Abstract
N6‐methyladenosine (m6A) is a dynamic and reversible RNA modification that has emerged as a crucial player in the life cycle of RNA, thus playing a pivotal role in various biological processes. In recent years, the potential involvement of RNA m6A modification in aging and age‐related diseases has gained increasing attention, making it a promising target for understanding the molecular mechanisms underlying aging and developing new therapeutic strategies. This Perspective article will summarize the current advances in aging‐related m6A regulation, highlighting the most significant findings and their implications for our understanding of cellular senescence and aging, and the potential for targeting RNA m6A regulation as a therapeutic strategy. We will also discuss the limitations and challenges in this field and provide insights into future research directions. By providing a comprehensive overview of the current state of the field, this Perspective article aims to facilitate further advances in our understanding of the molecular mechanisms underlying aging and to identify new therapeutic targets for aging‐related diseases.
Keywords: aging, disease, m6A, RNA methylation, senescence
Abbreviations
- 3'UTR
3' untranslated region
- AD
Alzheimer's disease
- AGO2
Argonaute 2
- ALKBH5
AlkB homolog 5
- Cas9
CRISPR‐associated protein 9
- CDKN1A
cyclin‐dependent kinase inhibitor 1A
- circRNA
circular RNA
- CRISPR
clustered regularly interspaced short palindromic repeats
- DGCR8
DiGeorge syndrome critical region 8
- DPSCs
dental pulp stem cells
- ERVs
endogenous retroviruses
- FF
follicular fluid
- FOS
Fos proto‐oncogene
- FTO
fat mass and obesity‐associated protein
- GCs
granulosa cells
- GLORI
glyoxal and nitrite‐mediated deamination of unmethylated adenosines
- HGPS
Hutchinson‐Gilford progeria syndrome
- IGF2BP1
insulin‐like growth factor 2 mRNA‐binding protein 1
- IGF2BP2
insulin‐like growth factor 2 mRNA‐binding protein 2
- LECs
lens epithelium cells
- LINE‐1
long interspersed element‐1
- m6A
N6‐methyladenosine
- m6A‐SAC‐seq
m6A‐selective allyl chemical labeling and sequencing
- METTL14
methyltransferase like 14
- METTL3
methyltransferase like 3
- miRNA
microRNA
- MIS12
MIS12 kinetochore complex component
- mRNA
messenger RNA
- MSCs
mesenchymal stem cells
- NF‐κB
nuclear factor‐kappa B
- NMDAR1
N‐methyl‐d‐aspartate receptor 1
- NPCs
nucleus pulposus cells
- NPNT
nephronectin
- PC12
rat pheochromocytoma cell line
- PD
Parkinson's disease
- PLK1
Polo‐like kinase 1
- Pth1r
parathyroid hormone receptor 1
- SA‐β‐gal
senescence‐associated β‐galactosidase
- SIRT1
Sirtuin 1
- TNF‐α
tumor necrosis factor‐alpha
- WS
Werner syndrome
- YTHDF
YTH domain‐containing family protein
1. INTRODUCTION
Aging is a complex biological process characterized by the progressive deterioration of physiological functions and is associated with increased susceptibility to various age‐related chronic diseases (Cai, Ji, et al., 2022; Cai, Song, et al., 2022; Kennedy et al., 2014; Ren et al., 2023; Sun, Li, & Kirkland, 2022). Deciphering the molecular mechanism of aging is crucial for developing effective interventions to combat aging‐associated degeneration and promote healthy aging (Cai, Ji, et al., 2022; Cai, Song, et al., 2022; Li, Xiong, et al., 2023; Liu et al., 2022; Wang et al., 2022; Zhang, Qu, et al., 2020). Similar to DNA and protein, RNA can also be decorated with a variety of chemical modifications, leading to a special layer of epigenetic regulation known as the epitranscriptome (McMahon et al., 2021; Zhao et al., 2017). Currently, over 170 types of RNA modifications have been discovered, among which N6 ‐methyladenosine (m6A) is reported as the most common and conserved modification in eukaryotic mRNA. RNA m6A modification is dynamically regulated by “writers” such as METTL3 and METTL14, “erasers” such as FTO and ALKBH5, and “readers” such as YTH family members and IGF2 binding proteins. These regulators function to control splicing, nuclear export, stability, and translation of target RNAs involved in various biological processes (Bai et al., 2023; Deng et al., 2018; Huang et al., 2020; Roignant & Soller, 2017). Recently, there has been increasing evidence for the involvement of m6A regulation in cellular senescence and other aging‐related processes (Bao et al., 2023; Casella et al., 2019; Jiapaer et al., 2022; Sun, Cheng, et al., 2022; Wu et al., 2022; Zhang & Xia, 2023). This offers a new perspective on understanding and combating aging and highlights the potential for targeting m6A regulation as a therapeutic strategy.
In this Perspective article, we provide an in‐depth overview of recent advances in m6A epitranscriptomic regulation during aging. Specifically, we will focus on the roles and mechanisms of m6A methyltransferases and demethylases in cellular senescence and tissue aging. We will examine how m6A regulation affects key players in cell cycle‐related events and tissue homeostasis. Finally, we will also discuss the current limitations of our understanding of m6A epitranscriptomic regulation during aging and highlight potential opportunities for future research.
