Abstract
Cardiovascular disease (CVD) is the major cause of mortality in autosomal dominant polycystic kidney disease (ADPKD) and contributes to significant burden of disease. The manifestations are varied, including left ventricular hypertrophy (LVH), intracranial aneurysms (ICAs), valvular heart disease, and cardiomyopathies; however, the most common presentation and a major modifiable risk factor is hypertension. The aim of this review is to detail the complex pathogenesis of hypertension and other extrarenal cardiac and vascular conditions in ADPKD drawing on preclinical, clinical, and epidemiological evidence. The main drivers of disease are the renin-angiotensin-aldosterone system (RAAS) and polycystin-related endothelial cell dysfunction, with the sympathetic nervous system (SNS), nitric oxide (NO), endothelin-1 (ET-1), and asymmetric dimethylarginine (ADMA) likely playing key roles in different disease stages. The reported rates of some manifestations, such as LVH, have decreased likely due to the use of antihypertensive therapies; and others, such as ischemic cardiomyopathy, have been reported with increased prevalence likely due to longer survival and higher rates of chronic disease. ADPKD-specific screening and management guidelines exist for hypertension, LVH, and ICAs; and these are described in this review.
Keywords: autosomal dominant polycystic kidney disease, cardiovascular disease, endothelial dysfunction, hypertension, intracranial aneurysms, valvular heart disease
ADPKD is the fourth most common cause of kidney failure worldwide and the most common monogenic cause of kidney impairment.1 ADPKD is caused by autosomal dominant inheritance of pathogenic mutations (or de novo mutations) in either PKD1 (∼78% of cases) or PKD2 (∼15% of cases), which results in impaired function of proteins polycystin-1 or polycystin-2 respectively, or rarely mutations in other genes (such as ALG5, ALG9, DNAJB11, GANAB, or IFT140) that have an effect on polycystin function.2,3 Polycystins have particular functional importance in renal tubular epithelial cells where its dysfunction leads to the hallmark finding of numerous, enlarging, fluid-filled cysts in the kidneys.1 Cystogenesis occurs sporadically and is hypothesized to be triggered by a reduction in total functional polycystin-1 below a certain “threshold” level (∼10%–30% below normal).4,5 The reduction may be due to a somatic mutation in the unaffected polycystin allele, localized kidney injury, environmental factors, and/or stochastic factors4,5
Loss of function of polycystin proteins in other cell types leads to extrarenal manifestations in ADPKD.1,6 In vascular endothelial cells, vascular smooth muscle cells (VSMCs), cardiomyocytes, and cardiac fibroblasts, the reduction in polycystin-1 contributes to early development of CVD.1,6 CVD is the most common cause of mortality and is a major cause of morbidity in patients with ADPKD.6, 7, 8 The cardiovascular manifestations present in the second and third decade of life, often prior to development of kidney impairment, and the subclinical manifestations such as endothelial dysfunction and arterial stiffness present earlier.7,9,10 The driving factors of CVD in ADPKD are enlarging cysts stimulating the RAAS and SNS combined with inherited dysregulation of vasoactive molecules (NO, ADMA, ET-1).6,7 In addition, ciliary-related abnormalities in cell development and cell-to-cell communication in vascular endothelial cells, smooth muscle cells and cardiomyocytes predispose to the development of arterial aneurysms and cardiac valvular disease.9,11 The development of vascular abnormalities are also hypothesized to be due to the “threshold” mechanism of disease; however, this level varies between different tissues and cell types, and is independent of kidney cystogenesis.4,12 CVD is a significant chronic condition that has acute and detrimental consequences. Changing clinical characteristics, increased patient education, and evolving therapies have resulted in increased patient survival but with the ongoing burden of high CVD risk.6 Clinicians and patients should be vigilant about screening and initiating management early to improve outcomes. This review discusses the epidemiological risk factors, pathogenesis, screening, and management of CVD in ADPKD. Hypertension is the most common manifestation, and its severity impacts other conditions; therefore, it will be discussed first, followed by cardiomyopathies (LVH, ischemic cardiomyopathy, and others), valvular heart disease (mitral valve prolapse [MVP] and mitral regurgitation [MR]), ICAs, and other vascular malformations.
Hypertension
Epidemiology and Clinical Associations
Hypertension is the most common presenting symptom of ADPKD, has a mean onset at 27 years old and occurs in 50%–70% of patients prior to decline in kidney function.6,7 Hypertension is also common in children with ADPKD, with a 2016 meta-analysis reporting a 20% prevalence (95% confidence interval 15%–27%).13 Hypertension is a major modifiable risk factor for the development of CVD and a key contributor to the development of LVH and albuminuria.14 For pregnant women with ADPKD, hypertension leads to a significantly greater risk of preeclampsia (54% vs. 8% in normotensive women with ADPKD, P < 0.001), maternal complications (∼85% vs. ∼30% in normotensive ADPKD, P < 0.001) and kidney function decline (0.8% of hypertensive ADPKD pregnancies progressed to kidney failure compared to 0% in the normotensive ADPKD and 0.0001% in the general population).15,16 In addition, the presence of hypertension is linked with increased severity of cystic kidney disease in children and adults.17 Cohort studies (including the large longitudinal CRISP cohort) of hypertensive patients with ADPKD have shown significantly greater kidney sizes, rates of kidney growth, decline in kidney function, and progression to kidney failure compared to normotensive patients with ADPKD.10,12,18, 19, 20, 21, 22. Together, these findings reflect the central role of hypertension in cardiovascular and kidney disease progression in ADPKD.
Pathogenesis of Hypertension
The pathogenesis of hypertension in ADPKD occurs through 2 main mechanisms: (i) hypertension driven by cystic growth and renal dysfunction and (ii) hypertension driven by vascular dysfunction due to abnormal polycystin function.23,24 The key downstream mediators of hypertension are the RAAS, NO, ADMA, ET, and the SNS.
Cystic Growth and Kidney Dysfunction as Drivers of Hypertension
Kidney cyst expansion leads to nephron obstruction and intrarenal microvascular ischemia stimulating the RAAS and SNS, and driving systemic hypertension.25 Cohort and histological studies have shown that both circulating and intrarenal renin, angiotensin II, and angiotensin-converting enzyme (ACE) levels are increased in ADPKD.26,27 Renin, aldosterone, and ACE activity was increased compared to patients with chronic kidney disease (CKD) and essential hypertension, implying an ADPKD-specific process contributing to RAAS overactivity (than renal dysfunction alone, another driver of RAAS activation).24,26,27 Similarly, RAAS overactivity is detected in hypertensive patients with ADPKD prior to the development of renal impairment.27,28 Focal ischemia of the parenchyma or the juxtaglomerular apparatus directly, stimulates renin secretion and RAAS activation.29 Graham et al.30 found additional renin secreting cells lining intrarenal vessels of ADPKD kidneys, which likely contribute to unregulated renin release and increased RAAS activity, particularly in patients with early or mild cyst burden. Graham et al.30 also found hyperplasia of juxtaglomerular apparatus in ADPKD kidneys, a feature also found in noncystic CKD, which likely plays a role in RAAS overactivity in late ADPKD when kidney dysfunction is established. RAAS is also a stimulator of angiogenesis and renal epithelial cell growth contributing further to cyst growth and the persistence of hypertension in vicious cycle (Figure 1).24,29
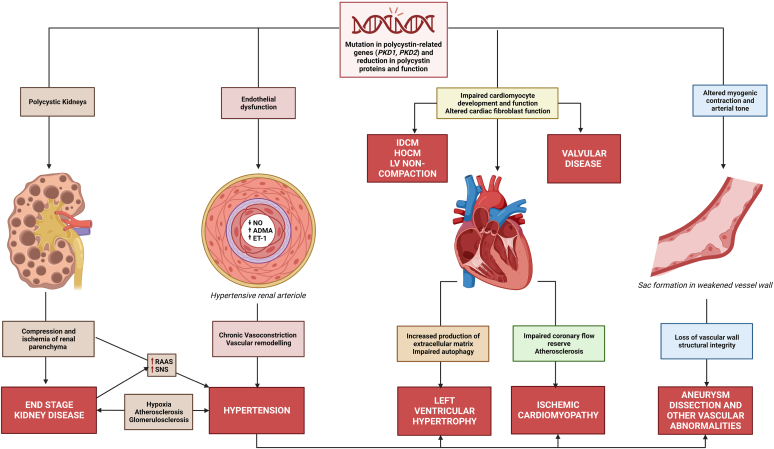
Figure 1.
Pathogenesis of cardiovascular disease in ADPKD. Inherited abnormalities and reduction of functional polycystin-1 and polycystin-2 proteins impact multiple organ systems. Kidney cyst growth activates the RAAS and SNS, driving hypertension and other cardiovascular abnormalities. Endothelial dysfunction and chronic vasoconstriction lead to hypertension and vascular abnormalities, including arterial stiffness, thickening of vessel walls and atherosclerosis. These vascular changes also contribute to other arterial abnormalities such as intracranial aneurysms, dissections, and coronary artery disease. Hypertension is also a key risk factor for development of LVH and together contribute to the risk of coronary ischemia, likely predisposing patients to ischemic cardiomyopathy. Hypertensive arterial injury and development of cardiomyopathy leads to impaired end organ perfusion and atheroma development, which in turn, leads to renal injury with glomerulosclerosis and further RAAS activation in a pathogenic cycle. ADMA, asymmetric dimethylarginine; ADPKD, autosomal dominant polycystic kidney disease; ET-1, endothelin-1; HOCM, hypertrophic obstructive cardiomyopathy; IDCM, idiopathic dilated cardiomyopathy; LVH, left ventricular hypertrophy; NO, nitric oxide; RAAS, renin-angiotensin-aldosterone system; SNS, sympathetic nervous system.
