Abstract
Chewing is widespread across vertebrates, including mammals, lepidosaurs, and ray-finned and cartilaginous fishes, yet common wisdom about one group—amphibians—is that they swallow food whole, without processing. Earlier salamander studies lacked analyses of internal kinematics of the tongue, analyses of muscle function, and sampled few individuals, which may have caused erroneous conclusions. Specifically, without tongue and food kinematics, intraoral behaviours are difficult to disambiguate. We hypothesized that ambystomatid salamanders use diverse intraoral behaviours, including chewing, and tested this hypothesis with biplanar videofluoroscopy, X-ray reconstruction of moving morphology, and fluoromicrometry. We generated musculoskeletal kinematic profiles for intraoral behaviours in Axolotls (Ambystoma mexicanum), including three-dimensional skeletal kinematics associated with feeding, for gape, cranial and pectoral girdle rotations, and tongue translations. We also measured muscle fibre and muscle–tendon unit strains for six muscles involved in generating skull, jaw and tongue kinematics (adductor mandibulae, depressor mandibulae, geniohyoid, sternohyoid, epaxialis and hypaxialis). A principal component analysis recovered statistically significant differences between behaviour cycles, classified based on food movements as either chewing or transport. Thus, our data suggest that ambystomatid salamanders use a previously unrecognized diversity of intraoral behaviours, including chewing. Combined with existing knowledge, our data suggest that chewing is a basal trait for tetrapods and jaw-bearing vertebrates.
This article is part of the theme issue ‘Food processing and nutritional assimilation in animals’.
Keywords: food processing, fluoromicrometry, salamander, elastic recoil, muscle strain, muscle–tendon unit
1. Introduction
Chewing, the action of hard and often tooth-bearing surfaces being brought together upon the food by repeated muscle shortening action [1], is generally considered critical for survival by facilitating food breakdown intraorally and nutritional assimilation in the gastrointestinal tract [2,3]. Accordingly, this intraoral behaviour is widespread across jaw-bearing vertebrates (Gnathostomata) [1,4,5] and has been studied specifically in mammals [6–12], lepidosaurs [1,13–17] and aquatic-feeding anamniotes including acanthopterygians, sarcopterygians and elasmobranchs [18–23]. However, common wisdom about amphibians suggests that they transport food intact to the oesophagus for swallowing [24–28], and thus omit food processing from their intraoral feeding behavioural repertoire.
The idea that amphibians—the closest extant clade of vertebrates to the tetrapod taxa that invaded land in the Devonian [29,30]—are unique among gnathostomes by not processing their food is unparsimonious, particularly when considering that both more basal, as well as more derived taxa process their food. Indeed, recent X-ray-based studies of several salamander species have generated evidence to the contrary [30–35] by revealing the presence of cyclic and rhythmic intraoral behaviours following food capture, and before swallowing [36]. However, these studies generally lacked the rigorous hypothesis-testing framework required to conclusively disambiguate food processing—chewing—from other intraoral behaviours, particularly intraoral food transport [20,37]. Hence, the idea that salamanders process their food represents a long-standing hypothesis that remains inconclusively tested and, therefore, we test that hypothesis here.
Previous studies of ambystomatid (mole) salamanders have used appropriate analyses to test this hypothesis but were technically limited by the use of light-based video to record external feeding behaviours [24–28]. Light-based video makes it difficult to obtain precise and accurate visualizations of internal musculoskeletal kinematics. Conclusions from those studies included that intraoral feeding kinematics were characterized by exceptionally high intraspecific (between-individual) variability. This conclusion might, at least in part, be driven by the incapability of disambiguating chewing and transport kinematics, mainly because movements of the hyoid (tongue) are partially concealed by external tissues of the mouth wall (suspensorium) and floor (throat).
Analyses of intraoral feeding behaviours in ambystomatid salamanders using electromyography have also concluded that food processing is omitted [24,26,38]. However, a recent study of jaw adductor muscle function in the Axolotl (Ambystoma mexicanum) revealed that the preparatory stretch of series elastic elements (formed by collagen within this muscle) during gape opening resulted in a time delay between the onset of activity in the jaw closer muscle and the onset of mouth closing (also evident in fig. 10 of [24]). Taken together, these data have shown that the function of this particular muscle–tendon unit (MTU) involves substantial series elastic action due to stretch and recoil of series elastic elements [31]. We hypothesized that variations in the delay caused by elastic action, between activation of muscles and movements of the skeleton, may enable different behaviors without detectable changes in the muscle activity pattern. Anatomical evidence suggests the presence of substantial collagenous elements in many muscles of the amphibian craniofacial system [39,40]. So, we predict a widespread influence of series elastic action, beyond the external jaw adductor muscle–tendon system, on intraoral feeding behaviours in this and other salamander species. We collected kinematics data that would allow us to determine the presence of elastic stretch and recoil [31] for other cranial muscles and sought to test the hypothesis that chewing and transport involve different contributions of elastic recoil in muscles that move the jaw and tongue during feeding.
Here, we present a detailed kinematics dataset, collected using X-ray reconstruction of moving morphology (XROMM; [41]) and fluoromicrometry [42], so as to include the 3D movements of the tongue that were missing from prior analyses, to analyse post-capture feeding kinematics in the Axolotl. Using an ordination approach (principal component analysis, PCA) coupled with analyses of variance (ANOVAs), we seek to determine if food processing is included in, or omitted from, the intraoral behavioural repertoire of this salamander species, with direct implications for our interpretation of the evolution of food processing across gnathostomes, and particularly across the fish–tetrapod split (see [43]).
2. Material and methods
(a) . Animal acquisition and training
Twelve adult female Axolotls (Ambystoma mexicanum; snout–vent length (SVL): 141.3 ± 24.9 mm; mean ± s.d.) were purchased from the Ambystoma Genetic Stock Center (Lexington, KY, USA). Animals were singly housed and maintained at the University of Massachusetts Lowell in 3 gallon tubs with modified Holtfreter's solution [44] that was continuously filtered by a small indwelling canister filter. Twice weekly, the animals were fed a mixed diet, consisting of pieces of Canadian nightcrawlers (worms), house crickets and feeder fishes. Food was presented from forceps to encourage feeding in a specific location, which is an essential requirement for X-ray reconstruction of moving morphology (XROMM) experiments. For the purpose of the present analysis, we focused on experiments using worms as food type, since a recent analysis showed that Axolotl feeding kinematics show a food type effect [31]. All animal care and experiment use complied with approved IACUC protocols from the University of Massachusetts Lowell (12-06-19-KON) and Harvard University (FAS; 20_09-03).
(b) . Surgical preparation for experiments
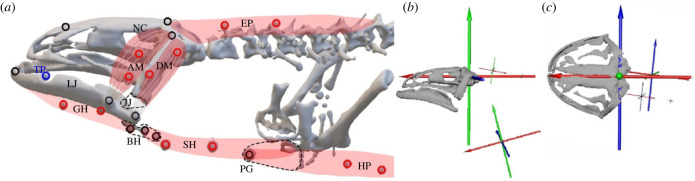
In preparation for XROMM experiments, all subjects were induced to a deep anaesthetic plane (benzocaine 1.25%; 2.5 g l−1 Holtfreter's solution) so that we could implant 22 marker spheres (0.5–1.0 mm diameter; figure 1a), made from tantalum, a radio-opaque and bio-inert metal (Abbott Ball Company, West Hartford CT, USA). Pairs of 0.8 mm markers were implanted using a trocar fitted with a stainless-steel plunger into the left bellies of adductor mandibulae externus (AMe, jaw closer), depressor mandibulae (DM, jaw opener), geniohyoid (GH, tongue protractor), sternohyoid (SH, tongue retractor), epaxialis (EP, dorsal body wall) and hypaxialis (HP, ventral body wall). Three 0.5 mm markers were pressed into pre-drilled holes of a slightly smaller diameter, drilled into the premaxilla and left/right squamosal to mark the neurocranium, another three 0.5 mm markers were implanted in the left lower jaw at the medial symphysis, at the adductor and depressor muscle attachment sites, and a 0.8 mm marker was lodged via trocar into the fibrocartilaginous tongue pad. The basihyal was marked using three 0.5 mm markers that were lodged via trocar in close association with this bone. Finally, a 1 mm laser-perforated marker was twist-tied to the left cleithral cartilage using fine steel wire (Medwire, Mount Vernon NY, USA) [45]. Skin incisions over the premaxilla, squamosal, caudal mandible aspect and cleithrum were no larger than 15 mm, closed with 5–0 absorbable (Vicryl) suture, and typically healed in 5 days. After marker implantation, subjects were fasted for 3 days to ensure markers remained lodged in place, owing to a concern that muscular force production during feeding could cause them to dislodge from their bony or muscular placement. All subjects were in vivo micro-CT scanned (nanoScan PET/CT, Mediso, Budapest, Hungary) to ensure marker implantation success and generate the skeletal models needed for the XROMM workflow (see below).
