Abstract
Background/Aims
Dilated intercellular spaces (DISs) facilitate the diffusion of noxious agents into the deep layers of the esophageal epithelium. The role of DIS in heartburn pathogenesis is still controversial. Therefore, we aim to reinvestigate DIS in an extensively evaluated group of patients and healthy controls (HCs).
Methods
We classified 149 subjects into the following groups: 15 HC, 58 mild erosive reflux disease (ERD), 17 severe ERD, 25 nonerosive reflux disease (NERD), 15 reflux hypersensitivity (RH), and 19 functional heartburn (FH). A total of 100 length measurements were performed for each patient’s biopsy.
Results
The overall intercellular spaces (ISs) value of gastroesophageal reflux disease (GERD) patients was higher than that of HC (P = 0.020). In phenotypes, mild ERD (vs HC [P = 0.036], NERD [P = 0.004], RH [P = 0.014]) and severe ERD (vs HC [P = 0.002], NERD [P < 0.001], RH [P = 0.001], FH [P = 0.004]) showed significantly higher IS. There was no significant difference between the HC, NERD, RH, and FH groups. The 1.12 μm DIS cutoff value had 63.5% sensitivity and 66.7% specificity in the diagnosis of GERD. There was a weak correlation (r = 0.302) between the IS value and acid exposure time, and a weak correlation (r = −0.359) between the IS value and baseline impedance. A strong correlation was shown between acid exposure time and baseline impedance (r = −0.783).
Conclusions
Since the IS length measurement had better discrimination power only in erosive groups, it is not feasible to use in daily routine to discriminate other nonerosive phenotypes and FH. The role of DIS in heartburn in nonerosive patients should be reconsidered.
Keywords: Electric impedance; Endoscopy, gastrointestinal; Extracellular space; Gastroesophageal reflux; Heartburn
Introduction
Gastroesophageal reflux disease (GERD) is one of the most common gastrointestinal disorders in the world, although its prevalence varies widely by geographic region.1 In a study recently published in Turkey, a country located between eastern and western cultures, the prevalence of GERD was found to be 22.8%.2
GERD shows a high level of heterogeneity in terms of its symptoms and pathophysiology.3 According to the commonly accepted pathogenesis of heartburn, exposure to acid reflux leads to dilated intercellular spaces (DIS) and tissue damage.4 Because of this damage, it is thought that subepithelial nerves may become more exposed to luminal content due to increased paracellular permeability in the esophageal epithelium without erosion in non-erosive reflux disease (NERD) patients.5 The same mechanism is accompanied by loss of mucosal integrity in erosive reflux disease (ERD) patients.5 DIS may play a role in increased tissue permeability and may be one of the early findings in the biopsies of GERD patients.6 However, it is controversial whether DIS provides an adequate explanation for the wide variety of pathophysiological mechanisms and different phenotypes of GERD. First, there are studies reporting that DIS could be found in healthy controls (HCs) and in other esophageal disorders apart from GERD.7 Visceral hypersensitivity may contribute to heartburn in addition to acid exposure in NERD patients.8 Recently, Woodland et al9 showed that epithelial afferent nerve fibers could be found superficially rather than at the level of DIS in NERD patients. There are also studies showing that cytokine-mediated mucosal inflammation may contribute to heartburn.10 These results indicate that DIS and paracellular permeability may not be as dominant as previously thought in explaining heartburn pathogenesis in NERD patients. Furthermore, visceral hypersensitivity seems to be very relevant in reflux hypersensitivity (RH) and possibly for functional heartburn (FH).3 Thus, studies that evaluate DIS in patients who are on the nonerosive spectrum might be important in explaining the pathophysiology of these phenotypes.
The importance of quantitative measurement of intercellular spaces (IS) in the diagnosis of GERD has been an intriguing research topic for many years. Quantitative studies with transmission electron microscopy (TEM) showed that NERD patients had IS as wide as ERD patients, and these 2 groups had wider IS than the control groups.11-13 Studies with light microscopy (LM) showed that ERD patients had a wider IS than NERD patients and that NERD patients had a wider IS than controls.13-15 In addition, the quantitative studies we mentioned above reported very different DIS cutoff values within a very wide range of specificity and sensitivity values confirmed with different diagnostic techniques.11-16
The conflicting results in the literature on the prevalence and the uncertain contribution of DIS in the pathophysiology of GERD raised some questions, such as whether the quantitative measurement of DIS with LM can differentiate GERD patients, especially FH and RH patients. Therefore, we aim to investigate the prevalence of DIS in different phenotypes of GERD and FH in an extensively evaluated group of patients and compared it to HC. We also aim to evaluate the correlation between the mean IS value, acid exposure time (AET) and baseline impedance (BI).
