Abstract
The SARS-CoV-2 has wreaked havoc globally and has claimed innumerable lives all over the world. The symptoms of this disease may range from mild influenza-like symptoms to severe acute respiratory distress syndrome with high morbidity and mortality. With improved diagnostic techniques and better disease understanding, an increased number of cases are being reported with extrapulmonary manifestations of this disease ranging from renal and gastrointestinal to cardiac, hepatic, neurological and haematological dysfunction. Subacute thyroiditis is a self-limiting and painful thyroid gland inflammation most often secondary to viral infections. We report a case of subacute thyroiditis in a 58-year-old gentleman presenting with a painful swelling in the neck who was subsequently detected to be positive for SARS-CoV-2. We seek to highlight the broad clinical spectrum of the COVID-19 by reporting probably the first case of subacute thyroiditis possibly induced by SARS-CoV-2 infection from India.
Keywords: thyroid disease, infectious diseases, thyroiditis
Background
The SARS-CoV-2 which emerged from Wuhan city, China, has been declared a pandemic by the WHO and has claimed innumerable lives till date. Majority of the cases present with respiratory symptoms, however, extrapulmonary manifestations of the COVID-19 are being increasingly reported, with possible acute kidney injury, pulmonary embolism, thromboembolic stroke, acute coronary disease, myocardial dysfunction, hepatocellular injury, neurological illnesses and dermatological manifestations as documented complications.1 2 Here, we report a case of a middle-aged gentleman who presented with subacute thyroiditis following COVID-19.
Case presentation
An immunocompetent 58-year-old gentleman, without any history of recent travel or contact with known or suspected SARS-CoV-2-positive patient, presented with pain in his throat accompanied by a low-grade fever for 2 days. He did not complain of anosmia, dysgeusia, rashes or any upper or lower respiratory symptoms. He was non-hypertensive and euthyroid, but diabetic for last 10 years on regular oral antihyperglycaemic agents. He did not have any significant family history of thyroid disease. As per his vaccination records, the patient was fully immunised against mumps, measles and rubella. On examination, he was febrile (temperature—99.6°F) with a pulse rate of 104 beats/min. His blood pressure was 124/78 mm Hg, respiratory rate 18/min, a random capillary blood glucose 117 mg/dL and oxygen saturation 99% on room air. A tender swelling (4 cm×5 cm) with a normal surface temperature and firm consistency was noted in the lower part of the front of his neck, with well-defined margins and normal overlying skin, clinically resembling a diffusely enlarged thyroid gland. The swelling displaced vertically on deglutition, but no movement could be observed on tongue protrusion. No signs of compression could be elicited. No lymph nodes were palpable. Other general and systemic examinations were unremarkable.
Over the next 2 days, the patient developed a high-grade intermittent fever (temperature—101.3°F) with tachycardia (pulse rate—116 bpm), but without any appreciable palpitations, excessive sweating or intensified physiological tremor. Apart from neck pain and increased frequency of stools, he did not have any complaints.
Investigations
Thyroid function tests showed decreased levels of thyroid stimulating hormone (TSH) with elevated serum T3 and T4. The salient laboratory investigations have been summarised in table 1.
Table 1.
Salient laboratory investigations
| Tests | Results | Normal range |
| Haemoglobin | 126 | 120–160 g/L |
| WBC | 8.85×109 | 4–11×109/L |
| Neutrophils | 73 | 40%–75% |
| Lymphocytes | 20 | 20%–40% |
| Platelet count | 276 | 150–450×109/L |
| ESR 1st hour | 110 | <30 mm |
| CRP | 16.6 | <10 mg/L |
| LDH | 224 | <248 U/L |
| Creatinine | 71 | 59–104 umol/L |
| ALT | 25 | 5–35 IU/L |
| AST | 27 | 5–35 IU/L |
| ALP | 193 | 110–310 IU/L |
| Fasting blood sugar | 102 | 75–110 mg/dL |
| TSH | <0.005 | 0.27–4.2 mIU/L |
| Serum T3 | 2.88 | 0.80–2.0 ng/mL |
| Serum T4 | 20.11 | 5.10–14.1 µg/dL |
ALP, Alkaline Phosphatase; ALT, Alanine transaminase; AST, Aspartate transaminase; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; TSH, thyroid stimulating hormone; WBC, white blood cells.
