Summary
The World Health Organization (WHO) South East Asia Region (SEAR) comprises 11 countries, which are one of the most culturally, topographically, and socially diverse areas worldwide, undergoing an epidemiological transition towards non-communicable diseases, including stroke and other cardiovascular diseases (CVDs). This region accounts for over 40% of the global stroke mortality. Few well-designed population-based epidemiological studies on stroke are available from SEAR countries, with considerable variations among them. Ischemic stroke, a common stroke subtype, has higher frequencies of intracerebral hemorrhage in many countries. Along with an aging population, the increased prevalence of risk factors such as hypertension, diabetes mellitus, tobacco and alcohol consumption, lack of physical activity, high ambient pollution, heat, and humidity contribute to the high burden of stroke in this region. SEAR's many unique and uncommon stroke etiologies include cerebral venous thrombosis, tuberculosis, dengue, scrub typhus, falciparum malaria, snake bite, scorpion sting, etc. Current data on stroke burden and risk factors is lacking, compelling an urgent need for high-quality hospital-level and population-level data in all SEAR countries. Strategies towards a consolidated approach for implementing improved stroke prevention measures, stroke surveillance, and established stroke systems of care are the path to bridging the gaps in stroke care.
Keywords: Stroke burden, Stroke risk factors, Unique etiologies
Introduction
Stroke continues to be one of the leading healthcare concerns globally. In 2019, it was the second leading cause of mortality and the third leading cause of mortality and morbidity combined.1 The past three decades have witnessed an increase in the total number of stroke-related disability-adjusted life years (DALYs) due to risk factors. There is significant heterogeneity in the distribution of stroke worldwide, with the bulk of the burden being borne by low- and middle-income countries (LMICs). According to the Global Burden of Disease (GBD) 2019 data, while both stroke-related mortality and DALYs showed a decrease in high-income countries (HICs), low-income countries (LICs) and /LMICs reflected substantial increases in these variables.1
The southeastern part of Asia, called the Southeast Asian region, comprises 11 member countries, namely Bangladesh, Bhutan, India, Indonesia, Maldives, Myanmar, Nepal, North Korea, Sri Lanka, Thailand, and Timor Leste, which come under the umbrella of the World Health Organization's South East Asia Region (SEAR) countries. Comprising three of the most highly populated countries of the world, the SEAR is one of the most culturally, topographically, and socially diverse areas worldwide. Like the rest of the world, this region is witnessing an epidemiological shift towards non-communicable diseases, including stroke and other cardiovascular diseases (CVDs). This region accounts for more than 40% of the global stroke mortality.2 In addition to an aging population, the increased prevalence of risk factors such as hypertension, diabetes, tobacco and alcohol consumption, lack of physical activity, high ambient pollution, and heat and humidity contribute to the high stroke burden in this region.1 These countries have higher rates of hemorrhagic strokes, cerebral venous thromboses, and intracranial atherosclerosis compared to HICs.3 In addition, many uncommon etiologies of stroke, such as tropical infections and toxins rarely seen in the Western world, are ubiquitous to this region.4
Although there exists some epidemiological data from different countries of the SEAR, these are primarily from hospital-based studies with wide variations in methodology and data analysis. The available literature reflects the paucity of well-designed population-based studies and the absence of established stroke surveillance systems and registries from this region. Moreover, there is a lack of overarching region-wide comprehensive data on the incidence and prevalence of stroke and stroke-related mortality and disability across the entire SEAR.5 Considering this, we conducted this review to summarize the available literature on the stroke burden in SEAR and identify future research priorities. This three-part series discusses the epidemiology of stroke and its risk factors, current stroke care systems, challenges, and opportunities for improvement in the SEAR countries.
Epidemiology
Incidence and prevalence
Data on stroke prevalence is more readily available than on stroke incidence, reflecting the lack of population-based data in the region. Most of the information available from different countries is based on hospital-based case series, with significant heterogeneity in methodologies, age-band inclusions, and data analysis techniques, making comparison between studies difficult. The GBD 2019 data estimates an overall incidence (crude numbers) of 1,328,397 and a prevalence of 10,423,817 per year in the SEAR. The highest incidence and prevalence are reported in India (1,291,245 and 9,650,716), followed by Indonesia (642,943 and 4,918,487) and Bangladesh (182,856 and 1,417,979). The highest rates of age-standardized stroke incidence, 293.3/100,000 (262.3–331.6), was reported from Indonesia in 2019.1 Another recent systematic review from India quoted crude incidence ranging from 108 to 172 per 100,000 people per year, crude prevalence from 26 to 757 per 100,000 people per year and one-month case fatality rates between 18 and 42%.6 Bhutan and Maldives report the lowest incidence and prevalence (576 and 4767) and (414 and 4108, crude numbers), respectively. Overall stroke-related mortality in the SEAR was 731,833, with the highest in Indonesia (331,349), followed by Myanmar (82,399) and Bangladesh (158,806).1 The INTERSTROKE study showed an overall case fatality rate of 15.3% and 12.3% in South and Southeast Asia, respectively.7 High stroke death rates in these countries could be due to variations in stroke incidence, severity, and quality of stroke care services. The number of DALYs lost due to stroke is the best index of stroke burden. Based on the GBD 2019 study,1 the DALYs following stroke was the highest in India (Total DALYs lost 17,332,326) followed by Indonesia (8,407,229) and Bangladesh (3,493,100), indicating the high burden of possibly more severe strokes and a lack of much-needed rehabilitation services in this region.
Stroke types and subtypes
Information on stroke types and subtypes is available from most countries based predominantly on findings from hospital-based studies. Acute ischemic stroke accounts for 80–85% of all strokes compared to hemorrhagic stroke, constituting around 20% of the stroke burden.3 Based on the GBD 2019 data, ischemic stroke was the most common type of stroke in the SEAR, with an incidence of 6.9/1000, followed by intracerebral hemorrhage (5.4/1000) and subarachnoid hemorrhage (0.93/1000). A multicenter study from Indonesia similarly reported a higher frequency of ischemic strokes (67.1%) than ICH (29.6%) in the country.8 However, a higher frequency of hemorrhagic strokes (19–46%) has been reported from many countries in Southeast Asia when compared to the data from the HICs.9,10 This may indicate the high prevalence and poor management of hypertension in these regions. Regional, cultural, and dietary variations, such as increased salt and saturated fats use in these countries, could also be contributory. Sampling bias related to hospital-based studies must also be considered while understanding these estimates.
