Abstract
Introduction
The aim was to investigate the risk, prevalence, and clinical characteristics of cerebral palsy among children born after assisted reproductive technology (ART) in Norway.
Material and methods
All liveborn children from 2002 to 2015 were included. Information was collected from the Medical Birth Registry of Norway, linked to the Norwegian Quality and Surveillance Registry for Cerebral Palsy as of December 31, 2022. Logistic regression analyses were used to calculate the prevalence of cerebral palsy per 1000 live births after ART and natural conception with birth year as covariate, crude odds ratios (OR) for cerebral palsy among children born after ART using children born after natural conception as reference, and OR adjusted for potential confounders, with 95% confidence intervals (CI). Potential mediators of the association were studied in stratified analyses. Descriptive statistics were used to compare proportions in clinical characteristics among children with cerebral palsy born after ART and natural conception.
Results
Among 833 645 livebirths, 23 645 children were born after ART and of the latter 97 were diagnosed with cerebral palsy. The overall prevalence of cerebral palsy after ART was 4.10 per 1000 live births (95% CI 3.36–5.00), decreasing from 7.79 per 1000 in 2002 to 3.55 in 2015. Compared with children born after natural conception, the OR for cerebral palsy was 2.01 (95% CI 1.63–2.47) adjusted for mother's age at birth, parity, and pre‐pregnancy health. When restricted to singletons born at term, the adjusted OR for cerebral palsy was 1.13 (95% CI 0.76–1.69). The distribution of cerebral palsy subtypes and the severity of gross and fine motor function and associated impairments did not differ significantly between children with cerebral palsy born after ART and natural conception.
Conclusions
Children born after ART had a risk of cerebral palsy that was twice that of children born after natural conception. The increased risk of cerebral palsy after ART is likely attributed to multiple pregnancies and preterm births. The prevalence of cerebral palsy after ART decreased significantly during the study period, despite an increased use of ART in the population. The distribution of clinical characteristics did not differ between children with cerebral palsy born after ART and those born after a natural conception, suggesting that the risk factors for, and causes of cerebral palsy were similar.
Keywords: assisted reproductive technology, cerebral palsy, clinical characteristics, multiple pregnancy, preterm births
There is still an increased risk of cerebral palsy associated with assisted reproductive technology (ART), mainly attributable to multiple pregnancies and children born preterm. Despite an increase in children born after ART, the prevalence of cerebral palsy after ART has decreased. The distribution of clinical characteristics may suggest that risk factors for, and causes of cerebral palsy are similar in children born after ART and natural conception.
Abbreviations
- ART
assisted reproductive technology
- CI
confidence intervals
- CP
cerebral palsy
- ICSI
intracytoplasmic sperm injection
- IVF
in vitro fertilization
- MBRN
Medical Birth Registry of Norway
- NorCP
Norwegian Quality and Surveillance Registry for Cerebral Palsy
Key message.
There is still an increased risk of cerebral palsy associated with assisted reproductive technology (ART), mainly attributable to multiple cpregnancies and children born preterm. Despite an increase in children born after ART, the prevalence of cerebral palsy after ART has decreased. The distribution of clinical characteristics suggests that risk factors for, and causes of cerebral palsy are similar in children born after ART and natural conception.
1. INTRODUCTION
Cerebral palsy (CP) is a group of disorders of motor development that lead to activity limitation. 1 The cause is a non‐progressive injury in the developing brain. 1 Based on the dominant neurological symptoms, CP is classified into spastic (unilateral or bilateral), dyskinetic, or ataxic CP subtypes. 2 The motor disturbances are often accompanied by associated impairments. 1 Known risk factors for CP are multiple pregnancies, preterm births, and intrauterine growth restriction. 3 Some risk factors are associated with a specific CP subtype, such as preterm birth being associated with spastic bilateral CP. 4 The prevalence of CP in high‐income countries (including Norway) in children born between 2010 and 2014 is 1.6 per 1000 live births, 5 significantly lower than 2.1 per 1000 in children born in the 1980s to 1990s. 6 The decline is attributed to improvements in antenatal, obstetric, and neonatal care. 5
Assisted reproductive technologies (ART) include in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) and subsequent embryo transfer. 7 Several studies have reported increased use of ART over the past three decades in Europe and the USA. 8 , 9 , 10 , 11 In Norway, approximately 5% of newborns are born after ART, with an increasing trend from around 1100 children in 2000 to 3100 in 2019. 12 The most common method of fertilization was IVF, whereas around 40% of children were conceived by ICSI. 10 Previously, ART has been associated with an increased risk of complications due to multiple pregnancies after the transfer of more than one embryo by IVF and ICSI. 13 , 14 , 15 However, from the beginning of the 2000s there have been advancements in ART treatment, including single embryo transfer, as well as improved embryo freezing and storage technology. This has led to a decrease in multiple pregnancies, and as a result, improvements in perinatal outcomes. 10 , 13 , 14 , 16 , 17 , 18 , 19 Nonetheless, singletons born after ART have an increased risk of adverse perinatal outcomes compared with those born after natural conception. 20
Several studies on children born in the 1980s to mid‐2000s have shown a moderately increased risk of CP after ART, primarily attributed to multiple pregnancies and preterm births. 21 , 22 , 23 , 24 , 25 Yet, some of the studies reported no increased risk of CP after ART in children born as a singleton at term. 22 , 24 , 25 A recent study reported a significant decrease in the risk of CP in children born 1990–2014 after ART in Sweden, Denmark, and Finland. 26 This was attributed to a reduction in multiple pregnancies and preterm births. The risk of CP after ART in children born in Norway has not been previously reported.
