Abstract
Achalasia is a major esophageal motor disorder featured by the altered relaxation of the esophagogastric junction in the absence of effective peristaltic activity. As a consequence of the esophageal outflow obstruction, achalasia patients present with clinical symptoms of dysphagia, chest pain, weight loss, and regurgitation of indigested food. Other less specific symptoms can also present including heartburn, chronic cough, and aspiration pneumonia. The delay in diagnosis, particularly when the presenting symptoms mimic those of gastroesophageal reflux disease, may be as long as several years. The widespread use of high-resolution manometry has permitted earlier detection and uncovered achalasia phenotypes which can have prognostic and therapeutic implications. Other tools have also emerged to help define achalasia severity and which can be used as objective measures of response to therapy including the timed barium esophagogram and the functional lumen imaging probe. Such diagnostic innovations, along with the increased awareness by clinicians and patients due to the availability of alternative therapeutic approaches (laparoscopic and robotic Heller myotomy, and peroral endoscopic myotomy) have radically changed the natural history of the disorder. Herein, we report the most recent advances in the diagnosis, classification, and management of esophageal achalasia and underline the still-grey areas that needs to be addressed by future research to reach the goal of personalizing treatment.
Keywords: Barium, Esophageal achalasia, Heller myotomy
Introduction
Esophageal achalasia is one of the most extensively studied primary motility disorder of the esophagus.1 It is considered to be a rare disease with an incidence of 1.99 cases per 100 000 and a prevalence of 27.1 per 100 000, with no gender or ethnicity distribution preference and a median age at diagnosis of 59 (interquartile range 43-75).2 However, most recent reports suggest a higher prevalence, which could be either the result of the improved diagnostic tools or a genuine increase in prevalence of the disorder and awareness by clinicians and patients due to the availability of alternative therapeutic approaches.3 The etiology of the disorder is still poorly characterized, but is likely to be multifactorial with genetic, infectious and autoimmune factors playing a pivotal role.4 On the other hand, the pathophysiology of the disorder is better understood, ultimately leading to the loss of function of the inhibitory component of the myenteric plexus of the lower esophageal sphincter (LES) and the esophageal body.5 Under normal physiological circumstances, the inhibitory neurons of the myenteric plexus are responsible for guaranteeing an adequate relaxation of the LES, as well as a normal coordination of the circular and longitudinal smooth muscles of the distal esophagus.6 As a consequence of the disruption of the inhibitory innervation, patients with achalasia develop an esophageal outflow obstruction caused by the impaired relaxation of the LES and absence of effective peristaltic smooth muscle contractions.7
From a clinical standpoint, these abnormalities translate into a clinical presentation that may vary, but almost invariably culminates into the presence of dysphagia for both solids and liquids. Dysphagia is indeed the most common presenting symptom and may initially be subtle and involve only solid foods; however, at diagnosis nearly all patients will have dysphagia to both solids and liquids.8 Together with dysphagia, patients will often complain of regurgitation, which generally occurs within few minutes from the meal and is characterized by the presence of non-acidic undigested food. On the other hand, it can also be reported as acidic with concomitant heartburn, often as a consequence of esophageal acidification following fermentation of food stasis, and thus be mistaken for gastroesophageal reflux disease (GERD); up to 75% of patient with achalasia can also present with heartburn.9-11 Chest pain can be meal-related or unrelated, and be present in more than half of patients, and in some, might not subside after treating the esophageal outflow obstruction. While other symptoms are undoubtedly the consequence of altered esophageal clearance, the pathophysiology of chest pain remains elusive and is likely multifactorial; activation of chemoreceptors due to food fermentation, esophagitis due to stasis, and activation of mechanoreceptors of the esophageal wall due to uncoordinated smooth muscle activity and/or visceral hypersensitivity can all be contributary.12-14 As a consequence of the inability for the bolus to progress into the stomach, patients often develop weight loss, which in some can be rapid and dramatic. Interestingly however, possibly because of the widespread availability of high calorie, low viscosity foods such as chocolates, sugary drinks and deserts, obese patients with achalasia are becoming increasingly more common and is estimated to be comprise 0.5-1.0% of the achalasia population,15 thus posing new challenges in diagnosis and management. Other less frequent sequalae include extraesophageal presentations of chronic cough or aspiration and pneumonia, sometimes related to the development of a sigmoid or megaesophagus due to decompensated late presentation of achalasia.
The most common scoring system used to assess severity of the disease is the standardized Eckardt symptom score (ESS), which encapsulates the 4 main presenting symptoms described: dysphagia, regurgitation, chest pain, and weight loss. A score of 0-3 is assigned to each symptom based on the frequency (none, with every meal, every day or occasional), or weight loss (increments of 5 kg), each assigned one point, with a maximum total score of 12.16 Although not originally intended to monitor the response to treatments, the ESS has been widely used in clinical practice to assess treatment outcomes. An ESS < 3 following treatment is considered a clinical success from a symptomatic standpoint;17 however there are discrepancies that need to be taken into account, such as regurgitation and chest pain being the consequence of post therapy reflux, and change in weight not being applicable during short interval follow-up.18
Diagnosis
Malignant or other benign esophageal disorders may mimic the initial presentation of esophageal achalasia. Indeed, dysphagia and regurgitation with weight loss may be the presenting symptoms of structural esophageal problems, including strictures and neoplastic esophageal diseases that require exclusion and necessitate a prompt endoscopic assessment.19
Endoscopy is not always decisive in diagnosing achalasia and may be undetected in up to two-thirds of patients.9,20 Nonetheless, apart from excluding other organic causes of dysphagia, endoscopy may allude to the presence of achalasia, especially in its late stage, with the presence of a dilated, food filled esophagus, mucosal edema, candida, and resistance to the passage of the scope through a non-relaxing LES.21,22 Other benign esophageal disorders that present with solid food dysphagia may be macroscopically recognizable on esophageal examination, including eosinophilic esophagitis (EoE), a common cause of non-malignant dysphagia which requires exclusion with the collection of differential esophageal biopsies.
