Abstract
The postantibiotic effect (PAE) following a 2-h exposure of Staphylococcus aureus NCTC 6571 to methicillin (5× the MIC) was investigated with fluorescent probes, 5-cyano-2,3-di-4-tolyl tetrazolium chloride (CTC), an indicator of respiratory activity, and the membrane potential-sensitive compound bis-(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC4(3)]. Counts of the numbers of CFU on solid agar correlated well with information gained from the CTC and DiBAC4(3) fluorescence intensity distributions obtained by flow cytometry and revealed that the postantibiotic effect was 3.1 h. Due to the capacity of flow cytometry to provide information on the heterogeneity of a bacterial population, both fluorescent probes identified the emergence of an active subpopulation 4 h after removal of the methicillin, indicating the recovery of a small percentage of the population. After removal of the methicillin and resuspension of the cells in methicillin-free medium, a further decrease in the respiratory activity and the membrane integrity of the population was observed, although the CFU counts hardly varied, indicating continued antibiotic-induced damage. Also, CTC fluorescence measurements identified numerous subpopulations during the PAE period; this suggests that the PAE is complex, with individual organisms exhibiting various degrees of recovery. Flow cytometry thus provides a rapid and sensitive alternative to traditional techniques that have been used to study PAE, with the added advantage that physiological changes can be detected as they arise.
Bacteria that survive exposure to an antimicrobial agent do not resume growth immediately after the drug is removed (4, 25). Rather, there is a period of recovery from the toxic effects, the duration of which depends on the bacterial strain, the type and concentration of antibiotic, and the exposure time (6). The persistent effects of penicillin on staphylococci was first described by Bigger (2) in 1944. A postantibiotic effect (PAE), as it is now termed, has since been reported for a wide range of both gram-positive and gram-negative bacteria (3, 8, 22). The evaluation of the PAE of new antimicrobial agents has been recommended in the published guidelines of the Infectious Diseases Society of America (5). Clinically, the PAE has important implications for antibiotic dosage regimens, especially when the concentration of the antibiotic may be allowed to fall below the MIC for the organism (16).
Flow cytometry has the capacity to analyze many characteristics of individual bacteria and can thus provide important information regarding the heterogeneity of a bacterial population (10). This provides an advantage over traditional techniques used to study PAE; those techniques only show whether or not the individual bacterium is capable of growth on solid agar. The physiological properties which may be studied by flow cytometry include respiratory activity, membrane potential, intracellular ion concentrations and pH, and many more (19). 5-Cyano-2,3-di-4-tolyl tetrazolium chloride (CTC) is a redox dye which is reduced by the respiratory electron transport chain to an insoluble, fluorescent formazan. It has been used as an indicator of the respiratory activities of Pseudomonas putida (18), Micrococcus luteus (9), and starved Escherichia coli (11). Bis-(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC4(3)] is an anionic membrane potential-sensitive fluorescent probe that enters cells with depolarized membrane potentials, where it binds to lipid-rich compounds (7). Among its various uses, DiBAC4(3) has recently been used in the development of a rapid flow cytometric antibiotic susceptibility assay for clinically important bacteria (14, 20).
This work has used CTC and DiBAC4(3) to provide an alternative technique to the study of the PAE of methicillin on Staphylococcus aureus in terms of respiratory activity and membrane potential.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strain used in this study was S. aureus NCTC 6571, supplied by Alan Paull of the Department of Medical Microbiology, University of Wales College of Medicine. Liquid cultures were grown in Trypticase soy broth (Oxoid) at 37°C in a shaking incubator (100 rpm), and viable counts were determined on Trypticase soy agar (Oxoid).
Staining procedures. (i) CTC.
A fresh solution of CTC (Park Scientific Ltd., Northampton, United Kingdom) was prepared for each experiment and was added to liquid cultures to give a final concentration of 5 mM. Incubation was at 37°C for 30 min in a shaking incubator (100 rpm).
(ii) DiBAC4(3).
A stock solution of DiBAC4(3) (Molecular Probes, Inc., Eugene, Oreg.) was prepared in 70% ethanol at a concentration of 1 mg/ml and was stored at −20°C. The dye was added directly to the liquid culture to give a final concentration of 1 μg/ml. Incubation was for 2 min at room temperature before flow cytometric analysis.
