Abstract
Introduction
Despite recognized geographic and sex-based differences in hemoglobin in the general population, these factors are typically ignored in patients with chronic kidney disease (CKD) in whom a single therapeutic range for hemoglobin is recommended. We sought to compare the distribution of hemoglobin across international nondialysis CKD populations and evaluate predictors of hemoglobin.
Methods
In this cross-sectional study, hemoglobin distribution was evaluated in each cohort overall and stratified by sex and estimated glomerular filtration rate (eGFR). Relationships between candidate predictors and hemoglobin were assessed from linear regression models in each cohort. Estimates were subsequently pooled in a random effects model.
Results
A total of 58,613 participants from 21 adult cohorts (median eGFR range of 17–49 ml/min) and 3 pediatric cohorts (median eGFR range of 26–45 ml/min) were included with broad geographic representation. Hemoglobin values varied substantially among the cohorts, overall and within eGFR categories, with particularly low mean hemoglobin observed in women from Asian and African cohorts. Across the eGFR range, women had a lower hemoglobin compared to men, even at an eGFR of 15 ml/min (mean difference 5.3 g/l, 95% confidence interval [CI] 3.7–6.9). Lower eGFR, female sex, older age, lower body mass index, and diabetic kidney disease were all independent predictors of a lower hemoglobin value; however, this only explained a minority of variance (R2 7%–44% across cohorts).
Conclusion
There are substantial regional differences in hemoglobin distribution among individuals with CKD, and the majority of variance is unexplained by demographics, eGFR, or comorbidities. These findings call for a renewed interest in improving our understanding of hemoglobin determinants in specific CKD populations.
Keywords: anemia, chronic kidney disease, geography, glomerular filtration rate, hemoglobin, sex
The prevalence of anemia in the general population varies substantially around the world due to multiple factors, including deficiency of iron and other micronutrients, acute and chronic infections, and hemoglobinopathies.1 Globally, the burden of anemia is higher in women compared to men, with the highest prevalence estimates in Sub-Saharan Africa and South Asia.2,3 Physiological, sex-based differences in hemoglobin concentration are recognized in the general adult population by having different reference ranges for men and women. The anticipated heterogeneity in hemoglobin values arising from these demographic factors is largely ignored in the setting of CKD. International guidelines suggest a narrow ‘acceptable’ range of hemoglobin in CKD irrespective of sex, geography, or other factors, principally with the aim of avoiding potential harms associated with therapeutic correction of hemoglobin to physiological levels.4, 5, 6
Within this treatment construct, there is an inherent assumption that individuals with CKD are similar in terms of hemoglobin distribution and determinants of hemoglobin. However, there is a paucity of information about geographic differences in hemoglobin distribution among CKD populations. Although there is an established relationship between eGFR and hemoglobin,7,8 there are limited data on other potential contributors to hemoglobin such as etiology of kidney disease, albuminuria, and chronic health conditions in people with CKD. It is also unclear if the level of kidney function modifies the association between hemoglobin and patient-level characteristics; for instance, if the difference in hemoglobin among men and women that is observed in the general population still holds when eGFR is reduced.
These knowledge gaps hinder our capacity to individualize the approach to anemia risk stratification, thresholds for initiation of therapy, and treatment goals for patients with CKD. Conversely, an improved awareness and understanding of hemoglobin variation in different CKD populations could enhance discussions with patients, as well as inform the future design of clinical trials of anemia therapy. In this collaborative initiative from the International Network of CKD Cohort Studies (iNET-CKD), we sought to evaluate geographic variation in hemoglobin distribution among individuals with CKD and investigate predictors of hemoglobin across participating cohorts with a particular focus on the contributions of sex, eGFR, and etiology of CKD.
Methods
Study Design
This cross-sectional study used a distributed network approach to meta-analyze individual participant data from each participating iNET-CKD cohort.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 Details of the cohorts, including study country, target population and recruitment years are provided in Supplementary Table S1. The iNET-CKD includes observational studies of individuals with varying severity of CKD with defined objectives and prospective data collection.30 The present analysis included study participants who had an eGFR below 60 ml/min per 1.73 m2 and complete data for a core set of variables including age, sex, and hemoglobin. Kidney transplant recipients and participants receiving dialysis at the time of hemoglobin measurement were excluded. The pediatric cohorts 4C (Europe), CKiD (US), and KNOW-Ped CKD (Korea) contributed to the descriptive phase of the analysis. The distributed network approach is similar to a 2-stage individual participant data meta-analysis; however, it uses standardized data collection and methods across cohorts, as opposed to using published data from previous studies.31 Participating investigators had the following options: of (i) providing deidentified individual-level data to a central hub at the University of British Columbia, Canada, for analysis or (ii) conducting the analysis and transferring the output to the central hub for pooled analysis (Supplementary Figure S1). For both options, a study protocol was sent to investigators. Ethical approval for the study was granted from the research ethics board at each participating site.
Variable Definitions
A variable dictionary was created to harmonize data extraction, coding, and labeling of variables (Supplementary Table S2). The first available hemoglobin value (in grams per liter, g/l) was used for each participant. Glomerular filtration rate was estimated by using the 2009 Chronic Kidney Disease Epidemiology Collaboration formula32 and using serum creatinine standardized to isotope dilution mass spectrometry. The bedside Schwartz equation was used to estimate glomerular filtration rate in pediatric cohorts.33 Albuminuria was measured by the albumin-to-creatinine ratio (ACR, in mg/g) and classified as per Kidney Disease Improving Global Outcomes stages as A1 (ACR <30 mg/g), A2 (ACR 30–299 mg/g), or A3 (ACR ≥ 300 mg/g). The closest value of eGFR or ACR within 3 months of the date of the index hemoglobin measurement was chosen. Iron saturation was measured in 9 adult cohorts. Etiology of kidney disease was classified as diabetic kidney disease, hypertension, glomerulonephritis, polycystic kidney disease, or ‘other’ based on a physician diagnosis or a kidney biopsy. A small number of cohorts did not collect data for specific etiologies such as polycystic kidney disease (Supplementary Table S2). In some instances, the ‘other’ category contained unknown or missing cases. Definitions for atheromatous cardiovascular disease, heart failure, and diabetes mellitus are provided in Supplementary Table S2. Body mass index (BMI) was calculated as weight (kg) divided by square height (m2) and categorized as <18.5, 18.5 to 24.9, 25.0 to 29.9, or ≥30.0. Smoking status was classified as current, former, or never. Use of erythropoiesis stimulating agent (ESA) therapy and renin-angiotensin-aldosterone-system (RAAS) inhibitors was ascertained as a yes/no exposure using medication records and/or Anatomical Therapeutic Chemical codes. Altitude was categorized as 1 to 499, 500 to 1000 and >1000 meters above sea level based on the location of residency or, if unavailable, the location of the center of study enrollment.
