Abstract
Endometrial inflammation is associated with reduced pregnancy per artificial insemination (AI) and increased pregnancy loss in cows. It was hypothesized that induced endometritis alters histotroph composition and induces inflammatory signatures on conceptus that compromise development. In Experiment 1, lactating cows were assigned to control (CON; n = 23) or to an intrauterine infusion of Escherichia coli and Trueperella pyogenes (ENDO; n = 34) to induce endometritis. Cows received AI 26 days after treatment, and the uterine fluid and conceptuses were collected on day 16 after AI. In Experiment 2, Holstein heifers were assigned to CON (n = 14) or ENDO (n = 14). An embryo was transferred on day 7 of the estrous cycle, and uterine fluid and conceptuses were recovered on day 16. Composition of histotroph and trophoblast and embryonic disc gene expression were assessed. Bacterial-induced endometritis in lactating cows altered histotroph composition and pathways linked to phospholipid synthesis, cellular energy production, and the Warburg effect. Also, ENDO reduced conceptus length in cows and altered expression of genes involved in pathogen recognition, nutrient uptake, cell growth, choline metabolism, and conceptus signaling needed for maternal recognition of pregnancy. The impact of ENDO was lesser on conceptuses from heifers receiving embryo transfer; however, the affected genes and associated pathways involved restricted growth and increased immune response similar to the observed responses to ENDO in conceptuses from lactating cows. Bacterial-induced endometrial inflammation altered histotroph composition, reduced conceptus growth, and caused embryonic cells to activate survival rather than anabolic pathways that could compromise development.
Keywords: conceptus development, dairy cow, induced endometritis, mRNA expression, histotroph metabolome
Bacterial-induced endometritis impairs conceptus development by altering histotroph composition and conceptus gene expression in dairy cattle.
Graphical Abstract
Graphical Abstract.
Introduction
Establishing and maintaining pregnancy require continuous reciprocal communication between the conceptus and the dam. In cows, interferon tau (IFNT), secreted from the conceptus in early pregnancy [1], serves as a signal that maintains the corpus luteum (CL) by suppressing the pulsatile release of prostaglandin F2α from the endometrium [2, 3]. Pregnancy maintenance in cattle requires continual progesterone secretion by the CL, and prevention of luteolysis coincides with elongation of the conceptus after hatching from the zona pellucida. At the onset of elongation, uterine histotroph is enriched with amino acids (AAs), lipids, saccharides, and other intermediate metabolites to support growth of the conceptus from a few millimeters to several centimeters [4–7]. Interestingly, induction of endometritis in dairy cows reduced the ability of oocytes to produce a morula in vitro [8], and morula quality and development of the pre-implantation conceptus were both compromised in dairy cows previously diagnosed with inflammatory diseases [9].
It is well established that inflammatory diseases of the uterus are associated with reduced pregnancy per artificial insemination (AI) in dairy cows [10]. The negative impact of uterine diseases on reproduction is related to not only reduced pregnancy per AI but also increased risk of pregnancy loss [9]. Indeed, maintenance of pregnancy is compromised in dairy cows previously diagnosed with inflammatory diseases even after disease resolution [9, 11]. Similarly, cows diagnosed with endometrial inflammation in the first few weeks postpartum had reduced maintenance of pregnancy after embryo transfer (ET) [9, 11], which suggests compromised uterine support of conceptus development. Conceptuses from cows previously diagnosed with disease are smaller and secrete less IFNT in the uterine fluid than conceptuses from cows without disease in early lactation [9], which might compromise the ability to maintain the CL. Therefore, it is reasonable to suggest that endometrial inflammation alters nutrient transport and the secretory activity of the endometrium, thus affecting histotroph composition, which can influence conceptus growth and development. Although the epidemiological link between uterine disease and fertility in dairy cows is strong, the underlying mechanisms remain elusive. Recently, a model for bacterial-induced endometritis in cows established a causative link between endometrial inflammation and reduced developmental capacity of oocytes and altered reproductive tissue transcriptome [8, 12].
Endometritis often follows metritis in cows, which results in dysbiosis of the uterine microflora with reduced microbial diversity and predominance of certain pathogens that include, among others, Escherichia coli and Trueperella pyogenes [13, 14]. Utero-pathogenic E. coli and T. pyogenes carry virulence factors that allow them to adhere to the endometrium, destroy the tissue architecture, promote immune cell migration and death, and establish the disease [13, 14]. Cows that suffer from endometritis have compromised reproduction presumably because of the inflammatory response and damage inflicted in the reproductive tract. In general, cows that suffered inflammatory diseases, including those that affect the reproductive tract, udder, gastrointestinal and respiratory tracts, in early lactation have conceptuses with molecular signatures relating to inflammatory response [9]. Upregulated pathways in conceptus cells included those involved in inflammation and immune activation [9]. Such molecular signatures suggest that the follicle and/or oocyte suffered alterations induced by disease that reflected the transcriptome of conceptus tissues [15]. Another possibility is that changes in uterine biology induced by components linked to endometrial inflammation, such as pathogen-associated molecular patterns or damage-associated molecular patterns, result in changes in the conceptus that reduce survival.
We hypothesized that bacterial-induced endometrial inflammation compromises conceptus development and growth by altering the histotroph composition and inducing molecular signatures on conceptus tissues relating to inflammatory response and reduced cell anabolism. The objectives of the present experiment were to investigate the impact of induced endometrial inflammation on the composition of histotroph, conceptus development, and mRNA expression of genes involved in inflammation, immune regulation, conceptus signaling, nutrient uptake, and metabolism.
Materials and methods
Ethical approval
The University of Florida Institutional Animal Care and Use Committee approved all the procedures used in the present experiment under protocol number 201508884 in both experiments described herein.
Experiment 1, lactating cows artificially inseminated
Bacterial culture and preparation of inoculants
E. coli MS499 and T. pyogenes MS249 were collected and isolated from cows with metritis and characterized previously [16, 17]. E. coli MS499 was cultured from frozen glycerol stocks on Luria–Bertani agar. The day before infusion into cows, a single colony was picked from the plate and inoculated into Luria–Bertani broth containing 1% tryptone, 0.5% yeast extract, and 1% sodium chloride. The culture was incubated overnight at 37 °C with shaking at 200 rpm. The overnight culture was diluted 1:100 into 50 mL of fresh Luria–Bertani broth and incubated 7 h to reach a stationary phase. After incubation, 1 mL of bacterial cells were collected by centrifugation at 10 000 × g for 5 min and washed with autoclaved phosphate buffer saline (PBS, 0.01 M of phosphate buffer and 0.154 M of sodium chloride, pH 7.40). Concentration of E. coli as colony-forming units (CFU) per milliliter of solution was determined using a spectrophotometer to measure light scattering at optical density of 600 nm [18]. There were five batches of inoculum prepared and diluted in PBS that resulted in a mean (±SD) of 5.20 ± 0.84 × 107 CFU/mL E. coli.
T. pyogenes MS249 was grown from frozen glycerol stocks on Trypticase Soy Blood agar at 37 °C for 48 h. The day before infusion into cows, a single colony was inoculated into Bacto Brain Heart Infusion broth (Thermo Fisher Scientific, Waltham, MA) supplemented with 5% fetal bovine serum (Thermo Fisher Scientific) and cultured at 37 °C with shaking at 200 rpm for 48 h to reach a stationary phase. The number of CFU/mL was evaluated as described for E. coli. There were five batches of inoculum prepared and diluted in PBS that resulted in a mean of 4.16 ± 0.76 × 107 CFU/mL T. pyogenes.
Sample size calculation
The sample size was calculated to allow sufficient experimental units to result in at least eight conceptuses per treatment for transcriptomic analyses. The assumptions were that 16 conceptuses would be sufficient to detect differences in mRNA expression between treatments based on data from previous experiments [4, 9]. It was also assumed that 80% of the cows would synchronize ovulation and, of those cows, 40% would have a recovered elongated conceptus on day 16 after insemination [9]. Thus, the sample size calculated was 50 experimental units. Seven additional cows were added to accommodate potential attrition during the experiment, or reduced conceptus recovery, particularly in cows treated with bacterial infusion.
Experimental design and treatments
The animal portion of the experiment was conducted from December 2020 to March 2021. The experiment was a randomized complete block design. All cows included in the experiment had a live singleton calf and had not been diagnosed with milk fever, retained placenta, metritis, displaced abomasum, or mastitis until the day of enrollment. Endometrial cytology was performed on day 20 ± 3 postpartum using a cytobrush (Medscand Medical, Cooper Surgical, Trumbull, CT) as previously described [19] to exclude cows with >18% polymorphonuclear leukocytes (PMN; calculated as proportion of endometrial epithelial cells, PMN, and mononuclear leukocytes). On day 20 ± 3 postpartum (Supplemental Figure S1A), cows received an i.m. injection of 100 μg of GnRH (OvaCyst, 50 μg/mL of gonadorelin diacetate tetrahydrate, Bayer HealthCare LLC, Whippany, NJ) and 57 Holstein cows with a CL detected by ultrasonography on day 26 ± 3 postpartum were enrolled in the experiment. The objective was to assure that all cows enrolled in the experiment had an ovulation by 26 DIM and that treatments were applied to cows in diestrus.
Cows in each weekly cohort were blocked by parity group, as primiparous or multiparous cows, and genomic breeding value for cow conception rate. Within block, cows were assigned randomly to remain as untreated controls (CON; n = 23) or to receive an intrauterine infusion of 5.20 × 108 CFU E. coli and 4.16 × 108 CFU T. pyogenes at 26 ± 3 days postpartum to induce endometrial inflammation (ENDO; n = 34). The rationale for not infusing vehicle in the uterine lumen of CON cows was to avoid vehicle-induced influx of PMN into the endometrium resulting in some cows presenting mucopurulent vaginal discharge [8, 20].
Intrauterine treatment in ENDO cows was performed as described by Piersanti et al. [20] with modification. No endometrial scarification was performed before bacterial infusion to avoid excessive endometrial inflammation induced by physical trauma. Cows were restrained and received a caudal epidural injection of 60 mg of lidocaine hydrochloride 2% (Aspen Veterinary Resources, Greeley, CO). The perineum and the exterior vulva were cleaned with Betadine soap (VEDCO, povidone-iodine 0.75% solution, St. Joseph, MO) followed by a spray of 70% ethanol. A Neilson catheter (45 cm; Supplies for Farmers, Lincolnshire, UK) covered in a sanitary sheath (IMV Technologies, Brooklyn Park, MN) was introduced into the reproductive tract and guided via transrectal palpation through the cervix to reach the uterine body. Once in the last ring of the cervix, the sheath was retracted to expose the catheter port and the solution was dispensed into the body of the uterus. The infusate was 30 mL total, 10 mL of PBS containing E. coli MS499, 10 mL of PBS containing T. pyogenes MS249, and a final 10 mL of PBS to flush the catheter. During the experimental period, cows did not receive any additional treatments or medication.
Endometrial cytology, rectal temperature, and serum concentrations of haptoglobin
Endometrial samples were collected for cytology on days 2 and 7 after enrollment and treatment administration (Supplemental Figure S1). The perineum and the exterior vulva were cleaned with Betadine soap followed by a spray of 70% ethanol. A cytobrush (Medscand Medical, Cooper Surgical, Trumbull, CT) was mounted on an adapted AI gun protected with a plastic chemise to collect endometrial cells [19]. The cytobrush was smeared on a clean glass slide, air-dried, and stained with Rapid-Chrome Kwik-Diff (Thermo Fisher Scientific). A total of 200 cells scattered throughout the slide, including endometrial epithelial cells, mononuclear leukocytes, and PMN were counted at 40× magnification (Nikon Instruments, Melville, NY), and the proportion of PMN was determined. Subclinical endometritis was defined as >10% PMN in endometrial cytology [19], and expressed as the proportion of PMN relative to the sum endometrial epithelial cells and leukocytes [PMN/(epithelial cells + PMN + mononuclear leukocytes)]. Rectal temperature (AG-102 thermometer, AG-Medix, Mukwonago, WI) was measured immediately before enrollment and then twice daily between 0600 and 0800 h and 1800 and 2000 h in the 7 days following enrollment.