Small vessel disease contributes largely to ischemic stroke etiology in the SEAR than in other regions and is probably due to the high prevalence of hypertension. Studies from India and Indonesia have reported hypertension prevalence of 19.9% and 26.7% among adults, respectively.9,11 Another characteristic finding is the higher prevalence of intracranial atherosclerotic disease, ranging from 20 to 53%, accounting for the high rates of recurrent stroke in the region. A 10-year-long hospital-based registry from South India documented large artery atherosclerosis (LAA) as the most common ischemic stroke subtype, with intracranial atherosclerosis accounting for 78.3% of the LAA subtype.9 This contrastingly differs from Western literature, where extracranial LAA dominates the LAA subtype of ischemic stroke mechanisms.
Furthermore, Cardioembolic strokes (CE) are less reported in this region compared to North American and European literature, where CE etiology is one of the most common causes of stroke. The Hyderabad stroke registry reports only 11% of CE strokes, while a study from Indonesia reported 2.1% of the strokes due to CE in their multicentric cohort.9 The Indo-US collaborative stroke project reported 29.9% of large-artery and almost 25% of cardiac mechanisms of stroke in their cohort, although only 4% of patients had atrial fibrillation.12 It is crucial to consider the differences in approach to duration, frequency, and extensiveness of long-term electrocardiographic monitoring in many of these regions while perceiving these estimates.13
Stroke risk factors
Age is an essential non-modifiable risk factor for stroke. According to the INTERSTROKE study, the mean age of stroke was 62.2 years overall compared to 59.6 and 56.6 in South and Southeast Asia, respectively.7 The proportion of young strokes (≤45 years) was also higher in these regions (15.8% and 18.3% patients, respectively) compared to 11.8% overall. About 61.6% of the total stroke DALYs were attributable to various risk factors such as high blood pressure, high body mass index, high blood glucose, high LDL cholesterol, dietary intake, alcohol use, smoking, low physical activity, air pollution, and low ambient temperature. Hypertension was the most common risk factor in Indonesia (69%), Timor Leste (60%), and Sri Lanka (56%). Dietary habits that contributed to the risk factors were seen in Timor Leste, Thailand, and Bangladesh populations. Low physical activity was seen among populations from Bhutan (1.9%), Maldives (1.7%), and Sri Lanka (1.4%).1 Recently, ambient particulate matter pollution was identified as a risk factor for stroke, with the highest PAR in Bangladesh (40.2%), followed by India (25.5%) and Thailand (22.3%), while household air pollution from solid fuels was seen in Timor Leste (32.6%), Myanmar (26.5%) and Nepal (23.2%). Low ambient temperature as a risk factor was seen in Bhutan (11.3%) and Nepal (9%). Smoking and second-hand smoke were high among populations from Maldives and Indonesia. Finally, cardiac causes were one of the critical risk factors in SEAR, which could be related to the high prevalence of rheumatic heart disease in some of these regions.7
Not many studies on genetic risk factors from the SEAR are available in the literature. An association was found between the risk of stroke and the single nucleotide polymorphisms (SNPs) SNP41 and SNP56 of a novel phosphodiesterase 4D (PDE4D) from a study in India.14 A meta-analysis of ischemic stroke from India, Sri Lanka, Bangladesh, and Pakistan revealed four genetic polymorphisms in PDE4D, ACE I/D, IL10, and MTHFR genes to have significant risk association with ischemic stroke in this cohort.15 Prevalence of high ApoE polymorphisms has also been shown to contribute to the high incidence of intracerebral hemorrhage in this region. We have summarized the prevalence of stroke risk factors and stroke sub-types from the SEAR in Table 1.
Table 1.
Shows the prevalence of stroke subtypes and risk factors in SEAR.
| Countries/Characteristics | Ischemic | Haemorrhagic | Risk factors |
|---|---|---|---|
| India | (46.5–84.8%)16 | 11.5–35%16 | Hypertension (30%), diabetes, dyslipidemia, tobacco use.16 |
| Nepal | 63%17 | 37%17 | Hypertension (29.6), diabetes mellitus (10.3), hypercholesterolemia (4.3), insufficient physical activity (4.1), obesity (3.7), smoking (24.1)18 |
| Bangladesh | 79.7%19,20 | ICH 15.7%19 SAH 4.6% |
Hypertension (79.2%), followed by dyslipidemia (38.9%), tobacco use in any form (37.2%), diabetes (28.8%), and ischemic heart disease (20.1%).19 uncontrolled hypertension, diabetes mellitus, smoking habit, and dyslipidemia.20 |
| Sri Lanka | 85.7%21 | 15.3%21 | IHD, BMI >23 kg/m2, history of TIA and arrhythmias, hypertension22 Hypertension (52–59%), diabetes mellitus (29–42%), dyslipidaemia (18–40%) and smoking (25–33%)23 |
| Bhutan | NA | NA | Hypertension (57%), diabetes mellitus (17.7%), smoking and substance abuse <0.5%.24 |
| Maldives | NA | NA | dietary risks, high blood pressure, and high body-mass index1 |
| Indonesia | 42.9%8 | 19.5%8 (SAH 1.4, ICH 18.5) | Hypertension (36–42%), diabetes mellitus, processed food consumption, salted fish consumption, insufficient physical activity (23.7%), cigarette smoking among men (76.2%)25 |
| Thailand | 80%26 | 20%26 | Hypertension (73.4%), diabetes, dyslipidemia, metabolic syndrome, and atrial fibrillation (40% of ischemic strokes)26 |
| Timor Leste | NA | NA | Hypertension, diabetes, dyslipidemia, obesity19 |
| North Korea | 60.9%27 | 24.1%27 (SAH 15%) | Hypertension, diabetes, dyslipidemia, obesity19 |
| Myanmar | 93.7%28 | 2.8%28 (CVT 3.6%) | Diabetes, hypertension, consumption of salty food, high usage of monosodium glutamate |
Unique etiological mechanisms of stroke in the SEAR
Many unique and uncommon etiologies of stroke, rarely seen in the HICs, are encountered in the SEAR. These include Cerebral venous thrombosis (CVT), tropical infections, toxins, and several other rare associations such as hookah (waterpipe) smoking and arsenic in well water. There are many unanswered questions regarding the causation, pathophysiology, and management of stroke due to these uncommon causes.
Cerebral venous thrombosis (CVT)
Cerebral venous thrombosis (CVT) is a rare type of stroke predominantly seen in the younger population and is traditionally thought to have a strong female predilection. There is a lack of population-based incidence surveys related to CVT from SEAR, and most of the literature from the region is from hospital-based registries and case series.