Materials and Methods
Classification of Subjects
A total of 149 subjects who were admitted to GERD Outpatient Clinic, Ege University, Turkey between 2016 and 2020 were included in this study. Turkish translated and validated Mayo Clinic GERD and quality of life questionnaires were used to evaluate the clinical status and quality of life of the patients. Patients who had typical GERD symptoms (heartburn and/or regurgitation) at least once a week were included. Patients who met the criteria in Table 1 were excluded. First, upper gastrointestinal endoscopy (UGE) was performed (Olympus GIF-H170; Olympus Medical Systems Corp, Tokyo, Japan). Proton pump inhibitors and histamine H2 receptor antagonists were stopped 2 weeks before UGE. All endoscopies were performed by the same physician, and 3 biopsies were taken from each patient 3-5 cm above the Z line from the squamous epithelium with Boston Scientific Radial Jaw 4 biopsy forceps (2.8 mm opening diameter). In the presence of erosions, biopsies were taken from nonerosive areas. The patients with erosions were classified as mild (grades A and B) and severe (grades C and D) ERD patients with UGE according to the Los Angeles classification.17 After UGE, solid-state 36-channel high-resolution esophageal manometry was performed to exclude motility disorders except pathologies related to GERD. Then, 24-hour pH-multichannel esophageal intraluminal impedance monitoring was performed (Ohmega; Laborie Medical Technologies Corp, Portsmouth, NH, USA). The catheter was placed 5 cm proximal to the lower esophageal sphincter. The catheter had 6 pairs of impedance electrodes at 3, 5, 7, 9, 15, and 17 cm above the lower esophageal sphincter and 1 pH sensors. Intraluminal impedance monitoring–pH tracings were analyzed with MMS software (Laborie Medical Technologies Corp, Portsmouth, NH, USA). The longest quiet area in the sleep period at night (at least 10 minutes) was measured, taken a mean and considered the resulting value as baseline impedance. The patients who had AET > 6% without erosion were classified as conclusive NERD patients according to Lyon Consensus.18 The patients who had typical GERD symptoms and AET < 4% without erosion were divided according to symptom association probability (SAP) and symptom index (SI).19 Patients who had both positive SAP (≥ 95%) and SI (≥ 50%) were classified as RH. Patients who had both negative SAP (< 95%) and negative SI (< 50%) were classified as FH. HC had normal UGE, 24-hour pH-impedance monitoring and high-resolution manometry while having no gastrointestinal symptoms or surgical history. Subjects were differentiated into 15 HC, 115 GERD patients (58 mild ERD, 17 severe ERD, 25 NERD, and 15 RH), and 19 FH.
Table 1.
Exclusion Criteria of Subjects
| Exclusion criteria |
|---|
|
GERD, gastroesophageal reflux disease.
Tissue Preparation and Intercellular Space Measurement
Specimens were fixed in neutral buffered 10% formalin, dehydrated with alcohol, cleared with xylene, and embedded in paraffin. Sections were cut at a thickness of 4 µm from paraffin blocks. Hematoxylin and eosin staining was performed.
For each specimen, in most well-oriented areas between the upper basal and lower prickle layers that had the most prominent intercellular enlargement,20 10 different hotspots without artifacts were chosen by 2 researchers in agreement and photographed with an Olympus DP72 camera at 1000× magnification under an oil lens with an Olympus BX51 light microscope. For each photograph, 10 perpendicular length measurements were performed continuously around the one epithelial cell that had the widest IS between adjacent epithelial cells (Supplementary Fig. 1, representative images of IS measurement).13,14,16 A total of 100 measurements were performed with CellSense Entry 1.7.1 (Olympus Corp) for each patient by 1 researcher blinded. No measurements were made around the epithelial cells adjacent to the vacuoles or lymphocytes or whose nuclei were not clearly visible.
Ethical Approval
The study was carried out with the ethical approval from the Clinical Research Ethics Committee, Ege University, Turkey (Approval No. 14-4.2/7 [27.05.2014] and 19-9/31 [10.09.2019]) with the written informed consent of the patients.
Statistical Methods
In the design of the study, we selected large effect size F = 0.40 (Cohen, one-way ANOVA), α error probability = 0.05 and β error probability = 0.2.21 For one-way ANOVA test with 6 groups, estimated required sample number was 15 via G Power version 3.1.9.7 software (Heinrich Heine University, Dusseldorf, Germany). IBM SPSS Statistics version 25 (IBM Corp, Armonk, NY, USA) was used for statistical analysis. The mean of 100 measurements was calculated for each subject and used for analysis. Normality was evaluated with the Shapiro–Wilk test when the group sample size was less than 50 and with the Kolmogorov–Smirnov test when the group sample size was more than 50.22 Categorical variables were compared with the Pearson chi-square test. Numerical variables were compared with the Mann–Whitney U test between the 2 groups that did not show a normal distribution. When comparing more than 2 groups, one-way ANOVA and Bonferroni multicomparison tests were used when they showed a normal distribution. The Kruskal–Wallis test and pairwise comparison test were used when more than 2 groups did not show a normal distribution. The Spearman correlation test was used for correlation analysis because the data were not normally distributed. Receiver operating characteristic (ROC) curve analysis was performed to determine the cutoff values, sensitivity and specificity. P < 0.05 was accepted for statistical significance. At the end of the study, effect size F was 0.505 (large effect size for one-way ANOVA test) and power of the study was 0.99.21
Results
Demography
The demographic characteristics of the subjects are shown in Table 2. There was a male predominance between HC and Severe ERD (Pearson chi-square = 12.22, P = 0.032) according to sex as expected. Severe ERD had higher age than HC (P = 0.041) and FH (P = 0.028) (Kruskal-Wallis H = 14.112, P = 0.015). In body mass index comparison Severe ERD was higher than HC (P = 0.008) and RH (P = 0.034), Mild ERD was higher than HC (P = 0.007) and RH (P = 0.039) (Kruskal-Wallis H= 24.53, P < 0.001). In mean nocturnal baseline impedance comparison (Kruskal-Wallis H = 70.68, P < 0.001), Severe ERD had the lowest mean (pairwise comparison: severe ERD vs HC [P < 0.001], vs mild ERD [P = 0.014], vs NERD [P = 0.008], vs RH [P < 0.001], vs FH [P < 0.001]). Mild ERD had the second lowest mean (pairwise comparison: mild ERD vs HC [P < 0.001], vs severe ERD [P = 0.014], vs RH [P = 0.005], vs FH [P < 0.001]). NERD had the third lowest mean (pairwise comparison: NERD vs HC [P = 0.001], vs FH [P = 0.020]).
Table 2.
