Abstract
Introduction
Effective strategies to prevent hemodialysis (HD) catheter dysfunction are lacking and there is wide variation in practice.
Methods
In this post hoc analysis of the REDUcing the burden of dialysis Catheter ComplicaTIOns: a national (REDUCCTION) stepped-wedge cluster randomized trial, encompassing 37 Australian nephrology services, 6361 participants, and 9872 catheters, we investigated whether the trial intervention, which promoted a suite of evidence-based practices for HD catheter insertion and management, reduced the incidence of catheter dysfunction, which is defined by catheter removal due to inadequate dialysis blood flow. We also analyzed outcomes among tunneled cuffed catheters and sources of event variability.
Results
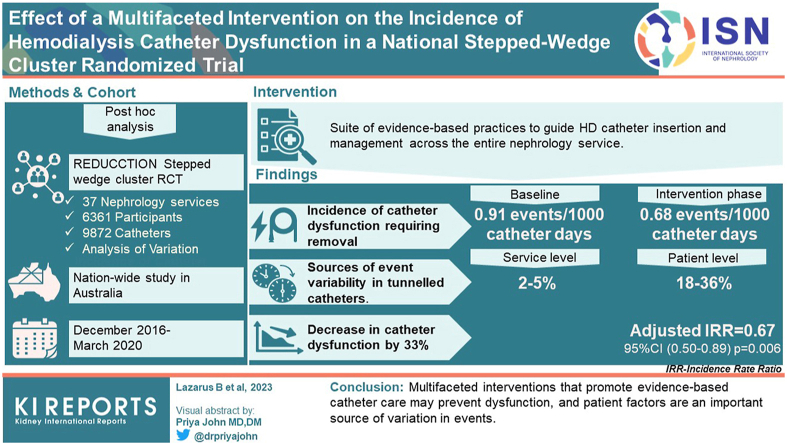
A total of 873 HD catheters were removed because of dysfunction over 1.12 million catheter days. The raw incidence was 0.91 events per 1000 catheter days during the baseline phase and 0.68 events per 1000 catheter days during the intervention phase. The service-wide incidence of catheter dysfunction was 33% lower during the intervention after adjustment for calendar time (incidence rate ratio = 0.67; 95% confidence interval [CI], 0.50–0.89; P = 0.006). Results were consistent among tunneled cuffed catheters (adjusted incidence rate ratio = 0.68; 95% CI, 0.49–0.94), which accounted for 75% of catheters (n = 7403), 97.4% of catheter exposure time and 88.2% of events (n = 770). Among tunneled catheters that survived for 6 months (21.5% of tunneled catheters), between 2% and 5% of the unexplained variation in the number of catheter dysfunction events was attributable to service-level differences, and 18% to 36% was attributable to patient-level differences.
Conclusion
Multifaceted interventions that promote evidence-based catheter care may prevent dysfunction, and patient factors are an important source of variation in events.
Keywords: CVC, dialysis, malfunction, randomized, renal, variance
Graphical abstract
Approximately 3 million people worldwide receive life-sustaining HD treatments for kidney failure, and the global incidence continues to increase.1 Central venous catheters (CVCs) are used to facilitate HD vascular access for between 60% to 80% of incident patients, 20% to 45% of prevalent patients, and almost 100% of patients with acute kidney injury (AKI) requiring dialysis.2, 3, 4, 5 Catheter dysfunction, defined as the “failure to maintain the prescribed extracorporeal blood flow required for adequate hemodialysis,”6 is a common complication that necessitates unplanned removal of between 10% and 30% of tunneled catheters despite the routine use of antithrombotic catheter locks.7, 8, 9 Dysfunction has been associated with worse patient outcomes and excess costs due to missed HD sessions; HD catheter-related bloodstream infections (HD-CRBSI); and hospitalizations and additional procedures to salvage, exchange, or replace the catheter.10, 11, 12, 13 Patients receiving HD have identified loss of functioning vascular access, including catheter dysfunction, as a key health priority.14
The best way to prevent HD catheter dysfunction is unknown. Observational studies have suggested that certain practices, such as right internal jugular venous site of insertion rather than left,15 use of imaging guidance at the time of insertion,16 and the use of tunneled rather than nontunneled HD catheters17 are associated with fewer mechanical complications. A variety of prophylactic therapeutic strategies, such as citrate locks and systemic anticoagulation, failed to reduce the risk of catheter dysfunction compared to standard heparin locks.13 Weekly thrombolytic catheter locks, with recombinant tissue plasminogen activator or urokinase, are not cost-effective and only have a modest impact on dysfunction.11,18,19 An absence of consensus guidelines may also contribute to the wide variation in catheter management practices20,21 and rates of dysfunction. Novel therapeutic strategies are required, and an understanding of the factors that mediate outcome variation may help to identify targets for future interventions.
Quality improvement interventions have sought to standardize catheter management practices to prevent HD-CRBSI,22, 23, 24 but it is not known whether such programs can influence the risk of catheter dysfunction. The multifaceted intervention implemented in the REDUCCTION approach trial sought to prevent HD-CRBSI,25,26 and promoted practices that may have improved catheter performance, such as prioritizing CVC insertion in the right internal jugular vein,15,27 ultrasound guidance during catheter insertion, and earlier transition from nontunneled to tunneled catheters.17,28 Antimicrobial lock solutions can also prevent the formation of biofilms,29 which may promote fibrin sheath formation,30 and act as a nidus for coagulation and thrombosis.31,32
We therefore conducted a post hoc analysis of the REDUCCTION trial to determine whether the intervention reduced the service-wide incidence of catheter dysfunction. In addition, we sought to quantify the extent to which service-level and patient-level differences contributed to unexplained variation in the number of catheter dysfunction events, to gain insight into possible targets for future interventions.
