Abstract
Introduction
Current use of combined hormonal contraceptives worsens glucose tolerance and increases the risk of type 2 diabetes mellitus at late fertile age, but the impact of their former use on the risk of glucose metabolism disorders is still controversial.
Material and methods
This was a prospective, longitudinal birth cohort study with long‐term follow‐up consisting of 5889 women. The cohort population has been followed at birth, and at ages of 1, 14, 31 and 46. In total, 3280 (55.7%) women were clinically examined and 2780 also underwent a 2‐h oral glucose tolerance test at age 46. Glucose metabolism indices were analyzed in former combined hormonal contraceptive users (n = 1371) and former progestin‐only contraceptive users (n = 52) and in women with no history of hormonal contraceptive use (n = 253).
Results
Compared with women with no history of hormonal contraceptive use, those who formerly used combined hormonal contraceptives for over 10 years had an increased risk of prediabetes (odds ratio [OR] 3.9, 95% confidence interval [CI]: 1.6–9.2) but not of type 2 diabetes mellitus. Former progestin‐only contraceptive use was not associated with any glucose metabolism disorders. The results persisted after adjusting for socioeconomic status, smoking, alcohol consumption, parity, body mass index and use of cholesterol‐lowering medication.
Conclusions
Former long‐term use of combined hormonal contraceptives was associated with a significantly increased risk of prediabetes in perimenopausal women, which potentially indicates a need of screening for glucose metabolism disorders in these women.
Keywords: combined hormonal contraception, glucose metabolism disorders, OGTT, prediabetes, progestin‐only contraception, type 2 diabetes mellitus
Former long‐term use of CHCs was associated with prediabetes in perimenopausal women, which potentially indicates a need of glucose metabolism screening in these women and emphasizes the importance of considering contraception alternatives other than CHCs at later fertile age.
Abbreviations
- BMI
body mass index
- CHC
combined hormonal contraceptive
- CI
confidence interval
- FPG
fasting plasma glucose
- NFBC1966
Northern Finland Birth Cohort 1966
- OGTT
oral glucose tolerance test
- OR
odds ratio
- POC
progestin‐only contraceptive
- T2DM
type 2 diabetes mellitus
Key message.
Former long‐term use of combined hormonal contraceptives was associated with prediabetes in perimenopausal women, which potentially indicates a need of glucose metabolism screening in these women and emphasizes the importance of considering contraception alternatives other than combined hormonal contraceptives at later fertile age.
1. INTRODUCTION
Combined hormonal contraceptives (CHCs) have been widely used for birth control since the early 1960s. The first‐ and second‐generation CHCs contained relatively high doses of ethinyl estradiol (EE, 50–150 μg) and progestins with androgenic properties, 1 whereas current, modern preparations consist of low‐dose EE (20–30 μg) and less androgenic or even antiandrogenic progestins. 2
The use of CHCs is often long‐term and is increasingly being prescribed for women over 40 years of age 3 , 4 as their use has been considered to be safe up to menopause in non‐smoking, healthy women with no known risk factors of cardiovascular disease. The available data suggest that the current use of CHCs increases the risk factors for impaired glucose tolerance as well as the risk of prediabetes and type 2 diabetes mellitus (T2DM) in women at late fertile age. 5 , 6 , 7 Moreover, some previous studies have also shown an association with former CHC use and impaired glucose metabolism or the development of prediabetes or T2DM, 5 , 6 , 8 while other studies have not revealed any association with impaired glucose metabolism. 9 , 10 Although the risk seems to decrease with time after discontinuation of CHCs, 5 , 6 it is unclear how long the unfavorable alterations persist. A large Swedish prospective, population‐based follow‐up study demonstrated a two‐fold increased risk for prediabetes in former CHC users. 5 Furthermore, a population‐based case–control study among Chinese women suggested a diminished risk of T2DM with time since the last use of CHCs. 6 Conversely, in the Nurse's Health Study cohort, the risk of T2DM was not dependent on the duration of former CHC use or the time interval since the last use of CHCs. 8
It is well recognized that prevalence of impaired glucose tolerance and T2DM is increased in women with polycystic ovary syndrome (PCOS). This risk is partly independent of obesity, but obesity exacerbates the risk even further. 11 CHCs are the first‐line treatment for clinical hyperandrogenism (hirsutism) and menstrual irregularities in PCOS, according to evidence‐based recommendations considering risks and benefits of CHC use. 12 Because of the beneficial effects of CHCs, it is expected that women with PCOS will use CHCs more frequently and for a longer period further impairing glucose metabolism.
