Abstract
Introduction
Several studies have investigated the impact of circulating complement-activating anti-human leukocyte antigen donor-specific antibodies (anti-HLA DSAs) on organ transplant outcomes. However, a critical appraisal of these studies and a demonstration of the prognostic value of complement-activating status over anti-HLA DSA mean fluorescence intensity (MFI) level are lacking.
Methods
We conducted a systematic review, meta-analysis and critical appraisal evaluating the role of complement-activating anti-HLA DSAs on allograft outcomes in different solid organ transplants. We included studies through Medline, Cochrane, Scopus, and Embase since inception of databases till May 05, 2023. We evaluated allograft loss as the primary outcome, and allograft rejection as the secondary outcome. We used the Newcastle-Ottawa Scale and funnel plots to assess risk of bias and used bias adjustment methods when appropriate. We performed multiple subgroup analyses to account for sources of heterogeneity and studied the added value of complement assays over anti-HLA DSA MFI level.
Results
In total, 52 studies were included in the final meta-analysis (11,035 patients). Complement-activating anti-HLA DSAs were associated with an increased risk of allograft loss (HR 2.77; 95% CI 2.33-3.29, p<0.001; I²=46.2%), and allograft rejection (HR 4.98; 95% CI 2.96-8.36, p<0.01; I²=70.9%). These results remained significant after adjustment for potential sources of bias and across multiple subgroup analyses. After adjusting on pan-IgG anti-HLA DSA defined by the MFI levels, complement-activating anti-HLA DSAs were significantly and independently associated with an increased risk of allograft loss.
Discussion
We demonstrated in this systematic review, meta-analysis and critical appraisal the significant deleterious impact and the independent prognostic value of circulating complement-activating anti-HLA DSAs on solid organ transplant risk of allograft loss and rejection.
Keywords: complement-activation, donor specific antibodies, anti-HLA, rejection, transplantation outcomes
1. Introduction
Antibody-mediated rejection has been identified as the main cause for allograft loss (1) and the prognostic role of circulating anti-human leukocyte antigen donor-specific antibodies (anti-HLA DSAs) has been extensively assessed across different solid organ transplants (2–5). One key characteristic of anti-HLA DSAs is their ability to undergo class-switch recombination and activate complement by fixing complement fractions. Several studies have been conducted to evaluate the impact of complement-activating anti-HLA DSAs on allograft outcomes. The reported results were heterogeneous with some studies demonstrating a strong association of complement-activating anti-HLA DSA with adverse allograft outcomes (6, 7) while others showed no or weak associations (8, 9).
As a consequence, our team previously performed a systematic review and meta-analysis to study the role of complement-activating anti-HLA DSAs on adverse allograft outcomes (10) and showed that circulating complement-activating anti-HLA DSAs increased the risk of allograft loss and rejection. However, since the publication of the review in May 2018, major studies assessing the effect of circulating complement-activating anti-HLA DSAs on allograft outcomes have been conducted (11, 12).
In addition, the quality and risk of bias of the previous and recent studies have not been evaluated and a critical appraisal remains to be performed. The Sensitization in transplantation: Assessment of Risk (STAR) working group have recently highlighted several gaps regarding whether ancillary complement-based assays (C1q, C3d, C4d) provide additional useful clinical information compared to mean fluorescence intensity (MFI) values provided by single antigen bead (SAB) pan-IgG assay (6, 8, 13, 14). Therefore, STAR working group recommends verify the role of complement binding assays in vivo as potential markers for adverse outcomes before recommending its use in clinical practice.
Therefore, the aim of this article was to provide a comprehensive up-to-date systematic review, meta-analysis and critical appraisal of studies testing the effect of circulating complement-activating anti-HLA DSAs on allograft outcomes and to evaluate and adjust for risk of bias.
2. Methods
This study was an incremental update of a systematic review and a meta-analysis (10), supplemented by a critical appraisal. The study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (15).
2.1. Data sources
A comprehensive search strategy was conducted on Medline, Cochrane, Scopus and Embase since inception of databases till January 31, 2018 (10). For the period between the closing date of the previous review (10) and May 05, 2023 we created a search strategy using a complementary combination of two PubMed search strategies: 1) narrow Boolean which consists of the main Medical Subject Heading (MeSH) for the population combined with the main MeSH for the intervention (see Supplementary for details), and 2) ranking strategy which consisted of screening all the studies listed under the “similar articles” feature on PubMed of the three largest and three newest studies included in the previous review (16). We opted for a PubMed-only database search for this period because the included articles of the previous review (10), whose search strategy was comprehensive and included multiple databases, were all indexed in PubMed (17). This search strategy was further complemented by a manual search for potential additional studies.
2.2. Study selection
The inclusion criteria were studies evaluating the effect of complement-activating anti-HLA DSAs on allograft loss and rejection in adult and paediatric solid organ transplant recipients. Two independent reviewers (SAA and AB) screened the titles and abstracts of the studies and any disagreement was resolved by consensus.
2.3. Data extraction
We collected the same data variables as the previous review: “author name, year of publication, study size, mean or median follow-up time, mean age of population, type of complement-activating anti-HLA DSA, comparison used (patients with complement-activating anti-HLA DSAs were either compared to patients without complement-activating anti-HLA DSAs, patients with non-complement activating anti-HLA DSAs detected, or a mixed group of patients without anti-HLA DSAs and with non-complement activating anti-HLA DSAs), effect sizes (HR and/or OR) and their 95% confidence intervals, potential confounding factors, and unadjusted and adjusted estimated risks of graft loss or graft rejection.” (10).
2.4. Critical appraisal
We used the Newcastle-Ottawa Scale (NOS) to assess the risk of bias in observational studies (18). A high NOS score (≥ 6) represents high methodological quality. Using this quality score, each study is judged on eight items which are divided into three components: selection of the study groups (up to four points), confounding variables adjustment quality (up to two points) and the outcome studied (up to three points). (see Supplementary for details).
Extraction of data and assessment of risk of bias was done by two independent reviewers (SAA and AB) and any disagreement was resolved by consensus.
2.5. Data synthesis and analysis
We performed the meta-analysis through a random-effects model with restricted maximum likelihood approach using an inverse-variance to incorporate a measure of the anticipated heterogeneity into the weight of the studies (19). The index group was complement-activating anti-HLA DSA positive patients. They were compared to either complement-activating anti-HLA DSA negative patients, anti-HLA DSA negative patients, or a mixed group of both. The pooled effect size, study weights and amount of study heterogeneity were represented by forest plots for allograft loss and rejection.
2.6. Statistical heterogeneity and small-study effects
We evaluated statistical heterogeneity using I² index which reflects the percentage of variability in the effect size caused by heterogeneity rather than by chance alone. An I² above 50% represented substantial heterogeneity (19).
We used a funnel plot to visually assess for the presence of small-size effects which occurs when smaller studies show different, often more pronounced effect size. We statistically assessed any asymmetry in the funnel plot with the Egger’s test (20). If this test was significant, we adjusted for small-study effects by using the precision-effect test (PET). This method provided an estimate of the effect size in a study with a hypothetical infinite sample size and thus eliminating small-study effects bias (21).
We tested for publication bias by using a contour-enhanced funnel plot (22). If a bias was observed, we adjusted by using the p-uniform* selection model which assumes that studies with statistically non-significant p-values are published with the same probability as statistically significant results (21).
2.7. Subgroup analyses
We performed the following subgroup analyses to address potential sources of heterogeneity in studies assessing graft loss:
2.7.1. High versus low methodological quality of studies
We separately meta-analysed higher quality studies (NOS scores ≥ 6) (23) versus lower quality studies (NOS score ≤ 5).
2.7.2. Comparator group used
We separately meta-analysed studies comparing complement activating anti-HLA DSA positive patients with complement activating anti-HLA DSA and studies comparing complement activating anti-HLA DSA positive patients with complement activating anti-HLA DSA negative patients and anti-HLA DSA negative patient.
2.7.3. Type of organ transplanted
We separately meta-analysed studies based on the type of the transplanted organ (kidney, liver, lung, heart, pancreas and intestine). We also separately meta-analysed kidney transplants versus all other organs based on the assumption that a low number of studies are available per organ.
2.7.4. Timing of antibody detection
We separately meta-analysed studies testing patients with preformed anti-HLA DSAs (defined as antibodies positive before or at the time of transplantation), de novo anti-HLA DSAs (defined as antibodies positive only after transplantation), or a combined group of de novo both.
2.7.5. Type of complement-activating capacity of antibodies
We separately meta-analysed anti-HLA DSA according to their C1q-, C3d-, or C4d-, binding capacity or according to their IgG subclass.
2.7.6. Thresholds for complement-activating anti-HLA DSA positivity
We separately meta-analysed studies that considered different MFI thresholds for complement-activating anti-HLA DSA positivity of 300, 500 or 1000.
2.8. Sensitivity analysis
We separately meta-analysed the newly identified studies since the publication of the previous review in 2018 and assessed the association of complement activating anti-HLA DSA with the risk allograft loss and allograft rejection.
2.9. Cumulative meta-analysis
We conducted a cumulative meta-analysis to show the change of hazard ratio of allograft loss as each study is added to the pool (24), which allowed to assess the stability of evidence i.e., whether additional studies change the overall effect of complement-binding anti-HLA DSAs on the outcome, and the sufficiency of evidence i.e., whether additional studies were needed to establish the same conclusion (25). The cumulative meta-analysis was represented on a forest-plot and the studies were arranged in a chronological order by year and month of publication.
2.10. Added prognostic value of complement-activating anti-HLA DSA status over anti-HLA DSA MFI level
We identified studies that showed a correlation between complement-activating anti-HLA DSA status and pan-IgG anti-HLA DSA defined by MFI levels. Then, we identified and separately meta-analysed studies that conducted multivariable analyses adjusting complement-activating anti-HLA DSA status on pan-IgG anti-HLA DSA defined by MFI levels to assess the prognostic value of complement-activating anti-HLA DSA over standard SAB pan-IgG assays.