Methods
Study Design and Population
Detailed reports of the REDUCCTION study design and the main findings have been published.25,26 In brief, 37 nephrology services in Australia were randomly assigned to 1 of 3 tranches. Covariate-constrained randomization was used to ensure that the average number of catheters inserted, among services during the baseline phase was balanced across each tranche.33 Further details regarding the randomization method are provided in the Supplementary Methods. The services were not advised of the nature of the intervention nor their allocated tranche until 6 weeks before the intervention was due to commence. The entire multifaceted intervention was implemented together as a single package at the level of the service, and each tranche of services implemented the intervention 6 months apart. All 37 services participated in both phases of the trial. Participants who were enrolled in the baseline phase could also participate during the intervention phase, either by continuing to use an HD catheter when the service implemented the intervention, or by using a new catheter during the intervention phase. Therefore, participants receiving chronic HD accumulated over time in each tranche. An individual catheter that was in situ during the baseline phase and remained in situ during the intervention phase contributed exposure time to both phases.
All patients within a nephrology service were eligible for inclusion if they received an HD CVC, either tunneled or nontunneled, on or after December 20, 2016 until March 31, 2020. Participants were not eligible for inclusion while their HD catheter was managed by the intensive care unit or another non-nephrology department. Participants were also excluded if they were less than 18 years old or chose to opt out of the study. Catheters with missing tunnel status or reason for removal were excluded from this analysis. Follow-up time started from the date on which the catheter came under the care of the nephrology service. The trial used a waiver or opt out approach to consent and was ethically approved across all jurisdictions.26
Intervention
All nephrology services started the study in the baseline phase, which involved no change to existing practice beyond the recording of patient and catheter information in the study database. Six weeks prior to transitioning to the intervention phase, the local physician and nurse champion were informed of the nature of the intervention package, commenced implementation training, and subsequently implemented it across the entire service.26 The multifaceted intervention was designed based on an independent literature review and sought to standardize catheter insertion, maintenance, and removal practices. Components included promoting the right internal jugular site of catheter insertion, ultrasound guided catheter placement, routine antimicrobial catheter prophylaxis, patient education, regular feedback of HD-CRBSI, and early removal of nontunneled, unused, or infected catheters.26 Each site confirmed the date of implementation, and specified which antimicrobial catheter locks or impregnated dressings were used. Adherence to handwashing and aseptic technique was not audited during individual patient encounters. Further details regarding the trial intervention are provided in the Supplementary Methods.
Study Measures
Participant characteristics were recorded at enrollment into the study and included age, gender, ethnicity, a diagnosis of diabetes mellitus, and the use of immunosuppressant medications. Catheter characteristics were recorded at the start of catheter exposure, and included tunnel status, site of insertion, department responsible for catheter insertion, and indication for catheter insertion as assessed by the treating clinician. Indications for insertion included AKI, commencement of chronic HD, transfer from peritoneal dialysis, complications of arteriovenous access, and other reasons. Baseline serum creatinine and other prognostic markers for kidney recovery were not measured; therefore, some participants who received HD for AKI may have had preexisting chronic kidney disease. The use of ultrasound guided insertion was only recorded during the intervention phase. All tunneled catheters were cuffed.
End Points
The primary outcome for this post hoc analysis was the service-wide incidence of catheter dysfunction. Catheter dysfunction was defined as removal of the catheter due to inadequate blood flow, as assessed by the treating clinician. In the original study protocol, catheter malfunction was defined as either treatment of the catheter with a thrombolytic agent or removal of the catheter due to an inability to maintain adequate blood flow during dialysis. However, during the trial, thrombolytic catheter treatments were not reliably measured and the patient-centered outcome of catheter dysfunction requiring removal was therefore used.14
Catheter exposure time commenced when the catheter came under the care of the nephrology service and ended on the day of removal, discharge from the service, or at the end of the study if not removed or discharged before this date. It was assumed that catheters were inserted at the start of the day and removed at the end of the day; therefore, catheters that were removed on the same day as insertion were in situ for 1 day.
Statistical Methods
Primary Analysis
Baseline data are presented as number (percentage), mean (SD), or median (interquartile range). The incidence of catheter dysfunction requiring removal was calculated by dividing the total number of events by the cumulative catheter exposure time in days. The effect of the intervention was analyzed by comparing the incidence of catheter dysfunction during the intervention phase to the incidence during the baseline phase across all 37 participating services. To account for correlations between the incidence of dysfunction during baseline and intervention phases for a given service, and underlying secular trends, a 2-level mixed effects Poisson regression model with a random effect for service and a fixed effect for calendar time, was used.34 Four calendar time periods were included, in which none, 1, 2, or all 3 tranches were assigned to the intervention.25,35 Overdispersion was not identified in a check of deviance and Pearson residuals. The intention-to-treat principle was followed. The date on which services were assigned to treatment intervention was used to allocate events to baseline or intervention phases.
Subgroup Analysis
Any potential beneficial intervention effect was hypothesized to be primarily observed among tunneled cuffed catheters owing to a greater level of involvement of the nephrology service in their placement and ongoing longer-term management, so tunneled catheters were prespecified as a clinical subgroup of interest. To examine the effect of the intervention in people with tunneled catheters, whether differences in catheter insertion site during the intervention phase influenced the effect, and sources of variation, a 3-level mixed effects Poisson regression model was used, with separate random effects for services and patients nested within services, and fixed effects for catheter and patient characteristics, and calendar time (Supplementary Figure S1).
Variance Partition Coefficients
Variation in the number of catheter dysfunction events was not fully explained by the covariates. The total unexplained variation was partitioned into that which was attributable to differences between services and differences between patients, to assess whether unmeasured differences at either level were associated with catheter dysfunction. The extent to which service-level and patient-level factors accounted for unexplained variation might have important implications for the design of future interventions to prevent dysfunction. For example, if a large proportion of variation was attributed to unmeasured patient-level factors, then elucidating those factors may enhance the prediction of catheter dysfunction and facilitate more targeted interventions and personalized management.