2. m6A REGULATION IN CELLULAR SENESCENCE
Cellular senescence, characterized by cell cycle arrest, is a critical hallmark and driver of tissue degeneration and aging. Emerging evidence has implicated RNA m6A regulation in the process of cellular senescence (Figure 1), which may act by altering the levels of cell cycle regulators. For instance, researchers have reported that METTL3/METTL14 catalyzes m6A modification in the 3'UTR of CDKN1A mRNA, which encodes the cyclin‐dependent kinase inhibitor p21, to promote cell cycle arrest in oxidative stress‐induced senescence of human cancer cells (Abbas & Dutta, 2009). Interestingly, knockdown of METTL3 or METTL14 decreased the protein level of p21 but had no effects on its overall mRNA level, thus demonstrating a role of m6A in the enhancement of p21 translation (Q. Li et al., 2017). The tumor suppressor p53, which functions as an upstream factor of p21 to induce cell cycle arrest (Engeland, 2022), is also involved in m6A‐associated regulation of cellular senescence. For example, in sulforaphane‐induced senescence of human cancer cells, upregulation of p53 and p21 concomitantly occurred with a reduction in the overall m6A abundance, which is also accompanied by alterations in global DNA methylation (Lewinska et al., 2017). This work implies a potential involvement of m6A regulation in chemical‐induced cellular senescence as well as a possible interplay between RNA modification and DNA methylation in this process, while the underlying mechanism remains unclear. Similarly, m6A‐mediated regulation of other cell cycle‐related factors has also been reported in human stem cell senescence. For example, knockdown of METTL3 led to accelerated senescence of human dental pulp stem cells (DPSCs), as indicated by increased staining of senescence‐associated β‐galactosidase (SA‐β‐gal) and disturbed cell cycle progression. This process was potentially mediated by the upregulation of PLK1, a critical cell cycle regulator, at both the mRNA and protein levels due to decreased m6A modification (Luo et al., 2021). Additionally, genetically modified human mesenchymal stem cell (MSC) models have been established to recapitulate two typical premature aging diseases, that is, Hutchinson‐Gilford progeria syndrome (HGPS) and Werner syndrome (WS). In both progeroid MSC models, downregulation of METTL3 was detected along with a global reduction in RNA m6A abundance during premature senescence. Moreover, CRISPR/Cas9‐mediated knockout of METTL3 accelerated senescence in wild‐type human MSCs, while overexpression of METTL3 rescued the premature senescence of progeroid MSCs. By profiling the m6A modification across the whole transcriptome, MIS12, another cell cycle modulator, whose methylation and expression levels were both diminished in senescent cells, was identified as a key downstream regulator of m6A in counteracting human MSC senescence. Further analysis identified that the m6A reader IGF2BP2 was involved in the recognition and stabilization of MIS12 mRNA (Wu et al., 2020). These findings offer novel insights into the epitranscriptomic regulation of human stem cell senescence and provide potential interventions by targeting cell cycle regulators to combat aging‐associated diseases.
FIGURE 1.
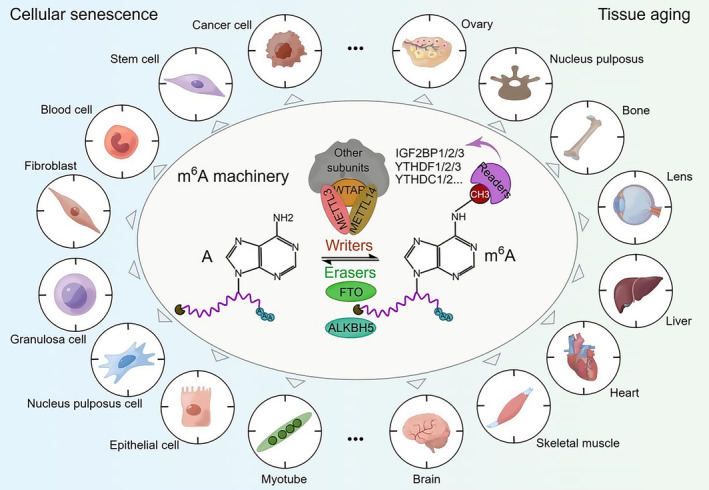
The m6A regulatory machinery and its association with senescence or aging in a variety of representative cell and tissue types. The schematic diagram is prepared by Figdraw.
RNA m6A modification has also been implicated in miRNA processing and subsequent gene expression modulation (Roundtree et al., 2017). Thus, it is worthwhile to investigate whether m6A can participate in the regulation of cellular senescence via miRNA‐related mechanisms. Indeed, in physiologically senescent human blood cells, m6A epitranscriptomic analysis identified a global decrease in the RNA methylation level when compared to that in young cells. Interestingly, a decrease in the methylation and expression level of AGO2 mRNA, which encodes a key enzyme in the RNA interference machinery, was observed in both physiologically senescent blood cells and replicatively senescent fibroblasts. Subsequently, researchers demonstrated that METTL3/METTL14‐mediated m6A modification promoted the stability of AGO2 mRNA and the subsequent expression of mature miRNAs. Meanwhile, knockdown of METTL3 or METTL14 accelerated the cellular senescence of fibroblasts (Min et al., 2018). Although this study couples senescence‐associated m6A regulation with miRNA processing, future mechanistic studies will be of great significance to address which m6A reader stabilizes methylated AGO2 mRNA and which miRNAs play a dominant role in counteracting senescence. m6A‐associated miRNA processing was also reported in human nucleus pulposus cell (NPC) senescence, as knockdown of METTL14 alleviated TNF‐α‐induced cell cycle arrest and senescence of NPCs via inhibiting the m6A and expression level of miR‐34a. Mechanistic insights identified an interaction between METTL14 and DGCR8 in promoting the processing of miR‐34a, which targets SIRT1 to accelerate NPC senescence (Zhu et al., 2021). These findings demonstrate the involvement of m6A‐mediated miRNA and its target mRNA regulation in cellular senescence. However, it is important to note that this field of research is still evolving, and further studies are needed to fully understand the underlying mechanisms and functional consequences.
Notably, m6A methyltransferases or demethylases also appear to regulate cellular senescence in an m6A‐independent manner. One example of such regulation is observed in oncogene‐induced senescence of human fibroblasts, where METTL3 and METTL14 facilitate chromatin remodeling via promoter‐enhancer looping, leading to the expression of senescence‐associated secretory phenotype (SASP) genes driven by NF‐κB p65. This regulation was independent of m6A modifications (Liu et al., 2021). Another intriguing finding is the interaction between METTL3 and METTL14 with Lamin A in both human and mouse cells. This interaction serves to ensure the correct positioning of nuclear speckles and protects these methyltransferases from proteasome‐mediated degradation (Zhang, Ao, et al., 2020). In this study, downregulation of METTL3 and METTL14 was associated with replicative senescence in normal human fibroblasts or premature senescence in fibroblasts from both HGPS patients and progeroid mice. Conversely, overexpression of METTL14 restored heterochromatin and nuclear organization, effectively alleviating cellular senescence. Although a specific role for m6A in this model was not declared in the study, it appears that METTL3 and METTL14 can antagonize cellular senescence independently of m6A modifications. Furthermore, FTO has also been implicated in the regulation of cellular senescence without m6A involvement. For instance, in human MSCs, depletion of FTO disrupted its interaction with MIS12, leading to a decrease in the level of MIS12 protein, rather than its mRNA form, and an accelerated senescence phenotype. Further evidence shows that FTO interacts with MIS12 and safeguards it from proteasomal degradation, although the detailed mechanism remains to be explored (Zhang et al., 2022). These findings highlight the significant role of m6A methyltransferases and demethylases, such as METTL3, METTL14, and FTO, in regulating cellular senescence. The evidence presented here demonstrates that their impact extends beyond traditional m6A modifications, emphasizing the need to explore alternative mechanisms through which these enzymes modulate cellular senescence pathways and providing valuable insights into the intricate nature of senescence regulation.
