Abstract
Introduction
Focal segmental glomerulosclerosis (FSGS) is a rare glomerular disease with high unmet clinical need. Interest in proteinuria as a surrogate end point for regulatory approval of novel treatments has increased. We assessed the relationship between achieving complete remission (CR) of proteinuria at least once during follow-up and long-term kidney outcomes.
Methods
This post hoc analysis included all patients enrolled in the DUET trial of sparsentan in FSGS and the open-label extension (OLE). Evaluations occurred every 12 weeks, including blood pressure (BP), edema, proteinuria, and kidney function. CR was defined as a urine protein/creatinine ratio ≤0.3g/g in a first morning urine sample.
Results
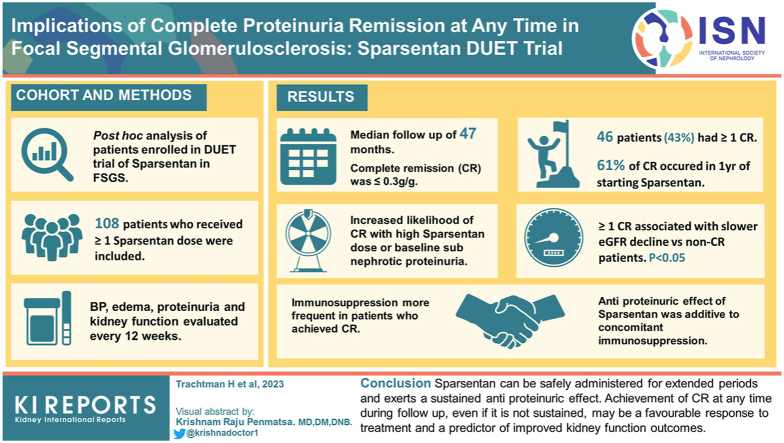
A total of 108 patients who received ≥1 sparsentan dose were included in this study. During a median follow-up of 47.0 months, 46 patients (43%) experienced ≥1 CR, 61% occurring within 12 months of starting sparsentan. There was an increased likelihood of CR with a higher sparsentan dose or baseline subnephrotic-range proteinuria. Achieving ≥1 CR was associated with significantly slower rate of estimated glomerular filtration rate (eGFR) decline versus non-CR patients (P < 0.05). Use of immunosuppressive agents was more frequent in patients who achieved a CR. However, the antiproteinuric effect of sparsentan was additive to that achieved with concomitant immunosuppressive treatment. No unanticipated adverse events occurred.
Conclusion
We conclude that sparsentan can be safely administered for extended periods and exerts a sustained antiproteinuric effect. Achievement of CR at any time during follow-up, even if it is not sustained, may be an indicator of a favorable response to treatment and a predictor of improved kidney function outcomes.
Keywords: complete remission, estimated glomerular filtration rate, focal segmental glomerulosclerosis, open-label extension, proteinuria, surrogate endpoint
Graphical abstract
FSGS results from initial injury to the podocytes and presents with a variable degree of proteinuria and often concomitant nephrotic syndrome.1 Although FSGS is a rare disease, ≥50% of patients with nephrotic-range proteinuria who are resistant to treatment will require kidney replacement therapy (KRT) within 5 to 10 years of diagnosis.2 FSGS accounts for 5% of adult and 12% of pediatric cases of end-stage kidney disease (ESKD).3,4 Moreover, the disease recurs in approximately 20% to 25% of pediatric patients who receive a kidney transplant.5,6
FSGS can be categorized as primary, genetic, or secondary.1,7, 8, 9, 10, 11 Primary FSGS has no definitive cause,1 but it is presumed to be related to circulating factor(s) that result in podocyte injury.8,9 FSGS of undetermined cause has been added as a subtype for patients who do not fit in the other 3 categories.7
Renin-angiotensin-aldosterone system inhibitors are prescribed routinely in all patients with FSGS and proteinuria. Patients who have nephrotic syndrome are generally given a trial of corticosteroids. Those with persistent proteinuria are then offered second-line immunosuppressive therapies (ISTs), such as calcineurin inhibitors, antimetabolites, and B-cell–directed intervention.12,13 These agents have limited efficacy and significant toxicity. There are no US Food and Drug Administration-approved therapies for FSGS. Assessment of clinical efficacy of novel treatments for FSGS is based on an improvement in hard clinical outcomes, namely, doubling of serum creatinine or progression to ESKD (i.e., eGFR <15 ml/min per 1.73 m2 or initiation of KRT). In rare glomerular diseases, these events occur in a limited number of patients during a clinical trial of feasible duration, and this impedes novel treatment trials.
To facilitate the performance of clinical trials for FSGS, there has been increased focus on the use of proteinuria as a surrogate end point to support regulatory approvals of novel therapies. Adult and pediatric patients with FSGS who achieve a sustained CR of proteinuria (i.e., normalization) have a favorable long-term prognosis.14, 15, 16, 17 In contrast, those who have no response have a high likelihood of progression to ESKD.14,16 The impact of a CR that is not sustained on clinical outcomes has not been as well investigated.
Sparsentan is a novel, selective, single-molecule dual endothelin angiotensin receptor antagonist that blocks the endothelin type A receptor and the angiotensin II subtype 1 receptor. In DUET, a phase 2 active-controlled, randomized clinical trial, administration of sparsentan for 8 weeks achieved greater reduction in proteinuria versus the active-control irbesartan.18 Patients who completed the double-blind phase (DB) were offered participation and treatment with sparsentan in an OLE, which is ongoing. During the OLE, a subset of patients achieved a sustained reduction in proteinuria compared to baseline while taking sparsentan.19, 20, 21, 22, 23 However, most patients had fluctuating levels of proteinuria with some who intermittently achieved CR of proteinuria. The clinical ramifications of this pattern of response are unclear. Therefore, the objectives of this post hoc analysis of the DUET study were to determine the following: (i) the proportion and characteristics of patients who achieved ≥1 CR at any time during the DUET study versus those who did not (non-CR) and the factors associated with CR and (ii) the relationship between achieving CR and the long-term trajectory of kidney function.
Methods
Study Design and Treatment
The phase 2 randomized, DB, active-control, dose-escalation DUET study (NCT01613118; registered June 6, 2012) enrolled patients in the United States and Europe following institutional review board or ethics committee approvals and in accordance with the Declaration of Helsinki. A complete description of the DUET study design has been published.18,24 In brief, enrolled patients had biopsy-proven FSGS, or documentation of a pathogenic genetic mutation associated with the lesion. Patients with a known secondary cause of FSGS were excluded. Eligibility criteria were as follows: (i) age 8 to 75 years old in the United States and 18 to 75 years old in Europe, (ii) screening urine protein-to-creatinine ratio (UP/C) ≥1.0 g/g, and (iii) eGFR >30 ml/min per 1.73 m2. At baseline, chronic IST, except cyclophosphamide and rituximab, was permitted if dosing was stable for 1 month before randomization.
For the DB, patients were initially assigned to a dose cohort and, within each cohort, randomized to receive either sparsentan (200, 400, or 800 mg/d) or the active control (irbesartan 300 mg/d) for 8 weeks. Patients who completed the 8-week DB were invited to enter the OLE and receive sparsentan. During the OLE, patients randomized to sparsentan continued with the same dose. Patients randomized to irbesartan received sparsentan at the dose assigned to the corresponding randomized cohort. Dose increases for efficacy and reductions for safety or tolerability were allowed and initiation or dose changes of IST were permitted during the OLE.