In our recent study from the same birth cohort, we showed that the current use of CHCs, but not of progestin‐only contraceptives (POCs), was associated with a two‐fold risk of prediabetes and a three‐fold risk of T2DM at age 46. 7 In the present study, we aimed to examine in the same population‐based cohort whether former CHC or POC use is associated with prediabetes and T2DM morbidity in women approaching menopause.
2. MATERIAL AND METHODS
2.1. Study design and participants
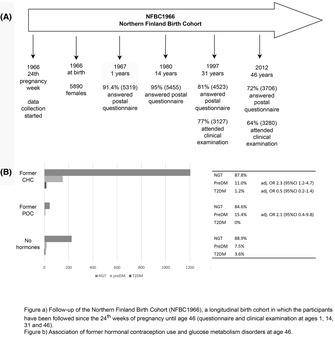
The study population comprised the Northern Finland Birth Cohort 1966 (Cohort NFBC1966 University of Oulu, Etsin‐service). The NFBC1966 is a unique, prospective, general population‐based cohort which includes all expected births in 1966 in the two northernmost provinces of Finland (Oulu and Lapland). The study population has been followed longitudinally since 24th gestational week and, so far, data has been collected at ages of at birth and 1, 14, 31 and 46. 13
In 2012–2014, at age 46 all women (n = 5123) received a large postal questionnaire and an invitation to clinical examination. A total of 72.4% of women (n = 3708) answered the questionnaire which included social background, education level (used as a proxy for socioeconomic status in this cohort), working life (workload, occupational health, economy), lifestyle factors (smoking, sleep, physical activity), health issues (medication, diseases, organ specific symptoms), quality of life and family history of diseases. The organ specific symptoms included questions on gynecological symptoms: number of pregnancies, infertility, pregnancy related diagnosis gestational diabetes or hypertension or pre‐eclampsia, menstruation cycle, menopause symptoms, diagnosis of endometriosis, myomas and PCOS, and former and current use of contraceptives. One of the questions concerned former use of hormonal contraceptives and length of use: less than 5 years, 5–10 years, or more than 10 years. From the answers to the questionnaire, we collected information on socioeconomic status (SES), smoking habits, use of alcohol, parity, family history of diabetes (parents, siblings and grandparents), previous diagnosis of gestational diabetes (GDM), diagnosis of PCOS and use of cholesterol‐lowering medication.
Altogether, 88.5% of these women participated in the clinical examination (n = 3280) at age 46. The examinations included anthropometric measurements and biological samples (blood, feces, urine, saliva and hair). In addition, several examinations were performed in subgroups of participants: brachial blood pressure, physical performance, 15‐lead electrocardiogram, heart rate variability test, pressure pain threshold and tolerance test, spirometry, cognitive test and objective measurements of physical activity and sleep. Also, 2780 of these 3280 women underwent a 2‐h 75 g oral glucose tolerance test (OGTT). A gynecological examination was not included in the clinical examination.
Diagnosis of PCOS was defined according to the postal questionnaire, which included the following question at age 46: “Have you ever been diagnosed as having polycystic ovaries and/or polycystic ovary syndrome (PCOS)?” Women who reported a diagnosis of PCO/PCOS by age 46 were considered women with PCOS (n = 181, 5.5%). We performed subanalyses to clarify the role of PCOS and former use of CHCs in abnormal glucose metabolism.
2.2. Former use of hormonal contraception
All women who attended clinical examinations and underwent OGTT, and reported former but not current use of hormonal contraception were included (N = 1423) and divided into two groups: (1) former CHC (including combined contraceptive oral pill, vaginal ring and transdermal patch) users (n = 1371) and (2) former POC (including hormone‐releasing intrauterine device, progestin‐only oral pill and subdermal capsule) users (n = 52). The group of former POC users was restricted to women who had used only POCs in their lifetime. Women who had used both CHCs and POCs were included in the group of former CHC users, meaning that the possible metabolic alterations caused by CHCs may have been diluted, as POCs are considered as metabolically relatively neutral. Women with no history of hormonal contraception use (n = 253) formed the reference group (Figure 1).
FIGURE 1.