3. m6A REGULATION IN TISSUE AGING
Either increased or decreased m6A modification has been reported in various tissues, contributing to degenerative progression and aging (Figure 1). To unravel the epitranscriptomic mechanisms underlying aging, it is crucial to understand the relationship between m6A dysregulation and its effects on different target RNAs, as well as the involvement of upstream regulatory enzymes in specific tissue types. In a study published in 2021, researchers identified downregulated FTO expression and an overall increase in RNA m6A modification in human follicular fluid (FF) and granulosa cells (GCs) from aged donors and murine ovaries from old mice (Sun et al., 2021). Shortly afterwards, another study claimed a similar dysregulation of m6A modification and FTO expression in GCs derived from aged human ovaries. Using in vitro cultured human ovarian granulosa cell lines, the researchers found that knockdown of FTO accelerated GC senescence, which was attenuated by FOS inhibition, identifying FOS mRNA as a key downstream effector of m6A. Further analysis revealed that IGF2BP2 was responsible for the stability of m6A‐tagged FOS mRNA (Jiang et al., 2021). Similarly, patients with intervertebral disc degeneration, a common aging‐associated disorder, exhibited elevated m6A modification levels in nucleus pulposus (NP) tissues, which positively correlated with METTL14 and TNF‐α expression (Zhu et al., 2021). Using human NPCs as a cellular model, the authors found that TNF‐α treatment induced upregulation of METTL14 and m6A and subsequent premature senescence. This can be reversed by METTL14 knockdown, potentially through a mechanism associated with miRNA regulation, as described earlier in Section 2 (Zhu et al., 2021). Taken together, these studies suggest that increased m6A deposition could contribute to aging acceleration in certain tissue types.
On the contrary, reduced m6A levels can also promote tissue degeneration or aging in certain contexts. For instance, conditional knockout of Mettl3 in mice led to reduced m6A levels and shortened lifespan, along with obvious degenerative alterations in bone, as characterized by decreased bone mass and increased marrow fat accumulation. Overexpression of Mettl3 prevented estrogen deficiency‐induced osteoporosis, an age‐related skeletal disorder, by facilitating the translation of Pth1r mRNA, which encodes a protein critical for bone formation, although the functional m6A reader of Pth1r mRNA remains uncharacterized (Wu et al., 2018). Additionally, m6A decline is associated with human lens aging (Li et al., 2020). For example, a study by Li et al. reported decreased m6A abundance in total circRNAs in lens epithelium cells (LECs) from patients with age‐related cataracts. This reduction was potentially due to the upregulated expression of ALKBH5 in LECs. The regulatory network for ALKBH5‐mediated m6A upregulation in this process remains to be clarified, but this study provides insights into the mechanism and potential target of ophthalmic degeneration disorders.
Recent studies have illustrated the m6A regulatory maps in multi‐tissue aging. Among them, our team profiled the m6A landscape in the liver, heart, and skeletal muscle during nonhuman primate aging, revealing a positive correlation between RNA m6A modification and gene expression homeostasis across tissues, as well as tissue type‐specific RNA methylation dynamics with aging (Wu et al., 2023). We found that skeletal muscle was most susceptible to m6A loss and METTL3 downregulation during aging, which was associated with age‐related phenotypes such as reduced fiber cross‐sectional area, augmented expression of inflammatory factors, decreased protein level of Lamin B1, and elevated apoptosis. Using human pluripotent stem cell‐derived myotubes as a cellular model, we identified METTL3 deficiency as a potential driver of skeletal muscle aging and NPNT as a key downstream factor of m6A in maintaining myotube homeostasis. Overexpression of METTL3 counteracted myotube senescence by upregulating NPNT in an m6A‐dependent manner, and IGF2BP1 was involved in NPNT mRNA stabilization. These findings provide epitranscriptomic insights into skeletal muscle aging and providing potential therapeutic targets to treat sarcopenia, another age‐associated disease commonly seen in the clinic. Previous studies found that METTL3 inhibition resulted in impaired skeletal muscle regeneration in mice (Liang et al., 2021) and that METTL3 knockout in mice led to muscle wasting and abrogated the overload‐induced hypertrophy (Petrosino et al., 2022), supporting our findings. Together, these findings suggest a geroprotective role of METTL3 in promoting skeletal muscle homeostasis and provide promising potential to develop novel interventions for the treatment of age‐related diseases, such as sarcopenia.
Apart from the aforementioned tissues, increasing evidence has pointed to an important role for m6A regulation in brain aging and neurodegenerative diseases. For example, Shafik et al. described increased m6A modification in brain aging in both mice and humans (Shafik et al., 2021). Unexpectedly, they detected a decrease in the m6A level in a mouse model of Alzheimer's disease (AD). Combined with a Drosophila AD model expressing human Tau, this study associated the inhibition of METTL3, METTL14, or YTHDF with enhanced Tau toxicity, thus hinting at a potential role of m6A in the regulation of AD pathogenesis. However, Castro‐Hernández et al. revealed a substantial reduction in m6A‐methylated transcripts in the brains of aged mice and human AD patients (Castro‐Hernández et al., 2023). They also provided evidence at the mechanistic level, which indicated that METTL3‐mediated m6A modification was related to the synthesis of synaptic proteins. Inconsistently, yet another study reported a global increase in m6A modification in the brains of AD mice, concomitant with the upregulated expression of METTL3 and downregulated expression of FTO (Han et al., 2020). The possible explanations for the inconsistency among these studies deserve further investigation. Altered m6A modification has also been involved in another neurodegenerative disease, that is, Parkinson's disease (PD). In a rat model of PD, a global reduction in RNA m6A levels was observed in the brain (Chen et al., 2019). Further analysis in 6‐hydroxydopamine‐treated PC12 cells revealed that FTO‐mediated m6A removal was associated with the upregulation of NMDAR1 expression, oxidative stress, and Ca2+ influx, which may contribute to dopaminergic neuron apoptosis and PD progression.