Study End Points
The post hoc analysis end points included the proportion of patients who achieved CR, defined as UP/C ≤0.3 g/g at any visit, CR duration, and factors associated with achieving CR. Occurrence of CR was recorded from study day 1 in patients randomized to sparsentan during the DB. In patients randomized to irbesartan, baseline for the current analysis was defined as day 56, when they transitioned to sparsentan at the start of the OLE. CR duration was determined as the time interval between the first CR visit and the last of consecutive visits with UP/C <0.6 g/g in conjunction with eGFR >15 ml/min per 1.73 m2. Following a UP/C ≤0.3 g/g, the upper limit of <0.6 g/g was used for sustained CR to allow for measurement variability in single sample UP/C. Patients were considered to have KRT if the study discontinuation reason included the terms “dialysis” or “transplant.” Patients were considered to have ESKD if the study discontinuation reason included the term “end-stage kidney disease” or consecutive eGFR values, at least 14 days apart, were <15 ml/min per 1.73 m2. Patients were considered to have a 40% reduction in eGFR if the lower level was confirmed in repeat testing ≥28 days apart.
Assessments and Procedures
Baseline characteristics were assessed in the DB, including patient age, self-reported selection of categories of race (Asian, Black, or African American, White, and Other for patient reports outside these categories), ethnicity (Hispanic/Latino and non-Hispanic/non-Latino), sex, and baseline values of BP, eGFR, and proteinuria. Patients were assessed at weeks 16 and 24 and at every 12 weeks thereafter during the OLE. The OLE evaluations included an interval history and focused physical examination with assessment of BP, edema, eGFR, and proteinuria.18,24 Renal-related IST use, including initiation and timing in relationship to CR status during the follow-up period, was adjudicated by review of individual patient documentation by 2 investigators (RK and HT).
Statistical Analyses
We present the clinical and laboratory data for all patients while they were receiving sparsentan regardless of original randomization in DUET. Data were analyzed from the first sparsentan dose in the DB or OLE through February 5, 2021. eGFR slope was calculated for the entire period of sparsentan treatment. Detailed description of the statistical methods is provided in the Supplementary Methods. Safety was examined by the overall incidence (i.e., frequency during the total study duration) of treatment-emergent adverse events (TEAEs), treatment-related TEAEs (confirmed by the site investigator), and serious adverse events.
Results
Patient Disposition
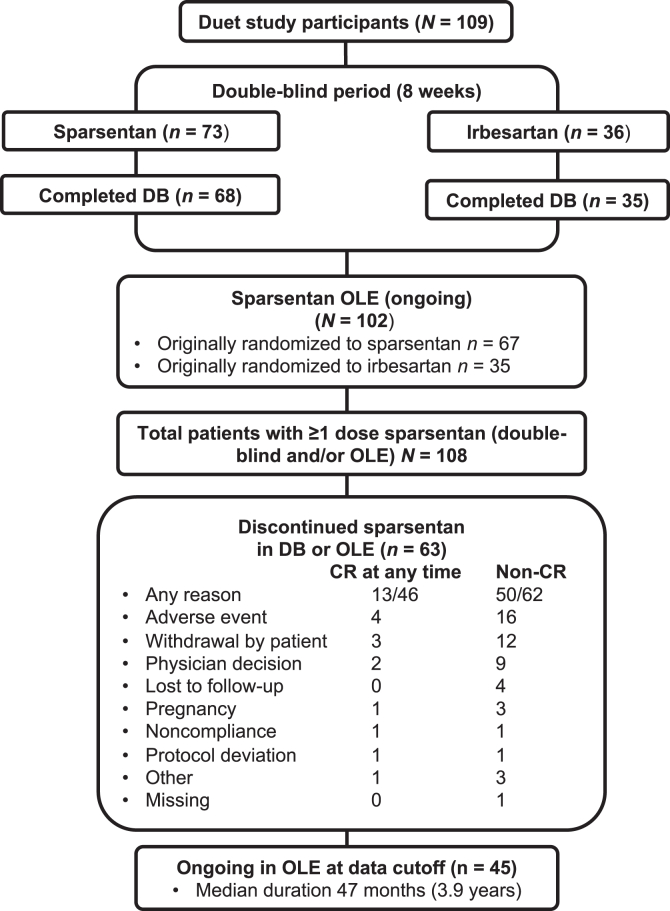
The DUET study enrolled 109 patients between April 2014 and April 2016, including 23 aged 8 to 18 years.18 This post hoc analysis included 108 patients who received ≥1 sparsentan dose in the DB and/or OLE. The median duration of sparsentan treatment at the OLE data cutoff was 3.9 years. Of the 73 patients randomized to sparsentan, 67 continued sparsentan in the OLE, whereas 35 of 36 patients randomized to irbesartan transitioned to sparsentan (Figure 1). At the OLE data cutoff, 45 patients remained on sparsentan treatment. During the OLE, no patients were treated with sodium-glucose cotransporter-2 inhibitors, other renin-angiotensin-aldosterone system inhibitor drugs, or endothelin receptor antagonists.
Figure 1.
This figure illustrates the patient disposition during the course of the DB and OLE of DUET. Duration of treatment is defined as the time between the date of first sparsentan dose and either data cutoff date (ongoing patients) or date of last dose (discontinued patients). DB, double-blind; OLE, open-label extension.
Proportion of Patients Achieving CR and Their Characteristics
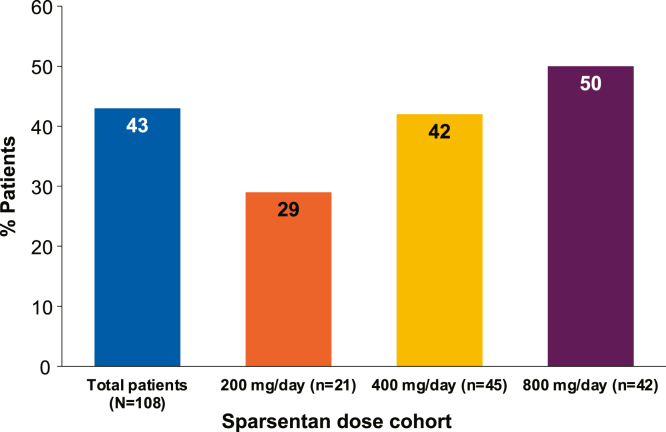
During the DB, 9 patients (8%) experienced CR while receiving sparsentan compared to 0 patients (0%) receiving irbesartan based on UP/C ≤0.3 g/g in a first morning urine sample. All other CRs occurred during the OLE. Forty-six patients (43%) experienced ≥1 CR (Figure 2). CR at ≥2 study visits occurred in 33 patients (31%) and at ≥3 study visits in 28 patients (26%; Supplementary Figure S1). In the CR group, 43% of patients achieved onset of CR within 6 months of starting sparsentan, and 61% of patients achieved onset of CR within 12 months of starting sparsentan. The median (interquartile range) cumulative duration of periods with a confirmed CR was 30.4 (11.2–54.8) months. Episodes of CR were also documented in patients with nephrotic-range proteinuria (≥3.5 g/g) at baseline (Supplementary Figure S1). Patients who were assigned to treatment with higher sparsentan doses (800 and 400 mg/d) tended to achieve CR more frequently than those assigned to the lower dose of 200 mg/d (Figure 2).