Flow cytometry.
Flow cytometry was performed on a Skatron Argus 100 high-pressure mercury arc lamp-based flow cytometer (Skatron Instruments Ltd., Newmarket, Suffolk, United Kingdom). CTC fluorescence was detected with a G1 filter block (Skatron) which provides an excitation wavelength of 520 to 560 nm and which transmits at 590 nm. DiBAC4(3) fluorescence was detected with a fluorescein isothiocyanate filter block (Skatron) which provides an excitation of 470 to 490 nm and which transmits at 520 to 550 nm. Acquisition of fluorescence data was gated by forward angle light scatter, which is an indication of cell size. Samples were allowed to run for 1 min before the acquisition of data for 5,000 cells. The performance of the machine was monitored daily with 1-μm fluorescent beads (Park Scientific Ltd.).
PAEs.
A total of 100 μl of an overnight culture of S. aureus was inoculated into Trypticase soy broth (25 ml in 100-ml flasks) and was allowed to grow for 3 h at 37°C. This resulted in a population of approximately 107 bacteria/ml. At this point, methicillin (Sigma, Poole, United Kingdom) was added to a concentration of 5 μg/ml and incubation was continued for a further 2 h. The antibiotic was then removed by centrifugation at 2,000 × g for 7 min, and the cells were washed twice in 20 mM HEPES buffer (pH 7.4) before being resuspended in prewarmed (37°C) methicillin-free broth. Samples for flow cytometric analysis and CFU counts were taken at time zero, after 1 and 2 h of exposure to antibiotic, and then at hourly intervals following removal of the antibiotic.
As controls, the following experiments were also set up. (i) One experiment was performed as described above, but following methicillin removal, the cells were resuspended in methicillin-containing medium. (ii) In another experiment the cells were incubated in methicillin-free broth, centrifuged, and then resuspended again in methicillin free-broth.
Counts of numbers of CFU.
Counts of the numbers of CFU were determined as outlined by Miles and Misra (17) on Trypticase soy agar after making an appropriate dilution series.
RESULTS
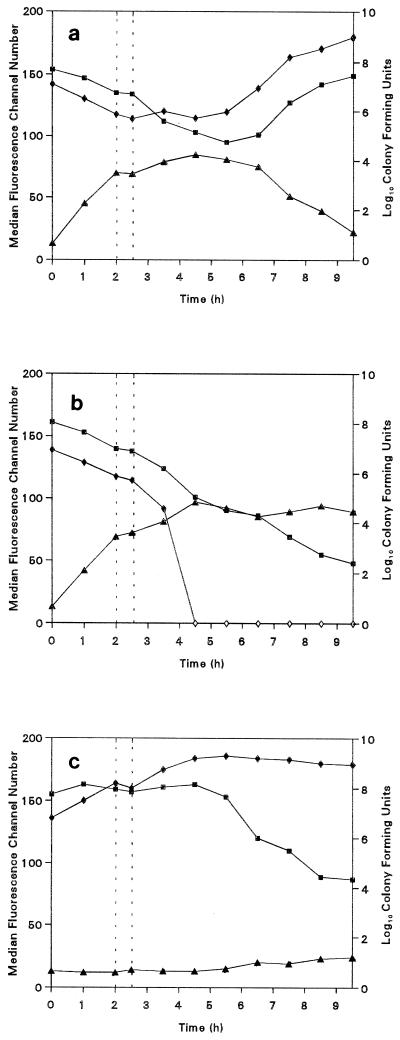
The PAE of methicillin after treatment of S. aureus in terms of viable counts, respiratory activity, and membrane potential is presented in Fig. 1a. CTC and DiBAC4(3) fluorescence is measured on an arbitrary scale of 0 to 256, each value of which corresponds to a channel number; thus, the median fluorescence intensity (the channel number above and below which 50% of the distribution can be found) provides an average value for the population as a whole. This measurement does not take into account the presence of multiple subpopulations, which are clearly evident at some stages, but represents an acceptable simplification of the large amount of data obtained. During the 2-h exposure of the cells to methicillin, a reduction in the CFU counts is paralleled by an increase in the DiBAC4(3) fluorescence, which indicates the membrane potential depolarization caused by antibiotic activity. Also, there is a decrease in the median CTC fluorescence intensity, indicating a decrease in the respiratory activity of the cells. After removal of the methicillin, a 3.1-h PAE was observed by obtaining CFU counts. This value was obtained by the method of Bundtzen et al. (3), who used the following equation: P = T − C, where P is the PAE, T is the time required for the treated cell number to increase by 1 log10 unit, and C is the time required for the control cell number to increase similarly. During this period the CFU count varied only slightly due to the antibiotic-induced inability of the cells to divide. However, after removal of the methicillin from the cells, respiratory activity and membrane integrity continue to decrease, suggesting further antibiotic-induced damage. The end of the PAE was marked by an increase in cell number, respiration, and membrane potential as the cells recover their integrity and activity and resume multiplication. As the culture enters the exponential phase of growth, as indicated by CFU counts, both the fluorescence intensities of CTC and DiBAC4(3) tend toward those values observed for organisms prior to methicillin exposure.