In addition, kidney cyst growth leads to stretching of the renal capsule and the release of neuromodulator signaling molecules from injured kidney tissue (such as bradykinin, neurokinin A, calcitonin gene-related peptide, substance P, and prostaglandins) leading to stimulation of surrounding renal sympathetic nerves and increased SNS activity.31, 32, 33 Angiotensin II also directly increases vascular sympathetic tone, leading to raised peripheral vascular resistance and blood pressure (Figure 1).32,33 This is reflected in the published literature, where measures of SNS activity in patients with ADPKD are significantly increased compared to noncystic CKD, essential hypertension, and healthy controls.32,34 In patients with ADPKD who are on dialysis, the morphological changes associated with SNS overactivity (increased renal nerve density and renal arterial sympathetic innervation) are significantly increased compared to non-ADPKD patients who are on dialysis, and these changes are thought to play a pivotal role in the persistence of hypertension in kidney failure.33,35
Chronic pain has an estimated prevalence of 50% to 60% in patients with ADPKD, and may contribute to SNS hyperactivity and hypertension.36, 37, 38 Moreover, chronic pain is reported as a cause of sleep disturbance in 16.8%–20.8% of patients with ADPKD.36,37 Pain may also contribute to poorer BP control through its potential role in loss of overnight decrease in BP and increase in early prewaking BP (a feature attributed to spikes in SNS overactivity) described in patients with ADPKD; however, the current evidence is circumstantial.38 Further studies are required to evaluate the impact of chronic pain, SNS overactivity, and elevated BP in ADPKD. This may be of additional importance to other cardiovascular manifestations because SNS overactivity independently contributes to the development of LVH, arterial remodeling, arrhythmias, and heart failure.39
Vascular Dysfunction as a Driver of Hypertension and CVD
Endothelial dysfunction is pathogenic precursor to chronic CVD and has been detected in patients with ADPKD prior to the development of hypertension or LVH.6 Endothelial dysfunction results in vascular abnormalities, including loss of flow-mediated dilation and increased arterial stiffness, which occur prior to hypertension, suggesting a role in its pathogenesis.40,41
Polycystins are expressed at high levels in the cilia of vascular endothelial cells where they sense fluid shear stress, detect minute changes to BP, and have a central role in flow-related endothelial response and performance.42, 43, 44, 45 In transgenic mice, endothelial PC-1 mediates Ca2+-dependent hyperpolarization and vasodilation through the activation of nitric oxide synthase (NOS) and intermediate or small-conductance K+ channels.46 PC-1 forms a complex with PC-2 which localizes to the plasma membrane of vascular endothelial cells.46,47 The loss of either PC-1 and/or PC-2 reduces flow-mediated vasodilation and an increase in BP.46, 47, 48 These conditional endothelial-specific knockout models provide evidence that CVD occurs prior to and independent of kidney impairment in ADPKD.46, 47, 48
However, as kidney impairment develops, mechanisms of hypertension common to other forms of CKD (such as increased RAAS activity and sodium retention) contribute to progression of CVD in ADPKD.49 This was demonstrated by endothelial-PKD1 knockout mice, where 5 of 6 nephrectomy exacerbated impaired flow-mediated dilation.48
Vascular endothelial cilia play a role in the regulation of cell division and endothelial-to-mesenchymal transition functions, which likely contributes to structural abnormalities such as vascular ectasias and MVP.50 In VSMCs primary cilia, PC-1 and PC-2 play a role in cell-extracellular matrix interaction and responses to mechanical stimulation.51 PC-2, localized to the plasma membrane on VSMCs, mediates sensing of intraluminal wall stretch, myocyte Ca2+ regulation, and modulation of myogenic tone.52,53 Cell culture and animal models have demonstrated that the quantity of PC-1 and PC-2, and their relative balance, is important to modulate their action on myocyte constriction and myogenic tone.45,54 The outcome of alterations of PC-2 in VSMCs in the current literature is conflicting. Studies of aortic arteries from PKD2+/− mice displayed exaggerated contraction to phenylephrine stimulus, whereas VSMC-specific PKD2 knockout mice developed vasodilation and reduced BP in mesenteric and hindlimb arteries.52,54 These differences may reflect variable expression between cell-specific and global constitutive knockout of PKD2.45,52 Although the exact mechanisms require further elucidation, polycystin expression and function is variable between cell types and these changes are important in the pathogenesis of CVD. Due to these abnormalities in polycystin function, key mediators of endothelial function NO, ADMA, and ET-1 are dysregulated (through the mechanisms described below and contribute to hypertension and vascular complications, including aneurysm, dissection, and atherosclerosis (Figure 1).50
Reduced Nitric Oxide
NO is a potent driver of endothelial-mediated vasodilation and in normal conditions, PC-1 and PC-2 modulate flow-stimulated Ca2+-dependent NOS activation, hyperpolarization, and relaxation in vascular endothelial cells.46,47 The reduction or inhibition of this process leads to vasoconstriction and increased BP.25,47 Zhang et al.55 detected a >70% reduction in NO metabolites in 2 separate human ADPKD cell lines. Other clinical and preclinical studies have detected this decrease in NO levels and NOS activity in ADPKD.55, 56, 57, 58 This reduction has been detected prior to establishment of hypertension or kidney impairment, and has a negative linear correlation with BP in hypertensive patients.56,59 Lorthioir et al.58 demonstrated a reduction in NO release and heat-stimulated flow-mediated dilation in normotensive patients with ADPKD compared to healthy controls, which improved with infusion of dopamine (stimulation of endothelial dopamine receptors restores Ca2+ influx in polycystin-deficient cells). Although in contrast to other studies, baseline plasma nitrite was higher in the ADPKD group compared with controls in this study, nitrite levels did not increase in response to heating as they did in controls, demonstrating a deficiency in NO production.58 These are supported by studies by Wang et al.,59 who demonstrated a reduction in NO metabolites and maximal acetylcholine-induced vasodilation (in normal conditions acetylcholine increases NO by stimulating intracellular calcium and activating NOS) in normotensive patients with ADPKD. The effect could not be overcome or further suppressed by an NO substrate (L-arginine) or a NOS inhibitor (NG-nitro-L-arginine methyl ester, or L-NAME) implying an inherent inactivity of endothelial NOS in ADPKD.57,59 Interestingly, ADPKD vessels responded to SIN-1, an exogenous NO-donor, to induce endothelial relaxation confirming that the endothelium in these normotensive patients were still able to respond to but unable to generate NO.59 Although a reduction in NO is a pathological feature in diabetes and CKD-related hypertension, is it unclear if it occurs early in these diseases as it does in ADPKD.59 Furthermore, there is persistence of NO deficiency until late stage ADPKD, with the development of characteristic sclerotic lesions in renal vessels (that are similar to those found in animals treated with NOS inhibitors), highlighting its pathogenic role in both early and late ADPKD.60
Increased ADMA and Oxidative Stress
ADMA is a potent NO-inhibitor and its levels are significantly increased in ADPKD compared to controls, including in patients with ADPKD with normal kidney function and BP.25,60 ADMA suppresses endothelial NO stimulating vasoconstriction, increasing peripheral vascular resistance and promoting atherosclerosis.25 ADMA is increased in all-cause CKD stage 3 onward, but is elevated early (CKD stage 1) in ADPKD.60 Even modest increases in ADMA levels (as demonstrated in the study above) can inhibit vasodilation and further impair NO production.60,61 Intravenous infusions of ADMA in healthy subjects significantly reduces renal blood flow and vasodilatory responses; and in CKD rats, ADMA infusion resulted in glomerular capillary loss and vascular sclerosis.62,63 Furthermore, elevated ADMA levels are a strong predictor of coronary vascular disease and mortality in patients with CKD.64,65 Further studies are required to evaluate the long-term impacts of ADMA; however, given its role in vascular dysfunction and the documented elevated levels, it is likely an important mediator of CVD in ADPKD.
The cause of increased ADMA is not certain but has been linked to increased oxidative stress and decreased clearance early ADPKD.25 Studies of patients with ADPKD with preserved kidney function demonstrated a significant increase in markers of oxidative stress (prostaglandin-2α, prostaglandin-D2, prostaglandin-E2, 8-isoprostane, and lipid peroxidation product 13-hydroxyoctadecadienoic acid), which are known to further decrease NO availability.25,60,66,67 In addition, elevated oxidative stress is associated with vascular dysfunction (as measured by impaired flow-mediated dilation) further supporting the interaction of these pathways and their contribution to disease.67
Increased Endothelin-1
ET-1 is an endothelial-derived systemic and intraglomerular mediator of blood flow.68 It acts as a vasoconstrictor in the renal cortex and a vasodilator in the renal medulla, and its actions are mediated by NO.69 Focal ischemia, hypoxia, and increased angiotensin II caused by cyst growth are strong stimulators of ET-1.69,70 It is speculated that ET-1 has a compensatory role in early ADPKD to maintain blood flow to medullary areas obstructed by cysts and to maintain sodium balance in the setting of tubular loss; however, this role becomes pathogenic as disease progresses, contributing to elevated BP and sodium retention, particularly in the setting of reduced NO mediation.68, 69, 70, 71
The imbalances of these endothelial mediators, vascular dysfunction, and consequences of cyst growth lead to the development and persistence of hypertension, kidney impairment, and CVD in a pathogenic cycle (Figure 1).63,72, 73, 74
Management of Hypertension
Detailed screening and therapeutic recommendations, including lifestyle and pharmacological interventions are presented in Table 1. Briefly, all patients with ADPKD should be screened for hypertension at the time of diagnosis and during follow-up, and managed with standard lifestyle principles, which include low sodium diet (<100 mmol/d), regular exercise, weight loss (if overweight), and smoking cessation. Sodium intake in ADPKD can be assessed using the scored salt questionnaires and recommended dietary changes are similar to other types of CKD.88, 89, 90 The first-line agents for antihypertensive therapy are ACE inhibitors (ACEi); or if not tolerated, angiotensin receptor blockers (ARB).91 The effectiveness of ACEi/ARB was tested in the landmark HALT-PKD randomized controlled trials which used by a 2-by-2 factorial design to evaluate a lower BP target of 95/60 to 110/75 mm Hg (vs. standard BP targets of 120/70 to 130/80 mm Hg), and combination of ACEi-ARB (vs. ACEi) alone on annual percentage change in total kidney volume.78 The results showed that lower BP targets were associated with slower kidney growth, lower left ventricular mass index and albuminuria.78 These beneficial effects were greatest in participants with large kidneys (≥ 75th percentile total kidney volume) and under the age of 30 years, participants with total kidney volume greater than the median, and in male participants.78 The combination therapy of ACEi-ARB did not alter the rate of annual total kidney volume increase or eGFR.
Table 1.
