Abstract
Introduction
The incidence and outcomes of kidney replacement therapy (KRT) have been well-studied in adults, but much less so in children. This study aimed to investigate the epidemiology and outcomes of KRT in children in Australia and New Zealand from 2000 to 2020.
Methods
Children aged <18 years initiating KRT in Australia and New Zealand between January 1, 2000 and December 31, 2020 and reported to the Australia and New Zealand Dialysis and Transplant Registry were included. Patient survival, technique-survival, and graft survival were analyzed by Cox regression analyses.
Results
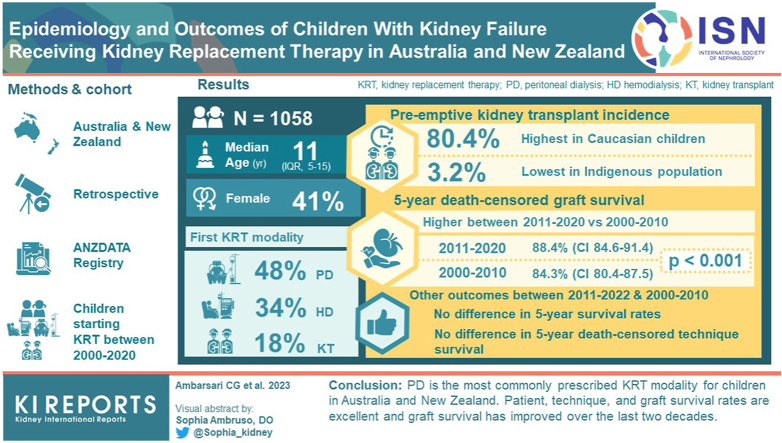
Overall, 1058 children (median [interquartile range (IQR)] age 11 [5–15] years, 41% female, 66% White) were followed-up with for a median period of 12.3 years. First KRT modalities were peritoneal dialysis (PD; 48%), hemodialysis (HD; 34%), and kidney transplantation (KT; 18%). Pre-emptive KT incidence was highest in Caucasian children (80.4%) and lowest in the Indigenous population (3.2%). There was no difference in 5-year patient survival rates between 2011 and 2020 (96.9%, 95% confidence interval [CI] 93.8–98.4) and the preceding decade, 2000–2010 (94.5%, 95% CI 90.4–96.8) (P = 0.79). There was no difference in 5-year death-censored technique survival between 2011 and 2020 (51.2%, 95% CI 39.1–62) and 2000–2010 (48.8%, 95% CI 40.5–56.6) (P = 0.27). However, 5-year derath-censored graft survival was significantly higher in 2011–2020 (88.4%, 95% CI 84.6–91.4) than in 2000–2010 (84.3%, 95% CI 80.4–87.5) (P < 0.001).
Conclusions
PD is the most commonly prescribed KRT modality for children in Australia and New Zealand. Patient-survival, technique-survival, and graft survival rates are excellent and graft survival has improved over the last 2 decades.
Keywords: hemodialysis, kidney failure, peritoneal dialysis, registry, survival, transplantation
Graphical abstract
Kidney failure is a devastating condition in children, and is associated with a 30-fold increased risk of mortality compared with healthy children.1 The prevalence of kidney failure requiring KRT among children is growing across the globe, by 5% per year since 2009 in the United States and by 1.9% per year since 2007 in European countries.2,3 Despite ongoing growth in the burden of kidney failure among children, studies of KRT epidemiology and outcomes have predominantly focused on adults, whereas children and adolescents have been less well-studied.
Recent adult studies suggest that improved patient care has led to improved survival rates. Moreover, KRT utilization has increased, particularly for KT.4, 5, 6, 7, 8, 9, 10, 11 However, it remains uncertain whether similar patterns and outcomes have been achieved among the pediatric population, given that the etiologies of kidney failure in children are notably different from those in adults.5,12, 13, 14, 15, 16, 17 Since the 1980s, improved patient care and KRT policies have resulted in the acceptance of more challenging patients into pediatric KRT programs, including very young patients, and prioritization of pediatric patients with kidney failure as KT recipients.1,16,18, 19, 20 Furthermore, PD has become the primary choice for dialysis for children and adolescents because of its better preservation of residual kidney function, tolerability, and cost-effectiveness.10,16,21, 22, 23, 24, 25
The aims of this study were to describe the epidemiology and outcomes of KRT in children and adolescents in Australia and New Zealand between 2000 and 2020 using data obtained from the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry.
Methods
The study was approved by the Princess Alexandra Hospital Human Research Ethics Committee (2021-79210) and was conducted and reported in accordance with STROBE guidelines.26
Data Source
Patient data were obtained from the ANZDATA Registry, which used real-time event-based reporting, and an annual survey, which recorded the status of all patients on KRT on December 31 of each year. Regarding consent and privacy provisions of the ANZDATA Registry, there was an opt-out approach for each individual. Registry conduct was in accordance with both the Australian and New Zealand National Privacy Principles.27 Only information with personal identifiers removed was released by the registry with permission granted by the ANZDATA Executive.
Study Population
All children aged <18 years commencing KRT between January 1, 2000 and December 31, 2020 were included in the study. Data collected included age at KRT initiation, sex, ethnicity, primary kidney disease, late referral (defined as referral to a pediatric nephrology unit within 3 months of commencing KRT), socioeconomic status (Australia only), remoteness of residence (Australia only), body weight, body height, and initial and subsequent KRT modalities. Initial treatment modalities were recorded as PD, HD, and KT.
Socioeconomic status was measured only for Australian children according to income, education, and occupation; and assessed based on the index of relative socioeconomic advantage and disadvantage (IRSAD). IRSAD was measured at the level of postcode and was derived from the 2016 census data on 21 census variables related to both advantage (e.g., proportion of people or households with high income, tertiary education, professional occupation, 3 or more cars, and 4 or more bedrooms) and disadvantage (e.g., proportion of people or households with low education level, low income, overcrowding, separated or divorced, unemployed, no internet connection, and 1 parent families, disabilities). Quartiles of IRSAD were used for analyses, with Q1 representing the most disadvantaged group and Q4 the most advantaged group.28,29
Remoteness of residence was evaluated among Australian children by the Accessibility/Remoteness Index of Australia (ARIA), which was developed by the Australian Government’s Department of Health and Aged Care.30 The scores were determined based on distance from towns and cities, access to goods and services, and opportunities for social interaction. A low ARIA score indicated high accessibility. ARIAs were defined as major cities (ARIA score 0–0.2), regional (ARIA score 0.2–5.92), and remote (ARIA score 5.92–15).30,31 A comparable socioeconomic status data set was collected in New Zealand (New Zealand Index of Multiple Deprivation)32 only from 2018 onward, and thus, was not used in this analysis.
Study Outcomes
The primary outcome was patient survival. Follow-up period was defined as time from dialysis initiation to death, censored at kidney function recovery lasting over 30 days, KRT modality switch, loss to follow-up, or end of study period (December 31, 2020), whichever occurred first. Death occurring within 30 days of transfer between modalities was assigned to the initial KRT modality. The secondary outcomes were mortality incidence and prevalence, KRT modality change, technique failure (censored for kidney function recovery, loss to follow-up, and end of study), graft failure, and association of KRT commencement decade with patient survival, technique failure, and graft failure. Technique failure was defined as transfer to a different dialysis modality for 30 days.33 Graft failure was defined as return to dialysis after receiving KT. Technique survival was defined as staying on the same initial KRT modality and was analyzed in the subpopulation of patients with dialysis and KT as initial KRT.
A sensitivity analysis of death as a competing event to technique failure and graft failure was performed. Furthermore, we performed a sensitivity analysis excluding children who received pre-emptive KT and children who started dialysis at age <5 years. We compared the results of these sensitivity analyses with the result of the main analysis.
Statistical Analysis
Patient characteristics were expressed as frequency and percentages for categorical variables, mean ± standard deviation for normally distributed continuous variables, and median (IQR) for nonnormally distributed continuous variables. Differences between categorical variables were assessed with the χ2 test, parametric continuous variables with Student’s t-test, and nonparametric continuous variables with Mann-Whitney U test. Kaplan-Meier plots were used to visualize survival probabilities and multivariable Cox proportional hazards regression models were used to evaluate association between survival outcomes and risk factors of interest. Adjustments were made for initial KRT modalities, age categories, sex, ethnicities, primary kidney diseases, late referral, and decade of KRT initiation, with IRSAD and ARIA specifically for Australian children. The proportionality assumption was examined visually with log-log survival plots. There were no significant first-order interactions identified. Analyses were performed using STATA statistical software package (version 17; StataCorp LP, College Station, TX). Z-score for anthropometric status were calculated with zanthro34 and zscore06 STATA package.35 P-values less than 0.05 were considered statistically significant.
Results
Overall, 1058 children (58.9% male) with a median age of 11 years (IQR, 5–15) started KRT for kidney failure in Australia and New Zealand. The median follow-up time was 12.3 years (IQR, 7.1–17.1). A large proportion started KRT on PD (48.1%), followed by HD (34%), and the rest were pre-emptive KT (17.9%) (Table 1 and Supplementary Figure S1). When examined by age, the most common initial KRT mode was PD for children aged 14 years and younger and HD for patients aged 15 years and older. Compared to 2000–2010, a greater proportion of younger children (0–4 years old) were admitted to the HD program in 2011–2020, from 8% to 21% (P < 0.05, Table 1).36,37
Table 1.