Additionally, although previous studies have suggested that the higher risk of CP associated with ART was mainly attributed to preterm births and multiples, it has also been discussed whether ART, the causes of subfertility, or both, may involve a higher risk for CP. 19 If this were the case, one might hypothesize that children with CP born after ART have dissimilar distributions of clinical characteristics compared with children with CP born after natural conception. Two previous studies in Western Australia and Denmark did not find evidence for such differences. 24 , 27
On this background, the main aim of this study was to explore the risk and prevalence of CP among Norwegian children born after ART since 2002. A secondary aim was to explore if the distribution of clinical characteristics differed between children with CP born after ART and natural conception.
2. MATERIAL AND METHODS
2.1. Study design and population
This study includes all Norwegian liveborn children between January 1, 2002 and December 31, 2015 who survived the first year of life. Information on ART, pregnancies, and subsequent births was collected from the Medical Birth Registry of Norway (MBRN), a compulsory national health registry established in 1967. 28 Information on ART conception was collected from the ART registration form introduced in 2002. Information from the MBRN was linked to the Norwegian Quality and Surveillance Registry for Cerebral Palsy (NorCP) using the unique personal identification number of each Norwegian citizen. The NorCP is a consent‐based national medical quality registry established in 2001, and records medical information on children with CP at the time of diagnosis, at age 5 years, and at age 15–17 years. 29 Information in this study was collected as of December 31, 2022 from the NorCP registration form at age 5 years, when the CP diagnosis was confirmed. 2 The NorCP includes 93% of children with CP born in Norway from 2002 to 2015. 29 , 30 This was ascertained through validation studies on all CP diagnosis codes in the specialized healthcare services. 29 , 30
2.2. Study variables
The main exposure was ART. 7 The ART methods were categorized as IVF, ICSI, both IVF and ICSI (in the few cases where the fertility specialists are initially unsure if they are going to use IVF or ICSI, the eggs are divided and half are fertilized using IVF and the other half using ICSI), and unknown. Main infertility causes were categorized by the MBRN as fallopian tube factor, endometriosis, ovarian factor, paternal factor, and other (>1 main infertility cause and unknown). Information on the mother's health before pregnancy (asthma, chronic hypertension and kidney disease, rheumatoid arthritis, epilepsy, and diabetes) and during pregnancy (preeclampsia, placental detachment) were categorized as yes/no. The mother's parity was categorized as nulliparous (no previous birth) and parous (≥1 previous births). Plurality was categorized as singletons and multiples (twins and above) and mother's age at time of birth as <25, 25–39, and ≥40 years. Information on the birth included fetal position categorized as normal and other (breech, transverse, deviating, and other), and gestational age as preterm (<37 weeks) and term (≥37 weeks). Among children born after ART, gestational age was determined using the embryo transfer date, and for children born after natural conception, it was determined from an ultrasound around week 18. If information from the ultrasound was missing, gestational age was determined from the first day of the last menstruation. Birthweight was categorized as <1000, 1000–1499, 1500–2499, and >2499 g, and Apgar score at 5‐minutes as 0–3 (low), 4–6 (moderate), and 7–10 (normal).
The primary outcome, CP, was defined according to Rosenbaum et al. 1 CP subtypes were classified according to the Surveillance of Cerebral Palsy in Europe guidelines as spastic unilateral, spastic bilateral, dyskinetic, ataxic, and mixed/unspecified. 2 Gross motor function was categorized using the Gross Motor Function Classification System 31 and fine motor function using the Manual Ability Classification System five‐level scales. 32 Epilepsy was defined as yes (a minimum of two unprovoked seizures after the neonatal period)/no and severe feeding difficulties as use of gastrostomy tube feeding yes/no. Severe hearing impairments was defined as yes (loss >70 dB before correction on the best ear)/no and visual impairments as yes (blind, i.e., <6/60 (<0.1) before correction on the best eye)/no. Speech ability was categorized using the Viking Speech Scale, 33 and cognitive function as normal, learning disorder (International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD‐10) F80‐81, F83), intellectual impairment (ICD‐10 F70‐73, F79 or clinical evaluation) or not testable.
2.3. Statistical analyses
Descriptive statistics were used to describe maternal and perinatal characteristics by conception type and plurality using numbers and proportions.
Logistic regression with birth year as covariate was used to estimate the prevalence of CP per 1000 live births after ART and natural conception, and for singletons and multiples separately. Non‐linear trends were accounted for using fractional polynomials with birth year as covariate.
Descriptive statistics were used to compare the number of children with and without CP born after ART and natural conception, as well as per term/preterm and combinations of term/preterm singletons and multiples separately. The numbers were used to calculate the prevalence of CP per 1000 live births with 95% confidence intervals (CI) after ART and natural conception, and per term/preterm and combinations of term/preterm singletons and multiples. Logistic regression analyses were used to calculate crude odds ratios (OR) for CP with 95% CI among children born after ART compared with children born after natural conception, and OR adjusted for potential confounders (Figure S1). Potential mediators of the association were studied in stratified analyses. Adjusting for possible mediators, including multiple pregnancies and preterm birth are in general not recommended. 34
Descriptive statistics were used to compare the total number of children born after ART with children with CP after ART, by ART method and main infertility cause. The numbers were used to calculate the prevalence of CP per 1000 live births with 95% CI per ART method and main infertility cause.
