Abstract
BAY 10-8888 is a cyclic β-amino acid that is related to cispentacin and that has antifungal activity. Candida albicans cells accumulated BAY 10-8888 intracellularly to a concentration about 200 that in the medium when grown in media with a variety of nitrogen sources. In complex growth medium, BAY 10-8888 transport activity was markedly reduced and was paralleled by a decrease in its antifungal activity. Uptake of BAY 10-8888 was mediated by an H+-coupled amino acid transporter with specificity for branched-chain amino acids (isoleucine, leucine, and valine) and showed a KT (Michaelis constant of the transport reaction) of 0.95 mM and a Vmax of 18.9 nmol × min−1 × 107 cells−1. Similar to the transport of natural amino acids in Saccharomyces cerevisiae, the transport of BAY 10-8888 into the cell was unidirectional. Efflux occurred by diffusion and was not carrier mediated. Inside the cell BAY 10-8888 inhibited specifically isoleucyl-tRNA synthetase, resulting in inhibition of protein synthesis and cell growth. Intracellular isoleucine reversed BAY 10-8888-induced growth inhibition. BAY 10-8888 was not incorporated into proteins. BAY 10-8888 inhibited isoleucyl-tRNA synthetase with the same concentration dependency as protein biosynthesis in intact cells assuming 200-fold accumulation.
In 1989, cispentacin [(1R,2S)-2-aminocyclopentane-1-carboxylic acid; Fig. 1], a cyclic β-amino acid isolated from Bacillus cereus (12) and, subsequently, from Streptomyces setonii (9), was described to have potent anti-Candida activity both in vitro and in a mouse model of systemic candidiasis (9, 12, 17, 18). At about the same time, aminocyclohexene carboxylic acids were designed as pyridoxal phosphate suicide inhibitors (1). One of those compounds [(1R,2S)-2-amino-3-cyclohexene-1-carboxylic acid; Fig. 1] showed activity against Candida albicans as well, and therefore, a derivatization program aimed at identifying cyclic β-amino acid derivatives with superior efficacy and good tolerability was started (1a). BAY 10-8888 [(1R,2S)-2-amino-4-methylene-cyclopentane-1-carboxylic acid; Fig. 1] showed in vitro activity against several Candida spp. (3a) and was chosen for further development.
FIG. 1.
Structures of the antifungal β-amino acids BAY 10-8888 [(1R,2S)-2-amino-4-methylenecyclopentane-1-carboxylic acid], cispentacin [(1R,2S)-2-aminocyclopentane-1-carboxylic acid], and BAY y 9379 [(1R,2S)-2-amino-3-cyclohexene-1-carboxylic acid].
Since fungi and humans are eukaryotes, selectivity is one of the main challenges in the development of new antifungal agents. Knowledge of the mode of action is crucial to distinguish toxic side effects which depend on the mode of action and, therefore, which are expected for all members of a certain class of compounds from those effects which are compound specific and which can be overcome by chemical derivatization. For the cyclic β-amino acid cispentacin, Capobianco and coworkers (2) suggested that the mode of action in C. albicans is interference with self-regulatory mechanisms of amino acid metabolism after accumulation via amino acid carriers. However, no information about the exact molecular target was provided.
Here, we show that BAY 10-8888 has a dual mode of action. First, it is actively accumulated by amino acid permeases. Second, inside the cell, BAY 10-8888 is a low-affinity inhibitor of isoleucyl-tRNA synthetase. Inhibition of isoleucyl-tRNA synthetase results in the disruption of protein biosynthesis and cell growth.
MATERIALS AND METHODS
Radioactive compounds.
Tritiated water ([3H]H2O; 1 mCi/ml), inulin-[14C]carboxylic acid (6.1 mCi/mmol), l-[14C]lysine (319 mCi/mmol, 160 μM), and l-[14C]isoleucine (313 mCi/mmol, 150 μM) were purchased from Amersham Buchler, Braunschweig, Germany. [14C]BAY 10-8888 (12 mCi/mmol) was provided by M. Radtke, Institute for Pharmacokinetics, Bayer AG. The radiochemical purity was >96%.
Materials.
Filter disks either were from Millipore, Eschborn, Germany, or were manufactured by Whatman, Kent, United Kingdom. tRNA from brewer’s yeast, tRNA from calf liver, and ATP were purchased from Boehringer, Mannheim, Germany. All other chemicals were obtained from Sigma, Deisenhofen, Germany. Nonlabelled BAY 10-8888 was prepared as described previously (1a) and was provided by J. Mittendorf, Institute for Chemistry. Fluconazole was synthesized at Bayer AG.
Organisms.
C. albicans BSMY 212 (ATCC 200498) was maintained by bimonthly transfer on Nervina agar (0.5% [vol/vol] glycerol, 0.5% Bacto Peptone, 0.5% sodium chloride, 4% malt extract, 2% Bacto Agar [pH 7.0]). Saccharomyces cerevisiae ATCC 46790 (a/α trp1/trp1 ura3/ura3 [rho+] [cir+]) was grown in minimal dextrose medium combined with supplement mixture (Bio 101, Inc., La Jolla, Calif.).
Overnight cultures in YPG medium (1.5% glucose, 1.0% peptone, 0.4% yeast extract, 0.05% K2HPO4, 0.05% MgSO4 · 7 H2O [pH 7.0]) were diluted 1:10 in fresh medium as indicated below and were incubated for about 4 h at 37°C to obtain logarithmic-phase culture cells. The following media were used: YNG medium (0.67% Bacto Yeast Nitrogen Base [Difco], 1.0% glucose [pH 7.0]), YNGW medium (0.67% Bacto Yeast Nitrogen Base without amino acids [Difco], 1.0% glucose [pH 7.0]), and YNGWA medium (0.67% Bacto Yeast Nitrogen Base without amino acids and ammonium sulfate [Difco], 1.0% glucose [pH 7.0]).
Estimation of intracellular water space in C. albicans.
Logarithmic-phase C. albicans cells grown in YNG or YPG medium at 37°C were harvested by centrifugation (at 1,850 × g for 10 min), washed once in ice-cold water, and resuspended in phosphate-buffered saline (PBS) at a final density of 109 cells/ml. A total of 100 μl of the cell suspension was mixed with 100 μl of a mixture of 10 μCi of inulin-[14C]carboxylic acid per ml and 10 μCi of [3H]H2O per ml in PBS. After incubation for 60 s the cells were pelleted by centrifugation (at 15,850 × g for 15 s). The supernatant was removed carefully, and the pellet was resuspended in 100 μl of 1% sodium dodecyl sulfate (SDS). For each sample 40 μl of the pellet suspension and supernatant was placed in duplicate into scintillation vials, and then 5 ml of scintillation fluid (Quickszint 401; Zinsser Analytic, Frankfurt, Germany) was added and the radioactivity was determined in a three-channel liquid scintillation counter (Beckmann LS 3801; Beckman Instruments, Munich, Germany). Channel settings were 0 to 400 for the detection of 3H counts and 400 to 670 for the detection of 14C counts. Activity was corrected for quenching with internal programs for quench compensation. The intracellular water space was calculated from the ratios of the 3H disintegrations per minute and the 14C disintegrations per minute in the supernatant and pellet as described previously (21). The data were evaluated under the assumptions described previously (3).
Transport measurements.