Demographics of children (0–18 years of age) with kidney failure by the first KRT modality in Australia and New Zealand, 2000–2020
|
Characteristics |
All (n = 1058) | First KRT modalities/Decade |
|||||
|---|---|---|---|---|---|---|---|
| 2000–2010 |
2011–2020 |
||||||
| PD (n = 249) | HD (n = 19 7) | KT (n = 90) | PD (n = 260) | HD (n = 163) | KT (n = 99) | ||
| Age (yrs), median (IQR) | 11 (5–t15) | 7 (2–13) | 15 (11–16) | 10 (6–15) | 9 (2–13) | 12 (7–16) | 12 (6–14) |
| 0–4 yrs old, n (%) | 252 (23.8) | 84 (33.7) | 15 (7.6) | 15 (16.7) | 86 (33.1) | 34 (20.9) | 18 (18.2) |
| 5–9 yrs old, n (%) | 218 (20.6) | 66 (26.5) | 25 (12.7) | 26 (28.9) | 51 (19.6) | 27 (16.6) | 23 (23.2) |
| 10–14 yrs old, n (%) | 287 (27.1) | 51 (20.5) | 51(25.9) | 25 (27.8) | 80 (30.7) | 46 (28.2) | 34 (34.3) |
| 15–18 yrs old, n (%) | 301 (28.5) | 48 (19.3) | 106 (53.8) | 24 (26.7) | 43 (16.5) | 56 (34.4) | 24 (24.2) |
| Sex (male), n (%) | 623 (58.9) | 141 (56.6) | 99 (50.3) | 61 (67.8) | 159 (61.2) | 90 (55.2) | 73 (73.7) |
| Weight-for-length (z score)a | |||||||
| Overweight or obese (> 1 SD) | 39 (26.9) | 16 (29.6) | 1 (20) | 0 (0) | 16 (25.8) | 4 (23.5) | 2 (40) |
| Normal (> −2 SD and <1) | 85 (58.6) | 28 (51.9) | 3 (60) | 2 (100) | 39 (62.9) | 10 (58.8) | 3 (60) |
| Wasted (< −2 SD) | 15 (10.3) | 7 (12.9) | 1 (20) | 0 (0) | 6 (9.7) | 1 (5.9) | 0 (0) |
| Severely wasted (< −3 SD) | 6 (2.2) | 3 (5.6) | 0 (0) | 0 (0) | 1 (1.6) | 2 (11.8) | 0 (0) |
| Body mass index (z score)b | |||||||
| Overweight or obese (> 1 SD) | 222 (24.5) | 41 (23.3) | 48 (27.6) | 21 (26.9) | 45 (21.1) | 46 (28.6) | 21 (20.4) |
| Normal (> −2 SD and <1) | 636 (70.3) | 123 (69.9) | 113 (64.9) | 55 (70.5) | 161 (75.6) | 108 (67.1) | 76 (73.8) |
| Wasted (< −2 SD) | 24 (2.7) | 7 (4) | 5 (2.9) | 1 (1.3) | 4 (1.9) | 4 (2.5) | 3 (2.9) |
| Severely wasted (<−3 SD) | 23 (2.5) | 5 (2.8) | 8 (4.6) | 1 (1.3) | 3 (1.4) | 3 (1.8) | 3 (2.9) |
| Ethnicity, n (%) | |||||||
| Caucasian | 701 (66.3) | 175 (70.3) | 117 (59.4) | 79 (87.8) | 163 (62.7) | 94 (57.7) | 73 (73.7) |
| Asian | 101 (9.6) | 25 (10.0) | 19 (9.6) | 2 (2.2) | 30 (11.5) | 21 (12.9) | 4 (4.0) |
| ATSI | 45 (4.3) | 10 (4.0) | 9 (4.6) | 0 | 14 (5.4) | 11 (6.8) | 1 (1.0) |
| Māori | 60 (5.7) | 19 (7.6) | 16 (8.1) | 1 (1.1) | 16 (6.2) | 7 (4.3) | 1 (1.0) |
| Pacific Islander | 49 (4.6) | 10 (4.0) | 17 (8.6) | 1 (1.1) | 11 (4.2) | 8 (4.9) | 2 (2.0) |
| Other | 102 (9.4) | 10 (4.0) | 19 (9.6) | 7 (7.8) | 26 (10.0) | 22 (13.5) | 18 (18.2) |
| Primary kidney diseases | |||||||
| CAKUT | 409 (38.7) | 98 (39.4) | 48 (24.4) | 64 (71.1) | 102 (39.2) | 43 (26.4) | 54 (54.6) |
| Cystic renal diseases | 100 (9.5) | 25 (10.0) | 11 (5.6) | 6 (6.7) | 31 (11.9) | 11 (6.8) | 16 (16.2) |
| Glomerulonephritis | 293 (27.7) | 74 (29.7) | 95 (48.2) | 6 (6.7) | 61 (23.5) | 55 (33.7) | 2 (2.0) |
| Other | 256 (24.2) | 52 (20.9) | 43 (21.8) | 14 (15.5) | 66 (25.4) | 54 (33.1) | 27 (27.3) |
| Late referral, n (%) | 242 (22.9) | 62 (24.9) | 67 (34.0) | 1 (1.1) | 59 (23.0) | 51 (32.1) | 2 (2.1) |
| Initial parent center state | |||||||
| Australia, n (%) | 850 (80.3) | 183 (73.5) | 164 (83.3) | 75 (83.3) | 201 (77.3) | 143 (87.7) | 84 (84.9) |
| New Zealand, n (%) | 208 (19.7) | 66 (26.5) | 33 (16.8) | 15 (16.7) | 59 (22.7) | 20 (12.3) | 15 (15.2) |
| Socio economic indexes for area (IRSAD), n = 847 IRSAD quartilesc |
|||||||
| 1, n (%) | 213 (25.2) | 46 (25.1) | 44 (26.8) | 19 (25.3) | 50 (24.9) | 43 (30.3) | 11 (13.4) |
| 2, n (%) | 212 (25) | 46 (25.1) | 35 (21.3) | 17 (22.7) | 60 (29.9) | 35 (24.7) | 19 (23.2) |
| 3, n (%) | 211 (24.9) | 47 (25.7) | 45 (27.4) | 18 (24.0) | 42 (20.9) | 43 (30.3) | 16 (19.5) |
| 4, n (%) | 211 (24.9) | 44 (24.0) | 40 (24.4) | 21 (28.0) | 49 (24.4) | 21 (14.8) | 36 (43.9) |
| Accessibility/Remoteness Index of Australia (ARIA), n = 847c | |||||||
| Major, n (%) | 615 (72.6) | 133 (72.7) | 124 (75.6) | 44 (58.7) | 141 (70.2) | 105 (73.9) | 68 (82.9) |
| Regional, n (%) | 214 (25.3) | 46 (25.1) | 33 (20.1) | 31 (41.3) | 56 (27.9) | 34 (23.9) | 14 (17.1) |
| Remote, n (%) | 18 (2.1) | 4 (2.2) | 7 (4.3) | 0 | 4 (2.0) | 3 (2.1) | 0 |
ATSI, Aboriginal and Torres Strait Islander; CAKUT, congenital anomalies of the kidneys and urinary tract; HD, hemodialysis; IRSAD, Index of Relative Socioeconomic Advantage and Disadvantage; IQR, interquartile range; KRT, kidney replacement therapy; KT, kidney transplantation; N/A, not available; PD, peritoneal dialysis.
Weight-for-length was used for patients aged < 2 years old.
Body mass index was used for patients aged ≥2 years old; There were 8 missing data in this age group Z-scores were calculated according to the World Health Organization 2006 growth chart36 and the Center for Disease Control 2000 reference values.37
Applicable only to Australia.
Across the decades, the proportions of pre-emptive KT incidences were similar across different Australian IRSAD quartiles. However, pre-emptive KT incidence remained the highest in Caucasian children (70%–80%), aged 5–14 years old (60%), children with congenital anomalies of the kidney and urinary tract as the primary cause of kidney failure (60%), and Australian children who lived in major cities (50%–80%, P < 0.05). No Australian patients who lived in remote areas ever received pre-emptive KT. Compared with 2000–2010, the proportion of children with a non-congenital anomalies of the kidney and urinary tract because of kidney failure receiving pre-emptive KT in 2011–2020, increased from 30% to 45% (P = 0.01, Table 1). All pre-emptive KT patients received transplantation from living donors.
Patient Survival on the First KRT Modalities
Mortality during the study was 8.7% (92/1058), with a median follow-up of 8.1 years (IQR, 3.5–13.2). Of these, 35% (32/92) of deaths occurred at the first KRT modality (18 on PD, 11 on HD, and 3 with KT, Supplementary Table S1). The overall mortality rate on the first KRT modality in this study was 1.2 (95% CI, 0.8–1.6) per 100 patient years. Across the decades, patients who received pre-emptive KT had consistently lower mortality rate compared with patients started on dialysis, 0.3 (95% CI, 0.1–0.9) versus 2.4 (95% CI, 1.5–3.8) in 2000–2010 and 0 versus 2.2 (95% CI, 1.2–3.9) in 2011–2020 (P < 0.05, Supplementary Table S2, Supplementary Figures S2 and S3). The most common causes of death on the first KRT modality within the 2 decades were cardiovascular disease (46.88%) and infections (21.88%) (P < 0.05, Supplementary Table S3).
Based on age at KRT initiation, infants had lower survival rates than other age groups, particularly 6 months and 1 year after KRT initiation. The highest survival was seen in children aged 10–14 years (Supplementary Table S4). Patients whose KRT was initiated at age <1 year had the shortest time to death at a median of 1 year (IQR, 0–1), whereas those who had the KRT initiation at age 10–14 years had the longest time to death at 9 years (3–11) (P < 0.05, Supplementary Table S5, Supplementary Figures S4–S9). Infection-related deaths were primarily noted in patients started on PD and aged <5 years (Supplementary Tables S3 and S5).
Among patients who died on PD as the last KRT modality, infections were the most common cause (40.63%), whereas cardiovascular problems were the most common cause (59.1%) for those who died on HD. Most patients who died on KT was because of other causes, namely severe metabolic acidosis, chronic respiratory failure, and vehicle accident. Approximately 56% of reported deaths on PD were in patients aged <5 years, whereas 61% of reported deaths on HD were aged >18 years (P < 0.05), with the most common cause of death being cardiovascular diseases in both groups (Supplementary Table S6).
