Summary
X-linked myotubular myopathy (XLMTM) is a severe congenital disease characterized by profound muscle weakness, respiratory failure, and early death. No approved therapy for XLMTM is currently available. Adeno-associated virus (AAV)-mediated gene replacement therapy has shown promise as an investigational therapeutic strategy. We aimed to characterize the transcriptomic changes in muscle biopsies of individuals with XLMTM who received resamirigene bilparvovec (AT132; rAAV8-Des-hMTM1) in the ASPIRO clinical trial and to identify potential biomarkers that correlate with therapeutic outcome. We leveraged RNA-sequencing data from the muscle biopsies of 15 study participants and applied differential expression analysis, gene co-expression analysis, and machine learning to characterize the transcriptomic changes at baseline (pre-dose) and at 24 and 48 weeks after resamirigene bilparvovec dosing. As expected, MTM1 expression levels were significantly increased after dosing (p < 0.0001). Differential expression analysis identified upregulated genes after dosing that were enriched in several pathways, including lipid metabolism and inflammatory response pathways, and downregulated genes were enriched in cell-cell adhesion and muscle development pathways. Genes involved in inflammatory and immune pathways were differentially expressed between participants exhibiting ventilator support reduction of either greater or less than 6 h/day after gene therapy compared to pre-dosing. Co-expression analysis identified similarly regulated genes, which were grouped into modules. Finally, the machine learning model identified five genes, including MTM1, as potential RNA biomarkers to monitor the progress of AAV gene replacement therapy. These findings further extend our understanding of AAV-mediated gene therapy in individuals with XLMTM at the transcriptomic level.
Keywords: X-linked myotubular myopathy, RNA sequencing, transcriptomic profiling, gene therapy, AAV, neuromuscular diseases, motor disorders
XLMTM is a severe congenital disease with no approved therapy. AAV-mediated gene replacement shows promise. We analyzed RNA-sequencing data from 15 XLMTM participants enrolled in ASPIRO (NCT03199469) before and after AAV dosing. This identified genes and pathways, enhancing our understanding of AAV-mediated gene therapy for XLMTM at the transcriptomic level.
Introduction
X-linked myotubular myopathy (XLMTM [MIM: 310400]) is a rare and life-threatening neuromuscular disease that predominantly affects the skeletal muscles. It is caused by mutations in the myotubularin gene (MTM1 [MIM: 300415]), responsible for myotubularin enzyme production.1 Myotubularin is required for the development and maintenance of skeletal muscle.2 The estimated incidence of XLMTM, which is inherited in an X-linked recessive pattern, is 1 in 40,000–50,000 newborn males.3 Individuals with XLMTM present with significant muscle weakness, hypotonia, and respiratory distress at birth.3,4,5,6
There is currently no cure for individuals diagnosed with XLMTM, with the majority of treatments directed toward the individualized management of symptoms.6 In many cases, the clinical complications of XLMTM in newborns are fatal, with an estimated mortality rate of 50% in the first two years of life.7 While some individuals with XLMTM can live into adolescence or even beyond, typically their survival is dependent on supportive care and, often, permanent mechanical ventilation.8 In recent years, gene replacement has been investigated as a potential therapy for individuals with genetically confirmed XLMTM.9 Previous studies have shown the efficacy of a gene therapy strategy based on the administration of an adeno-associated virus (AAV) vector carrying a normal copy of the MTM1 gene.9,10
Based on the efficacy of muscle-directed gene replacement therapy in murine and canine models of myotubular myopathy,11,12 Généthon collaborated with Astellas Gene Therapies (formerly Audentes Therapeutics, Inc., a company which specializes in the development of treatments for rare neuromuscular disorders) to initiate a gene therapy clinical trial program in participants with XLMTM. The program was initiated with the INCEPTUS study (ClinicalTrials.gov NCT02704273), a non-interventional, natural history study designed to prospectively characterize a control group of boys with XLMTM for the interventional study of resamirigene bilparvovec (AT132; rAAV8-Des-hMTM1).6 The interventional trial, ASPIRO (ClinicalTrials.gov NCT03199469), began in September 2017 and is evaluating the use of intravenously administered resamirigene bilparvovec, an investigational therapeutic MTM1 gene transfer therapy using a recombinant AAV8 vector to enhance muscle transduction and a muscle-specific promoter for tissue-directed expression.12 Importantly, the deaths of four dosed participants who had clinical evidence suggesting pre-existing cholestasis has highlighted the need to better understand the role of hepatobiliary disease in the natural history of XLMTM and the potential interaction of AAV-mediated gene therapy in this setting. To understand how AAV gene transfer therapy affects tissues on a molecular level and its potential risks and benefits, we conducted bulk RNA sequencing (RNA-seq) on 44 evaluable muscle biopsy samples (left gastrocnemius, right gastrocnemius, and vastus lateralis) from 15 individuals with XLMTM as part of the ASPIRO clinical trial. All participants were <5 years old, unless previously enrolled in INCEPTUS. Muscle biopsies were taken a few days prior to dosing (baseline) and at 24 weeks and 48 weeks after dosing. Transcriptomics profiling analysis was carried out on the biopsy samples, comparing between pre-dose and post dosing timepoints. Figure S1 outlines the three-tiered approach used to analyze the RNA-seq data, including differential expression analyses, gene co-expression analyses, and machine learning.
Material and methods
Study cohort and biopsy samples
ASPIRO is the first-in-human clinical study of a one-time intravenously administered investigational gene replacement therapy in individuals with genetically confirmed XLMTM. In total, 26 participants were enrolled, and 24 have received resamirigene bilparvovec. For each of the 15 participants whose samples were available at the time of this analysis, muscle biopsy was collected prior to dosing (baseline) and at 24 and 48 weeks after resamirigene bilparvovec administration (Table 1). A total of 44 biopsies were collected. Each participant had a muscle biopsy specified to be taken from the left lateral gastrocnemius (baseline), the right lateral gastrocnemius (24 weeks post-dosing), and the vastus lateralis (48 weeks post-dosing).
Table 1.
Key characteristics in ASPIRO study participants from whom muscle biopsy samples were obtained
| Characteristic | Participants, n = 15 |
|---|---|
| Age at dosing (range) | 8 months–6 years |
| Dose level of resamirigene bilparvovec (vg/kg) | |
| 1.3 × 1014 | 6/15 |
| 3.5 × 1014 | 9/15 |
| Ventilation support (mean h/day) | |
| Baseline | 22.5 |
| Week 24 | 12.7 |
| Week 48 | 6.7 |
| Biopsy samples available (time point) | |
| Left gastrocnemius (baseline) | 15/15 |
| Right gastrocnemius (week 24) | 15/15 |
| Vastus lateralis (week 48) | 14/15 |
| Transgene MTM1 mRNA levels, median (min-max) | |
| Resamirigene bilparvovec 1.3 × 1014 vg/kg | |
| Baseline | 0 |
| Week 24 | 55.6 (10.2–78.9) |
| Week 48 | 50.2 (6.5–113. 6) |
| Resamirigene bilparvovec 3.5 × 1014 vg/kg | |
| Baseline | 0 |
| Week 24 | 128.2 (9.85–322.1) |
| Week 48 | 90.5 (132.3–304.6) |
Transgene MTM1 mRNA levels are shown as a ratio relative to a reference gene (HEATR6).