Variance partition coefficients (VPCs) range from 0, where differences between clusters at that level do not account for any of the variation, to 1, where differences between clusters at that level account for all the unexplained variation.36 VPCs were calculated among tunneled cuffed catheters from the fully adjusted 3-level mixed effects Poisson model described in the subgroup analysis, which included participant and catheter characteristics, using the exact method for count data.36,37 As described by Austin et al.,36 VPCs for count data depend on the model covariates. In Poisson models of incidence rates, exposure time is included as an offset variable, but can also be viewed as an additional covariate with a regression coefficient that is always equal to 1.36 VPCs were therefore calculated for catheters with different durations of at-risk time, ranging from 1 to 365 days. The reference set of patient and catheter characteristics included male gender, 60 to 70 year-old age group, absence of diabetes mellitus, and right internal jugular vein catheter site. VPCs were calculated among catheters that survived for 6 months during the baseline phase, intervention phase, and for a range of plausible patient and catheter characteristics, including males aged less than 50 years, elderly females with diabetes mellitus, and catheters inserted in the left internal jugular vein. All tests were 2-sided with an alpha level of 0.05 and were performed using statistical software (Stata/BE, version 17.0, StataCorp LLC, College Station, TX and R, version 1.4, R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Participants and Catheter Characteristics
There were 198 participants (310 catheters) with a missing reason for catheter removal and 2 participants (2 catheters) with catheters that were not known to be tunneled or nontunneled; and these participants were excluded. In total, 6361 participants, 9872 unique catheters and 1.12 million catheter days were analyzed (Supplementary Figure S2). A total of 4198 (66%) participants used 1 catheter only, 1483 (23%) used 2 catheters, and 680 (11%) used 3 or more catheters (median = 1, interquartile range 1–2, range 1–15).
The characteristics of participants in the baseline phase only (n = 2388), the intervention phase only (n = 2800), or in both phases (n = 1173) are presented in Table 1. Among participants in both phases, the median number of catheters per participant was 2 (interquartile range 1–3, range 1–15). Characteristics of individual catheters that contributed exposure time to the baseline phase only (n = 4205), the intervention phase only (n = 4594), or both phases (n = 1073) are presented in Table 2. Of 3489 HD catheters that were required for kidney failure precipitated by AKI, 2076 (59.5%) were tunneled and 1413 (40.5%) were nontunneled. During the intervention phase, a smaller proportion of HD catheters were inserted for AKI. Ultrasound was used to guide insertion of 89.7% (n = 3117) of tunneled and 78.7% (n = 880) of nontunneled catheters during the intervention phase. Individual catheters that straddled both phases of the trial contributed a total of 185,313 catheter days to the baseline phase and 217,434 catheter days to the intervention phase. These catheters were predominantly long-term tunneled cuffed catheters required for maintenance HD and frequently placed in the right internal jugular vein. One hundred participants had separate catheters during the baseline and intervention phase, without an individual catheter that straddled both phases.
Table 1.
Baseline characteristics of patients during baseline, intervention or both phases
| Characteristic | Study Phase |
|||||
|---|---|---|---|---|---|---|
| Baseline only | Both phases | Intervention only | ||||
| Number of patients | 2388 | 1173 | 2800 | |||
| Mean age, yrs (SD) | 60.3 | (16.1) | 61.3 | (15.5) | 60.6 | (15.8) |
| Female, n (%) | 910 | (38.1%) | 508 | (43.3%) | 1,085 | (38.8%) |
| Ethnicity, n (%) | ||||||
| Asian | 198 | (8.3%) | 94 | (8.0%) | 228 | (8.1%) |
| First Nationsa | 229 | (9.6%) | 160 | (13.6%) | 348 | (12.4%) |
| Pacific Islander | 58 | (2.4%) | 34 | (2.9%) | 65 | (2.3%) |
| White | 1584 | (66.3%) | 704 | (60.0%) | 1796 | (64.1%) |
| Other or not recorded | 319 | (13.4%) | 181 | (15.4%) | 363 | (13.0%) |
| Diabetes mellitus, n (%) | 973 | (40.7%) | 596 | (50.8%) | 1213 | (43.3%) |
| Immunosuppressed, n (%) | 341 | (14.3%) | 123 | (10.5%) | 383 | (13.7%) |
Includes Aboriginal and Torres Strait Islander and Māori.
Table 2.