The first epidemiological study of hemiplegia due to stroke in South India by Abraham et al. showed that most strokes in young women occurred in the puerperal period.29 Other studies showed CVT occurring in 4.5/1000 obstetric admissions and contributing to 0.10% of all hospital admissions.30,31 The Asian Study of Cerebral Venous Thrombosis showed a lower age when compared to the Western CVT cases (Fig. 1).32 This is reflected in most Indian data with a mean age in the early 30s.33,34 However, in patients from Nepal and Thailand patients, the mean age was in the late 30s and early 40s.35,36
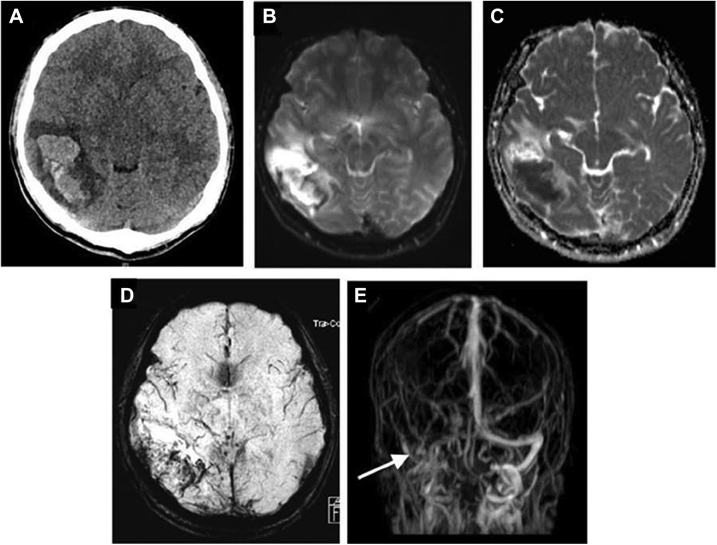
Fig. 1.
37-year-old gentleman who presented with acute onset incoherent speech and new onset headache of 1 day duration along with 1 episode of generalized tonic clonic seizures. There was a history of binge intake of alcohol prior to the episode. Non-contrast CT head reveals a hemorrhagic infarct in right temporoparietal region (A). MRI brain (B–E) shows heterogenous hyperdensity in the right temporoparietal region with hypodense areas. MR venogram (E) demonstrating lack of flow in the right transverse sinus.
Although CVT is traditionally a condition predominantly known to affect women, recent European studies show changes in this pattern.37 A few large studies from SEAR also show a similar trend with CVT cases in men outnumbering women.31,32,34 This declining shift in gender predilection over the last two decades in the SEAR seems to be mainly driven by decreased puerperal CVT cases, which constituted the majority of female CVTs in earlier studies.31,33 This could reflect the improved maternal health and mortality in the region over the years.38 Contrasting to the data from HICs where oral contraceptive pill (OCP) use is a major risk factor for CVT, in Asia, the puerperal period continues to be the leading risk factor for female CVT patients.32, 39 However, recent data from certain SEAR regions show that the use of OCPs is emerging to be a major contributor35 (Table 2).
Table 2.
Shows the papers on Unique etiologies of Stroke in SEAR.
| Author | Title | Limitations of the study |
|---|---|---|
| Cerebral venous thrombosis | ||
| Abraham J, Rao PS, Inbaraj SG, Shetty G, Jose CJ. | An epidemiological study of hemiplegia due to stroke in South India | In this epidemiological survey, suspected stroke patients were identified through interviews and history by trained field workers. A possible underestimation of the actual number of stroke cases. The etiological classification is not available |
| Bansal BC, Gupta RR, Prakash C. | Stroke during pregnancy and puerperium in young females below the age of 40 years as a result of cerebral venous/venous sinus thrombosis. | This is a case-control study with a small sample size. |
| Aaron S, Lakshmanan J, Sudarsanam TD, et al. | Cerebral venous thrombosis, seasonal trends, and climatic influence: a region-specific study. | This is a single-center observational study and thus cannot be generalized |
| Wasay M, Kaul S, Menon B, et al. | Asian study of cerebral venous thrombosis. | In this study there was a lack of central imaging review, nonuniformity of work-up, and a large number of dropouts at follow-up are major limitations of our study. CVT in children, though common in the region was not studied. |
| Narayan D, Kaul S, Ravishankar K, et al. | Risk factors, clinical profile, and long-term outcome of 428 patients of cerebral sinus venous thrombosis: insights from Nizam’s Institute Venous Stroke Registry, Hyderabad (India) | This is a registry from a single center and thus the findings cannot be representative of a larger, more diverse population |
| Kalita J, Chandra S, Kumar B, Bansal V, Misra UK. | Cerebral venous sinus thrombosis from a Tertiary Care Teaching Hospital in India | This is a single-center observational study with a small sample size. |
| Poungvarin N, Prayoonwiwat N, Ratanakorn D, et al. | Thai venous stroke prognostic score: TV-SPSS | This is a validation study of a venous stroke prognostic scoring tool |
| Ruuskanen JO, Kytö V, Posti JP, Rautava P, Sipilä JOT. | Cerebral venous thrombosis: Finnish nationwide trends | This is a retrospective study. Patient-level data on risk factors are unavailable. Main discharge diagnosis codes in the registry have been proven valid, but the coding of co-diagnoses is not as complete. |
| Aaron S, Lakshmanan J, Sudarsanam TD, et al. | Cerebral venous thrombosis, seasonal trends, and climatic influence: a region-specific study. | The data does not represent the true incidence or prevalence of CVT in this geographical area. |
| Aaron S, Alexander M, Maya T, et al. | Underlying prothrombotic states in pregnancy associated cerebral venous thrombosis | This study does not have a control group for the evaluation of thrombotic markers. |
| Baby P. | Traditional practice of fluid restriction among patients with puerperium associated cerebral venous thrombosis in rural India | The findings of this study do not ascertain causality between the restriction of fluids and CVT. |
| Das S, Chattopadhyay S, Munsi K, Basu S. | Scrub typhus with cerebral venous sinus thrombosis: a rare presentation. | This is a case report of a rare presentation and thus is not representative of this geographical area |
| Jena SS, Mathew A, Sanjith A, Ajith S, Nair BR, Prakash J. | Cerebral venous sinus thrombosis presentation in severe scrub typhus infection: a rare entity | This is a letter to the editor and is a case report of a rare presentation |
| Krishnan A, Karnad DR, Limaye U, Siddharth W. | Cerebral venous and dural sinus thrombosis in severe falciparum malaria | This is a case series reporting only three patients |
| Niyasom S, Sithinamsuwan P, Udommongkol C, Suwantamee J. | Dural sinus thrombosis in melioidosis: the first case report. | This is a case report and cannot be generalized to the geographic area |
