Abstract
Both teeth and the digestive tract show adaptations that are commonly interpreted in the context of trophic guilds—faunivory, herbivory and omnivory. Teeth prepare food for the digestive tract, and dental evolution focuses on increasing durability and functionality; in particular, size reduction of plant particles is an important preparation for microbial fermentative digestion. In narratives of digestive adaptations, microbes are typically considered as service providers, facilitating digestion. That the majority of ‘herbivorous’ (and possibly ‘omnivorous’) mammals display adaptations to maximize microbes' use as prey—by harvesting the microbes multiplying in their guts—is less emphasized and not reflected in trophic labels. Harvesting of microbes occurs either via coprophagy after separation from indigestible material by a separation mechanism in the hindgut, or from a forestomach by a ‘washing mechanism’ that selectively removes fines, including microbes, to the lower digestive tract. The evolution of this washing mechanism as part of the microbe farming niche opened the opportunity for the evolution of another mechanism that links teeth and guts in an innovative way—the sorting and cleaning of not-yet-sufficiently-size-reduced food that is then re-submitted to repeated mastication (rumination), leading to unprecedented chewing and digestive efficiency.
This article is part of the theme issue ‘Food processing and nutritional assimilation in animals’.
Keywords: rumination, merycism, microbial harvest, digesta separation mechanism, digesta washing
Mammals: the definite chewers [1, p.398].
Rumination clearly is not simply a subtle refinement of previously existing digestive strategies, but represents a fundamental innovation that sets ruminants apart from other herbivores [2, p.189].
1. Introduction: 1 + 1 = 2
The digestive system of mammals, like that of most other animals, can be characterized—in simplistic terms—as a sequence of mechanical and biochemical processing of the food [3,4]. First, food is processed with varying degrees of mastication and particle size reduction (comminution). Second, the food is digested by a series of enzyme cascades that either derive from the host animal (aut-enzymatic) or from symbiotic gut microbes (allo-enzymatic). The sequence of comminution and digestion may vary in detail across animals, e.g. salivary enzymes might already affect the food while it is still being masticated in mammals, or food may have been exposed to enzymatic action in a crop and proventriculus before being ground in the gizzard of birds. Nevertheless, the sequence is typically monodirectional, so that the effect of the first process impacts the second. Therefore, the relationship between the two processes can be summarized broadly as 1 + 1 = 2.
The better the food is comminuted into small particles (with a high surface-to-volume ratio), the faster the enzymatic digestion. This relationship is of little biological relevance for foods that do not pose substantial obstacles to enzymatic digestion, such as most animal tissue or marine plants [5], but has momentous implications for foods based on terrestrial plants. For terrestrial plants, with their fibrous (and lignified) cell walls, particle size reduction is the major determinant of the rate of digestion [6,7]. Therefore, particle size reduction ultimately affects the level of food intake, because higher food intake is typically linked to a shorter retention of digesta (food and substances added during digestion) in the gastrointestinal tract (GIT) [8]. Thus, the absence of efficient grinding chewing in reptiles [9] has been linked to their longer digesta retention and lower intake as compared to mammals [10], and within mammals, a compensation for low chewing efficacy by longer retention times has been postulated [11].
In this contribution, we selectively review aspects of the evolution of teeth and the GIT in mammals, at first mirroring the separate entities of these organ systems. This sets the stage for explaining how the digestive strategy of ruminants, which includes a largely unprecedented interplay of the GIT and chewing teeth, represents an innovation in mammals that merits the moniker of 1 + 1 = 3.
2. Tooth evolution in mammals
Dental (tooth) tissue is particularly resistant to decomposition and hence allows tracing changes over evolutionary time. In contrast to a widespread view that evolutionary adaptations do not follow a specific directional trajectory (e.g. [12], but see [13]), at least three major trajectories have been described in the evolution of the mammalian chewing apparatus that apply to many (but not necessarily all) mammalian taxa.
-
(i)
Towards higher dental durability, measured as the percentage of species with hypsodont (high-crowned) or hypselodont (ever-growing) teeth [14–16]. There is no doubt that these adaptations prolong tooth functionality, irrespective of whether wear is considered to be owing to intrinsic abrasives of the food, external abrasives such as dust or grit, or tooth-to-tooth contact [17].
-
(ii)
Towards a higher degree of chewing surface complexity [18,19], intuitively interpreted as an adaptation for increased chewing efficacy. This means, for example, cheek teeth designed for grinding in herbivores, or cheek teeth (the so-called ‘carnassials’) designed for cutting in carnivores. However, because there is a lack of empirical studies that relate actual chewing surface morphology to particle size reduction (with the rare exceptions of investigations on the effect of denture morphology on chewing efficacy in humans (e.g. [20]), this interpretation still rests on the modelling of the effect of tooth shape, or on circumstantial evidence like the observation that equids, with particularly complex enamel folds on the chewing surface of their cheek teeth [21], produce particularly small faecal particles for their body size [22].
-
(iii)
Towards transverse chewing movements that facilitate grinding and hence, putatively, increase chewing efficacy [23,24]; in this context, ‘transverse’ refers to any movement that is not just a simple orthal (up-and-down) movement of the jaws but contains an element that makes dental surfaces glide across each other. While this is again intuitive, empirical data linking chewing movement to chewing efficacy do not exist. The facilitation of these chewing movements includes several morphological adaptations, in particular a reduction in the anterior (incisor/canine) dentition in many herbivore lineages [25].
In summary, a directional trend towards a higher chewing efficacy appears as a common narrative in mammalian evolution.
3. Gastrointestinal tract evolution in mammals
(a) . No fossil record
In contrast to teeth, the poor systematic conservation of soft tissue in the fossil record basically prevents informed narratives on evolutionary trajectories in GIT morphology. Should one accept the volume of the reconstructed body cavity from mounted skeletons as a proxy for GIT volume, then one can postulate a trajectory of a reduction in GIT volume from synapsids to mammals, which has been interpreted in the context of a manoeuvrability arms race between predators and prey animals [26]. A trajectory of a reduction in GIT volume would have been supported by the mentioned trajectory of an increase in chewing efficacy. Individual comparisons between mammal taxa, e.g. juxtaposing equids (higher chewing efficacy, lower GIT volume and shorter digesta retention) and rhinoceroses (lower chewing efficacy, higher GIT volume and longer digesta retention) are tempting but lack the necessary measurements. Thus, we are mainly left with the GIT anatomy of extant mammals.
(b) . GIT and diet
We generally assume that there is congruence between the trophic niche (characterized e.g. as faunivory, omnivory and herbivory) and GIT anatomy [27], but quantitative analyses often do not yield straightforward results [28–30]. Actually, in spite of the commonplace narrative that GIT anatomy mirrors dietary adaptations [28,31,32], there have been historical warnings against this assumption [33,34].
Across terrestrial mammals, faunivores have smaller abdominal cavities [26] and a lesser functional GIT capacity [35], as well as shorter [29] and less complex [30] large intestines, but within narrower taxonomic clades, distinctions between trophic guilds are less evident or absent. For example, it has been noted that among the Carnivora, the more carnivorous felids all have a caecum, whereas the more omnivorous ursids, including the herbivorous panda, do not have one [36]. It is also noted that among the primates there is no relationship between diet and intestine length [37]; and that among rodents, the omnivorous Gliridae do not have a caecum whereas many insectivorous Muroidea do have one [38].
Stomach complexity is not related to diet across all or across terrestrial mammals [30], mainly owing to the fact that it is not only herbivorous foregut fermenters (see below) that have complex stomachs [39], but also the faunivorous cetaceans [40], and the muroid rodents for which no link to diet could be established so far [41].
(c) . GIT basic design and microbial colonization
The basic requirement for digestion is a location that can handle a (large) volume of food yet at the same time guarantee short distances between the food and the sites of enzyme secretion and nutrient absorption—which calls for a thin, long, tubular structure (the small intestine). For optimal function, food should be released continuously into this structure yet animals do not eat continuously, which accounts for an upstream storage site (the stomach). Re-absorption of fluid that is inadvertently part of the digestive process requires a downstream site where no substantial secretion occurs (the large intestine).
The ubiquity of microbes will inadvertently cause this structure to be populated by a microbiome—unless this is prevented by the host organism. The secretion of acid in the stomach and of digestive enzymes that will inadvertently digest the majority of microbes in the stomach and small intestine constrains the presence of high microbial density to mainly the hindgut (and possibly parts of the stomach upstream from acid secretion). Owing to short microbe generation times, a microbiome will evolve to thrive on the material that their host provides, and hence functional and possibly taxonomic convergence in the microbiome of animals using similar diets is expected [42]. Animals may evolve particular GIT adaptations for an increased hosting of a microbiome, e.g. because their digestive action can facilitate the use of a resource (in particular, plant cell walls) that cannot be digested by the host's own enzymes [43]. Because unlike aut-enzymatic secretion, microbes and the secretion of their allo-enzymes are not constrained to the GIT wall but occur in the midst of the ingested food, and because a certain residence time for microbial action is required, these sites are more voluminous, and absorption of microbial digestion products depends on peristalsis (mixing movements) ensuring that these products get into vicinity of the GIT walls. These voluminous GIT sites are often called ‘fermentation chambers' (because microbial digestion is also called fermentation) and are typically located in the hindgut, or additionally in a portion of the stomach upstream of the site of acid and enzyme secretion (foregut). An immense body of work on the respective merits of the shape of these chambers (as compared to reactor theory; e.g. [44]) and on the relative merits of foregut and hindgut fermentation exists (e.g. [43,45]).