Baseline characteristics of catheters during baseline, intervention or both phases
| Characteristic | Study Phase |
|||||
|---|---|---|---|---|---|---|
| Baseline only | Both phases | Intervention only | ||||
| Number of catheters | 4205 | 1073 | 4594 | |||
| Catheter type, n (%) | ||||||
| Tunneled | 2889 | (68.7%) | 1038 | (96.7%) | 3,476 | (75.7%) |
| Nontunneled | 1316 | (31.3%) | 35 | (3.3%) | 1,118 | (24.3%) |
| Venous insertion site, n (%) | ||||||
| R internal jugular | 2887 | (68.7%) | 859 | (80.1%) | 3,254 | (70.8%) |
| L internal jugular | 611 | (14.5%) | 151 | (14.1%) | 685 | (14.9%) |
| R femoral | 406 | (9.7%) | 16 | (1.5%) | 362 | (7.9%) |
| L femoral | 161 | (3.8%) | 8 | (0.7%) | 165 | (3.6%) |
| R subclavian | 73 | (1.7%) | 26 | (2.4%) | 72 | (1.6%) |
| L subclavian | 41 | (1.0%) | 6 | (0.6%) | 25 | (0.5%) |
| Other | 26 | (0.6%) | 7 | (0.7%) | 31 | (0.7%) |
| Reason for catheter insertion, n (%) | ||||||
| Acute kidney injury | 1675 | (39.8%) | 196 | (18.3%) | 1,618 | (35.2%) |
| Commence maintenance HD | 1270 | (30.2%) | 488 | (45.5%) | 1,679 | (36.5%) |
| AV access complication | 734 | (17.5%) | 203 | (18.9%) | 794 | (17.3%) |
| Transfer from PD | 451 | (10.7%) | 165 | (15.4%) | 427 | (9.3%) |
| Failing transplant | 25 | (0.6%) | 14 | (1.3%) | 24 | (0.5%) |
| Other | 50 | (1.2%) | 7 | (0.7%) | 52 | (1.1%) |
| Catheter proceduralist, n (%) | ||||||
| Interventional radiology | 2067 | (49.2%) | 638 | (59.5%) | 2,564 | (55.8%) |
| Critical care | 936 | (22.3%) | 47 | (4.4%) | 738 | (16.1%) |
| Nephrology | 622 | (14.8%) | 176 | (16.4%) | 687 | (15.0%) |
| Surgery | 509 | (12.1%) | 182 | (17.0%) | 492 | (10.7%) |
| Not known | 71 | (1.7%) | 30 | (2.8%) | 113 | (2.5%) |
AV, arteriovenous; HD, hemodialysis; PD, peritoneal dialysis; L, Left; R, Right.
Effect of the Intervention on Catheter Dysfunction
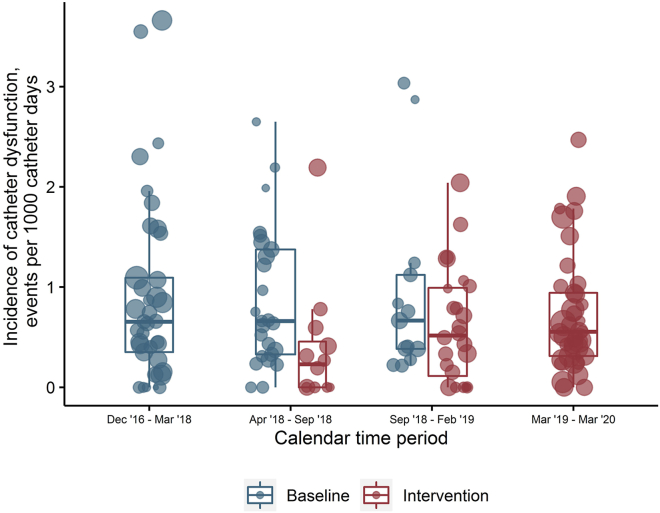
In total, 873 catheters were removed because of dysfunction over 1,120,385 catheter days, yielding an overall incidence of 0.78 events per 1000 catheter days. The raw incidence of catheter dysfunction requiring removal was 0.91 events per 1000 catheter days (95% CI, 0.82–0.99) during the baseline phase, and 0.68 events per 1000 catheter days (95% CI, 0.62–0.75) during the intervention phase (Table 3). During the equivalent calendar periods, services in the intervention phase had a lower median incidence of catheter dysfunction requiring removal than services in the baseline phase (Figure 1). After adjustment for calendar time and service-level clustering, the service-wide incidence of catheter dysfunction was 33% lower during the intervention period compared to the baseline period (incidence rate ratio = 0.67; 95% CI, 0.50–0.89; P = 0.006; Table 3).
Table 3.
Number of catheter dysfunction events, catheter days of exposure, and crude incidence of catheter dysfunction during the baseline and intervention phase, and the effect of the intervention on the service-wide incidence rate of catheter dysfunction adjusted for calendar time using 2-level Poisson regression with a random effect for service
| Baseline phase |
Intervention phase |
Effect of the intervention |
||||||
|---|---|---|---|---|---|---|---|---|
| Events, n | Catheter days | Incidence (95% CI)a | Events, n | Catheter days | Incidence (95% CI)a | Adjusted IRR | 95% CI | P-value |
| 438 | 481,863 | 0.91 (0.82–0.99) | 435 | 638,522 | 0.68 (0.62–0.75) | 0.67 | 0.50–0.89 | 0.006 |
CI, confidence interval; IRR, incidence rate ratio.
per 1000 catheter days.
Figure 1.
Grouped boxplot of the incidence rate of catheter dysfunction across nephrology services by calendar period. Size of data points reflects the relative number of catheter days at that service.
Subgroup Analysis Among Tunneled Cuffed Catheters
Overall, 7403 tunneled catheters accounted for 1.09 million catheter days (97.3% of the total exposure time), 88.2% of the total events in the study (n = 770) and were more frequently situated in the right internal jugular vein during the intervention phase compared to the baseline phase (Supplementary Table S1). During each phase, tunneled catheters were inserted mostly by interventional radiologists (67.9%), surgeons (15.3%), and nephrologists (12.2%), and rarely by critical care (2.2%). The raw incidence of catheter dysfunction requiring removal among tunneled catheters was 0.81 events per 1000 catheter days (95% CI, 0.73–0.90) in the baseline phase and 0.62 events per 1000 catheter days (95% CI, 0.56–0.69) in the intervention phase (Table 4).
Table 4.
Absolute number of catheters removed due to dysfunction, catheter days of exposure and crude incidence of catheter dysfunction among tunneled and nontunneled catheters during baseline and intervention phases
| Subgroup | Baseline phase | Intervention phase |
|---|---|---|
| Tunneled catheters | ||
| Events, n | 380 | 390 |
| Catheter days | 466,504 | 624,480 |
| Crude incidence, per 1000 catheter days | 0.81 | 0.62 |
| Nontunneled catheters | ||
| Events, n | 58 | 45 |
| Catheter days | 15,359 | 14,042 |
| Crude incidence, per 1000 catheter days | 3.78 | 3.20 |
After adjustment for secular trends, the incidence rate of tunneled catheter removals due to dysfunction was 35% lower during the intervention phase than during the baseline phase (incidence rate ratio = 0.65; 95% CI, 0.47–0.90; P = 0.009). This relationship persisted after adjusting for differences in insertion site, and other patient and catheter factors (Table 5). Model estimates of patient and catheter-level covariates are presented in Supplementary Table S2.