Screening and management of cardiovascular manifestations in ADPKD
| Hypertension |
Screening
|
Targets
|
Non-pharmacological interventions
|
Pharmacological interventions
|
| Left ventricular hypertrophy and other cardiomyopathies |
Screening
|
Management
|
| Valvular heart disease |
Screening
|
Management
|
| Intracranial aneurysms |
| Imaging should be done urgently for any patient with ADPKD and neurological symptoms (severe or atypical headache, history of transient ischemic attack, neurological deficits, cranial nerve palsy) because it may be due to an ICA or other intracranial vascular abnormality |
Screening
|
Management
|
| Abdominal Aortic Aneurysms |
| There are no ADPKD-specific guidelines for management of AAA and patients should be managed as per the general recommendations below.87 |
Screening
|
Management
|
AAA, abdominal aortic aneurysms; ABPM, ambulatory BP monitoring; ACEi, angiotensin-converting enzyme inhibitors; ADPKD, autosomal dominant polycystic kidney disease; ARB, angiotensin receptor blockers; BP, blood pressure; CT, computed tomography; CVD, cardiovascular disease; CKD, chronic kidney disease; ICA, intracranial aneurysm; IDCM, idiopathic dilated cardiomyopathy; LVH, left ventricular hypertrophy; MRI, magnetic resonance imaging; PKD, polycystic kidney disease; RAAS, renin-angiotensin-aldosterone system.
Cardiac Manifestations in ADPKD
Left Ventricular Hypertrophy and Other Cardiomyopathies
Epidemiology and Risk Factors of LVH
LVH is a serious complication of ADPKD and its presence increases the risk of premature death and major cardiovascular events (including arrhythmias and heart failure), especially when LVH coexists with hypertension.44,92 There is variability in the reported prevalence of LVH in ADPKD from 41% in a study in 1997 (Chapman et al.93), 21.4% in a 2019 study (Chen et al.92) and 3.9% in the HALT-PKD study (2006–2014).44,78,92, 93, 94 Furthermore, a recent retrospective study in February 2023 (Arjune et al.95) reported a 65% incidence of LVH in patients with ADPKD compared with 55% in controls. A key difference between these studies is the imaging modality; HALT-PKD used cardiac magnetic resonance imaging (the current gold-standard to measure ventricular dimensions) and the other studies used ultrasound echocardiography (with variability from advances in echocardiogram technology in the 26 years between the 2 studies). The lower prevalence of LVH in more recent studies may also be due to altered diagnostic definitions; increased and earlier screening; and importantly, increased use of RAAS blockers (5% in the 1997 study, 63.5% in the 2019 study, and >80% before commencement of HALT-PKD).78,92, 93, 94 Notably, when the same criteria for left ventricular mass in the HALT-PKD study was applied to the 2023 study, the incidence of LVH was found to be 5% (which is much more consistent with the HALT-PKD cohort).94,95 The lower incidence of LVH seen in recent studies is likely due to more effective introduction of antihypertensive therapy.92,94
Hypertension is a major risk factor in the development of LVH, and studies in ADPKD demonstrate a positive linear correlation with higher BPs and the presence of LVH.96 In children with ADPKD, elevated or borderline (above the 75th percentile) BP led to significant higher rates of LVH compared with children with lower BP.97,98 Particularly in “borderline” hypertensive patients, masked hypertension and loss of overnight decrease in systolic BP is a contributor to the development of LVH.98,99 Although LVH is a well-known consequence of hypertension, multiple studies of normotensive children and young adults with ADPKD have also shown increased rates of LVH compared with non-ADPKD controls, including those with similar 24-hour BP readings.24,92,93,100 Chen et al.92 reported a similar prevalence of LVH in hypertensive (22.4%) and normotensive (17.9%) groups. Chapman et al.93 reported a higher prevalence of LVH in normotensive ADPKD (23%) compared to healthy controls (16%); however, hypertensive ADPKD was significantly higher than both (48%). Therefore, hypertension is clearly a major driving factor for LVH in ADPKD; however, there are other predisposing mechanisms that lead to the increased prevalence of LVH in normotensive patients with ADPKD.
Kidney dysfunction and increased age are independent risk factors for the presence of LVH in ADPKD, similar to the non-ADPKD population.38,93,94 Total kidney volume is also associated with LVH, independent of BP and kidney function.92 Furthermore, high early morning or prewaking BP has been demonstrated in patients with ADPKD with LVH who did not have any other risk factors, suggesting that SNS overactivity may contribute to the development of LVH in ADPKD.38
Pathogenesis of LVH
The most commonly described trigger for LVH is an increase in volumetric burden on the left ventricle (usually due to hypertension or cardiac valvular disease) resulting in compensatory increase in myocardial hypertrophy leading to LVH. Over time, increased deposition of extracellular matrix and myocardial fibrosis results in ventricular stiffness and diastolic dysfunction.101 Angiotensin II, a mediator of this process, is overactive in ADPKD.24
The dysfunction of polycystins in experimental knockout models of PKD1 and PKD2 show significant cardiac abnormalities, and this may explain the incidence of LVH in normotensive patients with ADPKD. Primary cilia, expressing PC-1 and PC-2, are present in cardiomyocytes (particularly endocardial-facing) during early fetal development and contribute to cardiac left–right axis development, valvulogenesis, and myocardial regeneration; however, these cilia are no longer expressed in adult animal models.102,103 Instead, in adult rat cardiomyocytes, PC-1 localizes to the plasma membrane where it functions as a mechanosensor independently or in complex with PC-2.104 Studies of cardiomyocytes in PC-1 knockout mice demonstrate reduced L-type calcium channels and intracellular signaling, increased cell proliferation leading to cardiac wall thickening, and reduced cardiac contractility compared to controls.104,105 Primary cilia, which highly express PC-1 are also found in cardiac fibroblasts and these cells normally accumulate at the site of myocardial injury and regulate matrix deposition and fibrosis.106 Impairment of fibroblastic cilia results in increased cardiac hypertrophy and fibrosis.106 In the PKD1RC/RC mouse phenotype (which has a reduction but not complete loss of PC-1 expression), there is a dosage effect of PC-1 on the severity of the cardiac hypertrophy.107
PC-2 localizes to the sarcoplasmic reticulum and loss of PC-2 in murine cardiomyocytes leads to reduced cardiac shortening and cardiac dyssynchrony.108,109 Furthermore, decreased PC-2 alters cardiomyocyte beta-adrenergic pathways in murine PKD2+/− without hypertension or cystic disease, and these changes may contribute to high incidence of atrial fibrillation reported in patients with ADPKD.108,109 In addition, reduced PC-1 and PC-2 in ADPKD leads to dysregulation of mammalian target of rapamycin, an important regulator of autophagy, a process which maintains cardiomyocyte size, function, and structure.110 PC-2 also has a role in inducing and regulating autophagy via its Ca2+− conductance effects, independent of the mammalian target of rapamycin pathway.111 Preclinical PKD studies show that reduced autophagy leads to left ventricular hypertrophy, dilation and contractile dysfunction.44,110 These mechanisms together likely contribute to the development of LVH in ADPKD.
Management of LVH
Recommendations for management of LVH include rigorous BP control with use of a RAAS blocker as first-line therapy because ACEi and ARBs reverse the hypertrophic changes in the left ventricle (Table 1).76 The current focus of treatment is based on BP reduction; however, it is not clear if this alters cardiac outcomes, particularly given that there is a subset of patients with LVH without hypertension.92,93 In addition, in the large cohort HALT-PKD studies, the rate of serious cardiovascular events were unchanged despite a reduction in left ventricular mass index in the group with lower BP targets.78 Further longitudinal studies with serial imaging are required to characterize the effects of treatment in patients with LVH and ADPKD.
It should be noted that LVH is a structural finding and does not accurately reflect cardiac function, particularly in those patients without other manifestations of CVD.112 Therefore, the assessment of cardiac function should accompany investigations for LVH, including echocardiographic determination of ejection fraction and possible inclusion of newer methods to detect cardiac strain, such as 2-dimensional strain or global longitudinal strain.112,113 Furthermore, there are studies reporting increased cardiac strain in patients with ADPKD (detected by increased left ventricular global longitudinal strain on echocardiography) with normal ejection fractions and preserved kidney function.112,114 This subclinical sign of cardiac dysfunction is associated with decreased functional capacity (measured by 6-minute walk and timed up and go tests) and quality of life in CKD; however, further evaluation in ADPKD populations are required to determine its predictive long-term risk.113,115
Ischemic Cardiomyopathy
Ischemic heart disease is a major complication of CKD, but specific data in ADPKD is limited. Two large cohort studies, from China and Taiwan, reported a >2-fold higher incidence of myocardial infarction in patients with ADPKD, which was associated with increased severity of coronary disease and poorer cardiovascular outcomes compared with non-ADPKD controls.116,117 In particular, Yang et al.117 showed that those with ADPKD had a higher incidence of ST-elevation myocardial infarction (75% compared with 59%), sudden cardiac death (11.5% vs. 4.6%), need for coronary artery bypass grafting (7.7% vs. 5.4%) and higher overall mortality (13.5% vs. 6.2%). Most prior large ADPKD studies excluded patients with a history of ischemic heart disease and this may contribute to the relative paucity of data on ischemic cardiomyopathy in this group. However, clinical and preclinical studies show early vascular changes (as described above) lead to impairment of flow-mediated dilation and impaired coronary flow reserve in ADPKD.118 The presence of hypertension and LVH contributes to these vascular changes leading to the premature development of arterial stiffness and cardiac dysfunction.6,119 Increased levels of angiotensin II and ADMA also contribute to early atherosclerosis of coronary arteries.50,119 Furthermore, in normotensive patients with ADPKD with normal kidney function, coronary artery velocity reserve was significantly decreased and carotid intima-media thickness was increased compared to non-ADPKD controls, suggesting changes to coronary vasculature that predispose to ischemia occur at an early stage.119 Studies of human, mouse, and rat cardiac fibroblasts with loss of functional PC-1 demonstrate impaired myocardial healing from ischemic injury and alterations in scar architecture, increasing the risk of developing cardiomyopathy following myocardial infarction.106 As with ischemic cardiomyopathy from other causes, the management principles in ADPKD include classic CVD risk factor modification, treatment with RAAS blockers, beta-blockers, aspirin, statin, and revascularization where possible.83
Other Cardiomyopathies
Other rare cardiomyopathies are reported in ADPKD, including idiopathic dilated cardiomyopathy, which is characterized by left ventricular dilatation and systolic dysfunction; and hypertrophic obstructive cardiomyopathy, which is characterized by asymmetrical LVH and diastolic dysfunction.92 In a retrospective study of 667 patients with ADPKD who had echocardiographs, 58 had primary cardiomyopathy identified; idiopathic dilated cardiomyopathy was found in 5.8%, hypertrophic obstructive cardiomyopathy in 2.5%, and left ventricular noncompaction in 0.3%.120 PKD1 mutations were found in 100% (n = 2) of patients with noncompaction and those with PKD2 mutations had twice the expected incidence of idiopathic dilated cardiomyopathy (36.8%, n = 7), suggesting that polycystin expression impacts the pathogenesis of these conditions.120 Mutations in PKD2 are linked with impaired intracellular calcium flux leading to decreased cardiac contractility, thin ventricular walls, and dilated cardiomyopathy; and (as described earlier in this review) mutations in PKD1 can lead to cardiac hypertrophy and reduced myocyte fractional shortening, predisposing to the development of cardiomyopathies.107,109,121
Diastolic dysfunction is commonly reported in patients with ADPKD, usually in late disease stage with established kidney failure; however, it has also been reported in early disease prior to development of hypertension, LVH, and kidney impairment.122, 123, 124 As with the LVH, polycystin-related dysfunction of cardiomyocytes and vascular cells (as described earlier in this review) are likely involved in the pathogenesis of diastolic dysfunction.6 There is no specific ADPKD management guidelines for these cardiomyopathies and recommended therapy is described in Table 1.