(d) . Mammalian microbe farming
Although words like ‘microbial protein production’ are often part of the description of the GIT microbiome's functions, the fact that many mammals actually use the microbes in their GIT as food or ‘prey’ is, in our view, underemphasized (but see [4]). The classic description of trophic niches as faunivore, omnivore and herbivore blends out microbes as a major trophic niche. It is only among agricultural animal nutritionists that the relevance of foregut microbial matter for the nutrient and energy uptake of ruminants is well-known and incorporated into diet evaluation systems [46].
The use of GIT microbes as prey appears intuitive because of its unambiguous advantage. Owing to the size difference and the lack of ecological situations where microbes are lumped in distinct harvestable clusters (like social insects or krill), mammals cannot harvest microbes from the environment. But as stated before, microbes in the GIT are basically unavoidable. Therefore, mammals that use these microbes—which they carry inside themselves anyhow—as prey will have a broader set of dietary resources than animals that carry microbes but do not use them. Mammals can, so to speak, be microbe farmers [4]. This—possibly underemphasized—characteristic can be used to construe narratives, e.g. contributing to explanations for the demise in species diversity and the extinction of whole clades of the Perissodactyla. For this group, no adaptations for using their microbiome as a food source are known, in contrast to microbe farming clades such as the Ruminantia [47].
The location of the microbe-containing chamber (foregut or hindgut) is crucial in determining the respective adaptations necessary for microbe farming, owing to the unidirectional flow of intestinal contents from the stomach to the small and from the small to the large intestine: material, including microbes, in the large intestine is beyond the reach of the small intestine's digestive enzymes.
(i) . Hindgut microbe farming: coprophagy
Therefore, hindgut fermenters need to separate microbial matter from indigestible components in the hindgut, excrete these two fractions separately (as ‘hard versus soft’ faeces, or ‘faeces versus caecotrophs') and ingest the microbe-containing fraction in a process called ‘coprophagy’ or ‘caecotrophy’ (because the microbial fraction is retained in the caecum). Historically, the terms ‘caecotrophe’ and ‘caecotrophy’ have been linked to the ease of (mainly visual) distinction of the two faecal fractions. This led to some misclassification of animals that do use the strategy but without visually distinguishable yet nutritionally different faecal fractions [48]. We refer to this strategy as ‘(separation mechanism-based) coprophagy’; it is not necessarily compulsory but may be negligible under certain conditions of dietary nutrient provision [49]. The human reluctance to accept this strategy [4] is mirrored in historical sequences of publications that first claimed that a species did not practice coprophagy or considered it a pathology, then reported sporadic ‘normal’ observations, and finally accepted the strategy as a characteristic of the species, as exemplified in nutria (Mycastor coypus) [50–54], paca (Cuniculus paca) [55–59], capybara (Hydrochoerus hydochoeris) [60,61] or the gerbillinae [62–64].
A variety of colonic separation mechanisms have been described that either rely on the retrograde washing of large intestine contents, flushing the finest of particles, including microbes, backwards, or on the trapping and transport of microbes in specific folds of the intestinal wall [65,66]. In experiments where a dose of markers is fed to an animal and the marker concentration is measured subsequently in the faeces, these separation mechanisms are evident as recurring marker peaks due to repeated re-ingestion of a part of the marker that is contained in the caecotrophe (figure 1). Because the respective folds are common to basically all hystricomorph and muroid rodents investigated [40], it has been suggested that it may be more prudent to assume all members of this group practice coprophagy except for those in which it was conclusively shown they don't, rather than accepting it only for species in which it was actively documented [70]. Apart from these two rodent groups, coprophagy is assumed to occur in all lagomorphs [65], and has been described for individual other rodent [71,72] and marsupial species [73], and a primate genus [74], resulting in a large proportion of mammals being microbe farmers based on hindgut separation mechanisms. Whether the licking of the everted hindgut observed in many shrew species [e.g. 75] has a similar function remains to be investigated.
Figure 1.
Marker excretion patterns for a solute (liquid)-associated and a particulate marker in species with separation mechanism-based coprophagy and corresponding secondary marker excretion peaks (arrows) indicative of repeated marker ingestion via coprophagy. (a) rabbit (Oryctolagus cuniculus; lagomorph) with a wash-back separation mechanism; note the secondary peaks only for the liquid-associated marker [67]; (b) paca (Cuniculus paca; hystricomorph rodent) with a trap-based separation mechanism [68]; (c) steppe lemming (Lagurus lagurus; myomorph rodent) with a trap-based separation mechanism [69]. Icons by Jeanne Peter.
(ii) . Foregut microbe farming: digesta washing
By necessity, the microbes in any foregut will ultimately be flushed together with the food contents towards the stomach and small intestine and hence be digested, adding all mammals with a microbiome-colonized foregut among the microbe farmers. Muroid rodents with a microbiome-colonized foregut as well as a microbiome-colonized caecum and separation mechanism-based coprophagy [41] could be considered ‘dual-location farmers' in this respect. One could theoretically challenge the view that foregut fermenters are microbe farmers by claiming that this is an inadvertent but not desired side effect of these foreguts. However, many foregut-fermenting herbivores display physiological adaptations that give credence to the interpretation of a deliberate use of the microbiome as food. One set of adaptations is the presence of digestive enzymes required in particular for microbe digestion like lysozymes and ribonucleases [76,77]. More important for our argument, and for the evolution of rumination, is the adaptation of ‘digesta washing’.
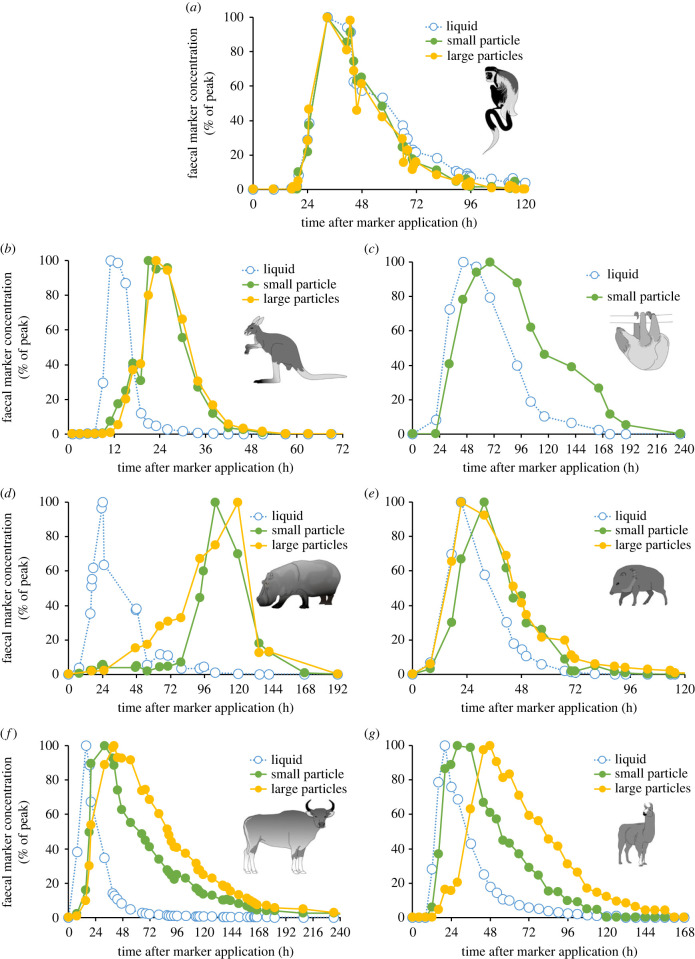
In all groups of herbivorous foregut fermenters investigated to date, with the exception of colobine monkeys, the liquid phase of the digesta moves faster than the particulate phase [78,79] (figure 2). Similar to the retrograde washing in the colonic separation mechanism of lagomorphs mentioned above, this forward washing will selectively remove fine particles and especially microbes from the foregut digesta to the lower digestive tract. The microbiome is thus constantly harvested, ensuring that it remains in a constant metabolic state of regrowth (rather than maintenance); the outflow of microbial mass is thus somewhat decoupled from the outflow of the other parts of the digesta. Both in vitro fermentation experiments (reviewed in [86]) and, to a more limited extent, in vivo studies in domestic ruminants (reviewed in [87]) showed that an enhanced fluid throughput increases microbial yield from fermentation chambers. The necessary fluid is provided via salivation.
Figure 2.
Marker patterns for a solute (liquid)-associated and one or two different-sized particulate markers in non-ruminating foregut fermenting herbivores: (a) black-and-white colobus (Colobus polykomos) [80]; (b) tammar wallaby (Macropus eugenii) [81]; (c) two-toed sloth (Cholepus didactylus) [82]; (d) hippopotamus (Hippopotamus amphibius) [83]; (e) collared peccary (Pecari tajacu) [80]; and in ruminating foregut fermenting herbivores: (f) banteng (Bos javanicus) [84]; (g) llama (Lama glama) [85]. Note that the liquid generally moves faster (except in (a)), and that particles of different size are excreted together (except in (f) and (g)). Icons by Jeanne Peter.