Table 5.
Effect of the intervention on the incidence of catheter dysfunction among tunneled catheters estimated from 3-level Poisson regression models, with random effects for service and patients nested within service, and fixed effects for calendar time and patient and catheter characteristics
| Effect of the intervention on catheter dysfunction incidence | Incidence rate ratio | 95% CI | P-value |
|---|---|---|---|
| Adjusted for calendar time | 0.65 | 0.47–0.90 | 0.009 |
| Adjusted for calendar time and catheter site | 0.68 | 0.49–0.94 | 0.019 |
| Adjusted for calendar time, catheter site, participant age, gender, diabetes mellitus, reason for catheter insertion, and department responsible for insertion | 0.68 | 0.49–0.94 | 0.019 |
CI, confidence interval.
VPCs
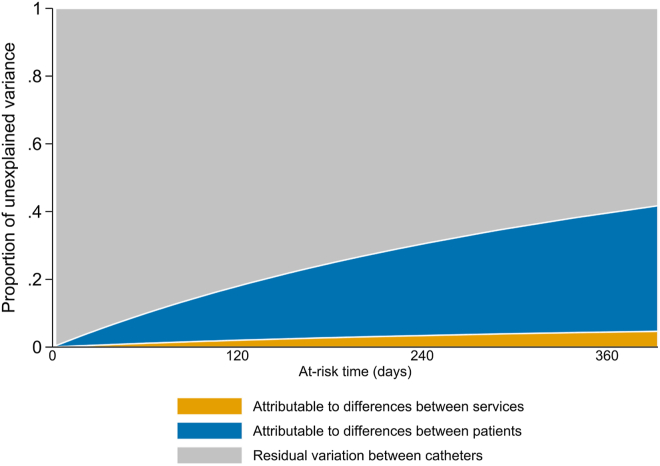
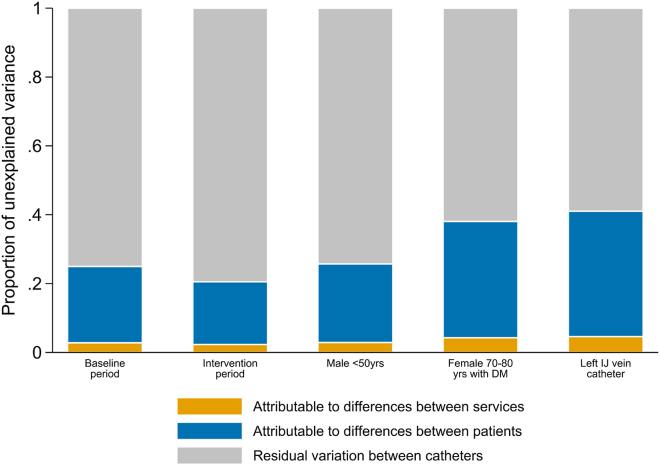
The VPCs among tunneled catheters (n = 7403) with at-risk times that ranged from 1 day to 365 days (92.7% of tunneled catheters), under standard covariate conditions, are presented in Figure 2. Among tunneled catheters that survived for 6 months (21.5% of tunneled catheters) during the baseline or intervention phase, between 2% and 5% of the total variation in the number of catheters with catheter dysfunction requiring removal was attributable to unmeasured differences between services, and 18%–36% was attributable to unmeasured differences between participants, depending on the patient or catheter characteristics (Figure 3). The remaining 59% to 79% was attributable to residual variation between catheters that was not accounted for by service-level or patient-level clustering.
Figure 2.
Variance partition coefficients among tunneled catheters with between 1 and 365 days of at-risk time, assuming standard covariates during the baseline phase. Standard covariates included male gender, 60–70 year-old age group, absence of diabetes mellitus, right internal jugular vein catheter site, catheter insertion by interventional radiology, and hemodialysis catheter required for chronic kidney failure.
Figure 3.
Variance partition coefficients among tunneled catheters that survived for 6 months (21.5% of catheters), during the baseline phase, intervention phase, and in a selected range of clinical scenarios. DM, diabetes mellitus; IJ, internal jugular; yrs, years.
Discussion
The multifaceted intervention implemented in this national, stepped-wedge, cluster randomized trial was associated with a 33% reduction in the service-wide incidence of catheter dysfunction, which persisted after accounting for underlying secular trends. Consistent results were observed among tunneled cuffed catheters, which accounted for most of the catheter exposure time, and after adjustment for measured differences in participant and tunneled catheter characteristics between phases. Our randomized data suggest that a multifaceted program of evidence-based catheter management practices in nephrology services may have secondary beneficial effects on HD catheter dysfunction.
We consider 2 plausible reasons for the observed relationship between the intervention and less catheter dysfunction. First, the intervention may have influenced catheter management practices that ultimately improved catheter tip position or prevented thrombosis. For example, greater utilization of imaging guidance during catheter insertion could have facilitated better positioning of the catheter tip.16 Antimicrobial catheter lock solutions may have prevented formation of microbial biofilms,29 which can promote thrombosis and fibrin sheath formation.30, 31, 32,38 Catheter dysfunction was also 3 times more common than HD-CRBSI, which provided more power to detect an intervention effect. Second, the intervention may have independently modified clinician behavior and catheter removal practices, independent of catheter performance. For example, during the intervention, clinicians might have tolerated lower flow rates, and persisted with alternative treatments for poor flow, rather than remove or exchange the CVC.