Figure 2.
This figure illustrates the percentage of patients who achieved ≥1 CR at any time in each sparsentan dose cohort that they were assigned to during the OLE. The percentage of patients shown is out of 100% at each dose level. The remaining percentage at each dose level represents the non-CR patients. The CR and non-CR groups did not differ in mean (454.1 vs. 406.8 mg/d, respectively) or median (399.8 vs. 399.9 mg/d, respectively) sparsentan dose. In the absence of randomization, descriptive analysis with no formal statistical testing was performed. CR, complete remission; OLE, open-label extension.
At baseline, CR and non-CR patients had comparable age, sex and race distribution, and BP (Table 1). There was a trend toward lower eGFR in non-CR patients. The proportion of patients with a history and/or current diagnosis of nephrotic syndrome was similar in the 2 groups. However, patients with ≥1 CR displayed significantly lower baseline proteinuria and a higher proportion were receiving IST.
Table 1.
Baseline demographics and clinical characteristics of CR and non-CR patients
| Characteristic | CR patients (any UP/C ≤ 0.3 g/g) (n = 46) |
Non-CR patients (no UP/C ≤ 0.3 g/g) (n = 62) |
P value |
|---|---|---|---|
| Age (yrs) | 0.292 | ||
| Mean (SD) | 38.8 (17.5) | 35.4 (15.8) | |
| Median (min, max) | 39.5 (8, 71) | 36.0 (8, 67) | |
| Age <18 yrs, n (%) | 8 (17.4) | 10 (16.1) | 0.862 |
| Sex, n (%) | 0.338 | ||
| Female | 18 (39.1) | 30 (48.4) | |
| Male | 28 (60.9) | 32 (51.6) | |
| Hispanic/Latino ethnicity, n (%) | 7 (15.2) | 12 (19.4) | 0.577 |
| Race, n (%) | 0.954 | ||
| Asian | 2 (4.3) | 4 (6.5) | |
| Black or African American | 7 (15.2) | 8 (12.9) | |
| White | 35 (76.1) | 47 (75.8) | |
| Othera | 2 (4.3) | 3 (4.8) | |
| Systolic BP, mean (SD)b | 129.6 (12.9) | 128.5 (12.0) | 0.637 |
| Diastolic BP, mean (SD)b | 80.5 (9.0) | 82.5 (8.7) | 0.249 |
| eGFR (ml/min per 1.73 m2) | 0.184 | ||
| Mean (SD) | 80.4 (38.6) | 70.0 (40.6) | |
| Median (min, max) | 72.9 (30, 189) | 55.6 (28, 212) | |
| Documented nephrotic syndrome, n (%) | 9 (19.6) | 14 (22.6) | 0.705 |
| UP/C (g/g)c | <0.001 | ||
| Mean (SD) | 2.5 (2.0) | 4.7 (3.4) | |
| Median (min, max) | 2.0 (0.3, 10.3) | 3.7 (0.8, 14.0) | |
| Geometric mean | 2.0 | 3.7 | |
| Any renal-related IST, n (%) | 20 (43.5) | 15 (24.2) | 0.034 |
| Steroids | 8 (17.4) | 9 (14.5) | |
| CNI | 11 (23.9) | 8 (12.9) | |
| MMF | 8 (17.4) | 5 (8.1) |
BP, blood pressure; CNI, calcineurin inhibitor; IST, immunosuppressive treatment; MMF, mycophenolate mofetil; UP/C, urinary protein-to-creatinine ratio.
Other race includes patient responses of multiracial, Hispanic only, Egyptian, and unknown.
Prior to first sparsentan dose.
n = 45 in any UP/C ≤ 0.3 g/g group.
Proteinuria
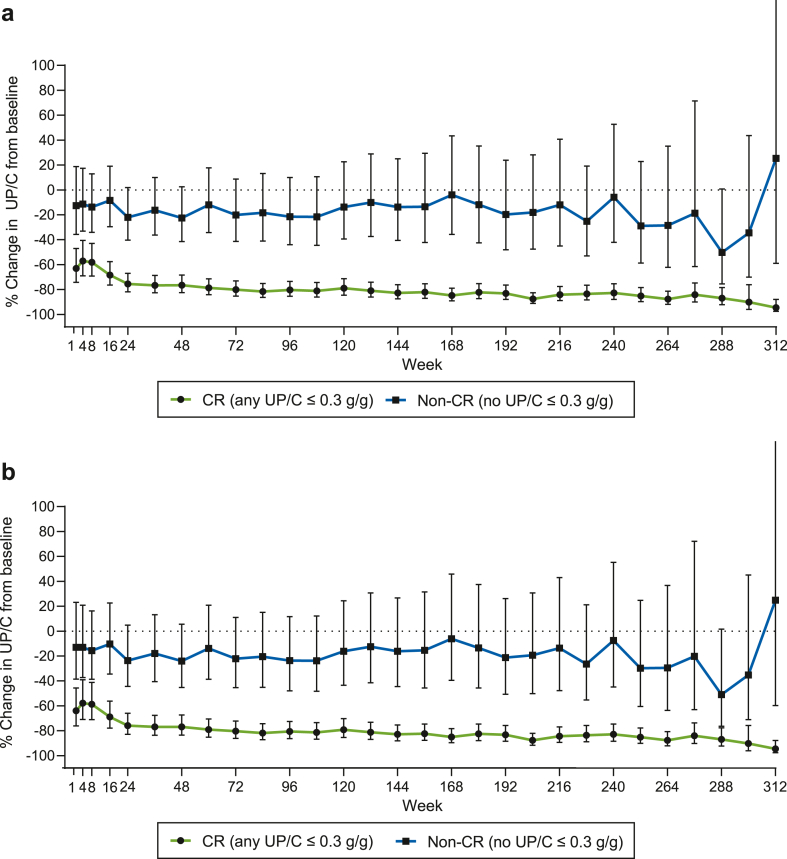
The long-term course of proteinuria during the OLE demonstrated a substantial and sustained reduction reaching approximately 80% in CR patients versus approximately 20% reduction in non-CR patients (Figure 3a). This difference persisted after adjustment for age, sex, race, ethnicity, nephrotic syndrome, baseline UP/C, and baseline eGFR (Figure 3b). Sustained decrease in proteinuria was also observed in non-CR patients, though it was not statistically significant (Figure 3). The quantitative changes in proteinuria without log transformation are provided in Supplementary Table S1.
Figure 3.