Figure 1.
Marker implantation scheme (a) and coordinate system approaches for XROMM (b,c). (a) A skeletal rendering with markers implanted in muscles (red; AM, DM, GH, SH, HP, EP), in skeletal tissue (black; NC, LJ, BH, PG) and in the tongue pad (blue; TP). (b,c) Neurocranial and jaw scans (OBJs) with the rostrocaudal cranial coordinate system axis parented to the cranio-vertebral joint. The cranial reference coordinate x-axis is parented to the anterior EP marker, and the pectoral girdle (PG) axis is parented to the pectoral girdle marker. Food and basihyal (BH) markers are imported, and the centroid of each marker is calculated using the vAv2 tool from the XROMM tool-shelf. Food and basihyal kinematics are extracted using the centroid marker with respect to the cranial axis. Pectoral girdle kinematics are extracted by using the pectoral girdle axis with respect to the cranial axis, and neurocranial (NC) kinematics are extracted by using the EP axis with respect to the cranial axis. For definition of abbreviations, see Material and Methods §2b.
(c) . Biplanar X-ray experiments
All implanted subjects were transported to the biplanar X-ray facility at the Concord Field Station (Harvard MCZ, Bedford, MA, USA) for experiments. Subjects were habituated to acrylic test tanks (910 × 70 × 150 mm L × W × H; 7 mm wall thickness) for 2 days. The tanks were custom-designed to fit within the visualization volume of two orthogonally arranged fluoroscopes (OEC 9600. GE, Boston, MA, USA) retrofitted with high-speed video cameras (Photron PCI-1024, Tokyo, Japan).
For feeding experiments, subjects were presented with pieces of Canadian nightcrawler (worm), cut to match the mouth-width of the subject, and implanted with three 1 mm steel markers so that intraoral food movements could be visualized (see §2f below). Food items were presented via plastic forceps, and post-capture feeding behaviours were imaged using an X-ray technique of 75–85 kVp and 2.5–3.0 mA with a fluoroscopic magnification factor of 2 and camera frame rate of 125 Hz (Photron Fastcam PCI-1024). All videos were recorded to PC hard drive via Photron FastCam Viewer (v. 1.3.40. Photron, Tokyo, Japan).
Although Axolotls have been shown to not modulate their feeding kinematics owing to satiation [46], we discontinued daily experiments after eight feeding trials for a given individual. Following the completion of experiments, subjects were euthanized by immersion in a benzocaine overdose (4%), exsanguinated via heart removal, and frozen for dissection to take morphological measurements.
Details of the selected trial subset, including numbers of behaviour cycles analysed, are listed in table 1. A total of 49 feeding trials were performed from 12 subjects, and a subset of 21 trials were selected for analyses based on the following inclusion criteria: (i) vigorous intraoral behaviours (thus excluding trials where the subjects remained quiescent following food capture), (ii) imaging of all markers in both camera views, (iii) avoidance of marker–marker occlusion during trials, (iv) marker reprojection errors below 0.6 pixels, and (v) appropriate marker implantations. Six subjects provided trial data that passed all our inclusion criteria.
Table 1.
Subjects, and intraoral feeding cycles (processing and transport) used for analyses. Chews and transports classified as described in figure 2.
| subject | trials recorded | processing cycles | transport cycles |
|---|---|---|---|
| 1 | 1 | 3 | 0 |
| 2 | 5 | 12 | 7 |
| 3 | 2 | 4 | 4 |
| 4 | 8 | 21 | 8 |
| 5 | 2 | 6 | 4 |
| 6 | 3 | 8 | 9 |
| total | 21 | 54 | 32 |
(d) . Video data conditioning
For each selected trial, the two camera views were de-distorted and spatially calibrated using direct linear transformation, and all markers were 3D-tracked in XMAlab (v.1.5.5) [47]. Micro-CT scans for all subjects were segmented in 3D Slicer (Cambridge, MA, USA) and 3D mesh models of the neurocranium, lower jaw and basihyal were imported into Maya (Autodesk, San Rafael CA, USA). We then used the XROMM workflow [41] to measure six-degrees-of-freedom skeletal kinematics, muscle strains and MTU strains for all behaviour cycles.
(e) . Coordinate systems for XROMM of neurocranium, lower jaw, basihyal and pectoral girdle
To measure time-varying rotations and translations of the neurocranium, lower jaw, basihyal (tongue) and pectoral girdle, a series of coordinate systems were required (figure 1b,c). We generated anatomical coordinate systems (ACSs) [48] using the XROMM tool-shelf for Maya [41]. Across subjects, the cranial ACS was positioned precisely at the centre of the cranio-vertebral joint, and the axes were rotated so that the red (x) axis aligned with the cranial rostrocaudal plane, the green (y) axis was aligned to be orthogonal and dorsoventral and the blue (z) axis was orthogonal along the medio-lateral cranial axis. Basihyal translations in 3D were measured by tracking the caudal-most basihyal marker with respect to the cranial ACS, using the ORel (relative motion) function in the XROMM tool-shelf. The same approach was applied for food translations and pectoral girdle rotations. Elevation and depression of the neurocranium were measured as an angle by creating an ACS on the rostral epaxial bead, and having the x-axis constantly aligned with the caudal epaxial bead, thus providing a measurable rostrocaudal axis down the animal's body. Using the ORel function, the rotation of the cranial ACS was then collected with respect to the epaxial ACS.
(f) . Determination of food movements and behavioural classification
Intraoral feeding behaviours were objectively distinguished by analysing food translations in the rostrocaudal plane (figure 2). For each cycle, total food translation in the rostrocaudal direction was measured. The underlying assumption for final classification was that a transport cycle would involve a substantial change in net food position in the caudal direction and that chews would involve little to no net change in food position. We found a natural cut-off between cycles where caudal food translation exceeded 0.25 of the standard deviation from the grand mean of net translation across all cycles analysed. Those cycles were identified as transports, whereas cycles that fell short of that value were identified as chews (figure 2; electronic supplementary material, videos S1A and S1B).
Figure 2.
Quantitative classification of intraoral behaviours. We used food movements, as visualized using biplanar videofluoroscopy, to classify intraoral behaviours. Time-varying position of food markers (foodx) was extracted with respect to the neurocranial long-axis in Maya. This feeding sequence illustrates three chew cycles, classified by small amounts of net rostrocaudal food translation (specifically translation <0.25 s.d. around the grand mean measured for all intraoral cycles). The two latter cycles with greater net rostrocaudal food translation (>0.25 s.d.) are classified as food transport behaviours.
(g) . Kinematic waveform conditioning and analyses
Unfiltered 3D (x,y,z) marker coordinates were exported to Igor Pro (v. 8.04.2, Wavemetrics, Lake Oswego, OR, USA) for further processing and measurement extraction. All 3D marker coordinates were smoothed using FIR interpolations (IgorPro). Gape was calculated as the distance between the premaxillary marker and the anterior-most lower jaw marker (see electronic supplementary material, figure S1 for a comparison of this approach with the joint coordinate system approach). Three-dimensional (x,y,z) coordinates for all muscle markers were filtered in XMALab, exported to IgorPro, and used for calculating length change and strain for the six muscles. In the few cases where one of the muscle markers of a pair was missing, confirmed to not be correctly placed, or moved outside the field of view in an X-ray frame, that missing marker was substituted for by using the closest skeletal marker. In these cases, we were not able to calculate time-varying muscle–tendon length (see below). By plotting all variables together in kinematics profiles for each trial (see example in figure 3), we identified basihyal protraction–retraction as the variable to use for identifying individual cycles, as it showed the most regularly cyclic motion of all kinematic variables. Therefore, all musculoskeletal kinematics were extracted with respect to the basibranchial protraction–retraction cycle. Each cycle was split into a preparatory and power-stroke phase, where peak basihyal excursion (maxima or minima) were centred at 50% cycle duration. Magnitudes of all variables (kinematics, muscle and food movement) were then extracted as maxima across both the preparatory and power-stroke phases.