Participants were grouped into two groups: A, those demonstrating improvements of ≥6 h/day reduction in ventilator support; and B, those who had <6 h/day reduction in ventilator support at week 24 or week 48 compared to baseline.
ASPIRO was conducted in accordance with International Conference on Harmonisation Good Clinical Practice guidelines, the Declaration of Helsinki, and Clinical Investigation of Medicinal Products in the Pediatric Population guidelines. Study protocols were approved by institutional review boards or ethics committees at each participating institution. Signed informed consent was obtained from the legal guardians of each participant.
RNA sequencing
An exploratory level RNA-seq analysis was conducted as part of the ASPIRO clinical trial. Frozen muscle biopsies were sent to Azenta Life Sciences (115 Corporate Blvd., South Plainfield, NJ, USA) for RNA extraction, RNA-seq library preparation, and sequencing. Total RNA was extracted using QIAGEN AllPrep DNA/RNA/miRNA Universal Kit, and the quality and quantity of RNA were assessed by Nanodrop, Qubit, and TapeStation. RNA-seq libraries were prepared using Illumina TruSeq Stranded mRNA Library Prep Kit with poly A selection and TruSeq RNA Unique Dual Indexes for sample multiplexing. ERCC (External RNA Control Consortium) RNA Spike-In Mixes (Thermo Fisher Scientific) were added during the library preparation to control for various sources of variability. Each sample was sequenced on one lane of an Illumina HiSeq 4000 (2 × 150 base pairs [bp]) to generate at least 300 million reads per sample.
Bioinformatic analyses
Quality control, read alignment, and expression quantification
Read quality was verified using the quality control checks of FastQC (v.0.11.9; http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Raw reads were trimmed for adapters and low-quality tails (quality <Q20) using BBDuk (v.38.82; https://jgi.doe.gov/data-and-tools/bbtools/). In addition, the first 10 nucleotides were force-trimmed for low quality. Reads shorter than 40 bp after trimming were removed. Reads were subsequently aligned to the human reference genome (GRCh38) using STAR13 (v.2.7.3a).
Raw gene counts were obtained in R using the featureCounts function of the Rsubread14 R package (v.2.0.1) and annotated using the UCSC hg38 reference genome. Signal quantification and normalization were carried out in R using the edgeR15 package (v.3.28.1). Raw read counts were normalized using trimmed mean normalization and transformed into counts per million mapped reads and fragments per kilobase per million mapped reads (FPKM). Only genes with >1 counts per million mapped reads in at least a few samples, equal to the lowest number of samples per time point, were retained for downstream analysis. Transgene expression was quantified using GATK’s ASEReadCounter16 (v.4.0.3.0). ASEReadCounter was applied to partition reads matching the transgene and endogenous sequences based on the different untranslated regions between the two. Quantification of MTM1 was reported as the ratio of the normalized transgene or endogenous read counts to the reference gene HEATR6 (HEAT repeat-containing protein 6).
Cell-type enrichment analysis
Cell-type enrichment analysis was performed in R using the xCellAnalysis wrapper of the xCell17 package (v.1.1.0; https://github.com/dviraran/xCell). This method uses an adaptation of the single sample Gene Set Enrichment Analysis (ssGSEA) algorithm and 489 built-in gene signatures generated from several public data sources (e.g., ENCODE, FANTOM5, Blueprint, IRIS, and GEO gene expression datasets) to calculate an enrichment score for each cell-type signature in the samples analyzed. As required by the algorithm, gene expression data were inputted as FPKM, while calibration parameters, spillover, and gene signatures were loaded from the xCell data object. To avoid overcompensation of the spillover compensation step, the analysis was run with a list of cell types that were expected to be in the mixture, such as adipocytes, astrocytes, chondrocytes, endothelial cells, ly (ly-6c) endothelial cells, measles virus endothelial cells, epithelial cells, fibroblasts, hepatocytes, keratinocytes, melanocytes, mesangial cells, myocytes, neurons, osteoblast, pericytes, preadipocytes, sebocytes, skeletal muscle, and smooth muscle. The significance of the enrichment scores (false discovery rate [FDR]-adjusted p value) was calculated using the xCellSignifcanceRandomMatrix function with 100 random permutations of the input data matrix.
Quantification of leukocyte subpopulations
To estimate the fraction of leukocyte subsets potentially present in the muscle biopsies, two deconvolution methods were applied to the gene expression data matrix: estimating the proportion of immune and cancer cells (EPIC)18 and CIBERSORT.19 EPIC was used to compare the level of expression of genes in a sample with a library of gene expression profiles from specific cell types and this information is used to predict the fraction of each cell type in the given sample (https://github.com/GfellerLab/EPIC). The EPIC function was used with cells and gene signatures of the BRef reference, i.e., the reference profiles obtained from immune cell samples of B cells, CD4 T cells, CD8 T cells, monocytes, natural killer cells, and neutrophils purified from PBMC or whole blood. All other cell types with no reference profile were classified under the label other cells. CIBERSORT implements a machine learning (ML) approach, called support vector regression (SVR), which provides the deconvolution of gene expression matrices from bulk mixtures through a combination of feature selection and mathematical optimization techniques. CIBERSORT was run online (http://cibersort.stanford.edu/) with LM22 (immune cell types) as the signature gene file and 1,000 permutations. Both EPIC and CIBERSORT were applied to the gene expression data from all 44 samples, quantified as FPKM values.
Exploratory data analysis
Principal component analysis (PCA) was performed using the function prcomp of the R stats package. PCA plots were generated using the autoplot function of the ggplot2 package. Global unsupervized clustering was performed using the function hclust of the R stats package, with Pearson correlation as distance metric and average agglomeration method. Gene expression heatmaps were generated using the function pheatmap of the R pheatmap package after row-wise standardization of the expression values. Before PCA and unsupervised clustering, genes with a coefficient of variation smaller than the 95th percentile of the coefficient of variation for the entire dataset were removed, to reduce the effect of noise from non-varying genes and retain only highly variable genes.
Differential gene expression analyses
Differential gene expression analysis was performed using the glmQLFTest function of the edgeR15 package (v.3.28.1). Gene expression levels in biopsies taken at the three time points were compared using a paired design to compare two conditions for each participant separately (week 24 vs. baseline, week 48 vs. baseline, week 24 vs. week 48) to exclude baseline differences among the participants. The paired designs were used to fit a generalized linear model and test for the treatment effect with the glmQLFit and glmQLFTest functions of edgeR. The results of our pairwise differential expression analyses were compared with those in canine12 and mouse20 models of the XLMTM phenotype.
Furthermore, at each biopsy time point, differential expression analysis was conducted between the group of participants who demonstrated a ≥6 h/day reduction in the requirement for ventilator support at weeks 24 or 48 compared to baseline and those who did not.