Further, recent evidence recognizes that EoE may also be associated with abnormal motor activity, some even exhibiting manometric patterns of achalasia and/or esophagogastric outflow obstruction (EGJOO).23 In a recent study, nearly 15% of patients with EoE who had a manometry study exhibited conclusive evidence for obstructive esophageal motor disorders, of which half (7.4%) would have been classified within the achalasia spectrum.24 The coexistence of these 2 rare disorders may not be coincidental; one hypothesis being that obstructive motor disorders may develop as a consequence of esophageal inflammation and release of myoactive and neuroactive eosinophil products, while on the other hand, esophageal stasis and fermentation itself may facilitate antigen presentation and esophageal eosinophilia that could resolve following treatment of the obstruction.23,24 Although this still elusive bidirectional association deserves further clarification, it is now apparent that dysphagia evaluation should prompt endoscopists to obtain esophageal biopsies in all patients, even in the absence of macroscopic lesions.25 High-resolution manometry (HRM) is the gold standard diagnostic tool in patients presenting with dysphagia and beholds relevant implications for classification and treatment. A proposed diagnostic algorithm of patients presenting with suspected achalasia is depicted in Figure 1.
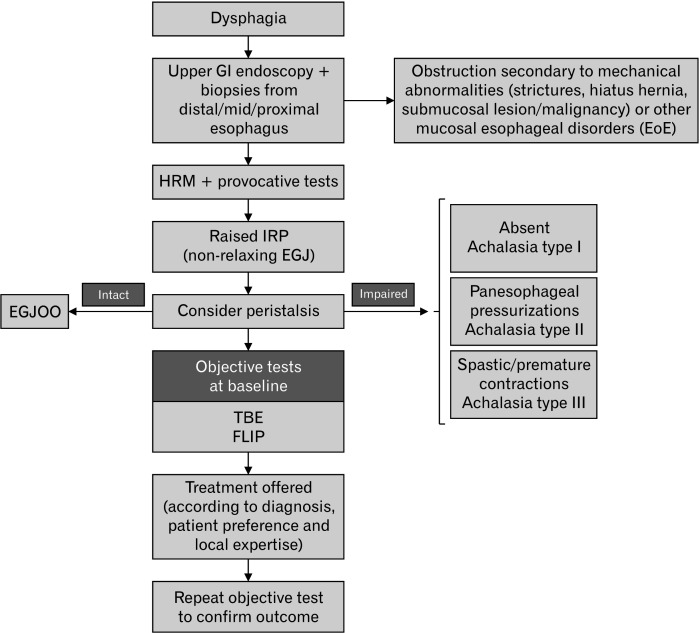
Figure 1.
Diagnostic algorithm in patients with suspected esophageal motility disorders. All dysphagia patients should be investigated with a prompt endoscopy to exclude obstruction due to mechanical/organic diseases, including esophageal cancer. Even with a normal endoscopic evaluation, differential esophageal biopsies should be obtained to exclude eosinophilic esophagitis (EoE). The presence of a disorder of the outflow obstruction is confirmed by the presence of a persistently raised integrated relaxation pressure (IRP) at supine and upright swallows and on provocative tests at high-resolution manometry (HRM) study. The presence and the severity of the obstruction should also be confirmed by additional diagnostic tests (functional luminal imaging probe [FLIP] and timed barium esophagogram [TBE]) prior to referring patients to treatment. EGJOO, esophagogastric junction outflow obstruction; GI, gastrointestinal.
Classification
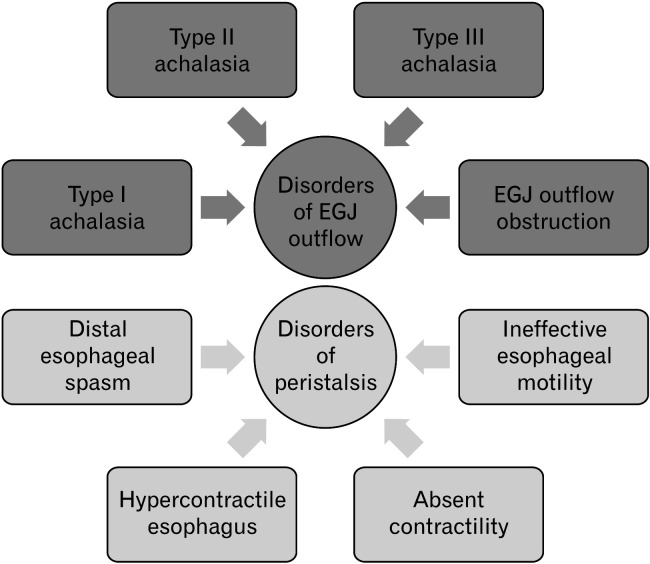
HRM is the gold standard tool to assess esophageal function in patients presenting with non-malignant dysphagia. HRM is not only a reliable diagnostic tool but can help guide management, particularly for patients with suspected achalasia or functional obstruction. The classification for esophageal motility disorders is the Chicago classification (CC) has recently been updated to its fourth iteration (CC v4.0) (Fig. 2).26 CC v4.0 has introduced a different protocol involving both supine and upright swallows, as well as additional provocative tests which attempt to challenge the esophagus, including multiple rapid swallow, rapid drink challenge, and solid swallows. Dysmotility is defined in a hierarchical manner by assessing for disorders of the outflow obstruction and then by evaluating problems of esophageal peristalsis, the rationale being that disorders of motility of the esophagogastric junction (EGJ) will influence motility of the esophageal body such that the latter can only be defined after the former has been addressed. Based on this classification, the key manometric parameter, which is the expression of the adequacy of the EGJ relaxation, is the integrated relaxation pressure (IRP), a measure of the pressure gradient between the esophageal body and the proximal stomach measured during the window of relaxation of a swallow. A persistently elevated IRP during water as well as during provocative tests (eg, a raised IRP during rapid drinking challenge) is conclusive for a disorder of the esophagogastric outflow. In the presence of normal esophageal body motility, this is defined as EGJOO. If esophageal body peristalsis is absent or spastic, achalasia is diagnosed.26,27
Figure 2.