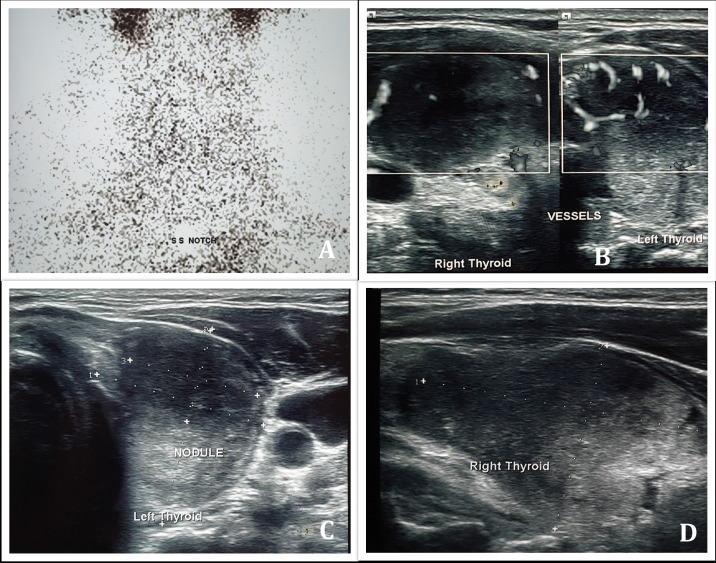
Radionuclide thyroid scan with technetium-99m showed poor and patchy radiotracer uptake, which rendered visualisation of the thyroid gland difficult, with high circulating and background radioactivities (figure 1A). These findings along with high erythrocyte sedimentation rate (ESR) were strongly indicative of subacute (deQuervain’s) thyroiditis. Ultrasonography (USG) of the thyroid gland revealed diffuse bilateral enlargement of thyroid with hypoechogenicity and increased vascularity on colour Doppler (figure 1B) and a solitary nodule in each lobe (figure 1C,D). Reverse transcriptase real-time qualitative polymerase chain reaction (RT-PCR) for SARS-CoV-2 from oropharyngeal and nasopharyngeal swabs turned out to be positive, thus, establishing a diagnosis of subacute thyroiditis possibly induced by COVID-19. Subsequent samples of nasopharyngeal and oropharyngeal swabs for other respiratory viruses such as respiratory syncytial viruses A and B, influenza A and B, parainfluenza viruses, metapneumovirus, rhinoviruses, adenoviruses and coxsackie viruses turned out to be negative. IgM against Epstein-Barr virus was negative.
Figure 1.
Radionuclide thyroid scan using 99m Tc04− showing poor and patchy uptake of radiotracer in the thyroid gland with high circulating and background radioactivities (A). Colour doppler showing increased vascularity of the thyroid gland (B). Ultrasonography of thyroid showing diffuse enlargement of the thyroid gland with hypo-echogenicity and a solitary nodule in each lobe (14×17 mm nodule in right and 19×14×26 mm nodule in left) (C,D).
Differential diagnosis
From the history, clinical examination and thyroid function tests; the possible causes of thyrotoxicosis presenting with a painful goitre were evaluated. Therefore, a differential of Grave’s disease, solitary toxic adenoma, toxic nodular goitre (Plummer’s disease), Hashimoto’s thyroiditis, deQuervain’s subacute thyroiditis and acute suppurative thyroiditis was considered. Antithyroid peroxidase and anti-TSH-R antibodies as well as blood cultures were negative, effectively excluding Grave’s disease, autoimmune thyroiditis and acute suppurative thyroiditis. The findings on USG, radionuclide thyroid scan along with high ESR were strongly indicative of subacute thyroiditis. Positive RT-PCR for SARS-CoV-2 along with negative screening for other respiratory viruses established the diagnosis of subacute thyroiditis possibly induced by SARS-CoV-2 infection.
Treatment
The patient was treated with a combination of analgesics, favipiravir and azithromycin along with zinc tablets and vitamin C capsules. He was given oral prednisolone at the dose of 30 mg/day which was gradually tapered over next 1 month and then stopped. Propranolol was also added at a dosage of 40 mg/day.
Outcome and follow-up
He had an initial uneventful recovery but has become hypothyroid in due course (TSH 21.290 mIU/L; T4 4.85 μg/dL; T3 0.73 ng/mL at 1 month after discharge). He has been advised to take oral levothyroxine supplementation (50 μg/day) with periodic monitoring of his thyroid profile.