| Korathanakhun P, Petpichetchian W, Sathirapanya P, Geater SL. | Cerebral venous thrombosis: comparing characteristics of infective and non-infective aetiologies: a 12-year retrospective study | This is a single-center study, with the possibility of selection bias towards severe cases. Patient follow-up was just until hospital discharge and thus, there was inadequate data to compare the long-term clinical outcome of infection-associated and non-infection-associated CVT. The retrospective nature of the study also resulted in a non-uniform method of neuroimaging studies. |
| Panagariya A, Maru A. | Cerebral venous thrombosis in pregnancy and puerperium–a prospective study | This is an observational study with a small sample size. |
| Soni N, Kumar S, Shimle A, Ora M, Bathla G, Mishra P. | Cerebrovascular complications in tuberculous meningitis-A magnetic resonance imaging study in 90 patients from a tertiary care hospital. | This is a retrospective study, with observations or selection bias with data from a tertiary care hospital, the absence of any control group, no matching CT evaluation, and the inclusion of descriptive statistics. |
| Tuberculosis | ||
| Fugate JE, Lyons JL, Thakur KT, Smith BR, Hedley-Whyte ET, Mateen FJ. | Infectious causes of stroke | This study emphasizes neurosyphilis and has limited information on stroke etiologies. |
| Wasay M, Khan M, Farooq S, et al. | Frequency and impact of cerebral infarctions in patients with tuberculous meningitis | This is a retrospective observational study. Brain images/angiography is not available in all patients. |
| Hsieh FY, Chia LG, Shen WC. | Locations of cerebral infarctions in tuberculous meningitis | This is an observational study with a small sample size and is thus not representative of the geography. |
| Tai MLS, Viswanathan S, Rahmat K, et al. | Cerebral infarction pattern in tuberculous meningitis | This is a descriptive study, and the findings cannot be extrapolated in other populations. Moreover, only descriptive statistics and chi-square analysis were performed. Another limitation was the absence of a control group. A case-control study may be useful. |
| Dengue | ||
| Sahu R, Verma R, Jain A, et al. | Neurologic complications in dengue virus infection: a prospective cohort study. | This a hospital-based enrolment of participants; patients with asymptomatic or mild dengue fever may not have reported to the health center. |
| Ngwe Tun MM, Muthugala R, Nabeshima T, et al. | Unusual, neurological and severe dengue manifestations during the outbreak in Sri Lanka, 2017 | This study describes all outcomes of dengue and is not stroke-specific |
| Araújo FMC, Araújo MS, Nogueira RMR et al. | Central nervous system involvement in dengue: a study in fatal cases from a dengue endemic area. | This study was not stroke-specific. Cerebro-spinal fluid (CSF), tissue, and blood samples were used to study the outcomes. |
| Kulkarni R, Pujari S, Gupta D. | Neurological manifestations of dengue fever. | All patients with dengue were not investigated and data was not recorded prospectively in a standard proforma. This might have led to some missing or inadequate information such as dengue CSF PCR in those with encephalitis. |
| Carod-Artal FJ, Wichmann O, et al. | Neurological complications of dengue virus infection. | This is not stroke-specific |
| Mathew S, Pandian JD. | Stroke in patients with dengue. | This study was a small case series reporting stroke in a small population of 3 patients |
| Sam JE, Gee TS, Nasser AW. | Deadly intracranial bleed in patients with dengue fever: a series of nine patients and review of literature | This is a small series study. |
| Basnayake BWMKE, Somaratne KGSK, Goonetilleke CU, Tilakaratna PMYI, Ranawaka UK. | Case report: dengue hemorrhagic fever with ischemic stroke | This is a case report. |
| Neurocysticercosis | ||
| Del Brutto OH. | Cysticercosis and cerebrovascular disease: a review | This is an old systematic review paper |
| Snakebite | ||
| Al-Sadawi M, Mohamadpour M, Zhyvotovska A, et al. | Cerebrovascular accident and snake envenomation: a scoping study. | This is a small series study. |
| Gawarammana I, Mendis S, Jeganathan K. | Acute ischemic strokes due to bites by Daboia russelii in Sri Lanka–first authenticated case series | This is not stroke-specific |
| Huang YK, Chen YC, Liu CC, Cheng HC, Tu AT, Chang KC. | Cerebral complications of snakebite envenoming: case studies | This is not stroke-specific |
| Scorpion sting | ||
| Bouaziz M, Bahloul M, Kallel H, et al. | Epidemiological, clinical characteristics and outcome of severe scorpion envenomation in South Tunisia: multivariate analysis of 951 cases. | This is a retrospective study with incomplete or inconsistent information. All patients with scorpion stings were not recruited. |
| Sarkar S, Bhattacharya P, Paswan A. | Cerebrovascular manifestations and alteration of coagulation profile in scorpion sting: a case series | In this study, vasospasm could not be proved by angiography. Protein C, Protein S, and antithrombin III estimation is not available. |
| Udayakumar N, Rajendiran C, Srinivasan AV. | Cerebrovascular manifestations in scorpion sting: a case series | The study is a case series and has a small sample size |
| Godoy DA, Badenes R, Seifi S, et al. | Neurological and systemic manifestations of severe scorpion envenomation | The study covers all neurological manifestations but not stroke-specific. |
| Squatting | ||
| Chakrabarti SD, Ganguly R, Chatterjee SK, Chakravarty A. | Squatting, blood pressure and stroke | The study is a case-control study with a small sample size. |
Some CVT risk factors may be unique for the SEAR. Fluid restriction during puerperium is a typical traditional practice in south India, especially in rural areas, and can be a modifiable risk factor for CVT in post-partum mothers.31,40 Anemia is another modifiable risk factor unique to CVT cohorts from the SEAR.32,33 High Serum homocysteine levels and alcohol consumption are other modifiable risks noted in many studies from the SEAR (Fig. 2).
Fig. 2.
Shows common risk factors for cerebral venous sinus thrombosis in Southeast Asiaregion.