Blood was sampled daily from the coccygeal vessels into evacuated tubes (Vacutainer, Becton Dickson, Franklin Lakes, NJ) containing no additive from days 0 to 6 relative to enrollment. Tubes were kept at ambient temperature for 30 min and then placed on ice and transported to the laboratory within 3 h of collection. Tubes were centrifuged at 2000 × g for 20 min for serum separation and then aliquoted and frozen at −20 °C until analysis. Concentrations of haptoglobin in serum were measured according to Makimura and Suzuki [21] using a standard curve designed using plasma with high and low concentrations of haptoglobin quantified using a commercial kit (Cow Haptoglobin ELISA, Hapt-11; Life Diagnostics Inc., West Chester, PA).
Estrous synchronization and AI
Cows had the estrous cycle and ovulation synchronized with the Double-Ovsynch protocol starting at on day 26 ± 3 postpartum (Supplemental Figure S1A), concurrent with treatment administration. Cows received an injection of 100 μg of GnRH, followed 7 days later by an i.m. injection of 25 mg of prostaglandin F2α (Lutalyse sterile solution, 5 mg/mL of dinoprost as tromethamine salt; Zoetis, Kalamazoo, MI), and another injection of GnRH on day 35 postpartum. Seven days later, the breeding Ovsynch started at 42 ± 3 days postpartum, and cows received two inseminations, the first in the morning on day 52 ± 3 postpartum, approximately 12 h after the final GnRH treatment and the second 12 h later (Supplemental Figure S1A). Cows were artificially inseminated with frozen–thawed semen from two Holstein sires with a sire conception rate > 2.0. The rationale to start the ovulation synchronization protocol early postpartum, on day 26 after calving, was to assure that cows would undergo conceptus collection before 70 days postpartum and still have time to have their estrous cycle resynchronized to receive an AI for the farm before 100 days postpartum.
Ovaries were scanned by ultrasonography concurrent with the last GnRH of the Double-Ovsynch protocol and again on days 2 and 7 of the subsequent estrous cycle to determine ovulation based on the disappearance of a follicle and appearance of a CL in the ipsilateral ovary. Synchronization was determined based on the presence of a pre-ovulatory follicle on the day of the final GnRH injection and subsequent disappearance of the follicle 2 days later with detection of a CL on day 7 in the same ovary of the pre-ovulatory follicle.
Histotroph sampling and conceptus collection
On day 16 after AI, uterine lumen fluid was sampled using a cytobrush [22], and conceptuses were collected by flushing the uterine horn ipsilateral to the CL [4]. The cows were restrained and received an epidural injection of 60 mg of lidocaine hydrochloride 2%. The perineum and the exterior vulva were cleaned with Betadine soap followed by a spray of 70% ethanol. The gun containing the cytobrush was introduced vaginally and positioned 4–5 cm past the uterine bifurcation in the uterine horn ipsilateral to the CL. The cytobrush was exposed and gently rotated twice to collect histotroph. Samples that had presence of blood based on visual appraisal were discarded. After removal from the tract, the cytobrush was immersed in a microcentrifuge tube containing 1 mL of PBS. Samples were kept on ice until processed. First, cells were separated from the brush by vortexing for 1 min. The brush was removed from the microcentrifuge tube, and the liquid phase was centrifuged at 400 × g for 7 min to remove cells and debris. The supernatant was aspirated and centrifuged again at 400 × g for 7 min. Then, the supernatant was snap-frozen in liquid N2 and stored at −80 °C for subsequent metabolomic analysis.
Following collection of histotroph sample, the uterine horn ipsilateral to the CL was flushed with 20 mL of PBS with 0.1% (v/v) of polyvinyl alcohol [4]. The fluid was examined for the presence of the conceptus under a stereoscope. The recovered fluid underwent centrifugation at 1000 × g for 10 min, snap-frozen in liquid N2, and stored at -80 °C for IFNT quantification. Up to five subsequent flushes of 45–50 mL of PBS were performed if no conceptus was recovered. The recovered conceptus was washed in PBS plus 0.1% polyvinyl alcohol, the morphology and length was recorded, and then, the embryonic disc was dissected to separate from the trophoblast. Embryonic disc and trophoblast were stored separately in RNAlater stabilization solution (Thermo Fisher Scientific) and then frozen at −80 °C until nucleic acid extraction.
Histotroph metabolome
Chemicals used in the assay included liquid chromatography-mass spectrophotometry (LC–MS) grade acetonitrile, methanol, isopropyl alcohol, and formic acid (≥99.0% purity; Thermo Fisher Scientific). The LC–MS grade ethanol, pyridine, and phenylisothiocyanate were purchased from Sigma-Aldrich (St. Louis, MO). Milli-Q water was used for the mobile phase (EMD Millipore, Billerica, MA).
Targeted metabolomics analyses were performed on the histotroph collected on day 16 using the commercially available MxP Quant500 kit (Biocrates Life Sciences, AG, Innsbruck, Austria) to identify and quantify 107 small molecules and 523 lipids. The assay was performed in samples from 8 CON and 12 ENDO pregnant cows that had an elongated conceptus recovered and had IFNT in the uterine fluid with a concentration > 2500 pg/mL. The histotroph sample was prepared as directed in the manufacturer’s instructions (Biocrates Life Sciences, AG, Innsbruck, Austria) for metabolomic analyses. In brief, histotroph samples and calibration standards were thawed on ice, mixed for 10 s, and centrifuged at 10 000 × g at 4 °C for 10 min. Calibration standards and quality controls were dissolved in 100 μL of H2O and mixed at 1200 rpm for 15 min. Twenty microliters of the sample and 10 μL of calibration standards, quality controls, and PBS were added to the 96-well plate. The plate was dried under N2 for 30 min. All samples and standards were mixed in a premix of phenylisothiocyanate at room temperature for 60 min for derivatization purposes and subsequently dried under N2 for 60 min. Samples were extracted in 5 mM ammonium acetate in methanol for 30 min using an orbital shaker, and the extracts were collected by centrifuging the preparation plate at 500 × g for 2 min. Sample extracts were diluted with H2O (1:1) for the liquid chromatography phase of the analysis. For the flow injection analysis, 50 μL of sample extract was mixed with 450 μL of the kit solvent on a separate plate. The liquid chromatography and flow injection analysis plates were sealed, mixed for 10 min at 600 rpm at room temperature, and placed into the thermostatically controlled autosampler for analysis.
Sample extracts were analyzed using an Acquity I-Class liquid chromatography unit coupled with a Waters Xevo-TQ-S (Waters Corporation, Milford, MA). Sample extracts were separated using the MxP Quant500 C18 column with an attached guard and precolumn mixer (Biocrates Life Sciences, AG, Innsbruck, Austria). The mobile phase consisted of A: H2O and 0.2% formic acid, B: MeCN, and 4-part 0.2% formic acid delivered at a flow rate of 0.8 mL/min with a gradient of B: 0–100% over 4.5 min. Eluent % B was increased to 1.0 mL/min flow rate and maintained at 100% for 30 s, followed by a rapid return to the initial conditions for 70 s to equilibrate the column. Both positive and negative mode gradient were 5.8 min long. The negative mode acquisition gradient differed from the positive mode with a difference in % B composition between 2.0 and 4.5 min. The injection volume was 5 μL for positive data acquisition and 15 μL for the negative run. Wash solvent composition consisted of equal volumes of H2O: MeOH: MeCN: IPA (v/v).
The MxP Quant 500 Kit offers direct flow injections for lipid analysis. An isocratic method was performed using the kit-provided solvent (290 mL MeOH: 1 ampule of flow injection analysis additives). The isocratic mobile phase (B: 100% MeOH) was delivered at a low flow rate of 0.03 mL/min. The injection volume was 20 μL for both positive and negative mode acquisitions. All data were extracted using the MetIDQ software following Biocrates’ instructions (Biocrates, Innsbruck, Austria). Three different concentration (low, mid, and high) ranges of quality control samples were provided by the manufacturer (Biocrates, Innsbruck, Austria).
Experiment 2, heifers receiving ET
Bacterial culture and preparation of inoculants
E. coli MS499 and T. pyogenes MS249 were cultured, and inoculum was prepared as described in Experiment 1. There was a single batch of inoculum prepared and diluted in PBS that resulted in 4.50 × 107 CFU/mL E. coli and 4.80 × 107 CFU/mL T. pyogenes.
Sample size calculation
The sample size was calculated to allow sufficient experimental units to result in eight conceptuses per treatment for transcriptomic analyses as previously described in Experiment 1. The assumption was that pregnancy per ET would be 60% in heifers and conceptus recovery would be 100%. Thus, the sample size calculated was 27 heifers, and 28 were enrolled in the experiment.
Experimental design and treatments
The animal portion of the experiment was conducted in the months of June and July of 2020. The experiment was a randomized complete block design. Holstein heifers weighing 448−592 kg received an injection of prostaglandin F2α followed 2 days later by an injection of GnRH to initiate an estrous cycle. Five days later, 28 heifers that had a CL detected by ultrasonography were blocked by body weight and, within block, assigned randomly to remain as control (CON; n = 14) or to receive an intrauterine infusion of 4.50 × 108 CFU E. coli and 4.80 × 108 CFU T. pyogenes on day 5 of the estrous cycle to induce endometrial inflammation (ENDO; n = 14) as described in Experiment 1. As detailed in Experiment 1, CON remained untreated to prevent vehicle-induced endometrial inflammation with influx of PMN into the endometrium, which could result in some animals presenting mucopurulent vaginal discharge [8, 20]. Previous experiments already compared the effects of infusing bacteria and vehicle or vehicle only on in vitro embryo production and reproductive tissue transcriptome [8, 12, 20].
Endometrial cytology, rectal temperature, and serum concentrations of haptoglobin
Endometrial samples were collected for cytology on day 2 after enrollment (Supplemental Figure S1B), and cells were evaluated as described in Experiment 1. Subclinical endometritis was defined as >5% PMN in the endometrial cytology. The reduced cut-off value for PMN used to categorize heifers as having endometrial inflammation was based on the fact that heifers have endometrium with a very small proportion of PMN [23]. Rectal temperature was measured once daily, in the morning, immediately before enrollment until day 6 after enrollment.
Blood was sampled daily from the coccygeal vessels into evacuated tubes (Vacutainer, Becton Dickson) containing no additive on days 0–6 relative to enrollment for serum separation. Serum was harvested and concentrations of haptoglobin was determined as described in Experiment 1.
Estrous synchronization
The estrous cycle and ovulation of heifers were synchronized for ET as previously described [24] and outlined in Supplemental Figure S1B. Ovaries were scanned by ultrasonography on the day of the last GnRH of the synchronization protocol and again on days 2 and 7 of the induced estrous cycle to determine ovulation as described in Experiment 1.
In vitro embryo production and transfer
Embryos were produced in vitro from oocytes harvested from Holstein ovaries and shipped in maturation medium at 38 °C (Simplot, Fresno, CA; n = 500). Oocytes were rinsed three times in 100 μL of CO2 equilibrated BO-IVF (IVF Bioscience, Falmouth, UK) medium. Five in vitro fertilization dishes (100 oocytes/dish) were prepared, one for each sire. Oocytes were fertilized with sperm from five Holstein sires with a sire conception rate > 2.0 to yield a final concentration of 2 × 106 sperm/mL and placed in a humidified incubator at 38.8 °C with 6% O2, 6% CO2, and balanced N2. After 22 h of fertilization, oocytes were rinsed in an oocyte wash medium (IVF Bioscience), and cumulus cells were removed by mechanical pipetting (Cooper Surgical, Trumbull, CT). Subsequently, oocytes were moved to 100 μL drops of BO-IVC embryo culture medium overlaid with light mineral oil (IVF Bioscience). Embryos were cultured in groups of 10–12 at 38.8 °C in a humidified environment of 6% O2 and 6% CO2 and balanced N2. Embryos were assessed for cleavage 3.5 days after fertilization. On day 7 after fertilization, a single grade 1 blastocyst was loaded into a 0.25 mL semen straw for fresh transfer in heifers on day 7 of the estrous cycle. Within a randomization block, heifers in both treatments received an embryo from a single sire. The embryo was transferred to the horn ipsilateral to the ovary bearing the CL.