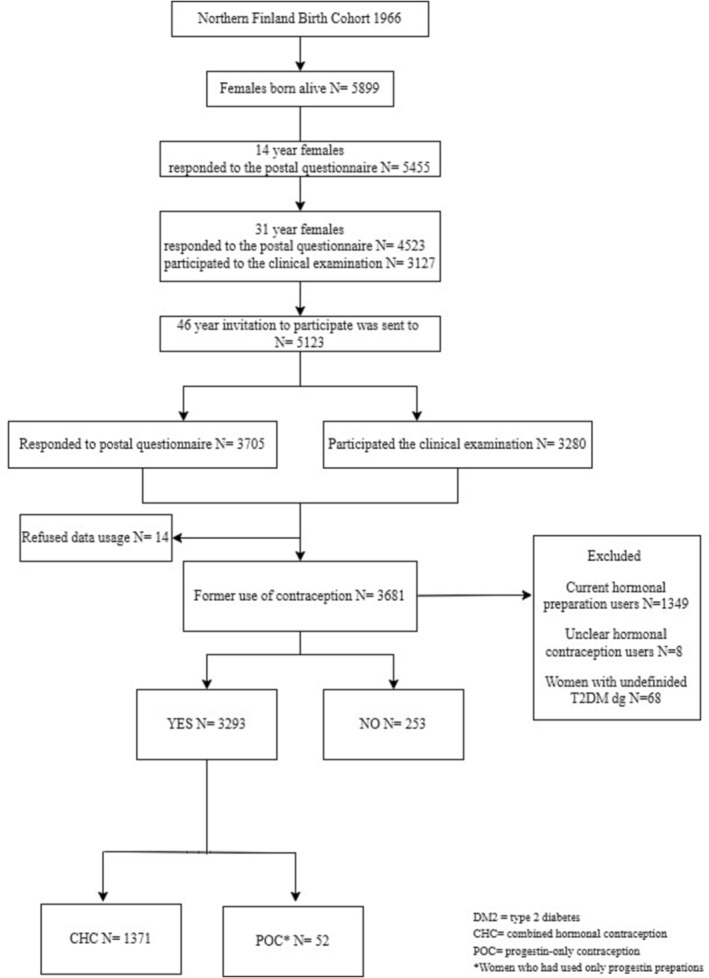
Flow chart of the study.
2.3. Anthropometric parameters
An experienced research nurse measured weight (kg) with a calibrated digital scale and height (cm) with a calibrated stadiometer. Body mass index (BMI) was calculated as the ratio of weight (kg) and to height squared (m2). Waist circumference (WC) was measured at the mid‐level between the lowest rib margin and the iliac crest in the clinical examinations.
2.4. Laboratory methods
At age 46, plasma glucose levels were analyzed by an enzymatic dehydrogenase method, and serum insulin levels by a chemiluminometric immunoassay (Advia 1800 and Advia Centaur XP, respectively, Siemens Healthcare Diagnostics). All samples collected during clinical examinations were analyzed in NordLab Oulu, a testing laboratory (T113) accredited by the Finnish Accreditation Service (FINAS) (EN ISO 15189).
2.5. Assessment of glucose metabolism disorders
After an overnight (12‐h) fasting, a 2‐h OGTT was performed in a total of 2780 women. Both serum insulin and plasma glucose levels were measured at baseline and at 30, 60 and 120 min after a 75‐g glucose intake. The glucose levels from fasting plasma (fP) and 2‐h OGTT samples from each time point were analyzed and categorized according to the WHO standards: normal glucose tolerance (NGT) was defined by a fasting glucose level of <6.1 mmoL/L and a 2‐h glucose level of <7.8 mmoL/L; impaired glucose tolerance (IGT) was defined by a fasting glucose level of <7.0 mmoL/L and a 2‐h glucose level of 7.8–11.0 mmoL/L impaired fasting glucose (IFG) was defined by an fasting glucose level of 6.1–6.9 mmoL/L and a 2‐h glucose level of <7.8 mmoL/L; and new T2DM was defined by an fasting glucose level of ≥7.0 mmoL/L or a 2‐h glucose level of ≥11.1 mmoL/L. 14 Prediabetes was defined by the presence of IFG or IGT. Diabetic medication for T2DM or a measured fasting capillary glucose level of >8.0 mmoL/L or diagnosis of type 1 diabetes (T1DM, n = 151) or undefined type of diabetes (n = 76) were exclusion criteria for OGTT examination. A previous diagnosis of T2DM was confirmed from hospital discharge documents and national drug registers of the Social Insurance Institution of Finland.
Fasting glucose (FPG) and insulin (FSI) values were used to calculate fasting indices: HOMA‐IR (homeostatic model assessment for insulin resistance) (FPG × FSI / 22.5) and HOMA‐2β index (homeostatic model assessment for beta‐cell function) ([20 × FSI] / [FPG ‐ 3.5] × 100). Glucose and insulin values in OGTTs were used to calculate insulin and glucose areas under the curve (glucose‐AUC and insulin‐AUC) and the Matsuda index was used for the whole‐body insulin sensitivity (ISI) (10 000 × ((FPG × FSI) × ((FPG + 30 min PG + 60 min PG + 120 min PG) / 4) × ((FSI + 30 min SI + 60 min SI + 120 min SI) / 4))). 15
Because we were not able to confirm the year of T2DM diagnoses or how the former use of CHCs or POCs was related to T2DM diagnoses, women with formerly diagnosed T2DM (n = 68) were excluded from the analysis. A total of 54 (79.4%) reported former use of CHCs, four (5.9%) reported former use of POCs and 10 (14.7%) had no history of hormonal contraception use.