In all comparisons, genes with an FDR-adjusted p ≤ 0.05 and absolute log2-fold change (log2FC) ≥2 were defined as differentially expressed (DE genes).
Functional enrichment analysis
DE genes from the pairwise differential expression analyses were further analyzed for enrichment using the Multiple Gene Lists function of Metascape (https://metascape.org/).21 For each gene list, functional enrichment analysis was carried out using ontologies from KEGG Pathway, GO Biological Processes, Reactome Gene Sets, Hallmark Gene Sets, Canonical Pathways, BioCarta Gene Sets, WikiPathways, and PANTHER Pathway. All genes in the genome were used as the enrichment background.
Gene co-expression network analysis
Gene co-expression network analysis (CNA) was applied to genes and samples to create scale-free networks based on Pearson correlation, using the tool Construction of co-expression Network Analysis-automated (CoCena2; https://github.com/MarieOestreich/hCoCena).22,23 The list of 2,148 unique genes with FDR ≤ 0.05 in at least one of the three comparisons (week 24 vs. baseline, week 48 vs. baseline, week 48 vs. week 24) and the list of 423 unique DE genes with both FDR ≤ 0.05 and absolute log2FC ≥ 2 were used as input. Pearson correlation was performed using the R package Hmisc (v.4.1-1). To increase data quality, only significant correlation values were kept (p < 0.05). The nodes were colored based on the group fold change, the mean under each condition versus the overall mean for each gene, for each group separately. Unbiased clustering was performed by applying all cluster algorithms implemented in the igraph (v.1.2.1) package sequentially 1,000 times. Genes assigned to more than five different clusters during the iterations received no cluster assignment. The mean group fold change expression for each cluster and condition was visualized in the Cluster/Condition heatmap. Clusters smaller than 10 genes were not shown.24
Machine learning
Prior to applying ML, gene selection and normalization were performed using the R packages DaMIRSEq (v.1.2.0) and MLSeq (v.2.2.1). The accuracy of seven different ML algorithms was evaluated prior to selecting the Support Vector Machine (SVM) model. The evaluated algorithms were Ensemble, Random Forest, Support Vector Machines, Naive Bayes, Linear Discriminant Analysis, Logistic Regression, and 3-Nearest Neighbors. SVM was the top performer across all and therefore is presented in the main text. The data were split into training and testing sets (70% for training, 30% for testing) to distinguish participants at baseline, at week 24 after dose, and at week 48 after dose. To prevent the inclusion of redundant features that may decrease the model performance during the classification step, a function that produces a pairwise absolute correlation matrix was applied. The FSelector package was used to rank features. Default parameters were used for all packages.
Results
Participants and biopsy samples
At the time of this analysis, 26 participants had been enrolled in ASPIRO, 24 of whom had received resamirigene bilparvovec. Of those 24 participants, 15 had a total of 44 evaluable muscle biopsy samples available for analysis (Table 1); one participant had muscle biopsy samples available at baseline and week 24 only. Muscle biopsy samples were not evaluable in the remaining participants due to missing samples (COVID-19-related reasons [MIM: 301051]) or due to the poor quality of the samples. Among the 15 participants, six received resamirigene bilparvovec at the 1.3 × 1014 vg/kg dose level, and nine were treated at the 3.5 × 1014 vg/kg dose level.
Quality control and MTM1 assessment
The transcriptomes of 44 evaluable muscle biopsy samples collected at three different timepoints (i.e., baseline and weeks 24 and 48 after dose) were sequenced and analyzed. Quality control assessments on the resulting data were performed, including an evaluation of adipose tissue contamination in the muscle biopsies and RNA degradation (3′ bias). Based on these assessments, four samples (baseline, n = 2; week 24, n = 1; week 48, n = 1) were excluded from the analysis (Figure S2); 40 samples were processed further (baseline, n = 13; week 24, n = 14; week 48, n = 13).
Exploratory analysis using PCA (not shown) and hierarchical unsupervised clustering performed on the gene expression levels of 668 highly variable genes demonstrated that the 40 samples did not separate into any relevant groups based on when they were obtained (baseline, week 24 after dose, or week 48 after dose), the dose of resamirigene bilparvovec administered, or the MTM1 mutation harbored by the participant (Figure 1A). MTM1 expression analysis revealed a statistically significant increase in RNA levels of MTM1 between baseline and weeks 24 and 48 (p < 0.0001 for both), confirming the transduction and expression of the resamirigene bilparvovec-derived transgene (Figure 1B). A dose-dependent effect of resamirigene bilparvovec on MTM1 mRNA levels was observed between low-dose vs. high-dose cohorts (Table 1).
Figure 1.
Quality control assessments and assessment of MTM1
(A) Exploratory analysis of the gene expression dataset. Hierarchical unsupervised clustering, performed on the gene expression levels of 668 highly variable genes, indicated that the 40 samples did not separate into any relevant group based on when they were obtained relative to dosing, the dose of resamirigene bilparvovec given, or the MTM1 mutation harbored by the participant.
(B) MTM1 expression in muscle biopsy samples. MTM1 RNA levels in biopsies at baseline and week 24 and week 48 after the administration of resamirigene bilparvovec (p values from one-way ANOVA test).
Differential gene expression analysis and enrichment
Differential expression analysis was performed on the 40 muscle biopsies (13 baseline, 14 weeks 24, and 13 weeks 48 samples) to identify transcriptomic changes induced by the administration of resamirigene bilparvovec, the results of which are shown in Table 2, Figure 2A, and Tables S1 and S2. A total of 191 genes were differentially expressed in the pairwise comparisons between week 24 and baseline data (68 up- and 123 down-regulated), using a threshold of FDR ≤ 0.05 and absolute log2FC ≥ 2. In the comparison between week 48 and baseline data, 334 DE genes were identified (75 up- and 259 down-regulated), using the same threshold. Interestingly, when comparing data between week 48 and week 24, only five genes were differentially expressed, implying that major transcriptomic changes induced by resamirigene bilparvovec occurred within 24 weeks post-dosing, while only minor changes happened between weeks 24 and 48.
Table 2.
Differentially expressed genes
| Gene expression data | Total DE genes | Upregulated genes | Downregulated genes |
|---|---|---|---|
| Week 24 vs. baseline | 191 | 68 | 123 |
| Week 48 vs. baseline | 334 | 75 | 259 |
| Week 48 vs. week 24 | 5 | 3 | 2 |
Number of differentially expressed (DE) genes in the comparisons of gene expression data from muscle biopsy samples at baseline, week 24, and week 48 after resamirigene bilparvovec dosing.
Figure 2.
Differential expression analysis
(A) Heatmap of gene expression profiles using DE genes from pairwise comparisons of data at week 24 and baseline, week 48 and baseline, and week 48 and week 24. Each column represents one separated biological sample. Genes are clustered using Pearson correlation as distance metric and average agglomeration method after row-wise standardization of the expression values. Samples are ordered according to time of biopsy and sample number.