4. Rumination
The presence of a digesta washing mechanism with copious amounts of fluid is a prerequisite for an additional function that evolved in the forestomach of ruminating herbivores—the ruminants and the camelids—that leads to an unprecedented chewing efficacy.
To explain this adaptation, it is first necessary to outline that a single chewing bout will always produce a bolus that consists of particles of different sizes—large and small ones [88]. Just ‘chewing more’ on the same bolus has limited potential for increasing comminution after a certain point, because bolus integrity might be compromised and make swallowing hazardous [88], and because of the principle of diminishing returns—with each single chew, the likelihood increases that of this particular bolus, particles will be affected that have already been reduced in size.
Nevertheless, some animals are known for ‘just chewing more’—they regurgitate some material from their (fore)stomach. This behaviour is referred to by the Greek term for ‘rumination’ as merycism [89], or as regurgitation and remastication or reingestion [90]. Both terms are also used for the behaviour when it is considered pathological, in humans and in captive great apes [91,92].
(a) . Merycism: rumination without sorting
Merycism doubtlessly occurs among mammals with a simple stomach in koalas (Phascolarctos cinereus) [93], and in mammals with a complex stomach in macropods (kangaroos) [94] and proboscis monkeys (Nasalis larvatus) [90]. While its causes remain obscure in macropods, it was associated with a compensation for tooth wear in koalas [95], with increasing leaf ingestion in proboscis monkeys [96] and generally with periods of higher food intake in koalas [97] and a proboscis monkey [90], in line with the general argument that finer particles can be digested faster and hence will allow higher food intakes. It has also been described in detail in the hyrax (Procavia capensis) [98] but also vehemently contested in this species [99], and also in some detail in capybaras [100]. Macropods [101], proboscis monkeys [96] and capybaras [22] produce comparatively fine faecal particles for their size, but current evidence rather points to characteristics of their dentition than to irregular merycism as the cause—in particular, because there is currently no evidence for a particle sorting mechanism in their forestomachs ([80,102] figure 2): markers attached to large and small particles are excreted in parallel by macropods and colobine monkeys.
(b) . Sorting mechanism-based rumination
It is the sorting mechanism in the forestomach of ruminants and camelids that makes these animals stand out. Again, while this mechanism is well-known in agricultural animal science for ruminants [103,104], it is probably less known among non-agricultural animal scientists [105]. When fermenting plant material is taken from the forestomach part of ruminants called the reticulorumen and put into a liquid medium, it separates nearly immediately into a floating portion of larger particles and a sedimenting portion of smaller particles, yielding a ‘stratification’ of the material [106–110]. This principle is used in ruminating mammals for a separation of forestomach contents: based on buoyancy characteristics (density), dense (small) particles are selectively expelled from the first compartments of the forestomach, and less dense (larger) particles are selectively retained at these sites [103,104]. Because buoyancy works alongside gravity, anatomical structures have to maintain a certain relation with gravity, making sternal rest the default resting position in ruminants and lying on the side particularly unlikely [111]. The selective retention can be demonstrated by the excretion patterns of markers attached to different-sized particles (figure 2) and occurs in both ruminants and camelids but not in nonruminant foregut fermenters [85]. In nonruminant foregut fermenters, a selective retention of large particles would not appear beneficial, given that these animals lack an option to efficiently reduce the size of these large particles. The selective particle passage and retention in ruminating mammals have also been demonstrated by analysing particle size at different sites in the digestive tract [108,109,112–114], or by the use of plastic markers of different size and density [115]. The latter also allow the demonstration that large particles are much more likely to be ruminated upon before leaving the forestomach than smaller particles [116]. The few large particles that escape the ruminant forestomach intact typically do so at the end of a feeding bout, when the forestomach is particularly full [116–118].
Thus, the material regurgitated and re-submitted to mastication in ruminants should be depleted of small particles. Investigations in domestic ruminants that collected forestomach content as well as regurgitated and swallowed boli have yielded varying results; in particular, reports on the particle size composition of regurgitated boli as compared to reticulorumen contents are often limited to a description of the large particle fraction, whereas it is particularly the mid-sized and fine particle fractions that are of interest. Some studies report that the bolus that is regurgitated already contains a higher proportion of large particles than the middle or lower reticulorumen contents [119,120], but there appears to be no unanimous consensus of whether regurgitated material originates from the upper layer of the reticulorumen contents or from the middle ones [104]. Because a part of the regurgitated bolus (the ‘tail bolus’) that contains mainly fine particles is immediately re-swallowed before rumination mastication is initiated, an additional concentration of larger particles occurs [104]. Thus, a combination of the stratification of reticulorumen contents and a recruitment of the regurgitated bolus from layers somewhat depleted in small particles, together with the further reduction of small particles due to the immediate re-swallowing of this fraction after regurgitation and before rumination, is responsible for ensuring that larger particles are selectively submitted to rumination. This sorting makes rumination into not just ‘more chewing on the same material’, but selective chewing on that fraction of the digesta that still requires comminution, and leads to the exceptionally small faecal particles in ruminants and camelids [2,22]. The teeth do their job, but in contrast to nonruminant mammals where they have to work on anything that is ingested, during rumination they work on pre-selected material. The sorting mechanism constantly removes that part of the digesta that does not require further comminution. On the one hand, this allows ruminating herbivores to retain larger particles selectively in the fermentation chamber without excessively blocking new food intake. On the other hand, the evident effect of this high degree of particle size reduction is the particularly high digestive efficiency observed in ruminants and camelids as compared to other similar-sized herbivores [2,121].
Additionally, the initial fermentation that occurs before digesta are submitted to rumination softens the material to some degree, so that less energy is required for comminution [104]. Even more, the sorting in a fluid medium washes off external abrasives like grit or dust, removing an important cause of dental wear from the material re-submitted to rumination [122–125]. Plausible consequences of these effects are a shift of the main load of mastication from ingestive mastication to ruminant mastication (to save chewing effort and minimize wear due to external abrasives) [126], a shifting from a less thorough, ‘sloppy’ chewing pattern during ingestion to a consistent, homogenous chewing pattern during rumination [127], or the fact that rumination contributes more to particle size reduction than ingestive mastication [128,129]. In morphological terms, ruminanting herbivores have slender jaws, suggestive of fewer chewing muscles, than nonruminant herbivores [130], a lower hypsodonty than equids in the same dietary niche [105,131], and complex enamel folding patterns yet not as complex as those of equids [21]; with respect to tooth wear, different wear traces between ruminant and nonruminant herbivores from the same habitat are expected due to the removal of external abrasives prior to rumination [132].
5. Conclusion
The forestomach types of ruminants and camelids offer the same benefits as a forestomach of non-ruminating herbivores: a fibre-digesting microbiome that may, at times, also be involved in the neutralisation of specific toxins, and that is additionally a farmed prey that can be harvested. However, the forestomachs of ruminants and camelids offer additional advantages that increase the chewing efficacy of the dentition, facilitating particularly high digestive efficiency, and that protect that dentition from wear caused by external abrasives. Trying to decide which of these benefits is more important may be an academic question, the answer to which might differ between ecological situations.
The forestomach sorting and washing mechanism is a convergent adaptation in camelids and ruminants, where different morphological structures fulfil similar physiological functions [114,133]. However, subtle differences in these morphologies exist, which particularly affect the size of the transitions between forestomach compartments; these are larger in ruminants [134], which is compatible with the finding that the forestomach system throughput, and hence food intake capacity, is higher in ruminants than in camelids [135]. Thus, ruminants can make full use of the intake-liberating effect of a high chewing efficacy [45]. It is tempting to link the many seeming advantages of the ruminant digestive tract with their enormous extant species diversity and the impression that they replaced both equids and camelids in many niches in the fossil record [136]. Even among the ruminants themselves, replacement sequences in the fossil record appear evident that could be explained by improvements in the forestomach system, such as the evolution of an additional compartment between the sorting chamber and the glandular stomach that reduces the fluid and buffering load of the digesta (the ‘omasum’) [137]. However, the example of the seeming replacement within the true ruminants, of giraffids by bovids and cervids [137], cautions against considering the digestive system as the only, or main, determinant of the evolutionary fate of taxa.
6. Outlook
Based on our narrative, several areas of fruitful future research suggest themselves. In terms of dental adaptations for chewing efficacy, studies that quantitatively assess the effect of chewing surface morphology and chewing movements under standardized conditions—similar to studies on denture morphology and chewing efficacy in humans (e.g. [20])—would be highly interesting to supplement the large body of speculative interpretations on dental function. Elucidating the mechanism, triggers and function of merycism in non-ruminating mammals would likely increase our understanding of rumination as well. Assessing species differences in the contribution of ingestive and ruminating mastication to overall chewing [138] among ruminants from niches of different diet abrasiveness might yield indications to which extent rumination also evolved as a protective mechanism against dental wear.