The final study population included 1371 former users of CHCs, 52 former users of POCs and 253 women who had no history of hormonal contraception use (Figure 1).
2.6. Statistical analyses
Characteristics between study groups were analyzed with oneway ANOVA. The independent samples student's t‐tests, for normally distributed variables, were used to compare differences between study groups. Variables with a skewed distribution were log‐transformed to obtain a normal distribution. Bonferroni correction was used because there were multiple t‐tests. Multivariate binary logistic regression models were used to investigate the associations with former use of the different hormonal contraceptives (CHCs and POCs) and glucose metabolism disorders (prediabetes and T2DM). Models were adjusted for SES, consumption of alcohol, smoking, parity, measured BMI and use of cholesterol‐lowering medication. The results are reported as odds ratios (ORs) with 95% confidence intervals (95% CIs) and as crude ORs and ORs adjusted for the covariates. IBM SPSS Statistics software (IBM Corporation, 1989, 2013) version 26.0 for Windows was used for all statistical analyses. The level of statistical significance was set at p ≤ 0.05.
To minimize possible bias due to non‐attendance to OGTT, we also compared the group of women reporting former CHC use who had participated in both clinical examinations and OGTT with the group of women reporting former CHC use and attended clinical examinations but not OGTT.
Ethics statement
The regional ethical committee of the Northern Ostrobotnia Hospital District approved the study (EETTMK 94/2011) on December 14, 2011. All participants of NFBC1966 gave informed consent for the use of their collected data for scientific purposes.
3. RESULTS
3.1. Baseline characteristics of the study groups
BMI and WC did not differ between former CHC and POC users and women with no history of hormonal contraception use. Former CHC users had had fewer deliveries than former POC users (p < 0.033) and women with no history of hormonal contraception use (p < 0.001). The number of deliveries was similar among former POC users and women with no history of hormonal contraception (Table 1).
TABLE 1.
Characteristics of study groups.
| Number of answers | CHC | Number of answers | POC | Number of answers | No hormones | p‐value | |
|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | n = 1371 | 26.3 ± 5.0 | n = 52 | 27.0 ± 5.7 | n = 253 | 26.9 ± 5.9 | 0.192 |
| Waist circumference (cm) | n = 1371 | 86.5 ± 12.7 | n = 52 | 88.4 ± 14.5 | n = 253 | 88.3 ± 14.4 | 0.089 |
| Number of deliveries | n = 1371 | 1.9 ± 1.3 | n = 52 | 2.6 ± 1.3 | n = 253 | 3.0 ± 3.6 | 0.000 |
| SES | n = 1236 | n = 47 | n = 221 | 0.013 | |||
| Professionals | n = 876 (71.1%) | n = 25 (53.2%) | n = 144 (66.5%) | ||||
| Skilled workers | n = 235 (19.0%) | n = 14 (29.8%) | n = 46 (20.8%) | ||||
| Unskilled workers | n = 92 (7.4%) | n = 5 (10.6%) | n = 20 (9.0%) | ||||
| Farmers | n = 30 (2.4%) | n = 3 (6.4%) | n = 8 (3.6%) | ||||
| Alcohol consumption | n = 1371 | n = 52 | n = 253 | 0.000 | |||
| Yes | n = 1095 (79.9%) | n = 39 (75.0%) | n = 133 (52.6%) | ||||
| No | n = 276 (20.1%) | n = 13 (25.0%) | n = 120 (47.4%) | ||||
| Smoking | n = 1287 | n = 49 | n = 236 | 0.659 | |||
| Yes | n = 216 (16.8%) | n = 6 (16.8%) | n = 37 (15.7%) | ||||
| No | n = 1071 (83.2%) | n = 43 (87.8% | n = 199 (84.3%) | ||||
| Cholesterol‐lowering medication | n = 1371 | n = 37 (2.7%) | n = 52 | n = 0 | n = 253 | n = 10 (4.0%) | 0.249 |
| GDM diagnoses | n = 1144 | n = 105 (9.2%) | n = 47 | n = 3 (6.4%) | n = 18 | n = 18 (9.7%) | 0.781 |
| Familial T2DM (including first degree relatives and grandparents) | n = 765 | n = 353 (46.1%) | n = 28 | n = 11 (39.2%) | n = 80 | n = 46 (57.5%) | 0.109 |
Note: Results are shown as mean ± standard deviation (SD).
