Abstract
During the global pandemic of COVID-19 accurate diagnosis of the infection by demonstrating SARS-CoV-2 viral RNA by PCR in specimens is crucial for therapeutic and preventative interventions. There have been instances where nasal and throat swabs have been negative despite the patient having typical clinical and radiological findings compatible with the disease. We report a case of a man in his late 50s, brought to the hospital following a cardiac arrest and prolonged unsuccessful resuscitation. The history was typical for COVID-19 with fever for 10 days and worsening shortness of breath. His throat and nasal swabs (after death) were negative for SARS-CoV-2. A limited diagnostic autopsy was performed after 27 days, and lung swabs confirmed presence of SARS-CoV-2. This case highlights the importance of lung swabs when initial upper respiratory tract swabs are negative and proves that the virus can be detected from dead human tissue almost a month later.
Keywords: infections, pathology
Background
SARS-CoV-2 virus has already claimed more than 900 000 deaths and infected 27 million people.1 The efforts to mitigate the spread of infection depend on accurate and early diagnosis of the disease.
Detection of viral RNA with reverse transcription-PCR remains the standard technique of confirming diagnosis.2 However, the sensitivity of the test remains uncertain and interpreting the result of a test for COVID-19 depends on two things: the accuracy of the test and the pre-test probability or estimated risk of disease before testing.3 4 Varying sensitivity rates have been reported for specimens obtained from different sites indicating potential diversity of the distribution of virus in different mucosal surfaces and parts of the respiratory tract system.5 6 The dynamics of virus shedding, viral load from different sites and time of infection have been thought to account for this variation in detection of the virus as for example virus being present in deeper respiratory specimens with advanced disease.7–9 However, more studies are required to fully understand the significance of sampling at different sites.
A negative PCR result needs to be interpreted in the context of this variability in viral shedding. A negative PCR assay could be due to a true negative (patient has not got the virus), sampling technique, timing of sampling and the viral load. Current practice is to repeat further nasopharyngeal swabs or if possible, to take deep respiratory samples if the first nasopharyngeal swab is negative. When a deceased patient is suspected to have COVID-19, current recommendation is to take two swabs each from upper and lower respiratory tracts.10
The degree to which live virus can survive in various environments and dead human tissue has been the subject of much debate since the beginning of this epidemic.11 This key piece of information can have a significant effect on a wide spectrum of areas from the safe handling of laboratory specimens to disease mitigation procedures and the disposal of the dead body.12 RNA has been recovered from the 1918 influenza epidemic using pathology museum samples and from lung tissue samples obtained from exhumed bodies from a mass grave in Alaska as late as 1997, though its viability is debatable.13 14 To date there has been no published data on the persistence of SARS-CoV-2 RNA in dead human tissue and we believe this is the first report to illustrate this finding.
Case presentation
A man in his late 50s was brought to the hospital by an ambulance. On arrival, the ECG showed he was in cardiac asystole. He had been receiving cardiopulmonary resuscitation for around 120 min but had remained in cardiac asystole (no cardiac output and absent cardiac electrical activity). His medical history revealed no previous comorbidities apart from hypercholesterolaemia. He has had a history of fever and shortness of breath for the previous 10 days. When he noticed the worsening of his shortness of breath, he contacted the emergency service but rapidly deteriorated while awaiting the arrival of the ambulance.
Investigations
A nose/throat swab taken within 24 hours of death was negative for COVID-19 by PCR.
The investigations carried out from the blood samples are summarised in table 1.
Table 1.
Results of the blood investigations at the time of resuscitation
| Investigation | Result |
| Full blood count | |
| White blood cells | 21.65×109/L |
| Neutrophil | 17.54×109/L (81%) |
| Lymphocytes | 1.95×109/L (9.0%) |
| Monocytes | 0.43×109/L (2%) |
| Promyelocytes | 0.87×109/L (4.0%) |
| Haemoglobin Platelets |
152 g/L 112×109/L |
| C reactive protein | 138 mg/L |
| Albumin Globulin |
20 g/L 53 g/L |
| Serum creatinine Blood urea |
230 μmol/L (eGFR-25 mL/min) 11.1 mmol/L |
eGFR, estimated glomerular filtration rate.
Blood investigations revealed leucocytosis and elevated C reactive protein which could be due to severe infection or acute distress following cardiac arrest and resuscitation. The patient had 4% promyelocytes which was unusual. A left-shifted myeloid series with immature promyelocytes and metamyelocyte has been described with severe COVID-19 infection. However, in this case a full blood picture has not been performed. Acute kidney injury also is commonly reported with severe COVID-19 infection.