4. CONCLUSION AND PERSPECTIVES
This Perspective article provides an overview of m6A regulation in cellular senescence and tissue aging, as well as a variety of age‐related disorders, focusing on the roles of m6A methyltransferases and demethylases and the underlying mechanisms. It offers epitranscriptomic insights into the molecular networks of aging and the development of potential therapeutic strategies. This encompasses the involvement of common regulatory pathways in distinct cellular and tissue contexts. Specifically, it has been consistently observed that abnormal expression of cell cycle‐related factors, mediated by m6A dysregulation, occurs in various types of human stem cells and cancer cells. This phenomenon may arise due to cell cycle arrest serving as a potentially universal hallmark of cellular senescence (Di Micco et al., 2021; Gorgoulis et al., 2019; López‐Otín et al., 2023; Ogrodnik et al., 2019; Wang et al., 2023), involving the expression control of a range of cell cycle regulators and persisting even across distinct cell types. The article also notes cell‐ or tissue‐specific effects of m6A regulation on aging‐related processes. As an example, our recent study reported a noticeable decline in both METTL3 expression and m6A modification specifically during the aging of primate skeletal muscles (Wu et al., 2023). Interestingly, this decline was not observed in aged livers or hearts, although both cardiac and skeletal muscles consist of postmitotic and highly differentiated myofibers. Although the underlying roots of such tissue specificity remain elusive, variations in cellular and molecular heterogeneity among different tissues may contribute to m6A‐associated aging regulation (He et al., 2020; Tian et al., 2023; Trapp et al., 2021; Yamamoto et al., 2022). Overall, we provide a comprehensive summary of m6A regulatory networks in aging at both the cellular and tissue levels, deepening the understanding of complex biological changes associated with senescence or aging. More importantly, the Perspective article also discusses the implications of m6A dysregulation in age‐related diseases such as osteoporosis and AD, highlighting the potential for the development of novel therapeutic strategies to ameliorate age‐related pathologies by targeting the m6A machinery.
Despite the valuable insights gained from m6A research, there are limitations and challenges that need to be addressed. For example, inconsistencies among studies on AD may be due to variations in research contexts and lack of standardized methodologies, highlighting the need for further investigations to ensure reproducibility and reliability in our understanding of m6A regulation during aging. Establishing uniform standards for animal or cell model construction, sample collection and processing, as well as guidelines for sequencing and analysis methodologies, may be helpful to address such issues. Looking ahead, there are several promising avenues for future research. First, uncovering more comprehensive and in‐depth mechanisms of m6A regulation in cellular senescence, aging or aging‐related diseases will provide valuable insights into the underlying molecular pathways and targets. For example, emerging studies have shown an important association of m6A in the regulation of retrotransposable elements including long‐interspersed element‐1 (LINE‐1) and endogenous retroviruses (ERVs), thus being involved in the homeostatic maintenance of stem cells (Chelmicki et al., 2021; Della Valle et al., 2022; Wei et al., 2022; Xu et al., 2021). Considering the critical involvement of LINE‐1 and ERVs in aging (Bi et al., 2020; De Cecco et al., 2019; Gorbunova et al., 2021; Liu, Liu, et al., 2023; Simon et al., 2019; Zhang et al., 2023), it would be very interesting to investigate whether m6A could regulate the activity of these retrotransposons in the aging process. Filling this knowledge gap can potentially lead to the novel development of targeted interventions to delay or mitigate age‐related processes. Second, exploring the broader landscape of m6A modifications across more tissues, cell types, and species will deepen our understanding of their regulatory functions during aging. This could be achieved through the combined application of advanced sequencing methods that enable high‐resolution profiling of m6A modifications at the single‐base or single‐cell level, such as m6A‐SAC‐seq (Hu et al., 2022), GLORI (Liu, Sun, et al., 2023), and single‐cell m6A‐seq (Li, Wang, et al., 2023; Yao et al., 2023). Finally, translating the findings from preclinical models and cellular systems to the clinic remains a crucial question. Further evaluation is needed to determine whether the mechanistic insights gained from model organisms and cellular models can be easily translated to humans and applied in clinical settings. A systematic research platform combining human stem cells, organoids, nonhuman primates, and clinical samples would be beneficial. Taken together, continued research focusing on addressing the current limitations and challenges in this field holds great potential for our understanding of the epigenetic regulation of aging and the development of novel therapeutics via targeting m6A regulatory machines to advance our ability to mitigate age‐related diseases.
AUTHOR CONTRIBUTIONS
G.H. L. and J.R. conceived the idea. Z.W. drafted the manuscript. All authors shared responsibility for editing both the form and content of this manuscript and approving the final version.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
We thank Lei Bai for her administrative assistance. This work was supported by the National Key Research and Development Program of China (2019YFA0802202, 2022YFA1103800, 2020YFA0804000, 2020YFA0112200, and 2020YFA0803401), the National Natural Science Foundation of China (32100937, 81921006, 92149301, 92168201, 32121001, and 31970597), CAS Project for Young Scientists in Basic Research (YSBR‐076), Beijing Natural Science Foundation (Z190019), New Cornerstone Science Foundation through the XPLORER PRIZE (2021‐1045), and CAS Special Research Assistant Program.
Wu, Z. , Ren, J. , & Liu, G.‐H. (2023). Deciphering RNA m6A regulation in aging: Perspectives on current advances and future directions. Aging Cell, 22, e13972. 10.1111/acel.13972
Contributor Information
Jie Ren, Email: renjie@big.ac.cn.
Guang‐Hui Liu, Email: ghliu@ioz.ac.cn.