Abbreviations: BMI, body mass index; CHC, combined hormonal contraception; GDM, gestational diabetes mellitus; POC, progestin‐only contraception; T2DM, type 2 diabetes mellitus; SES, socioeconomic status.
p‐value in one‐way ANOVA.
The well‐known risk factors for T2DM, that is, previously diagnosed GDM and family history of T2DM (grandparents, parents, siblings, and children) were asked as a part of the questionnaire in the clinical examinations. The prevalence of GDM and familial T2DM did not significantly differ between the study groups. There was no significant difference regarding the use of cholesterol‐lowering medication between the study groups.
The group of women who reported former use of CHCs and participated in clinical examinations without OGTT had significantly higher BMI (p < 0.001) and larger WC (p < 0.001) compared to those who also underwent OGTT (Table S1).
3.2. Glucose metabolism alterations in OGTTs
Former CHC users had lower fasting insulin levels (p = 0.034) and higher Matsuda index values (i.e., better whole‐body insulin sensitivity, p = 0.03) than women with no history of hormonal contraception use.
Former POC users had lower fasting glucose levels (p = 0.039) than women with no history of hormonal contraception use.
Glucose‐AUC and insulin‐AUC in the OGTTs, Matsuda index, HOMA‐2β and HOMA‐IR did not differ between the three groups, even after adjustment for BMI and WC (Table 2).
TABLE 2.
Glucose metabolic parameters in former CHC and POC users compared with no hormonal contraception users.
| CHC | POC | No hormones | p 1 | p 2 | p 3 | p‐value in one‐way ANOVA | |
|---|---|---|---|---|---|---|---|
| n | 1371 | 52 | 253 | ||||
| Fasting glucose (mmol/L) | 5.32 ± 0.5 | 5.19 ± 0.2 | 5.37 ± 0.5 | NS | NS | 0.039 | 0.078 |
| Fasting insulin (mU/L) | 8.62 ± 6.6 | 8.75 ± 5.2 | 9.9 ± 9.8 | NS | 0.034 | NS | 0.041 |
| AUC glucose 0–120 (mmol/L × min) | 13.17 ± 2.9 | 13.18 ± 2.8 | 13.23 ± 2.9 | NS | NS | NS | 0.962 |
| AUC insulin 0–120 (mU/L × min) | 121.81 ± 81.6 | 134.92 ± 95.8 | 134.42 ± 98.3 | NS | NS | NS | 0.073 |
| HOMA‐IR | 2.17 ± 2.2 | 2.05 ± 1.3 | 2.59 ± 3.2 | NS | NS | NS | 0.031 |
| HOMA‐2β | 93.5 ± 41.1 | 94.5 ± 45.5 | 97.9 ± 36.8 | NS | NS | NS | 0.770 |
| Matsuda index | 5.72 ± 3.1 | 5.82 ± 3.7 | 5.42 ± 3.2 | NS | 0.03 | NS | 0.390 |
Note: Results are shown as mean ± standard deviation (SD).
Abbreviations: AUC, area under the curve; CHC, combined hormonal contraception; HOMA‐IR, homeostasis model of assessment insulin resistance; HOMA‐2 β, homeostasis model of assessment beta cell function; POC, progestin‐only contraception.
p 1 = p‐value between CHC and POC users.
p 2 = p‐value between CHC and no history of hormonal contraception use.
p 3 = p‐value between POC and no history of hormonal contraception use.
The group of women who reported former use of CHCs and participated in clinical examinations without OGTT had significantly higher fasting glucose (p = 0.02), fasting insulin (p = 0.011) AUCglucose (p < 0.001) and AUCinsulin levels (p = 0.02). They also had higher HOMA‐IR (p = 0.02) and HOMA‐2β (p = 0.03) reflecting worse insulin‐sensitivity compared to those who also underwent OGTT (Table S1).
3.3. Prevalence of glucose metabolism disorders
Former use of CHCs for more than 10 years was associated with increased prediabetes risk, after adjusting for SES, BMI, parity, smoking, consumption of alcohol and use of cholesterol‐lowering medication (adjusted OR 3.9, 95% CI: 1.6–9.2) compared with no history of hormonal contraception. Former use of CHCs was not associated with T2DM (Table 3).
TABLE 3.