For transport measurements logarithmic-phase C. albicans cells grown in different media were pelleted by centrifugation and washed once with sterile PBS. The cell density was adjusted to 1.1 × 108/ml in transport medium. In the standard assay 180 μl of cells (2 × 107 cells) was equilibrated to 37°C for 3 min. Prior to the start of the reaction 10 μl of either PBS or a 20-fold-concentrated effector (inhibitor or competitor) solution was added and the mixture was incubated at 37°C for various periods. Uptake was started by adding 10 μl of 20-fold-concentrated [14C]BAY 10-8888 (radioactivity concentration range, 0.12 to 5 μCi/ml). After incubation at 37°C for various periods, uptake was stopped by the addition of 8 ml of ice-cold water. Cells were collected on cellulose mixed ester filter disks (pore size, 0.22 μm; GSTF; Millipore) and washed twice with 4 ml of ice-cold water. Air-dried filters were placed into scintillation vials, and after the addition of 5 ml of scintillation fluid the radioactivity was determined. To determine the [14C]BAY 10-8888 concentration in the supernatants, the cells were pelleted in a microcentrifuge (at 15,850 × g for 30 s) and 20 μl of the supernatant was processed as described above. For efflux measurements C. albicans cells were preloaded with [14C]BAY 10-8888 for 30 min at 37°C. Thereafter, the cells were pelleted by centrifugation (at 15,850 × g for 60 s) and the supernatant was removed. The pellet was washed once with 1.3 ml of ice-cold water, and the supernatant was removed entirely. The cells were resuspended in ice-cold efflux medium, and an aliquot was removed to determine the initial intracellular [14C]BAY 10-8888 concentration. After the addition of effector solution or PBS the cells were incubated at 37°C. At various time points aliquots were removed and processed as described above.
Susceptibility testing and growth inhibition.
C. albicans cells grown overnight in YPG medium at 28°C were diluted 1:50 in YNG medium (or some other medium, as indicated) and were incubated for 4 h at 28°C. An inoculum of 103 cells/ml was prepared, and 200 μl of the cell suspension was added to a microtiter plate containing serial dilutions of the test compound in 50 μl of PBS. Control incubations without test compound were included. The microtiter plate was incubated for 24 h at 37°C. Endpoint reading was performed by determination of the optical density at 540 nm. The 50% inhibitory concentration (IC50) was defined as the compound concentration reducing growth to 50% of the control value. IC90 was defined as the lowest compound concentration preventing visible growth. Susceptibility testing of S. cerevisiae was performed as described above with YNG medium supplemented with 75 μg of uridine per ml or minimal dextrose medium lacking tryptophan (Bio 101, Inc.). Endpoint reading was performed after 48 h of incubation at 37°C as described above.
To determine the intracellular BAY 10-8888 concentrations that inhibit growth, C. albicans cells grown overnight in YNG medium at 37°C were inoculated at a cell density of 2 × 106/ml in YNG medium or YNG medium containing 1 mM l-isoleucine and were incubated with shaking at 37°C. After 2 h, [14C]BAY 10-8888 was added to a final concentration of 50 μM. The final concentration of radioactivity was 0.05 μCi/ml. At different time points, samples were processed as described above and the intracellular BAY 10-8888 concentration was determined. The numbers of CFU were determined by plating an aliquot on Nervina agar at various time points.
Protein and nucleic acid labelling.
Logarithmic-phase C. albicans cells grown in YNG or YPG medium at 37°C were harvested by centrifugation (at 1,850 × g for 10 min), washed twice in ice-cold PBS, and resuspended in YNG medium at a density of 2.2 × 107 cells/ml. A total of 450 μl of this cell suspension was added to 50 μl of a 10-fold-concentrated inhibitor solution, and the mixture was incubated at 37°C for various periods. Cells were pulsed by the addition of 50 μl of l-[14C]lysine (final concentration, 20 μM, 0.15 μCi/ml) or [14C]adenine (final concentration, 45 μM, 1 μCi/ml). The l-[14C]lysine incorporation was stopped by adding 50 μl of a 10% SDS solution, 50 μl of bovine serum albumin solution (1 mg/ml in PBS), and 650 μl of 20% trichloroacetic acid (TCA) containing 2 mg of l-lysine per ml. The mixture was incubated for 15 min at 80°C, cooled to room temperature, and kept on ice for a further 30 min. [14C]adenine incorporation was stopped by adding 50 μl of a 10% SDS solution and 600 μl of 20% TCA containing 0.5 mg of adenine per ml. The mixture was incubated for 15 min at 90°C and cooled to room temperature. The samples were trapped on glass fiber filters (GF/C; Whatman). The filters were washed twice with 5 ml of ice-cold 10% TCA, 1 mg of l-lysine per ml, or 5 ml of 10% TCA–1 mg of adenine per ml and twice with 5 ml of ethanol. Thereafter, the filters were air dried and the radioactivity was determined.
Cell lysis and S100 preparation.
C. albicans cells grown overnight in YPG medium at 28°C were diluted 1:20 in YNG medium or 1:200 in YPG medium and were incubated overnight at 37°C with shaking. The cells were chilled on ice, harvested by centrifugation (at 1,850 × g for 10 min), and washed once with 0.4 volume of ice-cold lysis buffer (100 mM Tris-Cl, 1 mM EDTA, 1 mM dithiothreitol, 100 μg of phenylmethylsulfonyl fluoride per ml, 100 μg of Nα-p-tosyl-l-lysine chloromethylketone per ml, 2 μg of aprotinin per ml, 1 mM benzamidine, and 0.3 M KCl [pH 7.4]; freshly prepared). The washed pellets were resuspended in 3 ml of ice-cold lysis buffer/g of pellet. The Candida suspension was poured slowly into the same volume of liquid nitrogen. After the addition of 2 volumes of acid-washed glass beads (diameter, 425 to 600 μm; Sigma, Deisenhofen, Germany) the cells were broken by repeated cycles (about 15) of vigorous vortexing and cooling on ice (30 s of vortexing, 15 s of cooling). Thereafter, the beads were removed by centrifugation and were washed once with lysis buffer. The supernatants were pooled and centrifuged at 100,000 × g for 60 min. The supernatant from the centrifugation at 100,000 × g (S100) was quickly frozen in liquid nitrogen and was stored at −70°C.
Aminoacyl-tRNA synthetase activity.
The l-isoleucyl- and l-lysyl-tRNA synthetase activities in the S100 fraction were determined by a filtration method (5, 14). Briefly, C. albicans aminoacyl-tRNA synthetase activity was measured after the addition of the S100 extract to a reaction mixture containing 0.1 M Tris-Cl (pH 7.65), 0.1 M KCl, 10 mM MgSO4, 0.5 mM EDTA, 2 mM ATP, 15 μg of tRNA from brewer’s yeast per ml, and 9.6 μM l-[14C]isoleucine (3 μCi/ml) or 9 μM l-[14C]lysine (3 μCi/ml). After incubation for 1, 2, 3, and 4 min (alternatively, 2, 4, 6, and 8 min) at 37°C, 20 μl of the reaction mixture was removed and was spotted onto Whatman 3MM filter disks which had been soaked with 50 μl of 10% TCA and dried. The disks were washed once with ice-cold 10% TCA containing 1 mg of l-isoleucine per ml and 1 mg of l-lysine per ml and were placed in ice-cold 10% TCA containing 1 mg of l-isoleucine and l-lysine per ml for at least 15 min. Thereafter, the disks were washed for at least 10 min with ice-cold 5% TCA containing 1 mg of l-isoleucine and l-lysine per ml and were briefly rinsed with ethanol. The disks were dried under a heat lamp, and the amount of trapped radioactivity was determined as described above. Assays were performed in triplicate. Protein concentrations were determined by the bicinchoninic acid method (Pierce Chemical Company, Rockford, Ill.) after precipitation of an aliquot with 3 volumes of methanol. The results were expressed as specific activities (units per milligram of protein; 1 U is defined as the capacity to aminoacylate 1 nmol of tRNA per min under standard assay conditions).