With respect to mammalian microbe farming, investigations into the extent of microbe use, and the physiological adaptations for it, would be important. This includes demonstrating differences in saliva production, and assessing digesta passage not only using conventional solute and particle markers, but also markers specifically mimicking flow behaviour of microbes. We could compare physiological adaptations to foster or control a GIT microbiome. This could include the secretion of phosphorus (a major nutrient required for microbe growth) into [139], and the removal of calcium (that might render phosphorus unavailable by complexation) from the digesta [140]; the modulation of the microbiome by the degree of digesta washing [86] or peristalsis [141]; or any mechanism that determines the heritability of the GIT microbiome [142]. Possibly, ranking species by their urinary concentrations of purine derivates (from digestion and metabolism of nucleic acid-rich microbiota) [143,144], their adipose tissue concentrations of fatty acids of microbial origin [145], or microbial contribution to the isotope pattern of the essential amino acids in their red blood cells [146] could indicate the extent of microbe farming, but additional proxies would be welcome. Similarly, species could be ranked by quantifying adaptations to microbiota digestion, such as ribonuclease and lysozyme secretion in the GIT [76,77]; this could include assessing whether mammalian chitinases are involved in digesting the fungal parts of the GIT microbiome.
As model organisms among foregut fermenters, primates versus non-primates most likely represents a promising contrast due to the absence of digesta washing in primates [78]. Among small mammals, it may be difficult to find systems in which coprophagy can be reliably excluded; possibly, a comparison between selected sciuromorph and hystricomorph rodents, or between rodents and elephant shrews—ideally on plant-based diets using omnivorous species—would be promising. Among ruminants, the distinct difference in digesta washing in two relatively tractable species—cattle and common eland (Taurotragus oryx) [147]—offers a promising research model, but less pronounced yet distinct differences between sheep (or goats) and cattle [148] might provide an even more accessible model.
Ideally, properties identified in specific taxa should be checked for their universality among the taxa in question. This is done with the aim of linking these properties to these taxa's evolutionary history in the fossil record.
Acknowledgements
We thank the Society for Experimental Biology and The Company of Bilogists for session funding, Nicolai Konow and Callum Ross for inviting our contribution, and two reviewers for their constructive comments.
Ethics
No ethical approval was required for the writing of this review.
Data accessibility
No new original data were produced for this article.
Authors' contributions
M.C.: conceptualization, writing—original draft; J.F.: conceptualization, writing—review and editing; J.H.: conceptualization, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We have no competing interests.
Funding
M.C. was supported by SNSF (grant no. CRSII5_189970/1) during manuscript production.
References
- 1.Reilly SM, McBrayer LD, White TD. 2001. Prey processing in amniotes: biomechanical and behavioral patterns of food reduction. Comp. Biochem. Physiol. A 128, 397-415. ( 10.1016/S1095-6433(00)00326-3) [DOI] [PubMed] [Google Scholar]
- 2.Clauss M, Steuer P, Erlinghagen-Lückerath K, Kaandorp J, Fritz J, Südekum K-H, Hummel J. 2015. Faecal particle size: digestive physiology meets herbivore diversity. Comp. Biochem. Physiol. A 179, 182-191. ( 10.1016/j.cbpa.2014.10.006) [DOI] [PubMed] [Google Scholar]
- 3.Barboza PS, Parker KL, Hume ID. 2009. Integrative wildlife nutrition. Berlin, Germany: Springer. [Google Scholar]
- 4.Karasov WH, Martínez del Rio C. 2007. Physiological ecology: how animals process energy, nutrients, and toxins. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Lanyon JM, Sanson GD. 2006. Mechanical disruption of seagrass in the digestive tract of the dugong. J. Zool. 270, 277-289. ( 10.1111/j.1469-7998.2006.00135.x) [DOI] [Google Scholar]
- 6.Hummel J, Clauss M, Südekum K-H. 2020. Aspects of food comminution in ungulates and their consequences for energy budget. In Mammalian teeth - form and function (eds Martin T, von Koenigswald W), pp. 87-101. Munich, Germany: Dr. Friedrich Pfeil. [Google Scholar]
- 7.Bjorndal KA, Bolten AB, Moore JE. 1990. Digestive fermentation in herbivores: effect of food particle size. Physiol. Zool. 63, 710-721. ( 10.1086/physzool.63.4.30158172) [DOI] [Google Scholar]
- 8.Müller DWH, Codron D, Meloro C, Munn A, Schwarm A, Hummel J, Clauss M. 2013. Assessing the Jarman–Bell Principle: scaling of intake, digestibility, retention time and gut fill with body mass in mammalian herbivores. Comp. Biochem. Physiol. A 164, 129-140. ( 10.1016/j.cbpa.2012.09.018) [DOI] [PubMed] [Google Scholar]
- 9.Fritz J, Hummel J, Kienzle E, Streich WJ, Clauss M. 2010. To chew or not to chew: faecal particle size in herbivorous reptiles and mammals. J. Exp. Zool. A 313, 579-586. ( 10.1002/jez.629) [DOI] [PubMed] [Google Scholar]
- 10.Franz R, Hummel J, Müller DWH, Bauert M, Hatt J-M, Clauss M. 2011. Herbivorous reptiles and body mass: effects on food intake, digesta retention, digestibility and gut capacity, and a comparison with mammals. Comp. Biochem. Physiol. A 158, 94-101. ( 10.1016/j.cbpa.2010.09.007) [DOI] [PubMed] [Google Scholar]
- 11.Clauss M, Nunn C, Fritz J, Hummel J. 2009. Evidence for a tradeoff between retention time and chewing efficiency in large mammalian herbivores. Comp. Biochem. Physiol. A 154, 376-382. ( 10.1016/j.cbpa.2009.07.016) [DOI] [PubMed] [Google Scholar]
- 12.Benton MJ. 1987. Progress and competition in macroevolution. Biol. Rev. 62, 305-338. ( 10.1111/j.1469-185X.1987.tb00666.x) [DOI] [Google Scholar]
- 13.Clauss M, Müller DWH, Codron D. 2019. Within-niche pace of life acceleration as a fundamental evolutionary principle: a mammal pilot test case. Evol. Ecol. Res. 20, 385-401. ( 10.5167/uzh-186535) [DOI] [Google Scholar]
- 14.Tapaltsyan V, Eronen JT, Lawing AM, Sharir A, Janis C, Jernvall J, Klein OD. 2015. Continuously growing rodent molars result from a predictable quantitative evolutionary change over 50 million years. Cell Rep. 11, 673-680. ( 10.1016/j.celrep.2015.03.064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jernvall J, Fortelius M. 2002. Common mammals drive the evolutionary increase of hypsodonty in the Neogene. Nature 417, 538-540. ( 10.1038/417538a) [DOI] [PubMed] [Google Scholar]
- 16.von Koenigswald W. 2011. Diversity of hypsodont teeth in mammalian dentitions – construction and classification. Palaeontogr. A 294, 63-94. ( 10.1127/pala/294/2011/63) [DOI] [Google Scholar]
- 17.Schulz-Kornas E, et al. 2020. Everything matters: molar microwear texture in goats (Capra aegagrus hircus) fed diets of different abrasiveness. Palaeogeogr. Palaeoclimatol. Palaeoecol. 552, 109783. ( 10.1016/j.palaeo.2020.109783) [DOI] [Google Scholar]
- 18.Yamanaka A. 2022. Evolution and development of the mammalian multicuspid teeth. J. Oral Biosci. 64, 165-175. ( 10.1016/j.job.2022.03.007) [DOI] [PubMed] [Google Scholar]
- 19.Jernvall J, Hunter JP, Fortelius M. 1996. Molar tooth diversity, disparity, and ecology in Cenozoic ungulate radiations. Science 274, 1489-1492. ( 10.1126/science.274.5292.1489) [DOI] [PubMed] [Google Scholar]
