Abstract

Catechin is one of the best-known antioxidants and is reported to have some favorable physiological activities, including anti-cancer effects. We previously synthesized a catechin analog, planar catechin, which showed a 10-fold larger radical scavenging activity than (+)-catechin. However, the physiological effects of the planar catechin have remained unclear. In this study, we examined cytotoxicity and mitochondrial membrane potential after planar catechin treatment using a rat normal gastric mucosal cell line, RGM1, and its chemically induced cancer-like cell line, RGK1. Interestingly, the planar catechin showed remarkable cytotoxicity compared to (+)-catechin, with cancer cell specificity. Furthermore, the decrease in the mitochondrial membrane potential of cancer cells was observed at specific concentrations of the planar catechin. These results indicate that the planar catechin, possessing higher antioxidant activity, induces its anti-cancer effect through a decrease in the mitochondrial membrane potential and thus can be a promising agent for cancer treatment.
Keywords: planar catechin, (+)-catechin, mitochondria, anti-cancer effect
Redox imbalance derived from excess generation of reactive oxygen species (ROS) induces oxidative stress in the body, leading to many kinds of diseases, such as cardiovascular disease, neurodegenerative disease, and carcinogenesis.1 The ingestion of antioxidants is one of the most effective ways to eliminate the unwanted ROS and recover the redox balance. Recently, many types of antioxidants have been discovered and developed as supplements. Resveratrol is a polyphenol compound that is abundant in red wine and reported to suppress obesity and extend the lifespan in high-calorie-fed mice.2 Further, resveratrol is also reported to improve cardiovascular disease in animal models and show anti-cancer effects by nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway activation.3,4 Ascorbic acid, which is also known as vitamin C, also has antioxidant properties to scavenge ROS efficiently and is reported to improve the reproductive ability of superoxide dismutase 1-knockout mice.5,6 Moreover, not only low-molecular-weight compounds but also functional polymers possessing antioxidant ability have been developed to ameliorate the redox balance and cure diseases such as colitis and cancer.7,8 In addition, catechin, which is classified as a flavanol of the flavonoid family of polyphenols and rich in green tea, is also reported to have antioxidant and anti-cancer effects.9 However, regular green tea consumption does not reduce the risk of death with cancer and heart disease, although Japanese people often drink green tea. There are some case reports studying the effect of catechin compounds in green tea on cancer therapy, but no clear efficacy has been shown, unlike in cell experiments.10 Furthermore, bioavailability of catechin compounds in green tea is remarkably low in the human body, and less than 5% of the orally given dose of tea catechins reaches systemic circulation in rats.10 Therefore, development of more effective antioxidants is needed to prevent oxidative stress-related diseases including cancer and extend healthy life expectancy.
We have synthesized a catechin analog, planar catechin, which has stronger antioxidant capacity than (+)-catechin (see Figure 1 for structures).11 The planar catechin showed a 10-fold larger second-order-rate constant compared to (+)-catechin for the reaction with 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) solubilized in water by β-cyclodextrin in a phosphate buffer (0.1 M, pH 7.4) at 298 K.12 This result indicates that the planar catechin has a 10-fold higher antioxidant activity than (+)-catechin. In addition, the planar catechin showed a more remarkable radioprotective activity and suppressed apoptotic cell death of rat thymocytes induced by X-ray irradiation compared to (+)-catechin.12 However, the effect of planar catechin on cancer cells has not been investigated. We report herein the effects of the planar catechin with strong antioxidant capacity on cancer cells using a rat normal mucosal cell line, RGM1, and its cancer-like mutant cell line, RGK1.
Figure 1.
Chemical structures of (+)-catechin and planar catechin.
