Abstract
Since December 2019, coronavirus disease 2019 (COVID-19) has been an international public health emergency. The possibility of COVID-19 should be considered primarily in patients with new-onset fever or respiratory tract symptoms. However, these symptoms can occur with other viral respiratory illnesses. We reported a case of severe acute respiratory syndrome coronavirus 2 and influenza A virus coinfection. During the epidemic, the possibility of COVID-19 should be considered regardless of positive findings for other pathogens.
Keywords: infectious diseases, respiratory medicine
Background
In late 2019, a novel coronavirus, known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified as the cause of an outbreak of acute respiratory illness in Wuhan, China. Since then, there has been a rapid spread of the virus, leading to a global pandemic of coronavirus disease 2019 (COVID-19). We report a case of coinfection with SARS-CoV-2 and influenza A virus in a patient with pneumonia in Japan. The patient with both COVID-19 and influenza virus infection presented similar clinical characteristics with COVID-19 only. During the epidemic, the possibility of COVID-19 should be considered regardless of positive findings for other pathogens.
Case presentation
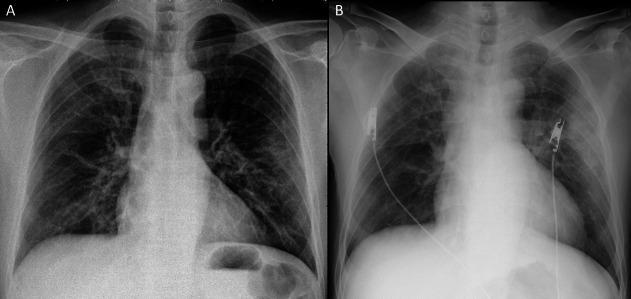
In April 2020, a 57-year-old Japanese man was admitted to our hospital with an 8-day history of fever and non-productive cough. Six days before admission, the patient presented first to general practitioners. The patient had a positive result for influenza A from a nasopharyngeal swab test. The patient was prescribed with laninamivir, but his symptoms did not improve. Two days before admission, the patient was referred to our hospital for a prolonged fever. Anteroposterior chest radiograph (figure 1A) found ground-glass opacities (GGO) in the left lung and he was treated with garenoxacin. Later, real-time reverse transcription-polymerase chain reaction (rRT-PCR) of his nasopharyngeal swab returned positive for SARS-CoV-2. His medical history included vasospastic angina and diabetes mellitus. His medications were sitagliptin and miglitol. He worked as customer service in a restaurant. There was no history of smoking, travel or any contact with sick people.
Figure 1.
Chest radiograph 2 days before admission (A) and on admission (B).
On admission, the patient reported of shortness of breath, anosmia and ageusia. Vital signs were as follows: temperature, 38.8°C; blood pressure 131/74, mm Hg; heart rate, 80 beats/min; respiratory rate, 20 breaths/min; and oxygen saturation, 91% on room air with desaturation on minimal exertion.
Investigations
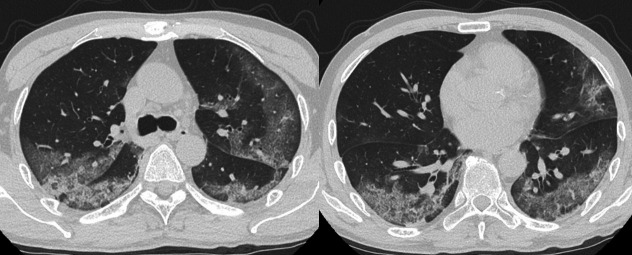
Complete blood count revealed white blood cells, 8.3×109/L (neutrophils, 86%; lymphocytes, 8%; and monocytes, 6%); haemoglobin 144 g/L; mean corpuscular volume, 96 fL; and platelets, 199×109/L. Serum laboratory test results were as follows: total protein, 6.5 g/dL; albumin, 3.1 g/dL; total bilirubin, 0.6 mg/dL; aspartate aminotransferase, 103 IU/L; alanine transaminase, 161 IU/L; g-glutamyl transferase, 114 IU/L; lactate dehydrogenase, 416 IU/L; blood urea nitrogen, 11 mg/dL; creatinine, 0.8 mg/dL; sodium, 136 mmol/L; potassium, 4.6 mmol/L; chloride, 97 mmol/L; glucose, 231 mg/dL; haemoglobin A1c, 7.2%; ferritin, 1695 ng/mL; C-reactive protein, 15.7 mg/dL; and procalcitonin,<0.1 ng/mL. Hepatitis B surface antigen and anti-hepatitis C virus were negative. An ECG was normal. Anteroposterior chest radiography (figure 1B) revealed consolidation in the left lung. Further evaluation with CT scanning of the chest (figure 2) found peripheral, bilateral, GGO with consolidation and visible intralobular lines (crazy-paving appearance). Sputum cultures for bacteria, fungus and acid-fast bacilli were all negative.
Figure 2.
Chest CT on admission.
Differential diagnosis
Initial considerations for this patient who presented acutely with fever and cough include infection with a common virus (rhinoviruses, non-SARS-CoV-2 coronaviruses and influenza virus) and community-acquired pneumonia. The rapid antigen testing and his clinical course make influenza pneumonia likely. Owing to the SARS-CoV-2 outbreak in Japan, COVID-19 also needs to be considered.