Figure 3.
Sample trial with time-varying skeletal kinematics (black), muscle fibre lengths (red), and muscle–tendon unit (MTU) lengths (blue). Grey column-pairs identify five intraoral cycles, split into the preparatory or expansive (light grey) and power-stroke or compressive (dark grey) phases. Note the limited caudal movement of food in the first three cycles (chews) and more substantial caudal movement of food in the last two (transport) cycles, as detailed in figure 2. Electronic supplementary material, video S2 shows an XROMM scene for the chewing sequence used here. ε, Strain, (L − LR)/LR. See Material and methods §2b for definitions of abbreviations.
(h) . Measurements of muscle fascicle and muscle–tendon unit strains
For muscle function during feeding, our goal was to determine if intraoral feeding behaviours (i) were characterized by different amounts of muscle strain, and (ii) showed evidence of different degrees of recoil action of series elastic elements [31], consisting of tendons or aponeuroses that have been shown to be prominent in the cranial muscles of many salamanders [39,40]. We used fluoromicrometry [31,42,45] to measure strain from jaw, tongue and axial muscles with recognized functions in feeding [31,49–52]. Muscle strain (ε) was measured as (L − LR)/LR or relative length change with respect to the resting muscle length (LR), which we determined from a sequence of video of each subject at rest and confirmed in the CT scans. Using the distance tool in Maya, we measured the time-varying MTU length as the origin-to-insertion span in four muscles (not possible for the axial muscles, where caudal bony insertions onto the vertebral column are ambiguous). Finally, time-varying series elastic stretch and recoil was calculated as ΔLTENDON = ΔLMTU − ΔLMUSCLE in IgorPro. Shortening strains for both muscle fibres and MTUs were expressed as positive based on the muscle physiological convention that shortening (concentric) contractions generate positive power and work for skeletal movement.
(i) . Choice and extraction of response variables
We used an ordination approach (PCA, see below) to determine if Axolotls use chewing food processing in addition to food transport. Ordination approaches such as PCA are limited in the number of response variables that one can factor per unit of analysis, which in our case was a cycle of intraoral behaviour. So, to limit our number of response variables, we focused our analysis on excursion magnitudes (e.g. peak excursions or muscle peak strain). Excursion magnitudes are generally considered more powerful for reflecting behavioural changes than changes in timing variables (e.g. onset and duration), which have been suggested to mainly reflect patterns of evolutionary change [53,54]. Preliminary analyses of our data indicated that there were only subtle differences between timing variables in the observed intraoral behaviours (i.e. predominately in gape timing, figure 4). Further motivated by our focus on a single species, we decided that timing variables were less important than excursion magnitudes and excluded the former from our analysis.
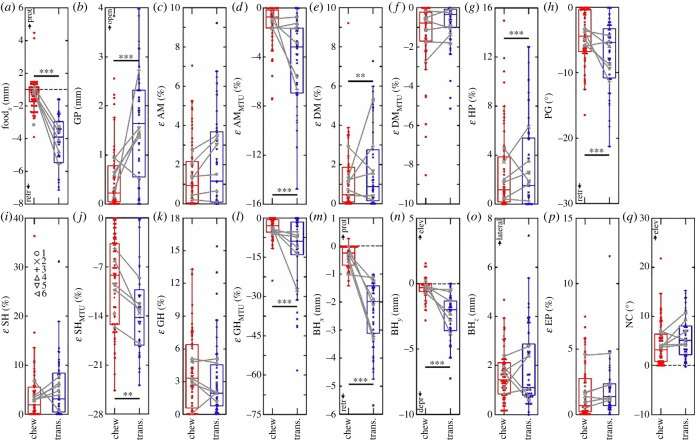
Figure 4.

Summary of kinematic differences between chew (red, dark) and transport (blue, light) cycles. Data are grand averages (lines) for all cycles with standard error measurements (whiskers) for the musculoskeletal kinematics variables: epaxial (EP) strain, neurocranial (NC) rotation, adductor mandibulae (AM) strain, gape (GP) change, depressor mandibulae (DM) strain, basihyal (BH) translations in three dimensions (x, rostrocaudal; y, dorsoventral; z, mediolateral), food rostrocaudal (foodx) movement, geniohyoid (GH) strain, sternohyoid (SH) strain, hypaxial (HP) strain and pectoral girdle (CL) rotation. Average length change for muscle–tendon units (MTUs) is indicated for AM, DM, GH and SH with dashed lines (error bars omitted for clarity). Positive muscle and MTU length change is shortening. Note the different axes scales (right) for GH and SH MTU strain. Cycles (chews, n = 62; transports, n = 36) were extracted based on the pattern of food protraction–retraction with peak extremes of BHx defining 50% cycle duration. Thus, 0–50% cycle duration represents the preparatory phase, and 50–100% represents the power-stroke phase of each cycle. ε, Strain, (L − LR)/LR.
(j) . Principal component analysis
We ran our PCA in Systat (v.12.0; Sigmaplot, Chicago, IL, USA) on the covariance matrix of our factored response variables, and constrained our subsequent analyses to the first three principal component axes with eigenvalues >1, following the ‘broken-stick’ approach for interrogating PCA Scree plots [55].
(k) . Hypothesis-testing and statistical design
A multivariate analysis of variance (MANOVA) on the response variables was used to detect stratified variation in the dataset. Then, to determine if chew and transport cycles differed statistically, we ran general linear models (GLMs) in Systat, on the principal component loadings for informative response variables. To determine if there were differences in muscle fibre and strains, and in skeletal kinematics, with respect to behaviours we ran GLMs on the individual response variables and factored individuals as random effects, behaviour as fixed effect. In cases where the behaviour term was not significant, but the individual term was, we also interrogated the nested effect of behaviour within individual to determine if the more fundamental statistical results were explained by the presence of inter-individual variability in kinematics or muscle function as previously suggested [24–28].
3. Results
(a) . Behavioural classifications of feeding cycles
We first analysed the amount of rostrocaudal translation of food (figure 2). This approach revealed a cut-off between cycles where caudal food translation exceeded or fell short of 0.25 of the standard deviation of the grand mean translation measured across all cycles analysed. Using this approach, we classified cycles that exceeded this cut-off as transport cycles (N = 36), as food was moved a significant distance towards the oesophagus. Conversely, cycles were classified as chews (N = 62) when food translation fell short of this cut-off, as food remained within the oropharynx for processing in the compressive phase of that or subsequent cycles (table 1).
(b) . Quantitative differences between cycles classified as different behaviours
With our classification of cycles based on the amount of caudal food translation (foodx, figure 4) (0.76 ± 0.99 mm, mean ± s.d., for chewing, and 5.78 ± 6.06 mm for transport; figure 5a), we used GLM analyses to discover statistically significant differences with respect to behaviour for nine of our 16 response variables. We present F-statistics from all GLM analyses in table 2.
Figure 5.
Summary plots of Axolotl kinematics and muscle variables for chew and transport cycles. Quartile box plots with median lines and whiskers indicating data ranges, and dot-density distribution of all cycle data using symbols to distinguish individuals showing magnitude differences for 16 variables between chew and transport behaviours. Grand means for each behaviour and individual are superimposed in grey symbols and lines to distinguish individual effects. Note that strain-normalization of muscle and muscle–tendon length changes (see figure 3) means that observed differences reflect true between-individual variability. Fibre strains (positive) indicate shortening and muscle–tendon strains (positive) indicate lengthening. Results from GLM analyses (table 2) are shown with ***p < 0.0001 and **p = 0.001. Horizontal dashed lines indicate zero when not at the plot-space upper or lower bound. For definition of abbreviations, please refer to Material and methods §2b.
Table 2.