Valvular Heart Disease
The patterns of cardiac valvular and structural heart disease vary as ADPKD progresses. In early disease, the most common valvular abnormality is MVP; and as disease progresses to kidney failure, mitral and aortic valve calcification and regurgitation are more common.122 Identification of these conditions are important because their presence can lead to systolic and diastolic dysfunction, heart failure, and (for MR in particular) arrhythmias.125
MVP is considered a classic extrarenal manifestation of ADPKD; however, recent reports of its prevalence vary between 0% and 26%.125, 126, 127, 128, 129, 130, 131 In a study in 2001 (Lumiaho et al. 126) of 109 patients with PKD1 mutations, MVP was found in 26%, which was significantly higher than unaffected relatives (14%) and unrelated healthy controls (10%). In this study, MVP was present in greater portion in younger and normotensive groups with no association with gender or kidney function.126 Hossack et al.127 reported a similar prevalence of MVP (26%) in a study in 1988 in a cohort that predominantly had PKD1 mutations detected by gene linkage. In a study in 1995 by Ivy et al.,130 MVP was prevalent in 12% of children with ADPKD (compared to 3% non-ADPKD affected children from the same families) and its presence was associated with more severe renal phenotype (>10 cysts). However, a recent study in 2022 by Savis et al.131 of 102 children and young adults with ADPKD, found 1 patient (0.98% prevalence) with MVP and 9 patients (8.8%) with changes that may represent early MVP changes or may be normal variation. Arjune et al.95 found 6 out of 141 patients (4%) with MVP and another 2 studies in adults did not find any patients with MVP (Saggar-Malik et al.128 and Miyamoto et al.125). Therefore, there is significant variability in the reported prevalence of MVP, and applicability of previous studies are limited by differences in genotype (greater portion of PKD1 mutations), disease stage, presence of other comorbidities, changes in echocardiography technology, and diagnosis guidelines.126,131,132 Further studies are needed to determine the true prevalence.
The presence of MVP in children, young adults, and normotensive patients suggests a connective tissue basis for its pathogenesis. Although the exact mechanism is still to be elucidated, likely mechanisms are: (i) polycystin-related ciliary dysfunction causing valvular cell growth abnormalities, altered valve geometry, myxomatous degeneration, and prolapse, or (ii) dysfunction of papillary muscle cell (which contain polycystin proteins) leading to abnormal valve biomechanics.6,129,131
MVP predisposes to MR because changes in the valve geometry can lead to separation of valve leaflets, particularly in the presence of increased left-sided preload as caused by hypertension and volume overload from kidney impairment and sodium retention.129 In keeping with this, unlike MVP, the presence of other valvular abnormalities are associated with later disease stages when there is significant kidney dysfunction and progression of CVD.133 Aortic valve calcification, mitral valve calcification, and MR were significantly more common findings in an ADPKD dialysis population (38%, 28%, and 8.6%, respectively) than MVP (4.3%).133 Lumiaho et al.126 also found a greater prevalence of grade 2 or 3 MR in PKD1 patients (12.8% vs. 2.7%) and this was associated with age, systolic BP, and impaired kidney function, with no cases of MR in patients with normal kidney function. In a recent study of 65 patients with ADPKD of which approximately 30% had MR, Miyamoto et al.125 found an increased prevalence of MR in those with PKD1 mutations over PKD2 or other ADPKD mutations (46.9% vs. 8.3% vs. 19.0%, respectively; P = 0.02); however, there were no other significant differences in prevalence of aortic regurgitation, mitral stenosis, or aortic stenosis between genotypes.125 Arjune et al.95 found a higher prevalence of 63% of MR in a study of 141 patients with ADPKD with CKD stages 1-4, although the authors have stipulated that most of these cases were of mild MR. Differences in diagnostic criteria may contribute to variability in the reported prevalence and uniform reporting guidelines in ADPKD would be beneficial in future research to evaluate the extent of these cardiac valvular manifestations.134
Current guidelines suggest echocardiographic evaluation of any cardiac murmur in ADPKD. Management of valvular abnormalities is based on the severity of patients’ overall condition and follows the principals of heart failure management (Table 1).84 There are no specific recommendations for ADPKD valvular disease.
Vascular Manifestations
Intracranial Aneurysms
Epidemiology and Risk Factors for ICAs
Compared to the general population, ICAs are found in higher prevalence in the ADPKD population (9%–12% vs. 2%–3%) and present at a younger age (∼40 years old vs. 51 years old).85,86,135,136 Rupture of an ICA and resulting subarachnoid hemorrhage is catastrophic, and leads to death (in 30%–60%) or significant neurological deficits.136, 137, 138 ICAs in patients with ADPKD are usually small (<5 mm in diameter) and found in the anterior circulation, with the exception of one large Finnish registry study where most ICAs were found in the middle cerebral artery, similar to the general population in Finland.136,137,139,140
The major known risk factor for the presence of ICAs in ADPKD is a positive personal or family history; and in this group, the prevalence of ICAs is reported as high as 22%.135 The classic risk factors for ICAs such as the presence of hypertension, smoking and older age, also apply to the ADPKD population.86 Furthermore, in studies from French and Japanese ADPKD cohorts, females were significantly more likely to develop aneurysms than males.136,139 In 2 Japanese studies, there was an association with more severe disease (reduced kidney function and larger kidney size) and the presence of ICAs.141,142 Patients with PKD1 mutations had a >2-fold increase in the risk of both ruptured and unruptured ICAs compared to those with PKD2 mutations, with no significant influence from the type or location of the mutation.136 These results from November 2022 are in contrast to with a previous case series from early 2003 that suggested that mutations in the 5’ region of PKD1 correlated with increased risk of ICAs and rupture.143
Pathogenesis of ICAs
The increased incidence of ICAs in patients with ADPKD is due to inherited dysfunction of vascular endothelial and smooth muscle cells and is compounded by hypertension and CVD. Impaired ciliary function results in the vessels’ inability to sense and react to fluid shear stress and loss of myogenic tone, leading to increased arterial wall stress, loss of structural integrity, sac formation, and expansion into an aneurysm (Figure 1).85,123,136,144
Management of ICAs
Given the high prevalence of ICAs in the ADPKD population, the decision to screen presymptomatic patients should be made in conjunction with the patient, taking into consideration their medical history and the potential for anxiety.85,86,145 Screening is recommended for patients with family or personal history of ICAs.85 Management should be decided in consultation with neurosurgery (Table 1).85
Other Vascular Malformations
The vessel wall abnormalities that lead to ICAs also predispose to the development of other vascular abnormalities. There have been case reports of dissections and aneurysms in most major vessels including aortic, coronary, carotid, cervicocephalic, and vertebral arteries.132,144 Abdominal aortic aneurysms are reported as high as 5% to 10% in patients with ADPKD (vs. 2%–4% in the general population) with case reports of rupture resulting in devastating consequences.138,146 Risk factors of hypertension, kidney dysfunction, age, and smoking predispose to abdominal aortic aneurysms formation and there are no specific recommendations for patients with ADPKD above the general guidelines (Table 1).138 Dolichoectasias, which is the elongation and dilation of an arterial segment that can predispose to dissection or rupture, has been reported with increased incidence in a study of 178 patients with ADPKD (2%–2.5% in ADPKD vs. 0.06% in the general population).147 Although these vascular malformations are a known feature of ADPKD, there have been no large registry, longitudinal, or systematic studies to determine the true burden of disease or provide specific screening guidelines. Expert opinion suggests that it is not unreasonable to extensively investigate patients with a strong personal or family history of vascular complications.144 Management is specific to the vascular characteristics and organ involved, and treatment decisions should be made in conjunction with surgical or interventional teams.