(B) Heatmap of enriched terms across input gene lists. The heatmap cells are colored based on p values; gray cells indicate a lack of enrichment for that term in the corresponding gene list. DE, differentially expressed; PPARs, peroxisome proliferator-activated receptor; ECM, extracellular matrix.
The DE genes identified in the week 24 vs. baseline or week 48 vs. baseline comparisons were subjected to functional gene enrichment analysis to identify the biological pathways and processes in which the DE genes are involved. Overall, upregulated DE genes were enriched in gene sets related to interferon gamma response, inflammatory response, and myotube differentiation, and downregulated genes were enriched in processes of cell-cell adhesion, extracellular matrix organization and regulation, and muscle tissue development (Figure 2B). A total of 27 genes were commonly upregulated and 78 genes were downregulated at both week 24 and week 48 compared with baseline (Figure S3). The commonly upregulated genes are involved in pathways related to inflammatory/immune response, such as the interferon alpha response and the interferon gamma response pathways. Recently, interferon gamma has been shown to be necessary for efficient skeletal muscle regeneration.25 The 78 downregulated genes are involved in apoptosis and apical junction pathways.
To investigate whether the transcriptomic changes associated with resamirigene bilparvovec correlated with clinical outcomes, we conducted differential expression analysis between participants who had a therapeutic benefit of ≥6 h/day reduction in ventilator support requirements at week 24 compared with baseline and those who did not; we carried out the same analysis using data for week 48 vs. baseline.
At baseline, all 15 participants required ventilator support for ≥12 h/day. At week 24 after resamirigene bilparvovec dosing, four of the 15 participants showed a reduction in ventilator support. At week 48, improvement of ≥6 h/day in ventilator support was achieved in 10 of the 15 participants. Overall reduction in ventilator support requirements was observed among participants who received resamirigene bilparvovec (Table 1). After week 48, all 15 participants achieved ventilator independence (0 h/day of ventilator support; data not shown).
Differential expression analysis at week 24 revealed that a total of 14 genes that were upregulated and 149 genes that were downregulated in participants who had a ventilator dependence improvement of ≥6 h/day compared with participants who had <6 h/day improvement (Figures S4A and S4B; Tables S3 and S4). At week 48, 1 upregulated gene and 1 downregulated gene were identified in participants who had ≥6 h/day reduction in ventilator support compared with those who did not. Gene set enrichment analysis for these comparisons indicated that the allograft rejection, interferon, and inflammatory response pathways were enriched at weeks 24 and 48 in participants who had a therapeutic improvement (≥6 h/day reduction in ventilator support), while angiogenesis, KRAS signaling, and protein secretion pathways were enriched in participants who did not have a therapeutic improvement in ventilator support at week 24 or week 48 (Figure S4C).
Comparisons with canine and mouse models of XLMTM
We compared the results of our pairwise differential expression analyses with those in canine and mouse models of XLMTM. Of the 95 genes identified by Dupont12 as showing a rescued expression profile in XLMTM dogs dosed with a single systemic infusion of rAAV8-cMTM1, 12 were shared within the set of 191 DE genes identified in the pairwise comparison between week 24 and baseline data in the ASPIRO study participants and 14 genes were shared with the 334 DE genes identified in week 48 compared to baseline (Figure S5A). A total of 11 genes were shared between these sets of 12 and 14 genes identified between the canine model and ASPIRO study data. Functional enrichment analyses of these genes showed their involvement in the Hedgehog signaling pathway, which has been shown to regulate angiogenesis and myogenesis during post-natal skeletal muscle regeneration.26
To compare our DE analyses with those reported in mouse models, we took the results from the RNA-seq mouse data generated by Volpatti et al.20 and compared the DE genes resulting from the comparison between Mtm1 knockout vs. wild-type mice20 with the DE genes we identified in the week 24 and week 48 analyses in ASPIRO participants. Several genes were found to be conserved across mouse and clinical study datasets and were similarly upregulated and downregulated (Figure S5B). As an example, five genes, including MTM1, were upregulated both in the wild-type mouse data and at week 24 and 48 in ASPIRO participants. These genes were MTM1, NOS1 (MIM: 163731), MYLK4, TFRC (MIM: 190010), and KCNA7 (MIM: 176268) (for week 24), and MTM1, RGS9BP (MIM: 607814), NOS1 (MIM: 163731), GPT2 (MIM: 138210), and KCNA7 (MIM: 176268) (for week 48). Gene set enrichment analyses showed that these genes were involved in early and late endosome pathways. A total of 19 common genes had similarly low expression in wild-type mice and at week 24 in ASPIRO participants, and 28 genes were downregulated in both wild-type mice and at week 48 in ASPIRO participants. These shared sets of downregulated genes were identified as being involved in apoptosis pathways.
Gene co-expression network analysis (CNA)
Classical approaches to analyze transcriptome data by using differential gene expression analysis based on sample groups defined by time points precluded dissection of the heterogeneity of the XLMTM muscle biopsy samples in this analysis. CNA, on the other hand, identifies regulated genes across samples, and then groups these genes into modules that can be explored for each participant sample individually or for the entire group of participants.27,28
As we found the largest number of DE genes at week 24 vs. baseline and week 48 vs. baseline, we decided to use CoCena223 on the participants’ data grouped by time point (baseline vs. week 24 vs. week 48). We ran two analyses, the first with the 2,148 genes with FDR ≤ 0.05 in all three time point comparisons and the second with the 423 genes obtained as the union of DE genes with both FDR ≤0.05 and absolute log2FC ≥ 2. For both analyses, the pipeline identified seven co-expression modules (Figure 3). Analysis of the modules revealed group-specific enrichment of co-expressed gene modules based on AAV-dosing time points. Functional enrichment analysis on each of the modules identified associated gene signatures displaying distinct functional characteristics (e.g., cellular responses, signaling pathways, etc.; Figure 4). These functional characteristics distinguish between samples before and after resamirigene bilparvovec administration. For example, the modules of upregulated genes after dosing (denoted by dark green, orchid, and steel blue in Figure 3A) were enriched in processes related to metabolite and energy metabolism, while the modules of downregulated genes (denoted by dark orange, gold, and maroon in Figure 3A) showed enrichment in apoptosis signaling, cell motility and adhesion-related processes, glycosylation of proteins, and processes related to collagens and extracellular matrix organization and regulation. Interestingly, the dark gray module (genes exclusively upregulated in week 24 samples, Figure 3A) showed almost homogeneous enrichment in interferon signaling and immune response mechanisms. These co-expression analysis findings agree with those of the functional enrichment analysis of pairwise and group DE genes.
Figure 3.
CoCena2 module detection in co-expression networks
(A) Gene modules identified by different cluster algorithms in the network were constructed using 2,148 unique genes with FDR ≤ 0.05 in all three comparisons (week 24 vs. baseline, week 48 vs. baseline, week 48 vs. week 24). Modules were color-coded for interpretability as dark green, orchid, steel blue, dark green, dark orange, gold, and maroon; colors were randomly assigned. Modules are visualized as clusters in a cluster-condition heatmap obtained using the Group Fold Change, i.e., the mean of each condition versus the overall mean for each gene and for each group separately.