This figure illustrates the percentage change in UP/C from baseline by (a) CR vs. non-CR and (b) CR vs. non-CR adjusted for age, sex, race, nephrotic syndrome, baseline UP/C, and baseline eGFR. Percent change (95% CI) of UP/C from baseline by CR versus non-CR groups was analyzed using MMRM. P ≤ 0.003 on each visit. CR, complete remission; eGFR, estimated glomerular filtration rate; MMRM, mixed model repeated measures; UP/C, urinary protein-to-creatinine ratio; CI, confidence interval.
eGFR
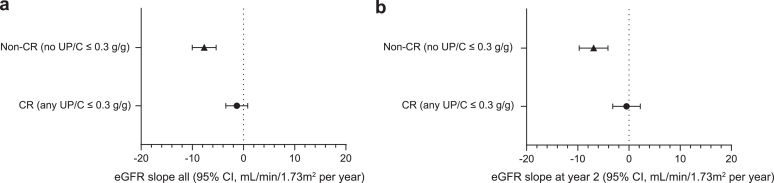
To test whether achieving ≥1 CR at any time had an impact on kidney function, we determined chronic eGFR slopes over the entire follow-up period in CR versus non-CR patients. Achieving ≥1 CR was associated with a significantly slower annual rate of decline in eGFR over the entire treatment period compared to non-CR patients (slope estimates −1.31 vs. −7.68, respectively; P < 0.001; Figure 4a). A substantial difference in chronic eGFR slopes between CR and non-CR patients was also observed during the first 2 years of the follow-up period (slope estimates −0.47 vs. −6.90, respectively; P = 0.002), the projected length of most clinical trials (Figure 4b). We determined the number of CR and non-CR patients who reached a clinically relevant end point, namely, KRT, ESKD, or 40% decrease in eGFR (Supplementary Table S2). Although the number of patients in each clinical end point category is small, there are consistently fewer patients in the CR versus non-CR group.
Figure 4.
This figure illustrates the chronic eGFR slope in CR versus non-CR patients for (a) the entire time period on sparsentan treatment and (b) at year 2 of sparsentan treatment. Chronic slope was analyzed using a random coefficients model with linear spline at 42 days. Slope estimates within the non-CR group: P < 0.001 (a and b). Slope estimates within the CR group: (a) P = 0.226 and (b) P = 0.726. CR, complete remission; eGFR, estimated glomerular filtration rate.
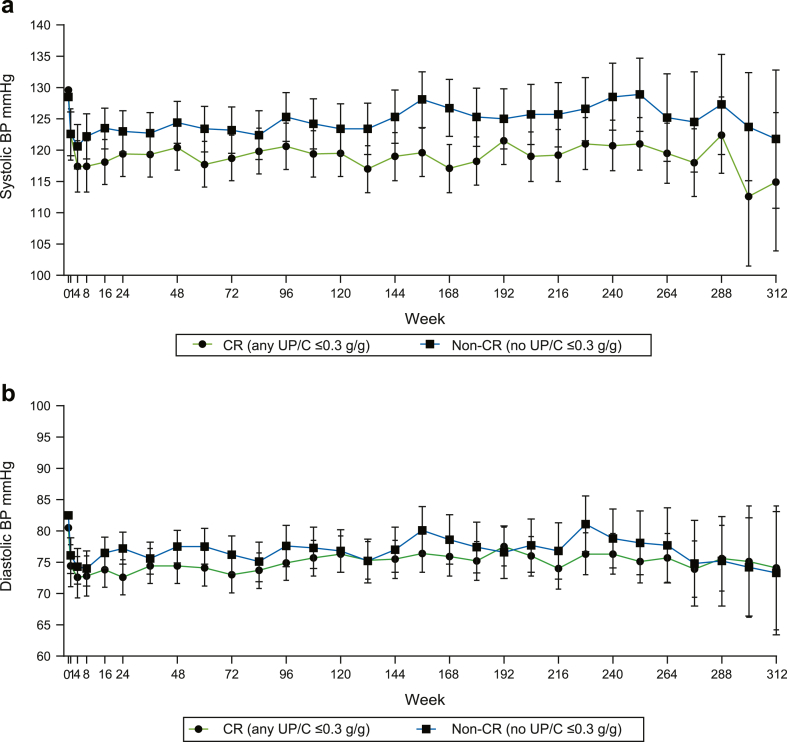
The average BP was determined across all visits for each patient. Overall, the mean systolic BP was 4.7 mm Hg lower in CR versus non-CR patients (P = 0.006; Table 2) and lower mean systolic BP levels were observed throughout the follow-up period (Figure 5a). There was no significant difference in overall mean diastolic BP between the CR and non-CR patients (P = 0.064; Table 2). Mean diastolic BP was similar in both groups at most study visits during the OLE (Figure 5b).
Table 2.
Average (95% CI) BP across all visits by CR group
| Characteristic | CR patients (any UP/C ≤ 0.3 g/g) | Non-CR patients (no UP/C ≤ 0.3 g/g) | Difference | P value |
|---|---|---|---|---|
| Systolic BP | 119.2 (116.8, 121.7) | 123.9 (121.6, 126.1) | −4.7 (−7.9, −1.4) | 0.006 |
| Diastolic BP | 74.4 (72.4, 76.4) | 76.9 (75.1, 78.7) | −2.5 (−5.2, 0.1) | 0.064 |
BP, blood pressure; CI, confidence interval; CR, complete remission; UP/C, urinary protein-to-creatinine ratio.
Figure 5.
This graph illustrates differences in BP between CR and non-CR patients in (a) systolic BP and (b) diastolic BP. Observed values are presented for baseline (week 0). All post-baseline visit BP values were derived as LS Mean (95% CI) at each visit from MMRM analysis. BP, blood pressure; CR, complete remission; MMRM, mixed model repeated measures; CI, confidence interval.
Renal-Related IST
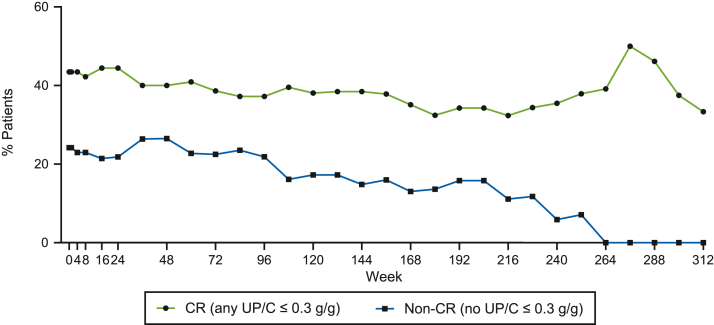
The therapeutic response to sparsentan may have been confounded by newly initiated, sustained, or modified dosing of IST. In fact, 9 patients achieved a CR following the initiation or intensification of IST. A lower proportion of non-CR patients were prescribed IST versus CR patients (Figure 6). Specifically, the proportions of patients receiving steroids, calcineurin inhibitors, or antimetabolites (mycophenolate mofetil, azathioprine) were higher in CR versus non-CR patients (Supplementary Figure S2).
Figure 6.
This graph illustrates the percentage of patients receiving renal-related IST by visit in CR and non-CR patients. CR, complete remission; IST, immunosuppressive treatment.
To assess the impact of IST in greater detail, we stratified CR and non-CR patients based on concomitant IST. We compared the baseline characteristics of CR patients with or without IST and non-CR with or without IST groups (Table 3). We also assessed the course of proteinuria and eGFR over the follow-up period in these subgroups. Similar to the overall cohort, age, sex, and race were not statistically different between the patient subgroups. CR patients with and without IST had lower baseline UP/C compared to non-CR patients. There was a trend toward a lower baseline eGFR in the non-CR/no IST patients.
Table 3.