REFERENCES
- Abbas, T. , & Dutta, A. (2009). p21 in cancer: Intricate networks and multiple activities. Nature Reviews. Cancer, 9(6), 400–414. 10.1038/nrc2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Y. , Li, K. , Peng, J. , & Yi, C. (2023). M6A modification: A new avenue for anti‐cancer therapy. Life Medicine, 2, lnad008. 10.1093/lifemedi/lnad008 [DOI] [Google Scholar]
- Bao, H. , Cao, J. , Chen, M. , Chen, M. , Chen, W. , Chen, X. , Chen, Y. , Chen, Y. , Chen, Y. , Chen, Z. , Chhetri, J. K. , Ding, Y. , Feng, J. , Guo, J. , Guo, M. , He, C. , Jia, Y. , Jiang, H. , … Liu, G.‐H. (2023). Biomarkers of aging. Science China Life Sciences, 66(5), 893–1066. 10.1007/s11427-023-2305-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, S. , Liu, Z. , Wu, Z. , Wang, Z. , Liu, X. , Wang, S. , Ren, J. , Yao, Y. , Zhang, W. , Song, M. , Liu, G. H. , & Qu, J. (2020). SIRT7 antagonizes human stem cell aging as a heterochromatin stabilizer. Protein & Cell, 11, 483–504. 10.1007/s13238-020-00728-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Y. , Ji, Z. , Wang, S. , Zhang, W. , Qu, J. , Belmonte, J. C. I. , & Liu, G.‐H. (2022). Genetic enhancement: A new avenue to combat aging‐related diseases. Life Medicine, 1, lnac054. 10.1093/lifemedi/lnac054 [DOI] [Google Scholar]
- Cai, Y. , Song, W. , Li, J. , Jing, Y. , Liang, C. , Zhang, L. , Zhang, X. , Zhang, W. , Liu, B. , An, Y. , Li, J. , Tang, B. , Pei, S. , Wu, X. , Liu, Y. , Zhuang, C. L. , Ying, Y. , Dou, X. , Chen, Y. , … Liu, G. H. (2022). The landscape of aging. Science China. Life Sciences, 65(12), 2354–2454. 10.1007/s11427-022-2161-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella, G. , Tsitsipatis, D. , Abdelmohsen, K. , & Gorospe, M. (2019). mRNA methylation in cell senescence. Wiley Interdiscip Rev RNA, 10(6), e1547. 10.1002/wrna.1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro‐Hernández, R. , Berulava, T. , Metelova, M. , Epple, R. , Peña Centeno, T. , Richter, J. , Kaurani, L. , Pradhan, R. , Sakib, M. S. , Burkhardt, S. , Ninov, M. , Bohnsack, K. E. , Bohnsack, M. T. , Delalle, I. , & Fischer, A. (2023). Conserved reduction of m(6)a RNA modifications during aging and neurodegeneration is linked to changes in synaptic transcripts. Proceedings of the National Academy of Sciences of the United States of America, 120(9), e2204933120. 10.1073/pnas.2204933120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelmicki, T. , Roger, E. , Teissandier, A. , Dura, M. , Bonneville, L. , Rucli, S. , Dossin, F. , Fouassier, C. , Lameiras, S. , & Bourc'his, D. (2021). M(6)a RNA methylation regulates the fate of endogenous retroviruses. Nature, 591(7849), 312–316. 10.1038/s41586-020-03135-1 [DOI] [PubMed] [Google Scholar]
- Chen, X. , Yu, C. , Guo, M. , Zheng, X. , Ali, S. , Huang, H. , Qie, S. , & Wang, J. (2019). Down‐regulation of m6A mRNA methylation is involved in dopaminergic neuronal death. ACS Chemical Neuroscience, 10(5), 2355–2363. 10.1021/acschemneuro.8b00657 [DOI] [PubMed] [Google Scholar]
- De Cecco, M. , Ito, T. , Petrashen, A. P. , Elias, A. E. , Skvir, N. J. , Criscione, S. W. , Caligiana, A. , Brocculi, G. , Adney, E. M. , Boeke, J. D. , Le, O. , Beauséjour, C. , Ambati, J. , Ambati, K. , Simon, M. , Seluanov, A. , Gorbunova, V. , Slagboom, P. E. , Helfand, S. L. , … Sedivy, J. M. (2019). L1 drives IFN in senescent cells and promotes age‐associated inflammation. Nature, 566(7742), 73–78. 10.1038/s41586-018-0784-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Valle, F. , Reddy, P. , Yamamoto, M. , Liu, P. , Saera‐Vila, A. , Bensaddek, D. , Zhang, H. , Prieto Martinez, J. , Abassi, L. , Celii, M. , Ocampo, A. , Nuñez Delicado, E. , Mangiavacchi, A. , Aiese Cigliano, R. , Rodriguez Esteban, C. , Horvath, S. , Izpisua Belmonte, J. C. , & Orlando, V. (2022). LINE‐1 RNA causes heterochromatin erosion and is a target for amelioration of senescent phenotypes in progeroid syndromes. Science Translational Medicine, 14(657), eabl6057. 10.1126/scitranslmed.abl6057 [DOI] [PubMed] [Google Scholar]
- Deng, X. , Su, R. , Weng, H. , Huang, H. , Li, Z. , & Chen, J. (2018). RNA N(6)‐methyladenosine modification in cancers: Current status and perspectives. Cell Research, 28(5), 507–517. 10.1038/s41422-018-0034-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Micco, R. , Krizhanovsky, V. , Baker, D. , & d'Adda di Fagagna, F. (2021). Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nature Reviews Molecular Cell Biology, 22(2), 75–95. 10.1038/s41580-020-00314-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeland, K. (2022). Cell cycle regulation: p53‐p21‐RB signaling. Cell Death and Differentiation, 29(5), 946–960. 10.1038/s41418-022-00988-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunova, V. , Seluanov, A. , Mita, P. , McKerrow, W. , Fenyö, D. , Boeke, J. D. , Linker, S. B. , Gage, F. H. , Kreiling, J. A. , Petrashen, A. P. , Woodham, T. A. , Taylor, J. R. , Helfand, S. L. , & Sedivy, J. M. (2021). The role of retrotransposable elements in ageing and age‐associated diseases. Nature, 596(7870), 43–53. 10.1038/s41586-021-03542-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis, V. , Adams, P. D. , Alimonti, A. , Bennett, D. C. , Bischof, O. , Bishop, C. , Campisi, J. , Collado, M. , Evangelou, K. , Ferbeyre, G. , Gil, J. , Hara, E. , Krizhanovsky, V. , Jurk, D. , Maier, A. B. , Narita, M. , Niedernhofer, L. , Passos, J. F. , Robbins, P. D. , … Demaria, M. (2019). Cellular senescence: Defining a path forward. Cell, 179(4), 813–827. 10.1016/j.cell.2019.10.005 [DOI] [PubMed] [Google Scholar]