(B) Same as in (A) for the network constructed using 423 unique gene symbols obtained from the union of the DE genes with both FDR ≤ 0.05 and absolute log2FC ≥ 2. Colors were randomly assigned (and differ from those assigned in panel A).
(C) Visualization of the CoCena2 co-expression network constructed as in (A). Network nodes are colored according to their cluster membership in the cluster-condition map and representative cluster-specific transcriptor factors (TF) are noted.
(D) Same as in (C) for the CoCena2 co-expression network constructed as in (B). CAN, co-expression network analysis; CoCena2, Construction of co-expression Network Analysis; DE, differentially expressed; FC, fold change; FDR, false discovery rate.
Figure 4.
Enrichment analysis of CNA clusters
Dot plot of the gene sets from the MSigDB Hallmarks collection most enriched in the clusters identified by the CNA analysis using the 2,148 unique genes with FDR ≤ 0.05 in all three comparisons (week 24 vs. baseline, week 48 vs. baseline, week 48 vs. week 24). The size of the dot is proportional to the GeneRatio and dots are color-coded based on their corresponding adjusted p values. Facet colors correspond to the colors of clusters in Figure 3. Numbers in the cluster labels correspond to the number of gene sets with p ≤ 0.05 in the hypergeometric test. CAN, co-expression network analysis; FDR, false discovery rate.
Machine learning
Based on the results of the DE genes analysis and the clustering of the participants according to co-expressed modules, we used the SVM model (Figure S6) on the transcriptomic data to distinguish between samples taken at baseline, 24 weeks after dosing, and 48 weeks after dosing, with the aim of predicting potential RNA biomarkers of XLMTM evolution following MTM1 gene therapy. The ML algorithm was able to predict baseline vs. week 24/week 48 samples. However, clear distinction between samples taken at week 24 and week 48 could not be made, as was similarly observed in the results of differential expression and co-expression analyses.
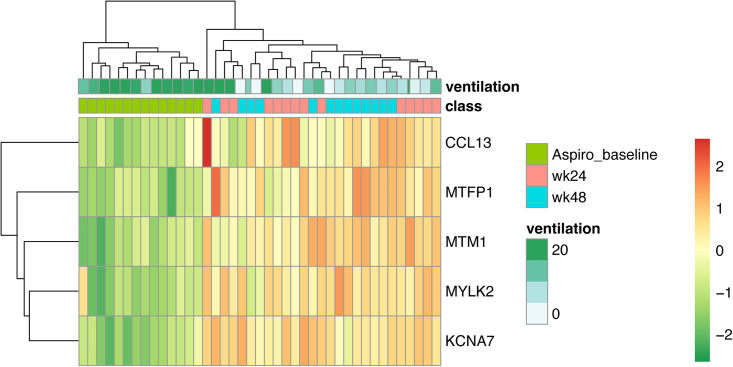
The genes identified by the algorithm as contributing the most to distinguishing muscle biopsy samples taken before and after dosing were KCNA7 (MIM: 176268), MYLK2 (MIM: 606566), MTM1 (MIM: 300415), CCL13 (MIM: 601391), and MTFP1 (MIM: 610235) (Figure 5).
Figure 5.
Hierarchical clustering
Dendrograms and heatmap generated using the expression values of the top predictors selected by FSelector function. Ventilation in hours/day.
Discussion
With the recent success of preclinical studies and clinical trials in gene therapy using AAV vectors,29,30,31 a better understanding of the processes by which disease phenotypes are impacted—and eventually rescued—is of considerable interest in the field of molecular medicine. Based on these successes, clinical trials employing AAV vectors have been initiated for neuromuscular disorders, including XLMTM.32,33 XLMTM is a devastating genetic disease characterized by profound muscle weakness and decreased muscle tone, which are usually evident at birth, and by early death.3,4,5 In addition to severe myopathy, individuals with XLMTM frequently exhibit liver dysfunction, including elevated liver enzymes and intrahepatic cholestasis,6,34,35,36 which is increasingly being recognized as complicating the presentation of the disease.6,37,38 XLMTM is caused by a mutation in MTM1 encoding the protein known as myotubularin.3,4,5 Mutations in MTM1 lead to low levels of functional myotubularin or its absence. Myotubularin is critical for the proper development, maintenance, and function of muscle tissue; however, the exact mechanisms by which it achieves these functions are not yet fully understood. Recent work suggests that myotubularin has a role in the maintenance of muscle structure, including the part of the muscle fiber responsible for excitation-contraction coupling.5
To better understand the transcriptomic changes induced by resamirigene bilparvovec in XLMTM, we conducted RNA-seq on 40 evaluable muscle biopsies (i.e., 13 at baseline, 14 at week 24, and 13 at week 48 samples) taken from 15 participants with XLMTM in ASPIRO. Interestingly, differential expression analysis between time points allowed for the elucidation of upregulated genes enriched in lipid metabolism processes and inflammatory response related to presence of MTM1 expression following gene therapy with resamirigene bilparvovec, while downregulated genes were enriched in cellular responses related to cell-cell adhesion, extracellular matrix organization and regulation, and muscle tissue development (Figure 2B). Moreover, gene set enrichment analysis on the significantly differentially expressed genes based on ventilator dependence data (Figure S4C) indicated an elevated expression of genes involved in myogenesis in week-24 biopsies, as well as an enrichment of genes related to the interferon pathways in biopsies obtained at week 24 and week 48. Conserved genes/pathways across mice and canine XLMTM models and individuals with XLMTM were also identified. Further research into these shared genes and pathways will help to identify key genes involved in muscle restoration after MTM1 transgene expression.
Alongside these molecular findings, MTM1 replacement therapy with resamirigene bilparvovec reduced ventilator dependence in our cohort of 15 boys with XLMTM (Table 1), with all achieving ventilator independence (i.e., 0 h/day ventilator support) by week 48. The latest results from the full ASPIRO study population show that 16 of 20 evaluable participants have achieved and maintained ventilator independence at a mean follow-up duration of 2.5 years after dose.39 Functional outcomes following resamirigene bilparvovec in the ASPIRO study also included significant improvements in respiratory muscle strength and motor function compared with untreated control subjects. Treated participants acquired and maintained several major motor milestones that were not achievable before dosing, including the ability to sit independently for >30 s and in some, the ability to walk independently.39 Collectively, these findings support the concept that MTM1 transfer has a direct beneficial effect on skeletal muscle function. Although our biopsy samples were limited to those obtainable from leg muscles of the study participants, we hypothesize that resamirigene bilparvovec also results in improvement in muscle function of the diaphragm. However, further research will be needed to establish and validate any firm correlations between observed clinical outcomes and the molecular effects of resamirigene bilparvovec in the associated muscles.