Demographic Characteristics of Subjects
| Subjects | HC | Mild ERD | Severe ERD | NERD | RH | FH |
|---|---|---|---|---|---|---|
| Sexa (n = 149) | n = 15 | n = 58 | n = 17 | n = 25 | n = 15 | n = 19 |
| Male/female (69/80) | 2/13 | 30/28 | 11/6 | 13/12 | 4/11 | 9/10 |
| Ageb (n = 149) | n = 15 | n = 58 | n = 17 | n = 25 | n = 15 | n = 19 |
| 43.4 ± 12.5 (mean ± SD, yr) | 38.8 ± 8.5 | 43.6 ± 11.7 | 52.8 ± 11.5 | 44.7 ± 15.1 | 39.1 ± 11.5 | 39.4 ± 11.1 |
| Body mass indexc (n = 149) | n = 15 | n = 58 | n = 17 | n = 25 | n = 15 | n = 19 |
| 27.0 ± 4.9 (mean ± SD, kg/m2) | 23.9 ± 2.9 | 28.4 ± 5.2 | 28.8 ± 4.1 | 27.3 ± 4.7 | 24.8 ± 4.5 | 25.3 ± 4.2 |
| MNBId (n = 140) (mean ± SD, Ω) | n = 15 | n = 51 | n = 15 | n = 25 | n = 15 | n = 19 |
| 2489.3 ± 529.1 | 1166.6 ± 726.0 | 428.5 ± 211.0 | 1235.8 ± 695.0 | 2120.3 ± 740.2 | 2228.3 ± 724.1 |
aPearson chi-square = 12.22, P = 0.032. Healthy control (HC) differs from severe erosive reflux disease (ERD).
bKruskal-Wallis H = 14.11, P = 0.015. Pairwise comparison: severe ERD vs HC (P = 0.041), vs functional heartburn (FH) (P = 0.028).
cKruskal-Wallis H = 24.53, P < 0.001. Pairwise comparison: severe ERD vs HC (P = 0.008), vs reflux hypersensitivity (RH) (P = 0.034). Mild ERD vs HC (P = 0.007), vs RH (P = 0.039).
dKruskal-Wallis H = 70.68, P < 0.001. Pairwise comparison: severe ERD vs HC (P < 0.001), vs mild ERD (P = 0.014), vs nonerosive reflux disease (NERD) (P = 0.008), vs RH (P < 0.001), vs FH (P < 0.001). Mild ERD vs HC (P < 0.001), vs severe ERD (P = 0.014), vs RH (P = 0.005), vs FH (P < 0.001). NERD vs HC (P = 0.001), vs FH (P = 0.020).
MNBI, mean nocturnal baseline impedance.
Mean Intercellular Space Value Comparison Between Healthy Controls and All Gastroesophageal Reflux Disease Patients
First, the mean IS value was compared between HC and all GERD patients (Table 3). GERD patients had a significantly wider IS than HCs (Mann–Whitney U = 544.5, P = 0.020).
Table 3.
Intercellular Space Values of Healthy Control and Gastroesophageal Reflux Disease Patients
| Groups | HC | GERD |
|---|---|---|
| n | 15 | 115 |
| Mean ± SD (μm) | 0.98 ± 0.23 | 1.15 ± 0.29 |
| Median (μm) | 1.01 | 1.19a |
| Minimum (μm) | 0.54 | 0.46 |
| Maximum (μm) | 1.38 | 1.69 |
aMann–Whitney U test, P = 0.020.
HC, healthy control; GERD, gastroesophageal reflux disease.
Mean Intercellular Space Value Comparison Between Healthy Controls, Gastroesophageal Reflux Disease Phenotypes, and Functional Heartburn
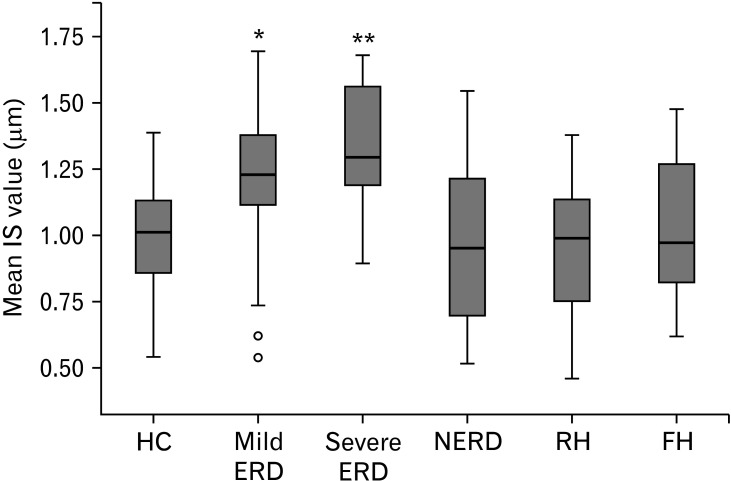
The mean IS values of HC, GERD phenotypes, and FH were compared. Since all groups showed a normal distribution, one-way ANOVA (F = 8.040, P < 0.001) and homogeneity of variances tests were performed (Levene = 1.216, P > 0.05). To compare the means, Bonferroni’s multiple comparison test was performed. Severe ERD patients had the highest mean (Table 4), which significantly differed from HC (P = 0.002), NERD (P < 0.001), RH (P = 0.001), and FH (P = 0.004). Mild ERD patients who had the second highest mean had significantly wider mean IS values than HC (P = 0.036), NERD (P = 0.004), and RH (P = 0.014). Interestingly, the other 4 groups (HC, NERD, RH, and FH) did not show any differences (P > 0.999) between groups. The boxplot of the phenotypes showed that the values of these 4 groups also tended to be distributed over an overlapping range (Fig. 1).
Table 4.