Our study also quantifies the sources of unexplained variation in tunneled catheter dysfunction events in Australia, which provides useful insights into the factors that mediate catheter dysfunction. Among tunneled cuffed catheters that survived for 6 months, a relatively small proportion of variation, between 2% and 5%, was attributable to service-level differences, which may have reflected service catheter volume and procedural experience, or local differences in management of poorly functioning HD catheters. A larger proportion of the unexplained variation, between 18% and 36%, was attributable to differences between patients within services, suggesting that there were some unmeasured, and potentially unknown patient factors that accounted for variation, and were associated with catheter dysfunction. Residual variation in the number of tunneled catheter removals due to dysfunction was also substantial. Future studies are required to better understand why some patients and catheters are more susceptible to catheter dysfunction, so that interventions can be directed toward high-risk groups and clinical decisions regarding vascular access modality can be individualized for patients.
Overall, 10.4% of all tunneled cuffed catheters were removed for dysfunction, and the raw incidence of tunneled catheter dysfunction requiring removal in our nation-wide cohort was 0.71 events per 1000 catheter days, which is less than has been previously reported internationally.12,39,40 These differences may reflect improvements to the design of catheters, the growing use of thrombolytic lock solutions, and other advances in catheter management over time, or regional differences in catheter management practices in Australia compared to other countries. More research is required to understand the international incidence of catheter dysfunction requiring removal, and the global variation in catheter management practices that may contribute to differences in dysfunction rates.
AKI was a common precipitant of kidney failure requiring HD via a CVC in Australian nephrology services. More than one-third of all HD catheters were inserted for AKI. Most HD catheters inserted for AKI were tunneled, which likely reflects a high prevalence of preexisting advanced chronic kidney disease among patients who received HD via nephrology services in Australia. In addition, patients with prolonged AKI who were recovering from critical illness in the intensive care unit, may have received a tunneled catheter prior to discharge to the nephrology service, to facilitate ongoing intermittent HD in the ward and general community. Further research is required to better understand the epidemiology of AKI in Australia.
Our study has notable strengths. REDUCCTION was a large, pragmatic HD trial that included the full spectrum of patients and catheters managed by nephrology services in Australia. Robust cluster randomization resulted in a good balance of service characteristics and facilitated internally valid estimates of the intervention effect. Patients also consider unplanned catheter failure to be an important outcome.14
However, this post hoc analysis has several limitations. First, catheter removal due to dysfunction was at the discretion of the treating nephrologist, without central adjudication, and no data pertaining to the severity of compromised blood flow necessitating catheter removal was collected. This definition is pragmatic and patient-centered; however, a standardized protocol for management of catheter dysfunction would reduce outcome variation due to different assessments between nephrologists and enhance international generalizability. Second, it is unknown whether services used prophylactic thrombolytic catheter locks during the intervention. Thrombolytic agents are expensive and are not publicly funded for prophylaxis in Australia. Third, the accumulation of prevalent catheters throughout the trial may have created a secular trend that favored the intervention. However, we adjusted for calendar time as a fixed effect, and services were cluster randomized; therefore, the underlying service-wide risk of catheter dysfunction within a given calendar period was probably balanced across tranches in the absence of the trial intervention. Fourth, audits were not conducted to assess the completeness of catheter enrollment, or the adherence to aseptic practices during individual patient encounters; however, the opt out and waiver approach to consent may have mitigated the possibility of missed catheters, and all sites confirmed the date of intervention implementation via their clinical champions. Finally, it was not possible to isolate the relative effect of individual intervention components because the intervention was implemented as a single package across the entire nephrology service.
In conclusion, our randomized data suggest that multifaceted interventions to promote evidence-based catheter care in nephrology services may prevent catheter dysfunction requiring removal. A substantial proportion of the variation in the number of catheter dysfunction events was attributable to patient-level differences, whereas only a small proportion was attributable to service-level differences. Further research is required to better predict which patients are most likely to get catheter dysfunction, which will inform personalized vascular access decisions and future interventions.
Appendix
Members of the REDUCCTION Investigators are Provided in Supplementary Appendix.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank all investigators, nephrology services, and patients for their participation in the trial.
The REDUcing the burden of dialysis Catheter ComplicaTIOns: a national approach (REDUCCTION) trial was supported by NHMRC Partnership grant (APP1103241), Department of Health and Human Services, Victoria, Queensland Health, and 22 other partners contributing in-kind and financial support as detailed in the Appendix. The funders had no input into the design, conduct, or publication of the study. Dr. Kotwal was supported by a MRFF Next Generation TRIP Fellowship (MRF1150335). Dr. Lazarus is supported by the NHMRC postgraduate research grant (APP2005174) and Monash Graduate Excellence Scholarship.
Data Sharing Statement
The individual patient data generated in the trial can be shared in accordance with the trial’s data sharing policy and in accordance with the local regulatory and ethical approval for the trial. The study protocol and statistical analysis plan have been published.
Trial Registration and Protocol
The trial was registered in the Australia and New Zealand clinical trials registry on the 23 June 2016 (ACTRN12616000830493).
Author Contributions
BL, SK, MG, and KP conceptualized the study; BL, KR, and KP were responsible for data analysis; BL wrote the original draft; SK and SC was responsible for project administration; BL, SK, MG, NG, SC, GT, and KP were responsible for methodology; all authors were responsible for the investigation and reviewed and edited the manuscript.
Footnotes
Supplementary Methods. Details of trial methods.
Figure S1. Illustration of multiple levels of clustering when catheter-level characteristics are included in assessing the intervention effect in a stepped-wedge cluster randomized trial.
Figure S2. Participant flow diagram for the post hoc analysis of the REDUCCTION national stepped-wedge cluster randomized trial.
Table S1. Baseline characteristics of tunneled catheters during baseline, intervention or both phases.