Baseline characteristics of patients by CR group and renal-related IST presence or absence
| Characteristic | CR/IST | CR/no IST | Non-CR/IST | Non-CR/no IST | P value |
|---|---|---|---|---|---|
| Age (yrs) | 0.260 | ||||
| N | 21 | 25 | 21 | 41 | |
| Mean (SD) | 36.4 (18.6) | 40.8 (16.6) | 31.1 (14.5) | 37.6 (16.1) | |
| Median (min, max) | 39.0 (8, 71) | 44.0 (11, 70) | 28.0 (11, 54) | 40.0 (8, 67) | |
| Age <18 yrs, n (%) | 5 (23.8) | 3 (12.0) | 4 (19.0) | 6 (14.6) | 0.720 |
| Sex, n (%) | 0.387 | ||||
| Female | 10 (47.6) | 8 (32.0) | 12 (57.1) | 18 (43.9) | |
| Male | 11 (52.4) | 17 (68.0) | 9 (42.9) | 23 (56.1) | |
| Ethnicity, n (%) | 0.565 | ||||
| Hispanic/Latino | 3 (14.3) | 4 (16.0) | 2 (9.5) | 10 (24.4) | |
| Not Hispanic/Latino | 18 (85.7) | 21 (84.0) | 19 (90.5) | 31 (75.6) | |
| Race, n (%) | 0.795 | ||||
| Asian | 0 | 2 (8.0) | 1 (4.8) | 3 (7.3) | |
| Black or African American | 3 (14.3) | 4 (16.0) | 1 (4.8) | 7 (17.1) | |
| White | 17 (81.0) | 18 (72.0) | 19 (90.5) | 28 (68.3) | |
| Othera | 1 (4.8) | 1 (4.0) | 0 | 3 (7.3) | |
| eGFR (ml/min per 1.73 m2) | 0.338 | ||||
| Mean (SD) | 74.9 (35.7) | 85.0 (41.1) | 76.7 (51.5) | 66.6 (33.9) | |
| Median (min, max) | 69.8 (30, 189) | 75.0 (39, 172) | 52.8 (28, 212) | 57.5 (28, 199) | |
| UP/C (g/g) | <0.001 | ||||
| N | 21 | 24 | 21 | 41 | |
| Mean (SD) | 2.8 (2.4) | 2.3 (1.5) | 5.5 (3.9) | 4.3 (3.1) | |
| Median | 2.3 | 1.8 | 4.3 | 3.2 | |
| Geometric mean | 2.0 | 1.9 | 4.5 | 3.4 | <0.001 |
| Min, Max | 0.3, 10.3 | 0.8, 7.0 | 1.6, 14.0 | 0.8, 12.0 | |
| Documented Nephrotic Syndrome, n (%)b | 5 (23.8) | 4 (16.0) | 7 (33.3) | 7 (17.1) | 0.421 |
CR, complete remission; eGFR, estimated glomerular filtration rate; IST, immunosuppressive treatment; UP/C, urinary protein-to-creatinine ratio.
Categorical variables were analyzed using chi-square test or Fisher exact test when 25% of the cells have expected counts <5. Continuous variables were analyzed using ANOVA.
Other race includes patient responses of multiracial, Hispanic only, Egyptian, and unknown.
Includes documented history of nephrotic syndrome or nephrotic syndrome at baseline.
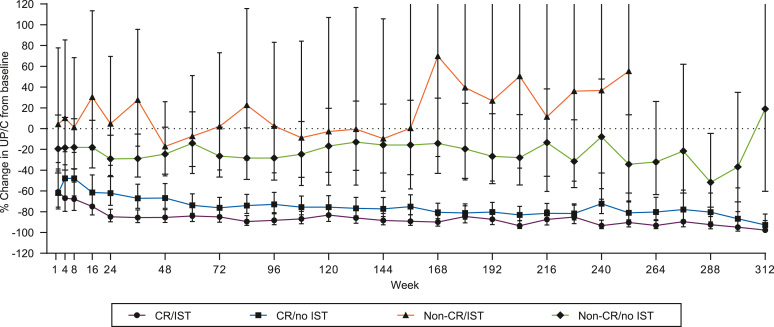
The greatest UP/C reduction was observed in CR/IST patients (Figure 7). However, a substantial and sustained reduction of UP/C was also observed in CR/no IST patients. A sustained decrease in proteinuria was also observed in non-CR patients, albeit to a lesser degree than in CR patients. Although there was some overlap in the level of proteinuria reduction in the CR and non-CR subgroups, the difference between these groups was sustained and consistently lower in CR patients throughout the OLE. In the non-CR patients, concomitant IST had no effect on proteinuria, which was comparable to patients with no IST after adjustment for IST use in the time interval between study visits.
Figure 7.
This graph illustrates the percentage change in UP/C from baseline by CR group and presence or absence of concomitant renal-related IST. Percent change of UP/C from baseline by CR versus non-CR groups and based on presence or absence of concomitant renal-related IST was analyzed using MMRM. P ≤ 0.006 for group comparisons by timepoint. CR, complete remission; IST, immunosuppressive treatment; MMRM, mixed model repeated measures; UP/C, urinary protein/creatinine ratio.
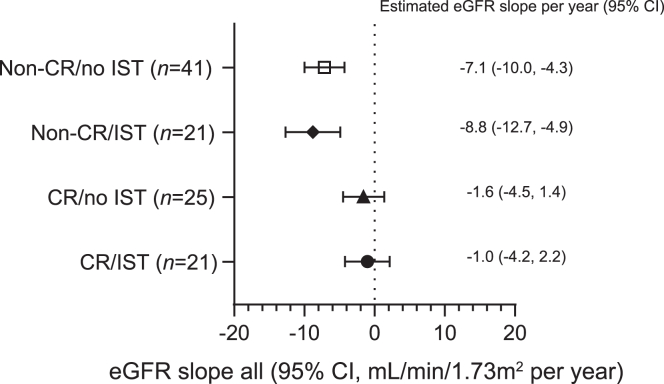
Chronic slopes of eGFR in the 4 patient subgroups corresponded to the degree of proteinuria reduction over time with the slowest decline in CR/IST patients (Figure 8). A significant attenuation of the decline in eGFR was also observed in CR/no-IST patients versus non-CR patients, again, in a model that was adjusted for administration of IST at any time.
Figure 8.
This figure illustrates chronic eGFR slope for the entire time period on sparsentan treatment by CR group and use of renal-related IST. Chronic slope was analyzed using a random coefficients model with linear spline at 42 days. CR, complete remission; eGFR, estimated glomerular filtration rate; IST, immunosuppressive treatment.