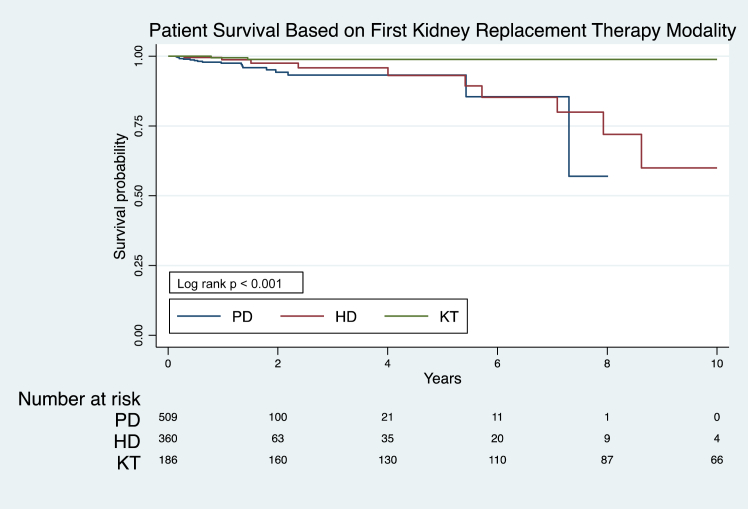
The overall patient survival rates among those who stayed on the first KRT modality at 1-year and 5-years were 98.3% (95% CI, 97–99) and 95.5% (95% CI, 93–97.1), respectively (Figure 1, Supplementary Table S7). Patient survival rates on the first KRT modality did not differ significantly across decades (Supplementary Table S8). However, patients who received pre-emptive KT consistently had significantly higher survival rates at 5, 10, and 15 years after KRT initiation than patients who were started on dialysis (P < 0.05). Among patients who started on dialysis, the association of better survival with PD compared with HD as the initial KRT modality was most pronounced within 6 months after KRT commencement (P < 0.05, Figure 1, Supplementary Table S7). Moreover, younger patients (aged <5 years) who started KRT with dialysis had consistently lower survival rates compared to older patients, 95.4% (95% CI, 91.0–97.7) versus 98.8% (95% CI, 96.8–99.6) at 1 year and 89.4% (95% CI, 81.6–94.0) versus 94.4% (95% CI, 86.8–97.7) at 5 years after initial KRT commencement, P < 0.05 (Supplementary Table S9). No improvements were observed in the survival of younger patients on dialysis over time (Supplementary Table S10).
Figure 1.
Patient survival on the first KRT modality: Australia and New Zealand Registry 2000–2020, log rank P < 0.001. HD, hemodialysis; KRT, kidney replacement therapy; KT, kidney transplantation; PD, peritoneal dialysis.
Compared with pre-emptive KT, having PD or HD as the initial KRT modality was associated with 2.4-fold (adjusted hazard ratio [aHR] 2.4, 95% CI 1.1– 5.7) and 3.6-fold (aHR 3.6, 95% CI 1.5–8.8) increases in mortality hazard, respectively (Table 2, Supplementary Figure S10). Age under 4 years at KRT initiation was associated with higher mortality, as was Māori ethnicity among Australian children (Table 2).38, 39, 40 Restricting the analysis model to include only patients started on dialysis produced similar results. However, dialysis modality (HD vs. PD) was not associated with increased mortality (aHR 1.2, 95% CI 0.4–3.4, P = 0.76; Supplementary Table S11). Compared with pre-emptive KT, having dialysis for more than 2 years before KT was associated with a 4.7-fold (aHR 4.7, 95% CI 1.6–13.5) increase in graft failure (Table 3).
Table 2.
Multivariable Cox proportional hazards analysis of mortality among children staying on the first KRT modality: Australia and New Zealand Registry 2000–2020
|
Parameter |
Multivariable-adjusted hazard ratio (95% CI) for Australia only |
Multivariable-adjusted hazard ratio (95% CI) for Australia and New Zealand |
|---|---|---|
| First KRT modality | ||
| Peritoneal dialysis vs. KT | 27.8 (4.6–168.3)a | 2.4 (1.1–5.7)a |
| Hemodialysis vs. KT | 21.2 (3.2–142.5)a | 3.6 (1.5–8.8)a |
| Period | ||
| 2011–2020 vs. 2000–2010 | 1.2 (0.4–3.2) | 0.9 (0.4–1.9) |
| Age at KRT | ||
| 5–9 vs. 0–4 yrs old | 0.2 (0.1–1.1) | 0.5 (0.2–0.8)a |
| 10–14 vs. 0–4 yrs old | 0.2 (0.1–0.8)a | 0.4 (0.2–0.7)a |
| 15–18 vs. 0–4 yrs old | 0.4 (0.1–1.6) | 0.5 (0.3–0.9)a |
ATSI, Aboriginal and Torres Strait Islander; CAKUT, congenital anomalies of the kidneys and urinary tract; HD, hemodialysis; KRT, kidney replacement therapy; KT, kidney transplantation; PD, peritoneal dialysis
Data from New Zealand alone was too limited to be shown separately.
Variables included in the model were selected based on background knowledge; these variables have been proven to be related to mortality in children receiving KRT.38, 39, 40
Statistically significant.
Table 3.
Crude and multivariable Cox proportional hazards analysis of graft failure and mortality comparing pre-emptive transplantation with effect of dialysis by vintage (time to transplant since dialysis initiation in months): Australia and New Zealand Registry 2000–2020
| Parameter | Crude hazard ratio (95% CI) | Multivariate-adjusted hazard ratio (95% CI) |
|---|---|---|
| Graft failure–death censored | ||
| Non pre-emptive vs. pre-emptive | ||
| 0–6 months vs. pre-emptive | 1.3 (0.8–2) | 1.1 (0.7–1.7) |
| 6–12 months vs. pre-emptive | 1.4 (0.9–2.1) | 1.2 (0.8–1.9) |
| 12–24 months vs. pre-emptive | 1.6 (1.1–2.4)a | 1.5 (0.9–2.2) |
| >24 months vs. pre-emptive | 2 (1.3–3)a | 1.7 (1.1–2.7)a |
| PD as first KRT vs. pre-emptive | ||
| 0–6 months vs. pre-emptive | 1.4 (0.8–2.2) | 1.3 (0.8–2.2) |
| 6–12 months vs. pre-emptive | 1.2 (0.7–2.1) | 1.1 (0.7–1.9) |
| 12–24 months vs. pre-emptive | 1.4 (0.9–2.2) | 1.5 (0.9–2.5) |
| >24 months vs. pre-emptive | 1.9 (1.2–3)a | 2.1 (1.2–3.6)a |
| HD as first KRT vs. pre-emptive | ||
| 0–6 months vs. pre-emptive | 1.1 (0.6–2) | 0.8 (0.5–1.5) |
| 6–12 months vs. pre-emptive | 1.6 (0.9–2.7) | 1.2 (0.7–2.2) |
| 12–24 months vs. pre-emptive | 2 (1.3–3.2)a | 1.4 (0.8–2.4) |
| >24 months vs. pre-emptive | 2.1 (1.3–3.5)a | 1.2 (0.6–2.4) |
| Mortality | ||
| Non-pre-emptive vs. pre-emptive | ||
| 0–6 months vs. pre-emptive | 1.2 (0.4–4.1) | 1.1 (0.3–3.8) |
| 6–12 months vs. pre-emptive | 1.6 (0.5–5.1) | 1.5 (0.5–5) |
| 12–24 months vs. pre-emptive | 1.3 (0.4–4) | 1.5 (0.5–4.6) |
| >24 months vs. pre-emptive | 3.6 (1.3–9.7)a | 4.7 (1.6–13.5)a |
| PD as first KRT vs. pre-emptive | ||
| 0–6 months vs. pre-emptive | 1.8 (0.5–6.4) | 2.4 (0.6–9.2) |
| 6–12 months vs. pre-emptive | 1.4 (0.3–5.6) | 1.5 (0.3–6.4) |
| 12–24 months vs. pre-emptive | 1.2 (0.3–4.5) | 1.7 (0.5–6.6) |
| >24 months vs. pre-emptive | 2.9 (0.9–9.4) | 4.5 (1.2–16.5)a |
| HD as first KRT vs. pre-emptive | ||
| 0–6 months vs. pre-emptive | 0.5 (0.1–4.4) | 0.3 (0.1–2.8) |
| 6–12 months vs. pre-emptive | 1.9 (0.5–7.6) | 1 (0.2–4.7) |
| 12–24 months vs. pre-emptive | 1.4 (0.3–5.5) | 0.8 (0.2–3.7) |
| >24 months vs. pre-emptive | 3.9 (1.3–11.7)a | 3.6 (0.9–14.6) |
CI, confidence interval; HD, hemodialysis; KRT, kidney replacement therapy; PD, peritoneal dialysis.
Statistically significant.
KRT Modality Changes/Sequential KRT Modality Utilization
Among 869 children commencing dialysis as the first KRT modality, 59.7% (519/869) received KT as their first change of treatment modality. Overall, 83.5% (726/869) of patients with dialysis at KRT commencement received KT after a median period of 1.3 years (IQR 0.7–2.7) (Supplementary Table S1). Among these, 28.5% (207/726) of patients switched between dialysis modalities (PD and HD) with a total of 281 changes before receiving KT (range of modality changes per patient, 1–6).
The prospect of receiving KT was similar among children started on PD and HD (aHR 0.9, 95% CI 0.8–1.1). However, lower KT probability was observed in children who started dialysis at age <5 years (aHR 0.7, 95% CI 0.6–0.9), age >14 years (aHR 0.7, 95% CI 0.5–0.8), and non-Caucasian ethnicities (aHR 0.6, 95% CI 0.5–0.7). Among those from non-Caucasian ethnicities, Māori children had the lowest chance of receiving KT (aHR 0.3, 95% CI 0.2–0.5). Overall, there were 1040 KTs in 915 patients, with 27.3% (250/915) of children returning to dialysis after their first KT. Furthermore, approximately 16.4% (41/250) of these graft failure episodes were observed in children who received KT pre-emptively (Supplementary Table S1).