- Han, M. , Liu, Z. , Xu, Y. , Liu, X. , Wang, D. , Li, F. , Wang, Y. , & Bi, J. (2020). Abnormality of m6A mRNA methylation is involved in Alzheimer's disease. Frontiers in Neuroscience, 14, 98. 10.3389/fnins.2020.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Memczak, S. , Qu, J. , Belmonte, J. C. I. , & Liu, G. H. (2020). Single‐cell omics in ageing: A young and growing field. Nature Metabolism, 2(4), 293–302. 10.1038/s42255-020-0196-7 [DOI] [PubMed] [Google Scholar]
- Hu, L. , Liu, S. , Peng, Y. , Ge, R. , Su, R. , Senevirathne, C. , Harada, B. T. , Dai, Q. , Wei, J. , Zhang, L. , Hao, Z. , Luo, L. , Wang, H. , Wang, Y. , Luo, M. , Chen, M. , Chen, J. , & He, C. (2022). M(6)a RNA modifications are measured at single‐base resolution across the mammalian transcriptome. Nature Biotechnology, 40(8), 1210–1219. 10.1038/s41587-02201243-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Weng, H. , & Chen, J. (2020). The biogenesis and precise control of RNA m(6)a methylation. Trends in Genetics, 36(1), 44–52. 10.1016/j.tig.2019.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Z. X. , Wang, Y. N. , Li, Z. Y. , Dai, Z. H. , He, Y. , Chu, K. , Gu, J. Y. , Ji, Y. X. , Sun, N. X. , Yang, F. , & Li, W. (2021). The m6A mRNA demethylase FTO in granulosa cells retards FOS‐dependent ovarian aging. Cell Death & Disease, 12(8), 744. 10.1038/s41419-021-04016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiapaer, Z. , Su, D. , Hua, L. , Lehmann, H. I. , Gokulnath, P. , Vulugundam, G. , Song, S. , Zhang, L. , Gong, Y. , & Li, G. (2022). Regulation and roles of RNA modifications in aging‐related diseases. Aging Cell, 21(7), e13657. 10.1111/acel.13657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, B. K. , Berger, S. L. , Brunet, A. , Campisi, J. , Cuervo, A. M. , Epel, E. S. , Franceschi, C. , Lithgow, G. J. , Morimoto, R. I. , Pessin, J. E. , Rando, T. A. , Richardson, A. , Schadt, E. E. , Wyss‐Coray, T. , & Sierra, F. (2014). Geroscience: Linking aging to chronic disease. Cell, 159(4), 709–713. 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinska, A. , Adamczyk‐Grochala, J. , Deregowska, A. , & Wnuk, M. (2017). SulforaphaneInduced cell cycle arrest and senescence are accompanied by DNA hypomethylation and changes in microRNA profile in breast cancer cells. Theranostics, 7(14), 3461–3477. 10.7150/thno.20657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Xiong, M. , Fu, X.‐H. , Fan, Y. , Dong, C. , Sun, X. , Zheng, F. , Wang, S.‐W. , Liu, L. , Xu, M. , Wang, C. , Ping, J. , Che, S. , Wang, Q. , Yang, K. , Zuo, Y. , Lu, X. , Zheng, Z. , Lan, T. , … Liu, G.‐H. (2023). Determining a multimodal aging clock in a cohort of Chinese women. Med. 10.1016/j.medj.2023.06.010. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Li, P. , Yu, H. , Zhang, G. , Kang, L. , Qin, B. , Cao, Y. , Luo, J. , Chen, X. , Wang, Y. , Qin, M. , Wu, J. , Huang, Y. , Zou, X. , Guan, H. , & Wang, Y. (2020). Identification and characterization of N6‐Methyladenosine CircRNAs and methyltransferases in the lens epithelium cells from age‐related cataract. Investigative Ophthalmology and Visual Science, 61(10), 13. 10.1167/iovs.61.10.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Li, X. , Tang, H. , Jiang, B. , Dou, Y. , Gorospe, M. , & Wang, W. (2017). NSUN2‐mediated m5C methylation and METTL3/METTL14‐mediated m6A methylation cooperatively enhance p21 translation. Journal of Cellular Biochemistry, 118(9), 2587–2598. 10.1002/jcb.25957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Wang, Y. , Vera‐Rodriguez, M. , Lindeman, L. C. , Skuggen, L. E. , Rasmussen, E. M. K. , Jermstad, I. , Khan, S. , Fosslie, M. , Skuland, T. , Indahl, M. , Khodeer, S. , Klemsdal, E. K. , Jin, K. X. , Dalen, K. T. , Fedorcsak, P. , Greggains, G. D. , Lerdrup, M. , Klungland, A. , … Dahl, J. A. (2023). Single‐cell m(6)A mapping in vivo using picoMeRIP‐seq. Nature Biotechnology. 10.1038/s41587-023-01831-7. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Y. , Han, H. , Xiong, Q. , Yang, C. , Wang, L. , Ma, J. , Lin, S. , & Jiang, Y. Z. (2021). METTL3Mediated m(6)a methylation regulates muscle stem cells and muscle regeneration by notch signaling pathway. Stem Cells International, 2021, 9955691. 10.1155/2021/9955691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, B. , Qu, J. , Zhang, W. , Izpisua Belmonte, J. C. , & Liu, G. H. (2022). A stem cell aging framework, from mechanisms to interventions. Cell Reports, 41(3), 111451. 10.1016/j.celrep.2022.111451 [DOI] [PubMed] [Google Scholar]
- Liu, C. , Sun, H. , Yi, Y. , Shen, W. , Li, K. , Xiao, Y. , Li, F. , Li, Y. , Hou, Y. , Lu, B. , Liu, W. , Meng, H. , Peng, J. , Yi, C. , & Wang, J. (2023). Absolute quantification of single‐base m(6)a methylation in the mammalian transcriptome using GLORI. Nature Biotechnology, 41(3), 355–366. 10.1038/s41587-022-01487-9 [DOI] [PubMed] [Google Scholar]