Reproductive tissues harvested
Heifers were euthanized on day 16 of the estrous cycle by penetrating captive bolt of the brain followed by exsanguination. Reproductive tracts were collected upon euthanasia, placed on ice, and processed within 1–2 h of slaughter. The uterine horn ipsilateral to the CL was clamped at the uterine bifurcation. The horn was flushed with 20 mL of PBS to collect uterine fluid and the potential conceptus. Additional flushes were performed as needed to recover the conceptus. The fluid of the first flush underwent centrifugation for 10 min at 1000 × g to remove debris, snap-frozen in liquid N2, and stored at -80 °C for IFNT quantification. The recovered conceptus was processed as described in Experiment 1 for later nucleic acid extraction.
Interferon-tau quantification
The concentration of IFNT in the uterine fluid was quantified by ELISA developed and validated in T. Hansen’s Laboratory (Colorado State University) as described in Dickson et al. [8]. Briefly, glycosylated recombinant bovine IFNT was purified from cultures of human HEK cells that were transformed with bovine IFNT cDNA (bTP509) and used to generate polyclonal antibodies in goats (Animal ID 51; 3.5 μg/mL) and in rabbits (Animal ID 5670; 9.6 μg/mL). In a sandwich ELISA, these antibodies were used as capture and biotinylated detection antibodies. The ELISA had a detection range of 7.8–500 pg/mL and a 66 pg/mL limit of detection. The intra-assay coefficient of variation was 0.2–2.2% for high-concentration (500 pg/mL), 0.3–2.8% for medium concentration (100 pg/mL), and 0–2.7% for low-concentration (20 pg/mL) recombinant bovine IFNT controls. The inter-assay coefficient of variation was 7.6, 6.6, and 6.3% for high-, medium-, and low-concentration controls, respectively. The ELISA specifically detects IFNT and does not cross-react with IFNW, IFNA/B, or IFNG. Samples were assayed undiluted or at dilutions in steer serum of 1:10, 1:100, 1:1000, 1:5000, or 1:10 000 to detect IFNT in the assay’s linear range. Operators were blind to the treatment of samples being assayed. Samples below the detection limit were assigned a value of 66 pg/mL. Samples with a concentration > 200 pg/mL were considered originating from a pregnant cow. The selected threshold was based on unpublished data in which non-inseminated cows had concentrations of IFNT <200 pg/mL in the uterine flush. In a group of 74 inseminated cows, the selected threshold of 200 pg/mL resulted in area under the receiver operating characteristic curve of 0.914 with 100% sensitivity, 82.8% specificity, and 93.2% accuracy to detect a recovered elongated conceptus on day 16 after AI (unpublished results).
Conceptus tissue RNA extraction and gene expression
Total RNA was extracted from trophoblast and embryonic disc using Trizol (TRIzol LS Reagent, Invitrogen, Waltham, MA). The tissues were placed in 800 μL of Trizol in a microtube containing zirconium oxide beads (CKMix, Bertin Corp. Thermo Fisher Scientific). Chloroform was added to establish a 20% solution. The tissue was homogenized (Precellys 24, Bertin Corp. Thermo Fisher Scientific) and then centrifuged at 15 000 × g for 15 min at 4 °C, and the colorless, aqueous supernatant containing RNA was transferred to a new microtube. An equal volume of 100% pure ethanol was added to the microtube, and samples were homogenized and then incubated for 3 min at room temperature. Ribonucleic acid was purified using the Quick-RNA 96 kit (Zymo Research, Irvine, CA) according to the manufacturer’s instructions. Purity and concentration were evaluated using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific). Samples had a mean (±SD) 260:280 nm ratio of 2.05 ± 0.03 and a 260:230 nm ratio of 2.12 ± 0.16.
The mRNA for a selected set of genes was quantified by the Fluidigm Biomark HD quantitative PCR microfluidic system (Fluidigm Co., San Francisco, CA). We used the CellsDirect™ One-Step qRT-PCR Kit (Thermo Fischer Scientific). A total 0.27 ng RNA was used in the rection. The detection chemistry was EvaGreen, and the PCR primers were designed by Fluidigm Delta Gene assays and synthesized by Fluidigm (Fluidigm Co.). Details of genes and primers are shown in Supplemental Table S1. Only genes that passed the quality control and had primer efficiency ranging from 96 to 104% were investigated. Genes investigated included those involved in pathogen recognition, cell signaling, inflammation and immune regulation, antioxidants, conceptus development and growth, nutrient uptake, and cellular metabolism. The housekeeping genes were ACTB, GAPDH, PGK1, RPL19, RPS9, and YWHAZ, and all were stable and did not differ between treatments. The geometric mean of the cycle threshold (Ct) values of all six housekeeping genes was calculated to determine the delta Ct (dCt). Statistical analyses were performed on dCt values as described by Steibel et al. [25]. Fold changes relative to trophoblast from CON treatment were calculated using the method described by Yuan et al. [26], whereby fold changes were calculated from least-squares means (LSM) difference according to the formula 2-ddCt, where dCt = Ct target gene − Ct geometric mean of housekeeping genes, and ddCt = dCt ENDO − dCt CON [27]. Heatmaps were generated to visualize the gene expression using the Heatmapper online tool [28].
Statistical analyses
Continuous data were analyzed by ANOVA with linear mixed-effects models using the MIXED procedure of SAS version 9.4 (SAS/STAT, SAS Institute Inc., Cary, NC). Normality of residuals and homogeneity of variance were examined for each continuous dependent variable analyzed after fitting the statistical models. Responses that violated the assumptions of normality were subjected to power transformation according to the Box-Cox procedure [29] using a macro for mixed models in SAS [30]. The LSM and standard errors of the means (SEM) were back-transformed for the presentation of results according to Jørgensen and Pedersen [31].
All statistical models included the fixed effects of treatment (CON vs. ENDO) and random effect of block. For analysis of conceptus mRNA expression, the models also included the fixed effects of type of tissue (trophoblast vs. embryonic disc), sire, and the interaction between treatment and tissue, and the random effect of the cow nested within treatment as the error term to test the effect of treatment. For responses with repeated measures over time within the same experimental unit, the statistical models also included the fixed effects of day of measurement, the interaction between treatment and day, a pre-treatment covariate, and the random effect of the cow nested within treatment. In the mixed-effects models with repeated measures, day was the term in the REPEATED statement, and the covariance structure was selected according to spacing between measurements and model fit assessed based on the smallest Akaike’s information criterion. When an interaction between treatment and day resulted in P ≤ 0.10, then means at different time points were partitioned using the SLICE command of SAS.
Categorical data, such as proportion of PMN in endometrial cytology and proportion of cows with subclinical endometritis, were analyzed by generalized linear mixed-effects models using logistic regression with the GLIMMIX procedure of SAS. The statistical models included the fixed effects of treatment and the proportion of PMN collected pre-enrollment as covariate, and the random effect of block. For proportion of pregnant cows, the models included the fixed effects of treatment and sire and the random effect of block. The adjusted probabilities of pregnancy per breeding or of subclinical endometritis and associated SE were computed using the inverse link function in SAS (ILINK) to return the estimates onto the scale of the data.
In all mixed-effects models, the Kenward–Roger method was used to approximate the denominator degrees of freedom for the F tests. Statistical significance was considered at P ≤ 0.05, and a tendency was considered at 0.05 < P ≤ 0.10.
All absolute metabolites concentrations were normalized to the respective cows’ total protein content in the histotroph and expressed as μM/g of protein. The mean (±SD) of total protein in the histotroph was 3.25 ± 2.34 g/L. Univariate t-tests with false discovery rate adjusted to P ≤ 0.10 were performed to compare concentrations of metabolites between two treatments using R (Version 4.1.3, R Development Core Team, 2022, https://www.r-project.org). Statistical significance was considered at P ≤ 0.05, and a tendency was considered at 0.05 < P < 0.10. All metabolomics data were processed and analyzed using the MetaboAnalyst 5.0 software [32]. Recommended statistical procedures for metabolomics analyses were followed according to previously published protocols [32–34]. Of the 630 analytes investigated in the MxP Quant500 kit platform, 122 were either frequently (>30%) below the detection limit or with more than 30% missing values and those were excluded from the all statistical analyses. The 20 histotroph samples analyzed with 508 metabolites identified generated 8931 quantified analytes of the 10,160 total. The 12.1% missing values (1229) were imputed by the k-nearest neighbor model with MetaboAnalyst (v5.0). Metabolite concentrations were normalized before the statistical analysis and metabolic pathway analysis to create a Gaussian distribution [32]. Log-transformation and auto scaling were used for metabolite values.
Principal component analysis (PCA), partial least squares-discriminant analysis (PLS-DA), quantitative enrichment analysis, and metabolic pathway analysis were performed via MetaboAnalyst (v5.0) using all identified analytes in the histotroph metabolome. In the PLS-DA model, a variable importance in the projection (VIP) plot was used to rank the metabolites based on their importance in discriminating ENDO from CON. The VIP score is a measure of the importance of each variable in the PLS-DA model and summarizes the contribution of each variable to the model. Metabolites with large VIP values, typically >1, are the most important in discriminating ENDO from CON. The quantitative enrichment analyses were performed in MetaboAnalyst (v5.0) by referring two databases; small molecule pathway database [35, 36] and the Kyoto Encyclopedia of Genes and Genome database [37] to evaluate the metabolic pathways altered by treatment.
Results
Experiment 1
Table 1 depicts the effects of treatment on endometrial polymorphonuclear cells, incidence of subclinical endometritis, rectal temperature, serum concentrations of haptoglobin, and conceptus development. Induced endometritis increased (P < 0.001) the proportion of PMN in the endometrial cytology and the risk of subclinical endometritis in both days 2 and 7 after enrollment. Treatment or the interaction between treatment and day did not affect the concentrations of haptoglobin in serum of cows. Of the 57 cows enrolled, 3 were sold by the farm and did not receive AI. Treatment did not affect synchronization of ovulation. Of the 54 inseminated cows, 43 had their uteri flushed. Of the 11 cows excluded, 7 cows did not have a synchronized ovulation, 1 had CL regression before day 16, and 3 cows were not flushed because of excessive manipulation of the reproductive tract to pass the catheter through the cervix. Twenty conceptuses were recovered, 8 from CON and 12 from ENDO cows; however, one ENDO conceptus was lost and not analyzed for the transcriptome profile. Treatment did not affect the proportion of pregnant cows on day 16 after AI, but ENDO cows produced shorter (P = 0.03) conceptuses compared with CON cows. Moreover, the concentration of IFNT in the uterine fluid of pregnant cows was less (P = 0.04) in ENDO than in CON cows.
Table 1.
Effect of treatment on endometrial polymorphonuclear cells, incidence of subclinical endometritis, rectal temperature, and conceptus development in lactating Holstein cows receiving AI
| Item | Treatment1 | |||
|---|---|---|---|---|
| CON | ENDO | SEM | P-value | |
| Polymorphonuclear cells,2 % | ||||
| Day 2 | 5.5 | 22.7 | 1.7 | <0.001 |
| Day 7 | 12.2 | 17.0 | 1.9 | 0.07 |
| Subclinical endometritis,2,3 % | ||||
| Day 2 | 14.3 | 88.0 | 6.8 | <0.001 |
| Day 7 | 30.0 | 73.3 | 10.2 | 0.008 |
| Rectal temperature, °C | 38.46 | 38.49 | 0.06 | 0.63 |
| Serum haptoglobin, μg/mL | 179.8 | 195.7 | 27.8 | 0.70 |
| Synchronized ovulation, % | 95.0 | 82.4 | 5.7 | 0.22 |
| Pregnant day 16,4 % | ||||
| All cows | 42.3 | 48.3 | 10.3 | 0.68 |
| Synchronized cows | 59.7 | 60.9 | 14.5 | 0.95 |
| Conceptus length, cm | 13.5 | 8.5 | 1.5 | 0.03 |
| Interferon-tau in uterine flush, ng/mL | 262.7 | 56.6 | 67.6 | 0.04 |
1Holstein cows at 26 ± 3 days postpartum were blocked by parity and genomic breeding value for cow conception rate, and, within block, they were assigned randomly to remain as controls (CON; n = 23) or to receive an intrauterine infusion of 5.20 × 108 CFU E. coli and 4.16 × 108 CFU T. pyogenes during the luteal phase to induce endometrial inflammation (ENDO; n = 34). Conceptuses recovered on day 16 of pregnancy from 8 CON and 12 ENDO cows.