Table S2. Relationship between patient-level and catheter-level covariates and the incidence of catheter dysfunction among tunneled catheters from 3-level Poisson regression models.
Supplementary Appendix. List of trial investigators.
Supplementary Material
Supplementary Methods. Details of trial methods.
Figure S1. Illustration of multiple levels of clustering when catheter-level characteristics are included in assessing the intervention effect in a stepped-wedge cluster randomized trial.
Figure S2. Participant flow diagram for the post hoc analysis of the REDUCCTION national stepped-wedge cluster randomized trial.
Table S1. Baseline characteristics of tunneled catheters during baseline, intervention or both phases.
Table S2. Relationship between patient-level and catheter-level covariates and the incidence of catheter dysfunction among tunneled catheters from 3-level Poisson regression models.
Supplementary Appendix. List of trial investigators.
References
- 1.Bello A.K., Okpechi I.G., Osman M.A., et al. Epidemiology of haemodialysis outcomes. Nat Rev Nephrol. 2022;18:378–395. doi: 10.1038/s41581-022-00542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Australia and New Zealand Dialysis and Transplant Registry, ANZDATA 42nd annual report 2019 (Data to 2018). Chapter 4: Haemodialysis. Accessed 1 June 2022. https://www.anzdata.org.au/report/anzdata-42nd-annual-report-2019/
- 3.Hussein W.F., Mohammed H., Browne L., Plant L., Stack A.G. Prevalence and correlates of central venous catheter use among haemodialysis patients in the Irish health system-a national study. BMC Nephrol. 2018;19:76. doi: 10.1186/s12882-018-0873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisoni R.L., Zepel L., Port F.K., Robinson B.M. Trends in US vascular access use, patient preferences, and related practices: an update from the US DOPPS practice monitor with international comparisons. Am J Kidney Dis. 2015;65:905–915. doi: 10.1053/j.ajkd.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Johansen K.L., Chertow G.M., Foley R.N., et al. US Renal Data System 2020 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2021;77(4 Suppl 1):A7–A8. doi: 10.1053/j.ajkd.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lok C.E., Huber T.S., Lee T., et al. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis. 2020;75(4 suppl 2):S1–S164. doi: 10.1053/j.ajkd.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Develter W., De Cubber A., Van Biesen W., Vanholder R., Lameire N. Survival and complications of indwelling venous catheters for permanent use in hemodialysis patients. Artif Organs. 2005;29:399–405. doi: 10.1111/j.1525-1594.2005.29067.x. [DOI] [PubMed] [Google Scholar]
- 8.Little M.A., Walshe J.J. A longitudinal study of the repeated use of alteplase as therapy for tunneled hemodialysis catheter dysfunction. Am J Kidney Dis. 2002;39:86–91. doi: 10.1053/ajkd.2002.29885. [DOI] [PubMed] [Google Scholar]
- 9.Poinen K., Quinn R.R., Clarke A., et al. Complications from tunneled hemodialysis catheters: a Canadian observational cohort study. Am J Kidney Dis. 2019;73:467–475. doi: 10.1053/j.ajkd.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths R.I., Newsome B.B., Leung G., Block G.A., Herbert R.J., Danese M.D. Impact of hemodialysis catheter dysfunction on dialysis and other medical services: an observational cohort study. Int J Nephrol. 2012;2012 doi: 10.1155/2012/673954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmelgarn B.R., Moist L.M., Lok C.E., et al. Prevention of dialysis catheter malfunction with recombinant tissue plasminogen activator. N Engl J Med. 2011;364:303–312. doi: 10.1056/NEJMoa1011376. [DOI] [PubMed] [Google Scholar]
- 12.Mokrzycki M.H., Lok C.E. Traditional and non-traditional strategies to optimize catheter function: go with more flow. Kidney Int. 2010;78:1218–1231. doi: 10.1038/ki.2010.332. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Ivany J.N., Perkovic V., Gallagher M.P., Woodward M., Jardine M.J. Anticoagulants and antiplatelet agents for preventing central venous haemodialysis catheter malfunction in patients with end-stage kidney disease. Cochrane Database Syst Rev. 2016;4:Cd009631. doi: 10.1002/14651858.CD009631.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viecelli A.K., Tong A., O’Lone E., et al. Report of the standardized outcomes in nephrology-hemodialysis (SONG-HD) consensus workshop on establishing a core outcome measure for hemodialysis vascular access. Am J Kidney Dis. 2018;71:690–700. doi: 10.1053/j.ajkd.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Shingarev R., Barker-Finkel J., Allon M. Natural history of tunneled dialysis catheters placed for hemodialysis initiation. J Vasc Interv Radiol. 2013;24:1289–1294. doi: 10.1016/j.jvir.2013.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal A.K., Haddad N., Boubes K. Avoiding problems in tunneled dialysis catheter placement. Semin Dial. 2019;32:535–540. doi: 10.1111/sdi.12845. [DOI] [PubMed] [Google Scholar]
- 17.Weijmer M.C., Vervloet M.G., ter Wee P.M. Compared to tunnelled cuffed haemodialysis catheters, temporary untunnelled catheters are associated with more complications already within 2 weeks of use. Nephrol Dial Transplant. 2004;19:670–677. doi: 10.1093/ndt/gfg581. [DOI] [PubMed] [Google Scholar]