Former CHC users association with prediabetes and new type 2 diabetes compared with no hormonal contraception users.
| Former CHC vs. never hormonal contraception use | |||||
|---|---|---|---|---|---|
| Duration of use | Any CHC use | CHC <5 years | CHC 5–10 years | CHC > 10 years | Never hormonal use |
| n | 1371 | 463 | 373 | 339 | 253 |
| PreDM n (%) | 151 (11.0%) | 49 (10.6%) | 38 (10.2%) | 39 (11.5%) | 19 (7.5%) |
| Crude OR (95% CI) | 1.4 (0.9–2.4) | 1.4 (0.8–2.5) | 1.4 (0.8–2.4) | 1.5 (0.9–2.7) | Ref |
| Adjusted* OR (95%CI) | 2.3 (1.2–4.7) | 1.7 (0.8–3.9) | 1.8 (0.8–4.1) | 3.9 (1.6–9.2) | Ref |
| NewT2DM n (%) | 17 (1.2%) | 7 (1.5%) | 5 (1.3%) | 3 (0.9%) | 9 (3.6%) |
| Crude OR (95% CI) | 0.4 (0.2–0.8) | 0.4 (0.2–1.2) | 0.4 (0.1–1.1) | 0.3 (0.1–0.9) | Ref |
| Adjusted* OR (95%CI) | 0.5 (0.2–1.4) | 0.6 (0.2–2.2) | 0.6 (0.2–2.8) | 0.5 (0.1–2.9) | Ref |
Abbreviations: CHC, combined hormonal contraception; OR, odds ratio; PreDM, prediabetes; T2DM, type 2 diabetes mellitus.
Adjusted with socioeconomic status, alcohol consumption, smoking, BMI, parity and cholesterol lowering medication.
A subanalysis including former CHC users only (not a combination of former POC and CHC users) yielded similar results; former use of CHCs for over 10 years was associated with a four‐fold risk of prediabetes (adjusted OR 4.1, 95% CI: 1.6–10.2) but not with T2DM (Table S2).
There were 181 women who reported PCOS diagnosis in the questionnaire at age 46. In total, 125 women reported former use of CHCs, and 31 participated in clinical examinations and underwent OGTT. Four women had formerly used CHCs over 10 years, 12 women 5–10 years and 13 women less than five years. The four women who had used CHCs more than 10 years were all normoglycemic in OGTT. Prediabetes was diagnosed in one woman who had formerly used CHCs for 5–10 years and in two women who reported former use of CHCs for less than five years. None had new T2DM. A subanalysis excluding women with PCOS diagnosis did not change the results, as former use of CHC for over 10 years was still associated with a four‐fold risk of prediabetes (adjusted OR 4.1, 95% CI: 1.6–10.2) and this was the only significant finding (data not shown).
Former use of POCs and no history of hormonal contraception were not associated with any glucose metabolism disorders (data not shown).
4. DISCUSSION
The results demonstrate that former long‐term use of CHCs for more than 10 years was associated with a 3.9‐fold risk of prediabetes (IGT or IFG) after adjustment for SES, BMI, alcohol consumption, smoking, parity and use of cholesterol‐lowering medication. However, former use of CHCs was not associated with an increased risk of T2DM. Moreover, former POC use was not associated with any glucose metabolism disorders. Although being overweight and obesity are known risk factors for prediabetes and T2DM, they did not explain our observations as BMI or WC did not significantly differ between the study groups.
The increased risk of prediabetes in former CHC users is in line with the results of a Swedish prospective, population‐based study including 4794 women aged 35–56 and showing that former CHC use was associated with a two‐fold risk of prediabetes. 5 This is a clinically important finding as up to 70% of prediabetic individuals eventually develop T2DM during their life. 16 Further, the cardiovascular risks of diabetes are well known as several meta‐analyses have shown that T2DM is associated with a two‐ to three‐fold risk for coronary heart disease and that women's risk exceeds that of men. 17 , 18 , 19 Moreover, a systematic review revealed that IFG is associated with a 1.24‐fold risk, and IGT with a 1.12‐fold risk, for cardiovascular disease. 20
However, the impact of the duration of CHC use regarding the risk of glucose metabolism disorders is controversial as some studies have shown no significant association between duration of use and glucose metabolism disorders, 10 , 21 whereas other studies have suggested a tendency towards an increased risk of T2DM with longer CHC use. 6 In the present study, the risk of prediabetes increased significantly only in women who had used CHCs for over 10 years. Variation in study design, contraceptive preparation used, BMI, ethnicity and, in some cases, inadequate sample sizes may explain the differences between studies. 22
In the present study, former POC use was not associated with prediabetes or new T2DM compared with women with no history of hormonal contraception use. This supports the results of earlier studies showing that POCs have no effect on glucose metabolism 23 or induce only minor and clinically non‐significant changes in insulin sensitivity. 24 , 25 , 26 All of these studies suggest that POCs may be safer than CHCs with regard to T2DM risk. Our present and previous results 7 indicate that POCs should be preferred over CHCs as contraception for women in their 40s and/or women with metabolic risk factors.