Overexpression of isoleucyl-tRNA synthetase in S. cerevisiae.
A 3.27-kb EcoRI-BamHI fragment from pILS (14) coding for isoleucyl-tRNA synthetase was blunt ended and cloned into the blunt-ended BamHI site of the yeast expression vector pG-1 (22). The resulting construct (pAL664) encodes yeast isoleucyl-tRNA synthetase under the control of the constitutive glyceraldehyde-3-phosphate dehydrogenase promoter (22). S. cerevisiae ATCC 46790 was transformed with pG-1 or pAL664 by using a yeast spheroplast transformation kit according to the manufacturer’s instructions (Bio 101, Inc.). Transformants were selected and propagated on minimal dextrose medium lacking tryptophan (Bio 101, Inc.) or YNG medium supplemented with 75 μg of uridine per ml.
Data analysis.
Data obtained from saturation and inhibition experiments were analyzed with the Prism program (GraphPad Software Inc., San Diego, Calif.) for microcomputers.
RESULTS
The structure of BAY 10-8888 and initial observations by Capobianco and coworkers (2) suggested that the antifungal activity of BAY 10-8888 may result from interference with the amino acid metabolism of C. albicans. Furthermore, the sensitivity of C. albicans to BAY 10-8888 was dependent on medium composition. The minimal inhibitory concentration (IC90) was 4 μg/ml (28 μM) in YNG medium, a minimal medium containing ammonium sulfate as the nitrogen source and glucose as the carbon source, and >64 μg/ml (>448 μM) in YPG medium, a complex medium containing yeast extract, peptone, and glucose. Therefore, we tested the influence of naturally occurring l-amino acids, their enantiomers, and related amino acids on the inhibitory concentration for 50% growth inhibition (IC50) of BAY 10-8888 against C. albicans. The amino acids which affected the IC50 of BAY 10-8888 are shown in Table 1. Concomitant incubation with 1 mM d-isoleucine, l-leucine, d-leucine, d-methionine, l-valine, or β-alanine increased the IC50 of BAY 10-8888 16-fold, while the addition of 1 mM l-isoleucine resulted in a 125-fold increase. All other l- and d-amino acids tested as well as d-norleucine, cis- and trans-hydroxyproline, pyroglutamic acid, l-ornithine, sarcosine, and citrulline did not affect the IC50 (data not shown). We also tested the influence of the corresponding α-keto acids of l-isoleucine (dl-α-keto-β-methylvaleric acid), l-leucine (α-ketoisocaproic acid), and l-valine (α-ketoisovaleric acid) at 1 mM concentrations on the IC50. The presence of 1 mM α-ketoisocaproic acid or 1 mM α-ketoisovaleric acid did not significantly influence the IC50 of BAY 10-8888 (the differences were below a factor of 2), whereas 1 mM dl-α-keto-β-methylvaleric acid increased the IC50 to 6.25 μg/ml. As shown in Table 1, the amino acids that decreased the sensitivity to BAY 10-8888 at least 10-fold belong to the class of branched-chain amino acids. To test whether competition for uptake was responsible for the decreased BAY 10-8888 sensitivity in the presence of branched-chain amino acids, we characterized the uptake of BAY 10-8888 in the presence of α-amino acids into C. albicans cells.
TABLE 1.
Effects of branched-chain amino acids and their respective α-keto acids on BAY 10-8888 susceptibility of C. albicans BSMY 212a
| Amino acid or α-keto | Concn (mM) | IC50b (μg/ml) |
|---|---|---|
| None | 0.8 | |
| β-Alanine | 1 | 12.5 |
| l-Isoleucine | 0.1 | 25 |
| l-Isoleucine | 1 | 100 |
| dl-α-Keto-β-methyl valeric acid | 1 | 6.25 |
| d-Isoleucine | 1 | 12.5 |
| l-Leucine | 0.1 | 8 |
| l-Leucine | 1 | 12.5 |
| α-Ketoisocaproic acid | 1 | 1.6 |
| d-Leucine | 1 | 12.5 |
| d-Methionine | 1 | 12.5 |
| l-Valine | 1 | 12.5 |
| α-Ketoisovaleric acid | 1 | 1.6 |
Logarithmic-phase cells grown at 28°C in YNG medium were incubated at a cell density of 103/ml in YNG medium at 37°C with twofold serial dilutions of BAY 10-8888 containing medium in the presence or absence of amino acid or α-keto acid. After 24 h the IC50 was determined.
The IC50 was defined as the test compound concentration reducing growth to 50% of the control value.
Inhibition of BAY 10-8888 uptake by α-amino acids.
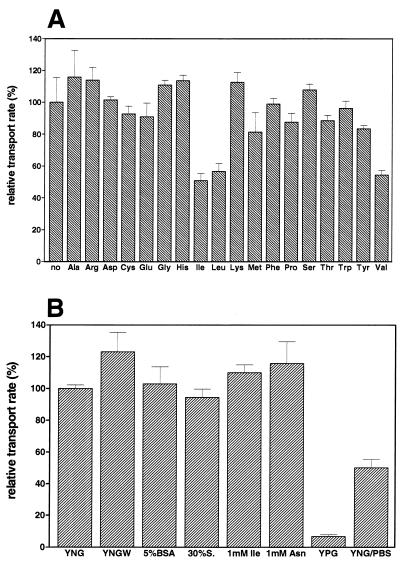
As shown in Fig. 2A only the branched-chain amino acids l-isoleucine, l-leucine, and l-valine reduced uptake to 51, 57, and 55% of the control value, respectively, when they were present at a 20-fold excess. This suggested that BAY 10-8888 is accumulated by an amino acid carrier specific for branched-chain amino acids. In contrast to their amino acid counterparts, the respective α-keto acids corresponding to isoleucine, leucine, and valine did not influence the uptake of BAY 10-8888 (data not shown). The d enantiomers of the proteinaceous amino acids isoleucine, leucine, and valine were also able to reduce the initial level of BAY 10-8888 uptake to 62, 66, and 79% of the control value, respectively, when the cells were preincubated with a 100-fold excess of the respective amino acid for 3 min (data not shown).
FIG. 2.
Inhibition of BAY 10-8888 uptake by l-amino acids (A) and effect of growth medium composition on BAY 10-8888 uptake (B). (A) C. albicans BSMY 212 cells grown in YNG medium were incubated with 50 μM [14C]BAY 10-8888 for 5 min in the presence of 1 mM the respective l-amino acid (tyrosine at 0.5 mM). Cells were processed as described in Materials and Methods. (B) C. albicans BSMY 212 cells were grown in different media at 37°C (YNG, YNG medium; YNGW, YNG medium without amino acids; 5% BSA, YNGWA medium containing 5% BSA as the nitrogen source; 30% serum [30% S.], YNGWA medium containing 30% fetal calf serum as the nitrogen source; 1 mM Ile, YNGWA medium containing 1 mM l-Ile as the nitrogen source; 1 mM Asn, YNGWA medium containing 1 mM l-Asn as the nitrogen source; YPG, YPG medium). Cells were harvested by centrifugation and were resuspended in growth medium, growth medium lacking the respective amino acid (cells grown in the presence of isoleucine or asparagine), or PBS (YNG/PBS). Thereafter, the cells were incubated with 50 μM [14C]BAY 10-8888 for 30 min and were processed as described in Materials and Methods. Relative transport rates are indicated. The error bars indicate standard deviations.