As classical approaches to analyze the transcriptome precluded dissection of the heterogeneity of the disease, we conducted a CNA. Using either DE genes with FDR ≤ 0.05 and absolute log2FC ≥ 2 or genes with FDR ≤0.05 only in the three comparisons, CNA detected clusters and specific gene signatures for each cluster related to up- or down-regulation in muscle biopsy samples from resamirigene bilparvovec-treated individuals at week 24 and week 48 compared with baseline. Pathways inferred from the functional enrichment analysis on the identified modules are detailed in Figure 4. These results suggest that changes in the transcriptional profile of muscle cells induced by resamirigene bilparvovec gene therapy are present at the earliest observation (week 24) and remain mostly unchanged until 48 weeks. However, it is possible that these findings are confounded by the inherent variability in transcriptomic profiles among different muscles in the body40 and the reduced power of the sample size (e.g., fewer biopsy samples were collected due to COVID-19-related restrictions). Interestingly, a few modules showed upregulation at week 24 and downregulation or a return to baseline at week 48 (e.g., orchid, steel blue, and dark gray modules in Figure 3A), suggesting a clear evolution of treatment response over time that might underly the continued improvement in participants beyond week 48.
Finally, using an ML approach we were able to extract genes that further distinguished baseline biopsy samples from those at weeks 24 and week 48. Importantly, the ML model identified MTM1, the gene associated with XLMTM, among the five genes contributing the most to distinguishing between samples taken before and after dosing (Figure 5). We hypothesized that there might be a relationship between XLMTM disease severity and the RNA levels of KCNA7, MYLK2, CCL13, and MTFP1. As MTM1 protein expression level does not reflect the severity of XLMTM phenotypes and no definitive studies have been performed to correlate disease severity with mutations in subdomains of the MTM1,41 the genes identified by this ML approach may prove to be informative in the study of XLMTM pathogenesis. Indeed, the other four genes identified by the model have been previously implicated in myopathies or immune responses. For example, MTFP1 is upregulated in dog models of XLMTM treated with AAV (rAAV8-cMTM1) compared with wild-type dogs.12 MTFP1 is a mitochondrial protein and downstream target of the phosphatidylinositol 3-kinase signaling pathway (see PIK3CA [MIM: 171834]) that plays a role in cell viability and mitochondrial dynamics.42 KCNA7 (Potassium Voltage-Gated Channel Subfamily A Member 7) is a protein-coding gene expressed preferentially in skeletal muscle, heart, and kidney and implicated in progressive familial heart block, photosensitive epilepsy, and ion channel muscle diseases.43,44 MYLK2 (Myosin Light-Chain Kinase 2) is a protein -oding gene associated with cardiomyopathy (MIM: 192600).45 Among its related pathways are the calcium signaling pathway and immune response CCR3 signaling in eosinophils. CCL13/MCP-4 is a CC family chemokine expressed in muscle tissues (Protein Atlas: https://www.proteinatlas.org/ENSG00000181374-CCL13) that is chemoattractant for eosinophils, basophils, monocytes, macrophages, immature dendritic cells, and T cells and can induce crucial immune-modulatory responses through its effects on epithelial, muscular, and endothelial cells. Recent studies have shown that skeletal muscle regeneration is highly dependent on the inflammatory response. A wide variety of innate and adaptive immune cells orchestrate the complex process of muscle regeneration.46 A number of studies have shown that CCL13 is involved in many chronic inflammatory diseases,47,48 in which it functions as a pivotal molecule in the selective recruitment of cell lineages to the inflamed tissues and their subsequent activation.47 Therefore, these results suggest the potential downstream events following AAV gene therapy that could help to explain the multifactorial, heterogeneous, and systemic nature of XLMTM. RNA biomarkers, such as those identified in this study, could also help to identify protein biomarkers known to be secreted in the blood, which would make monitoring the evolution of XLMTM after gene therapy easier.
The strengths of this study include the rich clinical data and undertaking one of the most extensive XLMTM RNA-seq profiling exercises conducted before and after gene therapy, which allowed us to identify transcriptomic effects of AAV dosing, shedding light on molecular mediators of disease heterogeneity. Although there are a few publicly available gene expression profiles of individuals with XLMTM, many of them are not annotated with ventilation support data and/or are from individuals who have not been treated with AAV gene therapy. Future research will include testing these findings in other study cohorts and expanding this work beyond transcriptomics to include a multi-omic approach. There are a few limitations that should be recognized. As the majority of participants within our cohort showed improvement in ventilator dependance following gene therapy treatment, it was challenging to identify transcriptomic differences between participants demonstrating therapeutic improvements and those who did not. Moreover, the study was limited by the use of biopsy samples from different muscles (vastus lateralis at week 48 and gastrocnemius muscle at baseline and week 24), which might increase variability, and from defined tissue areas at specific time points, which might not represent fully what happens in the whole tissue. The study was also limited by the absence of healthy control samples for comparison.
In summary, we present data from the largest cohort of gene therapy-treated individuals with XLMTM described to date who underwent RNA-seq. Participants in our cohort showed a marked improvement in functional outcome (i.e., reduced ventilator dependence) through 48 weeks after resamirigene bilparvovec administration. Having access to such a large amount of data is of the utmost importance to allow a comprehensive description and understanding of the disease. Further work to interrogate cross-platform expression data will be crucial to characterizing individuals with XLMTM as responsive and less-responsive to gene replacement therapy and to characterize at a molecular level the inflammatory and immune pathways that we observed from the transcriptomic data.
Acknowledgments
This work was supported by Astellas Gene Therapies (formerly Audentes Therapeutics). S.B. has a sponsored research agreement with Astellas Gene Therapies (formerly Audentes Therapeutics). The authors are grateful to all participants and their families for participating in the ASPIRO clinical trial.
Author contributions
Conceptualization and study design: S.B., F.U., F.M., M.J.S.-D., and H.-J.C. Data curation: G.A., O.R., A.G., and N.L. Data analysis: G.A., O.R., and A.G. Data analysis and interpretation: all authors. Critical review and revision of the manuscript: all authors.
Declaration of interests
G.A., P.P., F.V., and F.U. were formerly employees and/or stockholders of Astellas Gene Therapies (formerly known as Audentes Therapeutics). H.-J.C., M.J.S.-D., and N.L. are employees and/or stockholders of Astellas Gene Therapies (formerly known as Audentes Therapeutics). C.C. is a consultant for Astellas Gene Therapies (formerly known as Audentes Therapeutics). F.M. was formerly the senior vice president of translational science at Audentes Therapeutics. S.B. has a sponsored research agreement with Astellas Gene Therapies (formerly Audentes Therapeutics).
Published: September 5, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2023.08.008.
Contributor Information
Gaia Andreoletti, Email: andreolettigaia@gmail.com.
Fabrizia Urbinati, Email: urbinati@stanford.edu.