Statistical Analysis
To compare the distribution of hemoglobin across the cohorts, we report summary statistics (mean and SD) stratified by sex and eGFR category (<20, 20–29, 30–44, and 45–59 ml/min per 1.73 m2). In each of the adult cohorts, the association between hemoglobin and candidate predictor variables was examined in a series of linear regression models. To maximize cohort participation, the first set of models included a core set of variables that was available in all cohorts, including age (per 10-year increase), sex, year of hemoglobin measurement, and eGFR (per 10 ml/min per 1.73 m2 increase). An extended multivariable model incorporated comorbidities (cardiovascular disease, diabetes, and heart failure), BMI, albuminuria categories, and etiology of CKD. Due to interdependence between diabetes mellitus and etiology of CKD, estimates for etiology of CKD are presented separately for individuals with and without diabetes mellitus. Potential interactions were identified a priori between eGFR and the following variables: sex, age, and etiology of CKD. In an exploratory analysis, smoking status, use of RAAS inhibitors and altitude were added separately to the extended model. Only cohorts that collected data for each variable in a multivariable model could be included in that model. For categorical variables with missing values, a separate category was created to ensure that sequential models included the same individuals from each cohort, thus facilitating a better comparison between models. A complete case analysis was conducted as a sensitivity analysis. Covariate coefficient estimates from each cohort were subsequently pooled in a random effects meta-analysis. Heterogeneity in beta-coefficients was assessed using tau (the between-cohort SD) and the I2 statistic. To remove potential confounding from variable use of (or access to) ESA therapy in different countries, we repeated the analysis in patients unexposed to ESA treatment. All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 4.2.0 (R Core Team 2014, Vienna, Austria).
Results
Participant Characteristics
A total of 58,613 participants from 24 cohorts were included. Characteristics of included participants by cohort are summarized in Table 1. Three cohorts were pediatric cohorts including 1107 participants with median age ranging from 10 to 14 years. In the remaining 21 adult cohorts, median age ranged from 47 years in H3AKDN Enugu Site (Africa) to 84 years in CKDBIS (Germany). The majority of participants were male except for CKD-BIS and the primary care cohorts RRID (UK) and PROVALID (Europe), which had a slight majority of women. The proportion of participants with diabetes mellitus ranged from 7.5% in PSI BIND-NL (Netherlands) to 58% in CKDopps (US). One cohort (PROVALID) specifically recruited individuals in a primary care setting with diabetes. Kidney-specific characteristics are summarized in Table 2. Participants from pediatric cohorts had moderate to advanced CKD with median eGFR values of 26 (4C), 32 (KNOW-Ped CKD), and 45 ml/min per 1.73 m2 (CKiD). The median eGFR in adult CKD cohorts ranged between 17 ml/min per 1.73 m2 in EQUAL (Europe) and 49 ml/min per 1.73 m2 in RRID. The proportion of participants with severe albuminuria varied between 2.7% in NRHP (Uruguay) and 68% in CKDQLD (Australia). In general, albuminuria values were known for the majority of participants; however, missingness was higher in cohorts that recruited individuals with more advanced CKD (eGFR <30 ml/min per 1.73 m2). The etiology of CKD was unknown for a substantial number of participants in most cohorts. Where the cause was known (physician-diagnosed or biopsy-proven), the most common causes were hypertension and diabetic kidney disease, except for C-STRIDE (China), CKD-JAC (Japan), and H3AKDN Enugu Site, which had a relatively higher prevalence of glomerulonephritis. A total of 4947 participants (8.4%) were receiving ESA therapy (Table 1).
Table 1.
Participant characteristics by cohort
| Cohort | N | Age (Median, IQR) | Female (%) | BMI (%) |
DM (%) | CVD (%) | CHF (%) | ESA (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25–29.9 | ≥30 | Unknown | ||||||||
| CORE-CKD | 1312 | 64 (57, 69) | 34.5 | 2.3 | 43.4 | 37.3 | 15.7 | 1.3 | 50.8 | 16.5 | 1.0 | 2.5 |
| C-STRIDE | 2302 | 54 (43, 63) | 42.7 | 2.5 | 41.2 | 26.3 | 5.0 | 25.1 | 23.8 | 10.4 | 1.2 | 15.0 |
| ICKD | 3506 | 53 (44, 60) | 33.6 | 6.9 | 47.4 | 30.8 | 12.1 | 2.7 | 36.0 | 22.6 | 11.9 | 2.9 |
| KNOW-CKD | 1430 | 58 (50, 66) | 37.9 | 2.5 | 54.9 | 35.2 | 6.6 | 0.8 | 42.2 | 16.8 | 2.2 | 11.7 |
| CKD-JAC | 2494 | 63 (55, 70) | 36.3 | 6.9 | 55.5 | 22.3 | 5.0 | 10.3 | 38.4 | 22.8 | 4.8 | 13.8 |
| CKD.QLD | 1883 | 71 (61, 78) | 45.6 | 1.2 | 17.7 | 28.5 | 44.9 | 7.8 | 47.6 | 19.6 | 8.2 | 4.5 |
| CanPREDDICT | 2526 | 70 (61, 77) | 37.5 | 0.5 | 5.7 | 8.2 | 9.7 | 76.0 | 48.1 | 45.2 | 26.9 | 20.3 |
| CRIC | 4224 | 62 (55, 68) | 44.1 | 0.5 | 13.4 | 28.8 | 56.8 | 0.5 | 53.4 | 33.1 | 10.5 | 3.6 |
| CKDopps US | 2119 | 70 (61, 77) | 48.5 | 1.1 | 13.1 | 23.7 | 50.0 | 12.1 | 58.0 | 30.5 | 16.9 | 5.2 |
| CKDopps BR | 720 | 66 (57, 76) | 47.2 | 0.7 | 20.3 | 21.1 | 20.1 | 37.8 | 50.7 | 23.1 | 16.1 | 12.2 |
| NRHP | 19,288 | 73 (65, 79) | 42.1 | 0.6 | 16.8 | 28.7 | 29.3 | 24.5 | 37.1 | 28.2 | 9.4 | 7.6 |
| H3Africa | 338 | 47 (36, 59) | 47.9 | 1.2 | 8.3 | 8.6 | 1.2 | 80.8 | 19.5 | 2.4 | 0.9 | 2.7 |
| CKD-REIN | 2946 | 69 (61, 77) | 34.6 | 1.6 | 26.1 | 35.5 | 34.7 | 2.1 | 43.0 | 36.3 | 13.2 | 7.8 |
| RIISC | 790 | 65 (53, 76) | 36.6 | 0.9 | 22.7 | 31.4 | 40.6 | 4.4 | 40.3 | 35.3 | 0.0 | 6.6 |
| BIS | 777 | 84 (79, 88) | 52.3 | 0.4 | 25.4 | 45.6 | 28.7 | 0.0 | 31.0 | 55.3 | 46.5 | 0.5 |
| EQUAL | 1720 | 76 (71, 81) | 34.5 | 1.2 | 24.3 | 34.5 | 30.8 | 9.2 | 41.3 | 26.3 | 17.4 | 24.9 |
| GCKD | 3995 | 65 (57, 70) | 38.1 | 0.5 | 18.0 | 37.2 | 43.3 | 1.0 | 38.2 | 33.6 | 19.1 | 3.0 |
| CKDopps GERMANY | 2638 | 75 (67, 80) | 43.2 | 1.1 | 23.4 | 35.5 | 37.6 | 2.5 | 47.9 | 29.0 | 13.4 | 11.6 |
| PSI BIND-NL | 644 | 63 (54, 72) | 32.3 | 0.9 | 29.5 | 35.4 | 23.3 | 10.9 | 7.5 | 17.1 | 6.5 | 16.9 |
| RRID | 1184 | 76 (70, 81) | 53.7 | 0.3 | 19.3 | 42.4 | 38.0 | 0.0 | 20.3 | 19.4 | 4.5 | 0.0 |
| PROVALID | 670 | 68 (62, 74) | 58.5 | 0.0 | 12.8 | 32.4 | 53.6 | 1.2 | 100.0 | 42.7 | 5.8 | 7.3 |
| 4C | 651 | 12 (9, 14) | 33.8 | 8.1 | 69.6 | 11.7 | 10.6 | 0 | 0.6 | - | - | 23.5 |
| CKiD | 210 | 14 (11, 16) | 43.3 | - | - | - | - | 100 | 0.0 | 0.5 | 1.4 | 22.9 |
| KNOW-Ped CKD | 246 | 10 (5, 14) | 30.9 | 67.1 | 29.7 | 2.4 | 0.8 | 0 | 0.8 | - | 0.4 | 18.3 |
BMI, body mass index; CHF, congestive heart failure; CVD, cardiovascular disease; DM, diabetes mellitus; ESA, erythropoiesis stimulating agent; IQR, interquartile range.