Descriptive statistics were used to compare proportions in clinical characteristics among children with CP born after ART and natural conception. Pearson chi‐squared was used for nominal categorical and dichotomous variables, linear‐by‐linear association for ordinal continuous variables and Fisher's exact test where appropriate. Values of p below 0.05 were regarded as statistically significant.
Statistical analyses were performed using SPSS (IBM Corp. Released 2021; IBM SPSS Version 28.0) and Stata (StataCorp. 2021; Stata Statistical Software: Release 17).
2.4. Ethics statement
NorCP is governed by Regulations on Medical Quality Registers (FOR‐2019‐06‐21‐789). The Central Regional Ethics Committee for Medical and Healthcare Research approved this study (2011/754), including the linkage between the MBRN and NorCP.
3. RESULTS
Among 833 645 children born alive in Norway from 2002 to 2015, 23 645 were born after ART. Among the latter, 17 941 (75.9%) were singletons and 5704 (24.1%) were multiples. Figure 1 shows that the proportion of children born after ART increased from 2002 to 2015. Figure 1 also shows that the proportion of children born after ART as a singleton increased while the proportion of children born after ART as a multiple decreased. Among 810 000 children born after natural conception, 786 876 (97.1%) were singletons and 23 124 (2.9%) were multiples.
FIGURE 1.
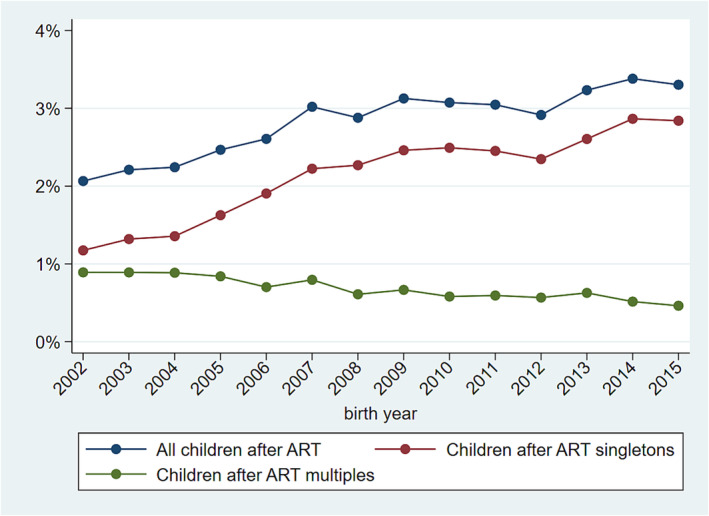
Trends in the proportion of children born after assisted reproductive technology (ART) in Norway.
3.1. Maternal and perinatal characteristics
Mothers treated with ART were in good general health before pregnancy (Table S1). Yet, they were somewhat older, and more often had preeclampsia and a multiple pregnancy than mothers who did not receive ART (Table S1). Children born after ART were more often born preterm (18.0%) and with a birthweight ≤2499 g (15.3%) than those born after natural conception (6.3% and 4.5%, respectively) (Table S1).
3.2. Risk and prevalence of CP among children born after ART and natural conception
Among the 23 645 children born after ART, 97 children were diagnosed with CP, and of the latter 44 (45.4%) were singletons and 53 (54.6%) were multiples. The overall prevalence of CP after ART was 4.10 per 1000 live births (95% CI 3.36–5.00). Figure 2A shows a decrease in the prevalence of CP among children born after ART from 7.79 per 1000 in 2002 to 3.55 per 1000 in 2015 (p < 0.001). The overall prevalence of children with CP born after ART as a multiple was 2.24 per 1000 live births (95% CI 1.71–2.93), decreasing significantly from 5.19 per 1000 in 2002 to 1.52 per 1000 in 2015 (p < 0.001), whereas the prevalence of children with CP after ART as a singleton remained stable around 1.9 per 1000 live births (95% CI 1.4–2.5; p = 0.477). Table 1 shows the prevalence of children with CP per 1000 live births per conception type stratified by gestational age and plurality.
FIGURE 2.