Scrub typhus caused by Orientia tsutsugamushi is endemic in the SEAR Region, which falls in the tsutsugamushi triangle. In recent years, CVT as a complication of scrub typhus infection has been reported from the SEAR region with increasing frequency.41,42
In addition, complications of other infective diseases endemic to the SEAR, such as falciparum malaria and meliodosis presenting as CVT, have been reported.43,44 Higher rates of infection associated with CVT (24.1%) have been reported from Thailand.45 Increase in the number of CVTs associated with higher ambient temperatures has been reported from South India, with consistent trends over 18 years.31
Consistent with global literature, the mortality associated with CVT has steadily decreased with better imaging modalities for early diagnoses and better treatment facilities in the SEAR. While earlier cohorts reported mortality rates around 18%,46,47 recent publications from the SEAR have shown a reduction in mortality, with some series reporting mortality rates even below 10%.33,36
Tuberculosis
Tuberculosis (TB) remains a significant health problem in the SEAR. Two-thirds of the global caseload of TB is clustered in eight countries, and four of them (India, Indonesia, Pakistan, and Bangladesh) are in the SEAR. The region accounted for 45% of the new cases of TB worldwide.48 Ischemic strokes are seen in 15–60% of cases with TB meningitis, and multiple infarcts are common.49,50 Leptomeningeal exudates encasing the walls of arteries and veins in the basal cisterns lead to endarteritis and resultant infarction. In addition, a hypercoagulable state is also thought to contribute.4 Majority of infarcts are seen in the supratentorial region and subcortical regions.49,50 Infarcts are thought to be predominantly (in up to 75%) located in a so-called 'tubercular zone' involving the head of the caudate nucleus, anteromedial thalamus, and anterior limb and genu of the internal capsule (Fig. 3).4,51 However, more recent MRI-based studies reveal that the 'ischaemic zone' of the lentiform nucleus, posterolateral thalamus, and posterior limb of the internal capsule is more likely to be involved.49,52 Older age and the presence of vascular risk factors are predictors of infarction in TBM.50 There is insufficient data on the benefits of steroid or antiplatelet treatment for preventing infarcts in TBM. What is more apparent is that the presence of cerebral infarction in patients with TBM is associated with higher mortality (up to 35%) and more severe disability (mRS 4–5 in 39%).49
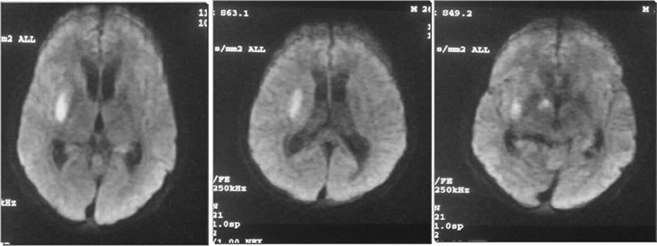
Fig. 3.
26-year-old male who presented with acute onset of inability to speak, left hemiparesis of 6 h duration with drowsiness. There was a history of loss of weight and appetite with generalized weakness of 2 months. MRI Brain revealed an area of true restriction in the right internal capsule of diffusion weighted images suggestive of an infarct. Patient underwent CSF examination which showed pleocytosis with 800 white blood cells (polymorphs 65%, lymphocytes 35%), raised proteins (120 mgs) and very low sugar levels (40 mgs). A TB PCR for Mycobacterium Tuberculosis was found positive on the CSF. He was further evaluated for an immunocompromised state and was found to be positive for HIV by Elisa and Western Blot assays.
Dengue
The SEAR contributed to more than half the global burden of dengue, and the case load in the region increased by 46% from 2015 to 2019.53 Several neurological manifestations are well recognized in dengue infection, but stroke is a rare complication. Stroke was not reported in several large case series detailing neurological involvement in dengue.54, 55, 56 Published literature is limited to isolated case reports and small case series, and available data suggest that stroke occurs in 0.14–0.26% of patients with dengue.57,58 Both ischemic stroke and different types of intracranial hemorrhage (subarachnoid, subdural, intraparenchymal) have been reported.
The pathophysiology and causative mechanisms of stroke in dengue are poorly understood. Increased capillary permeability, plasma leakage, platelet dysfunction, thrombocytopenia, coagulopathy, and vasculopathy are thought to be the underlying pathogenic mechanisms for hemorrhagic stroke.59,60 Postulated mechanisms of ischemic stroke include meningo-vasculitis and a transient hypercoagulable state following plasma leakage.61 The possibility of dengue in the setting of ischemic stroke needs to be considered in endemic regions as treatment options such as thrombolysis and antiplatelet treatment could lead to serious consequences with underlying dengue-related thrombocytopenia.61
Neurocysticercosis
Cysticercosis is a zoonotic parasitic infection with the pork tapeworm Taenia solium prevalent in several SEAR countries. Neurocysticercosis occurs due to the presence of the larval forms (cysticerci) in the brain parenchyma, subarachnoid space, or ventricles, leading to local inflammation and resultant leptomeningeal exudates.4 Inflammation leads to endarteritis, which can affect both large and small arteries and result in ischaemic stroke, intraparenchymal hemorrhage, and subarachnoid hemorrhage.4 Ischaemic stroke is reported in 4–12% of patients with neurocysticercosis and is commoner with subarachnoid neurocysticercosis (21–53%).4 Lacunar syndrome due to small vessel occlusion is thought to be the most common type of stroke.62
Snakebite
Snakebites are a common health problem in the SEAR region, and stroke is a rare complication, usually seen with vipers and elapids. In a scoping review of 83 cases of snakebite-associated stroke, 77% were ischaemic strokes, and 21% were intracranial hemorrhages; two-thirds were aged <50 years, mean delay to symptom onset was 24 h after snakebite, and mortality was 16.9%.63 Russell viper (Daboia russelli) is the most common snake species associated with stroke.63 In a study of ∼500 cases of Russell viper bites, nine ischaemic strokes and one ICH were reported.64
The underlying pathophysiologic mechanisms remain unclear. Due to Russell viper, multiple medium to large vessel territories were involved in infarctions, suggesting thrombosis as the possible underlying mechanism. Interestingly, watershed infarctions were not seen, which may result from hypotension due to hemorrhage, vasodilatation, or shock.64 Chemical compounds with prothrombotic properties in snake venom, such as snake C-type lectin-like proteins, SVSPs (snake-venom serine proteases), and PLA2 (phospholipase A2), are believed to cause ischaemic stroke and procoagulant metalloproteases and hemorrhaging to lead to consumptive coagulopathy and ICH.64,65
Scorpion sting
Scorpions are considered the most primitive arachnids in existence. Scorpion stings are a rare cause of stroke. In a Tunisian study of 951 cases of severe scorpion envenomation, 78% had neurological complications, but stroke was not reported.66 However, in two prospective studies from India, strokes were seen in 3/42 patients and 4/50 patients.67,68 Strokes are more commonly seen following stings by the Indian red scorpion (Mesobuthus tamulus).69 Majority of reported cases are ischaemic strokes, and multiple infarcts are common. The underlying pathophysiological mechanisms remain unclear, but possible mechanisms include cardiac embolism secondary to arrhythmias and myocardial dysfunction, hypertensive surge following autonomic storm, cerebral vasospasm, endothelial dysfunction, thrombosis and disseminated intravascular coagulation.69
Squatting
A low-seating toilet, which requires a squatting posture, is still used in many LMICs. A possible association of squatting and straining while defecating with a rise in blood pressure has been postulated as an underlying mechanism for stroke.70 An Indian study evaluated the clinical significance of this mechanism in 100 CT-proven stroke patients by correlating stroke onset with time, place, and activity and reported that more than 50% of strokes in their cohort occurred during early morning hours, with over a third of them while in toilets.