FIG. 1.
Viable counts (⧫), CTC median fluorescence channel number (▪), and DiBAC4(3) median fluorescence channel number (▴) for S. aureus NCTC 6571. (a) Organisms exposed to methicillin for 2 h and then resuspended in methicillin free medium; (b) organisms exposed to methicillin for 2 h and then resuspended in methicillin-containing medium; (c) organisms grown in methicillin-free medium. The vertical dotted lines represent the removal of the antibiotic by centrifugation.
Figure 1b indicates the changes in CFU counts, CTC fluorescence, and DiBAC4(3) fluorescence of cells resuspended in methicillin-containing medium. The time dependencies are similar to those in Fig. 1a, but after removal of the antibiotic, both the cell numbers and respiratory activities continue to decrease, whereas DiBAC4(3) fluorescence increases as the cells are damaged by the methicillin. Surprisingly, CTC fluorescence continued to decrease after the complete loss of organisms capable of colony formation. This may indicate a viable but nonculturable state of the cell (15).
The behavior of bacteria grown in methicillin-free medium is indicated in Fig. 1c. As expected, CFU counts increase with time, and both respiratory activity and membrane integrity show hardly any variations while the culture is in the exponential phase. After entry into the stationary phase, however, respiratory activity decreases and some membrane depolarization occurs.
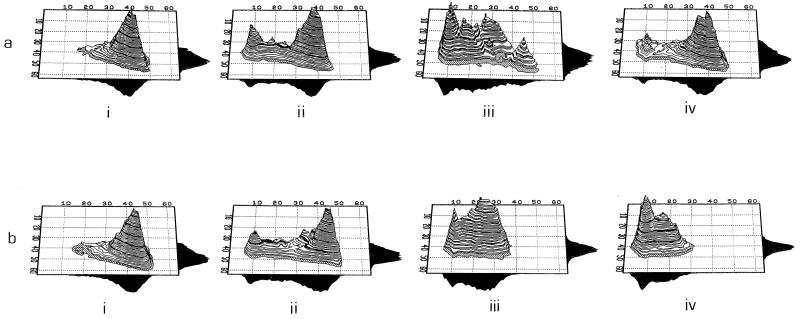
The dual-parameter histograms presented in Fig. 2 and 3 give three-dimensional perspectives of the population on the basis of the light scatter and fluorescence from individual cells; the vertical axis represents the relative numbers of organisms. This allows important aspects regarding the heterogeneity in the population to be recognized. The PAE, as observed by CTC fluorescence, is represented in Fig. 2. After 2 h of incubation in methicillin-containing medium, a peak of low fluorescence appeared; this reflects the portion of dead cells that showed little or no respiratory activity. It is also clear that a further two groups of organisms lie in an intermediate state between active and nonactive bacteria. This appears to reflect cells that have received nonlethal damage, from which they may have the potential to recover and they may resume multiplication. After 4 h of resuspension in methicillin-free medium, at least five subpopulations can be identified, and one of these subpopulations is actively respiring; this indicates the end of the PAE period. By 6 h, this population has entered the exponential phase and a large percentage of the population is actively respiring. In the case of those organisms which, after centrifugation, were resuspended in methicillin-containing medium, respiratory activity continues to decrease (Fig. 2b).
FIG. 2.