2Interaction between treatment and day after enrollment (P < 0.001).
3Based on >10% of polymorphonuclear cells in endometrial cytology.
4Pregnancy per AI on day 16 after insemination based on interferon-tau in the uterine flush fluid.
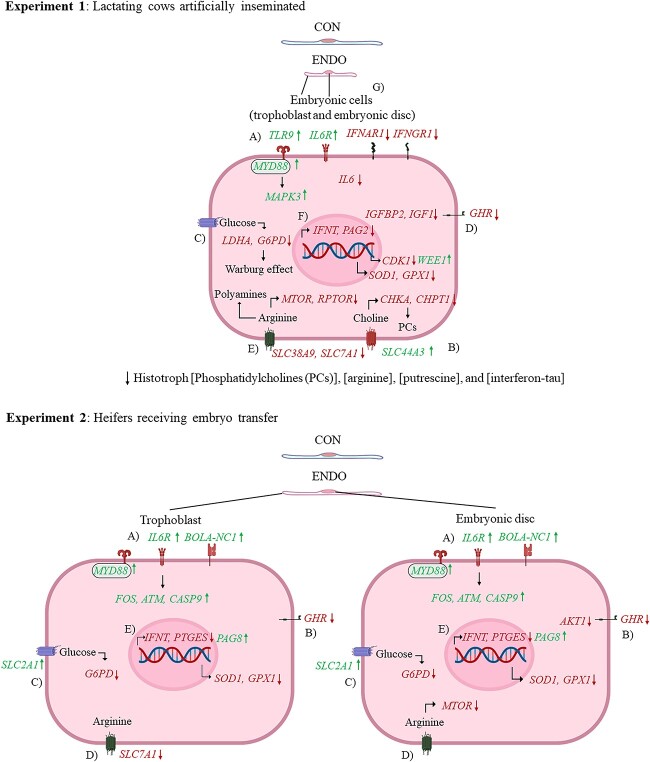
Table 2 depicts the relative expression of genes affected by treatment or by the interaction of treatment and tissue. The LSM and corresponding SEM for the dCt values of the genes affected either by treatment or by the interaction between treatment and conceptus tissue in lactating cows are depicted in Supplemental Table S2. Conceptuses from ENDO cows had increased (P ≤ 0.02) expression of a gene involved in pathogen recognition (TLR9), a gene member of the mitogen-activated protein kinase (MAPK3) family, and a cell cycle checkpoint kinase (WEE1). Consistent with reduced elongation, transcripts related to cell cycle progression (CDK1), embryo growth factors (GHR, IGFBP2, IGF1), glucose metabolism (LDHA, G6PD), AA transport (SLC38A9), mTOR pathway regulator (MTOR, RPTOR), and antioxidant defense (SOD1, GPX1) were less or tended to be less (P ≤ 0.06) expressed in conceptuses from ENDO compared with those from CON cows. Although ENDO tended to increase (P = 0.06) expression for the scavenger receptor (CD36) for fatty acid uptake and the cellular transporter for choline (SLC44A3), ENDO reduced or tended to reduce (P ≤ 0.06) expression of genes pertaining to phosphatidylcholine synthesis (CHKA, CHPTI) compared with CON. Furthermore, ENDO downregulated (P ≤ 0.03) genes involved in pregnancy signaling (IFNT, PAG2), and the gene that codes for aromatase (CYP19A1), but upregulated (P = 0.01) expression of hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1 (HSD3B1). A heatmap showing the differentially expressed genes in conceptus tissues was created using the fold-change relative to CON trophoblast to illustrate the patterns and intensities of change in mRNA expression according to treatment (Figure 1A).
Table 2.
Effect of treatment on mRNA expression of conceptus tissues recovered on day 16 of pregnancy in lactating Holstein cows receiving AI1
| Fold change3 | Trophoblast | Embryonic disk | P-value2 | ||||
|---|---|---|---|---|---|---|---|
| CON | ENDO | CON | ENDO | TRT | Tissue | TRT × tissue | |
| Pathogen recognition | |||||||
| TLR9 | 1.00 | 2.46 | 0.70 | 2.00 | < 0.01 | 0.24 | 0.76 |
| Cell signaling | |||||||
| MAPK3 | 1.00 | 1.41 | 0.94 | 1.22 | 0.02 | 0.33 | 0.68 |
| Cytokine and receptor | |||||||
| IL6 | 1.00 | 0.36 | 1.08 | 0.43 | 0.05 | 0.45 | 0.75 |
| IFNAR1 | 1.00 | 0.70 | 0.90 | 0.77 | 0.08 | 0.93 | 0.26 |
| IFNGR1 | 1.00 | 0.65 | 0.96 | 0.59 | 0.02 | 0.52 | 0.79 |
| Cell cycle | |||||||
| CDK1 | 1.00 | 0.91 | 1.11 | 0.92 | 0.06 | 0.40 | 0.47 |
| WEE1 | 1.00 | 2.76 | 0.32 | 1.88 | 0.02 | 0.18 | 0.49 |
| Apoptosis | |||||||
| BAD | 1.00 | 0.86 | 0.93 | 0.78 | 0.09 | 0.56 | 0.92 |
| Growth factor | |||||||
| GHR | 1.00 | 0.40 | 0.91 | 0.43 | 0.01 | 0.97 | 0.75 |
| IGFBP2 | 1.00 | 0.58 | 1.17 | 0.57 | 0.05 | 0.80 | 0.77 |
| IGF1 | 1.00 | 0.23 | 0.82 | 0.14 | < 0.01 | 0.39 | 0.74 |
| Antioxidants | |||||||
| SOD1 | 1.00 | 0.69 | 0.94 | 0.70 | 0.02 | 0.74 | 0.69 |
| GPX1 | 1.00 | 0.41 | 0.71 | 0.45 | 0.01 | 0.53 | 0.30 |
| Nutrient uptake | |||||||
| CD36 | 1.00 | 2.90 | 0.93 | 2.72 | 0.06 | 0.59 | 0.98 |
| SLC38A9 | 1.00 | 0.69 | 0.98 | 0.80 | 0.01 | 0.39 | 0.25 |
| SLC44A3 | 1.00 | 1.10 | 0.78 | 1.40 | 0.06 | 0.98 | 0.17 |
| Glucose metabolism | |||||||
| LDHA | 1.00 | 0.12 | 1.08 | 0.22 | < 0.01 | 0.44 | 0.52 |
| G6PD | 1.00 | 0.62 | 1.03 | 0.66 | 0.02 | 0.60 | 0.89 |
| Choline metabolism | |||||||
| CHKA | 1.00 | 0.46 | 0.88 | 0.51 | < 0.01 | 0.91 | 0.51 |
| CHPT1 | 1.00 | 0.77 | 0.86 | 0.72 | 0.06 | 0.07 | 0.41 |
| mTOR pathway | |||||||
| MTOR | 1.00 | 0.27 | 0.47 | 0.24 | < 0.01 | 0.15 | 0.27 |
| RPTOR | 1.00 | 0.67 | 1.08 | 0.69 | 0.03 | 0.45 | 0.64 |
| Conceptus signaling | |||||||
| IFNT | 1.00 | 0.56 | 1.22 | 0.62 | 0.01 | 0.08 | 0.55 |
| IFNT4 | 1.00 | 0.80 | 1.23 | 0.89 | 0.09 | 0.06 | 0.52 |
| PAG2 | 1.00 | 0.52 | 1.25 | 0.64 | 0.02 | 0.01 | 0.89 |
| Steroidogenesis | |||||||
| CYP19A1 | 1.00 | 0.35 | 1.19 | 0.30 | 0.03 | 0.91 | 0.42 |
| HSD3B1 | 1.00 | 1.28 | 0.34 | 0.70 | 0.02 | < 0.01 | 0.13 |
1Holstein cows at 26 ± 3 days postpartum were blocked by parity and genomic breeding value for cow conception rate, and, within block, they were assigned randomly to remain as controls (CON; n = 23) or to receive an intrauterine infusion of 5.20 × 108 CFU E. coli and 4.16 × 108 CFU T. pyogenes during the luteal phase to induce endometrial inflammation (ENDO; n = 34). Conceptuses recovered on day 16 of pregnancy and analyzed for transcriptome were 8 CON and 11 ENDO. Each conceptus was dissected and analyzed as a distal part of trophoblast and embryonic disc.
2TRT = effect of treatment (CON vs. ENDO); tissue = effect of conceptus tissue (trophoblast vs. embryonic disc); TRT × tissue = interaction between TRT and tissue.
3Fold change relative to the trophoblast tissue of CON.
4Fold change of IFNT was adjusted by the conceptus length in the statistical analysis.
Figure 1.
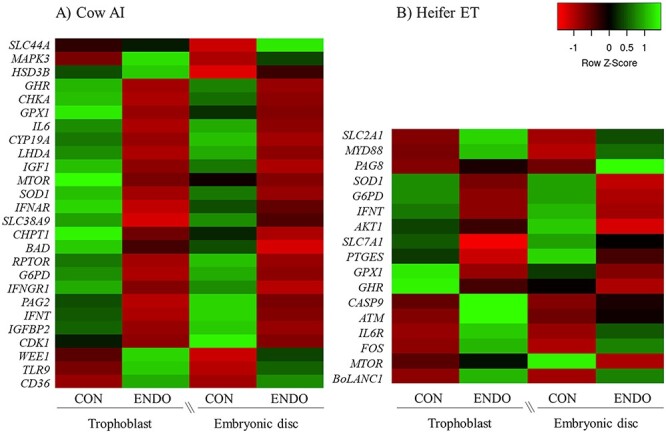
Effect of treatment on the mRNA expression of conceptus tissues from lactating dairy cows receiving AI (A) or heifers receiving ET (B). Conceptuses were recovered on day 16 of pregnancy and dissected into trophoblast and embryonic disc. Heat maps were generated including differently expressed genes (P ≤ 0.10) affected by treatment for the experiment with lactating cows receiving AI or for the experiment with heifers receiving ET. Genes were separated using the average linkage clustering method and following Euclidean distance measurement approach. The mRNA expression increases from red to green, and data based on genes were compared across the rows and presented on a Z-score-based scaling system.
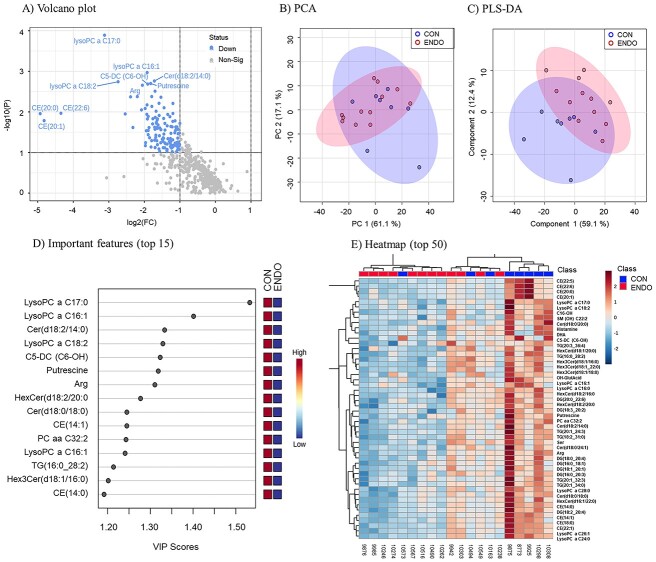
Treatment affected (P ≤ 0.05) the concentrations of 12 metabolites and tended (P < 0.10) to affect the concentrations of another 132 metabolites in the histotroph after adjusting for false discovery (Figure 2A). Specifically, 14 AAs, 9 biogenic amines, 1 fatty acid, 7 acylcarnitines, 11 lysophosphatidylcholines, 7 phosphatidylcholines, 36 ceramides and their derivatives, 14 cholesteryl esters, 22 diglycerides, and 23 triglycerides were all reduced in the ENDO compared with CON treatment. Table 3 depicts the mean (±SD) concentrations of the 50 metabolites most affected by treatment, the associated P-values after adjusting for the false discovery rate, the relative change in concentration in ENDO compared with CON, and the direction of change in concentration.