Table 2.
Description of kidney parameters by cohort
| Cohort | eGFR (Median, IQR) | eGFR (%) |
ACR (%) |
Etiology of CKD (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <20 | 20–30 | 30–45 | 45–59 | A1 | A2 | A3 | n/a | DKD | HTN | GN | PKD | Other | ||
| CORE-CKD | 36 (28, 47) | 5.0 | 25.8 | 38.4 | 30.8 | 26.0 | 22.9 | 41.3 | 9.8 | 36.0 | 24.6 | 5.3 | 3.0 | 31.0 |
| C-STRIDE | 33 (23, 44) | 18.3 | 24.5 | 34.5 | 22.7 | 12.5 | 27.2 | 49.8 | 10.6 | 14.7 | - | 41.7 | - | 43.6 |
| ICKD | 39 (33, 47) | 0.8 | 11.2 | 57.5 | 30.5 | 47.1 | 23.3 | 24.2 | 5.3 | 25.7 | 8.4 | 13.7 | 3.1 | 49.1 |
| KNOW-CKD | 33 (23, 45) | 20.0 | 22.7 | 32.0 | 25.2 | 8.8 | 28.3 | 58.1 | 4.8 | 31.3 | 23.4 | 29.9 | 9.6 | 5.9 |
| CKD-JAC | 33 (21, 45) | 22.3 | 21.4 | 31.1 | 25.3 | 8.9 | 24.2 | 57.3 | 9.5 | 21.5 | 19.8 | 41.7 | - | 17.0 |
| CKD.QLD | 32 (21, 42) | 20.8 | 24.6 | 35.4 | 19.2 | 16.4 | 8.0 | 68.2 | 7.5 | 24.0 | 21.1 | 9.3 | 2.8 | 42.7 |
| CanPREDDICT | 27 (21, 34) | 22.2 | 38.8 | 36.3 | 2.7 | 22.9 | 32.7 | 37.9 | 6.5 | 28.7 | 25.9 | 11.4 | 4.5 | 29.6 |
| CRIC | 44 (34, 52) | 2.2 | 15.1 | 37.2 | 45.5 | 27.2 | 21.0 | 27.2 | 24.6 | 27.9 | 16.6 | - | - | 55.5 |
| CKDopps US | 25 (18, 33) | 31.2 | 36.1 | 27.1 | 5.6 | 15.5 | 10.0 | 19.5 | 55.0 | 22.7 | 21.1 | 5.0 | 1.5 | 49.6 |
| CKDopps BR | 24 (17, 34) | 35.0 | 31.8 | 24.7 | 8.5 | 30.8 | 9.4 | 10.8 | 48.9 | 33.9 | 29.0 | 8.6 | 4.2 | 24.3 |
| NRHP | 37 (28, 46) | 10.5 | 19.8 | 42.9 | 26.8 | 96.4 | - | 2.7 | 0.9 | 11.0 | 52.1 | 3.8 | 1.4 | 31.7 |
| H3Africa | 23 (10, 38) | 42.6 | 17.2 | 26.9 | 13.3 | 46.7 | 13.0 | 40.2 | - | 19.8 | 5.6 | 34.0 | 2.4 | 38.2 |
| CKD-REIN | 31 (23, 41) | 16.2 | 30.0 | 37.9 | 15.9 | 25.1 | 28.6 | 37.9 | 8.4 | 20.3 | 20.9 | 17.5 | 5.6 | 35.7 |
| RIISC | 29 (22, 39) | 18.1 | 35.2 | 33.7 | 13.0 | 15.1 | 32.4 | 47.1 | 5.4 | 14.9 | 27.6 | 9.9 | 5.7 | 41.9 |
| BIS | 47 (37, 53) | 0.9 | 10.0 | 29.3 | 59.7 | 63.1 | 29.7 | 6.2 | 1.0 | - | - | - | - | 100.0 |
| EQUAL | 17 (14, 20) | 74.1 | 23.4 | 2.3 | 0.1 | - | 20.9 | 18.0 | 61.1 | 20.4 | 35.6 | 9.2 | 2.6 | 32.2 |
| GCKD | 42 (35, 50) | 1.0 | 11.1 | 46.3 | 41.6 | 43.6 | 29.7 | 25.0 | 1.7 | 16.3 | 23.5 | 15.4 | 3.9 | 40.8 |
| CKDopps GERMANY | 23 (19, 28) | 29.5 | 49.4 | 14.8 | 6.3 | 17.4 | 14.5 | 15.5 | 52.6 | 30.6 | 35.6 | 8.3 | 2.9 | 22.6 |
| PSI BIND-NL | 30 (21, 42) | 22.5 | 26.1 | 31.1 | 20.3 | - | - | - | 100 | 6.2 | 8.9 | 16.6 | 5.1 | 63.2 |
| RRID | 49 (42, 54) | - | 2.8 | 30.8 | 66.4 | 80.6 | 16.2 | 3.2 | - | 20.3 | - | - | - | 79.7 |
| PROVALID | 48 (39, 54) | 2.7 | 9.0 | 27.3 | 61.0 | 63.0 | 26.4 | 8.8 | 1.8 | 100.0 | - | - | - | - |
| 4C | 26 (17, 34) | 33.6 | 30.4 | 28.9 | 7.1 | 11.7 | 34.9 | 52.4 | 1.1 | |||||
| CKiD | 45 (37, 53) | 1.4 | 11.4 | 37.1 | 50.0 | - | - | - | 100 | |||||
| KNOW-Ped CKD | 32 (18, 47) | 27.6 | 17.1 | 25.2 | 30.1 | 59.8 | 10.6 | 29.7 | - | |||||
ACR, albumin to creatinine ratio; CKD, chronic kidney disease; DKD, diabetic kidney disease; eGFR, estimated glomerular filtration rate; GN, glomerulonephritis; HTN, hypertension; PKD, polycystic kidney disease.