Technique Failure
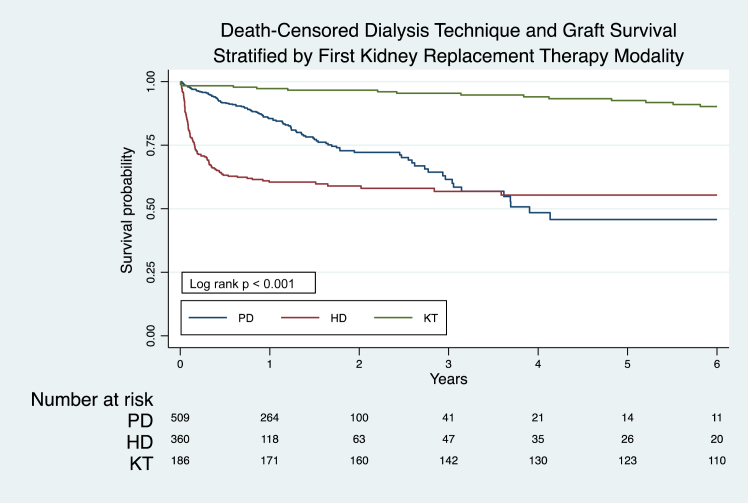
Among children who started on dialysis as the first KRT modality, 27.2% (237/869) experienced technique failure, with a median number of technique failure episodes of 1 (IQR, 1–2) per patient. The most common cause of technique failure in children who started on PD was infection (44.7% [46/102]), whereas for HD it was planned conversion to PD after acute HD (34.1% [46/135]) (Supplementary Table S12). The overall technique survival rates at 1, 2, and 5 years were 74.9% (95% CI, 71.6–77.9), 65.1% (95% CI, 60.8–69), and 49.9% (95% CI, 43.1–56.3), respectively (Supplementary Figure S10). Compared with patients who started on HD, patients who started on PD had significantly higher technique survival in the first 3 years (P < 0.05, Supplementary Table S13, Figure 2, Supplementary Figures S11 and S12). Furthermore, technique survival after initial KRT commencement did not significantly change across decades (Supplementary Table S14, Supplementary Figures S13–S20).
Figure 2.
Death-censored dialysis technique survival and graft survival stratified by the first KRT modality: Australia and New Zealand Registry 2000–2020, log rank P < 0.001.
HD, hemodialysis; KRT, kidney replacement therapy; KT, kidney transplantation; PD, peritoneal dialysis.
Poorer technique survival was associated with commencing HD as the first KRT (aHR 2.3, 95% CI, 1.7–3.1), having glomerulonephritis as the cause of kidney failure (aHR 1.6, 95% CI, 1.2–2.3), and being referred late to a pediatric nephrology unit (aHR 1.7, 95% CI, 1.3–2.2) (Table 4). Treating death as a competing risk yielded similar technique survival estimates and risk factors (Supplementary Tables S15–17).
Table 4.
Multivariable Cox proportional hazards analysis of death-censored technique failure among children receiving dialysis modalities (PD and HD) as the first KRT modality: Australia and New Zealand Registry 2000–2020
|
Parameter |
Multivariable-adjusted hazard ratio (95% CI) for Australia only |
Multivariable-adjusted hazard ratio (95% CI) for Australia and New Zealand |
|---|---|---|
| First KRT modality | ||
| Hemodialysis vs. PD | 2.7 (1.9–3.7)a | 2.3 (1.7–3.1)a |
| Period | ||
| 2011–2020 vs. 2000–2010 | 0.9 (0.7–1.2) | 1 (0.8–1.3) |
| Age at KRT | ||
| 5–9 vs. 0–4 years old | 1.1 (0.8–1.9) | 1.4 (0.9–2) |
| 10–14 vs. 0–4 years old | 0.7 (0.4–1) | 0.7 (0.5–1.1) |
| 15–18 vs. 0–4 years old | 0.6 (0.4–0.9)a | 0.6 (0.4–0.9)a |
| Gender | ||
| Female vs. male | 1.1 (0.8–1.4) | 1.1 (0.9–1.5) |
| Ethnicity | ||
| Asian vs. Caucasian | 0.8 (0.5–1.3) | 0.8 (0.5–1.3) |
| ATSIa vs. Caucasian | 0.9 (0.5–1.6) | 1 (0.6–1.7) |
| Māori vs. Caucasian | 1 (0.2–4.2) | 1.2 (0.7–2) |
| Pacific Islander vs. Caucasian | 1 (0.5–2) | 1.1 (0.7–1.9) |
| Other vs. Caucasian | 1 (0.6–1.6) | 1 (0.6–1.7) |
| Primary kidney diseases | ||
| Cystic diseases vs. CAKUT | 0.8 (0.4–1.4) | 0.7 (0.4–1.3) |
| Glomerulonephritis vs. CAKUT | 1.7 (1.2–2.5)a | 1.6 (1.2–2.3)a |
| Others vs. CAKUT | 1.1 (0.7–1.6) | 1 (0.7–1.5) |
| Late referral | 1.9 (1.4–2.6)a | 1.7 (1.3–2.2)a |
| Parent center state | ||
| New Zealand vs. Australia | 0.8 (0.6–1.2) | |
| Socio economic indexes for Area (IRSAD) | ||
| Quartile 2 vs. quartile 1 | 1 (0.7–1.4) | N/A |
| Quartile 3 vs. quartile 1 | 0.9 (0.6–1.4) | N/A |
| Quartile 4 vs. quartile 1 | 0.9 (0.6–1.4) | N/A |
| Accessibility/Remoteness Index of Australia (ARIA), | ||
| Regional vs. major | 1 (0.7–1.5) | N/A |
| Remote vs. major | 1.4 (0.6–3.2) | N/A |
ATSI, Aboriginal and Torres Strait Islander; CAKUT, congenital anomalies of the kidneys and urinary tract; HD, hemodialysis; KRT, kidney replacement therapy; KT, kidney transplantation; N/A, not available; PD, peritoneal dialysis.
Data from New Zealand alone was too limited to be shown separately.
Statistically significant.
Graft Failure
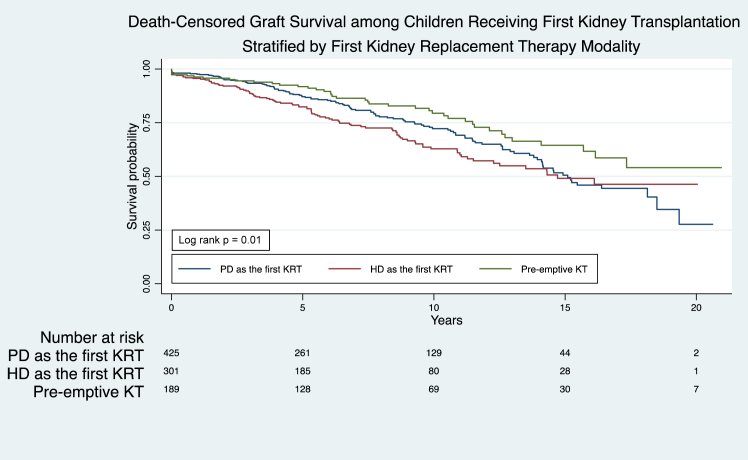
The overall 1-year, 5-year, 10-year, and 15-year graft survival rates on first KT were 97% (95% CI, 95.3–98), 86.5% (95% CI 83.9–88.7), 70.7% (95% CI 66.8–74.2), and 53.2% (95% CI 47.8–58.2), respectively. Patients with pre-emptive KT had the highest graft survival rates at 5 years and 10 years (92% [95% CI, 86.5–95.1] and 79.4% [95% CI, 71.2–85.6], respectively), followed by patients who had PD or HD at KRT initiation (P < 0.05, Supplementary Table S18,Figure 3). Compared with pre-emptive KT, having dialysis for more than 2 years before receiving KT was associated with a 1.7-fold (aHR 1.7, 95% CI 1.1–2.7) increase in graft failure (Table 3). Graft survival probability at 2 to 5 years after pre-emptive KT improved significantly across decades (P < 0.05, Supplementary Table S19, Supplementary Figures S21–S32). However, dialysis exposure before KT, age between 10 and 18 years at KT, and Aboriginal and Torres Strait Islander ethnicity (hereafter respectfully termed Indigenous Australians) were associated with an increased risk for graft failure. Compared with Caucasians, Asian ethnicity was associated with a decreased risk of graft failure, whereas Pacific Islander and Māori ethnicities were not associated with altered risks of graft failure (Table 5).
Figure 3.
Death-censored graft survival among children receiving the first KT, stratified by the first KRT modality (PD, HD, and KT): Australia and New Zealand Registry 2000–2020, log rank P = 0.01. HD, hemodialysis; KRT, kidney replacement therapy; KT, kidney transplantation; PD, peritoneal dialysis.
Table 5.
Multivariable Cox proportional hazards analysis of death-censored graft failure among children by the first KRT modality: Australia and New Zealand Registry 2000–2020
|
Parameter |
Multivariable-adjusted hazard ratio (95% CI) for Australia only | Multivariable-adjusted hazard ratio (95% CI) for Australia and New Zealand |
|---|---|---|
| First KRT modality | ||
| PD vs. KT | 1.1 (0.7–1.7) | 1.4 (0.9–2.1) |
| HD vs. KT | 1 (0.6–1.6) | 1.3 (0.9–2) |
| Period | ||
| 2011–2020 vs. 2000–2010 | 0.6 (0.4–0.9)a | 0.6 (0.4–0.9)a |
| Age at KRT | ||
| 5–9 vs. 0–4 years old | 0.9 (0.5–1.6) | 1.2 (0.7–1.9) |
| 10–14 vs. 0–4 years old | 1.8 (1.1–2.9)a | 2 (1.2–3.1)a |
| 15–18 vs. 0–4 years old | 2 (1.2–3.2) | 2.2 (1.4–3.5)a |
| Gender | ||
| Female vs. male | 1.1 (0.8–1.5) | 1.3 (0.9–1.7) |
| Ethnicity | ||
| Asian vs. Caucasian | 0.6 (0.3–1.2) | 0.5 (0.3–0.9)a |
| ATSIa vs. Caucasian | 2.7 (1.3–5.4)a | 2.6 (1.3–5.1)a |
| Māori vs. Caucasian | N/A | 0.7 (0.3–1.6) |
| Pacific Islander vs. Caucasian | 0.8 (0.2–2.5) | 1.4 (0.7–2.9) |
| Other vs. Caucasian | 1.2 (0.7–2) | 1.4 (0.8–2.2) |
| Primary kidney diseases | ||
| Cystic diseases vs. CAKUT | 1.5 (0.9–2.5) | 1.3 (0.8–2.1) |
| Glomerulonephritis vs. CAKUT | 1.3 (0.9–1.9) | 1.2 (0.8–1.7) |
| Others vs. CAKUT | 0.8 (0.5–1.3) | 0.8 (0.5–1.1) |
| Late referral | 0.8 (0.5–1.2) | 0.8 (0.5–1.1) |
| Parent center state | ||
| New Zealand vs. Australia | 1.3 (0.9–1.9) | |
| Socio economic indexes for Area (IRSAD) | ||
| Quartile 2 vs. quartile 1 | 0.9 (0.6–1.4) | N/A |
| Quartile 3 vs. quartile 1 | 0.9 (0.6–1.3) | N/A |
| Quartile 4 vs. quartile 1 | 0.7 (0.4–1.1) | N/A |
| Accessibility/Remoteness Index of Australia (ARIA), | ||
| Regional vs. major | 0.9 (0.6–1.3) | N/A |
| Remote vs. major | 1.6 (0.6–4.3) | N/A |
HD, hemodialysis; KRT, kidney replacement therapy; KT, kidney transplantation; N/A, not available; PD, peritoneal dialysis.