- Liu, P. , Li, F. , Lin, J. , Fukumoto, T. , Nacarelli, T. , Hao, X. , Kossenkov, A. V. , Simon, M. C. , & Zhang, R. (2021). M(6)A‐independent genome‐wide METTL3 and METTL14 redistribution drives the senescence‐associated secretory phenotype. Nature Cell Biology, 23(4), 355–365. 10.1038/s41556-021-00656-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Liu, Z. , Wu, Z. , Ren, J. , Fan, Y. , Sun, L. , Cao, G. , Niu, Y. , Zhang, B. , Ji, Q. , Jiang, X. , Wang, C. , Wang, Q. , Ji, Z. , Li, L. , Esteban, C. R. , Yan, K. , Li, W. , Cai, Y. , … Liu, G. H. (2023). Resurrection of endogenous retroviruses during aging reinforces senescence. Cell, 186(2), 287–304.e26. 10.1016/j.cell.2022.12.017 [DOI] [PubMed] [Google Scholar]
- López‐Otín, C. , Blasco, M. A. , Partridge, L. , Serrano, M. , & Kroemer, G. (2023). Hallmarks of aging: An expanding universe. Cell, 186(2), 243–278. 10.1016/j.cell.2022.11.001 [DOI] [PubMed] [Google Scholar]
- Luo, H. , Liu, W. , Zhang, Y. , Yang, Y. , Jiang, X. , Wu, S. , & Shao, L. (2021). METTL3‐mediated m(6)a modification regulates cell cycle progression of dental pulp stem cells. Stem Cell Research & Therapy, 12(1), 159. 10.1186/s13287-021-02223-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon, M. , Forester, C. , & Buffenstein, R. (2021). Aging through an epitranscriptomic lens. Nature Aging, 1(4), 335–346. 10.1038/s43587-021-00058-y [DOI] [PubMed] [Google Scholar]
- Min, K. W. , Zealy, R. W. , Davila, S. , Fomin, M. , Cummings, J. C. , Makowsky, D. , Mcdowell, C. H. , Thigpen, H. , Hafner, M. , Kwon, S. H. , Georgescu, C. , Wren, J. D. , & Yoon, J. H. (2018). Profiling of m6A RNA modifications identified an age‐associated regulation of AGO2 mRNA stability. Aging Cell, 17(3), e12753. 10.1111/acel.12753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrodnik, M. , Salmonowicz, H. , Jurk, D. , & Passos, J. F. (2019). Expansion and cell‐cycle arrest: Common denominators of cellular senescence. Trends in Biochemical Sciences, 44(12), 996–1008. 10.1016/j.tibs.2019.06.011 [DOI] [PubMed] [Google Scholar]
- Petrosino, J. M. , Hinger, S. A. , Golubeva, V. A. , Barajas, J. M. , Dorn, L. E. , Iyer, C. C. , Sun, H. L. , Arnold, W. D. , He, C. , & Accornero, F. (2022). The m(6)a methyltransferase METTL3 regulates muscle maintenance and growth in mice. Nature Communications, 13(1), 168. 10.1038/s41467-021-27848-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, J. , Song, M. , Zhang, W. , Cai, J.‐P. , Cao, F. , Cao, Z. , Chan, P. , Chen, C. , Chen, G. , Chen, H.‐Z. , Chen, J. , Chen, X.‐C. , Ci, W. , Ding, B.‐S. , Ding, Q. , Gao, F. , Gao, S. , Han, J.‐D. J. , He, Q.‐Y. , … Liu, G.‐H. (2023). The Aging Biomarker Consortium represents a new era for aging research in China. Nature Medicine. 10.1038/s41591-023-02444-y. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Roignant, J. Y. , & Soller, M. (2017). M(6)a in mRNA: An ancient mechanism for fine‐tuning gene expression. Trends in Genetics, 33(6), 380–390. 10.1016/j.tig.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Roundtree, I. A. , Evans, M. E. , Pan, T. , & He, C. (2017). Dynamic RNA modifications in gene expression regulation. Cell, 169(7), 1187–1200. 10.1016/j.cell.2017.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafik, A. M. , Zhang, F. , Guo, Z. , Dai, Q. , Pajdzik, K. , Li, Y. , Kang, Y. , Yao, B. , Wu, H. , He, C. , Allen, E. G. , Duan, R. , & Jin, P. (2021). N6methyladenosine dynamics in neurodevelopment and aging, and its potential role in Alzheimer's disease. Genome Biology, 22(1), 17. 10.1186/s13059-020-02249-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, M. , Van Meter, M. , Ablaeva, J. , Ke, Z. , Gonzalez, R. S. , Taguchi, T. , De Cecco, M. , Leonova, K. I. , Kogan, V. , Helfand, S. L. , Neretti, N. , Roichman, A. , Cohen, H. Y. , Meer, M. V. , Gladyshev, V. N. , Antoch, M. P. , Gudkov, A. V. , Sedivy, J. M. , Seluanov, A. , & Gorbunova, V. (2019). LINE1 Derepression in aged wild‐type and SIRT6‐deficient mice drives inflammation. Cell Metabolism, 29(4), 871–885.e875. 10.1016/j.cmet.2019.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. , Cheng, B. , Su, Y. , Li, M. , Ma, S. , Zhang, Y. , Zhang, A. , Cai, S. , Bao, Q. , Wang, S. , & Zhu, P. (2022). The potential role of m6A RNA methylation in the aging process and aging‐associated diseases. Frontiers in Genetics, 13, 869950. 10.3389/fgene.2022.869950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X. , Zhang, Y. , Hu, Y. , An, J. , Li, L. , Wang, Y. , & Zhang, X. (2021). Decreased expression of m(6)a demethylase FTO in ovarian aging. Archives of Gynecology and Obstetrics, 303(5), 1363–1369. 10.1007/s00404-020-05895-7 [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Li, Q. , & Kirkland, J. L. (2022). Targeting senescent cells for a healthier longevity: The roadmap for an era of global aging. Life Medicine., 1, 103–119. 10.1093/lifemedi/lnac030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, Y. E. , Cropley, V. , Maier, A. B. , Lautenschlager, N. T. , Breakspear, M. , & Zalesky, A. (2023). Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nature Medicine, 29(5), 1221–1231. 10.1038/s41591-02302296-6 [DOI] [PubMed] [Google Scholar]