- 20.Cardoso RG, Medeiros AKB, de Souza Leão R, de Moraes SLD, Carreiro ADFP. 2021. Impact of the occlusal morphology of artificial teeth on bimaxillary denture treatment in elderly individuals: a clinical trial. Int. J. Prostodont. 34, 309-316. ( 10.11607/ijp.7117) [DOI] [PubMed] [Google Scholar]
- 21.Famoso NA, Davis EB, Feranec RS, Hopkins SS, Price SA. 2016. Are hypsodonty and occlusal enamel complexity evolutionarily correlated in ungulates? J. Mamm. Evol. 23, 43-47. ( 10.1007/s10914-015-9296-7) [DOI] [Google Scholar]
- 22.Fritz J, Hummel J, Kienzle E, Arnold C, Nunn C, Clauss M. 2009. Comparative chewing efficiency in mammalian herbivores. Oikos 118, 1623-1632. ( 10.1111/j.1600-0706.2009.17807.x) [DOI] [PubMed] [Google Scholar]
- 23.Bhullar B-AS, Manafzadeh AR, Miyamae JA, Hoffman EA, Brainerd EL, Musinsky C, Crompton AW. 2019. Rolling of the jaw is essential for mammalian chewing and tribosphenic molar function. Nature 566, 528-532. ( 10.1038/s41586-019-0940-x) [DOI] [PubMed] [Google Scholar]
- 24.Grossnickle DM, Weaver LN, Jäger KR, Schultz JA. 2022. The evolution of anteriorly directed molar occlusion in mammals. Zool. J. Linn. Soc. 194, 349-365. ( 10.1093/zoolinnean/zlab039) [DOI] [Google Scholar]
- 25.Avedik A, Duque-Correa MJ, Clauss M. 2023. Avoiding the lockdown: morphological facilitation of transversal chewing movements in mammals. J. Morphol. 284, e21554. ( 10.1002/jmor.21554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clauss M, Nurutdinova I, Meloro C, Gunga H-C, Jiang D, Koller J, Herkner B, Sander PM, Hellwich O. 2017. Reconstruction of body cavity volume in terrestrial tetrapods. J. Anat. 230, 325-336. ( 10.1111/joa.12557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens CE, Hume ID. 1995. Comparative physiology of the vertebrate digestive system. New York, NY: Cambridge University Press. [Google Scholar]
- 28.Chivers DJ, Hladik CM. 1980. Morphology of the gastrointestinal tract in primates: comparisons with other mammals in relation to diet. J. Morphol. 166, 337-386. ( 10.1002/jmor.1051660306) [DOI] [PubMed] [Google Scholar]
- 29.Duque-Correa MJ, Codron D, Meloro C, McGrosky A, Schiffmann C, Edwards MS, Clauss M. 2021. Mammalian intestinal allometry, phylogeny, trophic level and climate. Proc. R. Soc. B 288, 20202888. ( 10.1098/rspb.2020.2888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langer P, Clauss M. 2018. Morphological adaptation of the eutherian gastrointestinal tract to diet. Vertebr. Zool. 68, 237-252. ( 10.5167/uzh-157906) [DOI] [Google Scholar]
- 31.Cuvier G. 1799–1805. Leçons d'anatomie comparée. Paris, France: Crochard. [Google Scholar]
- 32.Karasov WH, Martinez del Rio C, Caviedes-Vidal E. 2011. Ecological physiology of diet and digestive systems. Annu. Rev. Physiol. 73, 69-93. ( 10.1146/annurev-physiol-012110-142152) [DOI] [PubMed] [Google Scholar]
- 33.Jacobshagen E. 1937. Grundzüge des Innenreliefs vom Rumpfdarm der Wirbeltiere. Anatomischer Anzeiger 83, 241-261. [Google Scholar]
- 34.Harder W. 1951. Studien am Darm von Wild-und Haustieren. Anat. Embryol. 116, 27-51. [Google Scholar]
- 35.De Cuyper A, Meloro C, Abraham AJ, Müller DWH, Codron D, Janssens GPJ, Clauss M. 2020. The uneven weight distribution between predators and prey: comparing gut fill between terrestrial herbivores and carnivores. Comp. Biochem. Physiol. A 243, 110683. ( 10.1016/j.cbpa.2020.110683) [DOI] [PubMed] [Google Scholar]
- 36.McGrosky A, Navarrete A, Isler K, Langer P, Clauss M. 2016. Gross intestinal morphometry and allometry in Carnivora. Eur. J. Wildl. Res. 62, 395-405. ( 10.1007/s10344-016-1011-3) [DOI] [Google Scholar]
- 37.McGrosky A, Meloro C, Navarrete A, Heldstab SA, Kitchener AC, Isler K, Clauss M. 2019. Gross intestinal morphometry and allometry in primates. Am. J. Primatol. 81, e23035. ( 10.1002/ajp.23035) [DOI] [PubMed] [Google Scholar]
- 38.Gorgas M. 1967. Vergleichend-anatomische Untersuchungen am Magen-Darm-Kanal der Sciuromorpha, Hystricomorpha und Caviomorpha (Rodentia). Z. wiss. Zool. 175, 237-404. [Google Scholar]
- 39.Langer P. 1988. The mammalian herbivore stomach. Stuttgart, Germany/New York, NY: Gustav Fischer Verlag. [Google Scholar]
- 40.Langer P. 2017. Comparative anatomy of the gastrointestinal tract in eutheria: taxonomy, biogeography and food. Vol I: Afrotheria, Xenarthra and Euarchontoglires. Vol II: Laurasiatheria, General Discussion. Berlin, Germany: De Gruyter. [Google Scholar]
- 41.Steiner N, Clauss M, Martin LF, Imper C, Meloro C, Duque-Correa MJ. 2022. No news from old drawings? Stomach anatomy in muroid rodents in relation to body size and ecology. J. Morphol. 283, 1200-1209. ( 10.1002/jmor.21496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ley RE, et al. 2008. Evolution of mammals and their gut microbes. Science 320, 1647-1651. ( 10.1126/science.115572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens CE, Hume ID. 1998. Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol. Rev. 78, 393-427. ( 10.1152/physrev.1998.78.2.393) [DOI] [PubMed] [Google Scholar]
- 44.Jumars PA. 2000. Animal guts as nonideal chemical reactors: partial mixing and axial variation in absorption kinetics. Am. Nat. 155, 544-555. ( 10.1086/303334) [DOI] [PubMed] [Google Scholar]
- 45.Clauss M, Hume ID, Hummel J. 2010. Evolutionary adaptations of ruminants and their potential relevance for modern production systems. Animal 4, 979-992. ( 10.1017/S1751731110000388) [DOI] [PubMed] [Google Scholar]
- 46.Flachowsky F, et al. 2001. Empfehlungen zur Energie- und Nährstoffversorgung der Milchkühe und Aufzuchtrinder [Recommendations of energy and nutrient supply for dairy cows and breeding cattle] (eds Lebzien P, Schwarz J, Müller HL, Weigand E). Frankfurt, Germany: DLG Verlag [Google Scholar]
- 47.Clauss M, Codron D, Hummel J. 2023. Equid nutritional physiology and behavior: an evolutionary perspective. J. Equ. Veterin. Sci. 124, 104265. ( 10.1016/j.jevs.2023.104265) [DOI] [PubMed] [Google Scholar]
- 48.Hörnicke H, Björnhag G. 1980. Coprophagy and related strategies for digesta utilization. In Digestive physiology and metabolism in ruminants (eds Ruckebusch Y, Thivent P), pp. 707-730. Lancaster, UK: MTP Press. [Google Scholar]
- 49.Meredith AL, Prebble JL. 2017. Impact of diet on faecal output and caecotroph consumption in rabbits. J. Small Anim. Pract. 58, 139-145. ( 10.1111/jsap.12620) [DOI] [PubMed] [Google Scholar]
- 50.Kirner P. 1931. Über Koprophagie bei Nutria. Dt. Pelztierz. 6, 153. [Google Scholar]
- 51.Otto W. 1954. Über die Verdauung des Sumpfbibers [On the digestion of the nutria] (Myocastor coypus). Arch. Anim. Nutr. 4, 119-150. [Google Scholar]
- 52.Gosling LM. 1979. The twenty-four hour activity cycle of captive coypus (Myocastor coypus). J. Zool. 187, 341-367. ( 10.1111/j.1469-7998.1979.tb03374.x) [DOI] [Google Scholar]
- 53.Takahashi T, Sakaguchi E. 1998. Behaviors and nutritional importance of coprophagy in captive adult and young nutrias (Myocastor coypus). J. Comp. Physiol. B 168, 281-288. ( 10.1007/s003600050147) [DOI] [PubMed] [Google Scholar]
- 54.Takahashi T, Sakaguchi E. 2000. Role of the furrow of the proximal colon in the production of soft and hard feces in nutrias (Myocastor coypus). J. Comp. Physiol. B 170, 531-535. ( 10.1007/s003600000131) [DOI] [PubMed] [Google Scholar]
- 55.Kraus C, Gihr M, Pilleri G. 1970. Das Verhalten von Cuniculus paca (Rodentia, Dasyproctidae) in Gefangenschaft. Rev. Suisse Zool. 77, 353-388. [PubMed] [Google Scholar]
- 56.Matamoros Y. 1982. Notas sobre la biologia del tepezcuinte, Cuniculus paca en cautiverio. Brenesia 19/20, 71-82. [Google Scholar]
- 57.Pérez EM. 1992. Agouti paca. Mammal. Species 404, 1-7. ( 10.2307/3504102) [DOI] [Google Scholar]