Dual-parameter histograms of CTC fluorescence (x axis) against forward-angle light scatter (y axis) for S. aureus. (a) Organisms exposed to methicillin for 2 h and then washed and resuspended in methicillin free medium; (b) organisms exposed to methicillin and then washed and resuspended in methicillin-containing medium. The time for which data points are provided are 0 h, (i), immediately after resuspension (ii), 4 h after resuspension (iii), and 6 h after resuspension (iv).
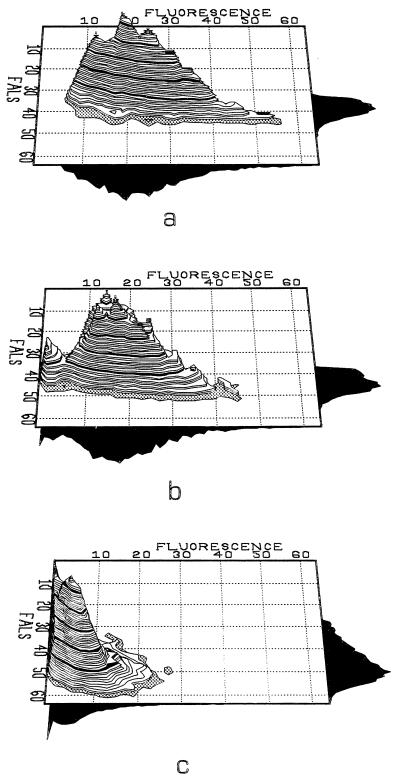
FIG. 3.
Dual-parameter histograms of DiBAC4(3) fluorescence (x axis) against forward-angle light scatter (FALS; y axis) for S. aureus treated for 2 h with methicillin immediately after resuspension in antibiotic-free medium (a) and 4 h (b) and 6 h (c) after resuspension in antibiotic-free medium.
The actively respiring subpopulation seen after 4 h is also apparent when DiBAC4(3) is used (Fig. 3). A small percentage of organisms is revealed to have repolarized membranes, as indicated by low fluorescence intensities. By 6 h, the fluorescence intensity was greatly reduced, indicating the recovery of the population, probably paralleled by lysis of the unrecoverable cells.
DISCUSSION
The use of flow cytometry to investigate PAE has a number of advantages over those traditional techniques, e.g., determination of the numbers of CFU on solid agar, which have been used. The CFU count shows only whether or not an organism is capable of growth and consequently provides only an average value for the whole population at a given time. Flow cytometry, however, by analyzing individual cells as they pass through the interrogating beam, will reveal variations in the population with respect to a specific property according to the type of fluorescent probe being used. Use of either CTC or DiBAC4(3) reveals clearly defined subpopulations during exposure of the culture to methicillin and the subsequent resuspension in antibiotic-free medium. Two proposed mechanisms of PAE are (i) limited persistence of drug at a bacterial binding site and (ii) drug-induced nonlethal damage. β-Lactam antibiotics, e.g., methicillin, are known to bind covalently to bacterial membrane proteins; some of these enzymes are essential for cell wall synthesis. The drug-enzyme complex can break down, resulting in the regeneration of enzyme active during cell wall synthesis (21). The continued decrease in respiratory activity and membrane integrity after removal of methicillin suggests persistence at the antibiotic binding sites, although it is probable that drug-induced nonlethal damage also has an effect. Not all work on PAE has revealed an invariable population count during the period of growth suppression. A gradual increase or decrease in the numbers of cells after drug removal but before the resumption of normal growth has been demonstrated (5). This may be due to errors in dilution during the viable counting procedure. In this study, the CFU count showed hardly any variation during the PAE, but the CTC fluorescence and membrane potential of the organisms continued to decline. This underlines the fact that information unavailable from CFU counts may be presented by flow cytometry. Furthermore, CTC fluorescence measurements identified several subpopulations during the recovery period, illustrating the complex nature of the PAE and the different effects of drugs on individual organisms. These subpopulations were also apparent by using DiBAC4(3) fluorescence measurements but were not as clearly defined as those gained with CTC. However, DiBAC4(3) successfully identified organisms which had recovered from the effects of methicillin and which had resumed multiplication. Wilson and Rolinson (24) claimed that individual cells within a culture show periods of growth suppression of between 20 min and 3 h. With flow cytometry, the presence of actively respiring organisms was only observed at 4 h after drug removal.