Effect of growth medium.
Figure 2B shows that the relative transport activity for BAY 10-8888 was also dependent on the source of the growth medium nitrogen. Relative transport activity was identical for cells grown in yeast nitrogen base-based media with ammonium sulfate (YNGW), ammonium sulfate and amino acids (YNG), 5% BSA, 30% fetal calf serum (30% serum), 1 mM isoleucine, or 1 mM asparagine as sources of nitrogen. Growth in medium containing peptone and yeast extract repressed BAY 10-8888 transport activity. Lack of energy sources during uptake of BAY 10-8888 (Fig. 2B, YNG/ PBS) reduced uptake by about 50%.
Active uptake of BAY 10-8888 in C. albicans.
To measure intracellular BAY 10-8888 concentrations, we determined the intracellular volume (water space) of C. albicans BSMY 212 by the double-labelling method described in Materials and Methods. The intracellular volume of 108 cells was 5.7 ± 1.8 μl (n = 13) for cells grown in YNG medium and 3.8 ± 1.0 μl (n = 14) for 108 cells grown YPG medium at 37°C. The morphological distribution of the populations was determined by light microscopy. For C. albicans BSMY 212 grown in YPG medium, 85% single cells and 15% pseudohyphae were observed. Cells grown in YNG medium were 50% single cells and 50% pseudohyphae.
As shown in Fig. 3A C. albicans cells grown in YNG medium at 37°C accumulated [14C]BAY 10-8888, which was added at a concentration of 50 μM (corresponding to 7.1 μg of BAY 10-8888 per ml, which is about twice the IC90), within 10 min up to an intracellular concentration of about 5 mM. This concentration was maintained for at least 50 min. In contrast, an intracellular concentration of 0.4 mM was measured after 30 min for cells grown in YPG medium. Uptake was identical in PBS instead of YPG medium (data not shown). Thus, the reduced uptake in YPG medium was not due to the compounds in the medium competing with BAY 10-8888 for uptake.
FIG. 3.
Uptake of BAY 10-8888. (A) C. albicans BSMY 212 cells grown in YNG or YPG medium at 37°C were incubated with 50 μM [14C]BAY 10-8888 in the same medium and were processed as described in Materials and Methods. The mean ± standard deviation intracellular concentration (BAY 10-8888in) is presented. (B) Uptake of [14C]BAY 10-8888 (1 μM to 8 mM) was measured at 37°C over a period of 3 min. Data represent means ± standard deviations. The dashed line represents a nonlinear fit. (Inset) Respective Hanes plot. (C) Dependence of intracellular (BAY 10-8888in) and medium (BAY 10-8888med) concentration on cell density. C. albicans BSMY 212 cells grown in YNG medium were incubated with 50 μM [14C]BAY 10-8888 for 30 min in the same medium at different cell densities. Thereafter, the cells were processed as described in Materials and Methods. Mean ± standard deviation intracellular and medium concentrations are shown. The dashed lines represent a nonlinear fit.
The dependency of BAY 10-8888 uptake on the concentration in the medium (BAY 10-8888med) during a 3-min incubation can be described by a Michaelis-Menten equation (Fig. 3B). Uptake was saturable with a KT (Michaelis constant of the transport reaction) of 0.95 mM and a Vmax of 18.9 nmol × min−1 × 107 cells−1. Assuming a dry weight similar to that of diploid S. cerevisiae (20 pg/cell [23]), this corresponds to 94.5 nmol/min per mg of cell (dry weight).
We used a cell density of 108/ml for our transport studies. At this cell density the medium concentration of BAY 10-8888 decreased from an initial concentration of 50 μM to one of 21 μM after 30 min (Fig. 3C). The decrease in the cell density resulted in an increase in the intracellular BAY 10-8888 concentration up to 14 mM at a cell density of 106 cells/ml (Fig. 3C), while the concentration in the medium remained essentially unchanged (50 μM). Overall, C. albicans BSMY 212 cells grown in YNG medium accumulated BAY 10-8888 to a concentration about 200 that in the medium.
Characterization of active transport.
The influence of several metabolic inhibitors and ionophores on BAY 10-8888 uptake is shown in Table 2. The proton ionophores carbonylcyanide-m-chlorophenylhydrazone (CCCP) and carbonylcyanide p-trifluoromethoxyphenylhydrazone reduced uptake to 7 and 25% of the control value when the cells were preincubated with 100 μM ionophore for 5 and 1 min, respectively. This suggested that uptake of BAY 10-8888 is proton linked. Metabolic inhibitors such as sodium cyanide and sodium azide reduced transport activity to 40 and 10% of the control value, respectively. The ionophores valinomycin and nigericin reduced the uptake of BAY 10-8888 to about 40% of the control value. N-Ethylmaleimide, which covalently modifies cysteine side chains, reduced uptake to 1.6% of the control value. As expected, the addition of SDS completely abolished BAY 10-8888 accumulation. The initial velocity of BAY 10-8888 uptake was measured at different pH values. The uptake showed a maximum at pH 6.0 and decreased to 6 and 8% of the control value at pH 4.0 and pH 9.0, respectively (data not shown).
TABLE 2.
Effects of metabolic inhibitors and ionophores on BAY 10-8888 uptakea
| Effector | Concn | Incubation time (min) | Uptake (% of control) | Uptake time (min) |
|---|---|---|---|---|
| CCCP | 0.1 mM | 5 | 7 ± 0.6 | 5 |
| FCCPb | 0.1 mM | 1 | 25 ± 2.5 | 5 |
| Sodium azide | 1 mM | 10 | 10 ± 0.7 | 5 |
| NaCN | 2 mM | 60 | 40 ± 10 | 5 |
| NEMc | 1 mM | 15 | 1.6 ± 0.1 | 5 |
| Valinomycin | 0.001 mM | 30 | 41 ± 6 | 5 |
| Nigericin | 0.003 mM | 30 | 38 ± 6 | 5 |
| SDS | 0.1% | 0 | 2.4 ± 0.6 | 5 |
| SDS | 0.1% | 0 | 14 ± 3 | 30 |
| NEM | 10 mM | 5 | 8 ± 0.1 | 30 |
| CCCP + sodium azide | 0.1 and 30 mM | 0 | 8.9 ± 0.1 | 30 |
C. albicans cells grown in YNG medium were incubated with the respective inhibitor for the indicated times. Thereafter, [14C]BAY 10-8888 was added and incubation was continued for the indicated times. Data are means ± standard deviations and are expressed as percentage of control uptake.
FCCP, carbonylcyanide p-trifluoromethoxyphenylhydrazone.
NEM, N-ethylmaleimide.
Efflux studies.
