI] [PubMed] [Google Scholar]
- 85.Mayerl CJ, Catchpole EA, Edmonds CE, Gould FDH, McGrattan KE, Bond LE, Stricklen BM, German RZ. 2020. The effect of preterm birth, recurrent laryngeal nerve lesion, and postnatal maturation on hyoid and thyroid movements, and their coordination in infant feeding. J. Biomech. 105, 109786. ( 10.1016/j.jbiomech.2020.109786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mayerl CJ, Myrla AM, Gould FDH, Bond LE, Stricklen BM, German RZ. 2021. Swallow safety is determined by bolus volume during infant feeding in an animal model. Dysphagia 36, 120-129. ( 10.1007/s00455-020-10118-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rendell L, Cantor M, Gero S, Whitehead H, Mann J. 2019. Causes and consequences of female centrality in cetacean societies. Phil. Trans. R. Soc. B 374, 20180066. ( 10.1098/rstb.2018.0066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gero S, Whitehead H. 2007. Suckling behavior in sperm whale calves: observations and hypotheses. Mar. Mamm. Sci. 23, 398-413. ( 10.1111/j.1748-7692.2007.00113.x) [DOI] [Google Scholar]
- 89.Johnson G, Frantzis A, Johnson C, Alexiadou V, Ridgway S, Madsen PT. 2010. Evidence that sperm whale (Physeter macrocephalus) calves suckle through their mouth. Mar. Mamm. Sci. 26, 990-996. ( 10.1111/j.1748-7692.2010.00385.x) [DOI] [Google Scholar]
- 90.Ridgway S, Kamolnick T, Reddy M, Curry C, Tarpley RJ. 1995. Orphan-induced lactation in Tursiops and analysis of collected milk. Mar. Mamm. Sci. 11, 172-182. ( 10.1111/j.1748-7692.1995.tb00516.x) [DOI] [Google Scholar]
- 91.Thexton AJ, Crompton AW, German RZ. 2012. EMG activity in hyoid muscles during pig suckling. J. Appl. Physiol. 112, 1512-1519. ( 10.1152/japplphysiol.00450.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Griffiths M. 1965. Rate of growth and intake of milk in a suckling echidna. Comp. Biochem. Physiol. 16, 383-392. ( 10.1016/0010-406X(65)90304-X) [DOI] [PubMed] [Google Scholar]
- 93.Smith KK. 2006. Craniofacial development in marsupial mammals: developmental origins of evolutionary change. Dev. Dyn. 235, 1181-1193. ( 10.1002/dvdy.20676) [DOI] [PubMed] [Google Scholar]
- 94.Carrier DR. 1996. Ontogenetic limits on locomotor performance. Physiol. Zool. 69, 467-488. [Google Scholar]
- 95.Steer KE, et al. In press. The function of the mammal extrinsic tongue musculature in the transition from suckling to drinking. Integr. Comp. Biol. ( 10.1093/icb/icad023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Langer P. 2008. The phases of maternal investment in eutherian mammals. Zoology 111, 148-162. ( 10.1016/j.zool.2007.06.007) [DOI] [PubMed] [Google Scholar]
- 97.Griffiths M, McIntosh DL, Coles REA. 1969. The mammary gland of the echidna, Tachyglossus aculeatus with observations on the incubation of the egg and on the newly-hatched young. J. Zool. 158, 371-386. ( 10.1111/j.1469-7998.1969.tb02155.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Crompton AW, Musinsky C, Bonaparte J, Bhullar B, Owerkowicz T. 2018. Evolution of the mammalian fauces region and the origin of suckling. Harvard Dash Repository. See https://nrs.harvard.edu/URN-3:HUL.INSTREPOS:37364482.
Data Availability Statement
This article has no additional data.