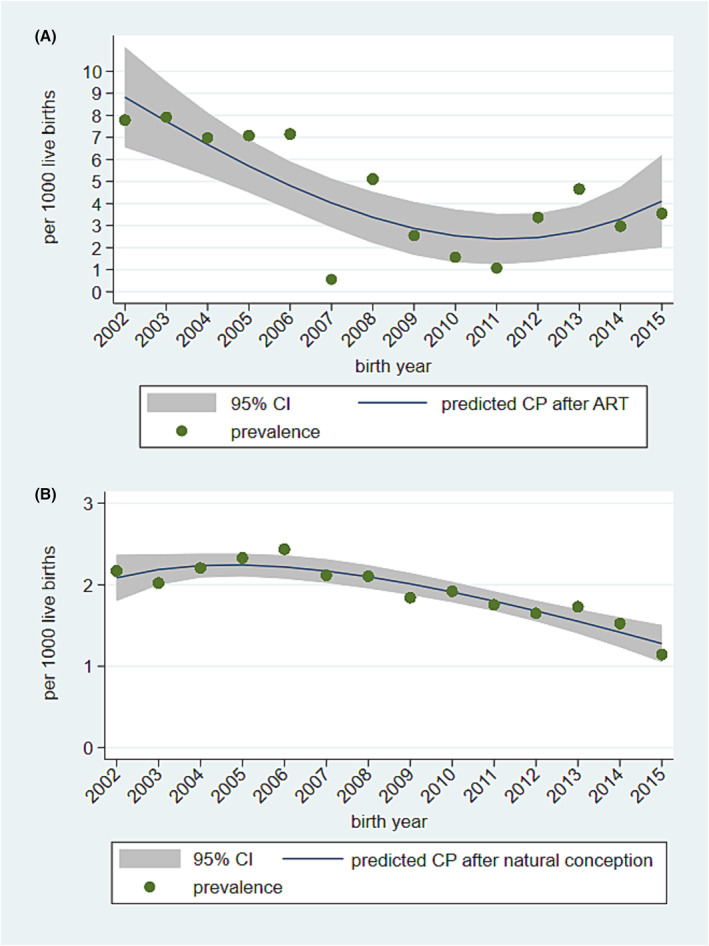
Trends in prevalence of children with cerebral palsy (CP) born after (A) assisted reproductive technology (ART) and (B) natural conception in Norway. Each point is the actual prevalence. The solid line represents predicted CP prevalence using logistic regression with fractional polynomials, and the shaded area denotes 95% confidence interval (CI).
TABLE 1.
Prevalence per 1000 livebirths and odds for cerebral palsy among children born in Norway from 2002 to 2015.
| Conception type | CP | Without CP | CP per 1000 LB | Crude OR | Adjusted OR* | |
|---|---|---|---|---|---|---|
| N | N | (95% CI) | (95% CI) | (95% CI) | ||
| Total population | ART | 97 | 23 548 | 4.10 (3.36–5.00) | 2.14 (1.74–2.63) | 2.01 (1.63–2.47) |
| Natural | 1556 | 808 444 | 1.92 (1.83–2.02) | |||
| Term born | ART | 31 | 19 354 | 1.60 (1.13–2.27) | 1.27 (0.89–1.81) | 1.20 (0.84–1.72) |
| Natural | 954 | 754 268 | 1.26 (1.19–1.35) | |||
| Term‐born singletons | ART | 25 | 16 479 | 1.52 (1.03–2.24) | 1.20 (0.84–1.72) | 1.13 (0.76–1.69) |
| Natural | 937 | 742 470 | 1.26 (1.18–1.35) | |||
| Term‐born multiples | ART | 6 | 2875 | 2.09 (0.96–4.55) | 1.45 (0.57–3.68) | 1.49 (0.57–3.91) |
| Natural | 17 | 11 798 | 1.44 (0.90–2.31) | |||
| Preterm‐born | ART | 66 | 4194 | 15.74 (12.39–19.97) | 1.33 (1.03–1.72) | 1.32 (1.02–1.71) |
| Natural | 591 | 49 995 | 11.82 (10.91–12.81) | |||
| Preterm‐born singletons | ART | 19 | 1418 | 13.40 (8.59–20.83) | 1.17 (0.74–1.85) | 1.15 (0.72–1.83) |
| Natural | 447 | 38 947 | 11.48 (10.47–12.58) | |||
| Preterm‐born multiples | ART | 47 | 2776 | 16.93 (12.76–22.44) | 1.30 (0.93–1.81) | 1.29 (0.92–1.81) |
| Natural | 144 | 11 048 | 13.03 (11.08–15.32) |
Abbreviations: ART, assisted reproductive technology; CI, confidence interval; CP, cerebral palsy, LB, live birth; OR, odds ratio.
Parity, mother's health before pregnancy (one or more of following conditions: asthma, chronic hypertension, chronic kidney disease, rheumatoid arthritis, epilepsy, diabetes mellitus) and mother's age at birth. Gestational age missing in 0.5% of children born by natural conception.
Among the 810 000 children born after natural conception, 1556 were diagnosed with CP, and of the latter 1395 (89.7%) were singletons and 161 (10.3%) were multiples. The overall prevalence of CP after natural conception was 1.92 per 1000 live births (95% CI 1.83–2.02). Figure 2B shows a decrease in the prevalence of CP after natural conception from 2.17 per 1000 in 2002 to 1.14 per 1000 in 2015 (p < 0.001).
The risk for CP among children born after ART was twice that in children born after natural conception (Table 1). When adjusted for mother's age at birth, pre‐pregnancy health, and parity in a multivariable analysis, the results were essentially unchanged (Table 1). Among singletons born at term or preterm, the odds for CP were marginally higher with confidence intervals on both sides of 1.0. After restriction to multiples, children born at term after ART had nearly 50% higher odds of CP compared with children born at term after natural conception (Table 1). Moreover, both overall and after restriction to multiples, children born preterm after ART had an approximately 30% higher odds of CP compared with children born preterm after natural conception (Table 1). Yet, among multiples born at term or preterm, confidence intervals were on both sides of 1.0.