Efflux was studied after the preloading of C. albicans cells with BAY 10-8888. The cells were incubated in YNG medium containing 50 μM BAY 10-8888 for 30 min. Thereafter, the cells were washed and resuspended in fresh YNG medium or YNG medium containing 1 mM l-isoleucine, and the intracellular BAY 10-8888 concentration was monitored (Fig. 4A). Initially, the intracellular concentration of BAY 10-8888 was 7.1 mM and it declined exponentially to 2.7 mM within 1 h. Thereafter, a slower decrease to 1.5 mM after an additional 17 h was observed. In cells, preloaded in PBS instead of YNG medium, the initial intracellular concentration of BAY 10-8888 was 4.15 mM, which decreased to 0.34 mM after 18 h. The addition of 1 mM l-isoleucine to the medium did not influence BAY 10-8888 efflux. In contrast to amino acid transporters from bacteria and mammalian cells, internalized amino acids do not efflux after deenergization of yeast cells (7, 8, 13). To test this hypothesis for BAY 10-8888, the influences of detergent, metabolic inhibitors in combination with proton ionophore and sulfhydryl-modifying agents on BAY 10-8888 efflux were tested over a period of 30 min (Fig. 4B), and the influence on efflux was compared to that on influx (Table 2). As expected, the addition of detergent (0.1% SDS) reduced the level of BAY 10-8888 uptake to below 15% of the untreated control value and resulted in the release of BAY 10-8888 from preloaded cells within 10 min. The addition of 30 mM sodium azide in combination with 0.1 mM CCCP reduced the level of uptake to 8% of the control value after 30 min, while the same treatment was not efficient in increasing the level of efflux of BAY 10-8888 from preloaded C. albicans cells. The cells retained 79% of the radioactivity retained by the untreated control. Furthermore, the larger amount of retained BAY 10-8888 in untreated cells may reflect reuptake of BAY 10-8888 instead of reduced efflux. This suggested that uptake of BAY 10-8888 is unidirectional or, at least, that the transport into the cell is strongly favored and efflux occurred in a carrier-independent manner. Treatment of C. albicans with 10 mM N-ethylmaleimide reduced uptake to below 9% of the control value by modification of the sulfhydryl groups of the transporter as well as other proteins (Table 2). The addition of 10 mM N-ethylmaleimide to cells which already received 30 mM sodium azide and 0.1 mM CCCP did not influence efflux (Fig. 4B).
FIG. 4.
Time course of BAY 10-8888 efflux. (A) Logarithmic-phase C. albicans cells grown at 37°C in YNG medium were preloaded with 50 μM [14C]BAY 10-8888 for 30 min. Cells preloaded in YNG medium were pelleted by centrifugation, washed once, and resuspended in YNG medium or YNG medium containing 1 mM isoleucine. Cells preloaded in PBS were resuspended in PBS or PBS containing 1 mM isoleucine. At the indicated time points, the intracellular concentration of BAY 10-8888 was determined. (B) Logarithmic-phase C. albicans cells grown at 37°C in YNG medium were preloaded with 50 μM [14C]BAY 10-8888 for 30 min. The cells were pelleted by centrifugation, washed once, and resuspended in YNG medium or YNG medium containing 0.1% SDS, 30 mM sodium azide, and 0.1 mM CCCP or 30 mM sodium azide, 0.1 mM CCCP, and 10 mM N-ethylmaleimide (NEM). At the indicated time points, the intracellular radioactivity was determined. The error bars indicate standard deviations. The dashed lines represent a nonlinear fit.
Reversion of BAY 10-8888-induced growth inhibition inside the cell by l-isoleucine.
As shown above, at 1 mM l-isoleucine the antifungal activity of BAY 10-8888 was reduced at least eightfold compared to the level of reduction obtained with any other amino acid tested. We therefore investigated whether l-isoleucine not only competed for uptake but was also able to reverse BAY 10-8888-induced growth inhibition by an intracellular mechanism. C. albicans cells from an overnight culture in YNG medium at 37°C were inoculated at a cell density of about 106 cell/ml in YNG medium and were equilibrated to 37°C for 2 h. Thereafter, either [14C]BAY 10-8888 (final concentration, 50 μM, 0.05 μCi/ml) or [14C]BAY 10-8888 (50 μM, 0.05 μCi/ml) and l-isoleucine (1 mM), l-isoleucine (1 mM), or PBS (no addition) were added, and growth was assessed by determining the numbers of CFU after 0, 1.5, 3, and 6 h (Fig. 5A). After a lag phase of about 3 h, the CFU count increased about 10-fold during the next 3 h in the control sample and the samples containing 1 mM l-isoleucine and 50 μM BAY 10-8888 or 1 mM l-isoleucine. In contrast, no growth was observed in the culture containing 50 μM BAY 10-8888 only. The intracellular concentrations of BAY 10-8888 were about 10 and 6.5 mM 1 and 2 h, respectively, after the addition of either 50 μM BAY 10-8888 only or 50 μM BAY 10-8888 and 1 mM l-isoleucine (Fig. 5B). Thus, the presence of a 20-fold excess of l-isoleucine did not influence the accumulation of BAY 10-8888 under equilibrium conditions. In cells incubated with BAY 10-8888 at a concentration of 50 μM the intracellular concentration decreased to 5.2 mM after 4 h and increased again to 9.5 mM after 6 h. In cells incubated with 50 μM BAY 10-8888 and 1 mM l-isoleucine the intracellular concentrations decreased to 2.2 and 3.7 mM after 4 and 6 h, respectively. The calculations of the values at 4 and 6 h were performed by taking into account the increase in the total cell volume during growth after 4 and 6 h. Growth was recorded by determining the numbers of CFU. The increase in the numbers of CFU may be higher than the increase in cell volume, and therefore, our calculated values may underestimate the BAY 10-8888 concentrations inside the cells. Overall, the intracellular BAY 10-8888 concentration was the same in the presence and absence of additional isoleucine in the medium. We also observed an absolute increase in the amount of radioactivity retained inside the cells at these time points (data not shown). It is evident from Fig. 5 that intracellular BAY 10-8888 at millimolar concentrations inhibits the growth of C. albicans and, furthermore, that this growth inhibition can be reversed by l-isoleucine.
FIG. 5.
l-Isoleucine reversion of BAY 10-8888-induced growth inhibition inside the cell. C. albicans BSMY 212 cells from an overnight culture in YNG medium at 37°C were equilibrated at a cell density of about 106 cell/ml in YNG medium to 37°C for 2 h. Thereafter, either [14C]BAY 10-8888 (final concentration, 50 μM; radioactivity concentration, 0.05 μCi/ml) or [14C]BAY 10-8888 (50 μM; 0.05 μCi/ml) and l-isoleucine (1 mM), l-isoleucine (1 mM), or PBS (no addition) were added and growth was recorded (A). In samples that received BAY 10-8888, the intracellular concentration was determined as described in Materials and Methods (B).
BAY 10-8888-induced inhibition of protein biosynthesis.
The reversion of BAY 10-8888-induced growth inhibition by l-isoleucine suggests that the intracellular target of BAY 10-8888 is part of the l-isoleucine metabolism. Endogenously synthesized l-isoleucine is charged with tRNA and is incorporated into proteins, while the carbon skeleton of externally acquired l-isoleucine can also be fed into the citric acid cycle after transamination to dl-α-keto-β-methylvaleric acid. Since dl-α-keto-β-methylvaleric acid was far less efficient than l-isoleucine in overcoming BAY 10-8888-induced growth inhibition (Table 1), a target within l-isoleucine biosynthesis or metabolism seemed less likely. We therefore investigated the influence of BAY 10-8888 on protein and nucleic acid biosynthesis as well as tRNA charging.