Conclusions and Future Directions
Longitudinal population studies in patients with ADPKD over the last decade have documented reduced rates of hypertension, LVH, and progression to kidney failure due to earlier diagnosis, engagement with healthcare professionals, and more rigorous CVD risk management.17,148,149 Despite this, CVD remains the main cause of mortality, and patients with ADPKD experience more severe cardiovascular events than the general population and have risk factors that are not optimally controlled.117,138,148 The pathogenesis of CVD is complex and evolves with progression of ADPKD during life. The clinical landscape of ADPKD therapies has changed with the introduction and increasing use of the vasopressin-2 receptor antagonist, tolvaptan, which is the first disease-modifying therapy that attenuates kidney cyst growth.150 In addition, there are promising phase 3 trials with repurposed drugs (e.g., cystic fibrosis transmembrane conductance regulator modulator GLPG2737 [NCT04578548], and metformin [NCT04939935]) and evolving novel therapies (such as micro RNA inhibitors RGLS8429, which is currently undergoing a phase 1b trial) targeted at reducing cyst burden and maintaining kidney function.151,152 It will be important to determine the impact of therapies that reduce cyst burden on CVD risk and outcomes, especially at different disease stages. The effect of statins to reduce oxidative stress and improve endothelial function have also been investigated in ADPKD.153 Statin trials so far have had contradictory results, with a 3-year randomized controlled trial showing reduction in total kidney volume in children and young adults; however, other studies such as secondary analysis of the HALT-PKD study showed no effect. Nevertheless, a current trial (NCT03273413) is underway and further test its effects.153, 154, 155
Other research strategies in CVD and ADPKD should include further elucidation of the factors leading to endothelial dysfunction, particularly because much of the research into some pathways, such as the role of ADMA, was published over a decade ago. In addition, because ADPKD-specific CVD can precede clinical kidney disease, development of therapies targeted at polycystin-related cardiovascular pathways are also required. Recently, dopamine receptor agonism was demonstrated to correct the reduction of Ca2+ influx, NO release, and flow-mediated vasodilation that occurs in ADPKD vascular endothelial cells and further research is required to determine its potential as a therapeutic target.58,156 Other novel therapies could include approaches to attenuating early endothelial dysfunction by acting on the ADMA pathway, reducing SNS overactivity, and improving cardiomyocyte function.
Ongoing long-term longitudinal studies are required to determine the impact of any intervention on attenuating CVD in patients with ADPKD. To facilitate this, standardized outcome measures in ADPKD trials would be beneficial. Recent comprehensive reviews of the clinical ADPKD literature found significant variability study reporting, particularly in composite outcomes.157,158 The 2020 international Delphi survey identified potential core outcome domains taking into account patient or care-giver and healthcare professionals’ priorities, which may be considered in developing standardized outcomes.159 Current therapeutic guidelines are appropriate and are based on general CVD and CKD recommendations; however, we await the release of the upcoming new version of the Kidney Disease: improving Global Outcomes ADPKD guidelines and determine the impact of recent trials on management recommendations.157
Disclosure
The authors declare no competing interests.
Acknowledgments
Funding
Funding provided by NHMRC (Project Grant 1164128), PKD Australia, and PS is supported by an ICPMR Jerry Koutts Scholarship for this work.
References
- 1.Chebib F.T., Torres V.E. Autosomal dominant polycystic kidney disease: core curriculum 2016. Am J Kidney Dis. 2016;67:792–810. doi: 10.1053/j.ajkd.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris P.C., Torres V.E. Polycystic kidney disease, autosomal dominant. https://www.ncbi.nlm.nih.gov/books/NBK1246/
- 3.Lemoine H., Raud L., Foulquier F., et al. Monoallelic pathogenic ALG5 variants cause atypical polycystic kidney disease and interstitial fibrosis. Am J Hum Genet. 2022;109:1484–1499. doi: 10.1016/j.ajhg.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong A.C., Harris P.C. A polycystin-centric view of cyst formation and disease: the polycystins revisited. Kidney Int. 2015;88:699–710. doi: 10.1038/ki.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallagher A.R., Germino G.G., Somlo S. Molecular advances in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:118–130. doi: 10.1053/j.ackd.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo I.Y., Chapman A.B., Kuo I.Y., Chapman A.B. Polycystins, ADPKD, and cardiovascular disease. Kidney Int Rep. 2019;5:396–406. doi: 10.1016/j.ekir.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ecder T. Cardiovascular complications in autosomal dominant polycystic kidney disease. Curr Hypertens Rev. 2013;9:2–11. doi: 10.2174/1573402111309010002. [DOI] [PubMed] [Google Scholar]
- 8.Turgut F., Oflaz H., Namli S., et al. Ambulatory blood pressure and endothelial dysfunction in patients with autosomal dominant polycystic kidney disease. Ren Fail. 2007;29:979–984. doi: 10.1080/08860220701641728. [DOI] [PubMed] [Google Scholar]
- 9.Helal I., Reed B., Mettler P., et al. Prevalence of cardiovascular events in patients with autosomal dominant polycystic kidney disease. Am J Nephrol. 2012;36:362–370. doi: 10.1159/000343281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fick-Brosnahan G.M., Tran Z.V., Johnson A.M., Strain J.D., Gabow P.A. Progression of autosomal-dominant polycystic kidney disease in children. Kidney Int. 2001;59:1654–1662. doi: 10.1046/j.1523-1755.2001.0590051654.x. [DOI] [PubMed] [Google Scholar]
- 11.de Chickera S., Akbari A., Levin A., et al. The risk of adverse events in patients with polycystic kidney disease with advanced chronic kidney disease. Can J Kidney Health Dis. 2018;5 doi: 10.1177/2054358118774537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman A.B., Guay-Woodford L.M., Grantham J.J., et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int. 2003;64:1035–1045. doi: 10.1046/j.1523-1755.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 13.Marlais M., Cuthell O., Langan D., Dudley J., Sinha M.D., Winyard P.J. Hypertension in autosomal dominant polycystic kidney disease: a meta-analysis. Arch Dis Child. 2016;101:1142–1147. doi: 10.1136/archdischild-2015-310221. [DOI] [PubMed] [Google Scholar]
- 14.Schrier R.W. Hypertension and autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2011;57:811–813. doi: 10.1053/j.ajkd.2011.02.379. [DOI] [PubMed] [Google Scholar]
- 15.McBride L., Wilkinson C., Jesudason S. Management of autosomal dominant polycystic kidney disease (ADPKD) during pregnancy: risks and challenges. Int J Womens Health. 2020;12:409–422. doi: 10.2147/ijwh.S204997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman A.B., Johnson A.M., Gabow P.A. Pregnancy outcome and its relationship to progression of renal failure in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1994;5:1178–1185. doi: 10.1681/asn.V551178. [DOI] [PubMed] [Google Scholar]
- 17.Schrier R.W., Brosnahan G., Cadnapaphornchai M.A., et al. Predictors of autosomal dominant polycystic kidney disease progression. J Am Soc Nephrol. 2014;25:2399–2418. doi: 10.1681/asn.2013111184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabow P.A., Chapman A.B., Johnson A.M., et al. Renal structure and hypertension in autosomal dominant polycystic kidney disease. Kidney Int. 1990;38:1177–1180. doi: 10.1038/ki.1990.330. [DOI] [PubMed] [Google Scholar]
- 19.Kistler A.D., Poster D., Krauer F., et al. Increases in kidney volume in autosomal dominant polycystic kidney disease can be detected within 6 months. Kidney Int. 2009;75:235–241. doi: 10.1038/ki.2008.558. [DOI] [PubMed] [Google Scholar]
- 20.Johnson A.M., Gabow P.A. Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J Am Soc Nephrol. 1997;8:1560–1567. doi: 10.1681/asn.V8101560. [DOI] [PubMed] [Google Scholar]
- 21.Seeman T., Dusek J., Vondrichová H., et al. Ambulatory blood pressure correlates with renal volume and number of renal cysts in children with autosomal dominant polycystic kidney disease. Blood Press Monit. 2003;8:107–110. doi: 10.1097/01.mbp.0000085762.28312.4a. [DOI] [PubMed] [Google Scholar]
- 22.Seeman T., Sikut M., Konrad M., Vondrichová H., Janda J., Schärer K. Blood pressure and renal function in autosomal dominant polycystic kidney disease. Pediatr Nephrol. 1997;11:592–596. doi: 10.1007/s004670050343. [DOI] [PubMed] [Google Scholar]
- 23.Chapman A.B., Stepniakowski K., Rahbari-Oskoui F. Hypertension in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:153–163. doi: 10.1053/j.ackd.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrier R.W. Renal volume, renin-angiotensin-aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2009;20:1888–1893. doi: 10.1681/ASN.2008080882. [DOI] [PubMed] [Google Scholar]
- 25.Raptis V., Loutradis C., Sarafidis P.A. Renal injury progression in autosomal dominant polycystic kidney disease: a look beyond the cysts. Nephrol Dial Transplant. 2018;33:1887–1895. doi: 10.1093/ndt/gfy023. [DOI] [PubMed] [Google Scholar]
- 26.Loghman-Adham M., Soto C.E., Inagami T., Cassis L. The intrarenal renin-angiotensin system in autosomal dominant polycystic kidney disease. Am J Physiol Ren Physiol. 2004;287:F775–F788. doi: 10.1152/ajprenal.00370.2003. [DOI] [PubMed] [Google Scholar]
- 27.Chapman A.B., Johnson A., Gabow P.A., Schrier R.W. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323:1091–1096. doi: 10.1056/nejm199010183231602. [DOI] [PubMed] [Google Scholar]
- 28.Torres V.E., Wilson D.M., Burnett J.C., Johnson C.M., Offord K.P. Effect of inhibition of converting enzyme on renal hemodynamics and sodium management in polycystic kidney disease. Mayo Clin Proc. 1991;66:1010–1017. doi: 10.1016/S0025-6196(12)61724-8. [DOI] [PubMed] [Google Scholar]
- 29.Wang D., Strandgaard S. The pathogenesis of hypertension in autosomal dominant polycystic kidney disease. J Hypertens. 1997;15:925–933. doi: 10.1097/00004872-199715090-00002. [DOI] [PubMed] [Google Scholar]
- 30.Graham P.C., Lindop G.B. The anatomy of the renin-secreting cell in adult polycystic kidney disease. Kidney Int. 1988;33:1084–1090. doi: 10.1038/ki.1988.115. [DOI] [PubMed] [Google Scholar]