There was no definitive difference in the prevalence of CP between children born after IVF vs ICSI, nor between ART‐conceived children with different parental causes of infertility (Table 2). Table 2 shows that ovarian factors were the main cause of infertility among mothers of children with CP born after ART compared with all ART‐conceived children, followed by endometriosis. Furthermore, the prevalence of maternal infertility causes was higher than paternal causes (Table 2).
TABLE 2.
Prevalence per 1000 livebirths for cerebral palsy among children born in Norway from 2002 to 2015, after various methods of assisted reproductive technology and main causes of infertility.
| CP after ART N | All after ART N | CP per 1000 LB (95% CI) | |
|---|---|---|---|
| ART methods | |||
| IVF | 62 | 13 086 | 4.74 (3.70–6.07) |
| ICSI | 31 | 10 190 | 3.04 (2.14–4.31) |
| IVF and ICSI | 2 | 152 | 13.16 (3.62–46.70) |
| Unknown | 2 | 217 | 9.22 (2.53–32.98) |
| Main infertility causes | |||
| Maternal | 48 | 8382 | 5.73 (4.32–7.58) |
| Fallopian tube factor | 17 | 3373 | 5.04 (3.15–8.06) |
| Endometriosis | 12 | 2093 | 5.73 (3.28–10.00) |
| Ovarian factor | 19 | 2916 | 6.52 (4.18–10.15) |
| Paternal | 29 | 9072 | 3.20 (2.23–4.59) |
| Other | 20 | 6191 | 3.23 (2.09–4.98) |
Abbreviations: ART, assisted reproductive technology; CP, cerebral palsy; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; LB, live birth.
3.3. Clinical characteristics among children with CP born after ART
We found no differences in the distribution of CP subtypes between children with CP born after ART and natural conception (Table 3). Among children with spastic CP, however, the proportion with bilateral CP born after ART (51 of 83; 61%) was higher than those born after natural conception (676 of 1372 children; 49%). Other clinical characteristics including gross and fine motor function and associated impairments (i.e., epilepsy, gastrostomy tube feeding, severe visual and hearing impairments, speech difficulties, and cognitive impairments) did not differ between children with CP born after ART and natural conception (Table 3).
TABLE 3.
Distribution of cerebral palsy subtypes, motor function levels and associated impairments among children born in Norway from 2002 to 2015 after assisted reproductive technology and natural conception.
| CP after ART N (%) | CP after natural conception N (%) | ||
|---|---|---|---|
| 97 (100) | 1556 (100) | p value | |
| CP subtype | 0.202 | ||
| Spastic unilateral | 32 (33.0) | 696 (44.7) | |
| Spastic bilateral | 51 (52.6) | 676 (43.4) | |
| Dyskinetic | 6 (6.2) | 100 (6.4) | |
| Ataxic | 6 (6.2) | 61 (3.9) | |
| Mixed/unspecified | 2 (2.1) | 23 (1.5) | |
| GMFCS | 0.480 | ||
| I | 45 (49.5) | 846 (55.4) | |
| II | 16 (17.6) | 238 (15.6) | |
| III | 8 (8.8) | 103 (6.7) | |
| IV | 10 (11.0) | 133 (8.7) | |
| V | 12 (13.2) | 208 (13.6) | |
| Total | 91 (100) | 1528 (100) | |
| Missing* | 6 (6.2) | 28 (1.8) | |
| MACS | 0.852 | ||
| I | 41 (45.6) | 616 (42.3) | |
| II | 16 (17.8) | 411 (28.2) | |
| III | 19 (21.1) | 160 (11.0) | |
| IV | 2 (2.2) | 97 (6.7) | |
| V | 12 (13.3) | 171 (11.8) | |
| Total | 90 (100) | 1455 (100) | |
| Missing* | 7 (7.2) | 101 (6.5) | |
| Epilepsy | 0.368 | ||
| Yes | 23 (25.3) | 429 (29.7) | |
| No | 68 (74.7) | 1015 (70.3) | |
| Total | 91 (100) | 1444 (100) | |
| Missing* | 6 (6.2) | 112 (7.2) | |
| Gastrostomy | 0.472 | ||
| Yes | 14 (15.7) | 187 (13.1) | |
| No | 75 (84.3) | 1244 (86.9) | |
| Total | 89 (100) | 1431 (100) | |
| Missing* | 8 (8.3) | 125 (8.0) | |
| Severe visual impairment | 0.452 | ||
| Yes | 4 (4.9) | 84 (6.0) | |
| No | 78 (95.1) | 1311 (94.0) | |
| Total | 82 (100) | 1395 (100) | |
| Missing* | 15 (15.5) | 161 (10.4) | |
| Severe hearing impairment | 0.302 | ||
| Yes | 3 (3.5) | 31 (2.2) | |
| No | 82 (96.5) | 1385 (97.8) | |
| Total | 85 (100) | 1416 (100) | |
| Missing* | 12 (12.4) | 140 (9.0) | |
| Viking speech scale | 0.532 | ||
| I | 43 (47.8) | 772 (54.2) | |
| II | 18 (20.0) | 227 (15.9) | |
| III | 14 (15.6) | 175 (12.3) | |
| IV | 15 (16.7) | 250 (17.6) | |
| Total | 90 (100) | 1424 (100) | |
| Missing* | 7 (7.2) | 132 (8.5) | |
| Cognitive function | 0.073 | ||
| Normal | 52 (61.2) | 687 (56.1) | |
| Learning disorder | 12 (14.1) | 176 (14.4) | |
| Intellectual impairment | 11 (12.9) | 281 (22.9) | |
| Not testable | 10 (11.8) | 81 (6.6) | |
| Total | 85 (100) | 1225 (100) | |
| Unable to determine from test | 0 (0) | 65 (4.2) | |
| Missing* | 12 (12.4) | 266 (17.1) |
Abbreviation: ART, assisted reproductive technology; CP, cerebral palsy; GMFCS, Gross Motor Function Classification System; MACS, Manual Ability Classification System.