Hierarchical classification for esophageal motility disorders according to Chicago classification version 4.0. According to the latest version of the Chicago classification, motility disorders can be classified into 2 main subcategories based on the manometric parameter, expressing the relaxation of the esophago-gastric junction, the integrated relaxation pressure. If raised, patients are categorized into the disorders of the esophagogastric junction (EGJ) outflow, comprising achalasia and EGJ outflow obstruction.
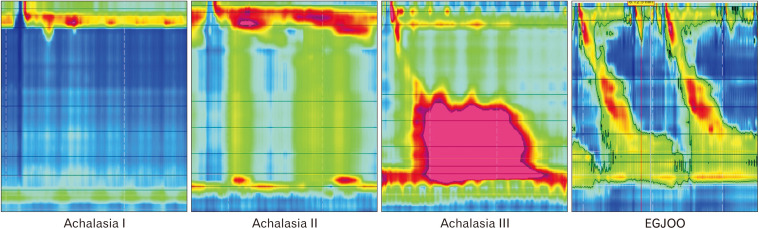
The pattern of esophageal body contractions observed at HRM allows for distinguishing 3 achalasia subtypes, which have relevant differences in terms of pathophysiology and management. The complete absence of peristaltic activity characterizes type I achalasia, a condition that is considered a later presentation whereby the esophagus has “decompensated” and has become dilated. By contrast, the occurrence of simultaneous longitudinal muscle contraction in a not yet dilated esophagus characterizes type II achalasia, which is manometrically is diagnosed by the presence of pan-esophageal pressurizations whereby there is lack of peristalsis but simultaneous pressure picked up from the HRM catheter sensors throughout the length of the esophagus.26 Finally, in type III achalasia, smooth muscle contractility is preserved, albeit associated with premature, spastic contractions (Fig. 3).26
Figure 3.
Representative pictures of achalasia subtypes and esophagogastric junction outflow obstruction (EGJOO) at high resolution manometry. In all conditions, the common manometric feature is the impaired relaxation of the esophago-gastric junction, as evaluated by a raised IRP. However, in achalasia peristalsis is never intact and it can be either absent (type I), with pan-esophageal pressurizations in at least 20% of wet swallows (type II) or with spastic/premature contractions (type III) in at least 20% of wet swallows (distal latency < 4.5 seconds), while EGJOO patients show normal/intact peristalsis.
Although the etiology is not yet clear, the manometric patterns are theorized to be the consequence of different pathophysiological mechanisms. In type III achalasia, it has been suggested that the underlying immune-mediated response leads to a loss of function of the inhibitory neurons without causing neuronal death; while type I and II achalasia subtypes are characterized by a progressive loss of myenteric neurons due to an immune-mediated cytotoxic effect.28-36 Further, classification into these 3 subtypes, as well as EGJOO may also have implications for the management, as will be discussed.37
EGJOO, with a non-relaxing LES but normal esophageal body motility could be the consequence of structural abnormalities; mucosal strictures (eg, EoE or peptic strictures), post-surgical obstruction (eg, tight fundoplication), or extra-luminal compression (eg, submucosal lesion). Alternatively, a non-relaxing LES could be due to a functional disorder isolated to the sphincter. In both, IRP will be raised so an endoscopy is crucial to exclude mucosal/structural pathology from a muscular non-relaxing valve. With regards to the latter, more restrictive criteria is required to secure a diagnosis of EGJOO as artefact from the catheter or clinically irrelevant findings are common; the diagnosis of EGJOO requires both an achalasia-like symptom presentation, a persistently raised IRP with single water swallows, as well as supportive evidence of outflow obstruction during complementary investigations, which could include provocative maneuvers during manometry (eg, free drinking or solids), barium swallow, or poor distensibility measured on EndoFlip (see below).38-40 Confirmation of idiopathic EGJOO can then permit consideration for achalasia-type therapies with similar symptomatic outcomes. It is still unclear if idiopathic EGJOO should be considered as being an early form achalasia or if it is a distinct clinical entity.38,41 Further studies are needed to clarify the most appropriate treatment and the long-term follow-up of this condition, as well as elucidate its relationship with achalasia and the underlying pathophysiological mechanisms.
Other Diagnostic Tools in Achalasia
In recent years, additional diagnostic tests have demonstrated relevant diagnostic and prognostic value in the assessment of achalasia, particularly when the results at HRM are dubious and/or inconclusive. Barium radiography has been used for decades in the assessment of dysphagia. Advantages are that this is a non-invasive, low-cost test which is widely available and can often evaluate esophageal anatomy and function. In achalasia, the classical “bird’s beak”’ sign can be observed at the standard barium swallow, as well as alterations in esophageal morphology, such as the mega- or sigmoid-shaped presentation of late-stage achalasia. However, in the absence of such advanced presentations, standard barium radiography has low diagnostic and prognostic yield in early stage or type III achalasia. Further, the lack of a standardized protocol, difference in image acquisition techniques and reporting terminology imply that the report is often subjective, heavily reliant on the operator experience and pattern recognition.42 To that end, more that 30% of achalasia cases can be missed with more than 50% lacking typical achalasia features.9,20
In the last 2 decades, the role of barium radiography has changed due to the introduction of the timed barium esophagogram (TBE), firstly described by de Oliveira et al.43 During this protocol, patients are invited to rapidly drink a fixed amount of barium contrast (150-200 mL) in the upright position, with multiple sequential X-ray films obtained, commonly at 1, 2, and 5 minutes after the ingestion. By defining the degree of barium retention in terms of height and the width during these timeframes, an objective assessment the degree of obstruction can be achieved.44 The technique has been used as a baseline and post therapeutic follow-up objective analysis of response to a high degree of accuracy.45 Under physiological circumstances, barium should be completely cleared from the esophagus within 1-2 minutes following ingestion.46 Retention of barium implies pathology and the higher the column, the lower is the esophageal clearance at each time point. In one study, a height of 5 cm at 1 minute or of 2 cm at 5 minutes showed a good sensitivity and specificity in identifying achalasia in a large cohort of dysphagia patients (sensitivity 86% and 80%, specificity 71% and 86%, respectively.47
In a recent study, Sanagapalli et al48 showed how esophageal stasis varies across subtypes of EGJ (Achalasia types I, II, and III, and EGJOO). At 1 and 5 minutes, the magnitude of residual barium was found to be grater for types I and II achalasia compared to type III and EGJOO, thus implying that the test is less sensitive in defining obstruction when esophageal body function is not aperistaltic. Further, when assessing outcome to therapy, change in barium column height or surface area was found to be most accurately correlated with treatment response rather than isolated barium column height.49 Recently, a computer-based code able to obtain an automatic analysis of TBE images has been described. The authors reported a sensitivity of 95% and a specificity of 93% for achalasia and a sensitivity of 85% and specificity of 87% for EGJOO, by measuring the dilated diameter index obtained in a retrospective cohort study comparing TBE and HRM findings.50 Finally, some authors have also suggested the usefulness of adding a solid component to the standard TBE protocol, in the forms of either a barium pill, marshmallow, or other solids, like bread. Although not standardized, a study comparing liquid and barium pill demonstrated a significant increase in the diagnostic yield both in achalasia and EGJOO.47