Summary statistics on behavioural and individual differences across variables. Italicized values are statistically significant at α = 0.05. MTU, muscle–tendon unit; n.a., not applicable. See Material and methods §2b for explanation of other abbreviations, and §2k for explanation of statistics.
| variable | chew ± s.d. | trans. ± s.d. | F1,84 | behaviour p-value | individual p-value | ind[behaviour] p-value |
|---|---|---|---|---|---|---|
| EP fibre strain (%) | 1.70 ± 2.00 | 1.80 ± 2.00 | 0.260 | 0.611 | 0.000 | n.a. |
| NC elevation (°) | 4.44 ± 4.40 | 5.86 ± 4.29 | 3.602 | 0.061 | 0.021 | n.a. |
| AM fibre strain (%) | 1.70 ± 2.20 | 2.10 ± 2.40 | 1.873 | 0.175 | 0.000 | n.a. |
| AM MTU strain (%) | −1.70 ± 2.20 | −5.20 ± 4.50 | 18.633 | 0.000 | 0.043 | 0.000 |
| gape (mm) | 0.56 ± 0.79 | 1.63 ± 1.16 | 23.975 | 0.000 | 0.262 | n.a. |
| DM fibre strain (%) | 1.20 ± 1.60 | 2.50 ± 3.10 | 8.422 | 0.005 | 0.005 | 0.130 |
| DM MTU strain (%) | −1.50 ± 2.20 | −1.80 ± 3.60 | 0.167 | 0.683 | 0.092 | n.a. |
| BH rostrocaudal (x, mm) | −0.35 ± 0.38 | −2.37 ± 1.22 | 133.304 | 0.000 | 0.001 | 0.000 |
| BH dorsoventral (y, mm) | −0.37 ± 0.74 | −2.44 ± 1.66 | 56.310 | 0.000 | 0.315 | n.a. |
| BH lateral (z, mm) | 0.80 ± 0.51 | 0.98 ± 0.84 | 2.246 | 0.137 | 0.045 | n.a. |
| food translation (mm) | −0.76 ± 0.99 | −5.78 ± 6.06 | 44.517 | 0.000 | 0.097 | n.a. |
| GH fibre strain (%) | 2.50 ± 2.30 | 2.20 ± 2.40 | 0.317 | 0.575 | 0.011 | n.a. |
| GH MTU strain (%) | −5.20 ± 5.60 | −20.30 ± 28.40 | 15.488 | 0.000 | 0.462 | n.a. |
| SH fibre strain (%) | 1.90 ± 2.90 | 2.70 ± 3.20 | 0.247 | 0.620 | 0.792 | n.a. |
| SH MTU strain (%) | −14.70 ± 9.20 | −13.40 ± 10.80 | 8.581 | 0.004 | 0.004 | 0.007 |
| HP fibre strain (%) | 0.70 ± 0.90 | 1.40 ± 1.90 | 7.549 | 0.007 | 0.047 | 0.010 |
| pectoral retraction (°) | −4.40 ± 3.97 | −7.30 ± 5.33 | 9.431 | 0.003 | 0.050 | n.a. |
Depression of the mandible (gape, figure 5b) during chew cycles (0.56 ± 0.79 mm) only reached approximately a third of the observed expansion during transport cycles (1.63 ± 1.16 mm) (p < 0.0001). Gape expansion was accompanied by stretch of the jaw adductor (AM, figure 5c,d) MTU to a significantly greater extent during transport cycles (−5.2 ± 4.5%) than during chew cycles (−1.7 ± 2.2%) (p < 0.0001). Differences in gape expansion related directly to less fibre shortening in the jaw depressor (DM, figure 5e,f) during chews (1.2 ± 1.6%) as compared with transport cycles (2.5 ± 3.1%) (p < 0.001).
Shortening contractions in the ventral axial musculature (HP, figure 5g) represent an alternative mechanism for gape expansion in vertebrates. We found consistent variation, with significantly more hypaxial fibre shortening in transport (0.7 ± 0.9%) than in chew cycles (1.4 ± 1.9%) (p < 0.01). Hypaxial shortening can act to retract the pectoral girdle (PG) (figure 5h) and we found significantly more pectoral girdle retraction during transports (7.3 ± 5.33°) than during chew cycles (4.4 ± 3.97°) (p < 0.01). As the hypaxial musculature shortened and retracted the pectoral girdle, the muscle–tendon units of the ventral tongue retractor (SH, figure 5i,j) and tongue protractor (GH, figure 5k,l), which suspend the tongue from the lower jaw were stretched significantly more during transports (SH: −13.4 ± 10.8%; GH: −20.3 ± 28.4%) than during chew cycles (SH: −7.7 ± 9.2%; GH: −5.2 ± 5.6%) (p < 0.01 and 0.0001, respectively). Increased oral and buccal expansion during transports, owing to greater jaw depression and pectoral girdle retraction respectively, was accompanied by the tongue (BH, figure 5m–o) undergoing significantly more retraction (BHx: −2.37 ± 1.22 mm) and depression (BHy: −2.44 ± 1.66%) during transport cycles than during chew cycles (BHx: −0.35 ± 0.38%; BHy: −0.37 ± 0.74%) (both p < 0.0001).
(c) . Statistical discrimination of feeding cycles
We ran a MANOVA on our dataset containing variables that captured fascicle and MTU strains in muscles of the jaw, the tongue, and the dorsal and ventral axes, 3D kinematics of the basihyal (tongue), and rotations of the neurocranium, jaw and pectoral girdle. This analysis identified significant variation in the dataset (Wilk's λ = 0.349; F17,79 = 8.651; p < 0.0001), which was stratified across our two behavioural classifications, ‘chew’ and ‘transport’. We then ran a PCA (figure 6) on the correlation matrix of the same dataset (table 3). This analysis returned three principal component axes that had eigenvalues exceeding 1.5. Together, these axes captured 49.1% of the total variation in the dataset (figure 6). GLM analysis on the principal component factor scores for each axis revealed that all three axes captured statistically significant differences between chew and transport cycles (see table 3 for GLM results).
Figure 6.
Scatter plots of (a) PC1 versus PC2, and (b) PC1 versus PC3 from a principal component analysis on 16 musculoskeletal variables. Variables analysed were fibre (FIB) and muscle–tendon unit (MTU) strains for adductor mandibulae (AM), depressor mandibulae (DM), geniohyoid (GH), sternohyoid (SH), and fibre strains for epaxialis (EP) and hypaxialis (HP), basihyal (BH) translations (x: rostrocaudal, y: dorsoventral, z: lateral), and rotations of the mandibular gape (GP), neurocranium (NC) and pectoral girdle (PG). Grey arrows are scaled to the principal component loading scores (table 3) to illustrate how variables segregate chew (red) and transport (blue) cycles along the first three principal component axes, all of which are statistically significant (GLM, table 3), and together explain 49.1% of the variation in the total dataset.
Table 3.
Principal component (PC) loading scores from a principal component analysis (PCA) on Axolotl intraoral behaviours comparing muscle and muscle–tendon unit (MTU) strain, and kinematics of chewing and transport. GLM analysis was used to determine if each PC was significant. Italic PC loadings (values >0.5) are mapped as eigenvectors in figure 5.
| response variable | PC1 | PC2 | PC3 |
|---|---|---|---|
| epaxial strain | 0.340 | −0.531 | −0.135 |
| neurocranial elevation | 0.443 | −0.453 | −0.460 |
| adductor mandibulae fibre strain | 0.405 | −0.283 | 0.422 |
| adductor mandibulae MTU strain | 0.651 | 0.048 | −0.047 |
| peak gape | 0.656 | 0.090 | −0.276 |
| depressor mandibulae fibre strain | 0.226 | 0.052 | 0.394 |
| depressor mandibulae MTU strain | 0.540 | −0.342 | −0.074 |
| basihyal rostrocaudal (x) | −0.698 | −0.413 | 0.405 |
| basihyal dorsoventral (y) | −0.667 | −0.576 | 0.095 |
| basihyal lateral (z) | 0.210 | 0.002 | 0.318 |
| geniohyoid fibre strain | 0.153 | −0.153 | 0.494 |
| geniohyoid MTU strain | 0.639 | 0.311 | 0.181 |
| sternohyoid fibre strain | 0.198 | 0.572 | −0.011 |
| sternohyoid MTU strain | 0.196 | −0.583 | 0.286 |
| hypaxial strain | 0.474 | 0.303 | 0.561 |
| pectoral girdle retraction | −0.707 | 0.470 | 0.309 |
| univariate F-statistics (d.f. = 1, 95) | F = 53.16 | F = 9.932 | F = 2.35 |
| p < 0.0001 | p = 0.002 | p = 0.018 | |
| variance explained | 24.13% | 13.70% | 11.33% |
(d) . Quantification of elastic recoil action
All four muscles of the jaw (AM, DM) and tongue (GH, SH)muscles with discrete bony origins and insertions showed evidence of elastic stretch (negative strain) and recoil action. For the jaw adductor (AM, figure 5c,d), we measured equivalent fascicle shortening and MTU lengthening during chewing (1.7 ± 2.2%) but significantly more MTU stretch (−5.2 ± 4.4%) compared with fibre shortening (2.1 ± 2.4%) during transport. Whereas the amount of stretch of the AM MTU differed significantly between chew and transport cycles (see above), the amount of AM fibre shortening was equivalent across behaviours (p = 0.18). For the jaw depressor (DM, figure 5e,f), the opposite was true, as fibre shortening strains differed significantly (see above) whereas the magnitude of MTU stretch was equivalent across behaviours (p = 0.68). For the tongue muscles (SH, figure 5i,j; GH, figure 5k,l), we measured substantial MTU stretch (see above) compared with muscle fascicle shortening (approx. 2–4%, table 2), and there were no significant differences in shortening strains for the fibres of the tongue muscles across behaviours (SH: p = 0.62; GH: p = 0.58).