Hemoglobin Distribution Among Cohorts
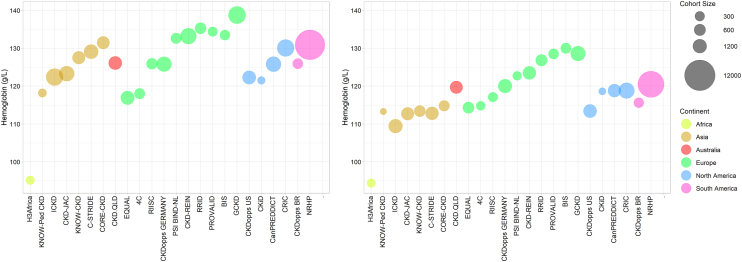
The distribution of hemoglobin across all participating cohorts is provided in Supplementary Table S3 and summarized graphically in Figure 1. Among pediatric cohorts, mean (SD) hemoglobin ranged from 113 (19) g/l to 119 (15) g/l in female participants, and from 118 (16) g/l to 122 (18) g/l in male participants. In adult cohorts, there was considerable variation in hemoglobin values in both men and women. For example, in women, the mean (SD) hemoglobin ranged from 94 (21) g/l to 130 (12) g/l. Even within the same world region, there was substantial variation in hemoglobin distribution. Among men participating in European cohorts, the mean (SD) hemoglobin varied between 117 (16) g/l and 139 (18) g/l. When comparing world regions, women from Asian cohorts tended to have a lower average hemoglobin than women from European cohorts, and this finding was broadly consistent within categories of eGFR (Supplementary Figure S2). With the exception of H3AKDN Enugu Site, men had a higher hemoglobin value, on average, compared to women from the same cohort. Nine adult cohorts collected data on iron saturation with values available for between 12.6% and 97.8% of participants (Supplementary Table S4). In these cohorts, the mean value of iron saturation ranged between 22.8% and 30.9%.
Figure 1.
Bubble plot showing the mean hemoglobin for men (left panel) and women (right panel) in each iNET-CKD cohort and grouped by world region.
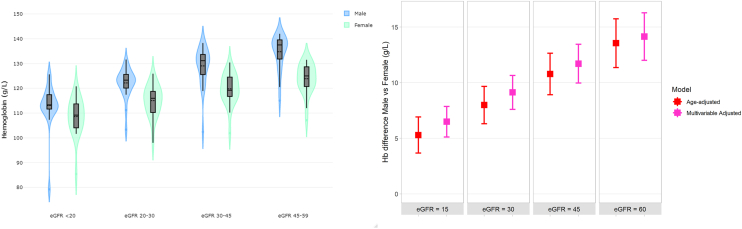
Sex Differences in Hemoglobin
To further illustrate differences in mean hemoglobin in men and women across the eGFR spectrum, data from all cohorts were pooled in a violin plot (Figure 2, left panel). In keeping with the bubble plots in Figure 1, mean hemoglobin values varied widely across the cohorts in both sexes and within strata of eGFR. The shape of the hemoglobin distribution was also different by sex, demonstrating a broader dispersion of values in women compared to men. Across all eGFR categories, women tended to have a lower hemoglobin value compared to men; however, the magnitude of these differences was smaller in more advanced CKD compared to earlier stages of CKD (Figure 2, right panel). Evidence of an interaction between sex and eGFR was consistent across cohorts with an overall P-value of <0.001 (Supplementary Table S5). For example, in women compared to men, mean hemoglobin was 13.5 (95% CI 11.4–15.7) g/l lower at an eGFR of 60 ml/min per 1.73 m2, 10.8 (95% CI 8.9–12.6) g/l lower at an eGFR of 45 ml/min per 1.73 m2, 7.9 (95% CI 6.3–9.7) g/l lower at an eGFR of 30 ml/min per 1.73 m2, and 5.3 (95% CI 3.7–6.9) g/l lower at an eGFR of 15 ml/min per 1.73 m2 (Figure 2, Supplementary Tables S6A–D). There was no attenuation in these estimates after multivariable adjustment.
Figure 2.
Violin plot of the distribution of mean hemoglobin values in participating iNET-CKD cohorts stratified by sex and category of eGFR (left panel), and estimates of sex-based differences in hemoglobin level from age-adjusted and multi-variable-adjusted models (right panel). A violin plot is a hybrid of a box plot and a kernel density plot. The boxes represent the median and interquartile range (IQR), and the whiskers represent 1.5 times the IQR. The kernel density estimation shows the shape of the distribution. Wider and narrower sections of the plot represent a higher and lower probability, respectively, that participating cohorts of the study will take on the given value. Cohorts with less than 10 participants in a given stratum of sex and eGFR category were excluded from the plot.
Differences in Hemoglobin by eGFR and Cause of CKD
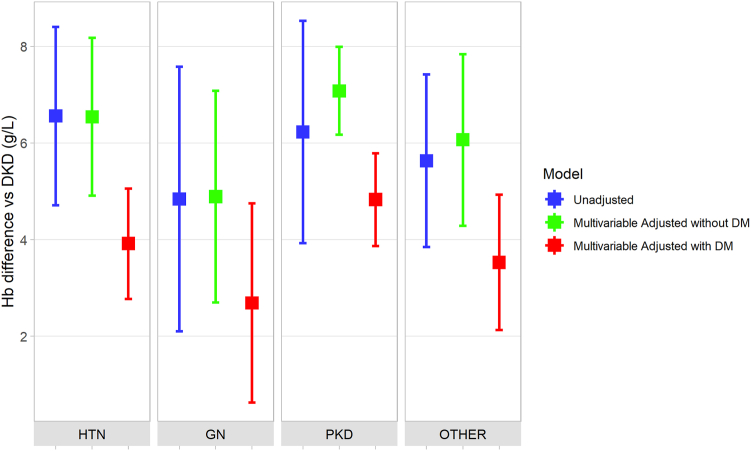
In men, each 10 ml/min per 1.73 m2 decrease in eGFR was associated with a 5.8 (95% CI 5.2–6.4) g/l decrease in mean hemoglobin. The corresponding estimate in women was 4.0 (95% CI 3.5–4.5) g/l. There was minimal change in these estimates after multivariable adjustment (Supplementary Table S7). Compared to diabetic kidney disease, glomerulonephritis (pooled mean difference 4.8 (95% CI 2.1–7.6) g/l), polycystic kidney disease (pooled mean difference 6.2 (95% CI 3.9–8.5) g/l) and hypertension (pooled mean difference 6.6 (95% CI 4.7–8.4) g/l) were all associated with higher mean hemoglobin values (Figure 3, Supplementary Table S8A). In a multivariable model, the pattern of these findings was consistent among individuals without diabetes mellitus, whereas there was some attenuation in disease-specific estimates among those with diabetes mellitus (Figure 3, Supplementary Tables S8B and C). There was no statistically significant interaction between eGFR and etiology of CKD based on the pooled analysis.
Figure 3.
Pooled estimates of the difference in mean hemoglobin according to the etiology of CKD based on meta-analysis of the unadjusted and multivariable-adjusted cohort-level estimates. For the multivariable-adjusted results, estimates are presented separately in individuals with and without diabetes mellitus. In each case, diabetic kidney disease (DKD) serves as the reference group. For example, in the case of glomerulonephritis (GN), the green estimate represents the mean difference in hemoglobin in non-diabetic individuals with GN compared to those with DKD; whereas, the red estimate represents the mean difference in hemoglobin in diabetic individuals with GN compared to those with DKD. DKD, diabetic kidney disease; DM, diabetes mellitus; GN, glomerulonephritis; HTN, hypertension; PKD, polycystic kidney disease.