In addition, to assess the added prognostic value of complement-activating anti-HLA DSA over EDTA treated SAB assays, we identified studies that pre-treated sera with ethylenediaminetetraacetic acid (EDTA) as means to overcome complement interference – a shortcoming of SAB assays caused by complement activation which usually results in underestimating or completely masking strong DSAs (26).
2.11. Added prognostic value of complement-activating anti-HLA DSA status over anti-HLA DSA class
We identified and separately meta-analysed studies that performed multivariable models adjusting complement-activating anti-HLA DSA status on DSA class to assess the independent prognostic value of complement-activating anti-HLA DSA.
The meta-analyses were conducted on R 4.1.1. All tests were two-sided, and a p-value lower than 0.05 was considered significant.
3. Results
3.1. Study identification
The search strategy identified 1,112 potential studies. After removing duplicates (n=91), studies with non-human data or not written in English (n=102), studies with non-solid organ transplant data (n=475), studies with non-complement binding anti-HLA DSAs (n=400), non-original articles (n=19), and studies with different outcomes or without hazard ratio/odds ratio (n=10), 15 new studies were identified, corresponding to 3,099 patients ( Figure 1 ). The previous review (10) included 37 studies, therefore, in this incremental update, 52 studies in total were included in the final meta-analysis, corresponding to 11,035 patients. A descriptive summary of all the included studies is shown in Table 1 .
Figure 1.
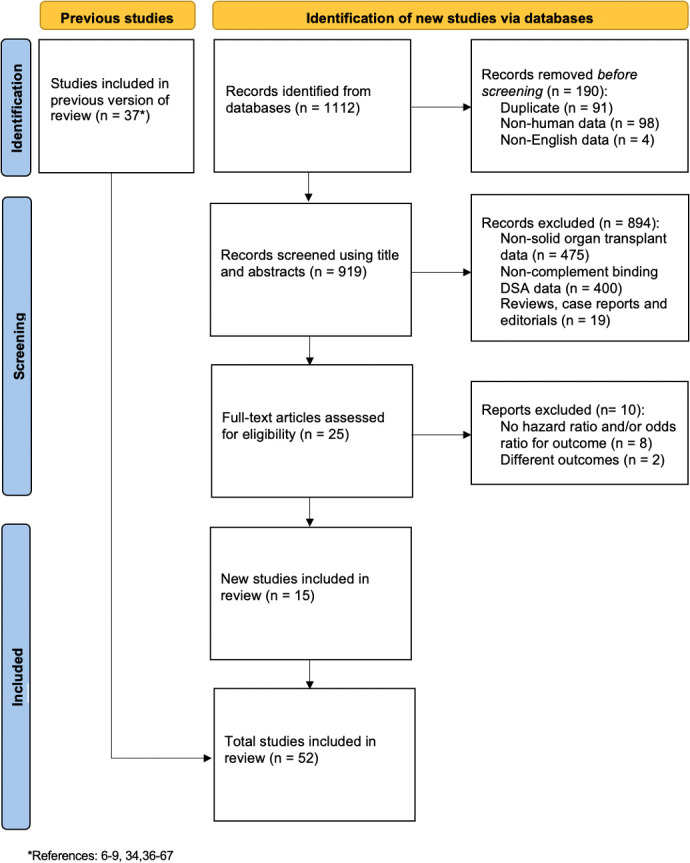
Flow chart summarizing study identification and selection process.
Table 1.
Description of the 52 included studies.
| First author (date of publication) |
Population | Study type | Period of inclusion |
Sample size | Type of C’ anti-HLA DSA | MFI threshold for DSA detection | MFI threshold for complement positivity |
EDTA pre-treatment | Outcome | Effect size (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Wahrmann et al. (2009) (27) | Retrospective, single-centre analysis of consecutive adult renal transplants selected based on the presence of pretransplant DSAs | Cohort | 2001-2002 | 338 | C4d | Not reported | Not reported | Not reported | Graft loss Rejection |
2.40 (0.90-6.00) 10.10 (3.20-31.00) |
| Hönger et al. (2010) (28) | Retrospective, single-centre analysis of consecutive adult renal transplant recipients with low levels of pretransplant DSAs | Cohort | 1999-2004 | 64 | C4d | >500 | Not reported | No | Rejection | 0.93 (0.25-3.44) |
| Sutherland et al. (2011) (29) | Retrospective, single-centre analysis of paediatric renal transplant recipients without DSAs at the time of transplantation | Cohort | 2000-2008 | 35 | C1q | >1000 | >450 | No | Graft loss | 5.80 (1.40-22.90) |
| Hönger et al. (2011) (30) | Retrospective, single-centre analysis of adult renal transplant recipients with high levels of DSAs pre transplant; recipients who developed AMR within 6 months | Cohort | 1999-2008 | 71 | IgG3 | >500 | Not reported | No | Rejection | 0.43 (0.17-1.12) |
| Smith et al. (2011) (31) | Retrospective, single-centre analysis of living heart transplant recipients after 1 year of transplantation without DSAs pre-transplant | Cohort | 1995-2004 | 243 | C4d | >1000 | Not reported | No | Graft loss | 3.02 (1.11-8.23) |
| Kaneku et al. (2012) (32) | Retrospective (2-centre) analysis of adult liver transplant recipients with liver biopsies showing chronic rejection and DSA analysis at the same time | Case-control | NC | 39 | IgG3 | >5000 | Not reported | No | Graft loss | 3.35 (1.39-8.05) |
| Bartel et al. (2013) (33) | Retrospective, single-centre analysis of 68 desensitized renal recipients who had been subjected to peri-transplant desensitization | Cohort | 1999-2008 | 68 | C4d | >500 | Not reported | No | Rejection | 10.10 (1.60-64.20) |
| Lawrence et al. (2013) (34) | Retrospective, single-centre study of consecutive renal transplant recipients | Cohort | 2005-2010 | 52 | C4d | >300 | Not reported | No | Rejection | 8.90 (1.20-65.86) |
| Crespo et al. (2013) (8) | Retrospective (2-left) analysis of renal transplant patients with pretransplant DSAs | Cohort | 2006-2011 | 355 | C1q | >2000 | >500 | Yes | Graft loss Rejection |
0.83 (0.17-4.14) 1.44 (0.23-9.11) |
| Loupy et al. (2013) (6) | Consecutive adult patients in a retrospective (2-left) analysis; unselected global population with DSA detection before or after renal transplantation | Cohort | 2004-2010 | 1,016 | C1q | >500 | >500 | No | Graft loss | 4.78 (2.69-8.49) |
| Freitas et al. (2013) (35) | Retrospective, single-centre analysis of renal transplant recipients selected on the basis of DSA detection during follow-up | Cohort | 1999-2012 | 203 | IgG3 | >1000 | >500 | No | Graft loss | 3.50 (1.30-9.50) |
| Arnold et al. (2014) (36) | Retrospective, single-centre analysis of renal transplant recipients without DSAs pre transplant and screened for de novo DSAs | Cohort | 1997-2007 | 274 | IgG3 | >1000 | >500 | No | Graft loss | 4.81 (1.65-14.03) |
| Smith et al. (2014) (37) | Retrospective, single-centre analysis of lung transplant recipients with pretransplant DSA detection | Cohort | 1991-2003 | 63 | C4d | 500 | Not reported | No | Graft loss | 6.43 (2.96-13.97) |
| Everly et al. (2014) (38) | Retrospective, single-centre analysis of primary renal transplant recipients without pretransplant DSA detection | Cohort | 1999-2006 | 179 | IgG3 | >1000 | >1000 | No | Graft loss | 2.48 (1.02-6.04) |
| O’Leary et al. (2015) (39) | Retrospective, single-centre analysis of ƒconsecutive patients with 1-year survival post liver transplantation; one group analysed pretransplant DSA effects, and another group analysed the impact of de novo DSAs | Cohort | 2000-2009 | 1,270 | C1q IgG3 |
>5000 | >500 | No | Graft loss Graft loss |
1.90 (1.62-3.45) 2.40 (1.82-5.75) |
| Wozniak et al. (2015) (40) | Retrospective, single-centre analysis of paediatric liver transplant patients who were either nontolerant, tolerant, or stable | Cohort | NC | 50 | C1q | >1000 | >1000 | No | Rejection | 4.30 (1.10-16.40) |
| Khovanova et al. (2015) (41) | Retrospective, single-centre analysis of HLA-incompatible desensitized renal transplant patients | Cohort | 2003-2012 | 80 | IgG3 | >1000 | Not reported | No | Graft loss | 2.09 (0.30-14.60) |
| Sicard et al. (2015) (14) | Retrospective analysis of consecutive (2-left) adult renal transplant patients who developed AMR | Cohort | 2004-2012 | 69 | C3d C1q |
>500 | Not reported | No | Graft loss Graft loss |
2.80 (1.12-6.95) 1.98 (0.95-4.14) |
|
Thammanichanond et al.
