Abstract
Staphylococcus-associated glomerulonephritis (SAGN) occurs as a complication of staphylococcal infection elsewhere in the body. Dermatomyositis (DM) can be associated with glomerulonephritis due to the disease per se. We report a case of a 40-year-old male patient with DM who presented with acute kidney injury, and was initially pulsed with methylprednisolone for 3 days, followed by dexamethasone equivalent to 1 mg/kg/day prednisolone. He was subsequently found to have SAGN on kidney biopsy along with staphylococcus bacteraemia and left knee septic arthritis. With proof of definitive infection, intravenous immunoglobulin 2 g/kg over 2 days was given and steroids were reduced. He was treated with intravenous vancomycin. With treatment, the general condition of the patient improved. On day 38, he developed infective endocarditis and died of congestive heart failure subsequently. Undiagnosed staphylococcal sepsis complicating a rheumatological disease course can lead to complications like SAGN, infective endocarditis and contribute to increased morbidity and mortality, as is exemplified by our case.
Keywords: connective tissue disease, acute renal failure
Background
Dermatomyositis (DM) belongs to the heterogeneous group of inflammatory myopathies, characterised by muscle weakness, skin rash and extramuscular manifestations, including pulmonary and cardiac disease. Renal involvement in DM is not uncommon and reported in one-fifth of patients in previous studies.1 2 The spectrum of renal involvement includes acute kidney injury (AKI), mainly due to myoglobin-induced acute tubular necrosis, chronic kidney disease and isolated proteinuria. Staphylococcus is notorious for causing disseminated and fatal infections, specially in immunocompromised settings. Glomerulonephritis (GN) is an uncommon complication of staphylococcal infection. We present a case of DM presenting with staphylococcal bacteraemia, septic arthritis and GN, complicated by infective endocarditis despite appropriate antibiotic coverage.
Case presentation
A 40-year-old male patient presented with complaints of difficulty in getting up from a squatting position and difficulty in performing overhead activities for the past 5 months. He also noticed a reddish skin rash over the periorbital area, and upper part of the chest and elbow. He had difficulty in swallowing both liquid and solid food along with difficulty in neck holding for the past 1 week. Urine output was decreased for last 24 hours. There was no history of high-grade fever, malar rash, oral ulcer or weight loss. He had been prescribed tablet methylprednisolone 4 mg/day for last 5 months.
Examination revealed a conscious and oriented patient. His pulse rate was 110/min and blood pressure was 130/70 mm Hg. He was tachypneic with a respiratory rate of 50/min and oxygen saturation of 95% on room air. Characteristic heliotrope rash, Gottron sign, ‘V’ sign and mechanics hand were present. There were bullous lesions over the posterior aspect of the left popliteal fossa and posterior aspect of the left ankle. There was arthritis of bilateral wrists, knees and ankles. Neurological examination revealed grade 2/5 power in muscles around the shoulder and hip, and neck muscle weakness. Deep tendon reflexes were normal in all limbs with flexor plantar response. Cranial nerves and sensory examination were normal. Gag reflex was preserved. Other systems revealed no abnormality.
Based on these clinical features, a diagnosis of DM with bulbar involvement and AKI along with cellulitis of left leg was entertained. The patient was given empirical antibiotics and pulse methylprednisolone 1 g for 3 days followed by intravenous dexamethasone (1 mg/kg/day prednisolone equivalent).
Investigations
Investigations revealed haemoglobin 90 g/L, white cell count 15.8×109 cells/L, platelet count 250 000/mm3, erythrocyte sedimentation rate 104 mm in first hour (Westergren method), C-reactive protein 280 mg/dL (n=0–6), creatine phosphokinase 767 IU/L (n=24–170), lactate dehydrogenase 586 IU/L (n=100–280), aspartate transaminase 93 IU/L (n=5–40) and alanine transaminase 47 IU/L (n=5–40). Screening for hepatitis B and C viruses, and HIV were negative. Fasting plasma glucose 98 mg/dL, blood urea 110 mg/dL, serum creatinine 2.2 mg/dL; urine routine examination showed 6–8 pus cells and 15–20 red blood cells per high power field with protein 30 mg%. Chest X-ray was normal and ultrasonography of the abdomen showed acute medical renal disease. Urine culture was sterile and 24-hour urinary protein was 1.43 g. Serum C3 was 27.3 mg/dL (n=90–180), C4 was 14.7 mg/dL (n=10–40) and anti-dsDNA <10 IU/mL (n<30). Antinuclear antibody by indirect immunofluorescence method on HEp-2 cells was 3+ fine speckled and extractable nuclear antigen screen was negative.