- 31.Riccio E., Esposito G., Franzone A., Imbriaco M., Santangelo M., Pisani A. Renal sympathetic-nerve ablation for uncontrolled hypertension in a patient with single-kidney autosomal dominant polycystic kidney disease. J Clin Hypertens (Greenwich) 2014;16:385–386. doi: 10.1111/jch.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein I., Ligtenberg G., Oey P.L., Koomans H.A., Blankestijn P.J. Sympathetic activity is increased in polycystic kidney disease and is associated with hypertension. J Am Soc Nephrol. 2001;12:2427–2433. doi: 10.1681/asn.V12112427. [DOI] [PubMed] [Google Scholar]
- 33.Rovella V., Scimeca M., Giannini E., et al. Morphological evaluation of sympathetic renal innervation in patients with autosomal dominant polycystic kidney disease. J Nephrol. 2020;33:83–89. doi: 10.1007/s40620-019-00612-3. [DOI] [PubMed] [Google Scholar]
- 34.Cerasola G., Vecchi M., Mulè G., et al. Sympathetic activity and blood pressure pattern in autosomal dominant polycystic kidney disease hypertensives. Am J Nephrol. 1998;18:391–398. doi: 10.1159/000013382. [DOI] [PubMed] [Google Scholar]
- 35.Converse R.L., Jr., Jacobsen T.N., Toto R.D., et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 36.Miskulin D.C., Abebe K.Z., Chapman A.B., et al. Health-related quality of life in patients with autosomal dominant polycystic kidney disease and CKD stages 1-4: a cross-sectional study. Am J Kidney Dis. 2014;63:214–226. doi: 10.1053/j.ajkd.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casteleijn N.F., Visser F.W., Drenth J.P., et al. A stepwise approach for effective management of chronic pain in autosomal-dominant polycystic kidney disease. Nephrol Dial Transplant. 2014;29(suppl 4):iv142–iv153. doi: 10.1093/ndt/gfu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yildiz A., Sag S., Gul C.B., et al. Morning blood pressure surge in early autosomal dominant polycystic kidney disease and its relation with left ventricular hypertrophy. Ren Fail. 2021;43:223–230. doi: 10.1080/0886022x.2020.1864403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mancia G., Grassi G., Giannattasio C., Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–728. doi: 10.1161/01.hyp.34.4.724. [DOI] [PubMed] [Google Scholar]
- 40.Clausen P., Feldt-Rasmussen B., Iversen J., Lange M., Eidemak I., Strandgaard S. Flow-associated dilatory capacity of the brachial artery is intact in early autosomal dominant polycystic kidney disease. Am J Nephrol. 2006;26:335–339. doi: 10.1159/000094402. [DOI] [PubMed] [Google Scholar]
- 41.Gul C.B., Yildiz A., Sag S., Oruc A., Ersoy A., Gullulu S. The effect of smoking on endothelial dysfunction in autosomal dominant polycystic kidney disease patients with preserved renal function. Ren Fail. 2021;43:1124–1129. doi: 10.1080/0886022x.2021.1949348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egorova A.D., Khedoe P.P., Goumans M.J., et al. Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ Res. 2011;108:1093–1101. doi: 10.1161/circresaha.110.231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nauli S.M., Kawanabe Y., Kaminski J.J., Pearce W.J., Ingber D.E., Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/circulationaha.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oto O.A., Edelstein C.L. The pathophysiology of left ventricular hypertrophy, beyond hypertension, in autosomal dominant polycystic kidney disease. Nephron. Published online July 27, 2022 doi: 10.1159/000525944. [DOI] [PubMed] [Google Scholar]
- 45.Sharif-Naeini R., Folgering J.H., Bichet D., et al. Polycystin-1 and −2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 46.MacKay C.E., Floen M., Leo M.D., et al. A plasma membrane-localized polycystin-1/polycystin-2 complex in endothelial cells elicits vasodilation. Elife. 2022;11 doi: 10.7554/eLife.74765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacKay C.E., Leo M.D., Fernández-Peña C., et al. Intravascular flow stimulates PKD2 (polycystin-2) channels in endothelial cells to reduce blood pressure. Elife. 2020;9 doi: 10.7554/eLife.56655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamzaoui M., Groussard D., Nezam D., et al. Endothelium-specific deficiency of Polycystin-1 promotes hypertension and cardiovascular disorders. Hypertension. 2022;79:2542–2551. doi: 10.1161/HYPERTENSIONAHA.122.19057. [DOI] [PubMed] [Google Scholar]
- 49.Ku E., Lee B.J., Wei J., Weir M.R. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis. 2019;74:120–131. doi: 10.1053/j.ajkd.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 50.Nauli S.M., Jin X., Hierck B.P. The mechanosensory role of primary cilia in vascular hypertension. Int J Vasc Med. 2011;2011 doi: 10.1155/2011/376281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu C.J., Du H., Wu J., et al. Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press Res. 2008;31:171–184. doi: 10.1159/000132462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bulley S., Fernández-Peña C., Hasan R., et al. Arterial smooth muscle cell PKD2 (TRPP1) channels regulate systemic blood pressure. Elife. 2018;7 doi: 10.7554/eLife.42628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qian Q., Hunter L.W., Li M., et al. Pkd2 haploinsufficiency alters intracellular calcium regulation in vascular smooth muscle cells. Hum Mol Genet. 2003;12:1875–1880. doi: 10.1093/hmg/ddg190. [DOI] [PubMed] [Google Scholar]
- 54.Qian Q., Hunter L.W., Du H., Ren Q., Han Y., Sieck G.C. Pkd2+/− vascular smooth muscles develop exaggerated vasocontraction in response to phenylephrine stimulation. J Am Soc Nephrol. 2007;18:485–493. doi: 10.1681/asn.2006050501. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J.Q.J., Saravanabavan S., Cheng K.M., et al. Long-term dietary nitrate supplementation does not reduce renal cyst growth in experimental autosomal dominant polycystic kidney disease. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang D., Iversen J., Wilcox C.S., Strandgaard S. Endothelial dysfunction and reduced nitric oxide in resistance arteries in autosomal-dominant polycystic kidney disease. Kidney Int. 2003;64:1381–1388. doi: 10.1046/j.1523-1755.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- 57.Wang D., Iversen J., Strandgaard S. Contractility and endothelium-dependent relaxation of resistance vessels in polycystic kidney disease rats. J Vasc Res. 1999;36:502–509. doi: 10.1159/000025693. [DOI] [PubMed] [Google Scholar]
- 58.Lorthioir A., Joannidès R., Rémy-Jouet I., et al. Polycystin deficiency induces dopamine-reversible alterations in flow-mediated dilatation and vascular nitric oxide release in humans. Kidney Int. 2015;87:465–472. doi: 10.1038/ki.2014.241. [DOI] [PubMed] [Google Scholar]
- 59.Wang D., Iversen J., Strandgaard S. Endothelium-dependent relaxation of small resistance vessels is impaired in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2000;11:1371–1376. doi: 10.1681/asn.V1181371. [DOI] [PubMed] [Google Scholar]
- 60.Wang D., Strandgaard S., Borresen M.L., et al. Asymmetric dimethylarginine and lipid peroxidation products in early autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2008;51:184–191. doi: 10.1053/j.ajkd.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 61.Vallance P., Leiper J. Cardiovascular biology of the asymmetric dimethylarginine: dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol. 2004;24:1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 62.Kielstein J., Simmel S., Bode-Böger S., et al. Subpressor dose asymmetric dimethylarginine modulates renal function in humans through nitric oxide synthase inhibition. Kidney Blood Press Res. 2004;27:143–147. doi: 10.1159/000078838. [DOI] [PubMed] [Google Scholar]
- 63.Ueda S., Yamagishi S-i, Matsumoto Y., et al. Involvement of asymmetric dimethylarginine (ADMA) in glomerular capillary loss and sclerosis in a rat model of chronic kidney disease (CKD) Life Sci. 2009;84:853–856. doi: 10.1016/j.lfs.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 64.Zoccali C., Bode-Böger S.M., Mallamaci F., et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–2117. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 65.Schächinger V., Britten M.B., Zeiher A.M. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 66.Klawitter J., Reed-Gitomer B.Y., McFann K., et al. Endothelial dysfunction and oxidative stress in polycystic kidney disease. Am J Physiol Ren Physiol. 2014;307:F1198–F1206. doi: 10.1152/ajprenal.00327.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nowak K.L., Wang W., Farmer-Bailey H., et al. Vascular dysfunction, oxidative stress, and inflammation in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2018;13:1493–1501. doi: 10.2215/cjn.05850518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raina R., Chauvin A., Vajapey R., Khare A., Krishnappa V. Endothelin-1 as a therapeutic target in autosomal dominant polycystic kidney disease. Clin Nephrol. 2019;91:370–379. doi: 10.5414/CN109598. [DOI] [PubMed] [Google Scholar]
- 69.Al-Nimri M.A., Komers R., Oyama T.T., Subramanya A.R., Lindsley J.N., Anderson S. Endothelial-derived vasoactive mediators in polycystic kidney disease. Kidney Int. 2003;63:1776–1784. doi: 10.1046/j.1523-1755.2003.00913.x. [DOI] [PubMed] [Google Scholar]
- 70.Kocyigit I., Eroglu E., Kaynar A.S., et al. The association of endothelin-1 levels with renal survival in polycystic kidney disease patients. J Nephrol. 2019;32:83–91. doi: 10.1007/s40620-018-0514-2. [DOI] [PubMed] [Google Scholar]
- 71.Giusti R., Neri M., Angelini D., et al. Plasma concentration of endothelin and arterial pressure in patients with ADPKD. Contrib Nephrol. 1995;115:118–121. doi: 10.1159/000424407. [DOI] [PubMed] [Google Scholar]
- 72.Theodorakopoulou M., Raptis V., Loutradis C., Sarafidis P. Hypoxia and endothelial dysfunction in autosomal-dominant polycystic kidney disease. Semin Nephrol. 2019;39:599–612. doi: 10.1016/j.semnephrol.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 73.Blankestijn P.J. Sympathetic hyperactivity in chronic kidney disease. Nephrol Dial Transplant. 2004;19:1354–1357. doi: 10.1093/ndt/gfh242. [DOI] [PubMed] [Google Scholar]