Other Covariate Associations With Hemoglobin
Cohort-specific and pooled estimates for the independent association between candidate predictor variables and hemoglobin from a multivariable linear regression model are provided in Supplementary Table S9. This analysis included 15 cohorts who had data available for all covariates in the extended model. Overall, each 10-year increase in age was associated with a modest reduction in mean hemoglobin of 0.6 (95% CI 0.27–0.99) g/l. The presence of diabetes mellitus was associated with a 2.5 (95% CI 1.5–3.5) g/l reduction in mean hemoglobin. Compared to individuals with a BMI of 18.5 to 24.9 kg/m2, those with a BMI ≥30 kg/m2 had, on average, a 5.2 (95% CI 3.9–6.6) g/l higher hemoglobin level. Heart failure was associated with a small reduction in hemoglobin (1.4 g/l, 95% CI 0.7–2.1). No statistically significant associations were found between hemoglobin level and coronary artery disease or categories of albuminuria. The proportion of variance (R2) in hemoglobin explained by this set of covariates, applied in the same way in all cohorts, ranged between 6.9% and 44.3%, representing a modest improvement in R2 compared to the simpler core model (Supplementary Table S10).
In an exploratory analysis, we evaluated the relationship between hemoglobin and smoking status (available in 11 cohorts), use of RAAS inhibitors (available in 13 cohorts) and altitude (available in 8 cohorts). These variables were added separately to the previously described extended model. Compared to nonsmokers, current smoking was associated with higher mean hemoglobin (2.1 g/l, 95% CI 1.1–3.1, Supplementary Table S11). Mean hemoglobin was also higher among those living at altitudes higher than 1000 meters compared to those living at sea level (5.4 g/l, 95% CI 0.5–10.4, Supplementary Table S12). The use of RAAS inhibitors was not consistently associated with a difference in hemoglobin (0.07 g/l, 95% CI −1.1 to 1.2, Supplementary Table S13).
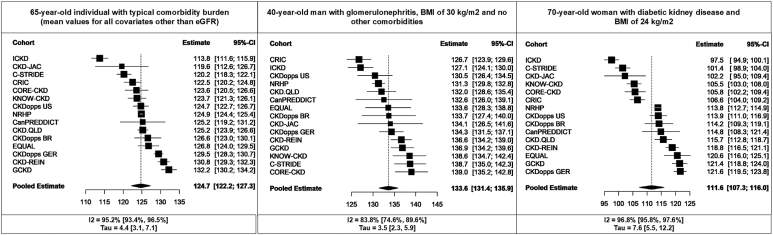
Expected Values of Hemoglobin Based on Specific Patient Characteristics
To illustrate the possible range in hemoglobin values among patients with CKD, even at the same level of eGFR, we used the extended regression model to generate estimates of hemoglobin conditional on specific patient characteristics (Figure 4). For all comparisons, eGFR was fixed at 34 ml/min per 1.73 m2 (overall mean in the study population), and other covariates were held at their mean values unless otherwise specified. The “typical” patient (based on mean values of covariates) across all cohorts was aged 65 years and had an expected hemoglobin value of 124.7 g/l (95% CI 122.2–127.3). A 40-year-old man with glomerulonephritis, a BMI of 30 kg/m2 and without diabetes, had an expected hemoglobin value of 133.6 g/l (95% CI 131.4–135.9), whereas a 70-year-old woman with diabetic kidney disease and a BMI of 24 kg/m2 had an expected hemoglobin value of 111.6 g/l (95% CI 107.3–116). Between-cohort heterogeneity accounted for a large proportion of variability in these estimates. For example, the pooled estimate for the last patient example had a tau of 7.6 g/l and I2 of 96.8%.
Figure 4.
Cohort-specific and pooled estimates for the expected value of hemoglobin in 3 hypothetical patients conditional on specific characteristics generated from the extended multivariable model (n = 15 cohorts). In all cases, eGFR is fixed at 34 ml/min per 1.73 m2. Unless otherwise specified, other covariates are held at their mean values. eGFR, estimated glomerular filtration rate.
Sensitivity Analysis
We repeated the analysis among participants unexposed to ESA therapy at the time of hemoglobin measurement. Estimates for mean differences in hemoglobin according to sex (Supplementary Table S14), etiology of CKD (Supplementary Table S15) and other covariates included in the extended multivariable model (Supplementary Table S16) were similar to those of the primary analysis. A complete case analysis (conducted in 10 cohorts who provided individual-level data) produced similar estimates to those of the primary analysis (Supplementary Table S17).
Discussion
In this study of over 58,000 individuals with CKD from 24 cohorts representing all major world regions, we observed wide variation in hemoglobin distribution internationally, both overall and within categories of eGFR. Women had a lower hemoglobin value, on average, compared to men in virtually all cohorts, and this difference was evident across the full range of eGFR. In multivariable regression models, we identified independent associations between hemoglobin and sex, eGFR, etiology of CKD, age, BMI, and the presence of heart failure and diabetes. Collectively, however, these variables explained only a minority of variance in hemoglobin.
To our knowledge, this is the first study to describe international differences in hemoglobin concentration in individuals with CKD and across the range of kidney function. The positive association between eGFR and hemoglobin has long been recognized.7 The largest and most contemporaneous study to demonstrate this association came from the CKD Prognosis Consortium (CKD-PC) and included 254,666 participants from 17 CKD cohorts.8 Across an eGFR range of 15 to 60 ml/min per 1.73 m2, the relationship between eGFR and hemoglobin was linear with a similar reduction in hemoglobin per unit decrease in eGFR that was observed in the present analysis. Similar to the CKD Prognosis Consortium study, we found minimal association of hemoglobin with participant age or the magnitude of albuminuria, and lower hemoglobin values among participants with diabetes compared to those without diabetes. Our study expands on the CKD Prognosis Consortium analysis by including a geographically more diverse population with CKD, with greater representation of people from African and Asian countries. We have also shown the potential contribution of the etiology of CKD to the level of hemoglobin, with higher values observed in individuals with hypertension, glomerulonephritis, and polycystic kidney disease compared to those with diabetic kidney disease. In their framework for diagnosing and classifying CKD, Kidney Disease Improving Global Outcomes recognizes the importance of ascertaining the etiology of CKD34; however, this is generally not considered in the assessment of different functions of the kidney. Taking these variables together, a younger male patient with glomerulonephritis and a BMI of 30 kg/m2 would be expected to have a hemoglobin of 134 g/l, whereas an older female patient with diabetic kidney disease and a BMI of 24 kg/m2 would be expected to have a hemoglobin of 112 g/l at the same eGFR value of 34 ml/min per 1.73 m2. This clinical example serves to demonstrate the anticipated variability in hemoglobin distribution based on patient-level characteristics outside of eGFR alone.