Percent of total number.
4. DISCUSSION
The prevalence of CP in children born after ART was approximately twice as high as after natural conception in Norwegian liveborn children 2002 to 2015. Yet, the absolute risk (4.1 per 1000 live births) was low. The increased risk was most likely attributed to the significantly higher proportion of multiple pregnancies and children born preterm. Among children born at term as singletons we found no increased risk for CP associated with ART. Despite a significant increase in the use of ART during the study period, the prevalence of CP after ART decreased. We found no differences in the distributions of CP subtypes, motor function and associated impairments between children with CP born after ART and natural conception.
A strength of this study is that information on ART, pregnancies, births, and CP were recorded prospectively and independently in the MBRN and NorCP. As of January 1, 2002, the Medical Birth Registry Regulation came into force, making notification of all pregnancies after week 12 mandatory, 28 increasing the completeness and quality of data reported to the MBRN. This included the requirement to report information on ART treatments that result in a pregnancy on the ART registration form introduced in 2002. 28 The information on CP collected from the NorCP has a documented high degree of completeness (93%) and is based‐on internationally recognized criteria and classification systems. 29 , 30 The remaining 7% of Norwegian children with CP not recorded in the NorCP was evenly distributed throughout the study period. 30
Our results suggested 100% higher odds for CP in children born after ART compared with natural conception, and the confidence intervals were well above 1.0. In addition, the ORs were similar, indicating that the available confounders had no strong impact on the associations. However, we cannot rule out that there may be other unidentified confounders that we did not investigate. We did not adjust for colliders (multiple pregnancies and gestational age), as this can introduce selection bias. Data on children who died or emigrated after the first year of life, but before receiving a CP diagnosis, are included in the population without CP. However, because of both improvements in perinatal outcomes, including stillbirths and infant mortality, after ART in Nordic populations and the low number of children who have emigrated from Norway, we believe that this has not influenced our results. 16 , 35
A limitation of this study is the lack of detailed information, an inherent feature of register‐based data. In particular this is a limitation regarding infertility causes and ART methods. Moreover, the confidence intervals regarding infertility causes and ART methods were wide and overlapping, and these results should be interpreted with caution.
We found that the prevalence of CP among Norwegian children born after ART from 2002 to 2015 was 4.1 per 1000 live births, double the prevalence of children born after natural conception (1.9 per 1000). Similar findings were published in a Nordic study (Sweden, Denmark, and Finland), reporting a prevalence of CP of 4.4 per 1000 children born after ART from 2003 to 2014. 26 The authors reported a marked decrease in the prevalence of CP associated with ART from 6.0 per 1000 in 2003–2006 to 3.4 in 2011–2014, compared with 7.8 per 1000 in 2002 to 3.6 in 2015 in our study. In an Australian study, the prevalence of CP among children born after ART was 7.2 per 1000 live births from 1994 to 2002 compared with 2.5 per 1000 after natural conception. 24 In our study, we also found an increased use of ART in Norway during 2002–2015, which is in line with several studies. 8 , 9 , 10 , 11 , 15 , 16 , 17 , 18 Also in line with previous studies, we found that singleton children born at term after ART had no increased risk of CP. 24 , 25 , 26 , 27
As shown in Table 2, there was no difference in the prevalence of CP among children born after IVF and ICSI. This finding is in line with two Danish studies. 22 , 36 Several studies have analyzed the risk of CP among children born after IVF and reported an increased risk of CP after IVF varying from 1.8 to 2.0, which fits well with our findings. 21 , 23
We did not find significant differences in CP subtypes, motor function, or associated impairments between children with CP born after ART and natural conception, consistent with two studies in Western Australia 24 and Denmark. 27
A likely explanation for the increased risk of CP among children born after ART was the large proportion of preterm births, in particular children born preterm as multiples. In support of this, we found no increased risk of CP after ART among singleton children born at term. This is also reflected in several studies where single embryo transfer in IVF became consensus in lieu of double embryo transfer, as well as better freezing techniques, which have resulted in a significant decrease in multiple and preterm births. 8 , 9 , 10 , 11 , 15 , 16 , 17 , 18 The reduction of multiples as a result of improved ART techniques, 8 , 9 , 10 , 11 , 15 , 16 , 17 , 18 as well as improvements in perinatal medicine in general, 37 most likely explain the decrease in the prevalence of CP among children born after ART, despite an increasing use of ART.