Safety
During the OLE, CR patients may have been exposed to more intensive sparsentan treatment because a higher proportion were in the 800 mg/d cohort and received concomitant IST. In addition, the number of patients who discontinued sparsentan overall or for any specific reason except nonadherence or protocol deviation was consistently lower in the CR versus non-CR patients (Figure 1). Specifically, discontinuation of sparsentan was noted in 81% of patients who never achieved a CR any time (50/62) versus 28% (13/46) who had CR. Therefore, further analyses assessed whether achieving CR was associated with a worse safety profile. Comparison of TEAEs (Supplementary Table S3) demonstrated similar overall incidence over the total study duration in CR and non-CR patients (93% vs. 95% of patients). The most common TEAEs with overall incidence ≥20% in either group were headache, edema peripheral, hyperkalemia, hypotension, nausea, diarrhea, and proteinuria, which were numerically more frequent in CR patients. The overall incidence of TEAEs indicating acute kidney injury tended to be more frequent in non-CR patients, and the overall incidence of fluid retention-associated TEAEs was comparable between CR (30%) and non-CR (29%) patients (Supplementary Table S3). No episodes of heart failure were reported during the OLE. Similarly, overall incidence of treatment-related TEAEs was not different between CR and non-CR patients (65% vs. 66%), with higher overall incidence of hyperkalemia and hypotension in the CR group (Table 4). Serious adverse events were more numerically frequent in CR than non-CR patients, driven mainly by chest pain, pneumonia, atrial fibrillation, and COVID-19 infection (4%–9% of patients) that were not, however, treatment-related (Supplementary Table S4).
Table 4.
Treatment-related TEAEs in >5% of patients in either CR or non-CR group
| TEAEs, n (%) | CR (any UP/C ≤ 0.3 g/g) (n = 46) |
Non-CR (no UP/C ≤ 0.3 g/g) (n = 62) |
|---|---|---|
| Any treatment-related TEAE | 30 (65) | 41 (66) |
| Treatment-related TEAEs in >5% of patients | ||
| Hyperkalemia | 12 (26) | 7 (11) |
| Hypotension | 10 (22) | 9 (15) |
| Blood creatinine increased | 5 (11) | 3 (5) |
| Dizziness | 4 (9) | 9 (15) |
| Headache | 4 (9) | 7 (11) |
| Nausea | 4 (9) | 6 (10) |
| Blood creatine phosphokinase increased | 4 (9) | 2 (3) |
| Anemia | 3 (7) | 4 (6) |
| Edema peripheral | 2 (4) | 5 (8) |
| Glomerular filtration rate decreased | 1 (2) | 7 (11) |
| Vomiting | 0 | 7 (11) |
| Acute kidney injury | 0 | 5 (8) |
CR, complete remission; TEAE, treatment-emergent adverse event.
TEAEs indicating acute kidney injury include the preferred terms blood creatinine increases, acute kidney injury, and glomerular filtration rate decreased. TEAEs indicating fluid retention include the preferred terms edema peripheral, edema fluid overload, joint effusion, joint swelling, and pleural effusion.
Discussion
In this report, we demonstrate that over a median sparsentan treatment period of nearly 4 years in the DUET trial OLE, 43% of patients achieved ≥1 CR, the majority of which occurred within 12 months of starting sparsentan. These patients had significantly lower proteinuria overall compared to non-CR patients. CR in response to sparsentan was not limited to patients with subnephrotic-range proteinuria but also occurred in patients with nephrotic-range proteinuria at baseline, including those with nephrotic syndrome. Second, the degree of proteinuria reduction in CR versus non-CR patients was paralleled by a slower rate of decline in kidney function, that is, the chronic slope of eGFR over the entire sparsentan treatment period. This was paralleled by a trend toward a lower incidence of hard renal end points in the CR versus non-CR patients. Although previous work has documented the beneficial effect of achieving a sustained CR in patients with FSGS, our study is the first to describe improved outcomes regarding preservation of kidney function in patients who experience ≥1 CR, even if transient, during follow-up. Our findings suggest that in addition to the percentage reduction in proteinuria, the nadir level achieved in response to treatment may also have implications for the trajectory of kidney function in patients with FSGS. Third, ISTs were administered in a higher proportion of patients with CR, and the lowest, near-normal levels of proteinuria throughout follow-up were observed in CR patients who received IST. However, a substantial and sustained proteinuria reduction was observed in CR patients with no IST, indicating that in some patients, sparsentan monotherapy can normalize proteinuria.
In accord with reports that IST may lower proteinuria,25,26 our data suggest that ISTs increase the likelihood of achieving long-term or short-term CR in a subset of patients with FSGS. In the DUET OLE, patients who received IST may have been deemed to have a better prognosis, based on the level of proteinuria, initial eGFR, or histopathology findings. Regardless of IST considerations, sparsentan had an additive antiproteinuric effect in patients who are treated with IST. The benefits of both interventions were greater in patients who achieved ≥1 CR at any time.
The antiproteinuric effect of sparsentan likely reflects its dual actions on the endothelin and angiotensin II signaling pathways.26,27 BP lowering may have contributed to the likelihood of achieving CR or slowing the rate of eGFR decline. However, the difference in systolic BP between CR and non-CR patients was established within 2 to 4 weeks after treatment initiation, was modest in degree, and remained stable throughout the follow-up period. It is unlikely to be a major factor in achieving the sustained reduction of proteinuria or even CR during the OLE.
There is increasing attention given to using proteinuria as a surrogate end point in clinical trials for glomerular diseases. No single criterion or set of criteria for changes in proteinuria has been defined for this purpose. The US Food and Drug Administration has called on the nephrology community to better define the quantitative relationship between reductions in proteinuria and kidney function outcomes for each specific glomerular disease. Progress has been made in membranous nephropathy and IgA nephropathy.28, 29, 30 However, gaps in knowledge persist for FSGS. The current data from the DUET OLE suggest that achieving CR can be evaluated as a continuous variable (e.g., duration of time with CR), as well as a categorical variable (e.g., “yes” or “no”) and broaden the use of CR in the evaluation of the efficacy of test therapies. The timing of achieving ≥1 CR in the DUET OLE is supportive of its potential use as an interim end point.
For proteinuria to be accepted as a stand-alone “probably likely” surrogate end point, changes in proteinuria need to be associated with a beneficial effect on how patients feel and/or function to obtain regulatory approval for a novel treatment. eGFR plays a central role because preservation of glomerular filtration rate and overall kidney function represents such an outcome. Our findings demonstrate that the slope of eGFR is lower in patients receiving sparsentan who achieve ≥1 CR at any time. This beneficial effect of achieving ≥1 CR on eGFR slope was noted after 2 years of follow-up and over the entire duration of the OLE. The difference in eGFR slope was mirrored by a decreased number of hard renal outcomes, namely, KRT, ESKD, or 40% decline in eGFR in patients who achieved ≥1 CR versus those who did not; this is consistent with the reported association between changes in eGFR slope and kidney function outcomes.31,32 Moreover, the impact on eGFR slope was observed regardless of treatment with IST. This suggests that sparsentan has an independent effect on proteinuria and the trajectory of eGFR.
Overall, sparsentan was well tolerated throughout the long-term OLE. The mean and median sparsentan dose did not differ between the CR and non-CR patient groups. There was less frequent discontinuation of sparsentan in patients who achieved ≥1 CR. There was no apparent adverse impact on overall safety or unanticipated toxicity of sparsentan. Some TEAEs were more common in CR patients, and others were more common in non-CR patients. This supports the conclusion that achieving a CR at any time does not heighten the risk of adverse events with sparsentan treatment. The higher incidence of hyperkalemia in patients who achieved CR has been reported for other agents that inhibit the RAAS axis. There was no difference in the safety profile of sparsentan with regard to the use of IST, suggesting that these 2 classes of drugs can be prescribed in combination in the treatment of patients with FSGS. In the absence of a standard-of-care comparator arm in the DUET OLE, we cannot define the incremental effect of sparsentan treatment on the occurrence of adverse events. However, our data provide real-world evidence about the use of the drug, and the findings should be reassuring to clinicians.