With significant proteinuria and low C3, the diagnosis of GN was considered. Renal biopsy revealed endocapillary proliferation and mesangial hypercellularity with predominant IgA deposition (figure 1A,B), suggestive of staphylococcus-associated GN (SAGN).
Figure 1.
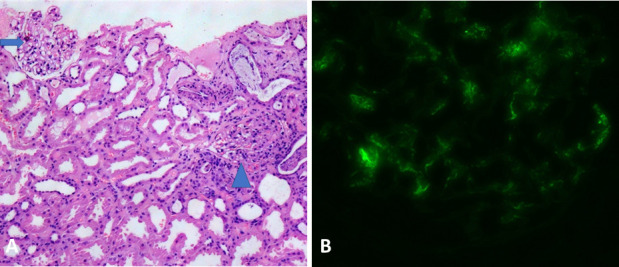
Histological examination of the renal biopsy specimen. (A) Light microscopy (H&E stain) showing two glomeruli, the left one is unremarkable (arrow) and the right one depicts segmental mesangial and endocapillary hypercellularity (arrowhead). (B) Immunofluorescence microscopy showing brightly immunofluorescent IgA deposits in the mesangium and focally along the capillary walls.
Outcome and follow-up
On day 5, he developed a significant tender left knee effusion, warranting aspiration and synovial fluid routine examination and microbiological culture. The analysis revealed 1 58 000 cells with culture showing growth of methicillin-resistant Staphylococcus aureus. Blood culture grew methicillin-sensitive S. aureus. He was continued on parenteral vancomycin as the isolated strains were sensitive and joint lavage was done. In view of his persistent muscle weakness and staphylococcal bacteraemia along with left knee septic arthritis, he was administered intravenous immunoglobulin 2 g/kg over 2 days and dexamethasone dose was reduced to 0.5 mg/kg/day of prednisolone equivalent. Gradually, his muscle weakness improved, left knee arthritis subsided, and he started walking with support. The laboratory parameters, including muscle enzymes, also showed improvement.
However, on day 38, the patient developed infective endocarditis with a 1.0 cm vegetation on the posterior mitral leaflet along with severe mitral regurgitation and moderate tricuspid regurgitation. Considering nosocomial infection and resistant strains, daptomycin and linezolid were added and surgical cardiac intervention was planned. On day 41, he succumbed to congestive heart failure.
Discussion
Renal involvement in DM commonly manifests as rhabdomyolysis-induced myoglobinuria, leading to renal insufficiency and chronic GN.3 Membranous nephropathy is the most common GN found in DM patients, although mesangioproliferative and diffuse proliferative GN and IgA nephropathy are also reported.3 4
Infections are a serious concern in the management of patients with inflammatory myopathies, with one study reporting major infections requiring >1 week antimicrobial treatment in 27.6% of patients.5 The common organisms isolated were S. aureus, Klebsiella, Escherichia coli, Salmonella and Mycobacterium. Age at onset >45 years, arthritis, interstitial lung disease and recent use of azathioprine or intravenous immunoglobulin were the associations with major infections in the above study. Our patient presented with arthritis and had used low-dose prednisolone for a prolonged period.