Intercellular Space Values of Healthy Control, Gastroesophageal Reflux Disease Phenotypes and Functional Heartburn
| Groups | HC | Mild ERD | Severe ERD | NERD | RH | FH |
|---|---|---|---|---|---|---|
| n | 15 | 58 | 17 | 25 | 15 | 19 |
| Mean (µm) | 0.98 | 1.22a | 1.35b | 0.98 | 0.96 | 1.02 |
| SD (µm) | 0.23 | 0.25 | 0.24 | 0.31 | 0.27 | 0.29 |
| Median (µm) | 1.01 | 1.22 | 1.29 | 0.95 | 0.99 | 0.97 |
| Minimum (µm) | 0.54 | 0.54 | 0.89 | 0.52 | 0.46 | 0.62 |
| Maximum (µm) | 1.38 | 1.69 | 1.68 | 1.54 | 1.37 | 1.47 |
Bonferroni multiple comparison: amild erosive reflux disease (ERD) vs healthy control (HC) (P = 0.036), nonerosive reflux disease (NERD) (P = 0.004), reflux hypersensitivity (RH) (P = 0.014), bsevere ERD vs HC (P = 0.002), NERD (P < 0.001), RH (P = 0.001), functional heartburn (FH) (P = 0.004).
Figure 1.
Boxplot of healthy control (HC), gastroesophageal reflux disease (GERD) phenotypes, and functional heartburn (FH). Bonferroni multiple comparison: *Mild ERD vs HC (P = 0.036), nonerosive reflux disease (NERD) (P = 0.004), and RH (P = 0.014). **Severe erosive reflux disease (ERD) vs HC (P = 0.002), NERD (P < 0.001), reflux hypersensitivity (RH) (P = 0.001), and FH (P = 0.004). Black lines inside the blue boxes show the median. Circles indicate outliers. IS, intercellular space.
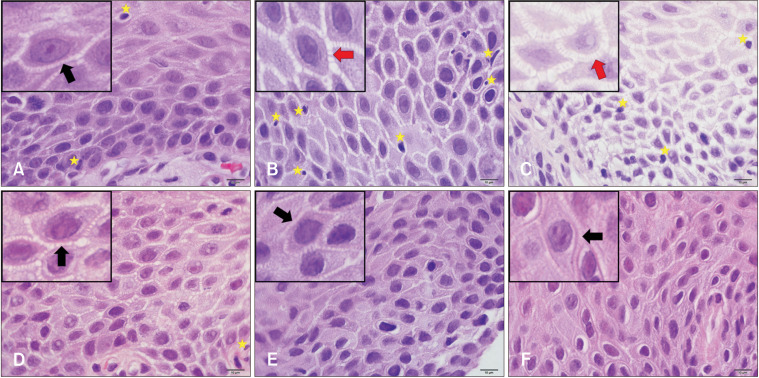
Histological examination demonstrated that DIS was the most prominent between the upper basal and lower prickle layers. Severe and mild ERD showed more prominent dilatation (Fig. 2).
Figure 2.
Histological images of healthy control (HC), gastroesophageal reflux disease phenotypes, and functional heartburn (FH) at 1000× magnification. (A) HC, (B) mild erosive reflux disease (ERD), (C) severe ERD, (D) nonerosive reflux disease, (E) reflux hypersensitivity, (F) FH. Small boxes on the upper left corner were zoomed with software to focus on one epithelial cell. Black arrows indicate intercellular spaces. Red arrowheads indicate dilated intercellular spaces. Yellow stars indicate lymphocytes. Bars = 10 μm.
Discrimination Power of the Mean Intercellular Space Value
ROC curve analysis was performed to evaluate the discrimination power of the mean DIS value in the diagnosis of GERD and different phenotypes. A summary of the results is shown in Table 5 (Supplementary Fig. 2, ROC curves). When the gold standard of diagnosis was accepted as AET > 6% (n = 78), the area under the curve (AUC) was 0.684 (P = 0.024). The 1.12 μm cutoff value had 64.1% sensitivity and 66.7% specificity with 90.9% positive and 26.3% negative predictive values. When the gold standard of diagnosis was accepted as esophagitis in UGE (n = 75), the AUC was 0.790 (P < 0.001). The 1.13 μm cutoff value had 73.3% sensitivity and 73.3% specificity with 93.2% positive and 35.5% negative predictive values. When evaluating the difference between all GERD patients (n = 115, mild and severe ERD, NERD, and RH) and controls, the AUC was 0.684 (P = 0.020). The 1.12 μm cutoff value had 63.5% sensitivity and 66.7% specificity with 93.6% positive and 19.2% negative predictive values. This cutoff value was able to discriminate 77.3% of erosive patients (mild and severe ERD) and 37.5% of nonerosive patients (NERD and RH). Achieving 100% specificity, the 1.39 μm cutoff value had 20% sensitivity with a 12.2% negative predictive value (Table 5).
Table 5.
Diagnostic Values of Different Cutoff Levels
| Comparison | Cutoff (μm) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| HC vs AET > 6% | 1.12 | 64.1% | 66.7% | 90.9% | 26.3% |
| HC vs esophagitis in UGE | 1.13 | 73.3% | 73.3% | 93.2% | 35.5% |
| HC vs all GERD | 1.12 | 63.5% | 66.7% | 93.6% | 19.2% |
| HC vs all GERD | 1.39 | 20.0% | 100.0% | 100.0% | 12.2% |
PPV, positive predictive value; NPV, negative predictive value; HC, healthy control; AET, acid exposure time; UGE, upper gastrointestinal endoscopy; GERD, gastroesophageal reflux disease.