(2016) (42) |
Retrospective, single-centre cohort study of patients with pre-transplant DSAs | Cohort | 2009-2013 | 48 | C1q | >1000 | >500 | No | Rejection | 2.20 (0.61-7.85) |
| Comoli et al. (2016) (43) | Retrospective analysis of consecutive paediatric recipients; single centre; first kidney transplant without any HLA antibodies in sera or at the time of transplantation | Cohort | 2002-2013 | 114 | C3d C1q C3d C1q |
>1000 | >500 | No | Rejection RejectionGraft loss Graft loss |
6.91 (2.78-17.18) 13.54 (4.95-36.99) 27.80 (5.61-137.72) 11.09 (2.25-54.64) |
| Yamamoto et al. (2016) (44) | Retrospective analysis of renal transplant patients with de novo DSAs and surveillance biopsies | Cohort | 2009-2013 | 43 | C1q | >1000 | >1000 | No | Rejection | 2.60 (0.12-53.90) |
| Calp-Inal et al. (2016) (7) | Retrospective analysis; single centre; consecutive renal transplant patients: Group 1 without pretransplant DSAs and Group 2 with a mix of pre-existing and de novo DSAs | Cohort | 2009-2012 | 284 | C1q | >1000 | >500 | No | Graft loss | 4.30 (1.10-16.50) |
| Malheiro et al. (2016) (45) | Retrospective, single-centre analysis of kidney transplant patients with DSAs pre transplant | Cohort | 2007-2012 | 60 | C1q | >1000 | >500 | Yes | Rejection | 16.80 (3.18-88.85) |
| Visentin et al. (2016) (46) | Retrospective, single-centre analysis of lung transplant patients with biopsy (with demonstration of rejection) and serum available | Cohort | 1999-2014 | 53 | C1q | >500 | >500 | No | Graft loss | 1.65 (0.68-3.97) |
| Kauke et al. (2016) (47) | Retrospective, single-centre analysis of patients selected based on renal biopsy-proven rejection during graft dysfunction or viremia with polyomavirus BK | Cohort | 2005-2011 | 611 | C3d C1q |
1000 | Not reported | No | Graft loss Rejection |
3.77 (1.40-10.16) 4.52 (1.89-10.37) |
| Bamoulid et al. (2016) (48) | Retrospective, single-centre analysis of renal transplant consecutive patients without DSAs pre transplant | Cohort | 2007-2014 | 59 | C1q | >1000 | >300 | No | Rejection Graft loss |
2.27 (1.05-4.91) 6.78 (0.86-53.50) |
| Fichtner et al. (2016) (49) | Retrospective, single-centre analysis of prospectively screened renal transplant paediatric patients, non-presensitised | Cohort | 1999-2010 | 62 | C1q | >500 | >300 | No | Graft loss | 6.35 (1.33-30.40) |
| Guidicelli et al. (2016) (50) | Retrospective, single-centre analysis of consecutive non-sensitized kidney transplant patients | Cohort | 1998-2005 | 346 | C1q | >500 | Not reported | Yes | Graft loss | 2.99 (0.94-10.27) |
| Lefaucheur et al. (2016) (51) | Retrospective analysis of consecutive patients (2-left); renal transplant patients were unselected | Cohort | 2008-2010 | 125 | IgG3 C1q |
>500 | >500 | No | Graft loss Graft loss |
4.80 (1.70-13.30) 3.60 (1.10-11.70) |
| Viglietti et al. (2017) (52) | Retrospective analysis of consecutive patients (2-left); renal transplant recipients were unselected | Cohort | 2008-2011 | 851 | IgG3 C1q |
>1000 | >500 | No | Graft loss Graft loss |
4.25 (1.88-9.61) 3.60 (1.71-7.59) |
| Wiebe et al. (2017) (53) | Retrospective analysis of consecutive adult and paediatric renal transplant patients, single centre; patients without pretransplant sensitization |
Cohort | 1999-2012 | 70 | C1q | >300 | >300 | Yes | Graft loss | 1.06 (0.50-2.40) |
| Moktefi et al. (2017) (9) | Retrospective analysis (2-left) of patients selected based on the development of acute renal AMR and the presence of DSAs | Cohort | 2005-2012 | 48 | C1q | >500 | >500 | No | Graft loss | 0.79 (0.25-2.44) |
| Sicard et al. (2017) (54) | Retrospective analysis of consecutive adult renal transplant patients (2-left) with unselected patients | Cohort | 2004-2012 | 52 | C3d | 500 | >500 | No | Graft loss | 3.71 (1.27-10.80) |
| Das et al. (2017) (55) | Retrospective, single-centre analysis of paediatric heart transplant without DSAs pre transplantation and at the time of transplantation | Cohort | 2005-2014 | 127 | C1q | >1000 | >500 | No | Graft loss | 3.20 (1.34-7.86) |
| Couchonnal et al. (2017) (56) | Retrospective analysis; single-centre analysis of consecutive paediatric liver transplant selected on the presence of DSAs during follow-up | Cohort | 1990-2014 | 100 | C3d | >500 | >500 | No | Graft loss | 4.12 (0.95-17.89) |
| Bailly et al. (2017) (57) | Retrospective analysis of multi-centre, prospective, randomized, double-blind, placebo-controlled, parallel-group trials; patients selected on the basis of renal AMR development and DSA detection; patients treated either with standard of care (PP plus IVIg) or rituximab plus standard of care | Cohort | 2008-2011 | 25 | C1q | >500 | >500 | Yes | Graft loss | 3.70 (0.80-17.00) |
| Molina et al. (2017) (58) | Retrospective analysis; single-centre analysis of consecutive adult kidney transplant patients selected on pretransplant DSA detection | Cohort | 1995-2009 | 389 | C1q | >1000 | >500 | No | Graft loss | 4.01 (2.33-6.92) |
| Lan et al. (2018) (11) | Retrospective multi-centre analysis of adult kidney transplant patients selected on the presence of preformed DSA | Cohort | Before 2005 | 896 | C3d | >500 | >500 | No | Graft loss | 1.04 (0.37-2.94) |
| Courant et al. (2018) (12) | Retrospective single-centre analysis of adult kidney transplant patients selected on pre-transplant DSA detection | Cohort | 2004-2013 | 192 | C1q C3d |
>500 | >300 | Yes | Graft loss Graft loss |
1.74 (0.94-3.21) 1.01 (0.51-1.98) |
| Brugière et al. (2018) (59) | Retrospective, three-centre analysis of consecutive adult lung transplant patients selected on the presence of DSA during follow-up | Cohort | 2009-2012 | 168 | C1q | >500 | >300 | Yes | Graft loss | 2.98 (1.33-6.66) |
| Kamburova et al. (2018) (60) | Retrospective multi-centre analysis of adult kidney transplant patients selected on the presence of preformed DSA | Cohort | 1995-2005 | 567 | C3d | >750 | Not reported | No | Graft loss | 1.02 (0.70-1.48) |
| Viglietti et al. (2018) (61) | Retrospective two-centre analysis of consecutive adult kidney transplant patients selected on the presence of DSA during follow-up | Cohort | 2008-2011 | 139 | C1q | >1000 | Not reported | No | Graft loss | 2.57 (1.29-5.12) |
| Lee H et al. (2018) (62) | Retrospective single-centre analysis of adult kidney transplant patients selected on the presence of de novo DSA | Cohort | 1988-2016 | 161 | C1q C3d C1q C3d |
>1000 | >1000 | Yes | Graft loss Graft loss Rejection Rejection |
2.90 (1.43-5.58) 2.82 (1.46-5.43) 18.5 (5.90-58.10) 8.10 (3.00-21.60) |
| Malheiro et al. (2018) (63) | Retrospective single-centre analysis of consecutive adult kidney transplant patients selected on the presence of DSA during follow-up | Cohort | 2008-2015 | 56 | C1q | >1000 | >500 | No | Graft loss | 3.94 (1.53-10.18) |
| Schinstock et al. (2018) (64) | Retrospective multi-centre analysis of adult solitary kidney transplant patients selected on the presence of DSA during follow-up | Cohort | 1998-2015 | 113 | C1q IgG3 |
>1000 | >1000 | No | Graft loss Graft loss |
5.90 (2.30-15.60) 3.80 (1.50-9.30) |
| Lee DR et al. (2018) (13) | Retrospective single-left analysis of adult solitary kidney transplant patients selected on the presence of DSA during follow-up | Cohort | 2013-2016 | 220 | C3d | >500 | >500 | No | Graft loss | 3.02 (1.52-12.12) |
| Babu et al. (2020) (65) | Retrospective multi-left analysis of adult solitary kidney transplant patients selected on the presence of DSA during follow-up | Cohort | 2005-2015 | 139 | C3d | Not reported | Not reported | No | Graft loss | 4.56 (1.46-14.4) |
| Vargas et al. (2020) (66) | Retrospective single-left analysis of adult solitary kidney transplant patients selected on the presence of DSA during follow-up | Cohort | 2003-2014 | 86 | C1q | >1000 | Not reported | No | Graft loss | 1.09 (0.42-2.78) |
| Zhang et al. (2018) (67) | Retrospective single-centre analysis of paediatric heart transplant patients selected on the presence of DSA during follow-up | Cohort | 2010-2013 | 176 | C3d | >1000 | >1000 | No | Rejection | 33.0 (8.10-138) |
| Cioni et al. (2019) (68) | Retrospective single-centre analysis of consecutive adult kidney transplant patients selected on the presence of de novo DSA | Cohort | 2002-2014 | 69 | C1q C3d |
>1000 | >500 | No | Rejection Rejection |
0.80 (0.20-3.10) 10.10 (1.50-68.30) |
| Hayde et al. (2020) (69) | Retrospective single-centre analysis of paediatric kidney transplant patients selected on the presence of de novo DSA | Cohort | 2009-2016 | 48 | C1q | >1000 | >500 | No | Rejection | 10.10 (2.00-51.80) |
| Pernin et al. (2020) (70) | Retrospective two-centre analysis of adult kidney transplant patients selected on the presence of de novo DSA | Cohort | 2014-2018 | 69 | IgG3 | >1000 | Not reported | Yes | Rejection | 11.15 (2.24-55.37) |
Effect sizes refer to HR for allograft loss and OR for rejection appearance.
C’, complement; C1q, complement component 1q; CI, confidence interval; DSA, donor-specific antibody; HR, hazard ratio; IgG3, immunoglobulin G3; OR, odds ratio.