SAGN complicating staphylococcal sepsis typically presents with haematuria, proteinuria and AKI, and is usually caused by methicillin-resistant S. aureus arising from the skin or visceral source.6 Rapidly progressive GN with nephrotic-range proteinuria has been reported in one-half of patients.7 The left leg cellulitis in our patient was the possible source, that subsequently disseminated to kidney and heart. The key pathogenic mechanisms of SAGN include staphylococcal superantigens causing uncontrolled activation of T and B cells with massive cytokines production and immune complex deposition. SAGN is characterised pathologically by mesangial and/or endocapillary proliferation with IgA-predominant or IgA-codominant immune complex deposits on immunofluorescence.6 This is in contrast to the endocapillary proliferation with IgG-dominant immune deposition in patients with postinfectious GN. Our patient had both mesangial hypercellularity and endocapillary proliferation with predominant IgA deposition. The differentiation from a primary IgA nephropathy is important as SAGN warrants an active search for an underlying infection and portends avoiding immunosuppressive medications.8 Hypocomplementaemia and IgA-predominant immune complex deposits in the mesangium and along the glomerular capillary walls in the presence of microbiological evidence for staphylococcus support the diagnosis of SAGN in our case. The goal of treatment is eradication of the underlying staphylococcal infection with appropriate antibiotics and surgical debridement if indicated. The prognosis is, however, guarded with significant proportion of patients progressing to end-stage renal disease with poor outcomes.
Our case highlights SAGN as a cause of renal involvement in a patient of DM, which is distinct from the known causes of renal involvement/GN in such patients. Undiagnosed staphylococcal sepsis complicating a rheumatological disease course (especially those on heavy immunosuppression) can lead to complications like SAGN and contribute to increased morbidity and mortality, as is exemplified by our case.
Learning points.
Staphylococcus-associated glomerulonephritis can mimic IgA nephropathy with predominant IgA deposition in renal biopsy.
The case also highlights the importance of detailed search for infections complicating the clinical course of patients with rheumatological diseases on immunosuppression.
Appropriate antibiotics and use of intravenous immunoglobulin when indicated, with judicious use of steroids would be imperative to improve outcomes in these patients.
Acknowledgments
We are thankful to Dr Kiranpreet Malhotra, Department of Pathology, Dr RML Institute of Medical Sciences, Lucknow, India, for providing us with the histopathology images of renal biopsy sample.
Footnotes
Twitter: @RasmiKGMU
Contributors: RRS: Data acquisition, drafting of manuscript and final approval. SP: Data acquisition, critical revision and final approval of the manuscript. APG: Data acquisition, critical revision and final approval of the manuscript. AW: Data interpretation, critical revision and final approval of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Couvrat-Desvergnes G, Masseau A, Benveniste O, et al. The spectrum of renal involvement in patients with inflammatory myopathies. Medicine 2014;93:33–41. 10.1097/MD.0000000000000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yen T-H, Lai P-C, Chen C-C, et al. Renal involvement in patients with polymyositis and dermatomyositis. Int J Clin Pract 2005;59:188–93. 10.1111/j.1742-1241.2004.00248.x [DOI] [PubMed] [Google Scholar]
- 3.Kronbichler A, Mayer G. Renal involvement in autoimmune connective tissue diseases. BMC Med 2013;11:95. 10.1186/1741-7015-11-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Civilibal M, Selcuk Duru N, Ozagari A, et al. Immunoglobulin A nephropathy associated with juvenile dermatomyositis. Pediatr Nephrol 2009;24:2073–5. 10.1007/s00467-009-1178-x [DOI] [PubMed] [Google Scholar]
- 5.Chen I-J, Tsai W-P, Wu Y-JJ, et al. Infections in polymyositis and dermatomyositis: analysis of 192 cases. Rheumatology 2010;49:2429–37. 10.1093/rheumatology/keq279 [DOI] [PubMed] [Google Scholar]
- 6.Wang S-Y, Bu R, Zhang Q, et al. Clinical, pathological, and prognostic characteristics of glomerulonephritis related to staphylococcal infection. Medicine 2016;95:e3386. 10.1097/MD.0000000000003386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wehbe E, Salem C, Simon JF, et al. IgA-dominant Staphylococcus infection-associated glomerulonephritis: case reports and review of the literature. NDT Plus 2011;4:181–5. 10.1093/ndtplus/sfr017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoskar AA, Nadasdy G, Plaza JA, et al. Staphylococcus infection-associated glomerulonephritis mimicking IgA nephropathy. Clin J Am Soc Nephrol 2006;1:1179–86. 10.2215/CJN.01030306 [DOI] [PubMed] [Google Scholar]