4. Discussion
Studies of feeding in ambystomatids and other salamanders have lacked intraoral measurements of 3D kinematics of the tongue and food or lacked the rigorous statistical analysis framework needed to disambiguate intraoral feeding behaviours. Here, we collected musculoskeletal kinematics using XROMM and fluoromicrometry during intraoral feeding behaviours in the Axolotl to demonstrate the use of food processing—chewing—in addition to transport as part of the intraoral behavioural repertoire of ambystomatid salamanders. Statistical differences between chewing and food transport were driven by functionally relevant differences in rostrocaudal and dorsoventral translations of the basihyal ‘tongue’ (figure 5m,n). Differences in jaw and pectoral kinematics were also uncovered, and explained by differences in shortening of muscles driving the kinematics of these skeletal modules. We also found evidence of elastic recoil action in the jaw and tongue muscles examined with differences in the contribution of elastic recoil to chewing and transport behaviors. In line with conclusions from previous studies of ambystomatid intraoral feeding behaviours [26,56,57], we also found evidence of pronounced intraspecific variability but not to the extent that the mechanical differences between chewing and transport behaviours were concealed.
(a) . Axolotls use statistically distinguishable chew and transport behaviours
The hypothesis that salamanders incorporate intraoral processing in addition to food transport was mainly motivated by the observation that chewing occurs in vertebrate taxa both basal and derived with respect to caudates [5,19,36], and further motivated by the crucial role of processing for food breakdown to aid nutritional assimilation in the gastrointestinal tract [58]. Moreover, several recent studies have drawn the conclusion of Lauder and co-workers [24–28], that salamanders do not chew, into question [30–35]. Although the use of X-ray-based kinematics approaches has allowed recent studies to measure internal kinematics of the basihyal or basibranchial components of the tongue module, our hypothesis remained untested owing to the lack of a rigorous statistical approach, which our study now provides.
The kinematics differences between chewing and transport reported here were limited to a few variables describing tongue, jaw and pectoral girdle movement (table 2 and figure 5b,h,m,n). Moreover, the two behaviours were only weakly segregated in principal component space (figure 6). Observations of Axolotl feeding suggest that intraoral feeding kinematics become amplified on harder-bodied food like insects and fishes. Such a food type effect was recently demonstrated for intraoral processing in another salamander [33] and may potentially polarize the distribution of chewing and transport kinematics across principal component space in future studies. However, despite our choice of a soft-bodied and relatively sedentary food type for this study, our statistical approach provided support for the hypothesis that at least some salamanders chew and encourages a similar approach in future studies aimed at broadening our understanding of intraoral behavioural diversity in Caudata.
(b) . Muscle and muscle–tendon function in the Axolotl feeding apparatus
We measured shortening strains of muscles driving motions of the jaw (AM, DM), tongue (GH, SH), skull (EP) and pectoral girdle (HP) that with few exceptions averaged less than 5% of resting length (table 2 and figure 5). These measurements fall within the strain-ranges of infra- and suprahyoid muscles in shark bite processing [59] and pig suckling and swallowing [60,61] and, perhaps unsurprisingly, fall short of the muscle strains measured via sonomicrometry or fluoromicrometry during high-powered suction feeding in fishes [49–51,59,62–64]. Despite that strain measurements remain much scarcer for craniofacial muscles during feeding than limb muscles during locomotion, available evidence suggests that muscles in vertebrate feeding systems undergo much more modest fibre shortening strains than those of limb systems. It has been argued that there are fundamental differences between jaw and limb motion systems, in terms of muscle moment-arm lengths, mechanical advantages and force vector-orientations [65]. However, hypotheses about muscle performance-related differences between these crucial survival systems will require more muscle strain measurements from the feeding systems of a greater diversity of vertebrates.
A prior analysis of the mechanical function of the Axolotl jaw adductor (AM) reported greater fibre shortening strains during chewing than those measured in the present study. In that study, the use of fish and cricket prey also revealed an effect of food hardness on the strain measurements [31]. The lower AM fibre strains in the present study likely owe to the food being softer-bodied worms that may require less fibre shortening to achieve the force production necessary to process food adequately prior to digestion.
(c) . Series elastic action in craniofacial muscles
We found evidence of series elastic recoil action in the jaw adductor (AM) during both chewing and food transport (figure 3), in line with our prior study of the mechanical function of this muscle [31]; as muscle fibres shortened, MTU length increased as the distance between the muscle origin and insertion increased (i.e. during jaw depression, figure 4). Opposing (shortening) strains of muscle fibres with respect to strain of the whole MTU is a hallmark of elastic energy storage that is well-documented in limb muscles, but less so in feeding muscles, especially those of small vertebrates moving in and through fluid [31,45,66–68]. Motivated by morphological evidence of prominent collagenous tissues in amphibian jaw and tongue systems [39,40], we sought to determine if feeding muscles other than AM showed signs of elastic recoil action. Our method for measuring MTU strain requires discrete muscle origins and insertions, which is not the case for myotomal muscles of the body wall (EP, HP). However, measurements were possible for all jaw and tongue muscles sampled (AM, DM, GH, SH), and all four muscles showed evidence of elastic recoil action.
Elastic recoil of the jaw depressor (DM) was previously reported in toads, which belong to the anuran sister group of caudates [68], but not previously for anamniotes that feed aquatically, such as lungfishes and salamanders. This is peculiar, considering the established role and clear benefits of elastic action in power amplification for initiating and accelerating gape expansion in power-demanding suction-feeding events [59,66,67]. We hypothesize that elastic recoil of the mandibular depressor muscle is widespread among tetrapods and warrants future investigation. For jaw muscles, elastic recoil during cyclic food processing and transport may confer several benefits, including shortening the period of mouth expansion to limit risks of food escape to increasing the power and work available for the processing power-stroke.
Elastic recoil was omnipresent for the muscles of the jaw and tongue investigated. However, statistically significant differences in the magnitude of MTU stretch between chewing and transport behaviours were only present for AM and the tongue retractor, SH (also commonly referred to as rectus cervicis in lungfishes and amphibians). The statistically significant result for SH was surprising, especially considering that this muscle has a highly variable contractile function across vertebrates, involving regional strain differences [61,69], variable electromyographic activation [4] and frequent lengthening contractions during feeding [20,50,61,70–72]. The labile function of SH highlights its multifunctional role [62], either involving active shortening to contribute power to hyoid movements, remaining isometric when active to transmit power from hypaxial contraction and pectoral girdle retraction to move the hyoid, or a combination, as seen in our study.
We found the most pronounced series elastic action in the tongue protractor (GH, figure 5l), reaching similar magnitudes to those of a functionally equivalent muscle analogue, the posterior intermandibularis during intraoral processing in the knifefish Chitala [73]. The considerable muscle–tendon strains observed in the Axolotl GH are also interesting considering the amount of sarcomere hyper-extension that has been suggested to be required in the anuran GH during ballistic tongue projection [74]. Contractile function of the tongue protractor and retractor musculature in aquatic feeding anamniotes is worthy of more detailed study systematically sampling a broader range of taxa.
Our results suggest that elastic recoil occurs in several muscles of the Axolotl feeding system and is involved in both processing and transport behaviours. However, more analyses are needed to determine the anatomical bases, mechanical function, and adaptive significance of jaw and tongue elastic recoil. Specific to ambystomatids, we predict that understanding changes in jaw and tongue muscle elastic recoil will be important to determine how muscle function is involved in generating alterations in feeding function across metamorphosis. This area of research has potential implications for our understanding of terrestrialization processes in general, and particularly for our understanding of the invasion of land by tetrapods [43].