Data from New Zealand alone was too limited to be shown separately.
Statistically significant.
Discussion
This study, comprising a large binational sample of pediatric patients with kidney failure, showed that overall 1-year and 5-year survival rates on KRT were excellent at 98.3% and 95.5%, respectively. These rates had not significantly changed over the last 2 decades and better survival was significantly associated with age >4 years and pre-emptive KT. Similarly, technique survival rates were excellent and stable over the last 2 decades. For KT, graft survival rates significantly improved over the last 2 decades and poorer graft survival rates were associated with dialysis exposure prior to KT, age between 10 and 18 years at the time of transplant, and Indigenous status. Better early transplant outcomes and a reduction in risk of early graft loss have improved graft survival in the last decade.18,19 The use of a tacrolimus/mycophenolate/prednisone regimen in the last decade, which was changed from a regimen of cyclosporine/mycophenolate/prednisone in the earlier decade, might have contributed to this early allograft loss risk reduction.41
The survival rates observed on KRT in Australia and New Zealand in this study were similar to those reported in other high-income countries, ranging from 97.6% to 99.2% for 1-year survival and 93% to 96.7% for 5-year survival.2,42, 43, 44 Furthermore, although a previous ANZDATA Registry study reported a significant improvement in pediatric KRT survival in Australia and New Zealand between 1963 and 2002,1 no further significant improvements in survival were observed over the last 20 years in the present study. Although overall survival rates of pediatric patients with kidney failure on KRT were excellent, their overall mortality rate remains at least 20-fold higher than that of the general pediatric populations.45
Similar to what has been previously reported in United States, United Kingdom, and European studies,44,46,47 we found that patients who started on dialysis had a higher risk of death than patients who received pre-emptive KT, even following adjustment for demographic and clinical factors. These findings may be related to dialysis-associated cardiovascular damage, access complications, infections, retarded linear growth, and poor cognitive development in children.48, 49, 50 A previous study from the United States involving over 7500 patients aged <18 years receiving KT demonstrated that children who received any dialysis modalities had a 2-fold higher risk for death (aHR 1.7, 95% CI 1.2–2.3) compared with children who received pre-emptive KT, regardless of the type of donor sources and transplant factors.51
Pre-emptive KT is a superior KRT option for children with kidney failure because it potentially avoids patient exposure to the adverse outcomes associated with dialysis.52 Its only caveat was that pre-emptive KT was not always possible, because it was generally delayed until the patients’ weight was 10 kg or higher.53 In our study, the minimum weight of a child having pre-emptive KT was 9.4 kg. Medical conditions, such as active glomerulonephritis, severe cardiac dysfunction, or malignancy may also preclude pre-emptive KT.54,55 Children who lived in regional or remote areas also had a 35% lower likelihood of receiving pre-emptive KT compared with children who lived in major cities where transplant centers were located.56 Furthermore, Australia's current practice of performing pediatric pre-emptive KT exclusively with living donor kidneys may have limited patients’ chances of accessing pre-emptive KT. Patients who did not have a living kidney donor at planned pre-emptive KT were left with dialysis modality as the sole life-saving alternative.
In the adult literature, PD was seen as an ideal bridge to KT due to its favorable profile in relation to patient survival, hemodynamic stability, infection risk, and preservation of residual kidney function and vascular access.54,55 In children, PD is particularly advantageous in facilitating regular school attendance and maintaining functional and complication-free vascular access in very small patients.57 However, we did not find any significant survival benefit associated with starting patients on PD compared with HD. This result agreed with those reported in previous similar-sized studies (<1000 patients) from Taiwanese, Korean, and Italian registries.58, 59, 60 In contrast, a propensity score-matched study of 3784 European children aged <19 years on dialysis showed that children who initially started on HD had a higher mortality risk compared with those on PD (propensity score 1.39, 95% CI 1.1–1.8), especially during the first year of dialysis (propensity score 1.8, 95% CI 1.0–2.4).42 Conversely, data from the United States, with >11,000 dialysis patients aged <21 years old, showed that PD was associated with a higher risk of death compared to HD, especially in patients starting at age >12 years (aHR 1.73, 95%CI 1.3–2.2, P < 0.001).61 Aside from having a larger cohort, study design and case-mix differences might explain the conflicting results between our study and the previous European and United States studies. First, the majority of patients in our study and the European cohort initiated HD using a central venous catheter, whereas the majority of patients aged >12 years in the United States study used arteriovenous fistulas or grafts.42,61,62 Second, the previous European study defined initial dialysis modality as treatment at day 30. Therefore, deaths occurring before day 30 were not counted. They also had a higher proportion of patients aged <5 years started on HD than in our study; 50% (278/555) versus 22% (49/219), respectively; and spent longer time on HD (1.76 years [95% CI 1.64–1.87] than those in our study, 1.1 years (95% CI 1.1–1.5).42
HD access type has been recognized as an essential modifier of dialysis modality and survival association. A previous study in adult patients reported that a survival advantage in patients with PD was only significant when comparing patients with HD who initiated with central venous catheter.63 Younger age at KRT initiation has also been reported to be associated with poorer patient survival and post KT graft survival.18,53,60 Furthermore, a Scottish study with 477 patients aged <18 years reported that patients staying on either PD or HD conferred a 20-fold increased risk of all-cause mortality compared with patients who received KT on follow-up, P <0.05.64 Similarly, previous work by Amaral et al.51 demonstrated that in the United States, patients <18 years old who stayed <12 and >18 months on dialysis experienced 60% (aHR 1.6, 95% CI 1.1–2.3) and 90% (aHR 1.9, 95% CI 1.3–2.7) increased risks of death, respectively, compared to patients who received pre-emptive KT. Nevertheless, most decision-making processes for choosing the initial KRT modality for children with kidney failure in clinical practice are multifactorial.25,65, 66, 67, 68 The clinicians will assess not only medical but also social factors, quality of life, patient and family preferences, and even contextual factors such as the location of residence.69 Therefore, strategies to increase patient survival on KRT should include choosing a particular dialysis modality and expediting KT for patients on dialysis. These strategies are particularly needed for non-Caucasians and patients commencing KRT under 5 years of age, because these patients were less likely to receive KT and had a higher mortality risk than the general cohort.18,47,70, 71, 72, 73
We observed a higher technique survival rate in patients who started on PD compared with HD. This result should be interpreted with caution, because 41% of HD technique failure causes were unknown, and 36% resulted from an initial plan to convert to PD after an acute HD (Supplementary Table S6).
Interestingly, only 3.2% (6/189) of children receiving pre-emptive KT were from Aboriginal and Torres Strait Islander, Māori, and Pacific Islander ethnicities. Although Indigenous peoples have a higher incidence of kidney failure and earlier age at CKD onset, their use of KRT was less than that of non-Indigenous peoples.56,70,74,75 The Indigenous population had a longer duration of dialysis before KT, lower frequency of receiving a live kidney donor transplant, a lower rate of pre-emptive KT, and a higher frequency of not receiving KT for their kidney failure treatment. Language barriers, remoteness, poor access to dialysis and transplant services, and health care professionals’ attitudes have contributed to the long waiting times and worse transplant outcomes in Indigenous populations. The disparities in KRT access among Indigenous peoples in Australia and New Zealand need to be addressed, because these also affect their survival.70
A key strength of our study is the large sample size and inclusion of all children with treated kidney failure from all units in Australia and New Zealand. Other strengths include prospective data collection, long follow-up duration with minimal loss to follow-up, and the use of robust statistical methodologies. These enabled us to provide valid and reasonably precise long-term survival estimates and identify modifiable and unmodifiable risk factors for death. However, the current study has limitations. The ANZDATA Registry does not record details of patients with kidney failure who are not treated with KRT. Attitudes among nephrologists toward offering KRT to very young children may vary considerably. Other information regarding details of treatment in the registry is limited. Data on parameters and interventions associated with morbidity and mortality, such as hemoglobin concentration, erythropoiesis-stimulating agents, lipid levels, blood pressure, growth hormone, cardiac function, mineral bone disorders, and vascular access peritoneal membrane characteristics, were not collected. The possibility of coding bias cannot be excluded.
Conclusion
Compared with previously published ANZDATA Registry data, KRT utilization increased in all age categories, with PD being the most commonly prescribed modality for Australasian children. Patient survival rates on KRT were excellent and remained stable over the last 20 years. Given that mortality rates in children with kidney failure on KRT remain 20-fold higher than in the general pediatric population, there is an exigent need to try to further improve outcomes in this vulnerable group.
Disclosure
CGA, EM, AF, LJK, and RL have declared no conflicting interests. DWJ has received consultancy fees, research grants, and speaker’s honoraria from Baxter Healthcare and Fresenius Medical Care, consultancy fees from AstraZeneca, Bayer and AWAK, speaker’s honoraria from ONO and BI & Lilly, and travel sponsorships from ONO and Amgen. He is a current recipient of an Australian National Health and Medical Research Council Leadership Investigator Grant. YC has received research grants and speaker’s honoraria from Baxter Healthcare and Fresenius Medical Care. She is a current recipient of an Australian National Health and Medical Research Council Emerging Leadership Investigator Grant, and the Advancing Queensland Clinical Research Fellowship.