Discussion
Subacute thyroiditis, though a relatively rare disease, is the most common cause of painful thyroiditis.3 The natural course of this disease usually involves an initial thyrotoxic phase followed by a hypothyroid phase with subsequent recovery to a euthyroid state. However, 30% patient may become hypothyroid post recovery from subacute thyroiditis.4 Although the exact pathogenesis is unknown, various viral aetiologies such as mumps, measles, coxsackie virus, adenovirus, influenza A and B as well as several other respiratory viruses have been implicated.5 Our patient presented with fever and a painful swelling in the neck, which on radionuclide scan revealed poor and patchy radiotracer uptake compatible with the diagnosis of subacute thyroiditis. The concomitant finding of high ESR further strengthened the diagnosis. Branchatella et al reported the first case of subacute thyroiditis secondary to COVID-19 worldwide.6 7 The majority of cases described in the recent literature have been post-COVID painful symptomatic subacute thyroiditis presenting from days to up to 5 weeks after diagnosis of COVID-19.8 Asymptomatic (non-painful) thyroid dysfunction has also been described in COVID-19 patients admitted to intensive care unit with severe disease.9 Mattar et al reported the first such case in an Asian population.10
SARS-CoV-2 has been reported to act through ACE 2 host cell receptor.11 Small intestine, testis, thyroid, kidney and heart tissues show higher ACE 2 expression compared with the lungs. Exaggerated innate and adaptive immune responses to COVID-19 may also induce a cytokine storm leading to immunopathological damage of tissues, thus suggesting possible mechanisms behind development of extrapulmonary manifestations of COVID-19.12 However, the presence of ACE 2 receptors in the thyroid and evidence of its expression in thyroid tissue do raise the possibility of thyroiditis arising from direct SARS-Cov2 infection of the thyroid.13 A substantial number of patients with SARS had alteration of follicular architecture of the thyroid gland.14
Till date, a handful of cases have emerged illustrating a possible association between SARS-CoV-2 infection and development of subacute thyroiditis either during or in the weeks following said infection. We add to the existing literature probably the first case of subacute thyroiditis as a complication of COVID-19 from India, to generate awareness among physicians and encourage prospective studies to further understand the mechanisms behind extrapulmonary manifestations of COVID-19.
Patient’s perspective.
I was very scared when I had fever and throat pain amidst this pandemic. But the reassurance and care I received from my doctors when I tested positive for COVID-19 were beyond my expectations. God bless them.
Learning points.
As the number of cases of COVID-19 are increasing worldwide, physicians should be cognizant with the less prevalent and extrapulmonary manifestations of this disease.
We emphasise the importance of considering the possible endocrinological manifestations of SARS-COV-2 infection.
Proposals for the exact mechanisms of subacute thyroiditis in COVID-19 are not fully understood and needs further research.
Subacute thyroiditis may present regardless of the severity of COVID-19, and therefore high index of suspicion is needed for its early diagnosis and prompt management.
Footnotes
Contributors: SG contributed to conception, initial drafting of manuscript, critical revision of content and final approval of manuscript. UC, AKR and AC contributed to patient management, conception, critical revision of content and final approval of manuscript. All authors are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Zheng KI, Feng G, Liu W-Y, et al. Extrapulmonary complications of COVID-19: a multisystem disease? J Med Virol 2020. doi: 10.1002/jmv.26294. [Epub ahead of print: 10 Jul 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017–32. 10.1038/s41591-020-0968-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fatourechi V, Aniszewski JP, Fatourechi GZE, et al. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab 2003;88:2100–5. 10.1210/jc.2002-021799 [DOI] [PubMed] [Google Scholar]
- 4.Alfadda AA, Sallam RM, Elawad GE, et al. Subacute thyroiditis: clinical presentation and long term outcome. Int J Endocrinol 2014;2014:794943. 10.1155/2014/794943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J 2009;6:5. 10.1186/1743-422X-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brancatella A, Ricci D, Viola N, et al. Subacute thyroiditis after Sars-COV-2 infection. J Clin Endocrinol Metab 2020;105:dgaa276:2367–70. 10.1210/clinem/dgaa276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brancatella A, Ricci D, Cappellani D, et al. Is subacute thyroiditis an underestimated manifestation of SARS-CoV-2 infection? Insights from a case series. J Clin Endocrinol Metab 2020;105:dgaa537. 10.1210/clinem/dgaa537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruggeri RM, Campennì A, Siracusa M, et al. Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones 2020;6. 10.1007/s42000-020-00230-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller I, Cannavaro D, Dazzi D, et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol 2020;8:739–41. 10.1016/S2213-8587(20)30266-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattar SAM, Koh SJQ, Rama Chandran S, et al. Subacute thyroiditis associated with COVID-19. BMJ Case Rep 2020;13:e237336. 10.1136/bcr-2020-237336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M-Y, Li L, Zhang Y, Wang XS, et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020;9:45. 10.1186/s40249-020-00662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol 2020;92:424–32. 10.1002/jmv.25685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rotondi M, Coperchini F, Ricci G, et al. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J Endocrinol Invest 2020;395. doi: 10.1007/s40618-020-01436-w. [Epub ahead of print: 06 Oct 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei L, Sun S, Xu C-H, et al. Pathology of the thyroid in severe acute respiratory syndrome. Hum Pathol 2007;38:95–102. 10.1016/j.humpath.2006.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]