Average hemoglobin values were particularly low among women in Asian and African cohorts compared to their European counterparts. This finding mirrors the patterns observed in global studies of anemia prevalence.2,3 This regional variation in hemoglobin distribution has not been well described in CKD; and this raises important questions about our understanding of the pathophysiology of anemia in CKD. In the general population, the proportion of prevalent anemia cases attributable to CKD varies significantly by world region, with a much higher attributable fraction observed in high-income Asia Pacific countries compared to South Asian or African countries.35 It was striking that all cohorts had a high proportion of unexplained variance in hemoglobin. Apart from the predictors of hemoglobin evaluated in the present study, there are other contributors to hemoglobin concentration that would be expected to vary by world region independently of CKD status. Anemia in African countries has been linked to a high prevalence of infections such as malaria, soil-transmitted helminthiasis, and schistosomiasis; whereas, genetic traits such as thalassemia and sickle cell disorders play an important role in the development of anemia in Africa and parts of central and South Asia.1,2 The same factors that contribute to variability in hemoglobin distribution internationally could potentially also influence response to anemia therapies, as has been postulated with the use of ESAs.36 This study represents an important first step in refining our understanding of regional determinants of hemoglobin in individuals with CKD, which has been identified as a key research priority by the International Society of Nephrology as part of its Closing the Gaps initiative.37,38
Across virtually all CKD cohorts, women had a lower hemoglobin compared to men. The magnitude of this difference became smaller with declining levels of eGFR, a finding which has also been observed in studies using measured glomerular filtration rate.39 The pooled analysis nonetheless showed that women continued to have a lower average hemoglobin down to an eGFR of 15 ml/min per 1.73 m2. This finding argues against the notion that physiological differences in hemoglobin concentration in men and women should be ignored in the presence of CKD, or that the same target range of hemoglobin should be sought regardless of sex. Previous studies have shown that women receiving dialysis require a higher dose of ESA to achieve the same target hemoglobin as men.40,41 Sex differences have been observed for other biomarkers with important implications for health, such as the threshold used for high sensitivity troponin in the diagnosis of myocardial infarction.42,43 Our understanding of sex differences in the epidemiology of kidney disease has advanced in recent years; however, many of the discrepancies observed between men and women in CKD outcomes remain unexplained.44 The sex-based differences in hemoglobin observed in this study call for research efforts to improve our understanding of the natural history and determinants of hemoglobin trajectory in women with CKD, and should stimulate debate about the uniform interpretation of hemoglobin values in individuals with CKD.
Strengths of this study include a large sample size with broad geographic representation of CKD cohorts, a diverse patient population in terms of demographics, etiology of CKD and range of kidney function, and the use of a standardized data dictionary with harmonization of study variables and methods. The findings should be interpreted in the context of potential limitations. Only a minority of cohorts had robust data for iron parameters, and data were not available for other potentially important variables such as hemoglobinopathies, inflammatory markers, malignancy, nutrition, or socioeconomic status. Similarly, data for smoking status, use of RAAS inhibitors, and altitude were not collected in all cohorts. There was likely misclassification of CKD etiology, which was based on either physician diagnosis or kidney biopsy. Some cohorts had a high proportion of “other” etiologies which could have included both unknown and missing cases. It was not possible to collect information about differences in laboratory measurement of hemoglobin; however, variation in hemoglobin assays is similarly unknown for registry data that contribute to global studies of anemia prevalence. Although the cross-sectional design facilitated inclusion of a larger number of cohorts, the findings are nonetheless based on a single hemoglobin value, and it would be more informative to evaluate differences in the longitudinal trajectory of hemoglobin values. This will be the focus of future work.
In conclusion, this collaborative study from the iNET-CKD cohorts demonstrated considerable variation in hemoglobin distribution among individuals with CKD from different world regions with more pronounced geographic differences in mean hemoglobin among women, mirroring findings from the general population. Our findings challenge the current dogma of interpreting hemoglobin values similarly in all patients with CKD, and call for a renewed interest in broadening our understanding of hemoglobin determinants in specific CKD populations.
Disclosure
Authors have received financial support from Agency Nationale de la Recherche, Akademie Niere, Akebia, Alexion, American Society of Nephrology, Amgen, Astellas, Astra Zeneca, AstraZeneca, Baxter, Baxter Healthcare, Bayer, Bayer AG, Biocon, Boehringer Ingelheim, Boehringer-Lilly, Cadilla, Cara Therapeutics, ChongKumDang, CSL Vifor Pharma, Fresenius Medical Care, GSK, Handok, Health system Research Institute, Thailand, International Society of Nephrology, Janssen, Kyowa Kirin Co Ltd, Lilly France, Merck, Merck Sharp & Dohme-Chibret, National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health, NephroPlus, Novo Nordisk, Otsuka Pharmaceutical, Pharmacosmos, Reata, Research of Korea Centers for Disease Control and Prevention of Republic of Korea, Roche, Sanofi Genzyme, University of British Columbia, Vertex and Vifor France. Authors have participated in data safety monitoring or advisory boards for Australian Kidney Clinical Trials Network, Bayer, Chinook, CSL Behring, Genentech, GSK, International Society of Nephrology Advocacy Group, National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health, Otsuka Pharmaceuticals and Vertex. Authors have participated in leadership and fiduciary roles for American Journal of Kidney Diseases, BC Renal, Chronic Kidney Disease-Mineral Gone Disease Working Group for European Renal Association, European Kidney Function Consortium, German Society of Nephrology, Kidney Improving Global Outcomes Working Group, Nephrology Dialysis Transplantation and University of British Columbia.
MC is supported by a KRESCENT New Investigator Award. Anna Richards is an employee of, and holds stocks and shares in GSK. All other authors declare no competing interests.
Acknowledgments
The authors thank all participants of the iNET-CKD cohort studies without whom this work would not be possible. This work was supported by an unrestricted grant from GSK, in-kind administrative support from the ISN, and in-kind analytical support from BC Renal.
Footnotes
Figure S1. Summary of data collection from participating iNET-CKD cohorts for pooled analysis.
Figure S2. Mean (SD) hemoglobin values in women (red circles) and men (blue circles) within categories of eGFR for each cohort and grouped by continent.
Table S1. Description of iNET-CKD cohorts.
Table S2. Variable definitions.
Table S3. Hemoglobin distribution (mean and SD) in each cohort, overall and within strata of sex and eGFR categories.
Table S4. Cohort-specific levels of iron saturation (mean and SD).
Table S5. Cohort-specific and pooled estimates for the interaction between eGFR and sex. Estimates represent the change in the slope of the relationship between eGFR and hemoglobin.
Table S6. Cohort-specific and pooled estimates for the mean difference in hemoglobin in women compared to men at different levels of eGFR.
Table S7. Cohort-specific and pooled estimates for the mean difference in hemoglobin per 10 ml/min per 1.73m2 increase in eGFR.
Table S8. Cohort-specific and pooled estimates for the mean difference in hemoglobin according to etiology of CKD.
Table S9. Cohort-specific and pooled estimates for the independent association between covariates and hemoglobin from the extended multivariable model.
Table S10. R-squared values for linear regression models applied in participating cohorts.
Table S11. Cohort-specific and pooled estimates for the independent association between smoking status and hemoglobin.
Table S12. Cohort-specific and pooled estimates for the independent association between altitude and hemoglobin.
Table S13. Cohort-specific and pooled estimates for the independent association between use of RAAS inhibitor and hemoglobin.
Table S14. Cohort-specific and pooled estimates for the mean difference in hemoglobin in women compared to men at different levels of eGFR in ESA-unexposed subgroup.