3.2. Study characteristics
In total, 31 (59.6%) studies originated from Europe, 13 (25.0%) from North America, 5 (9.6%) from the United Kingdom, and 3 (5.8%) from Asia. The majority of the patients were kidney recipients (n=8,746; 79.3%), followed by liver recipients (n=1,459; 13.2%), heart recipients (n=546; 4.9%), and lung recipients (n=284; 2.6%). No pancreas or intestine recipients were identified. Complement-activating anti-HLA DSAs were classified by their capacity to bind C1q (28 studies; 53.8%), C3d (12 studies; 23%), or C4d (6 studies; 11.5%) or by their IgG subclass composition (10 studies; 19%).
The median NOS score was 6 (IQR 3-9) with 1.5%, 13%, 15%, 30%, 28%, 13%, and 3% of studies having a NOS score of 3, 4, 5, 6, 7, 8, and 9, respectively. Details on the NOS scoring results are available in Supplementary Table 1 .
3.3. Outcomes
3.3.1. Risk for allograft loss
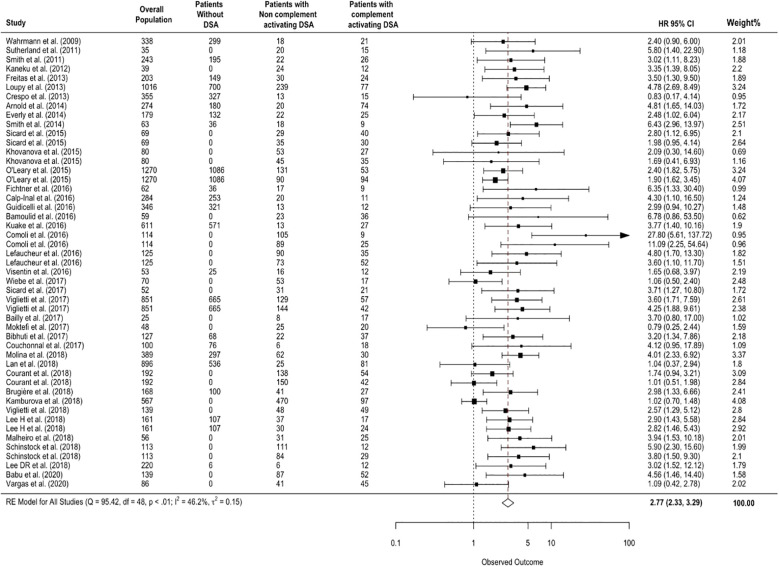
Patients with complement-activating anti-HLA DSAs had a 2.77-fold increase in risk for allograft loss (95% CI 2.33-3.29, p<0.001; I²=46.2%) compared to patients without complement-activating anti-HLA DSA, patients without anti-HLA DSAs, and a mixed group of both ( Figure 2 ).
Figure 2.
Association between circulating complement-activating anti-HLA DSAs and the risk of allograft loss. The figure shows the forest plot of the association between complement-activating anti-HLA DSAs and the risk of allograft loss for each complement binding study and overall (n = 49). Studies are listed by date of publication. Number of patients are listed in the 4 cohort columns. The black square-shaped boxes represent the HR for each individual study. The size of these boxes represents the weight of the study, and lines represent the 95% CI for individual studies. The diamond at the bottom represents the pooled HR. The number of patients in the overall population does not correspond to the sum of the different groups for the studies of Kaneku et al. (32) (3 patients), Sicard et al. (14) (4 patients), and Moktefi et al. (9) (3 patients) either because the data for these patients were missing or because they were not involved in the analysis. CI, confidence interval; DSA, donor-specific antibody; HLA, human leukocyte antigen; HR, hazard ratio.
3.3.2. Risk of allograft rejection
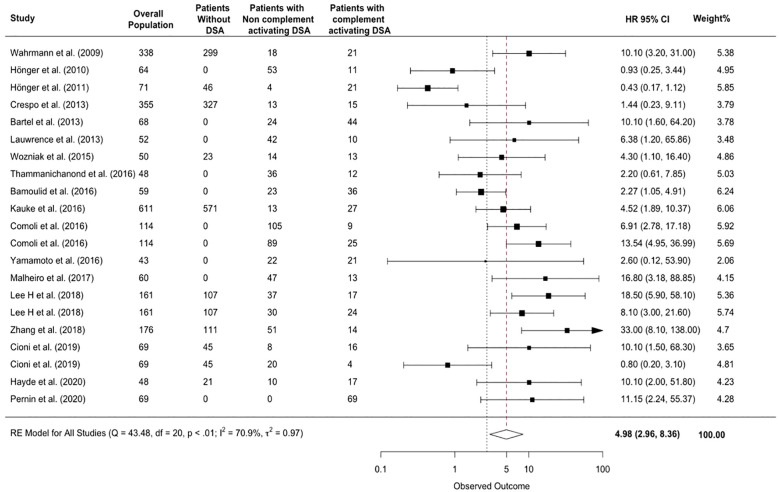
Patients with complement-activating anti-HLA DSAs had a 4.98-fold increase in risk of allograft rejection (95% CI 2.96-8.36, p<0.001; I²=70.9%) compared to patients without complement-activating anti-HLA DSA, patients without anti-HLA DSAs, and a mixed group of both ( Figure 3 ).
Figure 3.
Association between complement-activating anti-HLA DSAs and the risk of rejection. The figure shows the forest plot of the association between complement activating anti-HLA DSAs and the risk of rejection for each study and overall (n = 17). Studies are listed by date of publication. The black square-shaped boxes represent the HR for each individual study. The black square-shaped boxes represent the HR for each individual study. The size of these boxes represents the weight of the study, and lines represent the 95% CI for individual studies. The diamond at the bottom represents the overall HR. CI, confidence interval; DSA, donor-specific antibody; HLA, human leukocyte antigen; HR, hazard ratio.
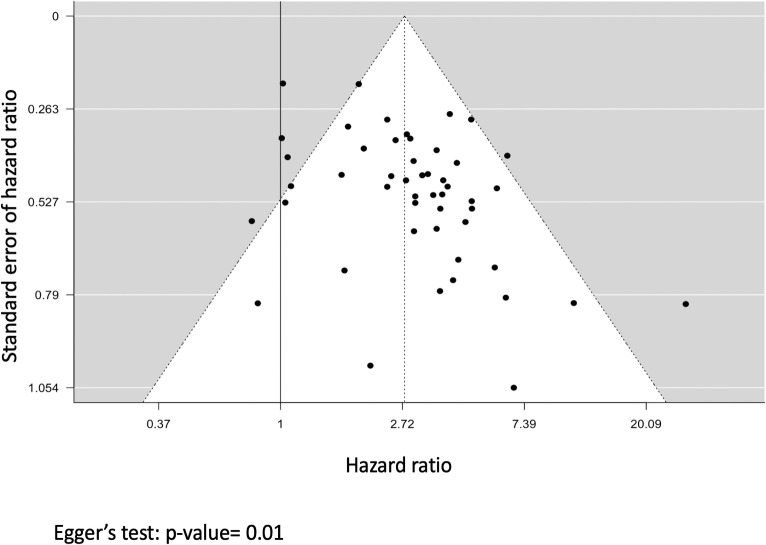
3.4. Small-study effects
Visually, the funnel plot presented in Figure 4 showed an asymmetry which was confirmed by Egger’s test (p=0.01) indicating the presence of small-study effects. When adjusting using the PET method, the hazard ratio remains significant (HR=1.5, p<0.001) indicating that in a hypothetical infinite sample size, complement-binding anti-HLA DSAs would still increase the risk for allograft loss ( Supplementary Figure 3 ).
Figure 4.
Funnel plot representing the analysis for small-study effects. Each black dot represents a study; the x-axis represents the study effect size (hazard ratio), and the y-axis represents the standard error of the hazard ratio. The dashed vertical line represents the overall risk estimate and the black line represents the no intervention effect.
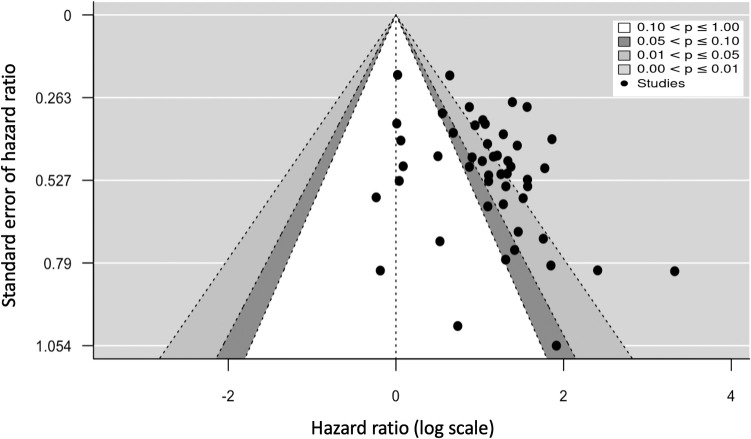
Publication bias, as a potential cause for small-study effects, was assessed using the contour-enhanced funnel plot presented in Figure 5 which showed that more studies lie in the statistically significant side of the graph. We adjusted for this bias by using the p-uniform* selection model which yielded a hazard ratio of 2.46 (p=0.01) indicating that taking into account studies with non-significant p-values, complement-binding anti-HLA DSAs would still increase the risk of allograft loss.
Figure 5.
Contour-enhanced funnel plot representing the analysis for publication bias according to the statistical significance of studies. Each black dot represents a study; the x-axis represents the study effect size (hazard ratio), and the y-axis represents the standard error of the hazard ratio.
3.5. Subgroup analysis
Table 2 summarizes the effect sizes for each subgroup.
Table 2.