Correlation Between Mean Intercellular Space Value, Acid Exposure Time, and Baseline Impedance
There was a weak positive correlation between the mean IS value and AET (Spearman’s rho = 0.302, P < 0.001 two-tailed). All participants were included in this analysis except for 2 severe and 6 mild ERD patients whose AET data were not at optimal quality (n = 141). Additionally, there was a weak negative correlation between the mean IS value and BI (Spearman’s rho = −0.359, P < 0.001 two-tailed). All participants were included in this analysis except for 2 severe and 7 mild ERD patients whose BI data were not at optimal quality (n = 140). There was an even weaker negative correlation between the mean IS value and BI (Spearman’s rho= −0.296, P = 0.001 two-tailed) when severe ERD patients were excluded (n = 125). There was a very strong negative correlation between AET and BI (Spearman’s rho= −0.783, P < 0.001 two-tailed). All participants were included in this analysis except for 3 severe and 8 mild ERD patients whose data were not appropriate for analysis as mentioned above (n = 138) (Supplementary Fig. 3, scatter diagrams).
Different Dilated Intercellular Space Cutoff Results in the Literature
A summary of the results is shown in Table 6.
Table 6.
Different Dilated Intercellular Space Cutoff Results in the Literature
| Study | Method | Subjects | Cutoff value | Results |
|---|---|---|---|---|
| Tobey et al11 | TEM, single length measurement | 13 controls 11 GERD |
2.4 µm | 73% sensitivity 100% specificity |
| Calabrese et al12 | TEM, mean of 100 length measurements | 12 controls 28 GERD 12 duodenal GERD |
0.74 µm | 100% sensitivity 100% specificity |
| Ribolsi et al16 | TEM and LM, mean of 100 length measurements | 12 controls 36 GERD |
TEM: 0.47 µm for distal esophagus | 100% sensitivity 100% specificity No significant correlation in patients between TEM and LM (r = 0.33) |
| Cui et al13 | TEM and LM, mean of 100 length measurements | 42 controls 119 GERD |
LM: 0.85 µm | 93.3% sensitivity 100.0% specificity High consistency between LM and TEM (Kappa = 0.691, P < 0.001) |
| Cui et al14 | LM, mean of 100 length measurements | 44 HC 297 GERD 224 non-GERD |
0.9 µm | 62.6% sensitivity 54.1% specificity |
| LM, histopathologic scores | histopathologic score > 3 | 71.7% sensitivity 47.4% specificity |
||
| Zhou et al15 | LM, mean of 100 length measurements | 352 GERD 284 non-GERD |
0.9 µm | 61.2% sensitivity 56.1% specificity |
| Altaf et al26 (in children) | TEM, mean of 100 measurements | 8 controls 35 NERD |
1.02 µm | 73.0% sensitivity 75.0% specificity |
| LM, area measurement | 8 controls 26 NERD |
11.1% area ratio | 96.0% sensitivity 75.0% specificity |
|
| 13.4% area ratio | 73.0% sensitivity 88.0% specificity |
|||
| Current study | LM, mean of 100 length measurements | 15 HC 115 GERD |
1.12 µm | 63.5% sensitivity 66.7% specificity |
TEM, transmission electron microscopy; GERD, gastroesophageal reflux disease; LM, light microscopy; HC, healthy control; NERD, nonerosive reflux disease.
Discussion
DIS are enlargements of the intercellular spaces of esophageal epithelium, which is thought to be one of the early signs of acid exposure in the tissue; however, the exact cause of formation was not clearly understood.23 Hopwood et al24 showed that DIS was prominently seen in the prickle layer of the esophagus in patients with esophagitis. There was damage in the desmosomes, which are cell junction complexes. However, it has been reported that DIS could also be found in asymptomatic control groups in approximately 14% with TEM and 20.4% with LM, indicating that DIS is not a specific marker for disease.7 In this study, we reconsidered whether quantitative IS length measurement under light microscopy, which is easily accessible, could be useful in the differential diagnosis of GERD phenotypes and FH. In particular, we investigated the importance of IS measurement for the RH and FH groups, which are more difficult to diagnose and possibly have different pathophysiologies.3
We performed the quantitative IS length measurement by taking a total of 100 measurements from 10 different areas per patient, similar to previous studies.11,14 As a result, we found that GERD patients in total had a higher mean IS value than HCs, similar to the results described in the literature.12,25 One of the most remarkable findings of our study was that NERD patients did not have a wider IS than HCs, which differs from the literature.11-15 In particular, quantitative studies with TEM have shown that NERD patients had IS as severe as ERD patients and clearly had wider IS than HC.11-13 In quantitative studies with light microscopy, the degree of dilatation in NERD patients is generally found to be lower than that in ERD patients and higher than that in HC.13-15 Due to the differences in TEM and LM tissue preparation processes, studies with TEM may show more prominent dilatation in NERD patients. In our study, we found that the mean IS value increased significantly as the severity of erosion increased in ERD patients compared to other groups (Table 4 and Fig. 1). However, there was no significant difference between the other groups (HC, NERD, RH, and FH). This result was also different from those of previous studies.14,15
In the literature, while the FH group was not different compared to controls in a study with TEM, they were similar to NERD patients and had a wider IS than controls in studies with LM.14,15,25 Cui et al14 reported that with LM, NERD patients, FH, and symptomatic controls had a larger IS than HC. However, they considered the SAP ≥ 95% criterion alone to be sufficient for GERD and did not create a separate subgroup for RH.14 This suggests that RH patients were evaluated within NERD.14 Similarly, in the study of Zhou et al15 with LM, SAP ≥ 95% was accepted as the NERD criterion alone. In our study, we did not accept the SAP value alone, and we evaluated patients without pathologic AET as RH if both SAP and SI were positive and not included in the NERD group. Although Vela et al25 found that FH patients had IS similar to HC with TEM, they did not evaluate RH in a separate subgroup. The reason why we found different results from the literature may be due to this difference in the grouping of the patients, apart from the TEM and LM tissue preparation processes. It should also be considered that there may be racial differences.