The functional lumen imaging probe (FLIP) is a novel complementary test that offers a valuable aid in diagnosing achalasia and EGJOO, particularly when HRM findings are inconclusive. The FLIP is an endoscopic device constituted by a catheter with 8-16 impedance electrodes and an inflatable balloon filled with a defined volume of fluid with known conductivity.51,52 By controlling the speed and volume of the balloon distension, this tool is able to measure the distensibility (distensibility index [DI]) of the lumen and/or the EGJ by simultaneously evaluating luminal diameters (cross sectional area) and intra-balloon pressure during distension.53 By these means, the FLIP allows to add relevant diagnostic data, particularly in the setting of the disorders of esophageal outflow, providing a reliable marker of resistance to bolus transit. Indeed, the FLIP has a higher specificity and sensibility in identifying EGJOO as compared to TBE and has been able to identify achalasia that could benefit from treatment, even when EGJ relaxation was considered normal by HRM.53-55 Moreover, it could guide treatment intraoperatively during surgical myotomy or peroral endoscopic myotomy POEM not only in achalasia, but also in EGJOO.56,57
Carlson et al58 have described esophageal motility responses to balloon distension in both healthy volunteers and achalasia patients. They have observed that anterograde contractions of the esophageal body represent the normal contractility response to balloon inflation in controls, while this response is aberrant in achalasia. Different motility responses, including retrograde contractions, occluding contractions, and sustained occluding contractions occurred in achalasia as compared to controls.59 By using machine-learning models, they have found that FLIP panometry could distinguish achalasia type III (spastic) vs types I and II (non-spastic) motility patterns with high level of accuracy.59
Treatment
Achalasia treatments aim to ameliorate EGJ obstruction and to allow, even in the absence of peristalsis, an effective emptying of the esophageal lumen, thus improving quality of life, reducing symptoms, and preventing achalasia complications and progression.
Current treatment options are mostly directed at disrupting the EGJ, but there is no superior treatment. Achalasia therapy should be tailored not only taking into account patient performance status, co-morbidities, symptoms presentation, age, and achalasia subtype, but also according to local expertise and therapeutic availability.
According to the American College of Gastroenterology clinical guidelines, endoscopic pneumatic dilatation (PD) is an effective non-surgical treatment option for patients with achalasia.13 PD is comprised of a minimally compliant balloon that is available in 3 sizes (30, 35, and 40 mm). In conjunction with fluoroscopy, whilst and under sedation, the balloon straddles the EGJ and is inflated such that the waste is seen fluoroscopically to be effaced, a marker of EGJ disruption. Recommendations for the best therapeutic outcomes is that this should be at least a 2-step graded process, with the first treatment usually starting at 30 mm in diameter with a planned second dilatation at 35 mm within 2-3 weeks. A further dilatation to 40 mm is sometimes also offered if symptoms persist.60 Using this graded methodology, the risk of iatrogenic perforation is reduced to 1-2% per procedure.60,61 As long as future dilatations in subsequent years are permissible if required as part of the protocol, PD achieves a clinical success in 83% of patients’ (defined as an ESS < 3),60 particularly in types I and II achalasia and in older patients. Predictive factors of symptomatic relapse include young age (< 40 years), male gender, single dilation (without a pre-planned second stretch within a few weeks), chest pain at baseline and LES basal pressure > 30 mmHg.62 Dilatation could also be useful in patients with recurrent symptoms after failure of surgical or endoscopic myotomy, in case remnant fibers require disrupting without having to subject patients to another myotomy.
The laparoscopic Heller myotomy (LHM) is a surgical myotomy of the LES muscle fibers without disruption of the mucosa that can be performed laparoscopically, and most recently with robotic surgery techniques.63 As acid reflux symptoms or indeed esophagitis can be commonplace after LES disruption, LHM is commonly followed by a partial posterior or anterior fundoplication (Dor or Toupet fundoplication).64 LHM is a highly effective treatment for achalasia, with success rates of over 90% especially in younger patients (< 40 years) with types I and II achalasia.65,66 However, LHM tends to exhibit a lower response rate in type III achalasia compared to types I and II (81% vs 90%; P = 0.010).66
POEM is an endoscopic alternative form of myotomy. After creating a submucosal tunnel from the distal esophagus through the LES and 2-3 cm into the cardia, transection of the muscularis propria is performed, with equally effective outcomes with an anterior or posterior approach.67
POEM has been demonstrated to be effective in treating all achalasia subtypes, but is superior to LHM in treating type III achalasia, likely reflecting the longer myotomy obtained with POEM as compared to LHM.68 The length of myotomy can be tailored based on the preoperative HRM findings or intraoperative EndoFlip assessment. Indeed EndoFlip is a useful mean for assessing treatment success, especially in POEM. The EGJ-DI could be measured both after procedure or during procedure, allowing to perform an additional myotomy if the DI after the first myotomy is inferior to 2.8, with a greater success rate at 12 months after POEM.69,70
One of the post-operative risks with POEM is the development of reflux disease as a fundoplication is not undertaken during the endoscopic approach. Recent studies have proven that short (< 3 cm) myotomy in POEM could reduce procedure time while decreasing the incidence of post-operative GERD. Short myotomy was found to be not inferior to long myotomy in terms of clinical success, adverse events and GERD incidence, with lower esophageal acid exposure time at the pH impedance monitoring.71,72
Nonetheless, a large metanalysis of 36 studies (2373 achalasia patients) treated with POEM suggested a high burden of GERD following this procedure, with abnormal pH testing in nearly half of treated patients.68 However, a recent study by Ponds et al73 found that although more common in the POEM than in PD group (49% vs 11% respectively; P < 0.001), reflux esophagitis was usually in the form of mild, grade A esophagitis, while only 8% had more severe grade C or D esophagitis. Further, following ambulatory pH monitoring, the median acid exposure time was not different between the POEM (7%) and PD (3%) group (P = 0.950).73 In the majority, regardless of whether treatment was undertaken with POEM, LHM, or PD, reflux symptoms commonly respond well to acid reducing therapy.74 It has been theorized that reflux symptoms following any form of achalasia therapy may, at least in part, be related to hypersensitivity rather than true reflux.75 Regardless, active follow-up should still be advised and any possible long-term consequence of GERD (eg, Barrett’s esophagus) should be promptly investigated, treated and surveillance offered regardless of the type of achalasia treatment.