The lack of published high-quality incidence, prevalence, and mortality data from this region may have limited our findings. In addition, most of the available literature from this region primarily represents urban areas or tertiary care centers. With a substantial proportion of the population in these countries inhabiting rural areas and the almost complete lack of rural data from the region, the epidemiological data reported may still be an underrepresentation. Although multi-national interaction and inputs from regional experts were utilized to supplement this deficiency where possible, these values may be the tip of the iceberg. Lastly, the searches were conducted only in 3 search engines; no registries, paid databases, or national databanks were searched, which may have limited the comprehensiveness of the findings.
Conclusions
Like other LMICs, the SEAR has witnessed increased stroke burden, mortality, and disability, with a significant rise in modifiable risk factors such as hypertension and dyslipidemia over the past two decades. There is a compelling need for high-quality hospital-level and population-level data, with comprehensive representation from rural regions. Distinctive etiologies less commonly encountered in the developed world, such as infections, snakebites, and scorpion stings, are specific to this region and contribute substantially to the stroke burden. The underlying mechanisms of cerebral venous thrombosis and infective etiologies such as tuberculosis and dengue towards stroke causation need to be considered while managing such patients. Contextualized interventions should include unique etiologies for each country while developing prevention strategies and improving the stroke care system in the region to reduce morbidity, mortality, and residual disability.
Contributors
JDP conceptualized the review; DBCG, RJI, PJV, NSC conducted literature search, data collection and analysis; JDP, IAS supervised data collection, compiled and wrote the first draft of the manuscript; MVP, SR, YVK wrote the section on burden and risk factors; SA, UKR wrote on the unique etiologies; NV, SA, MVP, UKR, IAS, SR, YVK, JDP revised the draft critically for important intellectual content. All authors have read and approved the final version of the manuscript.
Search strategy and selection criteria.
Literature searches were conducted in PubMed, Web of Science and Google Scholar databases for articles published between January 1, 1997 and December 31, 2022, describing epidemiology of stroke and its risk factors in South East Asia Region (SEAR) Countries. We used the search terms “Stroke” OR “Cerebrovascular disease” OR “Cerebrovascular Accident” OR “Acute Stroke” OR “Ischemic Stroke” OR “Intracerebral Hemorrhage” OR “stroke incidence” OR “stroke prevalence” OR “stroke mortality” OR “stroke disability” OR “stroke epidemiology” OR “stroke burden” OR “stroke risk factors” OR “cardiovascular risk factors” OR “stroke etiology”. Additional searches were also conducted to include articles describing effects of COVID-19 pandemic or vaccination on stroke from the SEAR countries. The search was restricted to South-East Asian region countries by using the Boolean operator AND with one of the following search terms: “South East Asia Region”, “SEAR”, “Bhutan”, “Bangladesh”, “India”, “Indonesia”, “Maldives”, “Myanmar”, “Nepal”, “North Korea”, “Sri Lanka”, “Thailand”, “Timor Leste”. Articles published in language other than English, or other than reviews or original research were excluded. Additional studies were identified through other sources including experts working in these countries and a hand search of search engines. A total of 4863 articles were identified, of which 388 met the screening criteria. Among these, 89 articles provided information on current burden and epidemiology of stroke, risk factors and etiologies specific to the SEAR which were included in the final analysis.
Declaration of interests
The authors declare no competing interests.
References
- 1.Feigin V.L., Stark B.A., Johnson C.O., et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulshreshtha A., Anderson L.M., Goyal A., Keenan N.L. Stroke in South Asia: a systematic review of epidemiologic literature from 1980 to 2010. Neuroepidemiology. 2012;38(3):123–129. doi: 10.1159/000336230. [DOI] [PubMed] [Google Scholar]
- 3.Ng J.C., Churojana A., Pongpech S., et al. Current state of acute stroke care in Southeast Asian countries. Interv Neuroradiol. 2019;25(3):291–296. doi: 10.1177/1591019918811804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fugate J.E., Lyons J.L., Thakur K.T., Smith B.R., Hedley-Whyte E.T., Mateen F.J. Infectious causes of stroke. Lancet Infect Dis. 2014;14(9):869–880. doi: 10.1016/S1473-3099(14)70755-8. [DOI] [PubMed] [Google Scholar]
- 5.Yadav J.K., Nepal G., Shing Y.K., Banerji R.R., Gajurel B.P. An opportunity to improve acute ischemic stroke care in the South Asian region through telestroke services. Ann Med Surg. 2021;72 doi: 10.1016/j.amsu.2021.103115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones S.P., Baqai K., Clegg A., et al. Stroke in India: a systematic review of the incidence, prevalence, and case fatality. Int J Stroke Soc. 2022;17(2):132–140. doi: 10.1177/17474930211027834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Donnell M.J., Chin S.L., Rangarajan S., et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388(10046):761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 8.Kusuma Y., Venketasubramanian N., Kiemas L.S., Misbach J. Burden of stroke in Indonesia. Int J Stroke. 2009;4(5):379–380. doi: 10.1111/j.1747-4949.2009.00326.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaul S., Alladi S., Jabeen S.A., et al. Intracranial atherosclerosis is the most common stroke subtype: ten-year data from hyderabad stroke registry (India) Ann Indian Acad Neurol. 2018;21(3):209–213. doi: 10.4103/aian.AIAN_86_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohammad Q.D., Habib M., Mondal B.A., et al. Stroke in Bangladeshi patients and risk factor. Mymensingh Med J. 2014;23(3):520–529. [PubMed] [Google Scholar]