A recent report from the Toronto Glomerulonephritis Registry Group has also examined the prognostic implication of achieving a CR in patients with FSGS.33 Among 203 (out of 435) adult patients who achieved ≥1 CR and were followed-up with for at least 12 months, 89 never relapsed and 114 experienced at least 1 relapse. Relapsers who ultimately ended in remission (n = 46) experienced 91% kidney survival versus 32% in those who ended in relapse (n = 68). The authors conclude that unless CR status is maintained and relapses are avoided, the risk of progressive disease remains high. There are a number of differences between this report and our observations, including the study design, namely, retrospective versus prospective, the time period of patient enrollment, the level of proteinuria, and the inclusion of 100% of the patients who had achieved CR. Jauhal et al.33 confirm the importance of a sustained CR. Unlike the present report, the patients who achieved ≥1 CR are not compared to those who never achieved a CR.
This study has several strengths. It represents a cohort of patients observed prospectively with a fairly standardized follow-up regimen and in whom the diagnosis of FSGS was established and managed based on current standards of care.7 The median follow-up period of 47 months, is considerable and sufficiently long to capture the occurrence of adverse clinical outcomes. The DUET OLE protocol is pragmatic in nature and provides real-world evidence of the benefits of sparsentan.
We acknowledge several limitations. The diagnosis of FSGS was based on review of biopsy reports or review of genetic test results. Proteinuria was measured in a single specimen. A substantial number of patients enrolled in the OLE had subnephrotic-range proteinuria at study entry. However, it would be a mistake to dismiss these patients as representing a milder FSGS phenotype because many of them required ongoing IST to maintain proteinuria in a manageable range. OLE studies by definition do not include a control arm; we cannot attribute changes in proteinuria to a specific component of the treatment regimen. Therefore, we are unable to define the contribution of sparsentan per se or IST because its use was not standardized. In several patients, initiation or changes in IST preceded CR. A patient is included in the CR group and/or IST use group if they experienced CR at any time and/or used IST at any time. Finally, in the absence of a control arm during the OLE, we are unable to make any definitive statement about the efficacy of sparsentan in achieving ≥1 CR. Nonetheless, these considerations do not undermine the importance of the finding that achieving ≥1 CR has favorable implications for the course of disease in patients with FSGS. This is buttressed by our observation that the majority of CR events occurred within 6 to 12 months of starting sparsentan. It is important to note that we compared patients who achieved ≥1 CR versus all other patients, which comprises those who reached partial remission as well as those who had no proteinuria response. The clinical implication of the standard definition of a partial remission, namely, a 50% reduction in proteinuria to a UP/C value <3.5 g/g, was not assessed in this study. Recently, Troost et al.16 developed a modified FSGS partial remission end point, namely >40% reduction in proteinuria to a UP/C value <1.5 g/g, that may improve the prognostic value of a proteinuria response that falls short of CR. Finally, the study population included patients with only modest levels of proteinuria. Although the likelihood of achieving ≥1 CR was greater in those with lesser degrees of proteinuria and better preserved eGFR, this outcome was also observed in those with nephrotic-range proteinuria. Further studies in other patient cohorts are needed to confirm the value of achieving ≥1 CR regardless of the baseline level of proteinuria and the timing of this response as an indicator of the course of disease in patients with FSGS.
In summary, in this post hoc analysis of the DUET trial OLE, we demonstrate that >40% of patients with FSGS who are on extended sparsentan treatment achieve ≥1 CR of proteinuria during follow-up. Achieving ≥1 CR is more likely in patients with lower baseline proteinuria or who receive IST. The antiproteinuric effect of sparsentan is sustained and is additive to any beneficial effect of IST. Achieving ≥1 CR on sparsentan is associated with better preservation of eGFR compared to patients who never achieve CR. We conclude that: (i) sparsentan can be safely administered for prolonged periods and exerts a sustained antiproteinuric effect that may result in intermittent normalization of proteinuria and (ii) achieving ≥1 CR, even if it is not sustained, maybe an indicator of a favorable response to a test therapy and a predictor of improved kidney function outcomes. We recommend further assessment of this index of proteinuria response in ongoing and planned clinical trials in FSGS.
Disclosure
HT reports being a consultant to and/or member of a data monitoring committee for Akebia, Chemocentryx, Goldfinch Bio Inc., Natera, Otsuka, Travere Therapeutics Inc., Aclipse, PhaseV, Boehringer-Ingelheim, and Walden. UD, JI, EM, KW, and RK are employees and stockholders of Travere Therapeutics, Inc.
Acknowledgments
This trial is registered on ClinicalTrials.gov (NCT01613118). Data included in this manuscript were previously presented in an oral presentation at ASN Kidney Week 2020 by Hogan et al. Editorial assistance was provided by Lynanne McGuire, PhD, and Stephen Bublitz, ELS, of MedVal Scientific Information Services, LLC (Princeton, NJ), which was funded by Travere Therapeutics, Inc. The DUET trial with open-label extension is funded by Travere Therapeutics, Inc.
Data Availability Statement
The data for the analyses described in this manuscript are available on request from Travere Therapeutics, Inc.
Author Contributions
HT and RK were responsible for the conception and design of the study, interpretation of the data, first draft, and subsequent revisions. UD, JI, EM, and KW were responsible for the design of the study, analysis and interpretation of the data, and manuscript revisions. All authors provided final approval for publication and agreed to be accountable for all aspects of the work.
Footnotes
Supplementary Methods.
Figure S1. Percentage of patients with ≥1, ≥2, or ≥3 CRs in (A) all patients and (B) patients with nephrotic-range proteinuria at baseline.
Figure S2. Percentage of patients receiving individual classes of renal-related IST by visit among CR vs. non-CR patients for (A) steroids; (B) CNI; and (C) MMF, azathioprine, and other.
Table S1. Percentage change in UP/C from baseline by CR vs. non-CR groups.
Table S2. KRT, ESKD, and reduction in eGFR in CR and non-CR patients.
Table S3. TEAEs in ≥10% of patients in either the CR or non-CR groups.
Table S4. Serious adverse events by CR and non-CR patients.
CONSORT Checklist.
Contributor Information
Howard Trachtman, Email: HowardTrachtman21@gmail.com.
Radko Komers, Email: radko.komers@travere.com.
Supplementary Material
Supplementary Methods.
Figure S1. Percentage of patients with ≥1, ≥2, or ≥3 CRs in (A) all patients and (B) patients with nephrotic-range proteinuria at baseline.
Figure S2. Percentage of patients receiving individual classes of renal-related IST by visit among CR vs. non-CR patients for (A) steroids; (B) CNI; and (C) MMF, azathioprine, and other.
Table S1. Percentage change in UP/C from baseline by CR vs. non-CR groups.
Table S2. KRT, ESKD, and reduction in eGFR in CR and non-CR patients.
Table S3. TEAEs in ≥10% of patients in either the CR or non-CR groups.
Table S4. Serious adverse events by CR and non-CR patients.
CONSORT Checklist.