In older patients who are unfit for surgery or endoscopic treatment with poor performance status, Botulinum toxin injection is a safe procedure with low rates of complications. Botulinum toxin is a presynaptic inhibitor of acetylcholine release, causing a short-term paralysis of the LES muscles with a reduction of 50% of the basal LES pressure76; however, its benefit dissipates over time, with a rapid symptom recurrence, especially in young patients.
End-stage achalasia occurs in less than 5% of patients and is characterized by a dilated (> 6 cm) and tortuous esophagus on barium swallow, leading to complications such as malnutrition, aspiration with pulmonary complications. Further, there is an increased risk of squamous carcinoma of the proximal esophagus. In exceptional circumstances, esophagectomy with gastric transposition or even colonic interposition could be indicated in patients that are considered fit for surgery.13 Alternatively, in patients who are at high preoperative risk, enteral nutrition to palliate symptoms and alleviate malnutrition complications with a percutaneous gastrostomy are a safer long-term option.
Conclusions
Over the last 2 decades, investigation, definition and treatment options for achalasia have advanced markedly. Tools such as HRM have permitted us not only to identify achalasia early, but also to define different phenotypes, which can have therapeutic implications. A new HRM based classification of motility disorders has emerged that has now entered its fourth iteration, encapsulating the definition of achalasia subtypes and EGJOO. There have also been advances in radiographic techniques such as the TBE as well as the introduction of the FLIP device as a surrogate measure of lower esophageal function distensibility, both of which have also helped define obstruction severity and objectively measure outcome. However, there remain grey areas that need to be addressed and deserve future research, which could help personalize therapy further.
Funding Statement
Financial support: None.
Footnotes
Conflicts of interest: None.
Author contributions: Marcella Pesce contributed to conception and design of the manuscript; Marta Pagliaro performed bibliographic search and wrote the first draft of the manuscript; Giovanni Sarnelli contributed to literature review and revised the manuscript; Marcella Pesce, Marta Pagliaro, Giovanni Sarnelli, and Rami Sweis contributed to manuscript revision and read, and approved the submitted version; and Rami Sweis provided overall oversight for the manuscript.
References
- 1.Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet. 2014;383:83–93. doi: 10.1016/S0140-6736(13)60651-0. [DOI] [PubMed] [Google Scholar]
- 2.Harvey PR, Coupland B, Mytton J, Evison F, Patel P, Trudgill NJ. Outcomes of pneumatic dilatation and heller's myotomy for achalasia in England between 2005 and 2016. Gut. 2019;68:1146–1151. doi: 10.1136/gutjnl-2018-316544. [DOI] [PubMed] [Google Scholar]
- 3.Gaber CE, Eluri S, Cotton CC, et al. Epidemiologic and economic burden of achalasia in the United States. Clin Gastroenterol Hepatol. 2022;20:342–352. e5. doi: 10.1016/j.cgh.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarnelli G, D'Alessandro A, Pesce M, Palumbo I, Cuomo R. Genetic contribution to motility disorders of the upper gastrointestinal tract. World J Gastrointest Pathophysiol. 2013;4:65–73. doi: 10.4291/wjgp.v4.i4.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savarino E, Bhatia S, Roman S, et al. Achalasia. Nat Rev Dis Primers. 2022;8:28. doi: 10.1038/s41572-022-00356-8. [DOI] [PubMed] [Google Scholar]
- 6.Pesce M, Sweis R. Advances and caveats in modern achalasia management. Ther Adv Chronic Dis. 2012;12:2040622321993437. doi: 10.1177/2040622321993437.12d1ec3ae1ef4a55b516ce5953ab75b4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gyawali CP. Achalasia: new perspectives on an old disease. Neurogastroenterol Motil. 2016;28:4–11. doi: 10.1111/nmo.12750. [DOI] [PubMed] [Google Scholar]
- 8.Laurino-Neto RM, Herbella F, Schlottmann F, Patti M. Evaluation of esophageal achalasia: from symptoms to the Chicago classification. Arq Bras Cir Dig. 2018;31:e1376. doi: 10.1590/0102-672020180001e1376.5d7f721392984f60a8668e3566b663a8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard PJ, Maher L, Pryde A, Cameron EW, Heading RC. Five year prospective study of the incidence, clinical features, and diagnosis of achalasia in Edinburgh. Gut. 1992;33:1011–1015. doi: 10.1136/gut.33.8.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponce J, Ortiz V, Maroto N, Ponce M, Bustamante M, Garrigues V. High prevalence of heartburn and low acid sensitivity in patients with idiopathic achalasia. Dig Dis Sci. 2011;56:773–776. doi: 10.1007/s10620-010-1343-x. [DOI] [PubMed] [Google Scholar]
- 11.Spechler SJ, Souza RF, Rosenberg SJ, Ruben RA, Goyal RK. Heartburn in patients with achalasia. Gut. 1995;37:305–308. doi: 10.1136/gut.37.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandolfino JE, Gawron AJ. Achalasia: a systematic review. JAMA. 2015;313:1841–1852. doi: 10.1001/jama.2015.2996. [DOI] [PubMed] [Google Scholar]