- 74.Norman J. Fibrosis and progression of autosomal dominant polycystic kidney disease (ADPKD) Biochim Biophys Acta. 2011;10:1327–1336. doi: 10.1016/j.bbadis.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rangan G.K., Lee V.W., Alexander S.I., Patel C., Tunnicliffe D.J., Vladica P. KHA-CARI autosomal dominant polycystic kidney disease guideline: screening for polycystic kidney disease. Semin Nephrol. 2015;35:557–564.e6. doi: 10.1016/j.semnephrol.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 76.Mallett A., Lee V.W., Mai J., Lopez-Vargas P., Rangan G.K. KHA-CARI autosomal dominant polycystic kidney disease guideline: pharmacological management. Semin Nephrol. 2015;35:582–589.e17. doi: 10.1016/j.semnephrol.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 77.Mei C.-L., Xue C., Yu S.-Q., et al. Executive summary: clinical practice guideline for autosomal dominant polycystic kidney disease in China. Kidney Dis (Basel) 2020;6:144–149. doi: 10.1159/000506288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schrier R.W., Abebe K.Z., Perrone R.D., et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2255–2266. doi: 10.1056/NEJMoa1402685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee V.W., Tunnicliffe D.J., Rangan G.K. KHA-CARI autosomal dominant polycystic kidney disease guideline: management of end-stage kidney disease. Semin Nephrol. 2015;35:595–602.e12. doi: 10.1016/j.semnephrol.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 80.Gimpel C., Bergmann C., Bockenhauer D., et al. International consensus statement on the diagnosis and management of autosomal dominant polycystic kidney disease in children and young people. Nat Rev Nephrol. 2019;15:713–726. doi: 10.1038/s41581-019-0155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chapman A.B., Devuyst O., Eckardt K.U., et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a kidney disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015;88:17–27. doi: 10.1038/ki.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kidney disease: improving global outcomes (KDIGO) CKD work group KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Inter Suppl. 2013;3:1–150. [Google Scholar]
- 83.Fihn S.D., Blankenship J.C., Alexander K.P., et al. ACC/AHA/AATS/PCNA/SCAI/STS Focused Update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2014;130:1749–1767. doi: 10.1161/CIR.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 84.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 85.Lee V.W., Dexter M.A., Mai J., Vladica P., Lopez-Vargas P., Rangan G.K. KHA-CARI autosomal dominant polycystic kidney disease guideline: management of intracranial aneurysms. Semin Nephrol. 2015;35:612–617.e20. doi: 10.1016/j.semnephrol.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 86.Sanchis I.M., Shukoor S., Irazabal M.V., et al. Presymptomatic screening for intracranial aneurysms in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2019;14:1151–1160. doi: 10.2215/cjn.14691218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Theivendran M., Chuen J. Updates on AAA screening and surveillance. Aust J Gen Pract. 2018;47:259–263. doi: 10.31128/AFP-07-17-4278. [DOI] [PubMed] [Google Scholar]
- 88.Wong A.T.Y., Munt A., Allman-Farinelli M., et al. Assessment of dietary sodium intake using the scored salt questionnaire in autosomal dominant polycystic kidney disease. Nutrients. 2020;12:3376. doi: 10.3390/nu12113376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vogt L., Waanders F., Boomsma F., de Zeeuw D., Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol. 2008;19:999–1007. doi: 10.1681/asn.2007060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Siervo M., Lara J., Chowdhury S., Ashor A., Oggioni C., Mathers J.C. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr. 2015;113:1–15. doi: 10.1017/s0007114514003341. [DOI] [PubMed] [Google Scholar]
- 91.Rangan G.K., Alexander S.I., Campbell K.L., et al. KHA-CARI guideline recommendations for the diagnosis and management of autosomal dominant polycystic kidney disease. Nephrol (Carlton) 2016;21:705–716. doi: 10.1111/nep.12658. [DOI] [PubMed] [Google Scholar]
- 92.Chen H., Watnick T., Hong S.N., Daly B., Li Y., Seliger S.L. Left ventricular hypertrophy in a contemporary cohort of autosomal dominant polycystic kidney disease patients. BMC Nephrol. 2019;20:386. doi: 10.1186/s12882-019-1555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chapman A.B., Johnson A.M., Rainguet S., Hossack K., Gabow P., Schrier R.W. Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1997;8:1292–1297. doi: 10.1681/asn.V881292. [DOI] [PubMed] [Google Scholar]
- 94.Perrone R.D., Abebe K.Z., Schrier R.W., et al. Cardiac magnetic resonance assessment of left ventricular mass in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:2508–2515. doi: 10.2215/cjn.04610511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arjune S., Grundmann F., Todorova P., et al. Cardiac manifestations in patients with autosomal dominant polycystic kidney disease (ADPKD): a single-center study. Kidney360. 2023;4:150–161. doi: 10.34067/kid.0002942022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martinez-Vea A., Valero A., Bardaji A. Left ventricular hypertrophy in hypertensive patients with autosomal dominant polycystic kidney disease: influence of blood pressure and humoral and neurohormonal factors. Am J Hypertens. 2000;13(suppl 2):299A. doi: 10.1016/s0895-7061(00)01079-7. [DOI] [PubMed] [Google Scholar]
- 97.Cadnapaphornchai M.A., McFann K., Strain J.D., Masoumi A., Schrier R.W. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int. 2008;74:1192–1196. doi: 10.1038/ki.2008.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marlais M., Rajalingam S., Gu H., Savis A., Sinha M.D., Winyard P.J. Central blood pressure and measures of early vascular disease in children with ADPKD. Pediatr Nephrol. 2019;34:1791–1797. doi: 10.1007/s00467-019-04287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wa L.K., Macnicol A.M., Watson M.L.T.C., Macnicol A.M., Watson M.L. Ambulatory blood pressure in hypertensive patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1997;12:2075–2080. doi: 10.1093/ndt/12.10.2075. [DOI] [PubMed] [Google Scholar]
- 100.Bardaji A., Vea A.M., Gutierrez C., Ridao C., Richart C., Oliver J.A. Left ventricular mass and diastolic function in normotensive young adults with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1998;32:970–975. doi: 10.1016/S0272-6386(98)70071-X. [DOI] [PubMed] [Google Scholar]
- 101.Kahan T., Bergfeldt L. Left ventricular hypertrophy in hypertension: its arrhythmogenic potential. Heart. 2005;91:250–256. doi: 10.1136/hrt.2004.042473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Djenoune L., Berg K., Brueckner M., Yuan S. A change of heart: new roles for cilia in cardiac development and disease. Nat Rev Cardiol. 2022;19:211–227. doi: 10.1038/s41569-021-00635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaur S., McGlashan S.R., Ward M.L. Evidence of primary cilia in the developing rat heart. Cilia. 2018;7:4. doi: 10.1186/s13630-018-0058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pedrozo Z., Criollo A., Battiprolu P.K., et al. Polycystin-1 is a cardiomyocyte mechanosensor that governs L-type Ca2+ Channel Protein Stability. Circulation. 2015;131:2131–2142. doi: 10.1161/CIRCULATIONAHA.114.013537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Altamirano F., Schiattarella G.G., French K.M., et al. Polycystin-1 assembles with Kv channels to govern cardiomyocyte repolarization and contractility. Circulation. 2019;140:921–936. doi: 10.1161/CIRCULATIONAHA.118.034731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Villalobos E., Criollo A., Schiattarella G.G., et al. Fibroblast primary cilia are required for cardiac fibrosis. Circulation. 2019;139:2342–2357. doi: 10.1161/CIRCULATIONAHA.117.028752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hopp K., Ward C.J., Hommerding C.J., et al. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest. 2012;122:4257–4273. doi: 10.1172/JCI64313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.DiNello E., Bovo E., Thuo P., et al. Deletion of cardiac polycystin 2/PC2 results in increased SR calcium release and blunted adrenergic reserve. Am J Physiol Heart Circ Physiol. 2020;319:H1021–H1035. doi: 10.1152/ajpheart.00302.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuo I.Y., Kwaczala A.T., Nguyen L., Russell K.S., Campbell S.G., Ehrlich B.E. Decreased polycystin 2 expression alters calcium-contraction coupling and changes β-adrenergic signaling pathways. Proc Natl Acad Sci U S A. 2014;111:16604–16609. doi: 10.1073/pnas.1415933111s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Atwood D.J., Pokhrel D., Brown C.N., et al. Increased mTOR and suppressed autophagic flux in the heart of a hypomorphic Pkd1 mouse model of autosomal dominant polycystic kidney disease. Cell Signal. 2020;74 doi: 10.1016/j.cellsig.2020.109730. [DOI] [PubMed] [Google Scholar]
- 111.Criollo A., Altamirano F., Pedrozo Z., et al. Polycystin-2-dependent control of cardiomyocyte autophagy. J Mol Cell Cardiol. 2018;118:110–121. doi: 10.1016/j.yjmcc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 112.Akpinar T.S., Kucukdagli P., Ozer P.K., et al. Subclinic arterial and left ventricular systolic impairment in autosomal dominant polycystic kidney disease with preserved renal functions. Int J Cardiovasc Imaging. 2022;38:271–278. doi: 10.1007/s10554-021-02389-8. [DOI] [PubMed] [Google Scholar]
- 113.Krishnasamy R., Hawley C.M., Stanton T., et al. Left ventricular global longitudinal strain is associated with cardiovascular risk factors and arterial stiffness in chronic kidney disease. BMC Nephrol. 2015;16:106. doi: 10.1186/s12882-015-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lassen M.C.H., Qasim A.N., Biering-Sørensen T., et al. Cardiac function assessed by myocardial deformation in adult polycystic kidney disease patients. BMC Nephrol. 2019;20:324. doi: 10.1186/s12882-019-1500-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Krishnasamy R., Hawley C.M., Stanton T., et al. Association between left ventricular global longitudinal strain, health-related quality of life and functional capacity in chronic kidney disease patients with preserved ejection fraction. Nephrology (Carlton) 2016;21:108–115. doi: 10.1111/nep.12557. [DOI] [PubMed] [Google Scholar]
- 116.Sung P.H., Chiang H.J., Yang Y.H., Chen C.J., Chiang J.Y., Yip H.K. An association between autosomal-dominant polycystic kidney disease and the risk of acute myocardial infarction in Asian population - results of a nationwide study. Oncotarget. 2017;8:19365–19375. doi: 10.18632/oncotarget.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang B., Wang Q., Wang R., Xu T. Clinical manifestation, management and prognosis of acute myocardial infarction in autosomal dominant polycystic kidney disease. Kidney Blood Press Res. 2018;43:1806–1812. doi: 10.1159/000495638. [DOI] [PubMed] [Google Scholar]