In the decision to recommend ART, one may take into account the higher risk for CP associated with ART. However, the absolute risk is low. When parents are concerned about the prognosis of their newborn baby, parents of a singleton can be reassured that the risk of CP is similar to that of other newborns. If the child after ART is born preterm, there is a marginally higher risk for CP compared with children born preterm after natural conception. However, in these cases the prognosis will likely depend more on the health status of the newborn and/or complications to treatment than on the use of ART.
The finding that the distribution of CP subtypes did not differ significantly between children born after ART and natural conception suggests that the already known general causes that lead to CP, also are the main causes of CP in children born after ART. The trend towards more children with the spastic bilateral CP subtype in the latter group is reasonable because preterm birth is associated with this CP subtype.
5. CONCLUSION
Despite the increased relative risk of CP among Norwegian children born after ART, the absolute risk was low. If a child of a mother who was treated with ART was born a singleton at term, there was no increased risk for CP. In addition, although there was a significant increase in the proportion of children born after ART, the prevalence of CP after ART decreased significantly. Thereby, the proportion of children born after ART without CP has increased. Moreover, the comparison of clinical characteristics suggests that the risk factors for, and causes of CP after ART and natural conception were similar.
AUTHOR CONTRIBUTIONS
HC, TV, GLA, KS and SJH conceived and designed the project. SJH and KS acquired the data. HC, TV, GLA and SJH analyzed the data, and all authors interpreted the data. HC, GLA, TV and SJH wrote the manuscript, and all authors revised and approved the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Supporting information
Figure S1: Directed acyclic graph describing potential causal relationship between ART and CP.
Table S1: Maternal and perinatal characteristics.
ACKNOWLEDGMENTS
We thank all children with CP and their families who agreed to participate in the Norwegian Quality and Surveillance Registry for Cerebral Palsy (NorCP), as well as all coordinators who collected signed consent forms and clinical data.
Carlsen H, Vik T, Andersen GL, et al. Cerebral palsy in children born after assisted reproductive technology in Norway: Risk, prevalence, and clinical characteristics. Acta Obstet Gynecol Scand. 2023;102:1450‐1458. doi: 10.1111/aogs.14663
REFERENCES
- 1. Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8‐14. [PubMed] [Google Scholar]
- 2. Cans C. Surveillance of cerebral palsy in Europe (SCPE): a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42:816‐824. [DOI] [PubMed] [Google Scholar]
- 3. MacLennan AH, Thompson SC, Gecz J. Cerebral palsy: causes, pathways, and the role of genetic variants. Am J Obstet Gynecol. 2015;213:779‐788. [DOI] [PubMed] [Google Scholar]
- 4. Himpens E, Van den Broeck C, Oostra A, Calders P, Vanhaesebrouck P. Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: a meta‐analytic review. Dev Med Child Neurol. 2008;50:334‐340. [DOI] [PubMed] [Google Scholar]
- 5. McIntyre S, Goldsmith S, Webb A, et al. Global prevalence of cerebral palsy: a systematic analysis. Dev Med Child Neurol. 2022;64:1494‐1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oskoui M, Coutinho F, Dykeman J, Jetté N, Pringsheim T. An update on the prevalence of cerebral palsy: a systematic review and meta‐analysis. Dev Med Child Neurol. 2013;55:509‐519. [DOI] [PubMed] [Google Scholar]
- 7. Zegers‐Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108:393‐406. [DOI] [PubMed] [Google Scholar]
- 8. Ferraretti AP, Nygren K, Andersen AN, et al. Trends over 15 years in ART in Europe: an analysis of 6 million cycles. Hum Reprod Open. 2017;2017:hox012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wyns C, Bergh C, Calhaz‐Jorge C, et al. ART in Europe, 2016: results generated from European registries by ESHRE. Hum Reprod Open. 2020;2020(3):hoaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Opdahl S, Henningsen AA, Bergh C, et al. Data resource profile: Committee of Nordic Assisted Reproductive Technology and Safety (CoNARTaS) cohort. Int J Epidemiol. 2020;49:365‐366f. [DOI] [PubMed] [Google Scholar]
- 11. Sunderam S, Kissin DM, Zhang Y, et al. Assisted reproductive technology surveillance‐United States, 2016. MMWR Surveill Summ. 2019;68:1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Norwegian directorate of health . Assisted Fertilization [Norwegian]. 2020. https://www.helsedirektoratet.no/statistikk/assistert‐befruktning#rapporter
- 13. Thurin A, Hausken J, Hillensjö T, et al. Elective single‐embryo transfer versus double‐embryo transfer in in vitro fertilization. N Engl J Med. 2004;351:2392‐2402. [DOI] [PubMed] [Google Scholar]
- 14. Bergh C, Kamath MS, Wang R, Lensen S. Strategies to reduce multiple pregnancies during medically assisted reproduction. Fertil Steril. 2020;114:673‐679. [DOI] [PubMed] [Google Scholar]