Figure 2.
Effect of treatment on the histotroph metabolome from lactating dairy cows on day 16 of pregnancy presented as: volcano plot (A); principal component analysis (B); partial least squares—discriminate analysis (C); 15 affected metabolites by treatment based on the VIP score (D); and heatmap of the 50 metabolites discriminating ENDO from CON treatment based on the VIP score (E). The histotroph was collected using the cytobrush technique on day 16 of pregnancy from 8 CON and 12 ENDO cows.
Table 3.
Effect of treatment on histotroph composition of the 50 metabolites most affected by treatment collected on day 16 of pregnancy in lactating Holstein cows receiving artificial insemination
| Metabolite2, μM/g of protein | Treatment1 | |||||
|---|---|---|---|---|---|---|
| CON | ENDO | P-value | Fold change (FC)3 | Log2 (FC) | ENDO/CON | |
| Lysophosphatidylcholine a C17:0 | 0.896 (1.313) | 0.103 (0.049) | 0.018 | 0.11501 | −3.12010 | Down |
| Hydroxyhexanoylcarnitine | 0.0004 (0.0002) | 0.0002 (0.0001) | 0.045 | 0.11420 | −3.13040 | Down |
| Lysophosphatidylcholine a C18:2 | 0.809 (1.325) | 0.121 (0.065) | 0.045 | 0.14984 | −2.73850 | Down |
| Arginine | 77.687 (83.225) | 18.691 (9.775) | 0.045 | 0.24059 | −2.05540 | Down |
| Lysophosphatidylcholine a C16:1 | 0.153 (0.124) | 0.051 (0.027) | 0.045 | 0.25820 | −1.95350 | Down |
| Putrescine | 11.231 (12.011) | 3.357 (3.147) | 0.045 | 0.28511 | −1.81040 | Down |
| Ceramides (d18:2/14:0) | 0.027 (0.024) | 0.008 (0.004) | 0.045 | 0.30396 | −1.71800 | Down |
| Phosphatidylcholine aa C32:2 | 0.780 (1.162) | 0.149 (0.090) | 0.052 | 0.19105 | −2.38800 | Down |
| Ceramides (d18:0/18:0) | 0.053 (0.072) | 0.011 (0.008) | 0.052 | 0.21789 | −2.19830 | Down |
| Cholesteryl esters (14:1) | 0.123 (0.112) | 0.036 (0.022) | 0.052 | 0.28853 | −1.79320 | Down |
| Hexosylceramides (d18:2/20:0) | 0.032 (0.027) | 0.010 (0.008) | 0.052 | 0.30788 | −1.69960 | Down |
| Lysophosphatidylcholine a C16:0 | 3.811 (2.645) | 1.316 (0.673) | 0.052 | 0.34534 | −1.53390 | Down |
| Cholesteryl esters (20:0) | 35.645 (64.958) | 1.170 (0.801) | 0.059 | 0.03281 | −4.92960 | Down |
| Cholesteryl esters (22:6) | 7.349 (12.495) | 0.361 (0.205) | 0.059 | 0.04914 | −4.34690 | Down |
| Cholesteryl esters (22:5) | 1.150 (1.421) | 0.198 (0.122) | 0.059 | 0.17211 | −2.53860 | Down |
| Ceramides (d18:0/20:0) | 0.0008 (0.0009) | 0.0002 (0.0001) | 0.059 | 0.19888 | −2.33000 | Down |
| Hexosylceramides (d16:1/22:0) | 0.033 (0.039) | 0.008 (0.005) | 0.059 | 0.23635 | −2.08100 | Down |
| Diglycerides (18:2_20:4) | 0.240 (0.301) | 0.055 (0.030) | 0.059 | 0.25494 | −1.97180 | Down |
| Cholesteryl esters (14:0) | 23.298 (28.078) | 6.137 (3.661) | 0.059 | 0.26342 | −1.92450 | Down |
| Cholesteryl esters (22:1) | 0.260 (0.303) | 0.069 (0.047) | 0.059 | 0.26523 | −1.91470 | Down |
| Triglycerides (20:1_32:3) | 0.111 (0.144) | 0.030 (0.013) | 0.059 | 0.26955 | −1.89140 | Down |
| Diglycerides (18:0_20:4) | 0.266 (0.282) | 0.077 (0.052) | 0.059 | 0.28919 | −1.78990 | Down |
| Triglycerides (20:1_34:0) | 0.137 (0.164) | 0.041 (0.018) | 0.059 | 0.29500 | −1.76120 | Down |
| Sphingomyelins (OH) C22:2 | 0.454 (0.338) | 0.140 (0.102) | 0.059 | 0.30910 | −1.69380 | Down |
| Lysophosphatidylcholine a C28:0 | 0.431 (0.352) | 0.135 (0.110) | 0.059 | 0.31421 | −1.67020 | Down |
| Lysophosphatidylcholine a C24:0 | 0.038 (0.029) | 0.016 (0.012) | 0.059 | 0.31713 | −1.65680 | Down |
| 3-Hydroxypalmitoylcarnitine | 0.002 (0.002) | 0.001 (0.001) | 0.059 | 0.32605 | −1.61680 | Down |
| Histamine | 1.476 (1.174) | 0.440 (0.319) | 0.059 | 0.32833 | −1.60680 | Down |
| Triglycerides (16:0_28:2) | 0.063 (0.045) | 0.020 (0.013) | 0.059 | 0.33040 | −1.59770 | Down |
| Diglycerides (21:0_22:6) | 0.002 (0.002) | 0.001 (0.001) | 0.059 | 0.36425 | −1.45700 | Down |
| Lysophosphatidylcholine a C26:1 | 0.073 (0.063) | 0.027 (0.018) | 0.059 | 0.36712 | −1.44570 | Down |
| Hydroxyglutaric acid | 4.399 (3.683) | 1.775 (1.728) | 0.059 | 0.39158 | −1.35260 | Down |
| Diglycerides (18:3_20:2) | 0.011 (0.007) | 0.004 (0.003) | 0.059 | 0.39433 | −1.34250 | Down |
| Hexosylceramides (d18:1/20:0) | 0.256 (0.133) | 0.105 (0.070) | 0.059 | 0.41124 | −1.28190 | Down |
| Hexosylceramides (d18:2/16:0) | 0.008 (0.003) | 0.004 (0.003) | 0.059 | 0.43613 | −1.19720 | Down |
| Trihexosylceramides (d18:1/16:0) | 1.923 (0.899) | 0.839 (0.640) | 0.059 | 0.43641 | −1.19620 | Down |
| Cholesteryl esters (20:1) | 7.572 (13.907) | 0.268 (0.157) | 0.060 | 0.03543 | −4.81880 | Down |
| Kynurenine | 4.879 (5.386) | 0.976 (1.127) | 0.060 | 0.20000 | −2.32190 | Down |
| Diglycerides (18:1_20:1) | 0.014 (0.023) | 0.002 (0.001) | 0.060 | 0.25627 | −1.96430 | Down |
| Triglycerides (20:1_24:3) | 0.194 (0.264) | 0.057 (0.036) | 0.060 | 0.29350 | −1.76860 | Down |
| Methylcrotonylcarnitine | 0.025 (0.034) | 0.007 (0.004) | 0.060 | 0.30484 | −1.71390 | Down |
| Docosahexaenoic acid | 7.040 (5.673) | 2.224 (1.217) | 0.060 | 0.31594 | −1.66230 | Down |
| Cholesteryl esters (18:0) | 4.405 (4.721) | 1.395 (0.867) | 0.060 | 0.31679 | −1.65840 | Down |
| Ceramides (d18:0/24:1) | 0.270 (0.323) | 0.087 (0.037) | 0.060 | 0.32046 | −1.64180 | Down |
| Hexosylceramides (d18:1/18:0) | 0.187 (0.243) | 0.060 (0.052) | 0.060 | 0.32073 | −1.64060 | Down |
| Diglycerides (16:0_18:1) | 2.680 (2.881) | 0.717 (0.468) | 0.060 | 0.32139 | −1.63760 | Down |
| Hexosylceramides (d16:1/24:0) | 0.022 (0.028) | 0.007 (0.004) | 0.060 | 0.32663 | −1.61430 | Down |
| Dihexosylceramides (d18:1/26:1) | 0.019 (0.020) | 0.006 (0.004) | 0.060 | 0.33928 | −1.55940 | Down |
| Diglycerides (16:0_20:3) | 0.383 (0.357) | 0.150 (0.118) | 0.060 | 0.39033 | −1.35720 | Down |
| Triglycerides (18:2_31:0) | 0.837 (0.772) | 0.351 (0.291) | 0.060 | 0.41915 | −1.25450 | Down |
1Holstein cows at 26 ± 3 days postpartum were blocked by parity and genomic breeding value for cow conception rate and, within block, they were assigned randomly to remain as controls (CON; n = 23) or to receive an intrauterine infusion of 5.20 × 108 CFU E. coli and 4.16 × 108 CFU T. pyogenes during the luteal phase to induce endometrial inflammation (ENDO; n = 34). The histotroph was collected using the cytobrush technique on day 16 of pregnancy from 8 CON and 12 ENDO cows.
2Metabolites were normalized with total protein quantified in the histotroph and listed according to the P-value adjusted for the false discovery rate at P ≤ 0.10.
3CON was the reference for comparison.
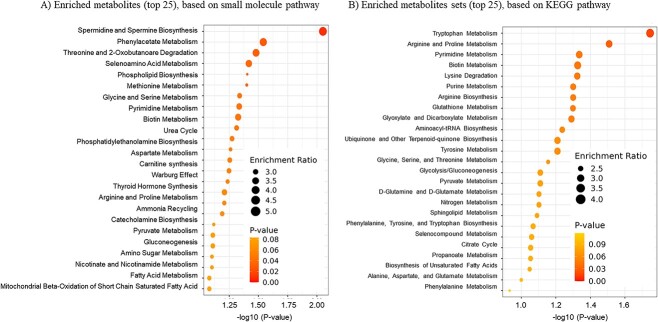
The PCA and PLS-DA analyses of the histotroph composition showed limited segregation of CON and ENDO cows on day 16 of pregnancy (Figure 2B and C). The 15 metabolites most affected by treatment are shown in the VIP score plot (Figure 2D). Lysophosphatidylcholine acyl C17:0 (LysPC a C17:0), LysPC a C16:1, ceramide (Cerd18:3/14:0), LysPC a C18:2, glutarylcarnitine (C5-DC), putrescine, and arginine were the compounds with the greatest impact discriminating ENDO from CON histotroph. The heatmap (Figure 2E) shows the metabolites discriminating histotroph from pregnant ENDO and CON cows based on the VIP score. Significantly altered histotroph metabolites and the 25 corresponding metabolic pathways affected by treatment are shown in Figure 3A and B. Several of the 25 identified pathways affected by treatment were common in the two databases.
Figure 3.
Summary plots for quantitative enrichment analysis based on small-molecule pathway databases (A) and the KEGG pathway (B). The histotroph was collected using the cytobrush technique on day 16 of pregnancy from 8 CON and 12 ENDO cows.
Experiment 2
Induced endometrial inflammation increased (P < 0.01) the proportion of PMN in the endometrial cytology, prevalence of subclinical endometritis, rectal temperature, and the concentration of haptoglobin in serum (Table 4). Of the 28 heifers enrolled, 3 did not have a synchronized ovulation, thus resulting in 13 CON and 12 ENDO heifers receiving ET. Treatment did not affect pregnancy per ET, length of recovered conceptuses, or concentration of IFNT in the uterine flush fluid. A total of 13 conceptuses were recovered, 8 from CON and 5 from ENDO heifers.
Table 4.