The further reduction in respiratory activity after the CFU counts had reached zero appears to reflect a viable but nonculturable state which may be of clinical importance. A similar state was recently described by Mason et al. (15) while assessing the antibacterial activity of ciprofloxacin.
A second advantage of flow cytometry over plate counts is the shorter time required. CFU counts require at least an overnight incubation, depending on the bacterial strain and the type of medium used. This period is a major problem and may not reflect the clinical situation under investigation (23). It has been claimed that counting of viable cells may overestimate antibacterial activity due to the loss of viability during the transfer of damaged cells from liquid to solid medium (13). By flow cytometry the cells can be analyzed and the results can be collected immediately following a short incubation [2 min for DiBAC4(3) and 30 min for CTC]). This allows the rapid investigation of variables that to date have been incompletely studied, e.g., the effects of inoculum size, growth phase, medium influence, and antibiotic synergism or antagonism on the PAE (1). CFU counts assume the development of a single colony from each organism; for some bacteria, e.g., E. coli, antibacterial activity results in a filament which contains many genomes and which will give rise to only a single colony (12). However, the use of flow cytometry would allow the study of PAE for filament-forming organisms because it can identify changes in cell size and shape by the way in which the cell scatters light. This is clearly shown in Fig. 2a, panel iii, in which the actively respiring population scattered light to a higher degree than the dead and damaged cells.
Antimicrobial activities in vivo are influenced by the time of exposure, the dosage regimen, and the host’s immune system (16). Bacteria showing a PAE phase have increased susceptibility to leukocyte activity (postantibiotic leukocyte enhancement). As a result the bactericidal process may continue for many hours after the concentration of the drug has fallen below the MIC. For example, the potentially toxic aminoglycosides produce a long PAE, and this may allow less frequent administration. With the increasing threat of multidrug-resistant bacteria, the efficient use of current antibiotics is extremely important. Flow cytometry therefore provides an alternative to standard methods for testing of the PAE and other antibacterial effects. It is rapid, sensitive, and capable of revealing important information regarding the heterogeneity of the population. Theoretically, antibiotic-induced bacterial damage which results in a depolarized membrane and decreased respiratory activity can be detected with CTC and DiBAC4(3), respectively, although this procedure needs to be tested with a range of different bacterial species. The availability of a wide variety of fluorescent probes allows the analysis of a large number of different characteristics which will facilitate the better understanding of the PAE phenomenon.
ACKNOWLEDGMENT
This work was funded by the Welsh Scheme for the Development of Health and Social Research (Welsh Office).
REFERENCES
- 1.Baquero, F., E. Culebras, C. Patron, J. C. Perez-Dias, J. C. Medrano, and M. F. Vicente. 1986. Postantibiotic effect of imipenem on gram-positive and gram-negative microorganisms. J. Antimicrob. Chemother. 18(Suppl. E):47–59. [DOI] [PubMed]
- 2.Bigger J W. The bactericidal action of penicillin on Staphylococcus aureus. Ir J Med Sci. 1944;227:533–568. [Google Scholar]
- 3.Bundtzen R W, Gerber A U, Cohn D, Craig W A. Postantibiotic suppression of bacterial growth. J Bacteriol. 1981;58:475–490. doi: 10.1093/clinids/3.1.28. [DOI] [PubMed] [Google Scholar]
- 4.Craig W A, Vogelman B S. The postantibiotic effect. Ann Intern Med. 1987;106:900–902. doi: 10.7326/0003-4819-106-6-900. [DOI] [PubMed] [Google Scholar]
- 5.Craig W A, Gudmundsson S. Postantibiotic effect. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 296–329. [Google Scholar]