Table S15. Cohort-specific and pooled estimates for the mean difference in hemoglobin according to etiology of CKD in ESA-unexposed subgroup.
Table S16. Cohort-specific and pooled estimates for the independent association between covariates and hemoglobin from the extended multivariable model in ESA-unexposed subgroup.
Table S17. Complete case analysis including 10 cohorts who provided individual-level data.
STROBE Statement.
Contributor Information
Mark Canney, Email: mcanney@toh.ca.
ISN iNET-CKD Investigators:
Curie Ahn, Stefan P. Berger, Fergus J. Caskey, Min Hyun Cho, Heeyeon Cho, Friedo W. Dekker, Vishal Diwan, Christiane Drechsler, Kai-Uwe Eckardt, Marie Evans, Alejandro Ferreiro, Jürgen Floege, Liliana Gadola, Hermann Haller, Kyung Hee Han, Helen G. Healy, Hiddo Lambers Heerspink, Marc Hemmelder, Thomas Hiemstra, Luuk Hilbrands, Seong Heon Kim, Pinkaew Klyprayong, Anna Köttgen, Florian Kronenberg, Veronica Lamadrid, Joo Hoo Lee, Patrick Mark, Matt Matheson, Eun Mi, Kajohnsak Noppakun, Peter Oefner, Thanachai Panaput, Young Seo Park, Hans-Ulrich Prokosch, André Reis, Pablo Rios, Laszlo Rosivall, Joris I. Rotmans, Alfred Sackeyfio, Pornpen Sangthawan, Matthias Schmid, Jae Il Shin, Ricardo Silavarino, Thomas Sitter, Claudia Sommerer, Maciej Szymczak, Claudia Torino, Janos Toth, Frans J. van Ittersum, Sree Krishna Venuthurupalli, Marianne C. Verhaar, Zaimin Wang, Christoph Wanner, Andrzej Wiecek, Gunter Wolf, Dick de Zeeuw, Luxia Zhang, Yuyan Zheng, Ming-Hui Zhao, and Robert Zietse
Appendix
List of Collaborating ISN iNET-CKD Investigators
Curie Ahn, Stefan P. Berger, Fergus J. Caskey, Min Hyun Cho, Heeyeon Cho, Friedo W. Dekker, Vishal Diwan, Christiane Drechsler, Kai-Uwe Eckardt, Marie Evans, Alejandro Ferreiro, Jürgen Floege, Liliana Gadola, Hermann Haller, Kyung Hee Han, Helen G. Healy, Hiddo Lambers Heerspink, Marc Hemmelder, Thomas Hiemstra, Luuk Hilbrands, Seong Heon Kim, Pinkaew Klyprayong, Anna Köttgen, Florian Kronenberg, Veronica Lamadrid, Joo Hoo Lee, Patrick Mark, Matt Matheson, Eun Mi, Kajohnsak Noppakun, Peter Oefner, Thanachai Panaput, Young Seo Park, Hans-Ulrich Prokosch, André Reis, Pablo Rios, Laszlo Rosivall, Joris I. Rotmans, Alfred Sackeyfio, Pornpen Sangthawan, Matthias Schmid, Jae Il Shin, Ricardo Silavarino, Thomas Sitter, Claudia Sommerer, Maciej Szymczak, Claudia Torino, Janos Toth, Frans J. van Ittersum, Sree Krishna Venuthurupalli, Marianne C. Verhaar, Zaimin Wang, Christoph Wanner, Andrzej Wiecek, Gunter Wolf, Dick de Zeeuw, Luxia Zhang, Yuyan Zheng, Ming-Hui Zhao, and Robert Zietse.
Supplementary Material
Figure S1. Summary of data collection from participating iNET-CKD cohorts for pooled analysis.
Figure S2. Mean (SD) hemoglobin values in women (red circles) and men (blue circles) within categories of eGFR for each cohort and grouped by continent.
Table S1. Description of iNET-CKD cohorts.
Table S2. Variable definitions.
Table S3. Hemoglobin distribution (mean and SD) in each cohort, overall and within strata of sex and eGFR categories.
Table S4. Cohort-specific levels of iron saturation (mean and SD).
Table S5. Cohort-specific and pooled estimates for the interaction between eGFR and sex. Estimates represent the change in the slope of the relationship between eGFR and hemoglobin.
Table S6. Cohort-specific and pooled estimates for the mean difference in hemoglobin in women compared to men at different levels of eGFR.
Table S7. Cohort-specific and pooled estimates for the mean difference in hemoglobin per 10 ml/min per 1.73m2 increase in eGFR.
Table S8. Cohort-specific and pooled estimates for the mean difference in hemoglobin according to etiology of CKD.
Table S9. Cohort-specific and pooled estimates for the independent association between covariates and hemoglobin from the extended multivariable model.
Table S10. R-squared values for linear regression models applied in participating cohorts.
Table S11. Cohort-specific and pooled estimates for the independent association between smoking status and hemoglobin.
Table S12. Cohort-specific and pooled estimates for the independent association between altitude and hemoglobin.
Table S13. Cohort-specific and pooled estimates for the independent association between use of RAAS inhibitor and hemoglobin.
Table S14. Cohort-specific and pooled estimates for the mean difference in hemoglobin in women compared to men at different levels of eGFR in ESA-unexposed subgroup.
Table S15. Cohort-specific and pooled estimates for the mean difference in hemoglobin according to etiology of CKD in ESA-unexposed subgroup.
Table S16. Cohort-specific and pooled estimates for the independent association between covariates and hemoglobin from the extended multivariable model in ESA-unexposed subgroup.
Table S17. Complete case analysis including 10 cohorts who provided individual-level data.
STROBE Statement.