Treatment
Ciclesonide inhaler (200 μg two times per day for 14 days), favipiravir (1800 mg two times per day at day 1, followed by 800 mg two times per day for a total duration of 14 days) and broad-spectrum antibiotics (meropenem and azithromycin) were initiated.
Outcome and follow-up
Soon after hospitalisation, the patient developed progressive hypoxaemia (oxygen saturation of 91% on 2 L/min via nasal cannula). The fever continued for the first 4 days of hospitalisation, but subsequently abated. At day 7 of hospitalisation, the patient’s dyspnoea and hypoxaemia improved. According to discharge criteria for confirmed COVID-19 cases in Japan (2 negative rRT-PCR tests from nasopharyngeal swabs at 24 hours interval and clinical improvement of signs and symptoms), he was discharged home 4 weeks after hospitalisation. Fortunately, because of the small number of patients with COVID-19 in our area, the patient was placed in a single-patient, negative-pressure room. All healthcare workers who enter the room of the patient wore personal protective equipment.
Discussion
Coinfection with SARS-CoV-2 and other respiratory viruses has been described, but the reported frequency is variable. Among 5700 patients hospitalised with COVID-19 in New York City, 42 patients (2.1%) had respiratory virus coinfection with enterovirus/rhinovirus (22/42), other coronavirus (7/42), respiratory syncytial virus (4/42), parainfluenza 3 (3/42), human metapneumovirus (2/42) and influenza A virus (1/42).1 In Northern California, the coinfection rate between SARS-CoV-2 and other respiratory pathogens was 20.7%.2 The coinfections were enterovirus/rhinovirus (6.9%), respiratory syncytial virus (5.2%), other Coronaviridae (4.3%), human metapneumovirus (1.7%) and influenza A virus (0.9%). There was no significant difference in the rates of SARS-CoV-2 infection in patients with and without other pathogens. The presence of a non-SARS-CoV-2 pathogen may not provide reassurance that a patient does not also have SARS-CoV-2. In Wuhan, China, 5 of 115 patients (4.35%) confirmed with COVID-19 were diagnosed with influenza virus infection, with 3 cases being of influenza A and 2 cases of influenza B.3 The clinical characteristics of patients with both COVID-19 and influenza virus infection were similar to those of COVID-19 cases.
Now, the National Institutes of Health COVID-19 treatment guidelines recommend remdesivir for hospitalised patients with severe COVID-19.4 However, at that time, no drug had been proven to be safe and effective for treating COVID-19. Therefore, with the consent of the patient, off-label use of ciclesonide and favipiravir was administered. In vitro studies have demonstrated that ciclesonide has good antiviral activity against SARS-CoV-2.5 Although the clinical effectiveness of ciclesonide in the treatment of COVID-19 was reported in some cases,6 there is no clinical trial to evaluate its antiviral activity in patients with COVID-19. Favipiravir is an RNA polymerase inhibitor that is available in some Asian countries for the treatment of influenza. In an open-label non-randomised control study, favipiravir was compared with lopinavir/ritonavir for the treatment of non-severe COVID-19.7 A shorter viral clearance time was found for favipiravir than lopinavir/ritonavir (median, 4 vs 11 days, p<0.001). At day 14 after treatment, the improvement rates on chest CT with favipiravir were significantly higher than lopinavir/ritonavir (91.4% vs 62.2 %, p=0.004). These data support further investigation with randomised clinical trials on the efficacy of favipiravir for the treatment of COVID-19.
Learning points.
There was no significant difference in rates of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with and without other pathogens.
SARS-CoV-2 mimics the clinical characteristics of the influenza virus.
During the epidemic, the possibility of COVID-19 should be considered regardless of positive findings for other pathogens.
Footnotes
Contributors: YK and SM wrote the initial manuscript and edited it. RY and TI supervised and edited the manuscript to its completion. All authors read and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA 2020. doi: 10.1001/jama.2020.6775. [Epub ahead of print: 22 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim D, Quinn J, Pinsky B, et al. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA 2020. doi: 10.1001/jama.2020.6266. [Epub ahead of print: 15 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding Q, Lu P, Fan Y, et al. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol 2020;382. doi: 10.1002/jmv.25781. [Epub ahead of print: 20 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institutes of health (NIH). coronavirus disease 2019 (COVID-19) treatment guidelines. NIH 2020. [PubMed] [Google Scholar]
- 5.Jeon S, Ko M, Lee J, et al. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents Chemother 2020;64. doi: 10.1128/AAC.00819-20. [Epub ahead of print: 04 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwabuchi K, Yoshie K, Kurakami Y, et al. Therapeutic potential of ciclesonide inahalation for COVID-19 pneumonia: report of three cases. J Infect Chemother 2020;26:625–32. 10.1016/j.jiac.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Q, Yang M, Liu D, et al. Experimental treatment with Favipiravir for COVID-19: an open-label control study. Engineering 2020. doi: 10.1016/j.eng.2020.03.007. [Epub ahead of print: 18 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]