- 13.Vaezi MF, Pandolfino JE, Yadlapati RH, Greer KB, Kavitt RT. ACG clinical guidelines: diagnosis and management of achalasia. Am J Gastroenterol. 2020;115:1393–1411. doi: 10.14309/ajg.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahrilas PJ, Boeckxstaens G. The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology. 2013;145:954–965. doi: 10.1053/j.gastro.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almogy G, Anthone GJ, Crookes PF. Achalasia in the context of morbid obesity: a rare but important association. Obes Surg. 2003;13:896–900. doi: 10.1381/096089203322618731. [DOI] [PubMed] [Google Scholar]
- 16.Slone S, Kumar A, Jacobs J, Velanovich V, Richter JE. Accuracy of achalasia quality of life and eckardt scores for assessment of clinical improvement post treatment for achalasia. Dis Esophagus. 2021;34:doaa080. doi: 10.1093/dote/doaa080. [DOI] [PubMed] [Google Scholar]
- 17.Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732–1738. doi: 10.1016/0016-5085(92)91428-7. [DOI] [PubMed] [Google Scholar]
- 18.Taft TH, Carlson DA, Triggs J, et al. Evaluating the reliability and construct validity of the Eckardt symptom score as a measure of achalasia severity. Neurogastroenterol Motil. 2018;30:e13287. doi: 10.1111/nmo.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schizas D, Theochari NA, Katsaros I, et al. Pseudoachalasia: a systematic review of the literature. Esophagus. 2020;17:216–222. doi: 10.1007/s10388-020-00720-1. [DOI] [PubMed] [Google Scholar]
- 20.El-Takli I, O'Brien P, Paterson WG. Clinical diagnosis of achalasia: how reliable is the barium x-ray? Can J Gastroenterol. 2006;20:335–337. doi: 10.1155/2006/193823.7261c152f1294ce9922865ce4245cde6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwakiri K, Hoshihara Y, Kawami N, et al. The appearance of rosette-like esophageal folds ("esophageal rosette") in the lower esophagus after a deep inspiration is a characteristic endoscopic finding of primary achalasia. J Gastroenterol. 2010;45:422–425. doi: 10.1007/s00535-009-0179-7. [DOI] [PubMed] [Google Scholar]
- 22.Gomi K, Inoue H, Ikeda H, et al. New endoscopic classification of the cardiac orifice in esophageal achalasia: Champagne glass sign. Dig Endosc. 2016;28:645–649. doi: 10.1111/den.12642. [DOI] [PubMed] [Google Scholar]
- 23.Spechler SJ, Konda V, Souza R. Can eosinophilic esophagitis cause achalasia and other esophageal motility disorders? Am J Gastroenterol. 2018;113:1594–1599. doi: 10.1038/s41395-018-0240-3. [DOI] [PubMed] [Google Scholar]
- 24.Ghisa M, Barberio B, Buda A, Savarino E. Eosinophilic esophagitis and achalasia: are we putting all the pieces together? Am J Gastroenterol. 2021;116:1759. doi: 10.14309/ajg.0000000000001268. [DOI] [PubMed] [Google Scholar]
- 25.Kim JP, Kahrilas PJ. How i approach dysphagia. Curr Gastroenterol Rep. 2019;21:49. doi: 10.1007/s11894-019-0718-1. [DOI] [PubMed] [Google Scholar]
- 26.Fox MR, Sweis R, Yadlapati R, et al. Chicago classification version 4.0© technical review: update on standard high-resolution manometry protocol for the assessment of esophageal motility. Neurogastroenterol Motil. 2021;33:e14120. doi: 10.1111/nmo.14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dervin H, Sweis R. Assessing the diagnostic yield of achalasia using provocative testing in high-resolution manometry. United European Gastroenterology J. 10:A93. doi: 10.1111/nmo.14668. [DOI] [PubMed] [Google Scholar]
- 28.Furuzawa-Carballeda J, Aguilar-León D, Gamboa-Domínguez A, et al. Achalasia--an autoimmune inflammatory disease: a cross-sectional study. J Immunol Res. 2015;2015:729217. doi: 10.1155/2015/729217.579bf4024a7a41f0bad713e0c10addd7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Giorgio R, Simone MP, Stanghellini V, et al. Esophageal and gastric nitric oxide synthesizing innervation in primary achalasia. Am J Gastroenterol. 1999;94:2357–2362. doi: 10.1111/j.1572-0241.1999.01357.x. [DOI] [PubMed] [Google Scholar]
- 30.Kilic A, Krasinskas AM, Owens SR, Luketich JD, Landreneau RJ, Schuchert MJ. Variations in inflammation and nerve fiber loss reflect different subsets of achalasia patients. J Surg Res. 2007;143:177–182. doi: 10.1016/j.jss.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 31.Moses PL, Ellis LM, Anees MR, et al. Antineuronal antibodies in idiopathic achalasia and gastro-oesophageal reflux disease. Gut. 2003;52:629–636. doi: 10.1136/gut.52.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaber CE, Cotton CC, Eluri S, Lund JL, Farrell TM, Dellon ES. Autoimmune and viral risk factors are associated with achalasia: a case-control study. Neurogastroenterol Motil. 2022;34:e14312. doi: 10.1111/nmo.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naik RD, Vaezi MF, Gershon AA, et al. Association of achalasia with active varicella zoster virus infection of the esophagus. Gastroenterology. 2021;161:719–721. e2. doi: 10.1053/j.gastro.2021.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Facco M, Brun P, Baesso I, et al. T cells in the myenteric plexus of achalasia patients show a skewed TCR repertoire and react to HSV-1 antigens. Am J Gastroenterol. 2008;103:1598–1609. doi: 10.1111/j.1572-0241.2008.01956.x. [DOI] [PubMed] [Google Scholar]