- 18.Bonkain F., Stolear J.C., Catalano C., et al. Prevention of tunneled cuffed catheter dysfunction with prophylactic use of a taurolidine urokinase lock: a randomized double-blind trial. PLoS One. 2021;16 doi: 10.1371/journal.pone.0251793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemmelgarn B.R., Manns B.J., Soroka S.D., et al. Effectiveness and cost of weekly recombinant tissue plasminogen activator hemodialysis catheter locking solution. Clin J Am Soc Nephrol. 2018;13:429–435. doi: 10.2215/CJN.08510817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran J.E., Ash S.R., ASDIN Clinical Practice Committee Locking solutions for hemodialysis catheters; heparin and citrate--a position paper by ASDIN. Semin Dial. 2008;21:490–492. doi: 10.1111/j.1525-139X.2008.00466.x. [DOI] [PubMed] [Google Scholar]
- 21.Winnicki W., Herkner H., Lorenz M., et al. Taurolidine-based catheter lock regimen significantly reduces overall costs, infection, and dysfunction rates of tunneled hemodialysis catheters. Kidney Int. 2018;93:753–760. doi: 10.1016/j.kint.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Lindberg C., Downham G., Buscell P., Jones E., Peterson P., Krebs V. Embracing collaboration: a novel strategy for reducing bloodstream infections in outpatient hemodialysis centers. Am J Infect Control. 2013;41:513–519. doi: 10.1016/j.ajic.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Rosenblum A., Wang W., Ball L.K., Latham C., Maddux F.W., Lacson E., Jr. Hemodialysis catheter care strategies: a cluster-randomized quality improvement initiative. Am J Kidney Dis. 2014;63:259–267. doi: 10.1053/j.ajkd.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Yi S.H., Kallen A.J., Hess S., et al. Sustained infection reduction in outpatient hemodialysis centers participating in a collaborative bloodstream infection prevention effort. Infect Control Hosp Epidemiol. 2016;37:863–866. doi: 10.1017/ice.2016.22. [DOI] [PubMed] [Google Scholar]
- 25.Kotwal S., Cass A., Coggan S., et al. Multifaceted intervention to reduce haemodialysis catheter related bloodstream infections: REDUCCTION stepped wedge, cluster randomised trial. BMJ. 2022;377 doi: 10.1136/bmj-2021-069634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotwal S., Coggan S., McDonald S., et al. REDUcing the burden of dialysis Catheter ComplicaTIOns: a national approach (REDUCCTION)-design and baseline results. Kidney360. 2020;1:746–754. doi: 10.34067/KID.0001132020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hryszko T., Brzosko S., Mazerska M., Malyszko J., Mysliwiec M. Risk factors of nontunneled noncuffed hemodialysis catheter malfunction. A prospective study. Nephron Clin Pract. 2004;96:c43–c47. doi: 10.1159/000076398. [DOI] [PubMed] [Google Scholar]
- 28.Ravani P., Gillespie B.W., Quinn R.R., et al. Temporal risk profile for infectious and noninfectious complications of hemodialysis access. J Am Soc Nephrol. 2013;24:1668–1677. doi: 10.1681/ASN.2012121234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shanks R.M., Sargent J.L., Martinez R.M., Graber M.L., O’Toole G.A. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol Dial Transplant. 2006;21:2247–2255. doi: 10.1093/ndt/gfl170. [DOI] [PubMed] [Google Scholar]
- 30.Lee T., Lok C., Vazquez M., Moist L., Maya I., Mokrzycki M. Minimizing hemodialysis catheter dysfunction: an ounce of prevention. Int J Nephrol. 2012;2012 doi: 10.1155/2012/170857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chauhan A., Bernardin A., Mussard W., et al. Preventing biofilm formation and associated occlusion by biomimetic glycocalyx like polymer in central venous catheters. J Infect Dis. 2014;210:1347–1356. doi: 10.1093/infdis/jiu249. [DOI] [PubMed] [Google Scholar]
- 32.Niyyar V.D., Lok C.E. Pros and cons of catheter lock solutions. Curr Opin Nephrol Hypertens. 2013;22:669–674. doi: 10.1097/MNH.0b013e328365ba53. [DOI] [PubMed] [Google Scholar]
- 33.Al-Jaishi A.A., Dixon S.N., McArthur E., Devereaux P.J., Thabane L., Garg A.X. Simple compared to covariate-constrained randomization methods in balancing baseline characteristics: a case study of randomly allocating 72 hemodialysis centers in a cluster trial. Trials. 2021;22:626. doi: 10.1186/s13063-021-05590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussey M.A., Hughes J.P. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28:182–191. doi: 10.1016/j.cct.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Kotwal S., Gallagher M., Rogers K., Di Tanna G.L. REDUcing the burden of dialysis Catheter ComplicaTIOns: a national approach (REDUCCTION) Statistical Analysis Plan. https://osf.io/gnw3u/ Published 2020. [DOI] [PMC free article] [PubMed]
- 36.Austin P.C., Stryhn H., Leckie G., Merlo J. Measures of clustering and heterogeneity in multilevel Poisson regression analyses of rates/count data. Stat Med. 2018;37:572–589. doi: 10.1002/sim.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leckie G., Browne W.J., Goldstein H., Merlo J., Austin P.C. Partitioning variation in multilevel models for count data. Psychol Methods. 2020;25:787–801. doi: 10.1037/met0000265. [DOI] [PubMed] [Google Scholar]
- 38.van Rooden C.J., Schippers E.F., Barge R.M., et al. Infectious complications of central venous catheters increase the risk of catheter-related thrombosis in hematology patients: a prospective study. J Clin Oncol. 2005;23:2655–2660. doi: 10.1200/JCO.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Grudzinski L., Quinan P., Kwok S., Pierratos A. Sodium citrate 4% locking solution for central venous dialysis catheters--an effective, more cost-efficient alternative to heparin. Nephrol Dial Transplant. 2007;22:471–476. doi: 10.1093/ndt/gfl606. [DOI] [PubMed] [Google Scholar]
- 40.Lok C.E., Appleton D., Bhola C., Khoo B., Richardson R.M. Trisodium citrate 4%-an alternative to heparin capping of haemodialysis catheters. Nephrol Dial Transplant. 2007;22:477–483. doi: 10.1093/ndt/gfl570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.