Differential diagnosis
In the context of the present coronavirus pandemic, his presentation was strongly suggestive of COVID-19 infection. However, the other infective aetiologies like viral and atypical pneumonias and non-infective causes such as pulmonary embolism, acute myocarditis and silent myocardial infarction leading to heart failure were possible explanations in a young patient presenting with cardiac arrest following a period of worsening shortness of breath. Clinically there were no evidence of deep vein thrombosis or peripheral oedema making the non-infective causes less likely.
Outcome and follow-up
Autopsy
A postmortem examination was requested by the HM Coroner to establish the cause of death. Due to administrative delays, there was a delay of 27 days. During this time, the body had remained in secure refrigeration at 4°C–6°C.
The history was sufficiently concerning to consider the nose/throat swab result to have been a ‘false negative’ result with regard to COVID-19 infection and it was agreed that we would undertake a limited chest incision in order to take a deep swab of lung tissue for a further COVID-19 test and, at the same time, obtain small samples of tissue from both lungs for histopathological examination.
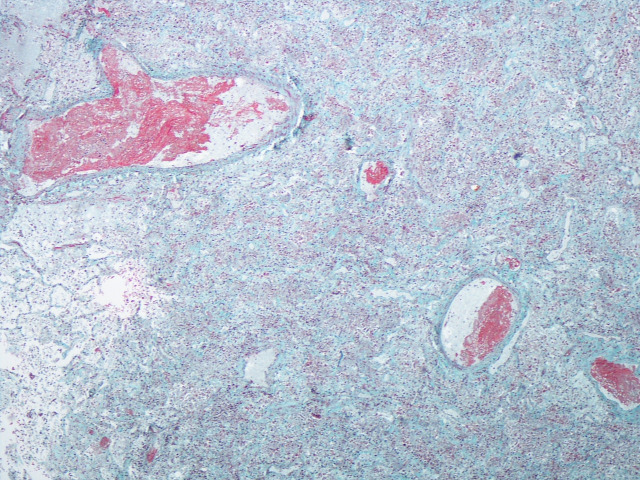
This second (lung tissue) swab detected the presence of SARS-CoV-2. Histological examination of the lung tissue showed features in both lungs consistent with severe diffuse alveolar damage together with intravascular microthrombi (figure 1). In the context of the current pandemic and the clinical history, the findings are consistent with the effects of COVID-19 infection. An external examination of the body was undertaken but a full internal examination was not performed as there was a clear cause of death.
Figure 1.
Histological section showing microthrombi in blood vessels and alveolar spaces filled with cellular debris. The features are compatible with diffuse alveolar damage although hyaline membranes are not yet formed. (Carstairs connective tissue stain) ×4 magnification.
There was clear histological evidence of severe acute diffuse alveolar damage in the lungs. In the present circumstances of the COVID-19 pandemic, in the absence of any other cause for diffuse alveolar damage, with a fairly typical clinical history and, finally, with a positive COVID-19 swab, we considered COVID-19-related lung injury as the most likely cause of his current presentation.
A full postmortem would have been unlikely to have revealed another significant cause for diffuse alveolar damage. Importantly, if the patient had developed acute left ventricular failure, for example from ischaemic heart disease and/or myocardial infarction or myocarditis, the lungs would have shown pulmonary oedema and vascular congestion, not diffuse alveolar damage.
Discussion
This case of an out-of-hospital cardiorespiratory arrest with high suspicion of COVID-19 highlights two important points.
First, it questions the validity and reliability of an initial negative nasopharyngeal swab in the diagnostic algorithm. The nose and throat swab may not in fact be a false negative result but, instead, it could be a ‘true negative’ result. This would imply that the virus has moved away from the upper respiratory tract mucosal surface and has gained access to lung parenchymal tissue where it is able to cause serious life-threatening damage. In turn, this brings into question the robustness underlying the COVID-19 testing strategy and the possible unfounded reassurance that will accompany a negative test result and the need for lower respiratory tract samples in cases of pneumonia. It also highlights the need for patients with ‘typical’ COVID-19 symptoms to seek assistance earlier in the course of their disease for closer medical surveillance and establishing a diagnosis.
Second, this case demonstrates that SARS-CoV-2 RNA is still detectable in lung tissue at least 4 weeks (in this example, 27 days) after death due to the virus. Survival of the virus on environmental surfaces has been the subject of much debate and has been shown to be dependent on the surface type. An experimental study using a SARS-CoV-2 strain reported viability on plastic for up to 72 hours, for 48 hours on stainless steel and up to 8 hours on copper and the virus has also been shown to remain viable and infectious in aerosols for hours.11 It is also likely that the virus persists and remains viable in deceased bodies hence the need to wear appropriate personal protective equipment when handling bodies of deceased persons and during postmortem examinations. However, presence of viral RNA does not always equate to viral viability or infectivity which could only be determined by viral culture.