A biomechanical model explaining musculoskeletal functional differences between transport and chewing in the Axolotl is presented in figure 7. Our data revealed particularly small fibre strains in jaw muscles, as compared with the tongue muscles, which are working against the distension of the oral volume that results from a fluid-filled cavity. As a consequence, MTU stretch increased in the tongue muscles during transport, when both the jaw depressor and the ventral musculature of the body wall (HP) generated more mouth opening than during chewing (see also [63]). We found considerable between-individual differences in other MTU and skeletal kinematics, yet the relatively small muscle fibre strains and larger MTU strains suggest that the principal input to kinematics of both chew and transport cycles is hypaxial strain driving pectoral and tongue kinematics contributing to food transport kinematics, and DM driving jaw kinematics with elastic recoil of the jaw adductor and the tongue retractor contributing to food processing kinematics. Thus, our data support earlier speculations that chewing evolved as a modulation of food transport, rather than modulation of the occluding phase of the suction-feeding strike [52].
Figure 7.
Musculoskeletal kinematics variables inform a biomechanical model of Axolotl chew and transport differences. A 3D rendering of an Axolotl computed tomography (CT) scan, with black circles indicating markers in the neurocranium (NC), lower jaw (LJ) and pectoral girdle (CL) elements (note that dashed lines outline the jaw joint (JJ), basihyal (BH) and pectoral girdle (PG), which are cartilaginous and therefore do not show up in the scan). Red circles indicate marker pairs in the epaxial (EP), jaw adductor (AM), jaw depressor (DM), tongue protractor (GH), tongue retractor (SH) and hypaxial (HP) muscles. The tongue pad (TP) marker is indicated by a blue circle. The yellow arched arrows indicate skeletal movements that are statistically more pronounced during transport as compared with chew cycles. Statistically greater shortening of fibres in DM and HP drives greater jaw depression to expand the oropharyngeal cavity during transport cycles. Jaw depression is augmented by a greater degree of pectoral girdle retraction, which stretches the tongue retractor and protractor muscles, the key function of which is to retract and depress the tongue (BH and TP) more during transport cycles than chew cycles. Electronic supplementary material, figure S2 shows the scan-rendering as a movable 3D figure, to better illustrate marker placements.
5. Conclusion
A statistically rigorous framework generated support for the hypothesis that ambystomatid Axolotls process food in addition to transporting it intraorally. Measuring intraoral food translations supported an objective classification system that determined differences in intraoral kinematics of the Axolotl jaw and tongue systems during chewing and transport cycles.
Our data support the fundamental idea that chewing is an ancestral behaviour that is conserved across jaw-bearing vertebrates, which is unsurprising considering its crucial function in food breakdown and to facilitate nutritional assimilation in the gut. Comparisons of MTU stretch and muscle fibre shortening showed that elastic recoil action occurs in both jaw and tongue muscles of the Axolotl, with potential implications on functional changes of these biomechanical modules with changes in feeding environment as salamanders terrestrialize.
Acknowledgements
This study formed part of the requirements for a UMass. Lowell BSc with Honors thesis by M.S. We thank Andrew Biewener and Beth Brainerd for X-ray time, Sam DeLap for assistance with developing analyses workflows, Ariel Camp and Elska Kazmarek for guidance on the XROMM workflow. U. Rizwan, J. Rozen, C. Pannesiti, S. Aldahabi, K. McCarthy and Brian Richard helped with digitizing, and Rick Hochberg and Daniel Schwarz for served as thesis committee members and provided comments on an earlier version of this manuscript.
Ethics
This work was carried out under Institutional Animal Care and Use committee approvals from UMass Lowell (12-06-19-KON) and Harvard University, FAS (20_09-03).
Data accessibility
Raw video data from this study is available through the XMAportal (XMAportal.org; study #BROWN47).
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
M.S.: data curation, formal analysis, investigation, methodology, resources, software, validation, visualization, writing—review and editing; M.R.-G.: investigation, resources, software, validation, visualization, writing—review and editing; Y.T.R.: data curation, formal analysis, investigation, writing—review and editing; N.K.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Conflict of interest declaration
The authors have no competing interests.
Funding
This study was funded by a start-up package from UML (to N.K.), two UML Kennedy College of Science fellowships and two UML Honors college scholarships (to M.S. and M.R.-G.), and an Urban Massachusetts Louis Stokes Alliance for Minority Participation in Research (NSF-HRD-1712771) award to M.R.-G. The Company of Biologists and the Society for Experimental Biology generously provided participation funding for the special session at SEB 2022 from which this paper arose.
References
- 1.Reilly SM, McBrayer LD, White TD. 2001. Prey processing in amniotes: biomechanical and behavioral patterns of food reduction. Comp. Biochem. Physiol. A 128, 397-415. ( 10.1016/S1095-6433(00)00326-3) [DOI] [PubMed] [Google Scholar]
- 2.Clauss M, Nunn C, Fritz J, Hummel J. 2009. Evidence for a tradeoff between retention time and chewing efficiency in large mammalian herbivores. Comp. Biochem. Physiol. A 154, 376-382. ( 10.1016/j.cbpa.2009.07.016) [DOI] [PubMed] [Google Scholar]
- 3.Lucas PW, Prinz JF, Agrawal KR, Bruce IC. 2002. Food physics and oral physiology. Food Quality Preference 13, 203-213. ( 10.1016/S0950-3293(00)00036-7) [DOI] [Google Scholar]
- 4.Konow N, Herrel A, Ross CF, Williams SH, German RZ, Sanford CPJ, Gintof C. 2011. Evolution of muscle activity patterns driving motions of the jaw and hyoid during chewing in gnathostomes. Integr. Comp. Biol. 51, 235-246. ( 10.1093/icb/icr040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross CF, Eckhardt A, Herrel A, Hylander WL, Metzger KA, Schaerlaeken V, Washington RL, Williams SH. 2007. Modulation of intra-oral processing in mammals and lepidosaurs. Integr. Comp. Biol. 47, 118-136. ( 10.1093/icb/icm044) [DOI] [PubMed] [Google Scholar]
- 6.Laird MF, Granatosky MC, Taylor AB, Ross CF. 2020. Muscle architecture dynamics modulate performance of the superficial anterior temporalis muscle during chewing in capuchins. Scient. Rep. 10, 6410. ( 10.1038/s41598-020-63376-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhullar B-AS, Manafzadeh AR, Miyamae JA, Hoffman EA, Brainerd EL, Musinsky C, Crompton AW. 2019. Rolling of the jaw is essential for mammalian chewing and tribosphenic molar function. Nature 566, 528-532. ( 10.1038/s41586-019-0940-x) [DOI] [PubMed] [Google Scholar]
- 8.Virot E, Ma G, Clanet C, Jung S. 2017. Physics of chewing in terrestrial mammals. Scient. Rep. 7, 43967. ( 10.1038/srep43967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross CF, Dharia R, Herring SW, Hylander WL, Liu Z-J, Rafferty KL, Ravosa MJ, Williams SH. 2007. Modulation of mandibular loading and bite force in mammals during mastication. J. Exp. Biol. 210, 1046-1063. ( 10.1242/jeb.02733) [DOI] [PubMed] [Google Scholar]