Supplemental information
Data and code availability
-
•
The code generated during this study are available at GitHub: https://github.com/gaiaandreoletti/XLMTM_paper
-
•
Researchers may request access to anonymized data supporting the current study at AGT_medinfo@astellas.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx
References
- 1.Laporte J., Guiraud-Chaumeil C., Tanner S.M., Blondeau F., Hu L.J., Vicaire S., Liechti-Gallati S., Mandel J.L. Genomic organization of the MTM1 gene implicated in X-linked myotubular myopathy. Eur. J. Hum. Genet. 1998;6:325–330. doi: 10.1038/sj.ejhg.5200189. [DOI] [PubMed] [Google Scholar]
- 2.Sacks N.C., Healey B.E., Cyr P.L., Slocomb T., James E., Beggs A.H., Graham R.J. Costs and health resource use in patients with X-linked myotubular myopathy: insights from US commercial claims. J. Manag. Care Spec. Pharm. 2021;27:1019–1026. doi: 10.18553/jmcp.2021.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laporte J., Blondeau F., Buj-Bello A., Mandel J.L. The myotubularin family: from genetic disease to phosphoinositide metabolism. Trends Genet. 2001;17:221–228. doi: 10.1016/s0168-9525(01)02245-4. [DOI] [PubMed] [Google Scholar]
- 4.Dowling J.J., Lawlor M.W., Das S. In: GeneReviews((R)) Adam M.P., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J.H., Gripp K.W., Amemiya A., editors. 1993. X-Linked Myotubular Myopathy. [PubMed] [Google Scholar]
- 5.Amburgey K., Tsuchiya E., de Chastonay S., Glueck M., Alverez R., Nguyen C.T., Rutkowski A., Hornyak J., Beggs A.H., Dowling J.J. A natural history study of X-linked myotubular myopathy. Neurology. 2017;89:1355–1364. doi: 10.1212/WNL.0000000000004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowling J.J., Müller-Felber W., Smith B.K., Bönnemann C.G., Kuntz N.L., Muntoni F., Servais L., Alfano L.N., Beggs A.H., Bilder D.A., et al. INCEPTUS Natural History, Run-in Study for Gene Replacement Clinical Trial in X-Linked Myotubular Myopathy. J. Neuromuscul. Dis. 2022;9:503–516. doi: 10.3233/JND-210781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beggs A.H., Byrne B.J., De Chastonay S., Haselkorn T., Hughes I., James E.S., Kuntz N.L., Simon J., Swanson L.C., Yang M.L., et al. A multicenter, retrospective medical record review of X-linked myotubular myopathy: The RECENSUS study. Muscle Nerve. 2018;57:550–560. doi: 10.1002/mus.26018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham R.J., Muntoni F., Hughes I., Yum S.W., Kuntz N.L., Yang M.L., Byrne B.J., Prasad S., Alvarez R., Genetti C.A., et al. Mortality and respiratory support in X-linked myotubular myopathy: a RECENSUS retrospective analysis. Arch. Dis. Child. 2020;105:332–338. doi: 10.1136/archdischild-2019-317910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Childers M.K., Joubert R., Poulard K., Moal C., Grange R.W., Doering J.A., Lawlor M.W., Rider B.E., Jamet T., Danièle N., et al. Gene therapy prolongs survival and restores function in murine and canine models of myotubular myopathy. Sci. Transl. Med. 2014;6:220ra10. doi: 10.1126/scitranslmed.3007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buj-Bello A., Fougerousse F., Schwab Y., Messaddeq N., Spehner D., Pierson C.R., Durand M., Kretz C., Danos O., Douar A.M., et al. AAV-mediated intramuscular delivery of myotubularin corrects the myotubular myopathy phenotype in targeted murine muscle and suggests a function in plasma membrane homeostasis. Hum. Mol. Genet. 2008;17:2132–2143. doi: 10.1093/hmg/ddn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mack D.L., Poulard K., Goddard M.A., Latournerie V., Snyder J.M., Grange R.W., Elverman M.R., Denard J., Veron P., Buscara L., et al. Systemic AAV8-mediated gene therapy drives whole-body correction of myotubular myopathy in dogs. Mol. Ther. 2017;25:839–854. doi: 10.1016/j.ymthe.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupont J.B., Guo J., Renaud-Gabardos E., Poulard K., Latournerie V., Lawlor M.W., Grange R.W., Gray J.T., Buj-Bello A., Childers M.K., Mack D.L. AAV-mediated gene transfer restores a normal muscle transcriptome in a canine model of X-linked myotubular myopathy. Mol. Ther. 2020;28:382–393. doi: 10.1016/j.ymthe.2019.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Y., Smyth G.K., Shi W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019;47:e47. doi: 10.1093/nar/gkz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castel S.E., Levy-Moonshine A., Mohammadi P., Banks E., Lappalainen T. Tools and best practices for data processing in allelic expression analysis. Genome Biol. 2015;16:195. doi: 10.1186/s13059-015-0762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aran D., Hu Z., Butte A.J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017;18:220. doi: 10.1186/s13059-017-1349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Racle J., Gfeller D. EPIC: A Tool to Estimate the Proportions of Different Cell Types from Bulk Gene Expression Data. Methods Mol. Biol. 2020;2120:233–248. doi: 10.1007/978-1-0716-0327-7_17. [DOI] [PubMed] [Google Scholar]
- 19.Newman A.M., Liu C.L., Green M.R., Gentles A.J., Feng W., Xu Y., Hoang C.D., Diehn M., Alizadeh A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volpatti J.R., Ghahramani-Seno M.M., Mansat M., Sabha N., Sarikaya E., Goodman S.J., Chater-Diehl E., Celik A., Pannia E., Froment C., et al. X-linked myotubular myopathy is associated with epigenetic alterations and is ameliorated by HDAC inhibition. Acta Neuropathol. 2022;144:537–563. doi: 10.1007/s00401-022-02468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y., Zhou B., Pache L., Chang M., Khodabakhshi A.H., Tanaseichuk O., Benner C., Chanda S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019;10:1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreoletti G., Lanata C.M., Trupin L., Paranjpe I., Jain T.S., Nititham J., Taylor K.E., Combes A.J., Maliskova L., Ye C.J., et al. Transcriptomic analysis of immune cells in a multi-ethnic cohort of systemic lupus erythematosus patients identifies ethnicity- and disease-specific expression signatures. Commun. Biol. 2021;4:488. doi: 10.1038/s42003-021-02000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oestreich M., Holsten L., Agrawal S., Dahm K., Koch P., Jin H., Becker M., Ulas T. hCoCena: horizontal integration and analysis of transcriptomics datasets. Bioinformatics. 2022;38:4727–4734. doi: 10.1093/bioinformatics/btac589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuit S., Salvagno C., Kapellos T.S., Hau C.S., Seep L., Oestreich M., Klee K., de Visser K.E., Ulas T., Schultze J.L. Transcriptional Signature Derived from Murine Tumor-Associated Macrophages Correlates with Poor Outcome in Breast Cancer Patients. Cell Rep. 2019;29:1221–1235.e5. doi: 10.1016/j.celrep.2019.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng M., Nguyen M.H., Fantuzzi G., Koh T.J. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 2008;294:C1183–C1191. doi: 10.1152/ajpcell.00568.2007. [DOI] [PubMed] [Google Scholar]