There are quite different cutoff values, sensitivities, and specificities in terms of the diagnostic value of the DIS (Table 6) in the literature. In light of these results, considerable differences between TEM and LM were observed. The cutoff values showed lower specificity and sensitivity as the number of patients in the study increased. Cui et al13,14 found that the 0.85 µm cutoff value in their first study with 161 participants had much better specificity and sensitivity than the 0.9 µm cutoff value in their later study with 565 participants. In the second study, they reached similar specificity and sensitivity values by scoring with the inclusion of histopathological findings other than DIS.14 In our study, the ROC analysis showed that the 1.12 μm cutoff value had 63.5% sensitivity and 66.7% specificity. These results were not satisfactory for diagnostic purposes. At the same time, this cutoff value had 93.6% positive and 19.2% negative predictive values, indicating that the presence of DIS may be more important than its absence. This cutoff value could discriminate erosives (mild and severe ERD) more than nonerosives (NERD and RH). Another finding that highlights the poor diagnostic value of DIS, we found that at 100.0% specificity, the cutoff value of 1.39 µm had only 20.0% sensitivity. In ROC analysis, when esophagitis was accepted as the gold standard in UGE, more significant sensitivity and specificity values were obtained compared to AET > 6% (Table 5). Since erosive patients could already be easily diagnosed with upper gastrointestinal endoscopy, length measurement of IS with LM, which requires a long time and effort, is not practical in routine clinical investigations.
However, the IS length measurement method, which is generally used, is highly dependent on the researcher due to its nature. However, studies using different methods may provide more precise quantitative measurement of IS. In their study on pediatric reflux patients, Altaf et al26 performed a conventional IS length measurement method with TEM and calculated the DIS area with LM with the assistance of software. In both methods, NERD patients had a significantly wider IS than the control group. While a 1.02 µm mean value had 73.0% sensitivity and 75.0% specificity by length measurement with TEM, an 11.1% DIS area ratio had 96.0% sensitivity and 75.0% specificity with LM.26 However, it could always be difficult to obtain a suitable biopsy specimen in area measurements, since artifacts, vacuoles, and lymphocyte dense areas may cause false wide IS. In the future, with the help of advanced software or artificial intelligence, IS measurement could not require the researcher, and precise measurements could be made with different methods.
The commonly accepted mechanism of heartburn in GERD patients is that the acid content in the lumen could more easily pass to the nerve endings lying in the deep layers of the esophagus.6 In GERD patients, acid exposure time in the lumen increases, while BI, which is a parameter of mucosal resistance, decreases.27 Consistent with the literature, we also obtained a strong negative correlation between AET and BI (Supplementary Fig. 3D). Additionally, loss of tissue integrity and formation of DIS have a facilitating effect on the paracellular permeability of acid.6 In line with this view, an increase in acid exposure and decrease in tissue resistance might be expected to result in a wider IS. However, contrary to this expectation, we found a weak correlation between AET and the mean IS value (Supplementary Fig. 3A). In the literature, while Xie et al28 found no significant relationship between the mean IS value and AET, Caviglia et al29 (distal r = 0.36, proximal r = 0.41) and Li et al30 (r = 0.32) found weak correlations. We also found a weak negative correlation between the mean IS value and BI (Supplementary Fig. 3B). Furthermore, when we excluded severe ERD, which already had a low BI and had the most severe DIS, from this correlation, the correlation was even weaker (Supplementary Fig. 3C). Xie et al28 found a weak (r = −0.230) correlation between BI and mean IS value, while Zhong et al31 found a strong (r = −0.637) correlation. These differences in correlation analyses may be due to differences in study designs, patient numbers and distributions. Sifrim et al32 showed that normal thresholds of pH-impedance monitoring are quite different by region. This is especially the case for Turkey, where some metrics, including BI, are particularly lower than other countries measured with the same equipment. The composition of the reflux content and tissue strength may contribute to the formation of DIS to varying degrees depending on the geographical region. In addition, there may be differences between studies, since prolonged exposure of esophageal tissue to acid-pepsin may increase DIS.
In light of our results, we hypothesize that the presence of DIS in the esophageal tissue may not be mandatory for heartburn symptoms, especially in nonerosive GERD subgroups (NERD and RH) and FH. Although these subgroups may have much lower acid permeability than erosive patients, they may give an exaggerated response. Weijenborg et al33 found that NERD patients had a higher acid perfusion sensitivity score than HCs, but there was no difference in transepithelial permeability and transepithelial electrical resistance. Additionally, Rinsma et al34 found similar permeability and transepithelial electrical resistance between NERD and HC. The rearrangement of nerve endings may play a role in the occurrence of the exaggerated response even in the presence of low acid permeability. Recently, Woodland et al9 showed that nerve fibers are located very close to the lumen and that the number of epithelial cell layers is reduced in NERD patients. Therefore, the distance that the acid in the lumen has to pass to reach the nerve ending is considerably reduced. In addition, GERD patients also have changes in receptor-mediated neuronal hypersensitivity. In the esophagus, differentiations in acid-sensitive receptors such as the transient receptor potential vanilloid 1 (TRPV1), the acid-sensing ion channel and the P2X receptor family contribute to visceral hypersensitivity.8 A study on human biopsies showed increased TRPV1 expression in ERD and NERD patients compared to controls.35 TRPV1 immunostained nerve fibers were also increased in patients with esophagitis.36 In an experimental chronic reflux esophagitis rat model, TRPV1 immunostaining was found to be increased in the dorsal root ganglion, spinal cord, and ganglion nodosum.37 The acid in the esophageal lumen could trigger cytokine-mediated inflammation, in addition to its own damage, and initiate a process that results in neuronal hypersensitivity.10
We found that only mild and severe ERD patients had increased mean IS values compared to other groups. Strikingly, there was no significant difference between NERD, RH, FH, and HC. Since the cutoff value was more powerful in the discrimination of erosive patients, we would not recommend the practical use of mean IS length measurement for conclusive diagnostic purposes. The increased mean IS value in erosive patients may support the permeability of the paracellular acid mechanism underlying the pathophysiology of heartburn in erosive patients. However, the fact that the mean IS value was not different in nonerosive phenotypes, FH and HC, and the weak correlation between mean IS value with AET and BI may indicate that low acid permeability may trigger heartburn without DIS. The role of DIS in the pathophysiology of heartburn in patients with reflux symptoms without endoscopic erosions should be reconsidered. In the future, evaluating DIS with more molecular and detailed studies, especially in nonerosive phenotypes, will contribute to explaining the pathophysiology of heartburn and reflux.