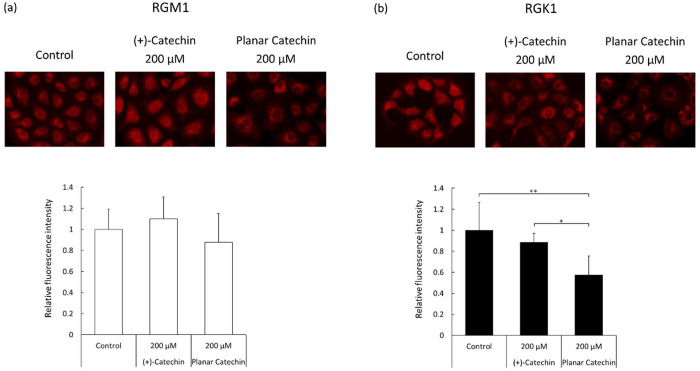
To achieve complete remission for cancer, many kinds of anti-cancer drugs, such as doxorubicin, cisplatin, and irinotecan, have been developed. Recently, new types of anti-cancer medicines based on inhibition of immune checkpoints have been approved and are being used in the clinic.13 However, some of anti-cancer drugs cause severe side effects to normal tissues, and it is still difficult to cure most malignant solid tumors completely. Antioxidants, such as flavonoid compounds, are thought to be promising agents for cancer treatment, because cancer cells overproduce ROS to activate many kinds of signal transduction, which are associated with proliferation and metastasis.14−16 Catechin is one of flavonoid compounds and is reported to have strong antioxidant ability and anti-tumor effects.9 The planar catechin, which we previously developed, has been reported to have a 10-fold stronger antioxidant capacity compared to (+)-catechin and shows outstanding cytotoxicity compared to the normal (+)-catechin, as can be seen in Figure 2. Notably, the cytotoxic effect of the planar catechin tends to be cancer cell dominant. These results suggest that the planar catechin may influence mitochondrial homeostasis, especially in RGK1 cancer cells, because mitochondria are one of the main organelles to generate ROS and the ROS levels in cancer cells tend to be elevated.17 We actually demonstrated cancer-cell-specific induction of mitochondrial dysfunction and decrease in the membrane potential by the planar catechin, as shown in Figure 3b. Some antioxidants are reported to suppress the growth of tumor cells, and we also reported that monascus purpureus, which is a red dye derived from yeast rice, induced cancer cellular apoptosis through scavenging mitochondrial ROS.18 The removal of mitochondrial ROS by monascus purpureus decreased the expression of acid ceramidase, which catalyzes the hydrolysis of ceramides to form sphingosine, leading to cellular proliferation. Thangavel et al. also reported that a nanoparticle-loading antioxidant inhibited acid ceramidase and the accumulation of ceramides in cancer cells promoted apoptotic cell death.19 In addition, mitochondrial ROS activate signal-related proteins, such as nuclear factor-kappa B (NF-κB) and tumor necrosis factor alpha (TNF-α), which are important for the survival of cancer cells.20,21 In contrast, the generation of ROS and activation of the signaling pathway in normal cells are not activated compared to those in cancer cells. Antioxidants have the possibility to alleviate the activation of the transcription factors and cytokines in cancer cells, and these differences between normal and cancer cells may be related to the cytotoxic selectivity of the planar catechin.
Figure 2.
Cell viability after (+)-catechin or the planar catechin treatment for 24 h, calculated by the water-soluble tetrazolium colorimetric assay. Statistical significance was tested by Tukey HSD. n = 6, mean ± SD, **p < 0.01.
Figure 3.
Mitochondrial membrane potential after treatment with (+)-catechin or the planar catechin, monitored using MitoTracker Red CMXRos. Representative images and bar graphs of relative fluorescence intensity in RGM1 (a) and RGK1 (b) are shown. Statistical significance was tested by Tukey HSD. n = 6, mean ± SD, *p < 0.05, **p < 0.01.
As mentioned above, the planar catechin has a notable antioxidant capacity. However, such powerful antioxidants can act as pro-oxidants because they also have strong reactivities with other kinds of oxidants. Carotenoids, which are known as possible antioxidants, also act as pro-oxidants, especially in cancer cells, and induce apoptosis via influencing mitochondria.22 Further, other kinds of strong antioxidants, such as N-acetylcysteine, have been reported to induce a paradoxical increase in mitochondrial oxidative stress through mitochondrial dysfunction, which is associated with reductive stress.23 The planar catechin may also act as a pro-oxidant and/or reductive stress inducer and cause apoptosis via mitochondrial dysfunction in cancer cells.