Acknowledgments
The authors are thankful to the patients and staff of the Australian and New Zealand kidney units for their contributions to the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). Data reported in this study were provided by ANZDATA. However, interpretation and reporting of these data are the responsibility of the authors and do not reflect the policy of the ANZDATA. Assistance with statistics was provided by Hardya Gustada Hikmahrachim, Fira Alyssa Gabriella Sinuraya, and Chika Carnation Tandri. The authors are also grateful to the International Society for Peritoneal Dialysis (ISPD) Asia Pacific Chapter for supporting CGA’s clinical fellowship in Princess Alexandra Hospital and Queensland Children’s Hospital, Brisbane, Australia in August to November 2021. This work was previously presented as a scientific poster at the ISPD Congress in August 2022 in Singapore.
Footnotes
Figure S1. Proportion of the first KRT modalities in children (0–18 years old) in Australia and New Zealand, 2000–2020, by year.
Figure S2. Overall patient survival in children receiving the first KRT modality.
Figure S3. Patient survival in children based on first KRT modality.
Figure S4. Patient survival in children receiving KRT based on age at KRT initiation.
Figure S5. Patient survival in children started on KRT at < 1 year old.
Figure S6. Patient survival in children started on KRT at 1–4 years old.
Figure S7. Patient survival in children started on KRT at 5–9 years old.
Figure S8. Patient survival in children started on KRT at 10–14 years old.
Figure S9. Patient survival in children started on KRT at 15–18 years old.
Figure S10. Dialysis patient survival related with history of technique failure (among all patients receiving PD and HD as the first KRT modality).
Figure S11. Overall technique survival in children receiving the first KRT modality.
Figure S12. Non-censored for death technique survival stratified by the first KRT modality.
Figure S13. Death-censored PD technique survival as the first KRT modality by decade.
Figure S14. Death-censored HD technique survival as the first KRT modality by decade.
Figure S15. Non-censored for death PD technique survival by decade.
Figure S16. Non-censored for death HD technique survival by decade.
Figure S17. Death-censored PD technique survival as the first KRT modality by age categories.
Figure S18. Death-censored HD technique survival as the first KRT modality by age categories.
Figure S19. Non-censored for death PD technique survival for PD as the first KRT modality by age categories.
Figure S20. Non-censored for death HD technique survival for HD as the first KRT modality.
Figure S21. Death-censored graft survival by age categories at time of transplantation.
Figure S22. Non-censored for death graft survival after pre-emptive KT by decade by age categories.
Figure S23. Death-censored graft survival after pre-emptive KT by decade.
Figure S24. Non-censored for death graft survival after pre-emptive KT by age categories at time of transplantation.
Figure S25. Death-censored dialysis technique survival and graft survival stratified by first KRT modality in children aged 0–4 years old.
Figure S26. Death-censored dialysis technique survival and graft survival stratified by first KRT modality in children aged 5–9 years old.
Figure S27. Death-censored dialysis technique survival and graft survival stratified by first KRT modality in children aged 10–14 years old.
Figure S28. Death-censored dialysis technique survival and graft survival stratified by first KRT modality in children aged 15–18 years old.
Figure S29. Non-censored for death dialysis technique survival and graft survival stratified by first KRT modality in children aged 0–4 years old.
Figure S30. Non-censored for death dialysis technique survival and graft survival stratified by first KRT modality in children aged 5–9 years old.
Figure S31. Non-censored for death dialysis technique survival and graft survival stratified by first KRT modality in children aged 10–14 years old.
Figure S32. Non-censored for death dialysis technique survival and graft survival stratified by first KRT modality in children aged 15–18 years old.
Table S1. Failures, death, and dialysis modality changes at the first KRT modality: Australia and New Zealand Registry 2000–2020.
Table S2. Death rates on the first KRT modality.
Table S3. Cause of death on the first KRT modality among pediatric KRT patients: Australia and New Zealand Registry 2000–2020.
Table S4. Comparison of patient survival among children receiving KRT, stratified by the age at KRT initiation: Australia and New Zealand Registry 2000–2020.
Table S5. Time to death, cause of death, and number of KRT sequences, by age at KRT initiation.
Table S6. Overall cause of death based on the last KRT modality among pediatric KRT patients: Australia and New Zealand Registry 2000–2020.
Table S7. Comparison of patient survival among children receiving PD, HD, and KT on the first KRT modality: Australia and New Zealand Registry 2000–2020.
Table S8. Patient survival among children staying on the first KRT modality, by decade.
Table S9. Comparison of patient survival among children who commenced dialysis at age < 5 years old and > 5 years old: Australia and New Zealand Registry 2000–2020.
Table S10. Patient survival among children aged < 5 years old staying on the first dialysis modality, by decade.
Table S11. Multivariable Cox proportional hazards analysis of mortality among children receiving dialysis modalities (PD and HD) as the first KRT: Australia and New Zealand Registry 2000–2020.
Table S12. Causes of technique failure in children with dialysis as the first KRT modality: Australia and New Zealand Registry 2000–2020.
Table S13. Death-censored technique survival among children receiving dialysis (PD and HD) as the first KRT modality: Australia and New Zealand Registry 2000–2020.
Table S14. Death-censored technique survival among children receiving dialysis (PD and HD) as the first KRT modality, by decade.
Table S15. Non-censored for death technique survival among children receiving dialysis (PD and HD) as the first KRT modality: Australia and New Zealand Registry 2000–2020.
Table S16. Non-censored for death technique survival among children receiving dialysis (PD and HD) as the first KRT modality, by decade.
Table S17. Multivariable Cox proportional hazards analysis of non-censored for death technique failure among children receiving dialysis modalities (PD and HD) as the first KRT: Australia and New Zealand Registry 2000–2020.
Table S18. Comparison of death-censored graft survival among children receiving first KT, stratified by the first KRT modality: Australia and New Zealand Registry 2000–2020.
Table S19. Death-censored graft survival among children receiving pre-emptive kidney transplantation, by decade.
Supplementary Material
Figure S1. Proportion of the first KRT modalities in children (0–18 years old) in Australia and New Zealand, 2000–2020, by year.
Figure S2. Overall patient survival in children receiving the first KRT modality.
Figure S3. Patient survival in children based on first KRT modality.
Figure S4. Patient survival in children receiving KRT based on age at KRT initiation.
Figure S5. Patient survival in children started on KRT at < 1 year old.
Figure S6. Patient survival in children started on KRT at 1–4 years old.
Figure S7. Patient survival in children started on KRT at 5–9 years old.
Figure S8. Patient survival in children started on KRT at 10–14 years old.
Figure S9. Patient survival in children started on KRT at 15–18 years old.
Figure S10. Dialysis patient survival related with history of technique failure (among all patients receiving PD and HD as the first KRT modality).
Figure S11. Overall technique survival in children receiving the first KRT modality.
Figure S12. Non-censored for death technique survival stratified by the first KRT modality.
Figure S13. Death-censored PD technique survival as the first KRT modality by decade.
Figure S14. Death-censored HD technique survival as the first KRT modality by decade.
Figure S15. Non-censored for death PD technique survival by decade.
Figure S16. Non-censored for death HD technique survival by decade.
Figure S17. Death-censored PD technique survival as the first KRT modality by age categories.
Figure S18. Death-censored HD technique survival as the first KRT modality by age categories.
Figure S19. Non-censored for death PD technique survival for PD as the first KRT modality by age categories.
Figure S20. Non-censored for death HD technique survival for HD as the first KRT modality.
Figure S21. Death-censored graft survival by age categories at time of transplantation.
Figure S22. Non-censored for death graft survival after pre-emptive KT by decade by age categories.
Figure S23. Death-censored graft survival after pre-emptive KT by decade.
Figure S24. Non-censored for death graft survival after pre-emptive KT by age categories at time of transplantation.
Figure S25. Death-censored dialysis technique survival and graft survival stratified by first KRT modality in children aged 0–4 years old.
Figure S26. Death-censored dialysis technique survival and graft survival stratified by first KRT modality in children aged 5–9 years old.
Figure S27. Death-censored dialysis technique survival and graft survival stratified by first KRT modality in children aged 10–14 years old.
Figure S28. Death-censored dialysis technique survival and graft survival stratified by first KRT modality in children aged 15–18 years old.
Figure S29. Non-censored for death dialysis technique survival and graft survival stratified by first KRT modality in children aged 0–4 years old.
Figure S30. Non-censored for death dialysis technique survival and graft survival stratified by first KRT modality in children aged 5–9 years old.
Figure S31. Non-censored for death dialysis technique survival and graft survival stratified by first KRT modality in children aged 10–14 years old.
Figure S32. Non-censored for death dialysis technique survival and graft survival stratified by first KRT modality in children aged 15–18 years old.
Table S1. Failures, death, and dialysis modality changes at the first KRT modality: Australia and New Zealand Registry 2000–2020.
Table S2. Death rates on the first KRT modality.
Table S3. Cause of death on the first KRT modality among pediatric KRT patients: Australia and New Zealand Registry 2000–2020.
Table S4. Comparison of patient survival among children receiving KRT, stratified by the age at KRT initiation: Australia and New Zealand Registry 2000–2020.
Table S5. Time to death, cause of death, and number of KRT sequences, by age at KRT initiation.
Table S6. Overall cause of death based on the last KRT modality among pediatric KRT patients: Australia and New Zealand Registry 2000–2020.
Table S7. Comparison of patient survival among children receiving PD, HD, and KT on the first KRT modality: Australia and New Zealand Registry 2000–2020.
Table S8. Patient survival among children staying on the first KRT modality, by decade.
Table S9. Comparison of patient survival among children who commenced dialysis at age < 5 years old and > 5 years old: Australia and New Zealand Registry 2000–2020.
Table S10. Patient survival among children aged < 5 years old staying on the first dialysis modality, by decade.
Table S11. Multivariable Cox proportional hazards analysis of mortality among children receiving dialysis modalities (PD and HD) as the first KRT: Australia and New Zealand Registry 2000–2020.
Table S12. Causes of technique failure in children with dialysis as the first KRT modality: Australia and New Zealand Registry 2000–2020.
Table S13. Death-censored technique survival among children receiving dialysis (PD and HD) as the first KRT modality: Australia and New Zealand Registry 2000–2020.
Table S14. Death-censored technique survival among children receiving dialysis (PD and HD) as the first KRT modality, by decade.
Table S15. Non-censored for death technique survival among children receiving dialysis (PD and HD) as the first KRT modality: Australia and New Zealand Registry 2000–2020.