Acknowledgements
A part of this study was presented at 28th United European Gastroenterology Week Virtual 2020 (OP206), at 37th National Gastroenterology Week 2020, Turkey (SS-011).
Funding Statement
Financial support: This study was supported by TUBITAK (Project ID 118S260) and Ege University Scientific Research Project Coordination Unit (Project ID: TGA-2021-22732).
Footnotes
Supplementary Materials
Note: To access the supplementary figures mentioned in this article, visit the online version of Journal of Neurogastroenterology and Motility at http://www.jnmjournal.org/, and at https://doi.org/10.5056/jnm22142.
Conflicts of interest: None.
Author contributions: Serhat Bor: study design; Volkan Gorgulu, Pelin Ergun, Sezgi Kipcak, Basak Doganavsargil, Serhat Bor: data acquisition; Volkan Gorgulu and Serhat Bor: statistical analysis; Volkan Gorgulu, Pelin Ergun, Sezgi Kipcak, Basak Doganavsargil, Daniel Sifrim, and Serhat Bor: manuscript preparation; and Basak Doganavsargil, Daniel Sifrim, and Serhat Bor: critical revision and supervision.
References
- 1.Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani-Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67:430–440. doi: 10.1136/gutjnl-2016-313589. [DOI] [PubMed] [Google Scholar]
- 2.Bor S, Kitapcioglu G, Kasap E. Prevalence of gastroesophageal reflux disease in a country with a high occurrence of Helicobacter pylori. World J Gastroenterol. 2017;23:525–532. doi: 10.3748/wjg.v23.i3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katzka DA, Pandolfino JE, Kahrilas PJ. Phenotypes of Gastroesophageal reflux disease: where rome, lyon, and montreal meet. Clin Gastroenterol Hepatol. 2020;18:767–776. doi: 10.1016/j.cgh.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ang D, Sifrim D, Tack J. Mechanisms of heartburn. Nat Clin Pract Gastroenterol Hepatol. 2008;5:383–392. doi: 10.1038/ncpgasthep1160. [DOI] [PubMed] [Google Scholar]
- 5.Barlow WJ, Orlando RC. The pathogenesis of heartburn in nonerosive reflux disease: a unifying hypothesis. Gastroenterology. 2005;128:771–778. doi: 10.1053/j.gastro.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Orlando RC. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Pract Res Clin Gastroenterol. 2010;24:873–882. doi: 10.1016/j.bpg.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Malenstein H, Farré R, Sifrim D. Esophageal dilated intercellular spaces (DIS) and nonerosive reflux disease. Am J Gastroenterol. 2008;103:1021–1028. doi: 10.1111/j.1572-0241.2007.01688.x. [DOI] [PubMed] [Google Scholar]
- 8.Miwa H, Kondo T, Oshima T, Fukui H, Tomita T, Watari J. Esophageal sensation and esophageal hypersensitivity - overview from bench to bedside. J Neurogastroenterol Motil. 2010;16:353–362. doi: 10.5056/jnm.2010.16.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodland P, Shen Ooi JL, Grassi F, et al. Superficial esophageal mucosal afferent nerves may contribute to reflux hypersensitivity in nonerosive reflux disease. Gastroenterology. 2017;153:1230–1239. doi: 10.1053/j.gastro.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Ustaoglu A, Nguyen A, Spechler S, Sifrim D, Souza R, Woodland P. Mucosal pathogenesis in gastro-esophageal reflux disease. Neurogastroenterol Motil. 2020;32:e14022. doi: 10.1111/nmo.14022. [DOI] [PubMed] [Google Scholar]
- 11.Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: a morphological feature of acid reflux--damaged human esophageal epithelium. Gastroenterology. 1996;111:1200–1205. doi: 10.1053/gast.1996.v111.pm8898633. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese C, Fabbri A, Bortolotti M, et al. Dilated intercellular spaces as a marker of oesophageal damage: comparative results in gastro-oesophageal reflux disease with or without bile reflux. Aliment Pharmacol Ther. 2003;18:525–532. doi: 10.1046/j.1365-2036.2003.01713.x. [DOI] [PubMed] [Google Scholar]
- 13.Cui R, Zhou L, Lin S, et al. The feasibility of light microscopic measurements of intercellular spaces in squamous epithelium in the lower-esophagus of GERD patients. Dis Esophagus. 2011;24:1–5. doi: 10.1111/j.1442-2050.2010.01083.x. [DOI] [PubMed] [Google Scholar]
- 14.Cui R, Zhang H, Zhou L, et al. Diagnostic value of dilated intercellular space and histopathologic scores in gastroesophageal reflux disease. Dis Esophagus. 2015;28:530–537. doi: 10.1111/dote.12256. [DOI] [PubMed] [Google Scholar]
- 15.Zhou LY, Wang Y, Lu JJ, et al. Accuracy of diagnosing gastroesophageal reflux disease by GerdQ, esophageal impedance monitoring and histology. J Dig Dis. 2014;15:230–238. doi: 10.1111/1751-2980.12135. [DOI] [PubMed] [Google Scholar]
- 16.Ribolsi M, Perrone G, Caviglia R, et al. Intercellular space diameters of the oesophageal epithelium in NERD patients: head to head comparison between light and electron microscopy analysis. Dig Liver Dis. 2009;41:9–14. doi: 10.1016/j.dld.2008.07.318. [DOI] [PubMed] [Google Scholar]