Effect sizes related to the different subgroup analyses.
| Subgroup analyses for allograft survival | Effect size | 95% CI | I2, p-value | |
|---|---|---|---|---|
| Effect of complement-activating anti-HLA DSAs in studies with high or low methodological quality | High-methodological quality studies NOS ≥6 | 2.79 | 2.33-3.35 | 45.7%, p<0.001 |
| Low-methodological quality studies NOS ≤ 5 | 2.46 | 1.28-4.70 | 60.5%, p<0.001 | |
| Effect of complement-activating anti-HLA DSAs in studies with different comparators used | Studies comparing index group and patients with non-complement-activating anti-HLA DSAs | 2.56 | 1.99-3.30 | 54.2%, p< 0.001 |
| Studies comparing index group and patients with non-complement-activating anti-HLA DSAs and without anti-HLA DSAs | 3.58 | 2.70-4.74 | 4.1%, p<0.001 | |
| Effect of complement-activating anti-HLA DSAs according to the type of solid organ transplant | Kidney transplantation studies only | 2.77 | 2.25-3.41 | 49.2%, p< 0.001 |
| Heart, lung, and liver transplantation studies | 2.74 | 2.03-3.69 | 29.2%, p<0.001 | |
| Effect of complement-activating anti-HLA DSAs according to the timing of antibody detection | Pre-existing DSAs | 2.56 | 1.99-3.30 | 56.2%, p< 0.001 |
| Pre-existing and de novo DSAs | 2.59 | 2.05-3.26 | 34.3%, p<0.001 | |
| De novo DSAs | 3.53 | 2.63-4.74 | 26.0%, p<0.001 | |
| Effect of complement-activating anti-HLA DSAs according to the type of test used for detecting complement-activating antibodies | C1q | 2.72 | 2.19-3.38 | 35.8%, p= 0.001 |
| C4d | 3.81 | 2.02-7.2 | 33.0%, p= 0.001 | |
| C3d | 2.50 | 1.48-4.25 | 73.1%, p< 0.001 | |
| IgG3 | 3.17 | 2.37-4.24 | 0.00%, p<0.001 | |
| Effect of complement-activating anti-HLA DSAs according to the MFI thresholds for complement-activating anti-HLA DSA positivity | 300 | 2.74 | 1.91-3.93 | 36.7%, p<0.001 |
| 500 | 2.29 | 2.29-3.82 | 47.8%, p<0.001 | |
| 1000 | 2.37 | 1.7-3.29 | 49.1%, p<0.001 | |
Effect sizes refer to HR for graft survival and OR for rejection appearance. The Index group refers to patients with complement-activating anti-HLA DSAs.
DSA, donor-specific antibody; C1q, complement component 1q; CI, confidence interval; HR, hazard ratio; HLA, human leukocyte antigen; I2, heterogeneity; IgG3, immunoglobulin G3; MFI, MFI, Mean fluorescent intensity, NOS, Newcastle-Ottawa scale; OR, odds ratio.
3.5.1. Effect of complement-activating anti-HLA DSAs in high methodological quality studies
Analysis done on high methodological quality studies (NOS≥6) showed a significantly increased risk of allograft loss in complement activating anti-HLA DSAs positive patients with a pooled HR of 2.79 (95% CI 2.33-3.35, p<0.001, I2 = 45.7%). Studies with lower methodological quality (NOS ≤ 5) also showed an increased risk of allograft loss with a HR of 2.46 (CI 1.28-4.70, p<0.001) however, as expected, the heterogeneity level between the lower methodological quality studies was higher (I2 = 60.5%).
3.5.2. Effect of the complement-activating anti-HLA DSAs using different comparators
The association between complement-activating anti-HLA DSAs and risk of allograft loss remained significant using different comparator groups. When comparing complement-activating anti-HLA DSAs positive patients to complement-activating anti-HLA DSAs negative patients, the pooled HR was 2.56 (95% CI 1.99-3.30, p<0.001, I2 = 54.2%). When comparing, complement- activating anti-HLA DSA positive patients to a mixed group of complement- activating anti-HLA DSA negative patients and anti-HLA DSA negative patients, the pooled HR was 3.58 (95% CI 2.70-4.74, p<0.001; I2 = 4.1%).
3.5.3. Effect of complement-activating anti-HLA DSAs according to the type of organ transplantation
Analysis done on kidney allograft recipients versus all other solid organ allograft recipients showed a significant increased risk of allograft loss with HRs of 2.77 (CI 2.25-3.41, p<0.001; I2 = 49.2%) and 2.74 (CI 2.03-3.69, p<0.001; I2 = 29.2%) respectively. Analysis specific to other organs showed an increased risk for allograft loss, however, the results were not statistically significant due to the low number of studies found ( Supplementary Table 2 ).
3.5.4. Effect of complement-activating anti-HLA DSAs according to the timing of antibody detection
Analysis according to the time of antibody detection all showed significant associations with the highest HR of 3.53 for de novo DSAs (CI 2.63-4.74, p<0.001; I2 = 26%).
3.5.5. Analysis according to the type of complement-activating antibodies
Analysis across the different types of complement-activating antibodies showed significant overall effect on allograft loss. The following groups were assessed: (i) C1q-binding capacity (HR 2.72; 95% CI 2.19-3.38, P<0.001; I2 = 35.8%), (ii) C4d-binding capacity (HR 3.81; 95% CI 2.02-7.20, p<0.001; I2 = 33%), (iii) C3d-binding capacity (HR 2.50; 95% CI 1.48-4.25, p<0.001; I2 = 73.1%), (iv) IgG3 subclass (HR 3.17; 95% CI 2.37-4.24, p<0.001; I2 = 0.0%).
3.5.6. Analysis according to MFI thresholds for complement-activating anti-HLA DSA positivity
6 (15.0%) studies used 300 as an MFI threshold for complement-activating anti-HLA DSA positivity, 1 (2.5%) study used 450, 21 (52.5%) studies used 500, 2 (5.0%) studies used 1000, and 11 (27.5%) studies did not provide the threshold value. The risk of allograft loss remained significantly increased at all complement-activating anti-HLA DSA positivity thresholds: (i) MFI 300 (HR 2.74; 95% CI 1.91-3.93, p<0.001; I2 = 36.7%), (ii) MFI 500 (HR 2.96; 95% CI 2.29-3.82, p<0.001; I2 = 47.8%), (iii) MFI 1000 (HR 2.37; 95% CI 1.7-3.29, p<0.001; I2 = 49.1%).
3.6. Sensitivity analysis
The separate meta-analysis of the 15 newly identified studies since the publication of the previous review in 2018 showed that patients with complement-activating anti-HLA DSAs had a 2.21-fold increase in risk for allograft loss (95% Cl 1.61-3.04; p<0.001; I2 = 58.8) ( Supplementary Figure 1 ) and a 8.87-fold increase in risk for allograft rejection (95% CI 3.64-21.6; p<0.001; I2 = 65.3%) compared to patients without complement-activating anti-HLA DSA, patients without anti-HLA DSAs, and a mixed group of both ( Supplementary Figure 2 ).
3.7. Cumulative meta-analysis
The cumulative meta-analysis showed the effect of adding new studies in a chronological order on the overall effect size ( Supplementary Figure 4 ). Starting at the second study in 2011 till the end of analysis, there is a consistent and statistically significant risk of allograft loss.
The cumulative meta-analysis demonstrated that adding new studies: i) narrowed the confidence intervals of the overall effect size, ii) reduced the already statistically significant p-values, iii) converged the overall effect size of complement- activating antibodies on allograft loss.
3.8. Added prognostic value of complement-activating anti-HLA DSA status over anti-HLA DSA MFI level on allograft loss
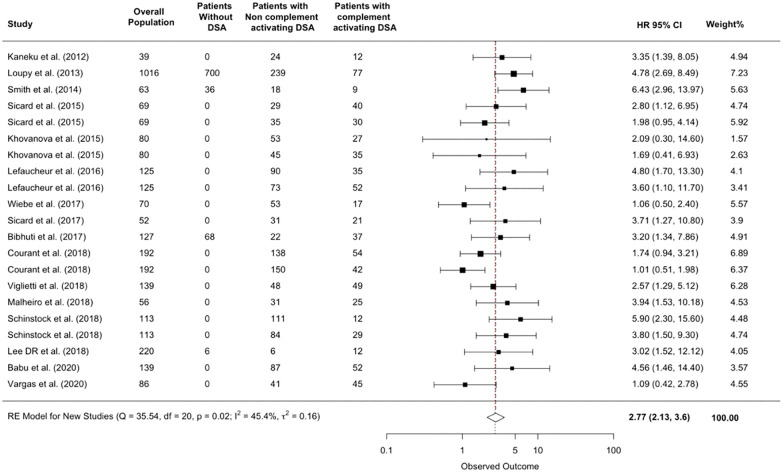
26 (50%) studies reported positive correlation between complement-activating anti-HLA DSA and pan-IgG anti-HLA DSA level defined by the MFI. 15 (37.5%) studies performed multivariable analyses adjusting complement-activating anti-HLA DSA status on pan-IgG anti-HLA DSA defined by the MFI levels as opposed to a linear univariable correlation analysis. The multivariable analysis demonstrated that complement-activating anti-HLA DSA’s presence was significantly and independently associated with an increased risk of allograft loss (HR 2.77; 95% CI 2.13-3.6, p=0.017; I2 = 45.4%) ( Figure 6 ).
Figure 6.
Added prognostic value of complement-activating anti-HLA DSA status over anti-HLA DSA MFI level on allograft loss. The figure shows the forest plot of the association between complement-activating anti-HLA DSAs and the risk of allograft loss for each c study that included the anti-HLA DSA MFI level in their evaluation of the prognostic value of complement-activating anti-HLA DSA. Number of patients are listed in the 4 cohort columns. The black square-shaped boxes represent the HR for each individual study. The size of these boxes represents the weight of the study, and lines represent the 95% CI for individual studies. The diamond at the bottom represents the pooled HR. CI, confidence interval; DSA, donor-specific antibody; HLA, human leukocyte antigen; HR, hazard ratio.
Seven (13.5%) studies pre-treated the sera of the studied population, or a sample of the studied population, with EDTA to uncover interfering substances and only 3 studies (5.8%) performed a multivariable analysis models adjusting complement-activating anti-HLA DSA status on EDTA treated pan-IgG anti-HLA DSA assays.
3.9. Added prognostic value of complement-activating anti-HLA DSA status over anti-HLA DSA class type
Among the 29 (55.8%) studies that used multivariate analysis to evaluate the risk of allograft loss, only three (5.8%) studies included DSA class as a predictive variable. Among these three studies, two showed that HLA class II DR was significantly associated with graft loss. Complement activating anti-HLA DSA remained independently associated with an increased risk for graft loss HR=3.76 (CI=2.33-6.06; p=0.626; I2 = 0%) however, the results were statistically insignificant due to the low number of studies that included DSA type in the multivariable models.
4. Discussion
4.1. Study overview
In this systematic review, meta-analysis, and critical appraisal including 11,035 solid organ recipients, we confirmed the increased risk of allograft failure and rejection associated with complement-binding anti-HLA DSAs. To the best of our knowledge, this is the first comprehensive systematic review and meta-analysis on the topic and the first in-depth critical appraisal assessing for the risk of bias, adjusting for it and providing several subgroup analyses to study the association of complement-binding anti-HLA DSAs with allograft outcomes. We also addressed the utility of complement-activating anti-HLA DSAs assessment over anti-HLA DSA MFI levels.
4.2. Subgroup analyses findings
This meta-analysis showed consistent results in multiple subgroup analyses. Complement-activating anti-HLA DSA were associated with an increased risk for allograft loss in higher quality studies, in different types of complement-activating anti-HLA DSAs (C1q, C3d, C4d and IgG3), at different times of evaluation for complement-activating anti-HLA DSA status (before and after transplantation) and at different MFI thresholds for complement-activating anti-HLA DSA positivity.
4.3. Cumulative meta-analysis findings
The cumulative meta-analysis further illustrated the significant overall effect of complement activating anti-HLA DSAs on allograft loss. Combining this finding with our findings from the subgroup analyses, we can perceive saturation of knowledge in particular in kidney transplant recipients and C1q evaluations. This is due to the fact that the majority of patients assessed were kidney recipients (78%) who were tested for C1q (54%) and therefore further research in this particular area could be redundant. However, there remains some areas that could benefit from further exploration, for instance, we did not identify any studies on the effect of complement-binding anti-HLA DSAs in pancreas and intestine transplants. In addition, more studies in liver, lung and heart recipients could be beneficial to confirm the initial findings by increasing the sample size and by comparing the risk of allograft loss across different organ transplants.
4.4. Added prognostic value of complement-activating anti-HLA DSA status over anti-HLA DSA MFI level
Several studies in this meta-analysis and in the literature (53, 71, 72) indicated a strong correlation between complement-activating antibody status and anti-HLA DSA MFI level. Interestingly, studies included in this meta-analysis that performed multivariable analyses for the assessment of the independent prognostic value of complement-activating anti-HLA DSA adjusted on pan-IgG anti-HLA DSA defined by the MFI levels, showed that the association between C1q, C3d, C4d-binding tests or IgG3 test and allograft lost was independent of anti-HLA DSA MFI levels.
Although the absence of DSA complement binding antibodies should not be considered as a lack of the harmful effects of DSA in vivo, our meta-analysis supports a clinical utility of performing complement-binding assays. Indeed, the clinical impact remains significantly associated with graft loss independent of anti-HLA DSA MFI levels.
In addition to the uncertain association between the MFI levels and the clinical significance of an antibody, SAB pan-IgG assay remains a semi-quantitative test and technical limitations have been raised such as significant variations in repeated testing, between different laboratories (73), and due to various interfering substances (74). In addition, even though some studies addressed interfering substances by pretreatment of sera with EDTA (12, 13, 50, 53, 59), several limitations were noted; the EDTA concentrations were inconsistent across the studies, two studies only pretreated a small sample of the studied populations (4-8 patients), and the prognostic advantage of EDTA treated sera over complement assays was not demonstrated.
Therefore, our study shows that the use of complement binding anti-HLA DSA in clinical practice, in complement to MFI levels, which remains gold standard, could enhance risk stratification.
4.5. Added prognostic value of complement-activating anti-HLA DSA status over anti-HLA DSA class
We could not show independent association of complement-activating anti-HLA DSA status over HLA-DSA class due insufficient data published so far (only 3 studies). Further studies should therefore investigate the independent impact of class I or class II anti-HLA DSA regardless of their ability to activate complement, but also investigate the clinical impact of class I versus class II complement-activating anti-HLA DSA.
4.6. Implications
This study addresses several gaps highlighted by the STAR working group including the strong evidence regarding the prognostic role of complement-activating anti-HLA DSA in allograft rejection and loss, in complement to HLA-DSA titre and MFI assessment. This strongly supports a potential role for this test in clinical practice. and encourages interventional research regarding the role of certain drugs that target complement-dependent cytotoxicity as a prophylaxis and/or treatment of antibody-mediated rejection and the value of a complement-activating anti-HLA DSA based strategy to monitor organ transplant patients to demonstrate clinical benefit and improvement of allograft survival.
4.7. Limitations
This study has the following limitations. First, we only included studies that provided a clear effect size for allograft loss or rejection (hazard or odds ratio). Second, No data was available from South America, Africa and Australia to reinforce the generalizability of the results. Third, all of the included studies were observational and retrospective. Finally, the review only included studies written in English.
5. Conclusion
The results of this systematic review, meta-analysis and critical appraisal support the significant and independent detrimental effects of complement-activating anti-HLA DSAs on allograft outcomes. This study highlights areas that need further exploration in complement-activating anti-HLA DSAs research, and encourages the clinical evaluation of complement-activating anti-HLA DSA testing to improve risk stratification and tailoring treatment regimens.
Data availability statement
The raw data supporting the conclusions of this article will be made available upon reasonable request.
Author contributions
S-AA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing- original draft, Writing- review & editing. MR: Formal analysis, Methodology, Supervision, Validation, Writing- review & editing. KL: Methodology, Supervision, Validation, Writing- review & editing. AB: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing- original draft, Writing- review & editing. J-LT: Validation, Writing- review & editing. OA: Methodology, Validation, Writing- review & editing. AL: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing- review & editing. CL: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing- review & editing.
Funding Statement
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1265796/full#supplementary-material
References
- 1. Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2012) 12(2):388–99. doi: 10.1111/j.1600-6143.2011.03840.x [DOI] [PubMed] [Google Scholar]
- 2. Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol (2012) 8(6):348–57. doi: 10.1038/nrneph.2012.81 [DOI] [PubMed] [Google Scholar]
- 3. Morrell MR, Pilewski JM, Gries CJ, Pipeling MR, Crespo MM, Ensor CR, et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant Off Publ Int Soc Heart Transplant (2014) 33(12):1288–94. doi: 10.1016/j.healun.2014.07.018 [DOI] [PubMed] [Google Scholar]
- 4. Ho EK, Vlad G, Vasilescu ER, de la Torre L, Colovai AI, Burke E, et al. Pre- and posttransplantation allosensitisation in heart allograft recipients: major impact of de novo alloantibody production on allograft survival. Hum Immunol (2011) 72(1):5–10. doi: 10.1016/j.humimm.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 5. O’Leary JG, Kaneku H, Susskind BM, Jennings LW, Neri MA, Davis GL, et al. High mean fluorescence intensity donor-specific anti-HLA antibodies associated with chronic rejection Postliver transplant. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2011) 11(9):1868–76. doi: 10.1111/j.1600-6143.2011.03593.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loupy A, Lefaucheur C, Vernerey D, Prugger C, van Huyen JPD, Mooney N, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med (2013) 369(13):1215–26. doi: 10.1056/NEJMoa1302506 [DOI] [PubMed] [Google Scholar]
- 7. Calp-Inal S, Ajaimy M, Melamed ML, Savchik C, Masiakos P, Colovai A, et al. The prevalence and clinical significance of C1q-binding donor-specific anti-HLA antibodies early and late after kidney transplantation. Kidney Int (2016) 89(1):209–16. doi: 10.1038/ki.2015.275 [DOI] [PubMed] [Google Scholar]
- 8. Crespo M, Torio A, Mas V, Redondo D, Pérez-Sáez MJ, Mir M, et al. Clinical relevance of pretransplant anti-HLA donor-specific antibodies: does C1q-fixation matter? Transpl Immunol (2013) 29(1–4):28–33. doi: 10.1016/j.trim.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 9. Moktefi A, Parisot J, Desvaux D, Canoui-Poitrine F, Brocheriou I, Peltier J, et al. C1q binding is not an independent risk factor for kidney allograft loss after an acute antibody-mediated rejection episode: a retrospective cohort study. Transpl Int Off J Eur Soc Organ Transplant (2017) 30(3):277–87. doi: 10.1111/tri.12905 [DOI] [PubMed] [Google Scholar]
- 10. Bouquegneau A, Loheac C, Aubert O, Bouatou Y, Viglietti D, Empana JP, et al. Complement-activating donor-specific anti-HLA antibodies and solid organ transplant survival: A systematic review and meta-analysis. PloS Med (2018) 15(5):e1002572. doi: 10.1371/journal.pmed.1002572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lan JH, Gjertson D, Zheng Y, Clark S, DeKAF Investigators. Reed EF, et al. Clinical utility of complement-dependent C3d assay in kidney recipients presenting with late allograft dysfunction. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2018) 18(12):2934–44. doi: 10.1111/ajt.14871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Courant M, Visentin J, Linares G, Dubois V, Lepreux S, Guidicelli G, et al. The disappointing contribution of anti-human leukocyte antigen donor-specific antibodies characteristics for predicting allograft loss. Nephrol Dial Transplant (2018) 33(10):1853–63. doi: 10.1093/ndt/gfy088 [DOI] [PubMed] [Google Scholar]
- 13. Lee DR, Kim BC, Kim JP, Kim IG, Jeon MY. C3d-binding donor-specific HLA antibody is associated with a high risk of antibody-mediated rejection and graft loss in stable kidney transplant recipients: A single-centre cohort study. Transplant Proc (2018) 50(10):3452–9. doi: 10.1016/j.transproceed.2018.06.037 [DOI] [PubMed] [Google Scholar]
- 14. Sicard A, Ducreux S, Rabeyrin M, Couzi L, McGregor B, Badet L, et al. Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J Am Soc Nephrol JASN (2015) 26(2):457–67. doi: 10.1681/ASN.2013101144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (2009) 339:b2535. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sampson M, de Bruijn B, Urquhart C, Shojania K. Complementary approaches to searching MEDLINE may be sufficient for updating systematic reviews. J Clin Epidemiol (2016) 78:108–15. doi: 10.1016/j.jclinepi.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 17. Garner P, Hopewell S, Chandler J, MacLehose H, Akl EA, Beyene J, et al. When and how to update systematic reviews: consensus and checklist. BMJ (2016) 354:i3507. doi: 10.1136/bmj.i3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ottawa Hospital Research Institute . (2021). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 19. Cochrane handbook for systematic reviews of interventions (2021). Available at: https://training.cochrane.org/handbook.
- 20. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Aert RCM, Wicherts JM, van Assen MALM. Publication bias examined in meta-analyses from psychology and medicine: A meta-meta-analysis. PloS One (2019) 14(4):e0215052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol (2008) 61(10):991–6. doi: 10.1016/j.jclinepi.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 23. McPheeters ML, Kripalani S, Peterson NB, Idowu RT, Jerome RN, Potter SA, et al. Closing the quality gap: revisiting the state of the science (vol. 3: quality improvement interventions to address health disparities). Evid ReportTechnology Assess (2012) 208.3):1–475. [PMC free article] [PubMed] [Google Scholar]
- 24. Lau J, Schmid CH, Chalmers TC. Cumulative meta-analysis of clinical trials builds evidence for exemplary medical care. J Clin Epidemiol (1995) 48(1):45–57; discussion 59-60. doi: 10.1016/0895-4356(94)00106-Z [DOI] [PubMed] [Google Scholar]
- 25. Mullen B, Muellerleile P, Bryant B. Cumulative meta-analysis: A consideration of indicators of sufficiency and stability. Sage Journals (2001) 27(11). doi: 10.1177/01461672012711006 [DOI] [Google Scholar]
- 26. Konvalinka A, Tinckam K. Utility of HLA antibody testing in kidney transplantation. J Am Soc Nephrol (2015) 26(7):1489–502. doi: 10.1681/ASN.2014080837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wahrmann M, Bartel G, Exner M, Regele H, Körmöczi GF, Fischer GF, et al. Clinical relevance of preformed C4d-fixing and non-C4d-fixing HLA single antigen reactivity in renal allograft recipients. Transpl Int Off J Eur Soc Organ Transplant (2009) 22(10):982–9. doi: 10.1111/j.1432-2277.2009.00912.x [DOI] [PubMed] [Google Scholar]
- 28. Hönger G, Wahrmann M, Amico P, Hopfer H, Böhmig GA, Schaub S. C4d-fixing capability of low-level donor-specific HLA antibodies is not predictive for early antibody-mediated rejection. Transplantation (2010) 89(12):1471–5. doi: 10.1097/TP.0b013e3181dc13e7 [DOI] [PubMed] [Google Scholar]
- 29. Sutherland SM, Chen G, Sequeira FA, Lou CD, Alexander SR, Tyan DB. Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr Transplant (2012) 16(1):12–7. doi: 10.1111/j.1399-3046.2011.01599.x [DOI] [PubMed] [Google Scholar]
- 30. Hönger G, Hopfer H, Arnold ML, Spriewald BM, Schaub S, Amico P. Pretransplant IgG subclasses of donor-specific human leukocyte antigen antibodies and development of antibody-mediated rejection. Transplantation (2011) 92(1):41–7. doi: 10.1097/TP.0b013e31821cdf0d [DOI] [PubMed] [Google Scholar]
- 31. Smith JD, Banner NR, Hamour IM, Ozawa M, Goh A, Robinson D, et al. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am J Transplant (2011) 11(2):312–9. doi: 10.1111/j.1600-6143.2010.03383.x [DOI] [PubMed] [Google Scholar]
- 32. Kaneku H, O’Leary JG, Taniguchi M, Susskind BM, Terasaki PI, Klintmalm GB. Donor-specific human leukocyte antigen antibodies of the immunoglobulin G3 subclass are associated with Chronic rejection and graft loss after liver transplantation. Liver Transpl (2012) 18(8):984–92. doi: 10.1002/lt.23451 [DOI] [PubMed] [Google Scholar]
- 33. Bartel G, Wahrmann M, Schwaiger E, Kikić Ž, Winzer C, Hörl WH, et al. Solid phase detection of C4d-fixing HLA antibodies to predict rejection in high immunological risk kidney transplant recipients. Transpl Int (2013) 26(2):121–30. doi: 10.1111/tri.12000 [DOI] [PubMed] [Google Scholar]
- 34. Lawrence C, Willicombe M, Brookes PA, Santos-Nunez E, Bajaj R, Cook T, et al. Preformed complement-activating low-level donor-specific antibody predicts early antibody-mediated rejection in renal allografts. Transplantation (2013) 95(2):341–6. doi: 10.1097/TP.0b013e3182743cfa [DOI] [PubMed] [Google Scholar]
- 35. Freitas MCS, Rebellato LM, Ozawa M, Nguyen A, Sasaki N, Everly M, et al. The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation (2013) 95(9):1113–9. doi: 10.1097/TP.0b013e3182888db6 [DOI] [PubMed] [Google Scholar]
- 36. Arnold ML, Ntokou IS, Doxiadis IIN, Spriewald BM, Boletis JN, Iniotaki AG. Donor-specific HLA antibodies: evaluating the risk for graft loss in renal transplant recipients with isotype switch from complement fixing IgG1/IgG3 to noncomplement fixing IgG2/IgG4 anti-HLA alloantibodies. Transpl Int (2014) 27(3):253–61. doi: 10.1111/tri.12206 [DOI] [PubMed] [Google Scholar]
- 37. Smith JD, Ibrahim MW, Newell H, Danskine AJ, Soresi S, Burke MM, et al. Pre-transplant donor HLA-specific antibodies: Characteristics causing detrimental effects on survival after lung transplantation. J Heart Lung Transplant (2014) 33(10):1074–82. doi: 10.1016/j.healun.2014.02.033 [DOI] [PubMed] [Google Scholar]
- 38. Everly MJ, Rebellato LM, Haisch CE, Briley KP, Bolin P, Kendrick WT, et al. Impact of IgM and IgG3 anti-HLA alloantibodies in primary renal allograft recipients. Transplantation (2014) 97(5):494–501. doi: 10.1097/01.TP.0000441362.11232.48 [DOI] [PubMed] [Google Scholar]
- 39. O’Leary JG, Kaneku H, Banuelos N, Jennings LW, Klintmalm GB, Terasaki PI. Impact of igG3 subclass and C1q-fixing donor-specific HLA alloantibodies on rejection and survival in liver transplantation. Am J Transplant (2015) 15(4):1003–13. doi: 10.1111/ajt.13153 [DOI] [PubMed] [Google Scholar]
- 40. Wozniak LJ, Hickey MJ, Venick RS, Vargas JH, Farmer DG, Busuttil RW, et al. Donor-specific HLA antibodies are associated with late allograft dysfunction after paediatric liver transplantation. Transplantation (2015) 99(7):1416–22. doi: 10.1097/TP.0000000000000796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khovanova N, Daga S, Shaikhina T, Krishnan N, Jones J, Zehnder D, et al. Subclass analysis of donor HLA-specific IgG in antibody-incompatible renal transplantation reveals a significant association of IgG4 with rejection and graft failure. Transpl Int (2015) 28(12):1405–15. doi: 10.1111/tri.12648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thammanichanond D, Wiwattanathum P, Mongkolsuk T, Kantachuvesiri S, Worawichawong S, Vallipakorn SA, et al. Role of pretransplant complement-fixing donor-specific antibodies identified by C1q assay in kidney transplantation. Transplant Proc (2016) 48(3):756–60. doi: 10.1016/j.transproceed.2015.12.116 [DOI] [PubMed] [Google Scholar]
- 43. Comoli P, Cioni M, Tagliamacco A, Quartuccio G, Innocente A, Fontana I, et al. Acquisition of C3d-binding activity by de novo donor-specific HLA antibodies correlates with graft loss in nonsensitised pediatric kidney recipients. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2016) 16(7):2106–16. doi: 10.1111/ajt.13700 [DOI] [PubMed] [Google Scholar]
- 44. Yamamoto T, Watarai Y, Takeda A, Tsujita M, Hiramitsu T, Goto N, et al. De novo anti-HLA DSA characteristics and subclinical antibody-mediated kidney allograft injury. Transplantation (2016) 100(10):2194–202. doi: 10.1097/TP.0000000000001012 [DOI] [PubMed] [Google Scholar]
- 45. Malheiro J, Tafulo S, Dias L, Martins LS, Fonseca I, Beirão I, et al. Determining donor-specific antibody C1q-binding ability improves the prediction of antibody-mediated rejection in human leucocyte antigen-incompatible kidney transplantation. Transpl Int (2017) 30(4):347–59. doi: 10.1111/tri.12873 [DOI] [PubMed] [Google Scholar]
- 46. Visentin J, Chartier A, Massara L, Linares G, Guidicelli G, Blanchard E, et al. Lung intragraft donor-specific antibodies as a risk factor for graft loss. J Heart Lung Transplant (2016) 35(12):1418–26. doi: 10.1016/j.healun.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 47. Kauke T, Oberhauser C, Lin V, Coenen M, Fischereder M, Dick A, et al. De novo donor-specific anti-HLA antibodies after kidney transplantation are associated with impaired graft outcome independently of their C1q-binding ability. Transpl Int (2017) 30(4):360–70. doi: 10.1111/tri.12887 [DOI] [PubMed] [Google Scholar]
- 48. Bamoulid J, Roodenburg A, Staeck O, Wu K, Rudolph B, Brakemeier S, et al. Clinical outcome of patients with de novo C1q-binding donor-specific HLA antibodies after renal transplantation. Transplantation (2017) 101(9):2165–74. doi: 10.1097/TP.0000000000001487 [DOI] [PubMed] [Google Scholar]
- 49. Fichtner A, Süsal C, Höcker B, Rieger S, Waldherr R, Westhoff JH, et al. Association of C1q-fixing DSA with late graft failure in paediatric renal transplant recipients. Pediatr Nephrol (2016) 31(7):1157–66. doi: 10.1007/s00467-016-3322-8 [DOI] [PubMed] [Google Scholar]
- 50. Guidicelli G, Guerville F, Lepreux S, Wiebe C, Thaunat O, Dubois V, et al. Non-complement–binding de novo donor-specific anti-HLA antibodies and kidney allograft survival. J Am Soc Nephrol (2016) 27(2):615–25. doi: 10.1681/ASN.2014040326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lefaucheur C, Viglietti D, Bentlejewski C, van Huyen JPD, Vernerey D, Aubert O, et al. IgG donor-specific anti-human HLA antibody subclasses and kidney allograft antibody-mediated injury. J Am Soc Nephrol (2016) 27(1):293–304. doi: 10.1681/ASN.2014111120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Viglietti D, Loupy A, Vernerey D, Bentlejewski C, Gosset C, Aubert O, et al. Value of donor–specific anti–HLA antibody monitoring and characterization for risk stratification of kidney allograft loss. J Am Soc Nephrol (2017) 28(2):702–15. doi: 10.1681/ASN.2016030368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wiebe C, Gareau AJ, Pochinco D, Gibson IW, Ho J, Birk PE, et al. Evaluation of C1q status and titer of de novo donor-specific antibodies as predictors of allograft survival. Am J Transplant (2017) 17(3):703–11. doi: 10.1111/ajt.14015 [DOI] [PubMed] [Google Scholar]
- 54. Sicard A, Meas-Yedid V, Rabeyrin M, Koenig A, Ducreux S, Dijoud F, et al. Computer-assisted topological analysis of renal allograft inflammation adds to risk evaluation at diagnosis of humoral rejection. Kidney Int (2017) 92(1):214–26. doi: 10.1016/j.kint.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 55. Das BB, Lacelle C, Zhang S, Gao A, Fixler D. Complement (C1q) binding de novo donor-specific antibodies and cardiac-allograft vasculopathy in pediatric heart transplant recipients. Transplantation (2018) 102(3):502–9. doi: 10.1097/TP.0000000000001944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Couchonnal E, Rivet C, Ducreux S, Dumortier J, Bosch A, Boillot O, et al. Deleterious impact of C3d-binding donor-specific anti-HLA antibodies after pediatric liver transplantation. Transpl Immunol (2017) 45:8–14. doi: 10.1016/j.trim.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 57. Bailly E, Anglicheau D, Blancho G, Gatault P, Vuiblet V, Chatelet V, et al. Prognostic value of the persistence of C1q-binding anti-HLA antibodies in acute antibody-mediated rejection in kidney transplantation. Transplantation (2018) 102(4):688–98. doi: 10.1097/TP.0000000000002002 [DOI] [PubMed] [Google Scholar]
- 58. Molina J, Navas A, Agüera ML, Rodelo-Haad C, Alonso C, Rodríguez-Benot A, et al. Impact of preformed donor-specific anti-human leukocyte antigen antibody C1q-binding ability on kidney allograft outcome. Front Immunol (2017) 8:1310. doi: 10.3389/fimmu.2017.01310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brugière O, Roux A, Le Pavec J, Sroussi D, Parquin F, Pradère P, et al. Role of C1q-binding anti-HLA antibodies as a predictor of lung allograft outcome. Eur Respir J (2018) 52(2):1701898. doi: 10.1183/13993003.01898-2017 [DOI] [PubMed] [Google Scholar]
- 60. Kamburova EG, Wisse BW, Joosten I, Allebes WA, van der Meer A, Hilbrands LB, et al. Pretransplant C3d-fixing donor-specific anti-HLA antibodies are not associated with increased risk for kidney graft failure. J Am Soc Nephrol (2018) 29(9):2279–85. doi: 10.1681/ASN.2018020205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Viglietti D, Bouatou Y, Kheav VD, Aubert O, Suberbielle-Boissel C, Glotz D, et al. Complement-binding anti-HLA antibodies are independent predictors of response to treatment in kidney recipients with antibody-mediated rejection. Kidney Int (2018) 94(4):773–87. doi: 10.1016/j.kint.2018.03.015 [DOI] [PubMed] [Google Scholar]
- 62. Lee H, Han E, Choi AR, Ban TH, Chung BH, Yang CW, et al. Clinical impact of complement (C1q, C3d) binding De Novo donor-specific HLA antibody in kidney transplant recipients. PloS One (2018) 13(11):e0207434. doi: 10.1371/journal.pone.0207434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Malheiro J, Santos S, Tafulo S, Dias L, Martins LS, Fonseca I, et al. Detection of complement-binding donor-specific antibodies, not IgG-antibody strength nor C4d status, at antibody-mediated rejection diagnosis is an independent predictor of kidney graft failure. Transplantation (2018) 102(11):1943–54. doi: 10.1097/TP.0000000000002265 [DOI] [PubMed] [Google Scholar]
- 64. Schinstock CA, Dadhania DM, Everly MJ, Smith B, Gandhi M, Farkash E, et al. Factors at de novo donor-specific antibody initial detection associated with allograft loss: a multicentre study. Transpl Int (2019) 32(5):502–15. doi: 10.1111/tri.13395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Babu A, Khovanova N, Shaw O, Griffin S, Briggs D, Krishnan NS, et al. C3d-positive donor-specific antibodies have a role in pretransplant risk stratification of cross-match-positive HLA-incompatible renal transplantation: United Kingdom multicentre study. Transpl Int Off J Eur Soc Organ Transplant (2020) 33(9):1128–39. doi: 10.1111/tri.13663 [DOI] [PubMed] [Google Scholar]
- 66. Gautier Vargas G, Olagne J, Parissiadis A, Joly M, Cognard N, Perrin P, et al. Does a useful test exist to properly evaluate the pathogenicity of donor-specific antibodies? Lessons from a comprehensive analysis in a well-studied single-centre kidney transplant cohort. Transplantation (2020) 104(10):2148–57. doi: 10.1097/TP.0000000000003080 [DOI] [PubMed] [Google Scholar]
- 67. Zhang Q, Hickey M, Drogalis-Kim D, Zheng Y, Gjertson D, Cadeiras M, et al. Understanding the correlation between DSA, complement activation, and antibody-mediated rejection in heart transplant recipients. Transplantation (2018) 102(10):e431. doi: 10.1097/TP.0000000000002333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cioni M, Nocera A, Tagliamacco A, Basso S, Innocente A, Fontana I, et al. Failure to remove de novo donor-specific HLA antibodies is influenced by antibody properties and identifies kidney recipients with late antibody-mediated rejection destined to graft loss – a retrospective study. Transpl Int (2019) 32(1):38–48. doi: 10.1111/tri.13325 [DOI] [PubMed] [Google Scholar]
- 69. Hayde N, Solomon S, Caglar E, Ge J, Qama E, Colovai A. C1q-binding DSA and allograft outcomes in pediatric kidney transplant recipients. Pediatr Transplant (2021) 25(2):e13885. doi: 10.1111/petr.13885 [DOI] [PubMed] [Google Scholar]
- 70. Pernin V, Beyze A, Szwarc I, Bec N, Salsac C, Perez-Garcia E, et al. Distribution of de novo Donor-Specific Antibody Subclasses Quantified by Mass Spectrometry: High IgG3 Proportion Is Associated With Antibody-Mediated Rejection Occurrence and Severity. Front Immunol (2020) 11:919. doi: 10.3389/fimmu.2020.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schaub S, Hönger G, Koller MT, Liwski R, Amico P. Determinants of C1q binding in the single antigen bead assay. Transplantation (2014) 98(4):387–93. doi: 10.1097/TP.0000000000000203 [DOI] [PubMed] [Google Scholar]
- 72. Yell M, Muth BL, Kaufman DB, Djamali A, Ellis TM. C1q binding activity of de novo donor-specific HLA antibodies in renal transplant recipients with and without antibody-mediated rejection. Transplantation (2015) 99(6):1151–5. doi: 10.1097/TP.0000000000000699 [DOI] [PubMed] [Google Scholar]
- 73. Reed EF, Rao P, Zhang Z, Gebel H, Bray RA, Guleria I, et al. Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2013) 13(7):1859–70. doi: 10.1111/ajt.12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zachary AA, Lucas DP, Detrick B, Leffell MS. Naturally occurring interference in Luminex assays for HLA-specific antibodies: characteristics and resolution. Hum Immunol (2009) 70(7):496–501. doi: 10.1016/j.humimm.2009.04.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available upon reasonable request.