- Trapp, A. , Kerepesi, C. , & Gladyshev, V. N. (2021). Profiling epigenetic age in single cells. Nature Aging, 1(12), 1189–1201. 10.1038/s43587-021-00134-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Yang, K. , Liu, X. , Wang, S. , Song, M. , Belmonte, J. C. I. , Qu, J. , Liu, G.‐H. , & Zhang, W. (2023). MAVS antagonizes human stem cell senescence as a mitochondrial stabilizer. Research, 6, 0192. 10.34133/research.0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K. , Liu, H. , Hu, Q. , Wang, L. , Liu, J. , Zheng, Z. , Zhang, W. , Ren, J. , Zhu, F. , & Liu, G. H. (2022). Epigenetic regulation of aging: Implications for interventions of aging and diseases. Signal Transduction and Targeted Therapy, 7(1), 374. 10.1038/s41392-022-01211-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, J. , Yu, X. , Yang, L. , Liu, X. , Gao, B. , Huang, B. , Dou, X. , Liu, J. , Zou, Z. , Cui, X. L. , Zhang, L. S. , Zhao, X. , Liu, Q. , He, P. C. , Sepich‐Poore, C. , Zhong, N. , Liu, W. , Li, Y. , Kou, X. , … He, C. (2022). FTO mediates LINE1 m(6)a demethylation and chromatin regulation in mESCs and mouse development. Science, 376(6596), 968–973. 10.1126/science.abe9582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Xie, L. , Wang, M. , Xiong, Q. , Guo, Y. , Liang, Y. , Li, J. , Sheng, R. , Deng, P. , Wang, Y. , Zheng, R. , Jiang, Y. , Ye, L. , Chen, Q. , Zhou, X. , Lin, S. , & Yuan, Q. (2018). Mettl3‐mediated m(6)a RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis. Nature Communications, 9(1), 4772. 10.1038/s41467-01806898-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z. , Lu, M. , Liu, D. , Shi, Y. , Ren, J. , Wang, S. , Jing, Y. , Zhang, S. , Zhao, Q. , Li, H. , Yu, Z. , Liu, Z. , Bi, S. , Wei, T. , Yang, Y. G. , Xiao, J. , Belmonte, J. C. I. , Qu, J. , Zhang, W. , … Liu, G.‐H. (2023). m6A epitranscriptomic regulation of tissue homeostasis during primate aging. Nature Aging., 3, 705–721. 10.1038/s43587-023-00393-2 [DOI] [PubMed] [Google Scholar]
- Wu, Z. , Shi, Y. , Lu, M. , Song, M. , Yu, Z. , Wang, J. , Wang, S. , Ren, J. , Yang, Y. G. , Liu, G. H. , Zhang, W. , Ci, W. , & Qu, J. (2020). METTL3 counteracts premature aging via m6A‐dependent stabilization of MIS12 mRNA. Nucleic Acids Research, 48(19), 11083–11096. 10.1093/nar/gkaa816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z. , Wang, S. , Belmonte, J. C. I. , Zhang, W. , Qu, J. , & Liu, G.‐H. (2022). Emerging role of RNA m6A modification in aging regulation. Current Medicine, 1(1), 1–4. 10.1007/s44194-022-00009-8 35673631 [DOI] [Google Scholar]
- Xu, W. , Li, J. , He, C. , Wen, J. , Ma, H. , Rong, B. , Diao, J. , Wang, L. , Wang, J. , Wu, F. , Tan, L. , Shi, Y. G. , Shi, Y. , & Shen, H. (2021). METTL3 regulates heterochromatin in mouse embryonic stem cells. Nature, 591(7849), 317–321. 10.1038/s41586-021-03210-1 [DOI] [PubMed] [Google Scholar]
- Yamamoto, R. , Chung, R. , Vazquez, J. M. , Sheng, H. , Steinberg, P. L. , Ioannidis, N. M. , & Sudmant, P. H. (2022). Tissue‐specific impacts of aging and genetics on gene expression patterns in humans. Nature Communications, 13(1), 5803. 10.1038/s41467-022-33509-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, H. , Gao, C. C. , Zhang, D. , Xu, J. , Song, G. , Fan, X. , Liang, D. B. , Chen, Y. S. , Li, Q. , Guo, Y. , Cai, Y. T. , Hu, L. , Zhao, Y. L. , Sun, Y. P. , Yang, Y. , Han, J. , & Yang, Y. G. (2023). scm(6)A‐seq reveals single‐cell landscapes of the dynamic m(6)a during oocyte maturation and early embryonic development. Nature Communications, 14(1), 315. 10.1038/s41467-023-35958-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Li, J. , Yu, Y. , Ren, J. , Liu, Q. , Bao, Z. , Sun, S. , Liu, X. , Ma, S. , Liu, Z. , Yan, K. , Wu, Z. , Fan, Y. , Sun, X. , Zhang, Y. , Ji, Q. , Cheng, F. , Wei, P. H. , Ma, X. , … Liu, G. H. (2023). Nuclear lamina erosioninduced resurrection of endogenous retroviruses underlies neuronal aging. Cell Reports, 42(6), 112593. 10.1016/j.celrep.2023.112593 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Ao, Y. , Zhang, Z. , Mo, Y. , Peng, L. , Jiang, Y. , Wang, Z. , & Liu, B. (2020). Lamin a safeguards the m(6) a methylase METTL14 nuclear speckle reservoir to prevent cellular senescence. Aging Cell, 19(10), e13215. 10.1111/acel.13215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , & Xia, J. (2023). N6‐Methyladenosine methylation of mRNA in cell senescence. Cellular and Molecular Neurobiology, 43(1), 27–36. 10.1007/s10571-021-01168-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Wu, Z. , Shi, Y. , Wang, S. , Ren, J. , Yu, Z. , Huang, D. , Yan, K. , He, Y. , Liu, X. , Ji, Q. , Liu, B. , Liu, Z. , Qu, J. , Liu, G. H. , Ci, W. , Wang, X. , & Zhang, W. (2022). FTO stabilizes MIS12 and counteracts senescence. Protein & Cell, 13, 954–960. 10.1007/s13238-022-00914-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Qu, J. , Liu, G. H. , & Belmonte, J. C. I. (2020). The ageing epigenome and its rejuvenation. Nature Reviews. Molecular Cell Biology, 21, 137–150. 10.1038/s41580-019-02045 [DOI] [PubMed] [Google Scholar]
- Zhao, B. S. , Roundtree, I. A. , & He, C. (2017). Post‐transcriptional gene regulation by mRNA modifications. Nature Reviews. Molecular Cell Biology, 18(1), 31–42. 10.1038/nrm.2016.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, H. , Sun, B. , Zhu, L. , Zou, G. , & Shen, Q. (2021). N6‐Methyladenosine induced miR‐34a5p promotes TNF‐α‐induced nucleus pulposus cell senescence by targeting SIRT1. Frontiers in Cell and Development Biology, 9, 642437. 10.3389/fcell.2021.642437 [DOI] [PMC free article] [PubMed] [Google Scholar]