References
- 1.Kassebaum N.J., Jasrasaria R., Naghavi M., et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–624. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens G.A., Finucane M.M., De-Regil L.M., et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e16–e25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLean E., Cogswell M., Egli I., Wojdyla D., de Benoist B. Worldwide prevalence of anaemia, WHO vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12:444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 4.KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 5.Pfeffer M.A., Burdmann E.A., Chen C.Y., et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 6.Singh A.K., Szczech L., Tang K.L., et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 7.Astor B.C., Muntner P., Levin A., Eustace J.A., Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2002;162:1401–1408. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- 8.Inker L.A., Grams M.E., Levey A.S., et al. Relationship of estimated GFR and albuminuria to concurrent laboratory abnormalities: an individual participant data meta-analysis in a global consortium. Am J Kidney Dis. 2019;73:206–217. doi: 10.1053/j.ajkd.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckardt K.U., Barthlein B., Baid-Agrawal S., et al. The German Chronic Kidney Disease (GCKD) study: design and methods. Nephrol Dial Transplant. 2012;27:1454–1460. doi: 10.1093/ndt/gfr456. [DOI] [PubMed] [Google Scholar]
- 10.Feldman H.I., Appel L.J., Chertow G.M., et al. The chronic renal insufficiency cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003;14(7 suppl 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 11.Oh K.H., Park S.K., Park H.C., et al. KNOW-CKD (KoreaN cohort study for Outcome in patients with Chronic Kidney Disease): design and methods. BMC Nephrol. 2014;15:80. doi: 10.1186/1471-2369-15-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eder S., Leierer J., Kerschbaum J., et al. A prospective cohort study in patients with type 2 diabetes mellitus for validation of biomarkers (PROVALID)-study design and baseline characteristics. Kidney Blood Press Res. 2018;43:181–190. doi: 10.1159/000487500. [DOI] [PubMed] [Google Scholar]
- 13.Furth S.L., Cole S.R., Moxey-Mims M., et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariani L., Stengel B., Combe C., et al. The CKD outcomes and practice patterns study (CKDopps): rationale and methods. Am J Kidney Dis. 2016;68:402–413. doi: 10.1053/j.ajkd.2016.03.414. [DOI] [PubMed] [Google Scholar]
- 15.Gao B., Zhang L., Wang H., Zhao M. Chinese cohort study of chronic kidney disease: design and methods. Chin Med J (Engl) 2014;127:2180–2185. [PubMed] [Google Scholar]
- 16.Imai E., Matsuo S., Makino H., et al. Chronic Kidney Disease Japan Cohort (CKD-JAC) study: design and methods. Hypertens Res. 2008;31:1101–1107. doi: 10.1291/hypres.31.1101. [DOI] [PubMed] [Google Scholar]
- 17.Jager K.J., Ocak G., Drechsler C., et al. The EQUAL study: a European study in chronic kidney disease stage 4 patients. Nephrol Dial Transplant. 2012;27(suppl 3):iii27–iii31. doi: 10.1093/ndt/gfs277. [DOI] [PubMed] [Google Scholar]
- 18.Kang H.G., Choi H.J., Han K.H., et al. KNOW-Ped CKD (KoreaN cohort study for outcomes in patients with pediatric CKD): design and methods. BMC Nephrol. 2016;17:35. doi: 10.1186/s12882-016-0248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar V., Yadav A.K., Gang S., et al. Indian chronic kidney disease study: design and methods. Nephrol (Carlton) 2017;22:273–278. doi: 10.1111/nep.12789. [DOI] [PubMed] [Google Scholar]
- 20.Levin A., Rigatto C., Brendan B., et al. Cohort profile: Canadian study of prediction of death, dialysis and interim cardiovascular events (CanPREDDICT) BMC Nephrol. 2013;14:121. doi: 10.1186/1471-2369-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navis G.J., Blankestijn P.J., Deegens J., et al. The biobank of Nephrological Diseases in the Netherlands cohort: the String of Pearls Initiative collaboration on chronic kidney disease in the university medical centers in the Netherlands. Nephrol Dial Transplant. 2014;29:1145–1150. doi: 10.1093/ndt/gft307. [DOI] [PubMed] [Google Scholar]
- 22.Osafo C., Raji Y.R., Burke D., et al. Human heredity and health (H3) in Africa kidney disease research network: a focus on methods in sub-Saharan Africa. Clin J Am Soc Nephrol. 2015;10:2279–2287. doi: 10.2215/CJN.11951214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Querfeld U., Anarat A., Bayazit A.K., et al. The cardiovascular comorbidity in Children with Chronic Kidney Disease (4C) study: objectives, design, and methodology. Clin J Am Soc Nephrol. 2010;5:1642–1648. doi: 10.2215/CJN.08791209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaeffner E.S., van der Giet M., Gaedeke J., et al. The Berlin initiative study: the methodology of exploring kidney function in the elderly by combining a longitudinal and cross-sectional approach. Eur J Epidemiol. 2010;25:203–210. doi: 10.1007/s10654-010-9424-x. [DOI] [PubMed] [Google Scholar]
- 25.Schwedt E., Sola L., Rios P.G., Mazzuchi N., National Renal Healthcare Program Improving the management of chronic kidney disease in Uruguay: a National Renal Healthcare Program. Nephron Clin Pract. 2010;114:c47–c59. doi: 10.1159/000245069. [DOI] [PubMed] [Google Scholar]
- 26.Shardlow A., McIntyre N.J., Fluck R.J., et al. Chronic kidney disease in primary care: outcomes after five years in a prospective cohort study. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stengel B., Combe C., Jacquelinet C., et al. The French Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) cohort study. Nephrol Dial Transplant. 2014;29:1500–1507. doi: 10.1093/ndt/gft388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stringer S., Sharma P., Dutton M., et al. The natural history of, and risk factors for, progressive chronic kidney disease (CKD): the Renal Impairment in Secondary care (RIISC) study; rationale and protocol. BMC Nephrol. 2013;14:95. doi: 10.1186/1471-2369-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venuthurupalli S.K., Hoy W.E., Healy H.G., Cameron A., Fassett R.G. CKD.QLD: establishment of a chronic kidney disease [CKD] registry in Queensland, Australia. BMC Nephrol. 2017;18:189. doi: 10.1186/s12882-017-0607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dienemann T., Fujii N., Orlandi P., et al. International Network of Chronic Kidney Disease cohort studies (iNET-CKD): a global network of chronic kidney disease cohorts. BMC Nephrol. 2016;17:121. doi: 10.1186/s12882-016-0335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dheri A.K., Kuenzig M.E., Mack D.R., et al. Meta-analysis of multi-jurisdictional health administrative data from distributed networks approximated individual-level multivariable regression. J Clin Epidemiol. 2022;149:23–35. doi: 10.1016/j.jclinepi.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz G.J., Munoz A., Schneider M.F., et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens P.E., Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Kidney disease: improving global outcomes chronic kidney disease guideline development work group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 35.Safiri S., Kolahi A.A., Noori M., et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: results from the Global Burden of Disease Study 2019. J Hematol Oncol. 2021;14:185. doi: 10.1186/s13045-021-01202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Nicola L., Locatelli F., Conte G., Minutolo R. Responsiveness to erythropoiesis-stimulating agents in chronic kidney disease: does geography matter? Drugs. 2014;74:159–168. doi: 10.1007/s40265-013-0175-3. [DOI] [PubMed] [Google Scholar]
- 37.Levin A., Tonelli M., Bonventre J., et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;390:1888–1917. doi: 10.1016/S0140-6736(17)30788-2. [DOI] [PubMed] [Google Scholar]
- 38.Bello A.K., Alrukhaimi M., Ashuntantang G.E., et al. Complications of chronic kidney disease: current state, knowledge gaps, and strategy for action. Kidney Int Suppl (2011) 2017;7:122–129. doi: 10.1016/j.kisu.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercadal L., Metzger M., Haymann J.P., et al. A 3-marker index improves the identification of iron disorders in CKD anaemia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madore F., Lowrie E.G., Brugnara C., et al. Anemia in hemodialysis patients: variables affecting this outcome predictor. J Am Soc Nephrol. 1997;8:1921–1929. doi: 10.1681/ASN.V8121921. [DOI] [PubMed] [Google Scholar]
- 41.Ifudu O., Uribarri J., Rajwani I., et al. Gender modulates responsiveness to recombinant erythropoietin. Am J Kidney Dis. 2001;38:518–522. doi: 10.1053/ajkd.2001.26842. [DOI] [PubMed] [Google Scholar]
- 42.Shah A.S., Griffiths M., Lee K.K., et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ. 2015;350:g7873. doi: 10.1136/bmj.g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 44.Carrero J.J., Hecking M., Chesnaye N.C., Jager K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14:151–164. doi: 10.1038/nrneph.2017.181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.