The addition of 8 μg of BAY 10-8888 per ml (56 μM) to a C. albicans cell suspension reduced the level of [14C]lysine incorporation to below 10% of the control value within 20 min and remained constantly low over the time period (90 min) investigated, while the concomitant addition of 1 mM l-isoleucine prevented BAY 10-8888-induced protein synthesis inhibition (Fig. 6A). No significant difference in the level of [14C]adenine incorporation between cells incubated in the absence or presence of 56 μM BAY 10-8888 or 56 μM BAY 10-8888 and 1 mM l-isoleucine could be detected (data not shown). This suggested that protein biosynthesis rather than nucleic acid metabolism was affected by BAY 10-8888.
FIG. 6.
Inhibition of protein biosynthesis and incorporation of BAY 10-8888 into proteins. (A) Logarithmic-phase C. albicans BSMY 212 cells grown in YNG medium at 37°C were incubated at a cell density of 107/ml in YNG medium (no addition), YNG medium containing 56 μM BAY 10-8888, or YNG medium containing 56 μM BAY 10-8888 and 1 mM l-isoleucine. After the indicated time points, a sample was taken and the level of [14C]lysine incorporation into TCA-precipitable material was measured for 10 min as described in Materials and Methods. The relative level of incorporation (the value at time zero minutes corresponds to 100%) is shown. (B) Duplicate samples of logarithmic-phase C. albicans BSMY 212 cells grown in YNG medium at 37°C were incubated at a cell density of 108/ml in YNG medium containing 50 μM [14C]BAY 10-8888 or 50 μM l-[14C]lysine for 30 min at 37°C. Thereafter, the intracellular concentrations of the respective amino acid and TCA-precipitable radioactivity were determined as described in Materials and Methods. The error bars indicate standard deviations.
To test whether BAY 10-8888 is charged to tRNA and is incorporated into protein, duplicate samples of C. albicans BSMY 212 were incubated with 50 μM [14C]BAY 10-8888 or 50 μM l-[14C]lysine for 30 min at 37°C. Thereafter, the intracellular amount of radioactivity, which could be precipitated by TCA and which was therefore attributable to either charged tRNA or to incorporation into protein, was determined (Fig. 6B). Within 30 min the intracellular amount of l-lysine increased to 8 mM, including the fraction incorporated into macromolecules. Approximately 50% of the accumulated l-lysine was incorporated into protein or was charged to tRNA. BAY 10-8888 was accumulated to an intracellular concentration of 6.3 mM; however, only as little as 2% of this amount could be precipitated by TCA. This indicated that little, if any, BAY 10-8888 was charged to tRNA and incorporated into proteins.
Inhibition of C. albicans isoleucyl-tRNA synthetase by BAY 10-8888.
We determined the specific activity of isoleucyl-tRNA synthetase in the S100 extract from C. albicans cells grown in YNG or YPG medium at 37°C using tRNA from brewer’s yeast as the substrate. The specific activities for isoleucyl-tRNA synthetase were 0.11 and 0.17 U/mg of protein for cells grown in YNG and YPG medium, respectively. These specific activities were of the same order of magnitude as those reported for crude extracts of S. cerevisiae (27). Isoleucyl-tRNA synthetase activity was inhibited by BAY 10-8888 at increasing concentrations (Fig. 7A). No difference was observed between cells grown in YNG medium and cells grown in YPG medium. In contrast, lysyl-tRNA synthetase activity, a different aminoacyl-tRNA synthetase, was not inhibited in the presence of BAY 10-8888 (data not shown). Using S100 extracts from cells grown in YNG medium as the source of isoleucyl-tRNA activity, we determined a Ki value for BAY 10-8888 by measuring synthetase activity in the presence of 0, 1, and 5 mM BAY 10-8888 with increasing isoleucine concentrations (1–20 μM). The Ki was calculated to be 1 mM (data not shown). As shown above, C. albicans BSMY 212 cells grown in YNG medium at a cell density of about 107/ml accumulated BAY 10-8888 about 200-fold within 30 min. We compared the concentration-dependent relative inhibition of isoleucyl-tRNA synthetase activity and protein biosynthesis taking into account a 200-fold accumulation of BAY 10-8888. As shown in Fig. 7B, inhibition of isoleucyl-tRNA synthetase activity and protein biosynthesis had identical dependencies on the BAY 10-8888 concentration. This suggested that BAY 10-8888-induced inhibition of isoleucyl-tRNA synthetase results in the inhibition of protein synthesis.
FIG. 7.
Correlation of inhibition of isoleucyl-tRNA synthetase activity with inhibition of protein synthesis. (A) Isoleucyl-tRNA synthetase activity was determined in an S100 extract from C. albicans cells grown in YNG or YPG medium at 37°C. (B) The concentration-dependent relative inhibition of isoleucyl-tRNA synthetase and protein biosynthesis activity were compared. Isoleucyl-tRNA synthetase activity was determined in an S100 extract from C. albicans cells grown in YNG medium at 37°C. The relative activity of protein biosynthesis was determined by incubation of logarithmic-phase C. albicans BSMY 212 cells grown in YNG medium at 37°C for 20 min at a cell density of 107/ml, with BAY 10-8888 used at a concentration 200-fold less than shown on the x axis. As indicated in Results, cells accumulate BAY 10-8888 about 200-fold under these conditions. Thereafter, the level of incorporation of [14C]lysine was determined as described in Materials and Methods. The dashed lines represent nonlinear fits assuming a Gaussian distribution. The error bars indicate standard deviations.
Decrease of BAY 10-8888 sensitivity by overexpression of isoleucyl-tRNA synthetase in S. cerevisiae.
Initial attempts to express S. cerevisiae isoleucyl-tRNA synthetase in C. albicans under the control of the actin promoter failed. This was most likely due to the unusual codon usage in C. albicans that prohibits expression of many S. cerevisiae genes in C. albicans (16, 24). We therefore tested the effect of overexpression of isoleucyl-tRNA synthetase on BAY 10-8888 sensitivity in S. cerevisiae. We cloned the coding region of S. cerevisiae isoleucyl-tRNA synthetase into plasmid pG-1, which allows constitutive expression of the synthetase under the control of the strong glyceraldehyde-3-phosphate dehydrogenase promoter. The resulting construct (pAL664) was introduced into S. cerevisiae ATCC 46790. Lysates prepared from cells grown under selective conditions were assayed for isoleucyl- and lysyl-tRNA synthetase activities. Compared to control transformants (pG-1), the specific activity of isoleucyl-tRNA synthetase was increased sevenfold in cells grown in YNG medium and eightfold in cells grown in minimal dextrose medium for transformants harboring plasmid pAL664 (Table 3). The activity of an unrelated aminoacyl-tRNA synthetase, lysyl-tRNA synthetase, remained unchanged. Sensitivity to BAY 10-8888 decreased two- and fourfold for cells overexpressing isoleucyl-tRNA synthetase grown in YNG medium and minimal dextrose medium, respectively. In contrast, the IC90 of fluconazole, an azole antifungal agent, was 16 μg/ml for both transformants.
TABLE 3.