- 17.Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the lyon consensus. Gut. 2018;67:1351–1362. doi: 10.1136/gutjnl-2017-314722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamasaki T, Fass R. Reflux hypersensitivity: a new functional esophageal disorder. J Neurogastroenterol Motil. 2017;23:495–503. doi: 10.5056/jnm17097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solcia E, Villani L, Luinetti O, et al. Altered intercellular glycoconjugates and dilated intercellular spaces of esophageal epithelium in reflux disease. Virchows Arch. 2000;436:207–216. doi: 10.1007/s004280050032. [DOI] [PubMed] [Google Scholar]
- 21.Kang H. Sample size determination and power analysis using the G*Power software. J Educ Eval Health Prof. 2021;18:17. doi: 10.3352/jeehp.2021.18.17.0dde9f70c871406b82494635a5a441fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra P, Pandey CM, Singh U, Gupta A, Sahu C, Keshri A. Descriptive statistics and normality tests for statistical data. Ann Card Anaesth. 2019;22:67–72. doi: 10.4103/aca.ACA_157_18.f8c78bf261b445208b0442d335e8c30a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dent J. Microscopic esophageal mucosal injury in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2007;5:4–16. doi: 10.1016/j.cgh.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Hopwood D, Milne G, Logan KR. Electron microscopic changes in human oesophageal epithelium in oesophagitis. J Pathol. 1979;129:161–167. doi: 10.1002/path.1711290402. [DOI] [PubMed] [Google Scholar]
- 25.Vela MF, Craft BM, Sharma N, Freeman J, Hazen-Martin D. Refractory heartburn: comparison of intercellular space diameter in documented GERD vs. functional heartburn. Am J Gastroenterol. 2011;106:844–850. doi: 10.1038/ajg.2010.476. [DOI] [PubMed] [Google Scholar]
- 26.Altaf MA, Ciecierega T, Szabo S, et al. Comparison of light and electron microscopy in measurement of esophageal intercellular space in children. J Pediatr Gastroenterol Nutr. 2014;59:232–236. doi: 10.1097/MPG.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 27.Kessing BF, Bredenoord AJ, Weijenborg PW, Hemmink GJ, Loots CM, Smout AJ. Esophageal acid exposure decreases intraluminal baseline impedance levels. Am J Gastroenterol. 2011;106:2093–2097. doi: 10.1038/ajg.2011.276. [DOI] [PubMed] [Google Scholar]
- 28.Xie C, Sifrim D, Li Y, Chen M, Xiao Y. Esophageal baseline impedance reflects mucosal integrity and predicts symptomatic outcome with proton pump inhibitor treatment. J Neurogastroenterol Motil. 2018;24:43–50. doi: 10.5056/jnm17032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caviglia R, Ribolsi M, Gentile M, et al. Dilated intercellular spaces and acid reflux at the distal and proximal oesophagus in patients with non-erosive gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2007;25:629–636. doi: 10.1111/j.1365-2036.2006.03237.x. [DOI] [PubMed] [Google Scholar]
- 30.Li YW, Sifrim D, Xie C, Chen M, Xiao YL. Relationship between salivary pepsin concentration and esophageal mucosal integrity in patients with gastroesophageal reflux disease. J Neurogastroenterol Motil. 2017;23:517–525. doi: 10.5056/jnm16178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong C, Duan L, Wang K, et al. Esophageal intraluminal baseline impedance is associated with severity of acid reflux and epithelial structural abnormalities in patients with gastroesophageal reflux disease. J Gastroenterol. 2013;48:601–610. doi: 10.1007/s00535-012-0689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sifrim D, Roman S, Savarino E, et al. Normal values and regional differences in oesophageal impedance-pH metrics: a consensus analysis of impedance-pH studies from around the world. Gut. 2021;70:1441–1449. doi: 10.1136/gutjnl-2020-322627. [DOI] [PubMed] [Google Scholar]
- 33.Weijenborg PW, Smout AJ, Verseijden C, et al. Hypersensitivity to acid is associated with impaired esophageal mucosal integrity in patients with gastroesophageal reflux disease with and without esophagitis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G323–G329. doi: 10.1152/ajpgi.00345.2013. [DOI] [PubMed] [Google Scholar]
- 34.Rinsma NF, Farré R, Troost FJ, et al. Exploration of the esophageal mucosal barrier in non-erosive reflux disease. Int J Mol Sci. 2017:18L1091. doi: 10.3390/ijms18051091.6c1912efd8474712b3560d9d786bd2d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guarino MP, Cheng L, Ma J, et al. Increased TRPV1 gene expression in esophageal mucosa of patients with non-erosive and erosive reflux disease. Neurogastroenterol Motil. 2010;22:746–751. e219. doi: 10.1111/j.1365-2982.2010.01514.x. [DOI] [PubMed] [Google Scholar]
- 36.Matthews PJ, Aziz Q, Facer P, Davis JB, Thompson DG, Anand P. Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur J Gastroenterol Hepatol. 2004;16:897–902. doi: 10.1097/00042737-200409000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Banerjee B, Medda BK, Lazarova Z, Bansal N, Shaker R, Sengupta JN. Effect of reflux-induced inflammation on transient receptor potential vanilloid one (TRPV1) expression in primary sensory neurons innervating the oesophagus of rats. Neurogastroenterol Motil. 2007;19:681–691. doi: 10.1111/j.1365-2982.2007.00947.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.