- 11.Venketasubramanian N., Yudiarto F.L., Tugasworo D. Stroke burden and stroke services in Indonesia. Cerebrovasc Dis Extra. 2022;12(1):53–57. doi: 10.1159/000524161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sylaja P.N., Pandian J.D., Kaul S., et al. Ischemic stroke profile, risk factors, and outcomes in India: the Indo-US Collaborative Stroke project. Stroke. 2018;49(1):219–222. doi: 10.1161/STROKEAHA.117.018700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsanos A.H., Bhole R., Frogoudaki A., et al. The value of transesophageal echocardiography for embolic strokes of undetermined source. Neurology. 2016;87(10):988–995. doi: 10.1212/WNL.0000000000003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munshi A., Roy S., Thangaraj K., Kaul S., Babu M.S., Jyothy A. Association of SNP41, SNP56 and a novel SNP in PDE4D gene with stroke and its subtypes. Gene. 2012;506(1):31–35. doi: 10.1016/j.gene.2012.06.079. [DOI] [PubMed] [Google Scholar]
- 15.Yadav S., Hasan N., Marjot T., et al. Detailed analysis of gene polymorphisms associated with ischemic stroke in South Asians. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0057305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.https://ncdirindia.org/all_reports/pbsrbook/resources/Factsheet.pdf
- 17.Shaik M., Keat Wei L., Gan S. Burden of stroke in Nepal. Int J Stroke. 2012;7:517–520. doi: 10.1111/j.1747-4949.2012.00799.x. [DOI] [PubMed] [Google Scholar]
- 18.Venketasubramanian N., Yoon B.W., Pandian J., Navarro J.C. Stroke epidemiology in South, East, and South-East Asia: a review. J Stroke. 2017;19(3):286–294. doi: 10.5853/jos.2017.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondal M.B.A., Hasan A.T.M.H., Khan N., Mohammad Q.D. Prevalence and risk factors of stroke in Bangladesh: a nationwide population-based survey. eNeurologicalSci. 2022;28 doi: 10.1016/j.ensci.2022.100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahidullah M., Sultana N., Dey S.K., Ahmed A., Ali S., Raknuzzaman M. Risk factors associated with various subtypes of ischemic stroke: a prospective study in BSMMU, Dhaka, Bangladesh. Neurosci Med. 2021;12(4):114–125. [Google Scholar]
- 21.Chang T., Gajasinghe S., Arambepola C. Prevalence of stroke and its risk factors in Urban Sri Lanka. Stroke. 2015;46(10):2965–2968. doi: 10.1161/STROKEAHA.115.010203. [DOI] [PubMed] [Google Scholar]
- 22.Ambawatte S.B., Weerathunga D.N., Dissanayake A., Somaratne S.C., Athukorala K., Wijewickrama P.S.A. Ischemic stroke subtypes: socio-demographic factors, risk factors, and outcomes in Southern Sri Lanka. Ethn Dis. 2021;31(4):509–518. doi: 10.18865/ed.31.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranawaka U.K., Venketasubramanian N. Stroke in Sri Lanka: how can we minimise the burden? Cerebrovasc Dis Extra. 2021;11(1):46–48. doi: 10.1159/000515890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Stroke Organization Burden and outcome of stroke in Bhutan. https://www.world-stroke.org/news-and-blog/blogs/burden-and-outcome-of-stroke-in-bhutan [cited 2023 June 20]
- 25.Setyopranoto I., Bayuangga H.F., Panggabean A.S., et al. Prevalence of stroke and associated risk factors in Sleman district of Yogyakarta Special Region, Indonesia. Stroke Res Treat. 2019;2019 doi: 10.1155/2019/2642458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suwanwela N.C. Stroke epidemiology in Thailand. J Stroke. 2014;16(1):1–7. doi: 10.5853/jos.2014.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung C.S., Park K.Y. Stroke care in Korea including North Korea. Int J Stroke. 2006;1(2):116–117. doi: 10.1111/j.1747-4949.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- 28.Ohnmar O., Kyaw M., Shwe Z.M., et al. The pattern and burden of neurological disorders: a systemic review of Neurology Department, Yangon General Hospital, Myanmar. Neurol Asia. 2020;25(4):555. [Google Scholar]
- 29.Abraham J., Rao P.S., Inbaraj S.G., Shetty G., Jose C.J. An epidemiological study of hemiplegia due to stroke in South India. Stroke. 1970;1(6):477–481. doi: 10.1161/01.str.1.6.477. [DOI] [PubMed] [Google Scholar]
- 30.Bansal B.C., Gupta R.R., Prakash C. Stroke during pregnancy and puerperium in young females below the age of 40 years as a result of cerebral venous/venous sinus thrombosis. Jpn Heart J. 1980;21(2):171–183. doi: 10.1536/ihj.21.171. [DOI] [PubMed] [Google Scholar]
- 31.Aaron S., Lakshmanan J., Sudarsanam T.D., et al. Cerebral venous thrombosis, seasonal trends, and climatic influence: a region-specific study. Ann Indian Acad Neurol. 2020;23(4):522–527. doi: 10.4103/aian.AIAN_409_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wasay M., Kaul S., Menon B., et al. Asian study of cerebral venous thrombosis. J Stroke Cerebrovasc Dis. 2019;28(10) doi: 10.1016/j.jstrokecerebrovasdis.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Narayan D., Kaul S., Ravishankar K., et al. Risk factors, clinical profile, and long-term outcome of 428 patients of cerebral sinus venous thrombosis: insights from Nizam’s Institute Venous Stroke Registry, Hyderabad (India) Neurol India. 2012;60(2):154–159. doi: 10.4103/0028-3886.96388. [DOI] [PubMed] [Google Scholar]
- 34.Kalita J., Chandra S., Kumar B., Bansal V., Misra U.K. Cerebral venous sinus thrombosis from a tertiary care teaching hospital in India. Neurologist. 2016;21(3):35–38. doi: 10.1097/NRL.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 35.Joshi R., Chaturbedi A., Shrestha P., Banjade U., Subedi R., Devkota U. Cerebral venous sinus thrombosis in a Tertiary Care Center in Nepal. Nepal J Neurosci. 2013;10:49–55. [Google Scholar]
- 36.Poungvarin N., Prayoonwiwat N., Ratanakorn D., et al. Thai venous stroke prognostic score: TV-SPSS. J Med Assoc Thai. 2009;92(11):1413–1422. [PubMed] [Google Scholar]
- 37.Ruuskanen J.O., Kytö V., Posti J.P., Rautava P., Sipilä J.O.T. Cerebral venous thrombosis: Finnish nationwide trends. Stroke. 2021;52(1):335–338. doi: 10.1161/STROKEAHA.120.031026. [DOI] [PubMed] [Google Scholar]
- 38.Registrar General of India (RGI) 2022. Status of IMR and MMR in India.https://pib.gov.in/pib.gov.in/Pressreleaseshare.aspx?PRID=1796436 [Google Scholar]
- 39.Aaron S., Alexander M., Maya T., et al. Underlying prothrombotic states in pregnancy associated cerebral venous thrombosis. Neurol India. 2010;58(4):555–559. doi: 10.4103/0028-3886.68676. [DOI] [PubMed] [Google Scholar]
- 40.Baby P. Traditional practice of fluid restriction among patients with puerperium associated cerebral venous thrombosis in rural India. Int J Med Public Health. 2021;11(1):38–39. [Google Scholar]