- 10.Williams SH, Vinyard CJ, Wall CE, Hylander WL. 2007. Masticatory motor patterns in ungulates: a quantitative assessment of jaw-muscle coordination in goats, alpacas and horses. J. Exp. Zool. A Ecol. Genet. Physiol. 307, 226-240. ( 10.1002/jez.362) [DOI] [PubMed] [Google Scholar]
- 11.Herring SW. 1993. Functional morphology of mammalian mastication. Am. Zool. 33, 289-299. ( 10.1093/icb/33.3.289) [DOI] [Google Scholar]
- 12.Gorniak GC, Gans C. 1980. Quantitative assay of electromyograms during mastication in domestic cats (Felis catus). J. Morphol. 163, 253-281. ( 10.1002/jmor.1051630304) [DOI] [PubMed] [Google Scholar]
- 13.Herrel A, Cleuren J, Vree F. 1996. Kinematics of feeding in the lizard Agama stellio. J. Exp. Biol. 199, 1727-1742. ( 10.1242/jeb.199.8.1727) [DOI] [PubMed] [Google Scholar]
- 14.So KKJ, Wainwright PC, Bennett AF. 1992. Kinematics of prey processing in Chamaeleo jacksonii: conservation of function with morphological specialization. J. Zool. 226, 47-64. ( 10.1111/j.1469-7998.1992.tb06126.x) [DOI] [Google Scholar]
- 15.Kraklau DM. 1991. Kinematics of prey capture and chewing in the lizard Agama agama (Squamata, Agamidae). J. Morphol. 210, 195-212. ( 10.1002/jmor.1052100208) [DOI] [PubMed] [Google Scholar]
- 16.Throckmorton GS. 1980. The chewing cycle in the herbivorous lizard Uromastix aegyptius (Agamidae). Arch. Oral Biol. 25, 225-233. ( 10.1016/0003-9969(80)90027-8) [DOI] [PubMed] [Google Scholar]
- 17.Jones MEH, O'higgins P, Fagan MJ, Evans SE, Curtis N. 2012. Shearing mechanics and the influence of a flexible symphysis during oral food processing in Sphenodon (Lepidosauria: Rhynchocephalia). Anat. Rec. 295, 1075-1091. ( 10.1002/ar.22487) [DOI] [PubMed] [Google Scholar]
- 18.Kolmann MA, Welch KC, Summers AP, Lovejoy NR. 2016. Always chew your food: freshwater stingrays use mastication to process tough insect prey. Proc. R. Soc. B 283, 20161392. ( 10.1098/rspb.2016.1392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gintof C, Konow N, Ross CF, Sanford CPJ. 2010. Rhythmic chewing with oral jaws in teleost fishes: a comparison with amniotes. J. Exp. Biol. 213, 1868-1875. ( 10.1242/jeb.041012) [DOI] [PubMed] [Google Scholar]
- 20.Konow N, Sanford CPJ. 2008. Is a convergently derived muscle-activity pattern driving novel raking behaviours in teleost fishes? J. Exp. Biol. 211, 989-999. ( 10.1242/jeb.013078) [DOI] [PubMed] [Google Scholar]
- 21.Bemis WE, Lauder GV. 1986. Morphology and function of the feeding apparatus of the lungfish, Lepidosiren paradoxa (Dipnoi). J. Morphol. 187, 81-108. ( 10.1002/jmor.1051870108) [DOI] [PubMed] [Google Scholar]
- 22.Lauder GV. 1980. Evolution of the feeding mechanism in primitive actionopterygian fishes: a functional anatomical analysis of Polypterus, Lepisosteus, and Amia. J. Morphol. 163, 283-317. ( 10.1002/jmor.1051630305) [DOI] [PubMed] [Google Scholar]
- 23.Laurence-Chasen JD, Ramsay JB, Brainerd EL. 2019. Shearing overbite and asymmetrical jaw motions facilitate food breakdown in a freshwater stingray, Potamotrygon motoro. J. Exp. Biol. 222, jeb197681. ( 10.1242/jeb.197681) [DOI] [PubMed] [Google Scholar]
- 24.Reilly SM, Lauder GV. 1991. Prey transport in the tiger salamander: quantitative electromyography and muscle function in tetrapods. J. Exp. Zool. 260, 1-17. ( 10.1002/jez.1402600102) [DOI] [Google Scholar]
- 25.Reilly SM, Lauder GV. 1990. The evolution of tetrapod feeding behavior: kinematic homologies in prey transport. Evolution 44, 1542-1557. ( 10.2307/2409336) [DOI] [PubMed] [Google Scholar]
- 26.Reilly SM, Lauder GV. 1989. Physiological bases of feeding behaviour in salamanders: do motor patterns vary with prey type? J. Exp. Biol. 141, 343-358. ( 10.1242/jeb.141.1.343) [DOI] [Google Scholar]
- 27.Thexton AJ, Wake DB, Wake MH. 1977. Tongue function in the salamander Bolitoglossa occidentalis. Arch. Oral Biol. 22, 361-366. ( 10.1016/0003-9969(77)90057-7) [DOI] [PubMed] [Google Scholar]
- 28.Bemis WE, Schwenk K, Wake MH. 1983. Morphology and function of the feeding apparatus in Dermophis mexicanus (Amphibia: Gymnophiona). Zool. J. Linn. Soc. 77, 75-96. ( 10.1111/j.1096-3642.1983.tb01722.x) [DOI] [Google Scholar]
- 29.Ahlberg PE. 2018. Follow the footprints and mind the gaps: a new look at the origin of tetrapods. Earth Environ. Sci. Trans. R. Soc. Edinb. 109, 115-137. ( 10.1017/S1755691018000695) [DOI] [Google Scholar]
- 30.Witzmann F, Brainerd EL, Konow N. 2019. Eye movements in frogs and salamanders—testing the palatal buccal pump hypothesis. Integr. Organism. Biol. 1, obz011. ( 10.1093/iob/obz011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rull M, Solomon J, Konow N. 2020. Elastic recoil action amplifies jaw closing speed in an aquatic feeding salamander. Proc. R. Soc. B 287, 32429804. ( 10.1098/rspb.2020.0428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarz D, Konow N, Roba YT, Heiss E. 2020. A salamander that chews using complex, three-dimensional mandible movements. J. Exp. Biol. 223, jeb220749. ( 10.1242/jeb.220749) [DOI] [PubMed] [Google Scholar]
- 33.Schwarz D, Gorb SN, Kovalev A, Konow N, Heiss E. 2020. Flexibility of intraoral food processing in the salamandrid newt Triturus carnifex: effects of environment and prey type. J. Exp. Biol. 223, jeb232868. ( 10.1242/jeb.232868) [DOI] [PubMed] [Google Scholar]
- 34.Schwarz D, Konow N, Porro LB, Heiss E. 2020. Ontogenetic plasticity in cranial morphology is associated with a change in the food processing behavior in Alpine newts. Front. Zool. 17, 34. ( 10.1186/s12983-020-00373-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heiss E, Schwarz D, Konow N. 2019. Chewing or not? Intraoral food processing in a salamandrid newt. J. Exp. Biol. 222, jeb.189886. ( 10.1242/jeb.189886) [DOI] [PubMed] [Google Scholar]
- 36.Richard BA, Spence M, Rull-Garza M, Tolosa Roba Y, Schwarz D, Ramsay JB, Laurence-Chasen JD, Ross CF, Konow N. . 2023. Rhythmic chew cycles with distinct fast and slow phases are ancestral to gnathostomes. Phil. Trans. R. Soc. B 378, 20220539. ( 10.1098/rstb.2022.0539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillis GB, Lauder GV. 1995. Kinematics of feeding in bluegill sunfish: is there a general distinction between aquatic capture and transport behaviors? J. Exp. Biol. 198, 709-720. ( 10.1242/jeb.198.3.709) [DOI] [PubMed] [Google Scholar]
- 38.Reilly SM, Lauder GV. 1988. Ontogeny of aquatic feeding performance in the eastern newt, Notophthalmus viridescens (Salamandridae). Copeia 1988, 87-91. ( 10.2307/1445926) [DOI] [Google Scholar]
- 39.Iordansky NN. 1994. Tendons of jaw muscles in Amphibia and Reptilia: homology and evolution. Russ. J. Herpetol. 1, 13-20. [Google Scholar]
- 40.Carroll RL, Holmes R. 1980. The skull and jaw musculature as guides to the ancestry of salamanders. Zool. J. Linn. Soc. 68, 1-40. ( 10.1111/j.1096-3642.1980.tb01916.x) [DOI] [Google Scholar]
- 41.Brainerd EL, et al. 2010. X-ray reconstruction of moving morphology (XROMM): precision, accuracy and applications in comparative biomechanics research. J. Exp. Zool. A Ecol. Genet. Physiol. 313, 262-279. ( 10.1002/jez.589) [DOI] [PubMed] [Google Scholar]
- 42.Camp AL, Astley HC, Horner AM, Roberts TJ, Brainerd EL. 2016. Fluoromicrometry: a method for measuring muscle length dynamics with biplanar videofluoroscopy. J. Exp. Zool. A 325, 399-408. ( 10.1002/jez.2031) [DOI] [PubMed] [Google Scholar]