Effect of treatment on endometrial polymorphonuclear leukocytes, rectal temperature, serum haptoglobin, conceptus length, and interferon-tau in the uterine fluid in Holstein heifers receiving embryo transfer
| Item | Treatment1 | |||
|---|---|---|---|---|
| CON | ENDO | SEM | P-value | |
| Polymorphonuclear cells,2 % | 0.8 | 12.3 | 1.5 | <0.01 |
| Subclinical endometritis,3 % | 20.5 | 93.4 | 9.3 | <0.01 |
| Rectal temperature, °C | 39.50 | 39.74 | 0.07 | <0.01 |
| Serum haptoglobin, μg/mL | 74.0 | 93.8 | 4.8 | <0.01 |
| Pregnant day 16,4 % | 59.6 | 57.2 | 15.6 | 0.91 |
| Conceptus length, cm | 6.03 | 5.63 | 1.48 | 0.86 |
| Interferon-tau in the flush, ng/mL | 29.9 | 12.4 | 24.9 | 0.61 |
1Holstein heifers were blocked by body weight and, within block, they were assigned randomly to remain as controls (CON; n = 14) or to receive an intrauterine infusion of 4.50 × 108 CFU E. coli and 4.80 × 108 CFU T. pyogenes during the luteal phase to induce endometrial inflammation (ENDO; n = 14). On day 7 of the estrous cycle, an in vitro produced fresh embryo was transferred and heifers were slaughtered 9 days later, corresponding to day 16 of pregnancy, for tissue recovery.
2Polymorphonuclear cells in endometrial cytology collected on day 2 after enrollment.
3Based on ≥5% of polymorphonuclear cells in endometrial cytology.
4Based on detection of interferon-tau in the uterine fluid.
Table 5 depicts the relative expression of genes affected by treatment or by the interaction between treatment and tissue. The LSM and corresponding SEM for the dCt values of the genes affected either by treatment or by the interaction between treatment and conceptus tissue in heifers are depicted in Supplemental Table S3. Induced endometrial inflammation either increased or tended to increase (P ≤ 0.10) expression of genes involved in pro-inflammatory signaling (MYD88), cytokine receptor (IL6R), transcription factors associated with stress response (FOS), apoptosis (ATM, CASP9), and immune function (BoLA-NC1). Also, ENDO tended (P = 0.10) to upregulate the expression of a glucose transporter (SLC12A1A), but either reduced or tended to reduce (P ≤ 0.06) the expression of genes involved with antioxidant effect (SOD1, GPX1), and reduced (P < 0.01) the expression of an AA transporter (SLC7A1) and a gene involved in glycolysis (G6PD). Consistent with the reduced expression of AA transporter, ENDO reduced (P = 0.01) the expression of the mTOR pathway gene (MTOR) in the embryonic disc, but not in the trophoblast. Conceptus from ENDO heifers had decreased (P < 0.01) expression of a gene involved in pregnancy recognition (IFNT) and tended (P = 0.09) to decrease prostaglandin E synthase (PTGES). The reduced expression of IFNT caused by ENDO tended (P = 0.09) to remain significant even after including conceptus length in the statistical model. A heatmap with the differentially expressed genes is depicted in Figure 1B.
Table 5.
Effect of treatment on mRNA expression of conceptus tissues recovered on day 16 of pregnancy in Holstein heifers receiving embryo transfer1
| Fold change3 | Trophoblast | Embryonic disc | P-value2 | ||||
|---|---|---|---|---|---|---|---|
| CON | ENDO | CON | ENDO | TRT | Tissue | TRT × tissue | |
| Cell signaling | |||||||
| AKT1 | 1.00a | 0.84ab | 1.19a | 0.63b | 0.02 | 0.66 | 0.09 |
| FOS | 1.00 | 2.36 | 0.85 | 2.13 | 0.01 | 0.58 | 0.90 |
| MYD88 | 1.00 | 1.20 | 0.96 | 1.15 | 0.01 | 0.43 | 0.99 |
| Cytokine receptor | |||||||
| IL6R | 1.00 | 2.32 | 1.00 | 1.89 | 0.01 | 0.60 | 0.59 |
| Immune function | |||||||
| BOLA-NC1 | 1.00 | 8.23 | 0.58 | 7.01 | 0.01 | 0.19 | 0.42 |
| Apoptosis | |||||||
| ATM | 1.00 | 3.63 | 1.15 | 1.80 | 0.03 | 0.37 | 0.18 |
| CASP9 | 1.00 | 3.25 | 0.82 | 1.51 | 0.07 | 0.22 | 0.47 |
| Growth factor | |||||||
| GHR | 1.00 | 0.49 | 0.59 | 0.31 | 0.10 | 0.16 | 0.90 |
| Antioxidants | |||||||
| SOD1 | 1.00 | 0.56 | 1.03 | 0.44 | 0.01 | 0.56 | 0.45 |
| GPX1 | 1.00 | 0.26 | 0.66 | 0.30 | 0.06 | 0.76 | 0.53 |
| Nutrient uptake | |||||||
| SLC2A1 | 1.00 | 1.27 | 0.97 | 1.16 | 0.10 | 0.55 | 0.77 |
| SLC7A1 | 1.00a | 0.29b | 1.18a | 0.81a | 0.01 | 0.02 | 0.06 |
| mTOR pathway | |||||||
| MTOR | 1.00bc | 1.82b | 3.68a | 0.35c | 0.11 | 0.71 | 0.01 |
| Glucose metabolism | |||||||
| G6PD | 1.00 | 0.53 | 1.03 | 0.47 | 0.01 | 0.82 | 0.70 |
| Conceptus signaling | |||||||
| IFNT | 1.00 | 0.52 | 1.12 | 0.48 | 0.01 | 0.91 | 0.68 |
| PAG8 | 1.00 | 1.25 | 1.05 | 2.00 | 0.08 | 0.21 | 0.30 |
| PTGES | 1.00 | 0.42 | 1.33 | 0.73 | 0.09 | 0.24 | 0.70 |
1Holstein heifers were blocked by body weight and, within block, they were assigned randomly to remain as controls (CON; n = 14) or to receive an intrauterine infusion of 4.50 × 108 CFU E. coli and 4.80 × 108 CFU T. pyogenes during the luteal phase to induce endometrial inflammation (ENDO; n = 14). On day 7 of the estrous cycle, an in vitro produced fresh embryo was transferred and heifers were slaughtered 9 days later, corresponding to day 16 of pregnancy, for tissue recovery. Conceptuses recovered on day 16 of pregnancy were 8 CON and 5 ENDO. Each conceptus was dissected and analyzed as distal part of trophoblast and embryonic disc.
2TRT = effect of treatment (CON vs. ENDO); tissue = effect of conceptus tissue (trophoblast vs. embryonic disc); TRT × tissue = interaction between TRT and tissue.
3Fold change relative to the trophoblast tissue of CON.
a,b,cCells with different superscripts in the same row differ (P < 0.05).
Discussion
The results of the experiments reported herein indicate that infusing a combination of E. coli and T. pyogenes caused endometrial inflammation weeks before breeding and impaired conceptus elongation with corresponding changes in expression of genes associated with growth and development. The impacts of induced endometritis on the conceptus tissues seemed more pronounced in Experiment 1 with lactating cows receiving AI. Conceptus transcriptome from ENDO-treated cows had signatures compatible with inflammatory response based on the upregulated genes linked to inflammation and immune surveillance, whereas genes that play roles in embryonic cell metabolism and favor growth and development were downregulated. Moreover, bacterial-induced endometrial inflammation downregulated genes that code for molecules involved in pregnancy signaling, providing evidence of impaired conceptus maternal crosstalk needed for maintenance of pregnancy. Similar findings have been observed in epidemiological studies in which conceptuses from cows diagnosed with disease before first AI were less developed and resulted in less IFNT content in uterine fluid [9]. Furthermore, cows diagnosed with disease before becoming pregnant to first AI had reduced upregulation of ISG15 expression in leukocytes on day 19 of gestation compared with pregnant cows that did not have disease before first AI [9]. The findings reported in the present experiment provide evidence that bacterial-induced endometrial inflammation is an important factor linking uterine disease and impaired embryo development, which helps explain the reduced maintenance of pregnancy in dairy cows that suffer from uterine diseases early postpartum [9, 11].
In agreement with the gene expression data, bacterial-induced endometritis perturbed the histotroph metabolome. The ENDO treatment induced changes in metabolic pathways related to cellular energy production and synthesis of DNA and cell membrane. Such alterations suggest that bacterial-induced endometrial inflammation affects growth and differentiation of embryonic cells during the pre-implantation period through altered supply of nutrients or altered secretion of histotroph [38]. The ENDO treatment upregulated genes involved in inflammation and downregulated genes involved in growth and development. Perturbations in expression of some of the investigated genes were compatible with the concurrent changes in the histotroph metabolome, thus supporting the idea that endometrial inflammation might limit support for conceptus growth. We hypothesize that inflammatory signals induced embryonic cells into pathways to prioritize survival at the expense of anabolic functions. Although a clear increase in the proportion of PMN in endometrial cytology was observed in ENDO compared with CON cows, the vaginal discharge was not evaluated in the present experiment. Thus, we cannot infer if the model used resulted in the degree of inflammation with purulent vaginal discharge typically observed in cows diagnosed with clinical endometritis [10]. Nevertheless, the increase in PMN in the endometrial cytology is compatible with that observed in dairy cows with naturally occurring endometrial inflammation diagnosed 5–6 weeks postpartum [19], and the latter is linked with reduced reproductive performance in lactating cows receiving AI or ET [11, 19].
Pregnancy maintenance is a critical regulatory event in eutherian mammals, in which the conceptus communicates its presence to the dam [2, 39]. Inadequacy in communication could lead to pregnancy failure either because of an incompetent conceptus or because of inability to prevent luteal regression during maintenance of the CL [7]. Interferon-tau is a key pregnancy recognition molecule in cattle required to prevent CL regression [2]. Interferon-tau reduces the expression of estrogen receptor alpha, which, in turn, suppresses the expression of oxytocin receptors on the uterine luminal epithelium [3]. The latter are critical steps blocking the pulsatile release of prostaglandin F2α [3]. Impaired conceptus elongation and reduced secretion of IFNT could affect pregnancy maintenance [39]. We observed reduced expression of IFNT in conceptus cells and concentration of IFNT in the uterine flush fluid in the ENDO treatment, suggesting that those conceptuses were less capable of transcribing the gene and/or had reduced machinery to synthesize IFNT. It is well described that IFNT content in uterine fluid reflects the length of the conceptus [4], and conceptuses from lactating cows in the ENDO treatment were smaller than those from CON cows. It is plausible that the reduced IFNT caused by induced endometritis compromises the conceptus-maternal crosstalk and contributes to the increased risk of pregnancy loss in cows with uterine disease [9, 11].
Although IFNT expression was downregulated in conceptuses from lactating cows and heifers treated with ENDO, the IFNT content in the uterine flush was only reduced by ENDO in lactating cows. Presumably, reduced IFNT content reflects less synthesis by the conceptus tissues and could be attributed to the retarded growth of the conceptus caused by endometrial inflammation [4]. The lack of effect of treatment on IFNT content in heifers receiving ET might be related to the inability of induced endometritis to have affected conceptus growth. Perhaps, the period of exposure of the embryo to the endometrium in heifers receiving ET, from day 7 to 16 of the estrous cycle, was not sufficient to alter conceptus development and ability to secrete IFNT. Regulation of expression of some genes is time- and tissue-dependent and in cows receiving AI the pre-ovulatory follicle, oocyte, oviduct, and endometrium could all have been affected by inflammation in ENDO cows [12, 15, 40]. Endometritis has a plethora of effects that influence numerous tissues in the reproductive tract, from the endometrium to the ovaries [15, 41]. In an experiment with dairy heifers using a similar protocol to induce endometritis, the authors showed marked differences in transcript expression of the endometrium, oviduct, and granulosa cells [12]. Spontaneous endometritis in lactating cows is known to affect not only the uterus but also follicle development, oocyte competence, fertilization, and early embryonic development [41]. Although ENDO did not affect conceptus length or IFNT content in the uterine flush on day 16 of pregnancy in heifers receiving ET, others have shown that transferring an embryo to cows previously diagnosed with uterine inflammation resulted in reduced maintenance of pregnancy [9, 11]. It is possible that lactating cows might respond differently to the endometrial insult compared with heifers, which might have different implications for conceptus development. Lactating cows are under very distinct nutritional and metabolic constraints compared with growing heifers. Negative nutrient balance and mobilization of body tissues are typical of cows in early lactation, whereas growing heifers are in positive nutrient balance. It is possible that the implications of induced endometrial inflammation with the model used herein is amplified during lactation in cows because of the physiological differences compared with growing heifers. Also, it is possible that the negative impacts of endometritis on maintenance of pregnancy, typically observed when cows received ET [9, 11], are not expressed in full by day 16 of gestation. Nevertheless, because endometritis can affect numerous reproductive tissues [12, 15, 40], it is not surprising that changes in day 16 conceptus seemed more remarkable in animals that received AI than those bred by ET. Furthermore, it is possible that the reduced IFNT transcription could be mediated by alterations in the mTOR pathway. Expression of mTOR was reduced in the trophoblast of conceptus from lactating cows bred by AI. Activation of mTOR enhances CDX2 expression in cells, and the latter is one of the trans-activators that regulate the IFNT gene transcription [42, 43]. Treatment with ENDO reduced conceptus MTOR expression, which could affect anabolic processes and result in less synthesis of IFNT.