- 15. Romundstad LB. Forty years of assisted reproductive technology. Tidsskr nor Laegeforen. 2019;139(2). [DOI] [PubMed] [Google Scholar]
- 16. Henningsen AA, Gissler M, Skjaerven R, et al. Trends in perinatal health after assisted reproduction: a Nordic study from the CoNARTaS group. Hum Reprod. 2015;30:710‐716. [DOI] [PubMed] [Google Scholar]
- 17. Karlström PO, Bergh C. Reducing the number of embryos transferred in Sweden‐impact on delivery and multiple birth rates. Hum Reprod. 2007;22:2202‐2207. [DOI] [PubMed] [Google Scholar]
- 18. Tiitinen A, Hydén‐Granskog C, Gissler M. What is the most relevant standard of success in assisted reproduction?: the value of cryopreservation on cumulative pregnancy rates per single oocyte retrieval should not be forgotten. Hum Reprod. 2004;19:2439‐2441. [DOI] [PubMed] [Google Scholar]
- 19. Berntsen S, Söderström‐Anttila V, Wennerholm UB, et al. The health of children conceived by ART: ‘the chicken or the egg?’. Hum Reprod Update. 2019;25:137‐158. [DOI] [PubMed] [Google Scholar]
- 20. Qin J, Liu X, Sheng X, Wang H, Gao S. Assisted reproductive technology and the risk of pregnancy‐related complications and adverse pregnancy outcomes in singleton pregnancies: a meta‐analysis of cohort studies. Fertil Steril. 2016;105:73‐e1‐6‐85. [DOI] [PubMed] [Google Scholar]
- 21. Källén AJ, Finnström OO, Lindam AP, Nilsson EM, Nygren KG, Olausson PM. Cerebral palsy in children born after in vitro fertilization. Is the risk decreasing? Eur J Paediatr Neurol. 2010;14:526‐530. [DOI] [PubMed] [Google Scholar]
- 22. Hvidtjørn D, Grove J, Schendel DE, et al. Cerebral palsy among children born after in vitro fertilization: the role of preterm delivery–a population‐based, cohort study. Pediatrics. 2006;118:475‐482. [DOI] [PubMed] [Google Scholar]
- 23. Lidegaard O, Pinborg A, Andersen AN. Imprinting diseases and IVF: Danish national IVF cohort study. Hum Reprod. 2005;20:950‐954. [DOI] [PubMed] [Google Scholar]
- 24. Goldsmith S, McIntyre S, Badawi N, Hansen M. Cerebral palsy after assisted reproductive technology: a cohort study. Dev Med Child Neurol. 2018;60:73‐80. [DOI] [PubMed] [Google Scholar]
- 25. Reid SM, Jaques AM, Susanto C, Breheny S, Reddihough DS, Halliday J. Cerebral palsy and assisted reproductive technologies: a case‐control study. Dev Med Child Neurol. 2010;52:e161‐e166. [DOI] [PubMed] [Google Scholar]
- 26. Spangmose AL, Christensen LH, Henningsen A‐KA, et al. Cerebral palsy in ART children has declined substantially over time: a Nordic study from the CoNARTaS group. Hum Reprod. 2021;36:2358‐2370. [DOI] [PubMed] [Google Scholar]
- 27. Hvidtjørn D, Grove J, Schendel D, et al. Multiplicity and early gestational age contribute to an increased risk of cerebral palsy from assisted conception: a population‐based cohort study. Hum Reprod. 2010;25:2115‐2123. [DOI] [PubMed] [Google Scholar]
- 28. Norwegian institute of public health . Medical Birth Registry of Norway, 16.12.2019. https://www.fhi.no/en/ch/medical‐birth‐registry‐of‐norway/medical‐birth‐registry‐of‐norway/
- 29. Andersen GL, Hollung SJ, Klevberg GL, Kløve N, Jahnsen R, Stadskleiv K. Norwegian quality and surveillance registry for cerebral palsy yearly report 2021 [Norwegian]. Sykehuset i Vestfold and Oslo Universitetssykehus. 2022;50‐52. [Google Scholar]
- 30. Hollung SJ, Vik T, Wiik R, Bakken IJ, Andersen GL. Completeness and correctness of cerebral palsy diagnoses in two health registers: implications for estimating prevalence. Dev Med Child Neurol. 2017;59:402‐406. [DOI] [PubMed] [Google Scholar]
- 31. Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the gross motor function classification system. Dev Med Child Neurol. 2006;48:424. [DOI] [PubMed] [Google Scholar]
- 32. Eliasson AC, Krumlinde‐Sundholm L, Rösblad B, et al. The manual ability classification system (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48:549‐554. [DOI] [PubMed] [Google Scholar]
- 33. Pennington L, Virella D, Mjøen T, et al. Development of the Viking speech scale to classify the speech of children with cerebral palsy. Res Dev Disabil. 2013;34:3202‐3210. [DOI] [PubMed] [Google Scholar]
- 34. Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. 2011;174:1062‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Statistics Norway . Emigration and re‐immigration of persons born in Norway [Norwegian]. Reports. 2021; https://www.ssb.no/en/befolkning/artikler‐og‐publikasjoner/emigration‐and‐re‐immigration‐of‐persons‐born‐in‐norway [Google Scholar]
- 36. Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995‐2006. Fertil Steril. 2010;94:1320‐1327. [DOI] [PubMed] [Google Scholar]
- 37. Hollung SJ, Vik T, Lydersen S, Bakken IJ, Andersen GL. Decreasing prevalence and severity of cerebral palsy in Norway among children born 1999 to 2010 concomitant with improvements in perinatal health. Eur J Paediatr Neurol. 2018;22:814‐821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Directed acyclic graph describing potential causal relationship between ART and CP.
Table S1: Maternal and perinatal characteristics.