- 58.Sabatini V, Paranhos De Costa MJR. 2001. Caecotrophy in pacas (Agouti paca). Mamm. Biol. 66, 305-307. [Google Scholar]
- 59.Guerra AL, Nogueira-Filho SLG, Mendes A, Souza Altino V, Clauss M, da Cunha Nogueira SS. 2018. Direct and indirect caecotrophy behaviour in paca (Cuniculus paca). J. Anim. Physiol. Anim. Nutr. 102, 1774-1782. ( 10.1111/jpn.12961) [DOI] [PubMed] [Google Scholar]
- 60.Hirakawa H. 2001. Coprophagy in leporids and other mammalian herbivores. Mammal Rev. 31, 61-80. ( 10.1046/j.1365-2907.2001.00079.x) [DOI] [Google Scholar]
- 61.Hirakawa H. 2002. Supplement: coprophagy in leporids and other mammalian herbivores. Mammal Rev. 32, 150-152. ( 10.1046/j.1365-2907.2002.00105.x) [DOI] [Google Scholar]
- 62.Otken CC, Scott CE. 1984. Feeding characteristics of Mongolian gerbils (Meriones unguiculatus). Lab. Anim. Sci. 34, 181-184. [PubMed] [Google Scholar]
- 63.Pei YX, Wang DH, Hume ID. 2001. Effects of dietary fibre on digesta passage, nutrient digestibility, and gastrointestinal tract morphology in the granivorous Mongolian gerbil (Meriones unguiculatus). Physiol. Biochem. Zool. 74, 742-749. ( 10.1086/322928) [DOI] [PubMed] [Google Scholar]
- 64.Khokhlova IS, Krasnov BR, Kuznetsov V, Sartor CE, Zan M, Salek L, Ghazaryan L, Kam M, Degen AA. 2005. Dietary intake and time budget in two desert rodents: a diurnal herbivore, Psammomys obesus, and a nocturnal granivore, Meriones crassus. Mammalia 69, 57-67. ( 10.1515/mamm.2005.005) [DOI] [Google Scholar]
- 65.Björnhag G, Snipes RL. 1999. Colonic separation mechanism in lagomorph and rodent species — a comparison. Zoosyst. Evol. 75, 275-281. ( 10.1002/mmnz.19990750208) [DOI] [Google Scholar]
- 66.Cork SJ, Hume ID, Faichney GJ. 1999. Digestive strategies of nonruminant herbivores: the role of the hindgut. In Nutritional ecology of herbivores (eds Jung HJG, Fahey GC), pp. 210-260. Savoy, IL: American Society of Animal Science. [Google Scholar]
- 67.Franz R, Kreuzer M, Hummel J, Hatt J-M, Clauss M. 2011. Intake, selection, digesta retention, digestion and gut fill of two coprophageous species, rabbits (Oryctolagus cuniculus) and guinea pigs (Cavia porcellus), on a hay-only diet. J. Anim. Physiol. Anim. Nutr. 95, 564-570. ( 10.1111/j.1439-0396.2010.01084.x) [DOI] [PubMed] [Google Scholar]
- 68.Guerra Aldrigui L, Nogueira-Filho SLG, Mendes A, Souza Altino V, Ortmann S, de Cunha Nogueira SS, Clauss M. 2018. Effect of different feeding regimes on cecotrophy behaviour and retention of solute and particle markers in the digestive tract of paca (Cuniculus paca). Comp. Biochem. Physiol. A 226, 57-65. ( 10.1016/j.cbpa.2018.08.013) [DOI] [PubMed] [Google Scholar]
- 69.Hagen KB, Müller DHW, Ortmann S, Kreuzer M, Clauss M. 2018. Digesta kinetics in two arvicoline rodents, the field vole (Microtus agrestis) and the steppe lemming (Lagurus lagurus). Mamm. Biol. 89, 71-78. ( 10.1016/j.mambio.2018.01.003) [DOI] [Google Scholar]
- 70.Clauss M, Besselmann D, Schwarm A, Ortmann S, Hatt J-M. 2007. Demonstrating coprophagy with passage markers? The example of the plains viscacha (Lagostomus maximus). Comp. Biochem. Physiol. A 147, 453-459. ( 10.1016/j.cbpa.2007.01.013) [DOI] [PubMed] [Google Scholar]
- 71.Richard PB. 1959. La caecotrophie chez le Castor du Rhône. C. R. Hebdom. Acad. Sci. 248, 1424-1426. [Google Scholar]
- 72.Kenagy GJ, Hoyt DF. 1980. Reingestion of feces in rodents and its daily rhythmicity. Oecologia 44, 403-409. ( 10.1007/BF00545245) [DOI] [PubMed] [Google Scholar]
- 73.Chilcott MJ, Hume ID. 1985. Coprophagy and selective retention of fluid digesta: their role in the nutrition of the common ringtail possum (Pseudocheirus peregrinus). Aust. J. Zool. 33, 1-15. ( 10.1071/ZO9850001) [DOI] [Google Scholar]
- 74.Hladik CM, Charles-Dominique P, Valdebouze P, Delort-Laval J, Flanzy J. 1971. La caecotrophie chez un Primate phyllophage du genre Lepilemur et les corrélations avec les particularités de son appareil digestif. C. R. Acad. Sci. Paris 272, 3191-3194. [PubMed] [Google Scholar]
- 75.Hirakawa H, Haberl W. 1998. The behaviour of licking the everted rectum in shrews (Soricidae, Insectivora). Acta Theriol. 43, 113-120. ( 10.4098/AT.ARCH.98-9) [DOI] [Google Scholar]
- 76.Stewart CB, Schilling JW, Wilson AC. 1987. Adaptive evolution in the stomach lysozymes of foregut fermenters. Nature 330, 401-404. ( 10.1038/330401a0) [DOI] [PubMed] [Google Scholar]
- 77.Havinga J, Beintema JJ. 1980. Pancreatic ribonucleases of mammals with ruminant-like digestion. Eur. J. Biochem. 110, 131-142. ( 10.1111/j.1432-1033.1980.tb04848.x) [DOI] [PubMed] [Google Scholar]
- 78.Müller DWH, Caton J, Codron D, Schwarm A, Lentle R, Streich WJ, Hummel J, Clauss M. 2011. Phylogenetic constraints on digesta separation: variation in fluid throughput in the digestive tract in mammalian herbivores. Comp. Biochem. Physiol. A 160, 207-220. ( 10.1016/j.cbpa.2011.06.004) [DOI] [PubMed] [Google Scholar]
- 79.Matsuda I, Espinosa-Gómez FC, Ortmann S, Sha JCM, Osman I, Nijboer J, Schwarm A, Ikeda T, Clauss M. 2019. Retention marker excretion suggests incomplete digesta mixing across the order primates. Physiol. Behav. 208, 112558. ( 10.1016/j.physbeh.2019.112558) [DOI] [PubMed] [Google Scholar]
- 80.Schwarm A, Ortmann S, Wolf C, Streich WJ, Clauss M. 2009. Passage marker excretion in red kangaroo (Macropus rufus), collared peccary (Pecari tajacu) and colobine monkeys (Colobus angolensis C. polykomos, Trachypithecus johnii). J. Exp. Zool. A 311, 647-661. ( 10.1002/jez.552) [DOI] [PubMed] [Google Scholar]
- 81.Munn AJ, Tomlinson S, Savage T, Clauss M. 2012. Retention of different-sized particles and derived gut fill estimate in tammar wallabies (Macropus eugenii): physiological and methodological considerations. Comp. Biochem. Physiol. A 161, 243-249. ( 10.1016/j.cbpa.2011.11.003) [DOI] [PubMed] [Google Scholar]
- 82.Vendl C, Frei S, Dittmann MT, Furrer S, Osmann C, Ortmann S, Munn A, Kreuzer M, Clauss M. 2016. Digestive physiology, metabolism and methane production of captive Linné's two-toed sloths (Choloepus didactylus). J. Anim. Physiol. Anim. Nutr. 100, 552-564. ( 10.1111/jpn.12356) [DOI] [PubMed] [Google Scholar]
- 83.Clauss M, Schwarm A, Ortmann S, Alber D, Flach EJ, Kühne R, Hummel J, Streich WJ, Hofer H. 2004. Intake, ingesta retention, particle size distribution and digestibility in the hippopotamidae. Comp. Biochem. Physiol. A 139, 449-459. ( 10.1016/j.cbpb.2004.10.002) [DOI] [PubMed] [Google Scholar]
- 84.Schwarm A, Ortmann S, Wolf C, Streich WJ, Clauss M. 2008. Excretion patterns of fluid and different sized particle passage markers in banteng (Bos javanicus) and pygmy hippopotamus (Hexaprotidon liberiensis). Comp. Biochem. Physiol. A 150, 32-39. ( 10.1016/j.cbpa.2008.02.022) [DOI] [PubMed] [Google Scholar]
- 85.Dittmann MT, Runge U, Ortmann S, Lang RA, Moser D, Galeffi C, Schwarm A, Kreuzer M, Clauss M. 2015. Digesta retention patterns of solutes and different-sized particles in camelids compared with ruminants and other foregut fermenters. J. Comp. Physiol. B 185, 559-573. ( 10.1007/s00360-015-0904-x) [DOI] [PubMed] [Google Scholar]
- 86.Pfau F, Südekum K-H, Breves G, Hünerberg M, Clauss M, Hummel J. 2021. Effects of dilution rate on fermentation characteristics of feeds with different carbohydrate composition incubated in the rumen simulation technique. Front. Anim. Sci. 2, 715142. ( 10.3389/fanim.2021.715142) [DOI] [Google Scholar]
- 87.Zhang X, Li Y, Terranova M, Ortmann S, Kehraus S, Gerspach C, Kreuzer M, Hummel J, Clauss M. 2023. Effect of induced saliva flow on fluid retention time, ruminal microbial yield and methane emission in cattle. J. Anim. Physiol. Anim. Nutr. 107, 769-782. ( 10.1111/jpn.13773) [DOI] [PubMed] [Google Scholar]