- 43.Schwarz D, Heiss E, Pierson TW, Konow N, Schoch RR. 2023. Using salamanders as model taxa to understand vertebrate feeding constraints during the late Devonian water-to-land transition. Phi. Trans. R. Soc. B 378, 20220541. ( 10.1098/rstb.2022.0541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coots PS, Seifert AW. 2015. Thyroxine-induced metamorphosis in the axolotl (Ambystoma mexicanum). In Salamanders in regeneration research: methods and protocols (eds Kumar A, Simon A), pp. 141-145. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 45.Konow N, et al. 2015. Spring or string: does tendon elastic action influence wing muscle mechanics in bat flight? Proc. R. Soc. B 282, 20151832. ( 10.1098/rspb.2015.1832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaffer HB, Lauder GV. 1985. Patterns of variation in aquatic ambystomatid salamanders - kinematics of the feeding mechanism. Evolution 39, 83-92. ( 10.2307/2408518) [DOI] [PubMed] [Google Scholar]
- 47.Knörlein BJ, et al. 2016. Validation of XMALab software for marker-based XROMM. J. Exp. Biol. 219, 3701-3711. ( 10.1242/jeb.145383) [DOI] [PubMed] [Google Scholar]
- 48.Gidmark NJ, Staab KL, Brainerd EL, Hernandez LP. 2012. Flexibility in starting posture drives flexibility in kinematic behavior of the kinethmoid-mediated premaxillary protrusion mechanism in a cyprinid fish, Cyprinus carpio. J. Exp. Biol. 215, 2262-2272. ( 10.1242/jeb.070516) [DOI] [PubMed] [Google Scholar]
- 49.Carroll AM, Wainwright PC. 2006. Muscle function and power output during suction feeding in largemouth bass, Micropterus salmoides. Comp. Biochem. Physiol. A 143, 389-399. ( 10.1016/j.cbpa.2005.12.022) [DOI] [PubMed] [Google Scholar]
- 50.Carroll AM. 2004. Muscle activation and strain during suction feeding in the largemouth bass Micropterus salmoides. J. Exp. Biol. 207, 983-991. ( 10.1242/jeb.00862) [DOI] [PubMed] [Google Scholar]
- 51.Camp A, Brainerd E. 2014. Role of axial muscles in powering mouth expansion during suction feeding in largemouth bass. J. Exp. Biol. 217, 1333-1345. [DOI] [PubMed] [Google Scholar]
- 52.Konow N, Sanford CPJ. 2008. Biomechanics of a convergently derived prey-processing mechanism in fishes: evidence from comparative tongue bite apparatus morphology and raking kinematics. J. Exp. Biol. 211, 3378-3391. ( 10.1242/jeb.023564) [DOI] [PubMed] [Google Scholar]
- 53.Alfaro ME, Herrel A. 2001. Introduction: major issues of feeding motor control in vertebrates. Am. Zool. 41, 1243-1247. ( 10.1093/icb/41.6.1243) [DOI] [Google Scholar]
- 54.Wainwright PC, Friel JP. 2001. Behavioral characters and historical properties of motor patterns. In The character concept in evolutionary biology (ed. GP Wagner), pp. 285-301. New York, NY: Academic Press. [Google Scholar]
- 55.Jackson DA. 1993. Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74, 2204-2214. ( 10.2307/1939574) [DOI] [Google Scholar]
- 56.Lauder GV, Shaffer HB. 1988. Ontogeny of functional design in tiger salamanders (Ambystoma tigrinum): are motor patterns conserved during major morphological transformations? J. Morphol. 197, 249-268. ( 10.1002/jmor.1051970302) [DOI] [PubMed] [Google Scholar]
- 57.Shaffer HB, Lauder GV. 1985. Aquatic prey capture in ambystomatid salamanders - patterns of variation in muscle-activity. J. Morphol. 183, 273-284. ( 10.1002/jmor.1051830304) [DOI] [PubMed] [Google Scholar]
- 58.Clauss M, Fritz J, Hummel J. 2023. Teeth and the gastrointestinal tract in mammals: when 1 + 1 = 3. Phil. Trans. R. Soc. B 378, 20220544. ( 10.1098/rstb.2022.0544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramsay JB, Wilga CD. 2017. Function of the hypobranchial muscles and hyoidiomandibular ligament during suction capture and bite processing in white-spotted bamboo sharks, Chiloscyllium plagiosum. J. Exp. Biol. 220, 4047-4059. ( 10.1242/jeb.165290) [DOI] [PubMed] [Google Scholar]
- 60.Holman SD, Konow N, Lukasik ST, German RZ. 2012. Regional variation in geniohyoid muscle strain during suckling in the infant pig. J. Exp. Zool. A 317, 359-370. ( 10.1002/jez.1729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Konow N, Thexton A, Crompton AW, German RZ. 2010. Regional differences in length change and electromyographic heterogeneity in sternohyoid muscle during infant mammalian swallowing. J. Appl. Physiol. 109, 439-448. ( 10.1152/japplphysiol.00353.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lomax JJ, Martinson TF, Jimenez YE, Brainerd EL. 2020. Bifunctional role of the sternohyoideus muscle during suction feeding in striped surfperch, Embiotoca lateralis. Integr. Organism. Biol. 2, obaa021. ( 10.1093/iob/obaa021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Camp AL, Olsen AM, Hernandez LP, Brainerd EL. 2020. Fishes can use axial muscles as anchors or motors for powerful suction feeding. J. Exp. Biol. 223, jeb225649. ( 10.1242/jeb.225649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Camp AL, Roberts TJ, Brainerd EL. 2018. Bluegill sunfish use high power outputs from axial muscles to generate powerful suction-feeding strikes. J. Exp. Biol. 221, jeb178160. ( 10.1242/jeb.178160) [DOI] [PubMed] [Google Scholar]
- 65.Granatosky MC, Ross CF. 2020. Differences in muscle mechanics underlie divergent optimality criteria between feeding and locomotor systems. J. Anat. 237, 1072-1086. ( 10.1111/joa.13279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aerts P, Osse JWM, Verraes W. 1987. Model of jaw depression during feeding in Astatotilapia elegans (Teleostei: Cichlidae): mechanisms for energy storage and triggering. J. Morphol. 194, 85-109. ( 10.1002/jmor.1051940108) [DOI] [PubMed] [Google Scholar]
- 67.Van Wassenbergh S, Strother JA, Flammang BE, Ferry-Graham LA, Aerts P. 2008. Extremely fast prey capture in pipefish is powered by elastic recoil. J. R. Soc. Interface 5, 285-296. ( 10.1098/rsif.2007.1124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lappin AK, Monroy JA, Pilarski JQ, Zepnewski ED, Pierotti DJ, Nishikawa KC. 2006. Storage and recovery of elastic potential energy powers ballistic prey capture in toads. J. Exp. Biol. 209, 2535-2553. ( 10.1242/jeb.02276) [DOI] [PubMed] [Google Scholar]
- 69.Ahn AN, Konow N, Tijs C, Biewener AA. 2018. Different segments within vertebrate muscles can operate on different regions of their force–length relationships. Integr. Comp. Biol. 58, 219-231. ( 10.1093/icb/icy040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Wassenbergh S, Herrel A, Adriaens D, Aerts P. 2005. A test of mouth-opening and hyoid-depression mechanisms during prey capture in a catfish using high-speed cineradiography. J. Exp. Biol. 208, 4627-4639. ( 10.1242/jeb.01919) [DOI] [PubMed] [Google Scholar]
- 71.Herrel A, Schaerlaeken V, Ross C, Meyers J, Nishikawa K, Abdala V, Manzano A, Aerts P. 2008. Electromyography and the evolution of motor control: limitations and insights. Integr. Compar. Biol. 48, 261-271. ( 10.1093/icb/icn025) [DOI] [PubMed] [Google Scholar]
- 72.Crompton AW, Cook P, Hiiemae K, Thexton AJ. 1975. Movement of the hyoid apparatus during chewing. Nature 258, 69-70. ( 10.1038/258069a0) [DOI] [PubMed] [Google Scholar]
- 73.Konow N, Camp AL, Sanford CPJ. 2008. Congruence between muscle activity and kinematics in a convergently derived prey-processing behavior. Integr. Comp. Biol. 48, 246-260. ( 10.1093/icb/icn045) [DOI] [PubMed] [Google Scholar]
- 74.Nishikawa KC, Kier WM, Smith KK. 1999. Morphology and mechanics of tongue movement in the African pig-nosed frog Hemisus marmoratum: a muscular hydrostatic model. J. Exp. Biol. 202, 771-780. ( 10.1242/jeb.202.7.771) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw video data from this study is available through the XMAportal (XMAportal.org; study #BROWN47).