Table S16. Non-censored for death technique survival among children receiving dialysis (PD and HD) as the first KRT modality, by decade.
Table S17. Multivariable Cox proportional hazards analysis of non-censored for death technique failure among children receiving dialysis modalities (PD and HD) as the first KRT: Australia and New Zealand Registry 2000–2020.
Table S18. Comparison of death-censored graft survival among children receiving first KT, stratified by the first KRT modality: Australia and New Zealand Registry 2000–2020.
Table S19. Death-censored graft survival among children receiving pre-emptive kidney transplantation, by decade.
References
- 1.McDonald S.P., Craig J.C. Australian and New Zealand Paediatric Nephrology Association. Long-term survival of children with end stage renal disease. N Engl J Med. 2004;350:2654–2662. doi: 10.1056/NEJMoa031643. [DOI] [PubMed] [Google Scholar]
- 2.United States renal data system USRDS annual data report. Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. https://adr.usrds.org/2021/end-stage-renal-disease/8-esrd-among-children-and-adolescents Published 2022.
- 3.Bonthuis M., Vidal E., Bjerre A., et al. Ten-year trends in epidemiology and outcomes of pediatric kidney replacement therapy in Europe: data from the ESPN/ERA-EDTA Registry. Pediatr Nephrol. 2021;36:2337–2348. doi: 10.1007/s00467-021-04928-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.See E.J., Hedley J., Agar J.W.M., et al. Patient survival on haemodiafiltration and haemodialysis: a cohort study using the Australia and New Zealand Dialysis and Transplant Registry. Nephrol Dial Transplant. 2019;34:326–338. doi: 10.1093/ndt/gfy209. [DOI] [PubMed] [Google Scholar]
- 5.Ambarsari C.G., Hidayati E.L., Trihono P.P., et al. Experience of the first 6 years of pediatric kidney transplantation in Indonesia: a multicenter retrospective study. Pediatr Transplant. 2020;24 doi: 10.1111/petr.13812. [DOI] [PubMed] [Google Scholar]
- 6.Scott T., Ethier I., Hawley C., et al. Burden of kidney failure from atheroembolic disease and association with survival in people receiving dialysis in Australia and New Zealand: a multi-centre registry study. BMC Nephrol. 2021;22:401. doi: 10.1186/s12882-021-02604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson G.J., Cho Y., Teixiera-Pinto A., et al. Long-term outcomes of patients with end-stage kidney disease due to membranoproliferative glomerulonephritis: an ANZDATA registry study. BMC Nephrol. 2019;20:417. doi: 10.1186/s12882-019-1605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.See E.J., Johnson D.W., Hawley C.M., et al. Risk predictors and causes of technique failure within the first year of peritoneal dialysis: an Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) study. Am J Kidney Dis. 2018;72:188–197. doi: 10.1053/j.ajkd.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Chen J.H.C., Johnson D.W., Hawley C., Boudville N., Lim W.H. Association between causes of peritoneal dialysis technique failure and all-cause mortality. Sci Rep. 2018;8:3980. doi: 10.1038/s41598-018-22335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambarsari C.G., Trihono P.P., Kadaristiana A., et al. Five-year experience of continuous ambulatory peritoneal dialysis in children: a single center experience in a developing country. Med J Indones. 2019;28:329–337. doi: 10.13181/mji.v28i4.3807. [DOI] [Google Scholar]
- 11.Doucet B.P., Cho Y., Campbell S.B., et al. Kidney transplant outcomes in elderly recipients: an Australia and New Zealand Dialysis and Transplant (ANZDATA) registry study. Transplant Proc. 2021;53:1915–1926. doi: 10.1016/j.transproceed.2021.06.024. [DOI] [PubMed] [Google Scholar]
- 12.ANZDATA registry [43rd Report] Australia and New Zealand Dialysis and Transplant Registry:Adelaide; Australia: 2022. Chapter 1: Incidence of Renal Replacement Therapy for End Stage Kidney Disease.https://www.anzdata.org.au/wp-content/uploads/2020/09/c01_incidence_2019_ar_2020_v1.0_20201111.pdf [Google Scholar]
- 13.Ambarsari C.G., Tambunan T., Pardede S.O., Rahman F.H.F., Kadaristiana A. Role of dipstick albuminuria in progression of paediatric chronic kidney disease. J Pak Med Assoc. 2021;71(suppl2):S103–S106. [PubMed] [Google Scholar]
- 14.Wente-Schulz S., Aksenova M., Awan A., et al. Aetiology, course and treatment of acute tubulointerstitial nephritis in paediatric patients: a cross-sectional web-based survey. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-047059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santoso D.N., Sinuraya F.A.G., Ambarsari C.G. Distal renal tubular acidosis presenting with an acute hypokalemic paralysis in an older child with severe vesicoureteral reflux and syringomyelia: a case report. BMC Nephrol. 2022;23:248. doi: 10.1186/s12882-022-02874-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ANZDATA Registry.43rd Report . Australia and New Zealand Dialysis and Transplant Registry,Adelaide; Australia: 2020. Chapter 12: Paediatric patients with end stage kidney disease requiring renal replacement therapy.https://www.anzdata.org.au/wp-content/uploads/2020/09/c12_paediatric_2019_ar_2020_v0.13_202102016.pdf [Google Scholar]
- 17.Ambarsari C.G., Trihono P.P., Kadaristiana A., et al. Low-dose maintenance intravenous iron therapy can prevent anemia in children with end-stage renal disease undergoing chronic hemodialysis. Int J Nephrol. 2020;2020 doi: 10.1155/2020/3067453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larkins N.G., Wong G., Alexander S.I., et al. Survival and transplant outcomes among young children requiring kidney replacement therapy. Pediatr Nephrol. 2021;36:2443–2452. doi: 10.1007/s00467-021-04945-9. [DOI] [PubMed] [Google Scholar]
- 19.Sypek M.P., Davies C.E., Le Page A.K., et al. Paediatric deceased donor kidney transplant in Australia: a 30-year review-What have paediatric bonuses achieved and where to from here? Pediatr Transplant. 2021;25 doi: 10.1111/petr.14019. [DOI] [PubMed] [Google Scholar]
- 20.Ambarsari C.G., Bermanshah E.K., Putra M.A., Rahman F.H.F., Pardede S.O. Effective management of peritoneal dialysis-associated hydrothorax in a child: a case report. Case Rep Nephrol Dial. 2020;10:18–25. doi: 10.1159/000506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bordador E.B., Johnson D.W., Henning P., et al. Epidemiology and outcomes of peritonitis in children on peritoneal dialysis in Australasia. Pediatr Nephrol. 2010;25:1739–1745. doi: 10.1007/s00467-010-1510-5. [DOI] [PubMed] [Google Scholar]
- 22.Ambarsari C.G., Hidayati E.L., Mushahar L., Kadaristiana A. Dressing versus non-dressing technique for long-term exit-site care in children on continuous ambulatory peritoneal dialysis: a single-center retrospective cohort study. Med J Indones. 2020;29:290–297. doi: 10.13181/mji.oa.204171. [DOI] [Google Scholar]
- 23.Karopadi A.N., Mason G., Rettore E., Ronco C. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant. 2013;28:2553–2569. doi: 10.1093/ndt/gft214. [DOI] [PubMed] [Google Scholar]
- 24.Obiagwu P.N., Sangweni B., Moonsamy G., Khumalo T., Levy C. Health-related quality of life in children and adolescents with end-stage renal disease receiving dialysis in Johannesburg. S Afr J Child Health. 2018;12 doi: 10.7196/SAJCH.2018.v12i2.1457. [DOI] [Google Scholar]
- 25.Ambarsari C.G., Cahyadi D., Sari L., et al. Late diagnosis of Lesch-Nyhan disease complicated with end-stage renal disease and tophi burst: a case report. Ren Fail. 2020;42:113–121. doi: 10.1080/0886022X.2020.1713805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ANZDATA Registry Data policies. https://www.anzdata.org.au/anzdata/services/data-policies/
- 27.Registry AaNZDaT Data policies. https://www.anzdata.org.au/anzdata/services/data-policies/ Published 2022.
- 28.Chan S., Cho Y., Koh Y.H., et al. Association of Socio-economic position with technique failure and mortality in Australian non-indigenous peritoneal dialysis patients. Perit Dial Int. 2017;37:397–406. doi: 10.3747/pdi.2016.00209. [DOI] [PubMed] [Google Scholar]
- 29.Australian Bureau of Statistics Census of population and housing: socio-economic indexes for areas (SEIFA), Australia. https://www.abs.gov.au/ausstats/abs@.nsf/mf/2033.0.55.001 Published 2022.
- 30.Zhao Y., You J., Wright J., Guthridge S.L., Lee A.H. Health inequity in the Northern Territory, Australia. Int J Equity Health. 2013;12:79. doi: 10.1186/1475-9276-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Department of Health Accessibility Remoteness Index of Australia (ARIA) remoteness Area (RA). Australian Government. https://www1.health.gov.au/internet/publications/publishing.nsf/Content/ARIA-Review-Report-2011∼ARIA-Review-Report-2011-2∼ARIA-Review-Report-2011-2-2-3 Published 2022.
- 32.The University of Auckland Deprivation and health geography within NZ. https://imdmap.auckland.ac.nz/download/ Published 2018.
- 33.Perl J., Davies S.J., Lambie M., Pisoni R.L., et al. The Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS): unifying efforts to inform practice and improve global outcomes in peritoneal dialysis. Perit Dial Int. 2016;36:297–307. doi: 10.3747/pdi.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vidmar S.I., Cole T.J., Pan H. Standardizing anthropometric measures in children and adolescents with functions for egen: update. The Stata Journal. 2013;13:366–378. doi: 10.1177/1536867X1301300211. [DOI] [Google Scholar]
- 35.Leroy J. ZSCORE06: Stata module to calculate anthropometric z-scores using the 2006 WHO child growth standards. Boston College Department of Economics. https://ideas.repec.org/c/boc/bocode/s457279.html Published 2022.
- 36.World Health Organization WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. https://www.who.int/tools/child-growth-standards/standards Published 2022.