At the time of presentation of this patient, antibody test was not available. The value of the antibody test in a patient presenting on day 10 of illness is not well established. IgG and IgM antibodies directed against SARS-CoV-2 can take 3 days to 3 weeks to appear. Therefore, variable sensitivity and specificity is seen within the first 14 days of infection. Currently serological studies are used for diagnosis of prior infection. However, a positive serology test can support the diagnosis of COVID-19 in a patient with a high clinical suspicion but negative PCR tests.15
We believe that this is the first time that the virus has been shown to be detectable in lung tissue 27 days after death.
Learning points.
In patients with high pre-test probability, a negative test should ideally be followed with a repeat specimen obtained from the lower respiratory tract. Due to the potential risk of obtaining such a sample by invasive methods (eg, endotracheal aspirate, bronchoscopy), we need further studies to establish the sensitivity of using safe non-invasive self-guided methods of collection of lower respiratory tract specimens.
The diagnostic protocol to be followed in an out-of-hospital patients who had cardiac arrest with suspected COVID-19 needs to include a limited autopsy with lung swabs to improve the diagnostic yield.
The confirmation of viral persistence of SARS-CoV-2 in human tissue for a prolonged period may have a significant effect on the handling of laboratory specimens as well as the disposal of the dead body and these protocols need to be reviewed to reflect this finding.
Acknowledgments
We thank Mr Lee Wood, coroner officer for the assistance provided. We thank the wife and the daughter of the patient for the cooperation at this difficult time.
Footnotes
Contributors: PS, CIK and PR equally contributed to the conception and design of the article; contributed to the design of the case report; contributed to the acquisition and analysis of the data; contributed to the interpretation of the data; and drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Next of kin consent obtained.
References
- 1.Coronavirus Update (Live): 27,761,512 Cases and 902,306 Deaths from COVID-19 Virus Pandemic - Worldometer. Available: https://www.worldometers.info/coronavirus/ [Accessed 9 Sep 2020].
- 2.Tang Y-W, Schmitz JE, Persing DH, et al. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol 2020;58. doi: 10.1128/JCM.00512-20. [Epub ahead of print: 26 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bullis SSM, Crothers JW, Wayne S, et al. A cautionary tale of false-negative nasopharyngeal COVID-19 testing. IDCases 2020;20:e00791. 10.1016/j.idcr.2020.e00791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson J, Whiting PF, Brush JE. Interpreting a covid-19 test result. BMJ 2020;369:m1808. 10.1136/bmj.m1808 [DOI] [PubMed] [Google Scholar]
- 5.Kojima N, Turner F, Slepnev V, et al. Self-Collected oral fluid and nasal swabs demonstrate comparable sensitivity to clinician collected nasopharyngeal swabs for Covid-19 detection. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020;323:1843–4. 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020;382:1177–9. 10.1056/NEJMc2001737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mawaddah A, Gendeh HS, Lum SG, et al. Upper respiratory tract sampling in COVID-19. Malays J Pathol 2020;42:23–35. [PubMed] [Google Scholar]
- 9.Zhang W, Du R-H, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect 2020;9:386–9. 10.1080/22221751.2020.1729071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanley B, Lucas SB, Youd E, et al. Autopsy in suspected COVID-19 cases. J Clin Pathol 2020;73:239–42. 10.1136/jclinpath-2020-206522 [DOI] [PubMed] [Google Scholar]
- 11.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020;382:1564–7. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC . Coronavirus disease 2019 (COVID-19), 2020. Available: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-postmortem-specimens.html [Accessed 16 May 2020].
- 13.Taubenberger JK, Reid AH, Krafft AE, et al. Initial genetic characterization of the 1918 "Spanish" influenza virus. Science 1997;275:1793–6. 10.1126/science.275.5307.1793 [DOI] [PubMed] [Google Scholar]
- 14.Taubenberger JK. The origin and virulence of the 1918 "Spanish" influenza virus. Proc Am Philos Soc 2006;150:86–112. [PMC free article] [PubMed] [Google Scholar]
- 15.Fang FC, Naccache SN, Greninger AL. The Laboratory Diagnosis of COVID-19-- Frequently-Asked Questions. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa742. [Epub ahead of print: 8 Jun 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]