Decreased susceptibility of S. cerevisiae to BAY 10-8888 by overexpression of isoleucyl-tRNA synthetasea
| Growth medium | Plasmid | IC90b (μg/ml) | Sp act (U/mg of protein [mean ± SD])
|
|
|---|---|---|---|---|
| Isoleucyl-tRNA synthetase | Lysyl-tRNA synthetase | |||
| YNG | pG-1 | 64 | 0.32 ± 0.09 | 0.76 ± 0.11 |
| pAL664 | 128 | 2.38 ± 0.52 | 0.71 ± 0.15 | |
| TRP | pG-1 | 32 | 0.35 ± 0.07 | 0.78 ± 0.08 |
| pAL664 | 128 | 2.90 ± 0.51 | 0.97 ± 0.15 | |
Logarithmic-phase cells grown at 30°C in YNG medium supplemented with 75 μg of uridine per ml (YNG) or minimal dextrose medium lacking tryptophan (TRP) were incubated at a cell density of 103/ml in the same medium at 37°C in the presence of BAY 10-8888. After 48 h the IC90 was determined. The specific activities of isoleucyl- and lysyl-tRNA synthetase in crude extract (S100) are given.
The IC90 was defined as the lowest concentration preventing visible growth.
DISCUSSION
We investigated the mode of action of BAY 10-8888, a cyclic β-amino acid with antifungal activity in C. albicans. Our data showed that BAY 10-8888 has a dual mode of action, namely, inhibition of isoleucyl-tRNA synthetase after concentrative uptake.
BAY 10-8888 serves as an artificial substrate and is accumulated by an H+-coupled symporter specific for branched-chain amino acids. In contrast, l-proline did not compete for BAY 10-8888 uptake, as has been shown for cispentacin (2, 10). This suggests that different β-amino acids use different carrier systems. With an apparent KT of 0.96 mM, BAY 10-8888 is a low-affinity substrate for its carrier. C. albicans has a high capacity for BAY 10-8888 transport, with uptake being 94.5 nmol/min per mg of cell (dry weight) at 37°C. This value is higher than those reported for the uptake of any other naturally occurring amino acid in S. cerevisiae (7). Accumulation of BAY 10-8888 is observed if cells are grown in a variety of nitrogen sources including ammonium ions, amino acids, protein, and serum. In addition, preliminary data indicate that BAY 10-8888 is also accumulated in C. albicans cells isolated from the kidneys of infected mice (data not shown). This strongly suggests that the BAY 10-8888 carrier is expressed during infection. The accumulation factor (200-fold) that we observed for BAY 10-8888 in C. albicans is of the same order of magnitude as the accumulation factor determined for the naturally occurring amino acid glycine in S. cerevisiae (7). At the lower cell densities (103/ml) used for susceptibility testing, the accumulation factor may be even higher. The high intracellular concentration necessary for growth inhibition suggests that BAY 10-8888 either interferes nonspecifically with cellular metabolism, as proposed for cispentacin (2), or has a low affinity for its intracellular target. Our data support the latter conclusion (see below). Carrier activity in medium (YPG) containing peptone and yeast extract as nitrogen sources is low and correlates with the low level of BAY 10-8888 accumulation and high IC90. This indicates that active accumulation is a prerequisite for the in vitro antifungal activity of BAY 10-8888, since at the same time no difference in the level of inhibition of the intracellular target isoleucyl-tRNA synthetase was observed between YPG medium and YNG medium.
In C. albicans about 10 amino acid transporters have been characterized by their different substrate specificities (4, 20). Nitrogen catabolite repression seems to be absent from C. albicans (28). Recently, a transporter for basic amino acids was cloned from C. albicans in complementation experiments with an S. cerevisiae mutant (25). This carrier showed a high degree of homology to both transporters for basic amino acids (Can1 and Lyp1) from S. cerevisiae (26), suggesting a high degree of conservation at the molecular level between these two species. The gene which encodes the transporter for BAY 10-8888 awaits further characterization.
In yeast, the accumulation of amino acids from the external medium into the cell occurs by unidirectional flux (7, 8, 13). This unidirectional flux is not related to the trapping of amino acids in the vacuole (19). We have shown that BAY 10-8888 is accumulated unidirectionally and leaves the cells only by diffusion, as is the case for proline in S. cerevisiae (8). Our evidence is based on the different effects of metabolic inhibitors in combination with protein ionophores and sulfhydryl blockers. While influx of BAY 10-8888 is strongly inhibited by these compounds, they have no effect on efflux. This may be important for the antifungal activity of BAY 10-8888, because deenergization as a result of the action of BAY 10-8888 does not lead to the immediate release of the highly accumulated compound through the uncoupled transporter and, subsequently, a decrease in its antifungal activity. Furthermore, efflux is independent from the presence of an excess of isoleucine in the medium. This indicates that the influx of isoleucine does not interfere with BAY 10-8888 efflux; i.e., changes in tissue or serum isoleucine levels will not interfere with the in vivo antifungal activity of BAY 10-8888.
Inside the cell, competitive inhibition of isoleucyl-tRNA synthetase is responsible for the antifungal activity of BAY 10-8888. Our conclusion is based on multiple lines of evidence. First, l-isoleucine is able to reverse BAY 10-8888-induced growth inhibition not only by competing with uptake but also by competing at the intracellular target. The compensatory effects of l-leucine and l-valine on growth inhibition by BAY 10-8888 are small compared to the effects of l-isoleucine. This can be explained either by competition at the carrier level or by an indirect influence on the intracellular l-isoleucine concentration. The effects of the d-amino acids may result from competition at the carrier level, because these amino acids are not incorporated into proteins. Second, BAY 10-8888 inhibits isoleucyl-tRNA synthetase from C. albicans competitively in a concentration-dependent manner. The Ki of about 1 mM, which is unusually high for an enzyme inhibitor, suggests that the interaction of isoleucyl-tRNA synthetase with BAY 10-8888 is not very specific. Nevertheless, overexpression of endogenous isoleucyl-tRNA synthetase in S. cerevisiae resulted in a small (two- to fourfold) but reproducible decrease in sensitivity to BAY 10-8888. The decrease in the sensitivity to BAY 10-8888 was comparable to the decrease in the sensitivity to azoles for a S. cerevisiae transformant overexpressing cytochrome P-450-dependent lanosterol 14-α-demethylase, the target of azole antifungal agents (11). In addition, a different aminoacyl-tRNA synthetase, lysyl-tRNA synthetase, is not inhibited by BAY 10-8888. Taking into account a 200-fold accumulation of BAY 10-8888, protein biosynthesis in intact C. albicans cells and isoleucyl-tRNA synthetase were inhibited by the same concentrations of BAY 10-8888. This suggests that charging of tRNA is a rate-limiting step in protein biosynthesis. An S. cerevisiae mutant showing temperature-sensitive protein biosynthesis was found to have a thermolabile isoleucyl-tRNA synthetase (6). This provides additional evidence that protein biosynthesis in yeasts is very sensitive to the loss or inhibition of isoleucyl-tRNA synthetase activity.
Inhibition of protein biosynthesis is the mode of action of several antibacterial agents successfully used in clinical practice (15). BAY 10-8888 is among the first of the antifungal drugs that block protein biosynthesis. The unique mode of action which differs from those of other antifungal compounds presently available or under development makes BAY 10-8888 a promising candidate for further clinical development.