In conclusion, the synthesized catechin analog, planar catechin, caused significant cell death compared to (+)-catechin, and the lethal effect was selectively induced in cancer cells at specific concentrations. A decrease in mitochondrial membrane potential in cancer cells was also observed with the planar catechin treatment. Thus, the planar catechin is likely to induce cancer cell death by targeting mitochondria. However, the mechanism for induction of cancer cell death and the relationship of the antioxidant ability of the planar catechin are still unknown. We are now further investigating these details.
Glossary
Abbreviations
- ROS
reactive oxygen species
- Nrf2
nuclear factor erythroid 2-related factor 2
- DPPH•
2,2-diphenyl-1-picrylhydrazyl radical
- NF-κB
nuclear factor-kappa B
- TNF-α
tumor necrosis factor alpha
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.3c00328.
Detailed description of experimental methods (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Brieger K.; Schiavone S.; Miller J.; Krause K. Reactive Oxygen Species: From Health to Disease. Swiss Med. Wkly. 2012, 142, 13659. 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- Baur J. A.; Pearson K. J.; Price N. L.; Jamieson H. A.; Lerin C.; Kalra A.; Prabhu V. V.; Allard J. S.; Lopez-Lluch G.; Lewis K.; Pistell P. J.; Poosala S.; Becker K. G.; Boss O.; Gwinn D.; Wang M.; Ramaswamy S.; Fishbein K. W.; Spencer R. G.; Lakatta E. G.; Le Couteur D.; Shaw R. J.; Navas P.; Puigserver P.; Ingram D. K.; de Cabo R.; Sinclair D. A. Resveratrol Improves Health and Survival of Mice on a High-Calorie Diet. Nature 2006, 444 (7117), 337–342. 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal R.; Deres L.; Toth K.; Halmosi R.; Habon T. The Effect of Resveratrol on the Cardiovascular System from Molecular Mechanisms to Clinical Results. Int. J. Mol. Sci. 2021, 22 (18), 10152. 10.3390/ijms221810152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi M.; Farkhondeh T.; Aschner M.; Samarghandian S. Resveratrol Mediates Its Anti-Cancer Effects by Nrf2 Signaling Pathway Activation. Cancer Cell Int. 2021, 21 (1), 579. 10.1186/s12935-021-02280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni O.; De Tullio M. C. Ascorbic Acid: Much More than Just an Antioxidant. Biochim. Biophys. Acta - Gen. Subj. 2002, 1569 (1–3), 1–9. 10.1016/S0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- Ishii N.; Homma T.; Lee J.; Mitsuhashi H.; Yamada K. I.; Kimura N.; Yamamoto Y.; Fujii J. Ascorbic Acid and CoQ10 Ameliorate the Reproductive Ability of Superoxide Dismutase 1-Deficient Female Mice. Biol. Reprod. 2020, 102 (1), 102–115. 10.1093/biolre/ioz149. [DOI] [PubMed] [Google Scholar]
- Vong L. B.; Tomita T.; Yoshitomi T.; Matsui H.; Nagasaki Y. An Orally Administered Redox Nanoparticle That Accumulates in the Colonic Mucosa and Reduces Colitis in Mice. Gastroenterology 2012, 143 (4), 1027–1036.e3. 10.1053/j.gastro.2012.06.043. [DOI] [PubMed] [Google Scholar]
- Koda Y.; Nagasaki Y. Design of Cysteine-Based Self-Assembling Polymer Drugs for Anticancer Chemotherapy. Colloids Surfaces B Biointerfaces 2022, 220 (Dec), 112909 10.1016/j.colsurfb.2022.112909. [DOI] [PubMed] [Google Scholar]
