Description
A previously healthy 13-year-old boy was hospitalised in April 2020 following 7 days of fever along with abdominal and thoracic pain, odynodysphagia and a new skin eruption first seen on the day of admission. Besides paracetamol, there were no other current treatments. Our patient’s mother developed anosmia and ageusia 3 weeks before and his father had flu-like symptoms at the same time. He had already been tested twice for severe acute respiratory syndrome coronavirus-2 (SARS-COV2) by nasopharyngeal swab in the days before his admission, with negative RT-PCR.
On clinical examination, diffuse abdominal tenderness and a rash were found. The rash consisted of four isolated round papular lesions on his left shoulder with a central dark red zone surrounded by a pale ring of oedema and an erythematous halo on the extreme periphery, compatible with the target lesions of erythema multiforme (EM).
Laboratory investigations showed elevated inflammatory signs (C reactive protein 265 mg/L, procalcitonin of 2.71 µg/L). The complete blood count showed lymphopaenia (0.93 g/L) and thrombocytopaenia (104 g/L). A full sepsis work-up was negative and apart from slightly elevated troponins (0199 µg/L), there were no signs for organ dysfunction. Chest x-ray and echocardiography were initially within normal limits and abdominal CT scan showed multiple peritoneal lymph nodes.
Given the severe inflammatory syndrome, an empiric antibiotic treatment with ceftriaxone was started.
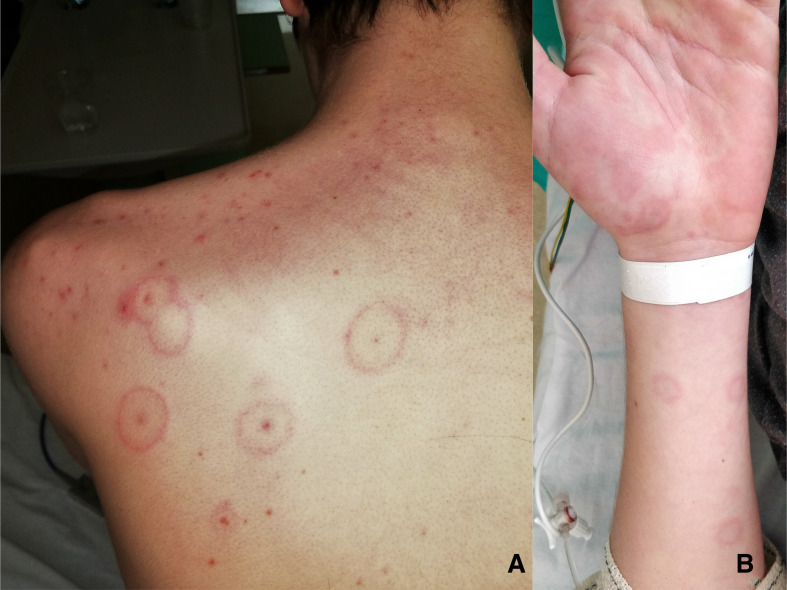
On day 2, the number of target lesions increased substantially with a generalised symmetrical distribution (figure 1A,B). The mucous membranes were involved in the form of isolated conjunctivitis. Suspecting Mycoplasma pneumoniae infection, antibiotic therapy was extended with azithromycin. On day 4, the patient developed symptoms and radiological signs of bibasal pneumonia on chest CT scan without any need for oxygen or respiratory support.
Figure 1.
The rash consisted of isolated round papular lesions with a central dark red zone surrounded by a pale ring of oedema and an erythematous halo on the back (A) and the extreme periphery (B), compatible with the target lesions of EM. EM, erythema multiforme.
SARS-COV-2 recent contact was confirmed by positive serology (IgA and IgG by ELISA, confirmed by immunofluorescence), while the most common infectious pathogens linked to EM (Mycoplasma pneumoniae, Epstein-Barr virus, Herpes simplex virus 1 and 2, adenovirus and parvovirus B19) were excluded.
The patient could be discharged on day 7 with complete resolution of clinical symptoms including EM. In a follow-up visit 3 weeks after being discharged, he was asymptomatic, and laboratory tests and echocardiography were normal.
Our case reflects the recently described cases of paediatric hyperinflammatory syndrome in children during COVID-19 pandemic.1–3 The WHO has defined this new syndrome associated with COVID-19 on 15 May 2020.4 It was called ‘Multisystem inflammatory syndrome in children and adolescents (MIS-C) temporally related to COVID-19’. Our patient completed this case definition,4 he presented more than 3 days of fever, a rash and non-purulent conjonctivitis, associated with features of myocardial dysfonction with elevated tropinin and acute gastrointestinal problems. He completed also the criteria of elevated markers of inflammation and the evidence of COVID-19 by serology, without other microbial cause of inflammation.
We conclude that our patient presented a multisystem inflammatory syndrome in children and adolescents (MIS-C) temporally related to COVID-19 associated with an erythema multiforme. To our knowledge, this is the first described case of EM in this context in paediatric age.
Patient’s perspective.
Before and during the hospitalization I was very tired, I couldn't walk because my muscles were very sore, it was hard to breathe and to eat because I had a sore throat. I vomited as soon as I had fever and I had abdominal pain at the beginning of my illness. During the hospitalization I was stressed and anxious because every day I saw the doctors who told me that they didn't know what was wrong with me.
Learning points.
The rash associated with the multisystem inflammatory syndrome in children and adolescents temporally related with COVID-19 could be an erythema multiforme (EM).
EM could be one of the first symptoms of this new syndrome.
Footnotes
Contributors: TB wrote the clinical case. FR and MM added corrections and made modifications. MR added corrections, made modifications and supervied TB to write.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Parental/guardian consent obtained.
References
- 1.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395:1607–8. 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deza Leon MP, Redzepi A, McGrath E, et al. COVID-19 associated pediatric multi-system inflammatory syndrome. J Pediatric Infect Dis Soc 2020. doi: 10.1093/jpids/piaa061. [Epub ahead of print: 22 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020;395:1771–8. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. Available: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 [Accessed 15 May 2020].