In lactating cows, both MTOR and the regulatory-associated protein of mTOR (RPTOR) were less expressed in ENDO compared with CON, whereas in heifers receiving ET, only MTOR expression was reduced by ENDO and the effect was observed in the embryonic disc. Conceptuses from ENDO heifers also had reduced expression of AKT1, and the latter is involved in insulin and mTOR signaling in cells that involve anabolism and tissue accretion. The mTOR protein kinase controls growth by balancing anabolic processes such as protein, lipid, and nucleotide synthesis and catabolic ones such as autophagy and proteasomal activity [44]. Increased supply of AA in the cellular pool activates the mTOR signaling and supports protein synthesis [45]. Expression of the SLC38A9 was reduced in conceptuses from ENDO cows. The SLC38A9 is a lysosomal membrane protein that mediates the transport of many essential AAs out of lysosomes in an arginine-mediated fashion to support growth [46]. Arginine concentration in the histotroph of ENDO cows was less than that in CON cows. Likely, decreased supply of arginine in the histotroph to be transferred to the cellular pool would limit the activation of the mTOR signaling pathway. Another possibility is that endometritis induces changes in the cells to favor synthesis of proteins required for immune activation concurrent with less AAs available for protein synthesis in anabolic pathways that are essential for cellular growth and conceptus development. Induced endometrial inflammation increased the expression of transcripts related to inflammation and immune activation (TLR9, MAPK3) in the conceptus recovered from ENDO cows. Perhaps the collective effect of altered histotroph composition and changes in cellular preference caused by endometrial inflammation downregulated anabolic pathways for growth and development and upregulated those involved in immune surveillance and activation. Changes in arginine uptake by the conceptus or supply by endometrial secretions in cows with induced inflammation could influence supply of polyamines that are critical for cellular growth and differentiation [47]. Putrescine, spermidine, and spermine are critical regulators of the proliferation, migration, and differentiation of trophectoderm cells during the peri-implantation period of pregnancy [48]. Reduced concentrations of arginine and putrescine in the histotroph from lactating cows with induced endometritis provides additional evidence of how inflammation might disturb the uterine environment to make is less conducive for maintenance of pregnancy in cattle.
Bacterial-induced endometritis upregulated the expression of the transcript for TLR9 in lactating cows receiving AI. Toll-like receptors respond to pathogen-associated molecular patterns and initiate a cellular cascade that mounts an inflammatory response [49]. Bacterial pathogen-associated molecular patterns such as lipopolysaccharides from Gram-negative bacteria, which activate the TLR2/TLR4 signaling pathway, and peptidoglycans from both Gram-positive and Gram-negative, which activate TLR2 signaling pathway, induce inflammatory responses on the host tissues. On the other hand, TLR9 is activated by bacterial DNA segments and restricted to areas of the DNA that have unmethylated CpG dinucleotides, abundant in prokaryotic DNA [49, 50]. It is possible that bacterial DNA residues might have persisted in the histotroph 26–42 days after treatment, which triggered the increase in expression of TLR9 in the conceptus tissues.
At the onset of conceptus elongation, the rate of embryonic cell division accelerates [7]. Cellular metabolism must be adjusted to meet the demands for nutrients needed to supply substrate to support the growth of embryonic cells. A vital characteristic of the fast-growing cancer cells is the ability to switch from oxidative phosphorylation to aerobic glycolysis consisting of increased glucose uptake and lactate production. This metabolic switch, known as the “Warburg effect,” provides readily available energy and substrates for accelerated growth [51]. Evidence suggests that metabolism of early bovine embryo is characterized by increased glucose uptake and lactate production [52], which mimic the “Warburg effect” observed in cancerous cells [53]. The conceptus recovered from ENDO cows showed decreased transcripts for lactate and NADPH biosynthesis (LDHA, G6PD). In agreement, quantitative enrichment pathway analyses of the metabolites in the histotroph revealed that glycolysis and pyruvate metabolism, along with the “Warburg effect,” were reduced in ENDO cows. We suggest that endometrial inflammation caused aberrations in gene transcription and associated metabolic pathways required for accelerated growth of the embryonic cells, thus compromising conceptus elongation.
Phosphatidylcholines are abundant phospholipids in mammalian cells and tissues [54, 55]. They are biosynthesized de novo either from choline obtained from the diet in numerous tissues or from ethanolamine through transmethylation reactions in the liver. Endometrial inflammation caused the conceptus tissues to downregulate transcription of genes involved in phosphatidylcholine synthesis (CHKA, CHPT1), and decreased lysophosphatidylcholine accumulation in the histotroph. Furthermore, ENDO reduced expression of the cell cycle progression gene CDK1 and increased the expression of the cell cycle checkpoint gene WEE1 compared with CON. Altogether, these data suggest that endometrial inflammation perturbed the transcripts required for anabolic pathways needed for cell division and growth, retarding conceptus development in cows. Interestingly, expression of the gene involved in the cellular uptake of choline (SLC44A3) was upregulated in conceptuses from ENDO-treated cows. Perhaps, conceptuses from ENDO cows attempted to compensate for the reduced phospholipid abundance in histotroph by upregulating genes involved in choline uptake. Another possibility is a change in the fate of choline by conceptuses from ENDO cows with increased oxidation to betaine. Betaine is a methyl donor, and DNA methylation is involved in regulation of gene expression, which could explain the reduced expression of genes involved in growth and development. A limitation of this experiment is that mechanisms involved in DNA methylation were not investigated. The suggestion that induced endometritis affects choline uptake by the conceptus and directs it to be utilized for DNA methylation requires further investigation.
Aside from the anti-luteolytic role of IFNT, it stimulates the expression of interferon-stimulated genes ISG15, MX2, and RTP4 among others in the glandular epithelium and stromal cells of the endometrium [56] and in blood leukocytes. Together with progesterone, IFNT increases the expression of genes required to transport nutrients into the uterine lumen, such as AA and glucose [57]. Bacterial-induced endometrial inflammation reduced expression of the arginine transporter SLC7A1 and the concentration of arginine in the histotroph of ENDO cows, which could affect conceptus growth and reduce IFNT production. Previous work using a similar model showed that induced endometrial inflammation upregulated pathways related to iNOS, TLR, and IL17 and downregulated the expression of ISG15 in pregnant cows [40]. Pregnancy upregulated expression of ISG15 in leukocytes on day 19 after AI, but the upregulation was less in pregnant cows diagnosed with disease before AI compared with pregnant cows that did not have disease before AI [9]. Lesser stimulation of endometrial and immune cells by IFNT is likely caused by reduced synthesis or response of the tissue to interferon. Independent of the mechanism through which it occurs, reduced IFNT could compromise uterine receptivity to pregnancy.
Induced endometrial inflammation reduced conceptus elongation by altering the transcripts involved in cellular growth and development. Inflammatory signals induced by endometrial inflammation altered cells’ metabolism to favor inflammatory signals and survival at the expense of anabolic pathways. Retarded conceptus growth and pregnancy recognition signals are potential mechanisms explaining the compromised establishment and maintenance of pregnancy in dairy cows that suffer from uterine diseases in early lactation. Overall, the impact of induced endometritis seemed to be lesser on conceptus tissues from heifers receiving ET relative to what was observed in cows receiving AI; however, the affected genes and associated pathways altered by inflammation involved restricted growth and increased immune response in both models studied herein. The present results suggest that endometrial inflammation caused by pathogenic bacteria may be responsible for much of the reproductive phenotype observed in cows after uterine disease.
Supplementary Material
Acknowledgments
The authors thank Select Sires and Alta Genetics for providing semen for this experiment. We extend our gratitude to Dr Michael Poindexter, Dr Nicolas DiLorenzo, Dr Tracy Scheffler, Dr Eliab Estrada, Dr Peter J. Hansen, Dr Achilles Vieira-Neto, Karla Ferreira, Daniella Heredia, Guido Exequiel Enriquez, and Sergei Sennikov (University of Florida) for their help with the conduct of the experiment and laboratory assays. The help of the staff of the University of Florida Dairy Unit (Gainesville, FL) and the North Florida Research and Education Center (Marianna, FL) is greatly appreciated.
Footnotes
† Grant Support: Research reported in this publication was partially supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD084316. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. AH was supported by a Fulbright PhD fellowship under Award Number PS00241264.
Contributor Information
Ali Husnain, Department of Animal Sciences, DH Barron Reproductive and Perinatal Biology Research Program, University of Florida, Gainesville, FL, USA.
Usman Arshad, Department of Animal Sciences, DH Barron Reproductive and Perinatal Biology Research Program, University of Florida, Gainesville, FL, USA.
Roney Zimpel, Department of Animal Sciences, DH Barron Reproductive and Perinatal Biology Research Program, University of Florida, Gainesville, FL, USA.
Eduardo Schmitt, Department of Animal Sciences, DH Barron Reproductive and Perinatal Biology Research Program, University of Florida, Gainesville, FL, USA.
Mackenzie J Dickson, Department of Animal Sciences, DH Barron Reproductive and Perinatal Biology Research Program, University of Florida, Gainesville, FL, USA.
Milerky C Perdomo, Department of Animal Sciences, DH Barron Reproductive and Perinatal Biology Research Program, University of Florida, Gainesville, FL, USA.
Mariana N Marinho, Department of Animal Sciences, DH Barron Reproductive and Perinatal Biology Research Program, University of Florida, Gainesville, FL, USA.
Nadia Ashrafi, Metabolomics Department, Beaumont Health, Royal Oak, MI, USA.
Stewart F Graham, Metabolomics Department, Beaumont Health, Royal Oak, MI, USA; Oakland University-William Beaumont School of Medicine, Rochester, MI, USA.
Jeanette V Bishop, Animal Reproduction and Biotechnology Laboratory, Department of Biomedical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO, USA.
Thomas R Hansen, Animal Reproduction and Biotechnology Laboratory, Department of Biomedical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO, USA.
Kwang C Jeong, Department of Animal Sciences, DH Barron Reproductive and Perinatal Biology Research Program, University of Florida, Gainesville, FL, USA.
Angela M Gonella-Diaza, North Florida Research and Education Center, University of Florida, Marianna, FL USA.
Ricardo C Chebel, Department of Large Animal Clinical Sciences, College of Veterinary Medicine, University of Florida, Gainesville, FL, USA.
I Martin Sheldon, Swansea University Medical School, Swansea University, Swansea, UK.
John J Bromfield, Department of Animal Sciences, DH Barron Reproductive and Perinatal Biology Research Program, University of Florida, Gainesville, FL, USA.
José E P Santos, Department of Animal Sciences, DH Barron Reproductive and Perinatal Biology Research Program, University of Florida, Gainesville, FL, USA.
Conflict of Interest
The authors have not stated any conflicts of interest.
Data availability
Data available upon request to the corresponding author.