- 41.Das S., Chattopadhyay S., Munsi K., Basu S. Scrub typhus with cerebral venous sinus thrombosis: a rare presentation. BMJ Case Rep. 2021;14(4) doi: 10.1136/bcr-2020-241401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jena S.S., Mathew A., Sanjith A., Ajith S., Nair B.R., Prakash J. Cerebral venous sinus thrombosis presentation in severe scrub typhus infection: a rare entity. Neurol India. 2014;62(3):308–310. doi: 10.4103/0028-3886.136991. [DOI] [PubMed] [Google Scholar]
- 43.Krishnan A., Karnad D.R., Limaye U., Siddharth W. Cerebral venous and dural sinus thrombosis in severe falciparum malaria. J Infect. 2004;48(1):86–90. doi: 10.1016/s0163-4453(03)00130-0. [DOI] [PubMed] [Google Scholar]
- 44.Niyasom S., Sithinamsuwan P., Udommongkol C., Suwantamee J. Dural sinus thrombosis in melioidosis: the first case report. J Med Assoc Thai. 2006;89(2):242–247. [PubMed] [Google Scholar]
- 45.Korathanakhun P., Petpichetchian W., Sathirapanya P., Geater S.L. Cerebral venous thrombosis: comparing characteristics of infective and non-infective aetiologies: a 12-year retrospective study. Postgrad Med J. 2015;91(1082):670–674. doi: 10.1136/postgradmedj-2015-133592. [DOI] [PubMed] [Google Scholar]
- 46.Panagariya A., Maru A. Cerebral venous thrombosis in pregnancy and puerperium--a prospective study. J Assoc Physicians India. 1997;45(11):857–859. [PubMed] [Google Scholar]
- 47.Nagaraja D., Haridas T., Taly A.B., et al. Puerperal cerebral venous thrombosis: therapeutic benefit of low dose heparin. Neurol India. 1999;47(1):43–46. [PubMed] [Google Scholar]
- 48.World Health Organisation . 2022. Global tuberculosis report.https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 [Google Scholar]
- 49.Soni N., Kumar S., Shimle A., Ora M., Bathla G., Mishra P. Cerebrovascular complications in tuberculous meningitis-A magnetic resonance imaging study in 90 patients from a tertiary care hospital. NeuroRadiol J. 2020;33(1):3–16. doi: 10.1177/1971400919881188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wasay M., Khan M., Farooq S., et al. Frequency and impact of cerebral infarctions in patients with tuberculous meningitis. Stroke. 2018;49(10):2288–2293. doi: 10.1161/STROKEAHA.118.021301. [DOI] [PubMed] [Google Scholar]
- 51.Hsieh F.Y., Chia L.G., Shen W.C. Locations of cerebral infarctions in tuberculous meningitis. Neuroradiology. 1992;34(3):197–199. doi: 10.1007/BF00596334. [DOI] [PubMed] [Google Scholar]
- 52.Tai M.L.S., Viswanathan S., Rahmat K., et al. Cerebral infarction pattern in tuberculous meningitis. Sci Rep. 2016;6 doi: 10.1038/srep38802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organisation . 2023. Dengue and severe dengue.https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue [Google Scholar]
- 54.Sahu R., Verma R., Jain A., et al. Neurologic complications in dengue virus infection: a prospective cohort study. Neurology. 2014;83(18):1601–1609. doi: 10.1212/WNL.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 55.Ngwe Tun M.M., Muthugala R., Nabeshima T., et al. Unusual, neurological, and severe dengue manifestations during the outbreak in Sri Lanka, 2017. J Clin Virol. 2020;125 doi: 10.1016/j.jcv.2020.104304. [DOI] [PubMed] [Google Scholar]
- 56.Araújo F.M.C., Araújo M.S., Nogueira R.M.R., et al. Central nervous system involvement in dengue: a study in fatal cases from a dengue endemic area. Neurology. 2012;78(10):736–742. doi: 10.1212/WNL.0b013e31824b94e9. [DOI] [PubMed] [Google Scholar]
- 57.Kulkarni R., Pujari S., Gupta D. Neurological manifestations of dengue fever. Ann Indian Acad Neurol. 2021;24(5):693–702. doi: 10.4103/aian.AIAN_157_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carod-Artal F.J., Wichmann O., Farrar J., Gascón J. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12(9):906–919. doi: 10.1016/S1474-4422(13)70150-9. [DOI] [PubMed] [Google Scholar]
- 59.Mathew S., Pandian J.D. Stroke in patients with dengue. J Stroke Cerebrovasc Dis. 2010;19(3):253–256. doi: 10.1016/j.jstrokecerebrovasdis.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Sam J.E., Gee T.S., Nasser A.W. Deadly intracranial bleed in patients with dengue fever: a series of nine patients and review of the literature. J Neurosci Rural Pract. 2016;7(3):423–434. doi: 10.4103/0976-3147.182777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Basnayake B.W.M.K.E., Somaratne K.G.S.K., Goonetilleke C.U., Tilakaratna P.M.Y.I., Ranawaka U.K. Case report: dengue hemorrhagic fever with ischemic stroke. Am J Trop Med Hyg. 2021;106(2):578–581. doi: 10.4269/ajtmh.21-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Del Brutto O.H. Cysticercosis and cerebrovascular disease: a review. J Neurol Neurosurg Psychiatry. 1992;55(4):252–254. doi: 10.1136/jnnp.55.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Sadawi M., Mohamadpour M., Zhyvotovska A., et al. Cerebrovascular accident and snake envenomation: a scoping study. Int J Clin Res Trials. 2019;4:133. https://www.graphyonline.com/archives/IJCRT/2019/IJCRT-133/index.php?page=abstract [Google Scholar]
- 64.Gawarammana I., Mendis S., Jeganathan K. Acute ischemic strokes due to bites by Daboia russelii in Sri Lanka - first authenticated case series. Toxicon. 2009;54(4):421–428. doi: 10.1016/j.toxicon.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Huang Y.K., Chen Y.C., Liu C.C., Cheng H.C., Tu A.T., Chang K.C. Cerebral complications of snakebite envenoming: case studies. Toxins. 2022;14(7):436. doi: 10.3390/toxins14070436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bouaziz M., Bahloul M., Kallel H., et al. Epidemiological, clinical characteristics and outcome of severe scorpion envenomation in South Tunisia: multivariate analysis of 951 cases. Toxicon. 2008;52(8):918–926. doi: 10.1016/j.toxicon.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 67.Sarkar S., Bhattacharya P., Paswan A. Cerebrovascular manifestations and alteration of coagulation profile in scorpion sting: a case series. Indian J Crit Care Med. 2008;12(1):15–17. doi: 10.4103/0972-5229.40944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Udayakumar N., Rajendiran C., Srinivasan A.V. Cerebrovascular manifestations in scorpion sting: a case series. Indian J Med Sci. 2006;60(6):241–244. [PubMed] [Google Scholar]
- 69.Godoy D.A., Badenes R., Seifi S., et al. Neurological and systemic manifestations of severe scorpion envenomation. Cureus. 2021;13(4) doi: 10.7759/cureus.14715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chakrabarti S.D., Ganguly R., Chatterjee S.K., Chakravarty A. Squatting, blood pressure and stroke. J Assoc Physicians India. 2002;50:382–386. [PubMed] [Google Scholar]