- 26.Straface G., Aprahamian T., Flex A., Gaetani E., Biscetti F., Smith R.C., Pecorini G., Pola E., Angelini F., Stigliano E., et al. Sonic hedgehog regulates angiogenesis and myogenesis during post-natal skeletal muscle regeneration. J. Cell Mol. Med. 2009;13:2424–2435. doi: 10.1111/j.1582-4934.2008.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banchereau R., Hong S., Cantarel B., Baldwin N., Baisch J., Edens M., Cepika A.M., Acs P., Turner J., Anguiano E., et al. Personalized Immunomonitoring Uncovers Molecular Networks that Stratify Lupus Patients. Cell. 2016;165:551–565. doi: 10.1016/j.cell.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aschenbrenner A.C., Mouktaroudi M., Krämer B., Oestreich M., Antonakos N., Nuesch-Germano M., Gkizeli K., Bonaguro L., Reusch N., Baßler K., et al. Disease severity-specific neutrophil signatures in blood transcriptomes stratify COVID-19 patients. Genome Med. 2021;13:7. doi: 10.1186/s13073-020-00823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller C., Flotte T.R. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- 30.Chu W.S., Ng J. Immunomodulation in Administration of rAAV: Preclinical and Clinical Adjuvant Pharmacotherapies. Front. Immunol. 2021;12:658038. doi: 10.3389/fimmu.2021.658038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell S., Bennett J., Wellman J.A., Chung D.C., Yu Z.F., Tillman A., Wittes J., Pappas J., Elci O., McCague S., et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hajba L., Guttman A. Recent Advances in the Analysis Full/Empty Capsid Ratio and Genome Integrity of Adeno-associated Virus (AAV) Gene Delivery Vectors. Curr. Mol. Med. 2020;20:806–813. doi: 10.2174/1566524020999200730181042. [DOI] [PubMed] [Google Scholar]
- 34.D'Amico A., Longo A., Fattori F., Tosi M., Bosco L., Chiarini Testa M.B., Paglietti M.G., Cherchi C., Carlesi A., Mizzoni I., Bertini E. Hepatobiliary disease in XLMTM: a common comorbidity with potential impact on treatment strategies. Orphanet J. Rare Dis. 2021;16:425. doi: 10.1186/s13023-021-02055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neese J.M., Yum S., Matesanz S., Raffini L.J., Whitworth H.B., Loomes K.M., Mayer O.H., Alcamo A.M. Intracranial hemorrhage secondary to vitamin K deficiency in X-linked myotubular myopathy. Neuromuscul. Disord. 2021;31:651–655. doi: 10.1016/j.nmd.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Gangfuss A., Schmitt D., Roos A., Braun F., Annoussamy M., Servais L., Schara-Schmidt U. Diagnosing X-linked myotubular myopathy - A German 20-year follow up experience. J. Neuromuscul. Dis. 2021;8:79–90. doi: 10.3233/jnd-200539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herman G.E., Kopacz K., Zhao W., Mills P.L., Metzenberg A., Das S. Characterization of mutations in fifty North American patients with X-linked myotubular myopathy. Hum. Mutat. 2002;19:114–121. doi: 10.1002/humu.10033. [DOI] [PubMed] [Google Scholar]
- 38.Molera C., Sarishvili T., Nascimento A., Rtskhiladze I., Muñoz Bartolo G., Fernández Cebrián S., Valverde Fernández J., Muñoz Cabello B., Graham R.J., Miller W., et al. Intrahepatic cholestasis is a clinically significant feature associated with natural history of X-linked myotubular myopathy (XLMTM): A case series and biopsy report. J. Neuromuscul. Dis. 2022;9:73–82. doi: 10.3233/JND-210712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shieh P., Kuntz N., Dowling J., Müller-Felber W., Blaschek A., Bönnemann C., Foley R., Saade D., Seferian A., Servais L., et al. O.06 Long term outcomes for X-Linked myotubular Myopathy (XLMTM) with gene replacement therapy, resamirigene bilparvovec: Preliminary results from ASPIRO. Neuromuscul. Disord. 2022;32:S44. doi: 10.1016/j.nmd.2022.07.012. [DOI] [Google Scholar]
- 40.Terry E.E., Zhang X., Hoffmann C., Hughes L.D., Lewis S.A., Li J., Wallace M.J., Riley L.A., Douglas C.M., Gutierrez-Monreal M.A., et al. Transcriptional profiling reveals extraordinary diversity among skeletal muscle tissues. Elife. 2018;7:e34613. doi: 10.7554/eLife.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawlor M.W., Pierson C.R. In: Pathobiology of Human Disease. McManus L.M., Mitchell R.N., editors. Elsevier; 2014. Congenital Myopathies; pp. 195–209. [Google Scholar]
- 42.Tondera D., Santel A., Schwarzer R., Dames S., Giese K., Klippel A., Kaufmann J. Knockdown of MTP18, a novel phosphatidylinositol 3-kinase-dependent protein, affects mitochondrial morphology and induces apoptosis. J. Biol. Chem. 2004;279:31544–31555. doi: 10.1074/jbc.M404704200. [DOI] [PubMed] [Google Scholar]
- 43.Abath Neto O., Tassy O., Biancalana V., Zanoteli E., Pourquié O., Laporte J. Integrative data mining highlights candidate genes for monogenic myopathies. PLoS One. 2014;9:e110888. doi: 10.1371/journal.pone.0110888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nerbonne J.M., Nichols C.G., Schwarz T.L., Escande D. Genetic manipulation of cardiac K(+) channel function in mice: what have we learned, and where do we go from here? Circ. Res. 2001;89:944–956. doi: 10.1161/hh2301.100349. [DOI] [PubMed] [Google Scholar]
- 45.Davis J.S., Hassanzadeh S., Winitsky S., Lin H., Satorius C., Vemuri R., Aletras A.H., Wen H., Epstein N.D. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001;107:631–641. doi: 10.1016/s0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 46.Ziemkiewicz N., Hilliard G., Pullen N.A., Garg K. The Role of Innate and Adaptive Immune Cells in Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2021;22:3265. doi: 10.3390/ijms22063265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mendez-Enriquez E., García-Zepeda E.A. The multiple faces of CCL13 in immunity and inflammation. Inflammopharmacology. 2013;21:397–406. doi: 10.1007/s10787-013-0177-5. [DOI] [PubMed] [Google Scholar]
- 48.Han J.H., Suh C.H., Jung J.Y., Nam J.Y., Kwon J.E., Yim H., Kim H.A. Association of CXCL10 and CXCL13 levels with disease activity and cutaneous manifestation in active adult-onset Still's disease. Arthritis Res. Ther. 2015;17:260. doi: 10.1186/s13075-015-0773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The code generated during this study are available at GitHub: https://github.com/gaiaandreoletti/XLMTM_paper
-
•
Researchers may request access to anonymized data supporting the current study at AGT_medinfo@astellas.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx