Abstract
Purpose
In recent years, immune checkpoint inhibitors have been used in combination with tyrosine kinase inhibitors and local therapies, creating a new era in treating hepatocellular carcinoma (HCC) with portal vein tumor thrombus (PVTT). However, the benefits of this triple therapy remain unclear. Thus, this study evaluated whether the combination of transarterial chemoembolization (TACE), lenvatinib, and programmed death-1 (PD-1) inhibitors (triple therapy) was effective and safe for unresectable HCC with main trunk portal vein tumor thrombus (Vp4).
Patients and Methods
This study enrolled patients receiving triple therapy at four institutions between August 2018 and April 2022. Patient characteristics and course of treatment were extracted from patient records. Tumors and tumor thrombus response were evaluated using an HCC-specific modified RECIST. Kaplan–Meier curve analysis demonstrated overall survival (OS) and progression-free survival (PFS). Adverse events (AEs) were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
Results
Median follow-up duration was 18 (4.0–26.3) months. Overall, 41 patients with HCC and Vp4 receiving first-line triple therapy were enrolled. The intrahepatic tumor objective response rate was 68.3%. The median OS was 21.7 (range, 2.8–30.5) months, whereas the median PFS was 14.5 (range, 1.3–27.6) months. Twelve patients received sequential resections. Resection was independently associated with favorable OS and PFS. Fever (31.7%), hypertension (26.8%), fatigue (24.4%), abnormal liver function (63.4%) and decreased appetite (21.9%) were the AEs frequently associated with treatment. No treatment-related mortality occurred.
Conclusion
TACE plus lenvatinib and PD-1 inhibition was effective and tolerable for treating unresectable HCC with Vp4, with a high tumor response rate and favorable prognosis.
Keywords: transarterial chemoembolization, lenvatinib, programmed death 1 inhibitor, hepatic cellular carcinoma, main trunk portal vein, triple therapy
Introduction
Hepatocellular carcinoma (HCC) is a major cause of cancer-associated mortality worldwide, with a poor prognosis.1 A dismal prognostic factor is portal vein tumor thrombus (PVTT), detected in approximately 10–60% of HCC patients at diagnosis and portends a median survival rate of 2.7–4.0 months without treatment.2
HCC with PVTT is considered an advanced disease, and systemic therapies, such as sorafenib and lenvatinib, are recommended as a first-line treatment by the Barcelona Clinic Liver Cancer (BCLC) and the European Association for the Study of the Liver (EASL).3 Hepatic arterial infusion chemotherapy is the preferred treatment according to Japanese and Chinese guidelines.4,5 However, survival benefits are modest.
HCC complicated by PVTT is a contraindication to transarterial chemoembolization (TACE) since it may disrupt the hepatic artery supply and induce ischemia-related post-TACE liver failure.6 However, certain patients can tolerate and benefit from TACE in cases of adequate liver function and sufficient blocking sites surrounding the collateral circulation.7–9 However, the therapeutic effects are often unsatisfactory. Recently, combined local and systemic treatment strategies that may improve outcomes were introduced for HCC with PVTT.
Over recent years, immune checkpoint inhibitors (ICIs) have been used, especially in combination with tyrosine kinase inhibitors (TKIs) and local therapies, entering a novel era in treating HCC complicated by PVTT. However, previous Phase III randomized controlled trials have often excluded HCC with Vp4. Therefore, ICIs still have unclear prognostic outcomes for HCC with Vp4.
According to our previous results, triple therapy (TACE and lenvatinib combined with programmed death-1 [PD-1] antibody immunotherapy) is effective and tolerable for HCC, even in patients with PVTT, and a high rate of conversion to surgery.10 Therefore, this study comprehensively analyzed the efficacy and safety of triple therapy in patients with HCC complicated by Vp4.
Materials and Methods
Patients
This retrospective study enrolled consecutive patients with HCC with Vp4 who received first-line triple therapy between August 2018 and April 2022 at the following four Chinese institutions: Fujian Provincial Hospital, First Affiliated Hospital of Fujian Medical University, Affiliated Hospital of Guilin Medical University, and Zhangzhou Affiliated Hospital of Fujian Medical University. The Institutional Ethics Committees of the above institutions approved the study protocol, and the study was carried out in accordance with the principles stated in the Declarations of Helsinki. All patients provided informed consent for use of their data in this research.
HCC diagnosis was made according to local and international guidelines, while Vp4 was diagnosed based on hyperenhancement within the thrombus on the arterial phase and with a subsequent washout on the portal vein and delayed phases and one low-attenuation intraluminal filling defect within the portal vein main trunk during the portal phase.11–13
The inclusion criteria were as follows: 1) aged 18–75 years; 2) Eastern Cooperative Oncology Group (ECOG) performance status (PS) score 0–1; 3) Child-Pugh class A and B; 4) HCC with Vp4 with triple therapy as first-line treatment; and 5) one or more target lesions with a measurable diameter. The exclusion criteria included: 1) use of additional anticancer treatments; 2) additional malignant diseases; 3) insufficient data; and 4) any previous anti-tumor treatments.
Procedures
Each patient was started on per oral lenvatinib (8 and 12 mg for body weights <60 and ≥60 kg, respectively) once a day and intravenous anti-PD-1 antibodies—camrelizumab (200 mg q3w), tislelizumab (200 mg q3w), or sintilimab (200 mg q3w) on day 4–14 after TACE depending on the patient’s symptoms and liver function. TACE was performed within 7 days of diagnosis via the femoral artery under local anesthesia. A microcatheter was introduced into the subsegmental or segmental feeding arteries. Iodized oil was blended with pirarubicin before injection in the target tumor arteries, the dose of pirarubicin was based on patient liver function, tumor size, vascularity, and body surface area. Subsequently, All tumor-feeding arteries were superselectively embolized using gelatin sponge particles until a significant reduction in arterial flow was observed. The amount of emulsion injected depended on tumor volume. If an artery–portal vein fistula was performed, it was occluded before mixture embolization by a spiral steel ring based on angiography images and the doctor’s experience. The treatment cycle was performed according to the evaluation of MRI/CT, tumor markers and liver test. Before and after TACE, lenvatinib and anti-PD-1 antibody administration was terminated for 3 days. Patients with untreated hepatitis B received an oral antiviral drug (entecavir or tenofovir) for the duration of anticancer therapy. When patients were evaluated as exhibiting drug resistance or unacceptable drug side effects, they were treated according to a multidisciplinary approach switching to second-line regimens, HAIC, or radiation therapy.
Clinical Assessments and Toxicity Evaluation
Tumor assessments were performed every 4–6 weeks. Upper abdominal contrast-enhanced computed tomography (CT) or magnetic resonance imaging were performed. Two radiologists with >6 years of experience in abdominal diagnosis performed a consensus evaluation of the images.
Tumor and tumor thrombus responses were evaluated in line with HCC-specific modified Response Evaluation Criteria in Solid Tumors (mRECIST): complete response of tumor thrombus was defined as total disappearance of enhancement inside portal vein thrombus.14 Thrombus regression was defined as an obvious reduction in length. The Common Terminology Criteria for Adverse Events (version 5.0) was employed for toxicity evaluation.
Indications and Procedures of Hepatic Resection
Once patients fulfilled the indication for surgery, curative-intent resection was performed. All other cases received treatment until tumor progression, withdrawal of consent, or intolerable toxic effects occurred. Preoperative assessments included blood tests and imaging. The indications for hepatic resection of HCC with Vp4 were as follows: (1) R0 resection achievable with adequate residual liver volume (40% and 30% of standard liver volume in liver cirrhosis and non-cirrhosis cases, respectively); (2) tumor thrombus regression at least near the bifurcation of the portal vein or complete response (CR); (3) absence of severe or persistent adverse events after triple therapy; (4) no extrahepatic metastasis; (5) absence of contraindications to hepatectomy.
Lenvatinib was stopped for 1 week or longer and anti-PD-1 antibodies for 3 weeks or longer in cases with selective hepatic resection. The surgical method depended on the response of the thrombus, including en bloc resection of the thrombus with the tumor when the thrombus regressed, was extracted from the portal vein with thrombus regression, or tumor resection alone without removal of the thrombus in patients with CR.
The Clavien–Dindo classification was employed for evaluating complications after surgery. We used the standard 7-point baseline sampling protocol for the resected tumor specimens,15 and the entire tumor was sampled once viable tumor cells were absent. Pathological CR (pCR) was defined as no viable residual tumor cells within the tumor and thrombus resected.
Postoperative Management
Lenvatinib and anti-PD-1 antibodies were re-administered 4 weeks postoperatively, after the patients had complete recovery from hepatectomy. Postoperative radiological examinations were conducted at 3-month intervals or tumor recurrence. If no evidence of tumor recurrence was found, we recommended lenvatinib combined with anti-PD-1 antibodies for 6 months postoperatively. In cases of post-recurrence, treatment was selected according to a multidisciplinary approach.
Here, overall survival (OS) was the primary study endpoint, while progression-free survival (PFS), surgery rate, and treatment-related adverse events (TRAEs) were secondary study endpoints.
Statistical Analysis
Continuous variables found to be non-normally distributed by the Kolmogorov–Smirnov test were represented by median (range), whereas categorical variables were represented by numbers (percentages). OS represented the duration between triple therapy initiation and patient death. Patients who died from causes other than hepatic disease were censored on their death date. PFS represented the duration between treatment initiation and patient death or disease progression. Survival rate was predicted at every time point by Kaplan–Meier curve analysis. A Cox regression model was utilized for multivariate regression. P<0.05 indicated statistical significance. Statistical Package for Social Sciences (SPSS) software (version 27 SPSS, Inc., Chicago, IL, USA) was employed for all statistical analyses.
Results
Patient Features
Between December 2018 and August 2022, 41 patients with HCC with Vp4 receiving first-line triple therapy at four institutions were enrolled (Table 1). All 41 patients (including 36 aged <65 and 5 aged ≥65 years; 37 males and four females) had hepatitis B virus, and most (78%) were classified as Child-Pugh A. The ECOG-PS scores in 95.1% of patients were 0–1. Most patients had tumor size >5 cm (90.2%) and multiple lesions (75.6%). Extrahepatic metastasis was noted in 7.3% of patients. Twelve patients (29.3%) underwent resection, while two required resections after achieving CR but refused surgery. Additionally, 6 (14.6%) patients were switched to second-line regimens due to drug resistance or drug side effects.
Table 1.
Baseline Demographic and Clinical Characteristics of Patients with VP4
| Number (N=41) | |
|---|---|
| Gender | |
| Female | 4 (9.8) |
| Male | 37 (90.2) |
| Age | n (%) |
| <65 | 36 (87.8) |
| ≥65 | 5 (12.2) |
| Child-Pugh | |
| A | 32 (78.0) |
| B | 9 (22.0) |
| ECOG-PS, score | |
| 0–1 | 39 (95.1) |
| 2 | 2 (4.9) |
| Platelet, (×109/L) | |
| <100 | 5 (12.2) |
| ≥100 | 36 (87.8) |
| Total bilirubin, µmol/L | |
| <34 | 38 (92.7) |
| ≥34 | 3 (7.3) |
| Albumin g/L | |
| <35 | 10 (24.4) |
| ≥35 | 31 (75.6) |
| ALT, IU/L | |
| <40 | 18 (43.9) |
| ≥40 | 23 (56.1) |
| AST, IU/L | |
| <40 | 8 (19.5) |
| ≥40 | 33 (80.5) |
| ALBI grade | |
| 1 | 12 (29.3) |
| 2 | 29 (70.7) |
| AFP, ng/mL | |
| <400 | 14 (34.1) |
| ≥400 | 27 (65.9) |
| Maximum tumor size, cm | |
| <5 | 4 (9.8) |
| ≥5 | 37 (90.2) |
| Anti-PD-1 antibodies | |
| Sintilimab | 15 (36.6) |
| Tislelizumab | 20 (48.8) |
| Camrelizumab | 6(14.6) |
| Tumor number | |
| Solitary | 10 (24.4) |
| Multiple | 31 (75.6) |
| Operation | |
| Yes | 12 (29.3) |
| No | 29 (70.7) |
| Extrahepatic metastases | |
| Yes | 3 (7.3) |
| No | 38 (92.7) |
| HBsAg | |
| Negative | 0 (0.00) |
| Positive | 41 (100.0) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; ALT, alanine transaminase; AST, aspartate transaminase; ALBI, albumin-bilirubin; AFP, ɑ-fetoprotein; HBsAg, hepatitis B surface antigen.
Anti-PD-1 antibodies utilized here were sintilimab (n = 15), tislelizumab (n = 20), and camrelizumab (n = 6).
Tumor Response and Patient Survival
Data were collected up to December 30, 2022, and patients were followed up for a median of 18 months. The intrahepatic tumor objective response rate (ORR) was 68.3% (28/41). Five CR, 23 partial response, seven stable disease, and six progressive disease cases were noted according to mRECIST. Regarding the tumor thrombus response, nine patients achieved CR, and fifteen experienced thrombus regression.
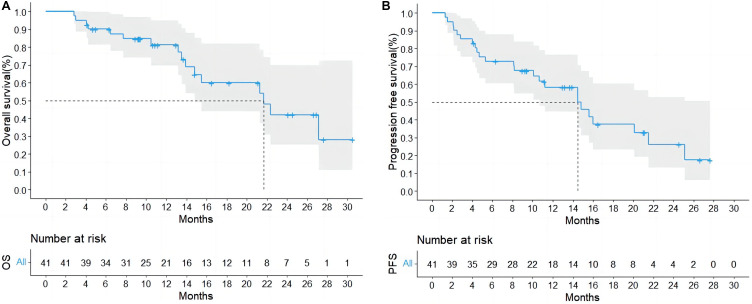
In total, twelve patients underwent surgical resection (surgical rate: 29.3%), and sixteen deaths were reported. In these cases. The median OS (mOS) and median PFS (mPFS) were 21.7 (range, 2.8–30.5) and 14.5 (range, 1.3–27.6) months, respectively (Figure 1A and B). All cases recovered well postoperatively.
Figure 1 .
Overall Survival (A) and Progression-Free Survival (B) for All Patients.
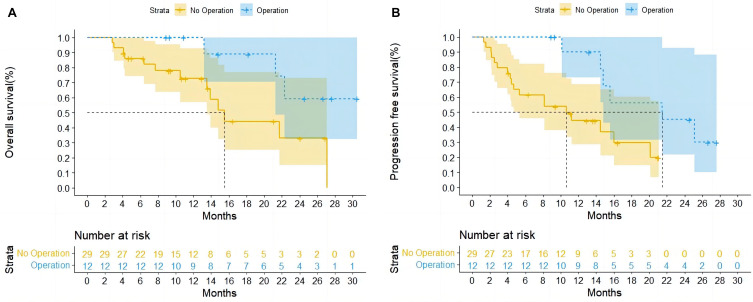
Upon multivariate regression, surgery showed independent relation to favorable OS (hazard ratio [HR]: 3.731; 95% confidence interval [CI], 1.038–13.407; P = 0.044) (Table 2) and PFS (HR, 3.492; 95% CI, 1.164–10.475; P = 0.026) (Table 3). According to Kaplan–Meier analysis, patients who underwent surgery had longer OS (mOS not reached vs 15.5 months) and PFS (mPFS 21.5 vs 10.7 months) than those who did not (Figure 2A and B).
Table 2.
Univariate and Multivariate Analysis of the Prognostic Factors for OS in All Patients
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender | 1.777 (0.126–2.522) | 0.453 | ||
| Male | ||||
| Female | ||||
| Age | 1.408 (0.561–2.512) | 0.655 | ||
| <65 | ||||
| ≥65 | ||||
| Child-Pugh | 0.642 (0.350–6.941) | 0.561 | ||
| A | ||||
| B | ||||
| ECOG-PS | 0.258 (0.430–2.719) | 0.218 | ||
| 0–1 | ||||
| 2 | ||||
| Platelet (10^9/L) | 0.998 (0.994–1.003) | 0.493 | ||
| ≥100 | ||||
| <100 | ||||
| Total bilirubin (µmol/L) | 0.981 (0.934–1.031) | 0.457 | ||
| ≥34 | ||||
| <34 | ||||
| Albumin (g/L) | 1.003 (0.895–1.124) | 0.956 | ||
| ≥35 | ||||
| <35 | ||||
| ALT (IU/L) | 0.908 (0.511–1.495) | 0.704 | ||
| ≥40 | ||||
| <40 | ||||
| AST (IU/L) | 0.737 (0.390–1.393) | 0.347 | ||
| ≥40 | ||||
| <40 | ||||
| ALBI grade | 1.051 (0.598–1.757) | 0.929 | ||
| ≥2 | ||||
| 1 | ||||
| AFP (ng/mL) | 0.300 (0.291–1.033) | 0.063 | ||
| ≥400 | ||||
| <400 | ||||
| Maximum tumor size (cm) | 0.543 (0.267–2.034) | 0.556 | ||
| ≥5 | ||||
| <5 | ||||
| Anti-PD-1 antibodies | 1.726 (0.267–3.864) |
0.732 | ||
| Sintilimab | ||||
| Tislelizumab | ||||
| Camrelizumab | ||||
| Tumor number | 1.910 (0.906–4.027) | 0.089 | ||
| Solitary | ||||
| Multiple | ||||
| Operation | 3.731 (1.038–13.407) | 0.044 | 3.731 (1.038–13.407) | 0.044 |
| Yes | ||||
| No | ||||
| Extrahepatic metastasis | 2.837 (0.327–4.583) | 0.344 | ||
| Yes | ||||
| No | ||||
Note: Bold values is to highlight the meaningful p-values.
Abbreviations: OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ALT, alanine transaminase; AST, aspartate transaminase; ALBI, albumin-bilirubin; AFP, ɑ-fetoprotein; HBsAg, hepatitis B surface antigen.
Table 3.
Univariate and Multivariate Analysis of the Prognostic Factors for PFS in All Patients
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender | 0.400 (0.134–1.196) | 0.101 | ||
| Male | ||||
| Female | ||||
| Age | 0.335 (0.813–3.671) | 0.155 | ||
| ≥65 | ||||
| <65 | ||||
| Child-Pugh | 0.740 (0.271–2.019) | 0.556 | ||
| A | ||||
| B | ||||
| ECOG-PS | 0.682 (0.306–1.520) | 0.350 | ||
| 0–1 | ||||
| 2 | ||||
| Platelet (×10^9/L) | 0.954 (0.216–4.213) | 0.951 | ||
| ≥100 | ||||
| <100 | ||||
| Total bilirubin (umol/L) | 0.909 (0.207–3.981) | 0.899 | ||
| ≥34 | ||||
| <34 | ||||
| Albumin (g/L) | 1.340 (0.451–3.988) | 0.598 | ||
| ≥35 | ||||
| <35 | ||||
| ALT (IU/L) | 0.204 (0.606–1.372) | 0.657 | ||
| ≥40 | ||||
| <40 | ||||
| AST (IU/L) | 0.989 (0.628–1.610) | 0.982 | ||
| ≥40 | ||||
| <40 | ||||
| ALBI grade (≥2 vs 1) | 0.894 (0.607–1.473) | 0.805 | ||
| ≥2 | ||||
| 1 | ||||
| AFP (ng/mL) | 1.316 (0.574–1.325) | 0.520 | ||
| ≥400 | ||||
| <400 | ||||
| Maximum tumor size (cm) | 0.940 (0.560–1.899) | 0.921 | ||
| ≥5 | ||||
| <5 | ||||
| Anti-PD-1 antibodies | 0.836 (0.256–1.632) | 0.687 | ||
| Sintilimab | ||||
| Tislelizumab | ||||
| Camrelizumab | ||||
| Tumor number | 0.340 (0.993–2.963) | 0.053 | ||
| Solitary | ||||
| Multiple | ||||
| Operation | 3.492 (1.164–10.475) | 0.026 | 3.492 (1.164–10.475) | 0.026 |
| Yes | ||||
| No | ||||
| Extrahepatic metastasis | 2.514 (0.554–11.411) | 0.332 | ||
| Yes | ||||
| No | ||||
Note: Bold values is to highlight the meaningful p-values.
Abbreviations: PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ALT, alanine transaminase; AST, aspartate transaminase; ALBI, albumin-bilirubin; AFP, ɑ-fetoprotein; HBsAg, hepatitis B surface antigen.
Figure 2 .
Overall Survival (A) and Progression-Free Survival (B) for Patients Who Underwent Surgery.
Surgical Procedure and Perioperative Findings
Median duration from triple therapy initiation to resection was 3.6 (range: 1.7–8.7) months in the 12 patients (median age of 55 (range: 37–75) years; one female) who underwent resection. Eleven cases were classified as Child-Pugh A, while one was Child-Pugh B. ECOG PS scores of eleven cases were 0, while the one case was 1. The median diameter of the largest tumor was 11.2 (range: 6.0–16.0) cm, and six patients had multiple lesions in the liver. The tumor thrombus was considered regressed in eight patients, and four patients experienced CR (complete necrosis). Five patients underwent en bloc tumor resection with the tumor thrombus, four underwent tumor thrombus extraction from the portal vein, and three underwent tumor resection without tumor thrombus extraction. Three patients had an intrahepatic tumor pCR, and seven had tumor thrombus pCR. Five cases had tumor recurrence following surgery (four with intrahepatic recurrence and one with pulmonary metastasis) (Table 4). No patients had intrahepatic tumor and tumor thrombus assessed as pCR recurrence at the data cutoff.
Table 4.
Characteristics of 12 Patients Before and After Surgery
| Patient No. | Tumor Thrombus Regression | Surgical Method for Thrombus | Intrahepatic Tumor pCR | Tumor Thrombus pCR | Recurrence | Recurrence Site |
|---|---|---|---|---|---|---|
| 1 | Yes | En bloc resection | No | No | No | |
| 2 | Yes | En bloc resection | No | No | No | |
| 3 | Yes | Extraction | No | No | Yes | Lung |
| 4 | Yes | Extraction | No | No | Yes | Liver |
| 5 | Yes | En bloc resection | Yes | Yes | No | |
| 6 | No | Not extraction | No | Yes | No | |
| 7 | Yes | En bloc resection | No | No | Yes | Liver |
| 8 | Yes | En bloc resection | Yes | Yes | No | |
| 9 | No | Not extraction | No | Yes | Yes | Liver |
| 10 | No | Not extraction | No | Yes | Yes | Liver |
| 11 | Yes | Extraction | Yes | Yes | No | |
| 12 | No | Extraction | No | Yes | No |
Abbreviation: pCR, pathological complete response.
Adverse Events
TRAEs are listed in Table 5. Thirty-five patients (85.3%) reported one or more TRAEs. Fever (31.7%), hypertension (26.8%), fatigue (24.4%), abnormal liver function (63.4%), and decreased appetite (24.4%) were frequently reported. Most patients had minor-to-moderate TRAEs, and no deaths were induced by toxicity. Four patients (9.8%) suffered from grade 4 TRAEs.
Table 5.
Treatment-Related Adverse Events
| Adverse Events | Any Grade n(%) | Grade 1–2 n(%) | Grade 3 n(%) | Grade 4 n(%) |
|---|---|---|---|---|
| Total | 35(85.3) | 28(68.3) | 7(17.0) | – |
| Fatigue | 10(24.4) | 8(19.5) | 2(4.9) | – |
| Decreased appetite | 10(24.4) | 7(17.1) | 3(7.3) | – |
| Fever | 13(31.7) | 12(29.3) | 1(2.4) | – |
| Nausea | 7(17.1) | 6(14.6) | 1(2.5) | – |
| Vomiting | 4(9.8) | 3(7.3) | 1(2.5) | – |
| Abdominal pain | 5(12.2) | 4(9.7) | 1(2.5) | – |
| Hand-foot syndrome | 6(14.8) | 4(9.8) | 1(2.5) | 1(2.5) |
| Diarrhea | 5(12.2) | 5(12.2) | – | – |
| Hypertension | 11(26.8) | 9(21.9) | 2(4.9) | – |
| Proteinuria | 5(12.2) | 3(7.2) | 1(2.5) | 1(2.5) |
| Skin rash | 7(17.1) | 5(12.1) | 1(2.5) | 1(2.5) |
| Thrombocytopenia | 6(14.6) | 6(14.6) | – | – |
| Hypothyroidism | 7(17.1) | 5(12.1) | 2(5.0) | – |
| Abnormal liver function | 26(63.4) | 21(51.2) | 4(9.7) | 1(2.5) |
Discussion
The HCC and Vp4 combination has an extremely poor prognosis. Tumor cells can disseminate via the bloodstream when a vascular invasion occurs, and blood supply reduction owing to tumor thrombus can aggravate remnant liver function, leading to limited treatment options, high recurrence rates after treatment, and worsened OS.16 In this multicenter retrospective study, triple therapy was beneficial in patients with HCC complicated by Vp4, facilitating potentially long-term PFS and OS. The mOS and mPFS were 21.7 and 14.5 months, respectively. 12 of 41 (29.3%) patients underwent sequential surgical resection. The tumor thrombus achieved CR in nine patients and regression in fifteen, which was better than the intrahepatic tumor response. This may be because drugs in the bloodstream act directly on the tumor thrombus. Concerning triple therapy safety, thirty-five patients (85.3%) cases had one or more TRAEs, while four patients (9.8%) suffered from grade 4 TRAEs, but without treatment-induced mortality. We propose this to be a candidate therapeutic option for patients with advanced HCC complicated by Vp4 with a tolerable profile.
Treating HCC and PVTT with TACE remains controversial because of liver failure and liver infarction risk. However, TACE is an effective treatment option for cases showing favorable liver function and sufficient collateral circulation surrounding portal vein occlusion, it is routinely used for patients with Vp4 in most Asian countries.9,17 It improved 6-months and 1-year OS compared with conservative treatment, with fewer fatal complications, even in patients with Vp4.6 With the emerging application of molecular targeting and ICI therapies, the treatment paradigm for HCC has changed significantly.
A retrospective analysis of PD-1 inhibitors combined with TKIs for primary HCC complicated by vascular invasion showed that 10 cases converted successfully and received resection.18 For downstaging and sequential radical resection, combined local and systemic therapies for patients with unresectable HCC showed substantial clinical benefits. The combination of TACE with TKI and anti-PD-1 antibodies in the treatment of unresectable HCC showed an ORR of 62.8–80.6%, mOS of 16.9–24 months, and mPFS of 7.3–11.4 months.19–24 Our previous multicenter retrospective study also showed that TACE plus lenvatinib and PD-1 inhibitor in treating HCC had high ORR and a high rate of conversion to surgical resection.10 The current study showed that triple therapy remained effective in patients with HCC, even in those with Vp4.
Prior phase III randomized control trials often excluded patients with Vp4. However, recent real-world studies have focused on these patients. Nakazawa et al compared sorafenib with radiotherapy for inoperable HCC complicated by PVTT based on propensity score analysis. Better survival was noted in the radiotherapy group than in the sorafenib group, and their median survival times were 10.9 and 4.8 months, respectively.25 When lenvatinib monotherapy was used to treat HCC with Vp4, the median OS was only 121 days if the degree of occlusion of the portal trunk was ≥70%.26 Moreover, a Phase II randomized trial found that the combination therapy group had a longer mOS (16.3 vs 6.5 months), mPFS (9.0 vs 2.5 months), and higher ORR (41% vs 3%) than the monotherapy group.27 A multicenter retrospective study compared whether camrelizumab plus apatinib was effective and tolerable among HCC cases with PVTT. It divided PVTT into three subgroups (types A–C, indicating PVTT in the main portal vein, branch of the first-order portal vein, and branches of the second- or lower-order portal veins, respectively). OS and PFS (5.8 and 5.0 months separately) of type A group was markedly decreased compared with the other groups.28 Our triple therapy had a longer mOS and mPFS than those reported in previous studies, which may be because >25% of included patients underwent an operation independently associated with a favorable OS and PFS by univariate and multivariate analyses. Triple therapy can be the candidate therapeutic option in treating Vp4.
HCC with PVTT is recognized to be an advanced-stage disease (BCLC-C), according to the BCLC staging system,29 and surgical resection is not recommended at this stage. However, experts from Eastern countries have recognized that in selected patients with HCC and PVTT, surgical treatment can improve quality of life and prolong survival.2,30,31 Most studies do not recommend surgery for Vp4 patients as they do not obtain survival benefits from resection.32 Our therapeutic strategy may reverse this situation. Triple therapy has shown good efficacy in patients with HCC combined with Vp4. 12 of 41 patients underwent sequential resection. As shown by the Kaplan–Meier survival curves, patients who underwent surgery had a prolonged OS and PFS compared to those without surgery, suggesting that surgical resection may extend the duration of the tumor response. Our triple therapy provides a potential opportunity for patients with HCC and Vp4 to receive curative surgery and indicates that this aggressive treatment is feasible and beneficial in such cases.
Thus far, indications for surgical resection and surgical approaches in patients with HCC combined with Vp4 who experience improvement with effective therapy have not been defined. We conducted a preliminary investigation of these patients. When the tumor thrombus regressed and CR was not achieved, the surgical approach was hepatic lobectomy or hemihepatectomy, consisting of en bloc resection of both the tumor and thrombus or tumor resection with thrombus extraction. When the thrombus achieved CR, we resected the tumor alone without removing the thrombus. There were no fatal postoperative complications or mortalities among the operative patients.
Here, among those who underwent surgery, tumor recurrence was diagnosed in five patients, including four intrahepatic recurrences and one pulmonary metastasis. This finding supports the idea that tumor cell metastasis mainly occurs through the portal vein. None of the three patients with CR of the tumor and thrombus developed metastasis. This finding suggests that patients with HCC and Vp4 responsive to triple therapy can undergo this radical curative therapy. However, none of the three patients with tumor and tumor thrombus complete necrosis showed metastasis or recurrence at the data cutoff, which showed that our surgical approach was effective in these patients.
This study has some limitations. First, this study was retrospective in nature, thus, the possibility of selection bias could not be completely avoided, and the sample size was relatively small. Therefore, this treatment strategy must be investigated in a prospective study to confirm our results. Second, the follow-up period was relatively short, and its long-term efficacy remains unknown. In cases undergoing resection, surgical approach remains controversial and requires further exploration. Based on the results currently available, triple therapy can serve as the preferred treatment for HCC patients with Vp4. Third, all cases had a hepatitis B virus etiology. For hepatitis B virus-related HCC cases, more benefits can be obtained from immune therapies compared with non-alcoholic steatohepatitis-induced HCC cases. Thus, whether this treatment is effective and safe for cases of other etiological factors, including hepatitis C infection or alcohol consumption, is unknown. Finally, the anti-PD-1 regimen was heterogeneous in our study; however, there is no evidence that anti-PD-1 antibody therapies have different therapeutic efficacy. The triple therapy combining TACE with lenvatinib, and anti-PD-1 immunotherapy is a promising therapy for HCC patients with Vp4.
Conclusion
This study preliminarily reported a triple therapy combining TACE with lenvatinib plus anti-PD-1, as an effective conversion strategy for unresectable HCC with Vp4. A high tumor response rate and improved prognosis were observed in these patients. Salvage surgery after conversion to triple therapy independently predicted OS and PFS.
Acknowledgments
We sincerely thank the researchers and study participants for their contributions towards this study.
Funding Statement
This study was financially supported by the Medical Innovation Project of Health and Family Planning Commission of Fujian Province (Grant number: 2022CXA002).
Abbreviations
BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; CR, complete response; CT, computed tomography; ECOG-PS, Eastern Cooperative Oncology Group – performance status; HCC, hepatocellular carcinoma; HR, hazard ratio; ICI, immune checkpoint inhibitor; mOS, median OS; mPFS, median PFS; ORR, objective response rate; OS, overall survival; pCR, pathologic complete response; PD-1, programmed death 1; PFS, progression-free survival; PVTT, portal vein tumor thrombus; RECIST, Response Evaluation Criteria in Solid Tumors; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor; TRAE, treatment-related adverse effect.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Institutional Ethics Committee of Fujian Medical University Research Ethics Committee in accordance with the 1975 Declaration of Helsinki. All patients signed written informed consent for their clinical data prior to the procedure.
Consent for Publication
All authors gave their consent for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 2.Cheng S, Chen M, Cai J, et al. Chinese Expert Consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus (2018 Edition). Liver Cancer. 2020;9(1):28–40. doi: 10.1159/000503685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL Conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421–430. doi: 10.1016/s0168-827(01)00130-1 [DOI] [PubMed] [Google Scholar]
- 4.Kokudo N, Takemura N, Hasegawa K, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49(10):1109–1113. doi: 10.1111/hepr.13411 [DOI] [PubMed] [Google Scholar]
- 5.Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi: 10.21037/hbsn-20-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13(1):60. doi: 10.1186/1471-230X-13-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun J, Shi J, Huang B, et al. The degree of hepatic arterial blood supply of portal vein tumor thrombus in patients with hepatocellular carcinoma and its impact on overall survival after transarterial chemoembolization. Oncotarget. 2017;8(45):79816–79824. doi: 10.18632/oncotarget.19767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ajit Y, Sudarsan H, Saumya G, et al. Transarterial chemoembolization in unresectable hepatocellular carcinoma with portal vein thrombosis: a perspective on survival. Oman Med J. 2014;29(6):430–436. doi: 10.5001/omj.2014.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258(2):627–634. doi: 10.1148/radiol.10101058 [DOI] [PubMed] [Google Scholar]
- 10.Wu JY, Yin ZY, Bai YN, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2021;8:1233–1240. doi: 10.2147/JHC.S332420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandrasegaran K, Tahir B, Nutakki K, et al. Usefulness of conventional MRI sequences and diffusion-weighted imaging in differentiating malignant from benign portal vein thrombus in cirrhotic patients. AJR Am J Roentgenol. 2013;201(6):1211–1219. doi: 10.2214/AJR.12.10171 [DOI] [PubMed] [Google Scholar]
- 12.Kudo M, Izumi N, Kubo S, et al. Report of the 20th nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res. 2020;50(1):15–46. doi: 10.1111/hepr.1343810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahringer-Kunz A, Steinle V, Duber C, et al. Extent of portal vein tumour thrombosis in patients with hepatocellular carcinoma: the more, the worse? Liver Int. 2019;39(2):324–331. doi: 10.1111/liv.13988 [DOI] [PubMed] [Google Scholar]
- 14.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 15.Cong WM, Bu H, Chen J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22(42):9279–9287. doi: 10.3748/wjg.v22.i42.9279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qadan M, Kothary N, Sangro B, Palta M. The treatment of hepatocellular carcinoma with portal vein tumor thrombosis. Am Soc Clin Oncol Educ Book. 2020;40:1–8. doi: 10.1200/EDBK_280811 [DOI] [PubMed] [Google Scholar]
- 17.Kim HC, Chung JW, Lee W, Jae HJ, Park JH. Recognizing extrahepatic collateral vessels that supply hepatocellular carcinoma to avoid complications of transcatheter arterial chemoembolization. RadioGraphics. 2005;25(suppl 1):S25–S39. doi: 10.1148/rg.25si055508 [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Hu B, Han J, et al. Surgery after conversion therapy with PD-1 inhibitors plus tyrosine kinase inhibitors are effective and safe for advanced hepatocellular carcinoma: a pilot study of ten patients. Front Oncol. 2021;11:747950. doi: 10.3389/fonc.2021.747950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Li Z, Zhang W, et al. Comprehensive treatment of trans-arterial chemoembolization plus lenvatinib followed by camrelizumab for advanced hepatocellular carcinoma patients. Front Pharmacol. 2021;12:709060. doi: 10.3389/fphar.2021.709060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju S, Zhou C, Yang C, et al. Apatinib plus camrelizumab with/without chemoembolization for hepatocellular carcinoma: a real-world experience of a single center. Front Oncol. 2021;11:835889. doi: 10.3389/fonc.2021.835889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teng Y, Ding X, Li W, Sun W, Chen J. A retrospective study on therapeutic efficacy of transarterial chemoembolization combined with immune checkpoint inhibitors plus lenvatinib in patients with unresectable hepatocellular carcinoma. Technol Cancer Res Treat. 2022;21:15330338221075174. doi: 10.1177/15330338221075174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai M, Huang W, Huang J, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13:848387. doi: 10.3389/fimmu.2022.848387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo J, Guo RP, Lai ECH, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18(2):413–420. doi: 10.1245/s10434-010-1321-8 [DOI] [PubMed] [Google Scholar]
- 24.Niu ZJ, Ma YL, Kang P, et al. Transarterial chemoembolization compared with conservative treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus: using a new classification. Med Oncol. 2012;29(4):2992–2997. doi: 10.1007/s12032-011-0145-0 [DOI] [PubMed] [Google Scholar]
- 25.Nakazawa T, Hidaka H, Shibuya A, et al. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 2014;14(1):84. doi: 10.1186/1471-230X-14-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukozu T, Nagai H, Matsui D, et al. Adaptation of lenvatinib treatment in patients with hepatocellular carcinoma and portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2022;89(1):11–20. doi: 10.1007/s00280-021-04359-2 [DOI] [PubMed] [Google Scholar]
- 27.Zheng K, Zhu X, Fu S, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: a randomized trial. Radiology. 2022;303(2):455–464. doi: 10.1148/radiol.211545 [DOI] [PubMed] [Google Scholar]
- 28.Yuan G, Cheng X, Li Q, et al. Safety and efficacy of camrelizumab combined with apatinib for advanced hepatocellular carcinoma with portal vein tumor thrombus: a multicenter retrospective study. Onco Targets Ther. 2020;13:12683–12693. doi: 10.2147/OTT.S286169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi: 10.1016/j.jhep.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kudo M, Kawamura Y, Hasegawa K, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10(3):181–223. doi: 10.1159/000514174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho MC, Hasegawa K, Chen XP, et al. Surgery for intermediate and advanced hepatocellular carcinoma: a consensus report from the 5th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2014). Liver Cancer. 2016;5(4):245–256. doi: 10.1159/000449336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng ZW, Guo RP, Zhang YJ, Lin XJ, Chen MS, Lau WY. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2012;118(19):4725–4736. doi: 10.1002/cncr.26561 [DOI] [PubMed] [Google Scholar]