- 35.Sodikoff JB, Lo AA, Shetuni BB, Kahrilas PJ, Yang GY, Pandolfino JE. Histopathologic patterns among achalasia subtypes. Neurogastroenterol Motil. 2016;28:139–145. doi: 10.1111/nmo.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy CA, Law R, Appelman HD, Chang AC, Korsnes S, Chen JW. Per-oral endoscopic myotomy biopsies of achalasia patients reveal schwann cell depletion in the muscularis propria. Clin Gastroenterol Hepatol. 2021;19:1294–1295. doi: 10.1016/j.cgh.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Kumbhari V, Tieu AH, Onimaru M, et al. Peroral endoscopic myotomy (POEM) vs laparoscopic heller myotomy (LHM) for the treatment of type III achalasia in 75 patients: a multicenter comparative study. Endosc Int Open. 2015;3:E195–E201. doi: 10.1055/s-0034-1391668.5be9932361974dfb97cfcffce3c74f5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanagapalli S, McGuire J, Leong RW, et al. The clinical relevance of manometric esophagogastric junction outflow obstruction can be determined using rapid drink challenge and solid swallows. Am J Gastroenterol. 2021;116:280–288. doi: 10.14309/ajg.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 39.van Hoeij FB, Smout AJ, Bredenoord AJ. Characterization of idiopathic esophagogastric junction outflow obstruction. Neurogastroenterol Motil. 2014;27:1310–1316. doi: 10.1111/nmo.12625. [DOI] [PubMed] [Google Scholar]
- 40.Carlson DA, Gyawali CP, Khan A, et al. Classifying esophageal motility by FLIP panometry: a study of 722 subjects with manometry. Am J Gastroenterol. 2021;116:2357–2366. doi: 10.14309/ajg.0000000000001532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biasutto D, Mion F, Garros A, Roman S. Rapid drink challenge test during esophageal high resolution manometry in patients with esophago-gastric junction outflow obstruction. Neurogastroenterol Motil. 2018;30:e13293. doi: 10.1111/nmo.13293. [DOI] [PubMed] [Google Scholar]
- 42.Levine MS, Rubesin SE, Laufer I. Barium studies in modern radiology: do they have a role? Radiology. 2009;250:18–22. doi: 10.1148/radiol.2501080806. [DOI] [PubMed] [Google Scholar]
- 43.de Oliveira JM, Birgisson S, Doinoff C, et al. Timed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasia. AJR Am J Roentgenol. 1997;169:473–479. doi: 10.2214/ajr.169.2.9242756. [DOI] [PubMed] [Google Scholar]
- 44.Neyaz Z, Gupta M, Ghoshal UC. How to perform and interpret timed barium esophagogram. J Neurogastroenterol Motil. 2013;19:251–256. doi: 10.5056/jnm.2013.19.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanagapalli S, Plumb A, Lord RV, Sweis R. How to effectively use and interpret the barium swallow: current role in esophageal dysphagia. Neurogastroenterol Motil. 2023;35:e14605. doi: 10.1111/nmo.14605. [DOI] [PubMed] [Google Scholar]
- 46.Kostic S, Andersson M, Hellström M, Lönroth H, Lundell L. Timed barium esophagogram in the assessment of patients with achalasia: reproducibility and observer variation. Dis Esophagus. 2005;18:96–103. doi: 10.1111/j.1442-2050.2005.00460.x. [DOI] [PubMed] [Google Scholar]
- 47.Blonski W, Kumar A, Feldman J, Richter JE. Timed barium swallow: diagnostic role and predictive value in untreated achalasia, esophagogastric junction outflow obstruction, and non-achalasia dysphagia. Am J Gastroenterol. 2018;13:196–203. doi: 10.1038/ajg.2017.370. [DOI] [PubMed] [Google Scholar]
- 48.Sanagapalli S, Plumb A, Sweis R. Timed barium swallow: esophageal stasis varies markedly across subtypes of esophagogastric junction obstruction. Neurogastroenterol Motil. 2022;34:e14322. doi: 10.1111/nmo.14322. [DOI] [PubMed] [Google Scholar]
- 49.Sanagapalli S, Plumb A, Maynard J, Leong RW, Sweis R. The timed barium swallow and its relationship to symptoms in achalasia: analysis of surface area and emptying rate. Neurogastroenterol Motil. 2020;32:e13928. doi: 10.1111/nmo.13928. [DOI] [PubMed] [Google Scholar]
- 50.Hajhosseini P, Forootan M, Shadbakht B, Bakhtavar K, Zali MR, Sedighi N. Novel description on esophageal timed barium swallow: a correlation between advanced parametrization and esophageal X-ray images. Gastroenterol Hepatol Bed Bench. 2022;15:366–376. doi: 10.22037/ghfbb.v15i4.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carlson DA. Functional lumen imaging probe: the FLIP side of esophageal disease. Curr Opin Gastroenterol. 2016;32:310–318. doi: 10.1097/MOG.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rao SS, Gregersen H, Hayek B, Summers RW, Christensen J. Unexplained chest pain: the hypersensitive, hyperreactive, and poorly compliant esophagus. Ann Intern Med. 1996;124:950–958. doi: 10.7326/0003-4819-124-11-199606010-00002. [DOI] [PubMed] [Google Scholar]
- 53.Carlson DA, Baumann AJ, Prescott JE, et al. Prediction of esophageal retention: a study comparing high-resolution manometry and functional luminal imaging probe panometry. Am J Gastroenterol. 2021;116:2032–2041. doi: 10.14309/ajg.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ponds FA, Bredenoord AJ, Kessing BF, Smout AJ. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol Motil. 2017;29:e12908. doi: 10.1111/nmo.12908. [DOI] [PubMed] [Google Scholar]