References
- 1.D’Agati V.D., Kaskel F.J., Falk R.J. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 2.Korbet S.M. Treatment of primary FSGS in adults. J Am Soc Nephrol. 2012;23:1769–1776. doi: 10.1681/ASN.2012040389. [DOI] [PubMed] [Google Scholar]
- 3.United States Renal Data System 2015 annual data report. Volume 2: ESRD in the United States: United States Renal Data System. https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/usrds/prior-data-reports/2015
- 4.Spino C., Jahnke J.S., Selewski D.T., Massengill S., Troost J., Gipson D.S. Changing the paradigm for the treatment and development of new therapies for FSGS. Front Pediatr. 2016;4:25. doi: 10.3389/fped.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudnicki M., Rudnicki M. FSGS recurrence in adults after renal transplantation. BioMed Res Int. 2016;2016 doi: 10.1155/2016/3295618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trachtman R., Sran S.S., Trachtman H. Recurrent focal segmental glomerulosclerosis after kidney transplantation. Pediatr Nephrol. 2015;30:1793–1802. doi: 10.1007/s00467-015-3062-1. [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group Kidney disease: improving global outcomes (KDIGO) glomerular diseases work group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100:S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Reiser J., Nast C.C., Alachkar N. Permeability factors in focal and segmental glomerulosclerosis. Adv Chronic Kidney Dis. 2014;21:417–421. doi: 10.1053/j.ackd.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg A.Z., Kopp J.B. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12:502–517. doi: 10.2215/CJN.05960616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadowski C.E., Lovric S., Ashraf S., et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2015;26:1279–1289. doi: 10.1681/ASN.2014050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hildebrandt F. Decade in review–genetics of kidney diseases: genetic dissection of kidney disorders. Nat Rev Nephrol. 2015;11:635–636. doi: 10.1038/nrneph.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethna C.B., Gipson D.S. Treatment of FSGS in children. Adv Chronic Kidney Dis. 2014;21:194–199. doi: 10.1053/j.ackd.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Kunz R., Friedrich C., Wolbers M., Mann J.F. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148:30–48. doi: 10.7326/0003-4819-148-1-200801010-00190. [DOI] [PubMed] [Google Scholar]
- 14.Troyanov S., Wall C.A., Miller J.A., Scholey J.W., Cattran D.C. Toronto Glomerulonephritis Registry Group. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16:1061–1068. doi: 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 15.Gipson D.S., Chin H., Presler T.P., et al. Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol. 2006;21:344–349. doi: 10.1007/s00467-005-2097-0. [DOI] [PubMed] [Google Scholar]
- 16.Troost J.P., Trachtman H., Nachman P.H., et al. An outcomes-based definition of proteinuria remission in focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2018;13:414–421. doi: 10.2215/CJN.04780517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Troost J.P., Trachtman H., Spino C., et al. Proteinuria reduction and kidney survival in focal segmental glomerulosclerosis. Am J Kidney Dis. 2021;77:216–225. doi: 10.1053/j.ajkd.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trachtman H., Nelson P., Adler S., et al. DUET: a phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J Am Soc Nephrol. 2018;29:2745–2754. doi: 10.1681/ASN.2018010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogan J., Diva U., Murphy E., et al. Complete remission of proteinuria in patients with focal segmental glomerulosclerosis treated with sparsentan, a dual endothelin and angiotensin receptor antagonist, in the DUET trial. J Am Soc Nephrol. 2020;31:55. [Google Scholar]
- 20.Tesar V., Trachtman H., Murphy E., Ferguson B., Komers R. Sun-037 no impact of newly initiated immunosuppressive therapy observed on long-term antiproteinuric effect of sparsentan in focal segmental glomerulosclerosis: interim 84-week analysis of the duet trial. Kidney Int Rep. 2019;4:S168–S169. doi: 10.1016/j.ekir.2019.05.432. [DOI] [Google Scholar]
- 21.Hogan J., Derebail V.K., Murphy E., et al. Long-term effects of sparsentan, a dual angiotensin and endothelin receptor antagonist in primary focal segmental glomerulosclerosis (FSGS): interim 84-week analysis of the DUET trial [abstract] J Am Soc Nephrol. 2018;29(suppl):61. [Google Scholar]
- 22.Trachtman H., Rychlik I., Haws R., et al. Newly administered immunosuppressive therapy (IST) has no impact on long-term antiproteinuric effect of sparsentan (SPAR), a dual angiotensin and endothelin receptor antagonist, in patients with primary focal segmental glomerulosclerosis (FSGS): interim analysis of the DUET trial [abstract] Nephrol Dial Transplant. 2018;33(suppl 1):i20. [Google Scholar]
- 23.Trachtman H., Rychlik I., Haws R.M., et al. Long-term effect of sparsentan (SPAR), a dual angiotensin and endothelin receptor antagonist, on proteinuria in patients with primary FSGS: interim analysis of the DUET trial. J Am Soc Nephrol. 2017;28:43–44. [Google Scholar]
- 24.Komers R., Gipson D.S., Nelson P., et al. Efficacy and safety of sparsentan compared with irbesartan in patients with primary focal segmental glomerulosclerosis: randomized, controlled trial design (DUET) Kidney Int Rep. 2017;2:654–664. doi: 10.1016/j.ekir.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caster D.J., Magalhaes B., Pennese N., et al. Efficacy and safety of immunosuppressive therapy in primary focal segmental glomerulosclerosis: a systematic review and meta-analysis. Kidney Med. 2022;4 doi: 10.1016/j.xkme.2022.100501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trachtman H. Emerging drugs for treatment of focal segmental glomerulosclerosis. Expert Opin Emerg Drugs. 2020;25:367–375. doi: 10.1080/14728214.2020.1803276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komers R., Plotkin H. Dual inhibition of renin-angiotensin-aldosterone system and endothelin-1 in treatment of chronic kidney disease. Am J Physiol Regul Integr Comp Physiol. 2016;310:R877–R884. doi: 10.1152/ajpregu.00425.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson A., Cattran D.C., Blank M., Nachman P.H. Complete and partial remission as surrogate end points in membranous nephropathy. J Am Soc Nephrol. 2015;26:2930–2937. doi: 10.1681/ASN.2015010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson A., Carroll K., Inker L.A., et al. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol. 2019;14:469–481. doi: 10.2215/CJN.08600718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inker L.A., Heerspink H.J.L., Tighiouart H., et al. Association of treatment effects on early change in urine protein and treatment effects on GFR slope in IgA nephropathy: an individual participant meta-analysis. Am J Kidney Dis. 2021;78:340–349.e341. doi: 10.1053/j.ajkd.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inker L.A., Heerspink H.J.L., Tighiouart H., et al. GFR slope as a surrogate end point for kidney disease progression in clinical trials: a meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol. 2019;30:1735–1745. doi: 10.1681/ASN.2019010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey A.S., Gansevoort R.T., Coresh J., et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75:84–104. doi: 10.1053/j.ajkd.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Jauhal A., Reich H.N., Hladunewich M., et al. Quantifying the benefits of remission duration in focal and segmental glomerulosclerosis. Nephrol Dial Transplant. 2023;38:950–960. doi: 10.1093/ndt/gfac238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for the analyses described in this manuscript are available on request from Travere Therapeutics, Inc.