- 37.Kuczmarski R.J., Ogden C.L., Guo S.S., et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1–190. [PubMed] [Google Scholar]
- 38.Chesnaye N.C., Schaefer F., Bonthuis M., et al. Mortality risk disparities in children receiving chronic renal replacement therapy for the treatment of end-stage renal disease across Europe: an ESPN-ERA/EDTA registry analysis. Lancet. 2017;389:2128–2137. doi: 10.1016/S0140-6736(17)30063-6. [DOI] [PubMed] [Google Scholar]
- 39.Schild R., Dupont S., Harambat J., et al. Disparities in treatment and outcome of kidney replacement therapy in children with comorbidities: an ESPN/ERA Registry study. Clin Kidney J. 2023;16:745–755. doi: 10.1093/ckj/sfad008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chesnaye N.C., van Stralen K.J., Bonthuis M., Harambat J., Groothoff J.W., Jager K.J. Survival in children requiring chronic renal replacement therapy. Pediatr Nephrol. 2018;33:585–594. doi: 10.1007/s00467-017-3681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Francis A., Johnson D.W., Melk A., et al. Survival after kidney transplantation during childhood and adolescence. Clin J Am Soc Nephrol. 2020;15:392–400. doi: 10.2215/cjn.07070619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chesnaye N.C., Schaefer F., Groothoff J.W., et al. Mortality risk in European children with end-stage renal disease on dialysis. Kidney Int. 2016;89:1355–1362. doi: 10.1016/j.kint.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 43.UK Renal Registry . 2020. UK Renal Registry 22nd annual report – data to 31/12/2018.https://ukkidney.org/audit-research/annual-report/22nd-annual-report-data-31122018 Bristol, UK. [Google Scholar]
- 44.UK Kidney Association 23rd Annual Report - data to 31/12/2019. https://ukkidney.org/audit-research/annual-report/23rd-annual-report-data-31122019
- 45.The State of Queensland (Queensland Family and Child Commission) 2017. Australian and New Zealand Child Death Statistics.https://www.qfcc.qld.gov.au/sites/default/files/2022-06/Australia%20and%20New%20Zealand%20child%20death%20statistics%202017.pdf [Google Scholar]
- 46.Chavers B.M., Molony J.T., Solid C.A., Rheault M.N., Collins A.J. One-year mortality rates in US children with end-stage renal disease. Am J Nephrol. 2015;41:121–128. doi: 10.1159/000380828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chesnaye N., Bonthuis M., Schaefer F., et al. Demographics of paediatric renal replacement therapy in Europe: a report of the ESPN/ERA-EDTA registry. Pediatr Nephrol. 2014;29:2403–2410. doi: 10.1007/s00467-014-2884-6. [DOI] [PubMed] [Google Scholar]
- 48.Warady B.A., Chadha V. Chronic kidney disease in children: the global perspective. Pediatr Nephrol. 2007;22:1999–2009. doi: 10.1007/s00467-006-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harambat J., Ekulu P.M. Inequalities in access to pediatric ESRD care: a global health challenge. Pediatr Nephrol. 2016;31:353–358. doi: 10.1007/s00467-015-3263-7. [DOI] [PubMed] [Google Scholar]
- 50.Palupi-Baroto R., Hermawan K., Murni I.K., et al. High fibroblast growth factor 23 as a biomarker for severe cardiac impairment in children with chronic kidney disease: a single tertiary center study. Int J Nephrol Renovasc Dis. 2021;14:165–171. doi: 10.2147/IJNRD.S304143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amaral S., Sayed B.A., Kutner N., Patzer R.E. Preemptive kidney transplantation is associated with survival benefits among pediatric patients with end-stage renal disease. Kidney Int. 2016;90:1100–1108. doi: 10.1016/j.kint.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bock M.E., Cohn R.A. Pre-emptive kidney transplantation--just do it. Pediatr Transplant. 2010;14:561–564. doi: 10.1111/j.1399-3046.2010.01347.x. [DOI] [PubMed] [Google Scholar]
- 53.Hogan J., Bacchetta J., Charbit M., et al. Patient and transplant outcome in infants starting renal replacement therapy before 2 years of age. Nephrol Dial Transplant. 2018;33:1459–1465. doi: 10.1093/ndt/gfy040. [DOI] [PubMed] [Google Scholar]
- 54.Tang M., Li T., Liu H. A comparison of transplant outcomes in peritoneal and hemodialysis patients: a meta-analysis. Blood Purif. 2016;42:170–176. doi: 10.1159/000446272. [DOI] [PubMed] [Google Scholar]
- 55.Tang S.C.W., Lai K.N. Peritoneal dialysis: the ideal bridge from conservative therapy to kidney transplant. J Nephrol. 2020;33:1189–1194. doi: 10.1007/s40620-020-00787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Francis A., Didsbury M., Lim W.H., et al. The impact of socioeconomic status and geographic remoteness on access to pre-emptive kidney transplantation and transplant outcomes among children. Pediatr Nephrol. 2016;31:1011–1019. doi: 10.1007/s00467-015-3279-z. [DOI] [PubMed] [Google Scholar]
- 57.Warady B.A., Schaefer F., Bagga A., et al. Prescribing peritoneal dialysis for high-quality care in children. Perit Dial Int. 2020;40:333–340. doi: 10.1177/0896860819893805. [DOI] [PubMed] [Google Scholar]
- 58.Lin H.H., Tsai C.W., Lin P.H., et al. Survival analysis of pediatric dialysis patients in Taiwan. Nephrol (Carlton) 2012;17:621–627. doi: 10.1111/j.1440-1797.2012.01613.x. [DOI] [PubMed] [Google Scholar]
- 59.Chang H.J., Han K.H., Cho M.H., et al. Outcomes of chronic dialysis in Korean children with respect to survival rates and causes of death. Korean J Pediatr. 2014;57:135–139. doi: 10.3345/kjp.2014.57.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vidal E., van Stralen K.J., Chesnaye N.C., et al. Infants requiring maintenance dialysis: outcomes of hemodialysis and peritoneal dialysis. Am J Kidney Dis. 2017;69:617–625. doi: 10.1053/j.ajkd.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 61.Arhuidese I.J., Wanogho J., Faateh M., Aji E.A., Rideout D.A., Malas M.B. Hemodialysis and peritoneal dialysis access related outcomes in the pediatric and adolescent population. J Pediatr Surg. 2020;55:1392–1399. doi: 10.1016/j.jpedsurg.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 62.ANZDATA Registry. 42nd Report . Australia and New Zealand Dialysis and Transplant Registry, Adelaide; Australia: 2019. Chapter 12: Paediatric patients with end stage kidney disease requiring renal replacement therapy.https://www.anzdata.org.au/wp-content/uploads/2019/09/c12_paediatric_2018_v1.0_20200205.pdf [Google Scholar]
- 63.Perl J., Wald R., McFarlane P., et al. Hemodialysis vascular access modifies the association between dialysis modality and survival. J Am Soc Nephrol. 2011;22:1113–1121. doi: 10.1681/ASN.2010111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galiyeva D.B., Jackson C.A., Wild S.H., et al. Long-term all-cause mortality and cardiovascular outcomes in Scottish children after initiation of renal replacement therapy: a national cohort study. Pediatr Nephrol. 2020;35:677–685. doi: 10.1007/s00467-019-04430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ambarsari C.G., Sindih R.M., Saraswati M., Trihono P.P. Delayed admission and management of pediatric acute kidney injury and multiple organ dysfunction syndrome in children with multiple wasp stings: a case series. Case Rep Nephrol Dial. 2019;9:137–148. doi: 10.1159/000504043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaspar C.D., Bholah R., Bunchman T.E. A review of pediatric chronic kidney disease. Blood Purif. 2016;41:211–217. doi: 10.1159/000441737. [DOI] [PubMed] [Google Scholar]
- 67.Watson A.R., Hayes W.N., Vondrak K., et al. Factors influencing choice of renal replacement therapy in European paediatric nephrology units. Pediatr Nephrol. 2013;28:2361–2368. doi: 10.1007/s00467-013-2555-z. [DOI] [PubMed] [Google Scholar]
- 68.Ambarsari C.G., Hidayati E.L., Hasan I., Grace A., Oswari H. Successful treatment of hepatitis C virus infection using direct-acting antiviral agents (DAAs) in adolescents with kidney transplantation: a case series. Int J Nephrol Renovasc Dis. 2020;13:139–146. doi: 10.2147/IJNRD.S248632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Favel K., Dionne J.M. Factors influencing the timing of initiation of renal replacement therapy and choice of modality in children with end-stage kidney disease. Pediatr Nephrol. 2020;35:145–151. doi: 10.1007/s00467-019-04391-8. [DOI] [PubMed] [Google Scholar]
- 70.Chaturvedi S., Ullah S., LePage A.K., Hughes J.T. Rising incidence of end-stage kidney disease and poorer access to kidney transplant among Australian Aboriginal and Torres Strait Islander children and young adults. Kidney Int Rep. 2021;6:1704–1710. doi: 10.1016/j.ekir.2021.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ku E., McCulloch C.E., Grimes B.A., Johansen K.L. Racial and ethnic disparities in survival of children with ESRD. J Am Soc Nephrol. 2017;28:1584–1591. doi: 10.1681/ASN.2016060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tjaden L.A., Noordzij M., van Stralen K.J., et al. Racial disparities in access to and outcomes of kidney transplantation in children, adolescents, and young adults: results from the ESPN/ERA-EDTA (European society of Pediatric Nephrology/European Renal Association-European Dialysis and transplant association) registry. Am J Kidney Dis. 2016;67:293–301. doi: 10.1053/j.ajkd.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 73.Mitsnefes M.M., Laskin B.L., Dahhou M., Zhang X., Foster B.J. Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990-2010. JAMA. 2013;309:1921–1929. doi: 10.1001/jama.2013.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grace B.S., Kara T., Kennedy S.E., McDonald S.P. Racial disparities in pediatric kidney transplantation in New Zealand. Pediatr Transplant. 2014;18:689–697. doi: 10.1111/petr.12322. [DOI] [PubMed] [Google Scholar]
- 75.Majoni S.W., Dole K., Hughes J.T., Pain C. Review of current pathways to wait-listing for kidney transplantation for Aboriginal and Torres Strait Islander peoples with end-stage kidney disease in the Top End of Northern Australia. Aust Health Rev. 2021;45:185–193. doi: 10.1071/AH20011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.