ACKNOWLEDGMENTS
We gratefully acknowledge G. Munack, MPI für Experimentelle Medizin, Göttingen, Germany, for providing the S. cerevisiae isoleucyl-tRNA synthetase gene. We thank S. Badock and A. Ludwig for excellent technical assistance.
REFERENCES
- 1.Babczinski, P. Unpublished results.
- 1a.Bremm, K., R. Endermann, F. Kunisch, M. Matzke, K.-G. Metzger, H. Militzer, J. Mittendorf, and M. Plempel. May 1993. EP patent 571,870.
- 2.Capobianco J, Zakula D Z, Coen M L, Goldman R C. Anti-Candida activity of cispentacin: the active transport by amino acid permeases and possible mechanisms of action. Biochem Biophys Res Commun. 1993;190:1037–1044. doi: 10.1006/bbrc.1993.1153. [DOI] [PubMed] [Google Scholar]
- 3.Fasoli M O F, Kerridge D. Uptake of pyrimidines and their derivatives into Candida glabrata and Candida albicans. J Gen Microbiol. 1990;136:1475–1481. doi: 10.1099/00221287-136-8-1475. [DOI] [PubMed] [Google Scholar]
- 3a.Geschke, F. U., A. Schmidt, and W. Schönfeld. Unpublished results.
- 4.Gupta P, Mahanty S K, Ansari S, Prasad R. Transport of acidic amino acids in Candida albicans. J Met Vet Mycol. 1992;30:27–34. [PubMed] [Google Scholar]
- 5.Hampel A E, Enger M D, Ritter P O. Aminoacyl-tRNA synthetases from cultured CHO cells. Methods Enzymol. 1979;59:229–234. doi: 10.1016/0076-6879(79)59086-7. [DOI] [PubMed] [Google Scholar]
- 6.Hartwell L H, McLaughlin C S. Mutants of yeast with temperature-sensitive isoleucyl-tRNA synthetases. Proc Natl Acad Sci USA. 1968;59:422–428. doi: 10.1073/pnas.59.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horak J. Amino acid transport in eucaryotic microorganisms. Biochim Biophys Acta. 1986;864:223–256. doi: 10.1016/0304-4157(86)90001-8. [DOI] [PubMed] [Google Scholar]
- 8.Horak J, Rihova L. l-Proline transport in Saccharomyces cerevisiae. Biochim Biophys Acta. 1982;691:144–150. doi: 10.1016/0005-2736(82)90223-1. [DOI] [PubMed] [Google Scholar]
- 9.Iwamoto T, Tsujii E, Ezaki M, Fujie A, Hashimoto S, Okuhara M, Kohsaka M, Imanaka H. FR109615, a new antifungal antibiotic from Streptomyces setonii: taxonomy, fermentation, isolation, physico-chemical properties and biological activity. J Antibiot. 1990;43:1–7. doi: 10.7164/antibiotics.43.1. [DOI] [PubMed] [Google Scholar]
- 10.Jethwaney D, Hofer M, Khaware R K, Prasad R. Functional reconstitution of a purified proline permease from Candida albicans: interaction with the antifungal cispentacin. Microbiology. 1997;143:397–404. doi: 10.1099/00221287-143-2-397. [DOI] [PubMed] [Google Scholar]
- 11.Kirsch D R, Lai M H, O’Sullivan J O. Isolation of the gene for cytochrome P450L1A1 (lanosterol 14 alpha-demethylase) from Candida albicans. Gene. 1988;68:229–237. doi: 10.1016/0378-1119(88)90025-x. [DOI] [PubMed] [Google Scholar]
- 12.Konishi M, Nishio M, Saitoh K, Miyaki T, Oki T, Kawaguchi K. Cispentacin, a new antifungal antibiotic. I. Production, isolation, physico-chemical properties and structure. J Antibiot. 1989;42:1749–1755. doi: 10.7164/antibiotics.42.1749. [DOI] [PubMed] [Google Scholar]
- 13.Kotyk A, Rihova L. Transport of α-aminoisobutyric acid in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972;288:380–389. doi: 10.1016/0005-2736(72)90259-3. [DOI] [PubMed] [Google Scholar]
- 14.Munack G. Ph.D. thesis. Braunschweig, Germany: University of Braunschweig; 1994. [Google Scholar]
- 15.Murray P R, Kobayashi G S, Pfaller M A, Rosenthal K S. Medical microbiology. London, United Kingdom: Mosby-Year Book, Inc.; 1994. [Google Scholar]
- 16.Ohama T, Suzuki T, Mori M, Osawa S, Ueda T, Watanabe K, Nakase T. Non-universal decoding of the leucine codon CUG in several Candida species. Nucleic Acids Res. 1993;21:4039–4045. doi: 10.1093/nar/21.17.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohki H, Inamoto Y, Kawabata K, Kamimura T, Sakane K. Synthesis and antifungal activity of FR109615 analogs. J Antibiot. 1991;44:546–549. doi: 10.7164/antibiotics.44.546. [DOI] [PubMed] [Google Scholar]
- 18.Oki T, Hirano M, Tomatsu K, Numata K, Kamei H. Cispentacin, a new antifungal antibiotic. II. In vitro and in vivo antifungal activities. J Antibiot. 1989;42:1756–1762. doi: 10.7164/antibiotics.42.1756. [DOI] [PubMed] [Google Scholar]
- 19.Opekarova M, Caspari T, Tanner W. Unidirectional arginine transport in reconstituted plasma-membrane vesicles from yeast overexpressing CAN1. Eur J Biochem. 1993;211:683–688. doi: 10.1111/j.1432-1033.1993.tb17596.x. [DOI] [PubMed] [Google Scholar]
- 20.Prasad R. Nutrient transport in Candida albicans, a pathogenic yeast. Yeast. 1987;3:209–221. doi: 10.1002/yea.320030402. [DOI] [PubMed] [Google Scholar]
- 21.Rottenberg H. The measurement of membrane potential and delta pH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- 22.Schena M, Picard D, Yamamoto K. Vectors for constitutive and inducible gene expression in yeast. Methods Enzymol. 1991;194:389–398. doi: 10.1016/0076-6879(91)94029-c. [DOI] [PubMed] [Google Scholar]
- 23.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 24.Sumiyama H, Ohkuma M, Masuda Y, Park S-M, Ohta A, Takagi M. In vivo evidence for non-universal usage of the codon CUG in Candida maltosa. Yeast. 1995;11:43–52. doi: 10.1002/yea.320110106. [DOI] [PubMed] [Google Scholar]
- 25.Sychrova H, Chevallier M-R. Transport properties of a C. albicans amino-acid permease whose putative gene was cloned and expressed in S. cerevisiae. Curr Genet. 1993;24:487–490. doi: 10.1007/BF00351710. [DOI] [PubMed] [Google Scholar]
- 26.Sychrova H, Souciet J-L. CAN1, a gene encoding a permease for basic amino acids in Candida albicans. Yeast. 1994;10:1647–1651. doi: 10.1002/yea.320101214. [DOI] [PubMed] [Google Scholar]
- 27.Van der Haar F. Purification of aminoacyl-tRNA synthetases. Methods Enzymol. 1979;59:257–267. doi: 10.1016/0076-6879(79)59088-0. [DOI] [PubMed] [Google Scholar]
- 28.Verma R S, Prasad R. Absence of derepression of amino acids transport in Candida. Biochem Int. 1983;7:707–717. [PubMed] [Google Scholar]