References
- 1. Godkin JD, Bazer FW, Moffatt J, Sessions F, Roberts RM. Purification and properties of a major, low molecular weight protein released by the trophoblast of sheep blastocysts at day 13–21. J Reprod Fert 1982; 65:141–150. [DOI] [PubMed] [Google Scholar]
- 2. Spencer TE, Bazer FW. Ovine interferon tau suppresses transcription of the estrogen receptor and oxytocin receptor genes in the ovine endometrium. Rapid Commun 1996; 137:1144–1147. [DOI] [PubMed] [Google Scholar]
- 3. Spencer TE, Bazer FW. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci 2002; 7:d1879–d1898. [DOI] [PubMed] [Google Scholar]
- 4. Ribeiro ES, Greco LF, Bisinotto RS, Lima FS, Thatcher WW, Santos JEP. Biology of preimplantation conceptus at the onset of elongation in dairy cows. Biol Reprod 2016; 94:97–18. [DOI] [PubMed] [Google Scholar]
- 5. Simintiras CA, Sánchez JM, McDonald M, Martins T, Binelli M, Lonergan P. Biochemical characterization of progesterone-induced alterations in bovine uterine fluid amino acid and carbohydrate composition during the conceptus elongation window. Biol Reprod 2019; 100:672–685. [DOI] [PubMed] [Google Scholar]
- 6. Simintiras CA, Sánchez JM, McDonald M, Lonergan P. The biochemistry surrounding the bovine conceptus elongation. Biol Reprod 2019; 101:328–337. [DOI] [PubMed] [Google Scholar]
- 7. Spencer TE, Forde N, Lonergan P. Insights into conceptus elongation and establishment of pregnancy in ruminants. Reprod Fert Devel 2017; 29:84–100. [DOI] [PubMed] [Google Scholar]
- 8. Dickson MJ, Piersanti LR, Ramirez-Hernandez R, de Oliveira EB, Bishop JV, Hansen TR, Ma Z, Jeong KC, Santos JEP, Sheldon MI, Block J, Bromfield JJ. Experimentally induced endometritis impairs the developmental capacity of bovine oocytes. Biol Reprod 2020; 103:508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ribeiro ES, Gomes G, Greco LF, Cerri RLA, Vieira-Neto A, Monteiro PLJ Jr, Lima FS, Bisinotto RS, Thatcher WW, Santos JEP. Carryover effect of postpartum inflammatory diseases on developmental biology and fertility in lactating dairy cows. J Dairy Sci 2016; 99:2201–2220. [DOI] [PubMed] [Google Scholar]
- 10. LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, Johnson WH. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J Dairy Sci 2002; 85:2223–2236. [DOI] [PubMed] [Google Scholar]
- 11. Edelhoff INF, Pereira MHC, Bromfield JJ, Vasconcelos JLM, Santos JEP. Inflammatory diseases in dairy cows: risk factors and associations with pregnancy after embryo transfer. J Dairy Sci 2020; 103:11970–11987. [DOI] [PubMed] [Google Scholar]
- 12. Horlock AD, Piersanti RL, Ramirez-Hernandez R, Yu F, Ma Z, Jeong KC, Clift MJD, Block J, Santos JEP, Bromfield JJ, Sheldon IM. Uterine infection alters the transcriptome of the bovine reproductive tract three months later. Reproduction 2020; 160:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bicalho ML, Machado VS, Oikonomou G, Gilbert RO, Bicalho RC. Association between virulence factors of Escherichia coli, Fusobacterium necrophorum, and Arcanobacterium pyogenes and uterine diseases of dairy cows. Vet Microbiol 2012; 157:125–131. [DOI] [PubMed] [Google Scholar]
- 14. Galvão KN, Bicalho RC, Jeon SJ. Symposium review: the uterine microbiome associated with the development of uterine disease in dairy cows. J Dairy Sci 2019; 102:11786–11797. [DOI] [PubMed] [Google Scholar]
- 15. Bromfield JJ, Santos JEP, Block J, Williams RS, Sheldon IM. Physiology and endocrinology symposium: uterine infection: linking infection and innate immunity with infertility in the high-producing dairy cow. J Anim Sci 2015; 93:2021–2033. [DOI] [PubMed] [Google Scholar]
- 16. Goldstone RJ, Talbot R, Schuberth HJ, Sandra O, Sheldon IM, Smith DG. Draft genome sequence of Escherichia coli MS499, isolated from the infected uterus of a postpartum cow with metritis. Genome Announc 2014; 2:e00217–e00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldstone RJ, Amos M, Talbot R, Schuberth HJ, Sandra O, Sheldon IM, Smith DG. Draft genome sequence of Trueperella pyogenes, isolated from the infected uterus of a postpartum cow with metritis. Genome Announc 2014; 2:e00217–e00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stevenson K, McVey AF, Clark IBN, Swain PS, Pilizota T. General calibration of microbial growth in microplate readers. Sci Rep 2016; 6:38828. 10.1038/srep38828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kasimanickam R, Duffield TF, Foster RA, Gartley CJ, Leslie KE, Walton JS, Johnson WH. Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology 2004; 62:9–23. [DOI] [PubMed] [Google Scholar]
- 20. Piersanti RL, Zimpel R, Molinari PCC, Dickson MJ, Ma Z, Jeong KC, Santos JEP, Sheldon IM, Bromfield JJ. A model of clinical endometritis in Holstein heifers using pathogenic Escherichia coli and Trueperella pyogenes. J Dairy Sci 2019; 102:2686–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Makimura SC, Suzuki N. Quantitative determination of bovine serum haptoglobin and its elevation in some inflammatory diseases. Jpn J Vet Sci 1982; 44:15–21. [DOI] [PubMed] [Google Scholar]
- 22. Silva FACC, da Silva GF, Vieira BS, Neto AL, Rocha CC, Turco EGL, Nogueira GP, Pugliesi G, Binelli M. Peri-estrus ovarian, uterine, and hormonal variables determine the uterine luminal fluid metabolome in beef heifers. Biol Reprod 2021; 105:1140–1153. [DOI] [PubMed] [Google Scholar]
- 23. Pascottini OB, Hostens M, Dini P, Van Eetvelde M, Vercauteren P, Opsomer G. Prevalence of cytological endometritis and effect on pregnancy outcomes at the time of insemination in nulliparous dairy heifers. J Dairy Sci 2016; 99:9051–9056. [DOI] [PubMed] [Google Scholar]
- 24. Lima FS, Ribeiro ES, Bisinotto RS, Greco LF, Martinez N, Amstalden M, Thatcher WW, Santos JEP. Hormonal manipulation in the 5-day timed artificial insemination protocol to optimize estrous cycle synchrony and fertility in dairy heifers. J Dairy Sci 2013; 96:7054–7065. [DOI] [PubMed] [Google Scholar]
- 25. Steibel JP, Poletto R, Coussens PM, Rosa GJ. A powerful and flexible linear mixed model framework for the analysis of relative quantification RT-PCR data. Genomics 2009; 94:146–152. [DOI] [PubMed] [Google Scholar]
- 26. Yuan JS, Reed A, Chen F, Stewart CN Jr. Statistical analysis of real-time PCR data. BMC Bioinformatics 2006; 7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 2001; 25:402–408. [DOI] [PubMed] [Google Scholar]
- 28. Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A, Wishart DS. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res 2016; 44:W147–W153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Box GEP, Cox DR. An analysis of transformations. J R Stat Soc B 1964; 26:211–243. [Google Scholar]
- 30. Piepho H-P. Data transformation in statistical analysis of field trials with changing treatment variance. Agron J 2009; 101:865–869. [Google Scholar]
- 31. Jørgensen E, Pedersen AR. How to Obtain Those Nasty Standard Errors from Transformed Data—and Why They Should Not Be Used. Page 20 in Biometry Research Unit—Internal Report 7. Aarhus University, The Faculty of Agricultural Sciences; 1998. Accessed Aug. 01, 2022. https://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.47.9023 [Google Scholar]
- 32. Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res 2009; 37:W652–W660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res 2015; 43:W251–W257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pang Z, Zhou G, Ewald J, Chang L, Hacariz O, Basu N, Xia J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat Protoc 2022; 17:1735–1761. [DOI] [PubMed] [Google Scholar]
- 35. Frolkis A, Knox C, Lim E, Jewison T, Law V, Hau DD, Liu P, Gautam B, Ly S, Guo AC, Xia J, Liang Y, et al. SMPDB: the small molecule pathway database. Nucleic Acids Res 2010; 38:D480–D487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jewison T, Su Y, Disfany FM, Liang Y, Knox C, Maciejewski A, Poelzer J, Huynh J, Zhou Y, Arndt D, Djoumbou Y, Liu Y, et al. SMPDB 2.0: big improvements to the small molecule pathway database. Nucleic Acids Res 2014; 42:D478–D484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 2000; 28:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes’ compromises conceptus survival and elongation. Reproduction 2002; 124:289–300. [PubMed] [Google Scholar]
- 39. Thatcher WW, Hansen PJ, Gross TS, Helmer SD, Plante C, Bazer FW. Antiluteolytic effects of bovine trophoblast protein 1. J Reprod Fertil Suppl 1989; 37:91–99. https://europepmc.org/article/med/2810237. [PubMed] [Google Scholar]
- 40. Dickson MJ, Bishop JV, Hansen TR, Sheldon IM, Bromfield JJ. The endometrial transcriptomic response to pregnancy is altered in cows after uterine infection. PloS One 2022; 17:e0265062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gilbert RO. The effects of endometritis on the establishment of pregnancy in cattle. Reprod Fert Develop 2012; 24:252–257. [DOI] [PubMed] [Google Scholar]
- 42. Fan H-B, Zhai Z-Y, Li X-G, Gao C-Q, Yan H-C, Chen Z-S, Wang X-Q. CDX2 stimulates the proliferation of porcine intestinal epithelial cells by activating the mTORC1 and Wnt/β-catenin signaling pathways. Int J Mol Sci 2017; 18:2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ezashi T, Imakawa K. Transcriptional control of IFNT expression. Reproduction 2017; 154:F21–F31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism and disease. Cell 2017; 168:960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hara K, Yonezawa K, Weng Q-P, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem 1998; 273:14484–14494. [DOI] [PubMed] [Google Scholar]
- 46. Wyant GA, Abu-Remaileh M, Wolfson RL, Chen WW, Freinkman E, Danai LV, Heiden MGV, Sabatini DM. mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Cell 2017; 171:642–654.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J 1998; 336:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang X, Frank JW, Little DR, Dunlap KA, Satterfield MC, Burghardt RC, Hansen TR, Wu G, Bazer FW. Functional role of arginine during the peri-implantation period of pregnancy. I. Consequences of loss of function of arginine transporter SLC7A1 mRNA in ovine conceptus trophectoderm. FASEB J 2014; 28:2852–2863. [DOI] [PubMed] [Google Scholar]
- 49. Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2001; 2:675–680. [DOI] [PubMed] [Google Scholar]
- 50. Dalpke A, Frank J, Peter M, Heeg K. Activation of toll-like receptor 9 by DNA from different bacterial species. Infect Immun 2006; 74:940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Warburg O. On the origin of cancer cells. Science 1956; 123:309–314. [DOI] [PubMed] [Google Scholar]
- 52. Thompson JG, Partridge RJ, Houghton FD, Cox CI, Leese HJ. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J Reprod Fert 1996; 106:299–306. [DOI] [PubMed] [Google Scholar]
- 53. Smith DG, Sturmey RG. Parallels between embryo and cancer cell metabolism. Biochem Soc Trans 2013; 41:664–669. [DOI] [PubMed] [Google Scholar]
- 54. Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res 2008; 49:1187–1194. [DOI] [PubMed] [Google Scholar]
- 55. van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 2008; 9:112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bazer FW, Burghardt RC, Johnson GA, Spencer TE, Wu G. Interferons and progesterone for establishment and maintenance of pregnancy: interactions among novel cell signaling pathways. Reprod Biol 2008; 8:179–211. [DOI] [PubMed] [Google Scholar]
- 57. Bazer FW, Burghardt RC, Johnson GA, Spencer TE, Wu G. Mechanisms for the establishment and maintenance of pregnancy: synergies from scientific collaborations. Biol Reprod 2018; 99:225–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon request to the corresponding author.