- 55.Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of esophageal motility utilizing the functional lumen imaging probe. Am J Gastroenterol. 2016;111:1726–1735. doi: 10.1038/ajg.2016.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsing LC, Choi K, Jung KW, et al. The predictive value of intraoperative esophageal functional luminal imaging probe panometry in patients with achalasia undergoing peroral endoscopic myotomy: a single-center experience. J Neurogastroenterol Motil. 2022;28:474–482. doi: 10.5056/jnm21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beveridge CA, Triggs JR, Thanawala SU, et al. Can FLIP guide therapy in idiopathic esophagogastric junction outflow obstruction? Dis Esophagus. 2022;35:doab077. doi: 10.1093/dote/doab077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carlson DA, Kou W, Lin Z, et al. Normal values of esophageal distensibility and distension-induced contractility measured by functional luminal imaging probe panometry. Clin Gastroenterol Hepatol. 2019;17:674–681. e1. doi: 10.1016/j.cgh.2018.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carlson DA, Kou W, Rooney KP, et al. Achalasia subtypes can be identified with functional luminal imaging probe (FLIP) panometry using a supervised machine learning process. Neurogastroenterol Motil. 2021;33:e13932. doi: 10.1111/nmo.13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moonen A, Annese V, Belmans A, et al. Long-term results of the European achalasia trial: a multicentre randomised controlled trial comparing pneumatic dilation versus laparoscopic heller myotomy. Gut. 2016;65:732–739. doi: 10.1136/gutjnl-2015-310602. [DOI] [PubMed] [Google Scholar]
- 61.Richter JE, Boeckxstaens GE. Management of achalasia: surgery or pneumatic dilation. Gut. 2011;60:869–876. doi: 10.1136/gut.2010.212423. [DOI] [PubMed] [Google Scholar]
- 62.Khashab MA, Vela MF, Thosani N, et al. ASGE guideline on the management of achalasia. Gastrointest Endosc. 2020;91:213–227. e6. doi: 10.1016/j.gie.2019.04.231. [DOI] [PubMed] [Google Scholar]
- 63.Milone M, Manigrasso M, Vertaldi S, et al. Robotic versus laparoscopic approach to treat symptomatic achalasia: systematic review with meta-analysis. Dis Esophagus. 2019;32:1–8. doi: 10.1093/dote/doz062. [DOI] [PubMed] [Google Scholar]
- 64.Torres-Villalobos G, Coss-Adame E, Furuzawa-Carballeda J, et al. Dor Vs toupet fundoplication after laparoscopic heller myotomy: long-term randomized controlled trial evaluated by high-resolution manometry. J Gastrointest Surg. 2018;22:13–22. doi: 10.1007/s11605-017-3578-8. [DOI] [PubMed] [Google Scholar]
- 65.Boeckxstaens GE, Annese V, des Varannes SB, et al. Pneumatic dilation versus laparoscopic heller's myotomy for idiopathic achalasia. N Engl J Med. 2011;364:1807–1816. doi: 10.1056/NEJMoa1010502. [DOI] [PubMed] [Google Scholar]
- 66.Andolfi C, Fisichella PM. Meta-analysis of clinical outcome after treatment for achalasia based on manometric subtypes. Br J Surg. 2019;106:332–341. doi: 10.1002/bjs.11049. [DOI] [PubMed] [Google Scholar]
- 67.Rodríguez de Santiago E, Mohammed N, Manolakis A, Shimamura Y, Onimaru M, Inoue H. Anterior versus posterior myotomy during poem for the treatment of achalasia: systematic review and meta-analysis of randomized clinical trials. J Gastrointestin Liver Dis. 2019;28:107–115. doi: 10.1055/s-0039-1681414. [DOI] [PubMed] [Google Scholar]
- 68.Akintoye E, Kumar N, Obaitan I, Alayo QA, Thompson CC. Peroral endoscopic myotomy: a meta-analysis. Endoscopy. 2016;48:1059–1068. doi: 10.1055/s-0042-114426. [DOI] [PubMed] [Google Scholar]
- 69.Jain AS, Carlson DA, Triggs J, et al. Esophagogastric junction distensibility on functional lumen imaging probe topography predicts treatment response in achalasia-anatomy matters! Am J Gastroenterol. 2019;114:1455–1463. doi: 10.14309/ajg.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holmstrom AL, Campagna RAJ, Cirera A, et al. Intraoperative use of FLIP is associated with clinical success following POEM for achalasia. Surg Endosc. 2021;35:3090–3096. doi: 10.1007/s00464-020-07739-6. [DOI] [PubMed] [Google Scholar]
- 71.Nabi Z, Ramchandani M, Sayyed M, et al. Comparison of short versus long esophageal myotomy in cases with idiopathic achalasia: a randomized controlled trial. J Neurogastroenterol Motil. 2021;27:63–70. doi: 10.5056/jnm20022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghazaleh S, Beran A, Khader Y, et al. Short versus standard peroral endoscopic myotomy for esophageal achalasia: a systematic review and meta-analysis. Ann Gastroenterol. 2021;34:634–642. doi: 10.20524/aog.2021.0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ponds FA, Fockens P, Lei A, et al. Effect of peroral endoscopic myotomy vs pneumatic dilation on symptom severity and treatment outcomes among treatment-naive patients with achalasia: a randomized clinical trial. JAMA. 2019;322:134–144. doi: 10.1001/jama.2019.8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Awaiz A, Yunus RM, Khan S, Memon B, Memon MA. Systematic review and meta-analysis of perioperative outcomes of peroral endoscopic myotomy (POEM) and laparoscopic heller myotomy (LHM) for achalasia. Surg Laparosc Endosc Percutan Tech. 2017;27:123–131. doi: 10.1097/SLE.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 75.Ponds FA, Oors JM, Smout AJPM, Bredenoord AJ. Reflux symptoms and oesophageal acidification in treated achalasia patients are often not reflux related. Gut. 2021;70:30–39. doi: 10.1136/gutjnl-2020-320772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoogerwerf WA, Pasricha PJ. Pharmacologic therapy in treating achalasia. Gastrointest Endosc Clin N Am. 2001;11:311–24. vii. doi: 10.1016/S1052-5157(18)30073-4. [DOI] [PubMed] [Google Scholar]