- 6.Eagle H, Musselman A D. The slow recovery of bacteria from the toxic effects of penicillin. J Bacteriol. 1949;58:475–490. doi: 10.1128/jb.58.4.475-490.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epps D E, Wolfe M L, Groppi V. Characterization of the steady-state and dynamic fluorescent properties of the potential-sensitive dye bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4(3)) in model systems and cells. Chem Phys Lipids. 1994;69:137–150. doi: 10.1016/0009-3084(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 8.Gerber, A. U., and W. A. Craig. 1981. Growth kinetics of respiratory pathogens after short exposure to ampicillin and erythromycin in vitro. J. Antimicrob. Chemother. 8(Suppl. C):81–91. [DOI] [PubMed]
- 9.Kaprelyants A S, Kell D B. The use of 5-cyano-2,3-ditodyl tetrazolium chloride and flow cytometry for the visualisation of respiratory activity in individual cells of Micrococcus luteus. J Microbiol Methods. 1993;17:115–122. [Google Scholar]
- 10.Lloyd D. Flow cytometry: a technique waiting for microbiologists. In: Lloyd D, editor. Flow cytometry in microbiology. London, United Kingdom: Springer-Verlag; 1993. pp. 1–9. [Google Scholar]
- 11.López-Amorós R, Mason D J, Lloyd D. Use of two oxonols and a fluorescent tetrazolium dye to monitor starvation of Escherichia coli in sea water. J Microbial Methods. 1995;22:165–176. [Google Scholar]
- 12.Lorian V, Ernst J, Amaral L. The post-antibiotic effect defined by bacterial morphology. J Antimicrob Chemother. 1989;23:485–491. doi: 10.1093/jac/23.4.485. [DOI] [PubMed] [Google Scholar]
- 13.Mackenzie F M, Gould I M. Postantibiotic effect: a review. J Antimicrob Chemother. 1993;32:519–537. doi: 10.1093/jac/32.4.519. [DOI] [PubMed] [Google Scholar]
- 14.Mason D J, Allman R, Stark J M, Lloyd D. Rapid estimation of bacterial susceptibility with flow cytometry. J Microsc. 1994;176:8–16. doi: 10.1111/j.1365-2818.1994.tb03494.x. [DOI] [PubMed] [Google Scholar]
- 15.Mason D J, Power E G M, Talsania T, Phillips I, Gant V A. Antibacterial action of ciprofloxacin. Antimicrob Agents Chemother. 1995;39:2752–2758. doi: 10.1128/aac.39.12.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald P J, Craig W A, Kunin C M. Persistent effects of antibiotics on Staphylococcus aureus after exposure for limited periods of time. J Infect Dis. 1977;135:217–227. doi: 10.1093/infdis/135.2.217. [DOI] [PubMed] [Google Scholar]
- 17.Miles A A, Misra S S, Irwin J O. The estimation of the bactericidal properties of the blood. J Hyg. 1938;38:732–749. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez G G, Ishiguro D P, Ridgway H F. Use of a fluorescent redox probe for direct visualisation of actively respiring bacteria. Appl Environ Microbiol. 1992;58:1801–1808. doi: 10.1128/aem.58.6.1801-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro H M. Practical flow cytometry. 2nd ed. New York, N.Y: Alan R. Liss, Inc.; 1988. [Google Scholar]
- 20.Suller M T E, Stark J M, Lloyd D. A flow cytometric study of antibiotic-induced damage and evaluation as a rapid antibiotic susceptibility test for methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1997;41:77–83. doi: 10.1093/jac/40.1.77. [DOI] [PubMed] [Google Scholar]
- 21.Tomasz A. From penicillin binding proteins to the lysis and death of bacteria: a 1979 review. Rev Inect Dis. 1979;1:134–167. doi: 10.1093/clinids/1.3.434. [DOI] [PubMed] [Google Scholar]
- 22.Vogelman, B. S. and W. A. Craig. 1985. Postantibiotic effects. J. Antimicrob. Chemother. 15(Suppl. A):37–46. [DOI] [PubMed]
- 23.Webster, C., K. Ghazanfar, and R. Slack. 1988. Sub-inhibitory and post-antibiotic effects of spiramycin and erythromycin on Staphylococcus aureus. J. Antimicrob. Chemother. 22(Suppl. B):33–39. [DOI] [PubMed]
- 24.Wilson D A, Rolinson G N. The recovery period following exposure of bacteria to penicillins. Chemotherapy (Basel) 1979;25:14–22. doi: 10.1159/000237817. [DOI] [PubMed] [Google Scholar]
- 25.Zhanel G G, Hoban D J, Harding K M. The post-antibiotic effect: a review of in vitro and in vivo data. Ann Pharmacother. 1991;25:153–162. doi: 10.1177/106002809102500210. [DOI] [PubMed] [Google Scholar]