- 88.Prinz JF, Lucas PW. 1997. An optimization model for mastication and swallowing in mammals. Proc. R. Soc. B 264, 1715-1721. ( 10.1098/rspb.1997.0238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barker S, Brown GD, Calaby JH. 1963. Food regurgitation in the macropodidae. Aust. J. Sci. 25, 430-432. [Google Scholar]
- 90.Matsuda I, Murai T, Clauss M, Yamada T, Tuuga A, Bernard H, Higashi S. 2011. Regurgitation and remastication in the foregut-fermenting proboscis monkey (Nasalis larvatus). Biol. Lett. 7, 786-789. ( 10.1098/rsbl.2011.0197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parry-Jones B. 1994. Merycism or rumination disorder. A historical investigation and current assessment. Br. J. Psychiatr. 165, 303-314. ( 10.1192/bjp.165.3.303) [DOI] [PubMed] [Google Scholar]
- 92.Hill SP. 2018. 'Regurgitation and reingestion’ (R/R) in great apes: a review of current knowledge. Int. Zoo Yb. 52, 62-78. ( 10.1111/izy.12204) [DOI] [Google Scholar]
- 93.Logan M. 2001. Evidence for the occurence of rumination-like behaviour, or merycism, in koalas (Phascolarctos cinereus). J. Zool. (Lond.) 255, 83-87. ( 10.1017/S0952836901001121) [DOI] [Google Scholar]
- 94.Vendl C, Munn A, Leggett K, Clauss M. 2017. Merycism in western grey (Macropus fuliginosus) and red kangaroos (Macropus rufus). Mamm. Biol. 86, 21-26. ( 10.1016/j.mambio.2017.03.005) [DOI] [Google Scholar]
- 95.Logan M. 2003. Effect of tooth wear on the rumination-like behavior, or merycism, of free-ranging koalas (Phascolarctos cinereus). J. Mammal. 84, 897-902. ( 10.1644/BBa-002) [DOI] [Google Scholar]
- 96.Matsuda I, et al. 2014. Faecal particle size in free-ranging primates supports ‘rumination’ strategy in the proboscis monkey (Nasalis larvatus). Oecologia 174, 1127-1137. ( 10.1007/s00442-013-2863-9) [DOI] [PubMed] [Google Scholar]
- 97.Logan M, Sanson GD. 2003. The effects of lactation on the feeding behaviour and activity patterns of free-ranging female koalas (Phascolarctos cinereus). Aust. J. Zool. 51, 415-428. ( 10.1071/ZO03017) [DOI] [Google Scholar]
- 98.Hendrichs H. 1965. Vergleichende Untersuchung des Wiederkauverhaltens [Comparative study of rumination behaviour]. Biol. Zentr.bl. 84, 681-751. [Google Scholar]
- 99.Sale JB. 1966. Daily food consumption and mode of ingestion in the hyrax. J. East Afr. Nat. Hist. Soc. 25, 215-224. [Google Scholar]
- 100.Lord RD. 1994. A descriptive account of capybara behavior. Stud. Neotrop. Fauna Environ. 29, 11-22. ( 10.1080/01650529409360912) [DOI] [Google Scholar]
- 101.Schwarm A, Ortmann S, Fritz J, Rietschel W, Flach EJ, Clauss M. 2013. No distinct stratification of ingesta particles and no distinct moisture gradient in the forestomach of nonruminants: the wallaby, peccary, hippopotamus, and sloth. Mamm. Biol. 78, 412-421. ( 10.1016/j.mambio.2013.04.001) [DOI] [Google Scholar]
- 102.Matsuda I, et al. 2015. Excretion patterns of solute and different-sized particle passage markers in foregut-fermenting proboscis monkey (Nasalis larvatus) do not indicate an adaptation for rumination. Physiol. Behav. 149, 45-52. ( 10.1016/j.physbeh.2015.05.020) [DOI] [PubMed] [Google Scholar]
- 103.Lechner-Doll M, Kaske M, von Engelhardt W. 1991. Factors affecting the mean retention time of particles in the forestomach of ruminants and camelids. In Physiological aspects of digestion and metabolism in ruminants (eds Tsuda T, Sasaki Y, Kawashima R), pp. 455-482. San Diego, CA: Academic Press. [Google Scholar]
- 104.Kennedy PM. 2005. Particle dynamics. In Quantitative aspects of ruminant digestion and metabolism (eds Dijkstra J, Forbes JM, France J), pp. 123-156. Wellingford, UK: CAB International. [Google Scholar]
- 105.Sanson GD. 2023. Reassessing assumptions about the evolution of herbivore teeth. PNAS 120, e2219060120. ( 10.1073/pnas.2219060120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sutherland TM. 1988. Particle separation in the forestomach of sheep. In Aspects of digestive physiology in ruminants (eds Dobson A, Dobson MJ), pp. 43-73. Ithaca, NY: Cornell University Press. [Google Scholar]
- 107.Hummel J, Südekum K-H, Bayer D, Ortmann S, Hatt J-M, Streich WJ, Clauss M. 2009. Physical characteristics of reticuloruminal contents of cattle in relation to forage type and time after feeding. J. Anim. Physiol. Anim. Nutr. 93, 209-220. ( 10.1111/j.1439-0396.2008.00806.x) [DOI] [PubMed] [Google Scholar]
- 108.Clauss M, Fritz J, Bayer D, Nygren K, Hammer S, Hatt J-M, Südekum K-H, Hummel J. 2009. Physical characteristics of rumen contents in four large ruminants of different feeding type, the addax (Addax nasomaculatus), bison (Bison bison), red deer (Cervus elaphus) and moose (Alces alces). Comp. Biochem. Physiol. A 152, 398-406. ( 10.1016/j.cbpa.2008.11.009) [DOI] [PubMed] [Google Scholar]
- 109.Clauss M, Fritz J, Bayer D, Hummel J, Streich WJ, Südekum K-H, Hatt J-M. 2009. Physical characteristics of rumen contents in two small ruminants of different feeding type, the mouflon (Ovis ammon musimon) and the roe deer (Capreolus capreolus). Zoology 112, 195-205. ( 10.1016/j.zool.2008.08.001) [DOI] [PubMed] [Google Scholar]
- 110.Lechner I, Barboza P, Collins W, Fritz J, Günther D, Hattendorf B, Hummel J, Südekum K-H, Clauss M. 2010. Differential passage of fluids and different-sized particles in fistulated oxen (Bos primigenius f. taurus), muskoxen (Ovibos moschatus), reindeer (Rangifer tarandus) and moose (Alces alces): rumen particle size discrimination is independent from contents stratification. Comp. Biochem. Physiol. A 155, 211-222. ( 10.1016/j.cbpa.2009.10.040) [DOI] [PubMed] [Google Scholar]
- 111.Pucora E, Schiffmann C, Clauss M. 2019. Resting postures in terrestrial mammalian herbivores. J. Mammal. 100, 552-563. ( 10.1093/jmammal/gyz044) [DOI] [Google Scholar]
- 112.Clauss M, Fritz J, Tschuor A, Braun U, Hummel J, Codron D. 2017. Dry matter and digesta particle size gradients along the goat digestive tract on grass and browse diets. J. Anim. Physiol. Anim. Nutr. 101, 61-69. ( 10.1111/jpn.12505) [DOI] [PubMed] [Google Scholar]
- 113.Lechner-Doll M, von Engelhardt W. 1989. Particle size and passage from the forestomach in camels compared to cattle and sheep fed a similar diet. J. Anim. Physiol. Anim. Nutr. 61, 120-128. ( 10.1111/j.1439-0396.1989.tb00091.x) [DOI] [Google Scholar]
- 114.Idalan N, Martin LF, Clauss M. 2019. Physical characteristics of gastrointestinal content of llama (Lama glama). J. Anim. Physiol. Anim. Nutr. 103, 1015-1022. ( 10.1111/jpn.13116) [DOI] [PubMed] [Google Scholar]
- 115.Clauss M, Lechner I, Barboza P, Collins W, Tervoort T, Südekum K-H, Codron D, Hummel J. 2011. The effect of size and density on the mean retention time of particles in the reticulorumen of cattle (Bos primigenius f. taurus), muskoxen (Ovibos moschatus) and moose (Alces alces). Br. J. Nutr. 105, 634-644. ( 10.1017/S0007114510004101) [DOI] [PubMed] [Google Scholar]
- 116.Lauper M, Lechner I, Barboza P, Collins W, Hummel J, Codron D, Clauss M. 2013. Rumination of different-sized particles in muskoxen (Ovibos moschatus) and moose (Alces alces) on grass and browse diets, and implications for rumination in different ruminant feeding types. Mamm. Biol. 78, 142-152. ( 10.1016/j.mambio.2012.06.001) [DOI] [Google Scholar]
- 117.Findeisen E, Südekum K-H, Hummel J, Clauss M. 2021. Increasing food intake in domestic goats (Capra hircus): measured effects on chewing intensity are probably driven by escape of few, large particles from the forestomach. Comp. Biochem. Physiol. A 257, 110972. ( 10.1016/j.cbpa.2021.110972) [DOI] [PubMed] [Google Scholar]