Interestingly, former CHC users tended to have a better metabolic profile according to WC, fasting insulin levels and insulin sensitivity compared with women with no history of hormonal contraception use. This finding may be explained by the lower BMI and fewer number of deliveries in the CHC group as well as a prescription bias towards a preferential use of CHCs in women free of metabolic risks. It is notable that despite their more beneficial metabolic profile, these women still displayed a significant increased risk of prediabetes.
The greatest strength of this study resides in the characteristics of the study population. The NFBC1966 data set provided a unique opportunity to investigate the association between former hormonal contraception use and glucose metabolism disorders in a large non‐selected population of perimenopausal women. The study population was extremely homogenous, as all women were 46 years old and Caucasian.
The study had also limitations, including the fact that the data on former hormonal contraception use and no history of hormonal contraception use were based on self‐reporting, which may have led to information bias, even though these self‐reported answers were confirmed during the clinical examinations. Furthermore, we lacked information on the reason for and the time of cessation of hormonal contraception use, which may have affected the results. The group of former POC users was relatively small because we excluded all women who had also used CHCs at any point in their life. Therefore, any exclusive conclusions on association with former POC use and glucose metabolism disorders cannot be made. Furthermore, the composition of different CHC preparations was not available and therefore we could not compare the risk of glucose metabolism alterations between different CHC generations or preparations. Former CHC users who underwent OGTT had a better metabolic profile (lower BMI and WC values, and lower levels of fasting glucose and insulin compared to former CHC users who did not undergo OGTT) than former CHC users who did not attend OGTT. It is therefore possible that including these women into our analyses would have strengthened our results, that is, former CHC users would have shown stronger association with glucose metabolism disorders. We were not able to identify all women with PCOS, because PCOS diagnosis was self‐reported, which may have affected the results. Former CHC users with PCOS had higher measured BMI and WC and more impaired glucose metabolism (higher levels of fasting glucose, insulin AUCglucose and AUCinsulin values) and worse insulin‐sensitivity (higher HOMA‐IR and lower Matsuda indexes). However, the relatively small number of CHC users with PCOS did not allow us to estimate whether former use of CHCs would be a greater risk for glucose metabolism disorders in PCOS women compared to the group of women without PCOS. Of note, our results did not change after exclusion of women with PCOS, suggesting that former use of CHCs is a risk for glucose metabolic disorders in a general population.
5. CONCLUSION
Former long‐term use of CHC was associated with an increased risk of prediabetes in the studied group of perimenopausal women. Although this may also be explained by other factors the observations could potentially indicate a need of screening for glucose metabolism disorders in these women. Furthermore, the present results emphasize the importance of considering contraception alternatives other than CHCs, especially in women with cardiovascular risk factors.
AUTHOR CONTRIBUTIONS
M‐EM, JT and LMP planned the study. M‐EM, TN, JJ and JA conducted the statistical analyses. M‐EM, TP, JT and LMP wrote the manuscript. MEM created the figures and tables. All of the authors reviewed and approved of the final manuscript.
FUNDING INFORMATION
NFBC1966 received financial support from University of Oulu grant no. 24000692, Oulu University Hospital grant no. 24301140, ERDF European Regional Development Fund grant no. 539/2010 A31592. The Sigrid Jusélius Foundation, the Academy of Finland, Oulu and Helsinki University Hospital Research Funds, Oulu University Medical Research Center.
CONFLICT OF INTEREST STATEMENT
The authors explicitly state that there are no conflicts of interest in connection with this article.
Supporting information
Table S1.
Table S2.
ACKNOWLEDGMENTS
We thank all cohort members and researchers who participated in the study. We also wish to acknowledge the work of the NFBC1966 project center.