- Musial C.; Kuban-Jankowska A.; Gorska-Ponikowska M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21 (5), 1744. 10.3390/ijms21051744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almatroodi S. A.; Almatroudi A.; Khan A. A.; Alhumaydhi F. A.; Alsahli M. A.; Rahmani A. H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25 (14), 3146. 10.3390/molecules25143146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara K.; Nakanishi I.; Kansui H.; Sugiyama E.; Kimura M.; Shimada T.; Urano S.; Yamaguchi K.; Miyata N. Enhanced Radical-Scavenging Activity of a Planar Catechin Analogue. J. Am. Chem. Soc. 2002, 124 (21), 5952–5953. 10.1021/ja0178259. [DOI] [PubMed] [Google Scholar]
- Sekine-Suzuki E.; Nakanishi I.; Imai K.; Ueno M.; Shimokawa T.; Matsumoto K. I.; Fukuhara K. Efficient Protective Activity of a Planar Catechin Analogue against Radiation-Induced Apoptosis in Rat Thymocytes. RSC Adv. 2018, 8 (19), 10158–10162. 10.1039/C7RA13111A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares C. M.; Van Allen E. M.; Drake C. G.; Allison J. P.; Hu-Lieskovan S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients?. Am. Soc. Clin. Oncol. Educ. B 2019, 39, 147–164. 10.1200/EDBK_240837. [DOI] [PubMed] [Google Scholar]
- Grigalius I.; Petrikaite V. Relationship between Antioxidant and Anticancer Activity of Trihydroxyflavones. Molecules 2017, 22 (12), 2169. 10.3390/molecules22122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravishankar D.; Rajora A. K.; Greco F.; Osborn H. M. I. Flavonoids as Prospective Compounds for Anti-Cancer Therapy. Int. J. Biochem. Cell Biol. 2013, 45 (12), 2821–2831. 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Arfin S.; Jha N. K.; Jha S. K.; Kesari K. K.; Ruokolainen J.; Roychoudhury S.; Rathi B.; Kumar D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10 (5), 642. 10.3390/antiox10050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan L. B.; Chandel N. S. Mitochondrial Reactive Oxygen Species and Cancer. Cancer Metab. 2014, 2, 17. 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa H.; Ito H.; Matsui H. Monascus Purpureus Induced Apoptosis on Gastric Cancer Cell by Scavenging Mitochondrial Reactive Oxygen Species. J. Clin. Biochem. Nutr. 2017, 61 (3), 189–195. 10.3164/jcbn.17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavel S.; Yoshitomi T.; Sakharkar M. K.; Nagasaki Y. Redox Nanoparticles Inhibit Curcumin Oxidative Degradation and Enhance Its Therapeutic Effect on Prostate Cancer. J. Controlled Release 2015, 209, 110–119. 10.1016/j.jconrel.2015.04.025. [DOI] [PubMed] [Google Scholar]
- Chen A. C. H.; Arany P. R.; Huang Y.-Y.; Tomkinson E. M.; Sharma S. K.; Kharkwal G. B.; Saleem T.; Mooney D.; Yull F. E.; Blackwell T. S.; Hamblin M. R. Low-Level Laser Therapy Activates NF-KB via Generation of Reactive Oxygen Species in Mouse Embryonic Fibroblasts. PLoS One 2011, 6 (7), e22453 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S.; Badana A. K.; G M. M.; G S.; Malla R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13, 117727191875539 10.1177/1177271918755391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.; Song M. H.; Oh J. W.; Keum Y. S.; Saini R. K. Pro-oxidant Actions of Carotenoids in Triggering Apoptosis of Cancer Cells: A Review of Emerging Evidence. Antioxidants 2020, 9 (6), 532. 10.3390/antiox9060532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris E.; Micallef P.; Paul A.; Palsdottir V.; Enejder A.; Bauzá-Thorbrügge M.; Olofsson C. S.; Wernstedt Asterholm I. Antioxidant Treatment Induces Reductive Stress Associated with Mitochondrial Dysfunction in Adipocytes. J. Biol. Chem. 2019, 294 (7), 2340–2352. 10.1074/jbc.RA118.004253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.