- 118.Hummel J, Scheurich F, Ortmann S, Crompton LA, Gerken M, Clauss M. 2018. Comparative selective retention of particle size classes in the gastrointestinal tract of ponies and goats. J. Anim. Physiol. Anim. Nutr. 102, 429-439. ( 10.1111/jpn.12763) [DOI] [PubMed] [Google Scholar]
- 119.Kovács PL, Südekum K-H, Stangassinger M. 1997. Effects of intake level of a mixed diet on chewing activity and on particle size of ruminated boli, ruminal digesta fractions and faeces of steers. Reprod. Nutr. Dev. 37, 517-528. ( 10.1051/rnd:19970503) [DOI] [PubMed] [Google Scholar]
- 120.Kennedy PM. 1985. Effect of rumination on reduction of particle size of rumen digesta by cattle. Austr . J. Agric. Res. 36, 819-828. ( 10.1071/AR9850819) [DOI] [Google Scholar]
- 121.Foose TJ. 1982. Trophic strategies of ruminant versus nonruminant ungulates. PhD Thesis, University of Chicago, Chicago, IL. [Google Scholar]
- 122.Hatt J-M, Codron D, Müller DWH, Ackermans NL, Martin LF, Kircher PR, Hummel J, Clauss M. 2019. The rumen washes off abrasives before heavy-duty chewing in ruminants. Mamm. Biol. 97, 104-111. ( 10.1016/j.mambio.2019.06.001) [DOI] [Google Scholar]
- 123.Hatt J-M, Codron D, Ackermans NL, Martin LF, Richter H, Kircher PR, Gerspach C, Hummel J, Clauss M. 2020. The effect of the rumen washing mechanism in sheep differs with concentration and size of abrasive particles. Palaeogeogr. Palaeoclimatol. Palaeoecol. 550, 109728. ( 10.1016/j.palaeo.2020.109728) [DOI] [Google Scholar]
- 124.Hatt J-M, Codron D, Richter H, Kircher PR, Hummel J, Clauss M. 2021. Preliminary evidence for a forestomach washing mechanism in llamas (Lama glama). Mamm. Biol. 101, 941-948. ( 10.1007/s42991-021-00142-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Valerio SO, Hummel J, Codron D, Hatt J-M, Clauss M. 2022. The ruminant sorting mechanism protects teeth from abrasives. Proc. Natl Acad. Sci. USA 119, e2212447119. ( 10.1073/pnas.2212447119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luginbuhl J-M, Pond KR, Burns JC, Russ JC. 1989. Eating and ruminating behavior of steers fed coastal bermudagrass hay at four levels. J. Anim. Sci. 67, 3410-3418. ( 10.2527/jas1989.67123410x) [DOI] [PubMed] [Google Scholar]
- 127.Dittmann MT, Kreuzer M, Runge U, Clauss M. 2017. Ingestive mastication in horses resembles rumination but not ingestive mastication in cattle and camels. J. Exp. Zool. A 327, 98-109. ( 10.1002/jez.2075) [DOI] [PubMed] [Google Scholar]
- 128.McLeod MN, Minson DJ. 1988. Large particle breakdown by cattle eating ryegrass and alfalfa. J. Anim. Sci. 66, 992-999. ( 10.2527/jas1988.664992x) [DOI] [PubMed] [Google Scholar]
- 129.Trudell-Moore J, White RG. 1983. Physical breakdown of food during eating and rumination in reindeer. Acta Zool. Fenn. 175, 47-49. [Google Scholar]
- 130.Fletcher TM, Janis CM, Rayfield EJ. 2010. Finite element analysis of ungulate jaws: can mode of digestive physiology be determined? Palaeontol. Electron. 13, 21A. [Google Scholar]
- 131.Damuth J, Janis CM. 2011. On the relationship between hypsodonty and feeding ecology in ungulate mammals, and its utility in palaeoecology. Biol. Rev. 86, 733-758. ( 10.1111/j.1469-185X.2011.00176.x) [DOI] [PubMed] [Google Scholar]
- 132.Mihlbachler MC, Campbell D, Ayoub M, Chen C, Ghani I. 2016. Comparative dental microwear of ruminant and perissodactyl molars: Implications for paleodietary analysis of rare and extinct ungulate clades. Palaeobiol. 42, 98-116. ( 10.1017/pab.2015.33) [DOI] [Google Scholar]
- 133.Clauss M, Hofmann RR. 2014. The digestive system of ruminants, and peculiarities of (wild) cattle. In Ecology, evolution and behaviour of wild cattle: implications for conservation (eds Melletti M, Burton J), pp. 57-62. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 134.Pérez W, König HE, Jerbi H, Clauss M. 2016. Macroanatomical aspects of the gastrointestinal tract of the alpaca (Vicugna pacos) and dromedary (Camelus dromedarius). Vertebr. Zool. 66, 419-425. ( 10.3897/vz.66.e31574) [DOI] [Google Scholar]
- 135.Dittmann MT, Hummel J, Runge U, Galeffi C, Kreuzer M, Clauss M. 2014. Characterising an artiodactyl family inhabiting arid habitats by its metabolism: low metabolism and maintenance requirements in camelids. J. Arid Environ. 107, 41-48. ( 10.1016/j.jaridenv.2014.04.005) [DOI] [Google Scholar]
- 136.Janis CM, Gordon IJ, Illius AW. 1994. Modelling equid/ruminant competition in the fossil record. Hist. Biol. 8, 15-29. ( 10.1080/10292389409380469) [DOI] [Google Scholar]
- 137.Clauss M, Rössner GE. 2014. Old world ruminant morphophysiology, life history, and fossil record: exploring key innovations of a diversification sequence. Ann. Zool. Fenn. 51, 80-94. ( 10.5735/086.051.0210) [DOI] [Google Scholar]
- 138.Baumont R, Doreau M, Ingrand S, Veissier I. 2006. Feeding and mastication behaviour in ruminants. In Feeding in domestic vertebrates: from stucture to behaviour (ed. Bels V), pp. 241-262. Wallingford, UK: CAB International. [Google Scholar]
- 139.Breves G, Schröder B. 1991. Comparative aspects of gastrointestinal phosphorus metabolism. Nutr. Res. Rev. 4, 125-140. ( 10.1079/NRR19910011) [DOI] [PubMed] [Google Scholar]
- 140.Liesegang A, Burger B, de Vries de Heekelingen T, Schroeter-Vogt C, Hatt J-M, Kowalewski MP, Clauss M. 2023. Rabbits (Oryctolagus cuniculus) increase caecal calcium absorption at increasing dietary calcium levels. J. Anim. Physiol. Anim. Nutr. (online). ( 10.1111/jpn.13880) [DOI] [PubMed] [Google Scholar]
- 141.Dittmann MT, et al. 2016. Influence of ruminal methane on digesta retention and digestive physiology in non-lactating dairy cattle. Br. J. Nutr. 116, 763-773. ( 10.1017/S0007114516002701) [DOI] [PubMed] [Google Scholar]
- 142.Grieneisen L, et al. 2021. Gut microbiome heritability is nearly universal but environmentally contingent. Science 373, 181-186. ( 10.1126/science.aba5483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen XB, Ørskov ER. 2004. Research on urinary excretion of purine derivatives in ruminants: past, present and future. In Estimation of microbial protein supply in ruminants using urinary purine derivatives (eds Makkar HPS, Chen XB), pp. 180-210. Amsterdam, The Netherlands: Kluwer Accademic Publishers. [Google Scholar]
- 144.Belenguer A, Balcells J, Fondevila M, Abecia L, Solanas E. 2008. Alternative methodologies to estimate ingestion of caecotrophes in growing rabbits. Livestock Sci. 115, 13-19. ( 10.1016/j.livsci.2007.06.002) [DOI] [Google Scholar]
- 145.De Cuyper A, Winkler D, Tütken T, Janssens GPJ, Clauss M. 2020. Fatty acids of microbial origin in the perirenal fat of rats (Rattus norvegicus domestica) and guinea pigs (Cavia porcellus) fed various animal- and plant-based diets. Lipids 55, 341-351 ( 10.1002/lipd.12240) [DOI] [PubMed] [Google Scholar]
- 146.Besser AC, Manlick PJ, Blevins CM, Takacs‐Vesbach CD, Newsome SD. 2023. Variation in gut microbial contribution of essential amino acids to host protein metabolism in a wild small mammal community. Ecol. Lett. 26, 1359-1369. ( 10.1111/ele.v26.8) [DOI] [PubMed] [Google Scholar]
- 147.Hejcmanová P, Ortmann S, Stoklasová, L, Clauss M. 2020. Digesta passage in common eland (Taurotragus oryx) on a monocot or a dicot diet. Comp. Biochem. Physiol. A 246, 110720. ( 10.1016/j.cbpa.2020.110720) [DOI] [PubMed] [Google Scholar]
- 148.Pfau F, Clauss M, Hummel J. 2023. Is there a difference in ruminal fermentation control between cattle and sheep? A meta-analytical test of a hypothesis on differential particle and fluid retention. Comp. Biochem. Physiol. A 277, 111370. ( 10.1016/j.cbpa.2023.111370) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new original data were produced for this article.