Mosorin M‐E, Ollila M‐M, Nordström T, et al. Former long‐term use of combined hormonal contraception and glucose metabolism disorders in perimenopausal women: A prospective, population‐based cohort study. Acta Obstet Gynecol Scand. 2023;102:1488‐1495. doi: 10.1111/aogs.14636
REFERENCES
- 1. Godsland IF, Crook D, Wynn V. Low‐dose oral contraceptives and carbohydrate metabolism. Am J Obstet Gynecol. 1990;163:348‐353. [DOI] [PubMed] [Google Scholar]
- 2. Sitruk‐Ware R, Nath A, Mishell DR. Contraception technology: past, present and future. Contraception. 2013;87:319‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baldwin MK, Jensen JT. Contraception during the perimenopause. Maturitas. 2013;76:235‐242. [DOI] [PubMed] [Google Scholar]
- 4. Allen RH, Cwiak CA, Kaunitz AM. Contraception in women over 40 years of age. CMAJ. 2013;185:565‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deleskog A, Hilding A, Östenson CG. Oral contraceptive use and abnormal glucose regulation in Swedish middle aged women. Diabetes Res Clin Pract. 2011;92:288‐292. [DOI] [PubMed] [Google Scholar]
- 6. Rosenthal AD, Shu XO, Jin F, et al. Oral contraceptive use and risk of diabetes among Chinese women. Contraception. 2004;69:251‐257. [DOI] [PubMed] [Google Scholar]
- 7. Mosorin ME, Haverinen A, Ollila MM, et al. Current use of combined hormonal contraception is associated with glucose metabolism disorders in perimenopausal women. Eur J Endocrinol. 2020;183:619‐626. [DOI] [PubMed] [Google Scholar]
- 8. Rimm EB, Manson JE, Stampfer MJ, et al. Oral contraceptive use and the risk of type 2 (non‐insulin‐dependent) diabetes mellitus in a large prospective study of women. Diabetologia. 1992;35:967‐972. [DOI] [PubMed] [Google Scholar]
- 9. Chasan‐Taber L, Willett WC, Stampfer M, et al. A prospective study of Oral contraceptives ana NIDDM among U.S. women. Diabetes Care. 1997;20:330‐335. [DOI] [PubMed] [Google Scholar]
- 10. Troisi RJ, Cowie CC, Harris MI. Oral contraceptive use and glucose metabolism in a national sample of women in the United States. Am J Obstet Gynecol. 2000;183:389‐395. [DOI] [PubMed] [Google Scholar]
- 11. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta‐analysis. Hum Reprod. 2010;16:347‐363. [DOI] [PubMed] [Google Scholar]
- 12. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nordström T, Miettunen J, Auvinen J, et al. Cohort profile: 46 years of follow‐up of the northern Finland birth cohort 1966 (NFBC1966) . Int J Epidemiol. 2022;50:1786‐1787j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539‐553. [DOI] [PubMed] [Google Scholar]
- 15. Matsuda, M , DeFronzo, RA . Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462‐1470. [DOI] [PubMed] [Google Scholar]
- 16. Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high‐risk state for diabetes development. Lancet. 2012;379:2279‐2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee JY, Ku SY, Kim SH, Hwang SS, Lee HW, Park SM. Oral contraceptive use and measurable cardiovascular risk factors in Korean women aged 20‐50 years: the fourth Korean National Health and nutrition examination survey 2007‐2009 (KNHANES IV). Gynecol Endocrinol. 2013;29:707‐711. [DOI] [PubMed] [Google Scholar]
- 18. Kanaya AM, Grady D, Barrett‐Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus a meta‐analysis. Arch Intern Med. 2002;162:1737‐1745. [DOI] [PubMed] [Google Scholar]
- 19. Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta‐analysis of 37 prospective cohort studies. BMJ. 2006;332:73‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ford ES, Zhao G, Li C. Pre‐diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310‐1317. [DOI] [PubMed] [Google Scholar]
- 21. Furman BL. Impairment of glucose tolerance produced by diuretics and other drugs. Pharmacol Ther. 1981;12:613‐649. [DOI] [PubMed] [Google Scholar]
- 22. Lopez LM, Grimes DA, Schulz KF. Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus. Cochrane Database Syst Rev. 2019;2019(11):CD006133.pub5. [DOI] [PubMed] [Google Scholar]
- 23. Koopersmith TB, Lobo RA. Insulin sensitivity is unaltered by the use of the Norplant® subdermal implant contraceptive. Contraception. 1995;51:197‐200. [DOI] [PubMed] [Google Scholar]
- 24. Kahn HS, Curtis KM, Marchbanks PA. Effects of injectable or implantable progestin‐only contraceptives on insulin‐glucose metabolism and diabetes risk. Diabetes Care. 2003;26:216‐225. [DOI] [PubMed] [Google Scholar]
- 25. Biswas A, Viegas OAC, Coeling Bennink HJT, Korver T, Ratnam SS. Implanon® contraceptive implants: effects on carbohydrate metabolism. Contraception. 2001;63:137‐141. [DOI] [PubMed] [Google Scholar]
- 26. Konje JC, Otolorin EO, Ladipo OA. The effect of continuous subdermal levonorgestrel (Norplant) on carbohydrate metabolism. Am J Obstet Gynecol. 1992;166:15‐19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.