- 118.Ecder T., Schrier R.W. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat Rev Nephrol. 2009;5:221–228. doi: 10.1038/nrneph.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Turkmen K., Oflaz H., Uslu B., et al. Coronary flow velocity reserve and carotid intima media thickness in patients with autosomal dominant polycystic kidney disease: from impaired tubules to impaired carotid and coronary arteries. Clin J Am Soc Nephrol. 2008;3:986–991. doi: 10.2215/cjn.02330607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chebib F.T., Hogan M.C., El-Zoghby Z.M., et al. Autosomal dominant polycystic kidney patients may be predisposed to various cardiomyopathies. Kidney Int Rep. 2017;2:913–923. doi: 10.1016/j.ekir.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kuo I.Y., Duong S.L., Nguyen L., Ehrlich B.E. Decreased polycystin 2 levels result in non-renal cardiac dysfunction with aging. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Martinez-Vea A., Bardaji A., Valero A., et al. M003: cardiac involvement in autosomic dominant polycystic kidney disease (ADPKD): an hypertensive heart disease. Am J Hypertens. 2000;13(suppl 2):299A–300A. doi: 10.1016/s0895-7061(00)01080-3. [DOI] [Google Scholar]
- 123.Spinelli L., Giugliano G., Esposito G. Cardiac involvement in autosomal dominant polycystic kidney disease. Cardiogenetics. 2021;11:39–49. doi: 10.3390/cardiogenetics11020006. [DOI] [Google Scholar]
- 124.Oflaz H., Alisir S., Buyukaydin B., et al. Biventricular diastolic dysfunction in patients with autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68:2244–2249. doi: 10.1111/j.1523-1755.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 125.Miyamoto R., Sekine A., Fujimaru T., et al. Echocardiographic findings and genotypes in autosomal dominant polycystic kidney disease. Kidney Dis (Basel) 2022;8:246–252. doi: 10.1159/000520300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lumiaho A., Ikäheimo R., Miettinen R., et al. Mitral valve prolapse and mitral regurgitation are common in patients with polycystic kidney disease type 1. Am J Kidney Dis. 2001;38:1208–1216. doi: 10.1053/ajkd.2001.29216. [DOI] [PubMed] [Google Scholar]
- 127.Hossack K.F., Leddy C.L., Johnson A.M., Schrier R.W., Gabow P.A. Echocardiographic findings in autosomal dominant polycystic kidney disease. N Engl J Med. 1988;319:907–912. doi: 10.1056/NEJM198810063191404. [DOI] [PubMed] [Google Scholar]
- 128.Saggar-Malik A.K., Missouris C.G., Gill J.S., Singer D.R., Markandu N.D., MacGregor G.A. Left ventricular mass in normotensive subjects with autosomal dominant polycystic kidney disease. BMJ. 1994;309:1617–1618. doi: 10.1136/bmj.309.6969.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nieman A., Wang C., Wang C., Beck T., Harvey A., Norris R.A. Mitral valve prolapse and its motley crew-syndromic prevalence, pathophysiology, and progression of a common heart condition. J Am Heart Assoc. 2021;10 doi: 10.1161/jaha.121.020919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ivy D.D., Shaffer E.M., Johnson A.M., Kimberling W.J., Dobin A., Gabow P.A. Cardiovascular abnormalities in children with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;5:2032–2036. doi: 10.1681/ASN.V5122032. [DOI] [PubMed] [Google Scholar]
- 131.Savis A., Simpson J.M., Kabir S., Peacock K., Beardsley H., Sinha M.D. Prevalence of cardiac valvar abnormalities in children and young people with autosomal dominant polycystic kidney disease. Pediatr Nephrol. 2023;38:705–709. doi: 10.1007/s00467-022-05500-w. [DOI] [PubMed] [Google Scholar]
- 132.Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:173–180. doi: 10.1053/j.ackd.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 133.Bardají A., Martinez-Vea A., Valero A., et al. Cardiac involvement in autosomal-dominant polycystic kidney disease: a hypertensive heart disease. Clin Nephrol. 2001;56(3):211–220. [PubMed] [Google Scholar]
- 134.Kuo I.Y. Defining cardiac dysfunction in ADPKD. Kidney360. 2023;4:126–127. doi: 10.34067/kid.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Flahault A., Joly D. Screening for intracranial aneurysms in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2019;14:1242–1244. doi: 10.2215/cjn.02100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lefèvre S., Audrézet M.-P., Halimi J.-M., et al. Diagnosis and risk factors for intracranial aneurysms in autosomal polycystic kidney disease: a cross-sectional study from the Genkyst cohort. Nephrol Dial Transplant. 2022;37:2223–2233. doi: 10.1093/ndt/gfac027. [DOI] [PubMed] [Google Scholar]
- 137.Irazabal M.V., Huston J., Kubly V., et al. Extended follow-up of unruptured intracranial aneurysms detected by presymptomatic screening in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:1274–1285. doi: 10.2215/cjn.09731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Luciano R.L., Dahl N.K. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): considerations for routine screening and management. Nephrol Dial Transplant. 2013;29:247–254. doi: 10.1093/ndt/gft437. [DOI] [PubMed] [Google Scholar]
- 139.Matsuura R., Honda K., Oki R., Hamasaki Y., Doi K., Nangaku M. Screening and management for intracranial aneurysms in Japanese patients with ADPKD. Kidney Int Rep. 2022;7:1893–1896. doi: 10.1016/j.ekir.2022.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nurmonen H.J., Huttunen T., Huttunen J., et al. Polycystic kidney disease among 4,436 intracranial aneurysm patients from a defined population. Neurology. 2017;89:1852–1859. doi: 10.1212/wnl.0000000000004597. [DOI] [PubMed] [Google Scholar]
- 141.Kataoka H., Akagawa H., Yoshida R., et al. Impact of kidney function and kidney volume on intracranial aneurysms in patients with autosomal dominant polycystic kidney disease. Sci Rep. 2022;12 doi: 10.1038/s41598-022-22884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yoshida H., Higashihara E., Maruyama K., et al. Relationship between intracranial aneurysms and the severity of autosomal dominant polycystic kidney disease. Acta Neurochir (Wien) 2017;159:2325–2330. doi: 10.1007/s00701-017-3316-8. [DOI] [PubMed] [Google Scholar]
- 143.Rossetti S., Chauveau D., Kubly V., et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. Lancet. 2003;361:2196–2201. doi: 10.1016/s0140-6736(03)13773-7. [DOI] [PubMed] [Google Scholar]
- 144.Perrone R.D., Malek A.M., Watnick T. Vascular complications in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2015;11:589–598. doi: 10.1038/nrneph.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rinkel G.J., Ruigrok Y.M. Preventive screening for intracranial aneurysms. Int J Stroke. 2022;17:30–36. doi: 10.1177/17474930211024584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takagi H., Umemoto T. Abdominal aortic aneurysm and autosomal-dominant polycystic kidney disease. Kidney Int. 2005;67 doi: 10.1111/j.1523-1755.2005.091_2.x. 376-376. [DOI] [PubMed] [Google Scholar]
- 147.Schievink W.I., Torres V.E., Wiebers D.O., Huston J. Intracranial arterial dolichoectasia in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1997;8:1298–1303. doi: 10.1681/asn.V881298. [DOI] [PubMed] [Google Scholar]
- 148.Gorriz J.L., Arroyo D., D’Marco L., et al. Cardiovascular risk factors and the impact on prognosis in patients with chronic kidney disease secondary to autosomal dominant polycystic kidney disease. BMC Nephrol. 2021;22:110. doi: 10.1186/s12882-021-02313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alam A., Perrone R.D. Left ventricular hypertrophy in ADPKD: changing demographics. Curr Hypertens Rev. 2013;9:27–31. doi: 10.2174/1573402111309010005. [DOI] [PubMed] [Google Scholar]
- 150.Müller R.-U., Messchendorp A.L., Birn H., et al. An update on the use of tolvaptan for autosomal dominant polycystic kidney disease: consensus statement on behalf of the ERA Working Group on Inherited Kidney Disorders, the European Rare Kidney Disease Reference Network and Polycystic Kidney Disease International. Nephrol Dial Transplant. 2021;37:825–839. doi: 10.1093/ndt/gfab312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee E.C., Valencia T., Allerson C., et al. Discovery and preclinical evaluation of anti-miR-17 oligonucleotide RGLS4326 for the treatment of polycystic kidney disease. Nat Commun. 2019;10:4148. doi: 10.1038/s41467-019-11918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bais T., Gansevoort R.T., Meijer E. Drugs in clinical development to treat autosomal dominant polycystic kidney disease. Drugs. 2022;82:1095–1115. doi: 10.1007/s40265-022-01745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cadnapaphornchai M.A., George D.M., McFann K., et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2014;9:889–896. doi: 10.2215/cjn.08350813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Brosnahan G.M., Abebe K.Z., Rahbari-Oskoui F.F., et al. Effect of statin therapy on the progression of autosomal dominant polycystic kidney disease. A secondary analysis of the HALT PKD trials. Curr Hypertens Rev. 2017;13:109–120. doi: 10.2174/1573402113666170427142815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fassett R.G., Coombes J.S., Packham D., Fairley K.F., Kincaid-Smith P. Effect of pravastatin on kidney function and urinary protein excretion in autosomal dominant polycystic kidney disease. Scand J Urol Nephrol. 2010;44:56–61. doi: 10.3109/00365590903359908. [DOI] [PubMed] [Google Scholar]
- 156.Abdul-Majeed S., Nauli S.M. Dopamine receptor Type 5 in the primary cilia has dual chemo- and mechano-sensory roles. Hypertension. 2011;58:325–331. doi: 10.1161/HYPERTENSIONAHA.111.172080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jdiaa S.S., Husainat N.M., Mansour R., et al. A systematic review of reported outcomes in ADPKD studies. Kidney Int Rep. 2022;7:1964–1979. doi: 10.1016/j.ekir.2022.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sautenet B., Cho Y., Gutman T., et al. Range and variability of outcomes reported in randomized trials conducted in patients with polycystic kidney disease: a systematic review. Am J Kidney Dis. 2020;76:213–223. doi: 10.1053/j.ajkd.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 159.Cho Y., Rangan G., Logeman C., et al. Core outcome domains for trials in autosomal dominant polycystic kidney disease: an international Delphi survey. Am J Kidney Dis. 2020;76:361–373. doi: 10.1053/j.ajkd.2020.01.005. [DOI] [PubMed] [Google Scholar]