Abstract
Human neutrophils contain two structurally distinct types of antimicrobial peptides, β-sheet defensins (HNP-1 to HNP-4) and the α-helical peptide LL-37. We used radial diffusion assays and an improved National Committee for Clinical Laboratory Standards-type broth microdilution assay to compare the antimicrobial properties of LL-37, HNP-1, and protegrin (PG-1). Although generally less potent than PG-1, LL-37 showed considerable activity (MIC, <10 μg/ml) against Pseudomonas aeruginosa, Salmonella typhimurium, Escherichia coli, Listeria monocytogenes, Staphylococcus epidermidis, Staphylococcus aureus, and vancomycin-resistant enterococci, even in media that contained 100 mM NaCl. Certain organisms (methicillin-resistant S. aureus, Proteus mirabilis, and Candida albicans) were resistant to LL-37 in media that contained 100 mM NaCl but were susceptible in low-salt media. Burkholderia cepacia was resistant to LL-37, PG-1, and HNP-1 in low- or high-salt media. LL-37 caused outer and inner membrane permeabilization of E. coli ML-35p. Chromogenic Limulus assays revealed that LL-37 bound to E. coli O111:B4 lipopolysaccharide (LPS) with a high affinity and that this binding showed positive cooperativity (Hill coefficient = 2.02). Circular dichroism spectrometry disclosed that LL-37 underwent conformational change in the presence of lipid A, transitioning from a random coil to an α-helical structure. The broad-spectrum antimicrobial properties of LL-37, its presence in neutrophils, and its inducibility in keratinocytes all suggest that this peptide and its precursor (hCAP-18) may protect skin and other tissues from bacterial intrusions and LPS-induced toxicity. The potent activity of LL-37 against P. aeruginosa, including mucoid and antibiotic-resistant strains, suggests that it or related molecules might have utility as topical bronchopulmonary microbicides in cystic fibrosis.
The ability of neutrophils to ingest and kill bacteria and fungi is an important component of innate immunity. The microbicidal prowess of human neutrophils emanates from oxidative and nonoxidative mechanisms. The former results from activation of an enzyme complex that oxidizes NADPH to produce copious amounts of superoxide (5), whose dismutation yields hydrogen peroxide, which can form stronger oxidants by reacting with myeloperoxidase (15).
The nonoxidative mechanisms of human neutrophils are mediated by antimicrobial peptides and proteins stored within its various cytoplasmic granules. Cathepsin G, azurocidin (also called CAP37), BPI (also called CAP57), and defensins are restricted to the primary (azurophil) granules, which also contain myeloperoxidase, elastase, and proteinase 3 (10, 29, 35). Lactoferrin and hCAP-18 (the precursor of LL-37) are restricted to the neutrophil’s secondary (specific) granules (40). Lysozyme, another antimicrobial molecule, occurs in both primary and secondary granules (10, 29). Whereas azurophil granule contents are delivered preferentially to intracellular phagolysosomes, the specific granule contents are largely secreted extracellularly.
The precursor of LL-37 is a 19.3-kDa prepropeptide (13) which, after losing its signal sequence, is called hCAP-18 (23). The cathelin domain of hCAP-18 (22, 36) places it within the cathelicidin family (51). Like other cathelicidins found in porcine, bovine, rabbit (51), and mouse (9), neutrophils, hCAP-18’s cathelin domain is highly conserved and precedes the domain that encodes an antimicrobial peptide. Human hCAP-18 is expressed constitutively within neutrophils (40) and the testes (1) and is inducibly expressed by keratinocytes (8).
We compared the antimicrobial properties of three peptides: human LL-37, human defensin HNP-1, and porcine protegrin-1 (PG-1) (21). Protegrins are stored within the granules of porcine neutrophils as cathelin-containing precursors, whose proteolytic processing by elastase releases the microbicidal protegrin domain (34, 52). Similar elastase-mediated processing was also shown for bovine cathelicidins (39). The primary structures of the precursors of LL-37 and PG-1 are shown in Fig. 1. Also shown in Fig. 1 is CAP-18, an extensively studied rabbit cathelicidin that encodes an α-helical antimicrobial peptide similar to LL-37. The structures of HNP-1 and other defensins have been described in recent reviews (10, 49).
FIG. 1.
Primary sequences of cathelicidins. The sequences of rabbit CAP-18, human hCAP-18 (the precursor of LL-37), and prepro-PG-1 are shown. Identical residues are connected by vertical lines, and similar residues are connected by dots.
MATERIALS AND METHODS
Peptides.
LL-37 was synthesized at a 0.25-mmol scale with a Perkin-Elmer ABI 431 A synthesizer with prederivatized polyethylene glycol polystyrene serine resin (PerSeptive Biosystems, Framingham, Mass.), FastMoc chemistry, and single coupling for all residues. After its purification by reversed-phase high-pressure liquid chromatography, the peptide appeared to be homogeneous by capillary zone electrophoresis and had a mass of 4,493.16 by electrospray-mass spectrometry (expected mass, 4,493.3). HNP-1 was purified from human neutrophils as described previously (16), and protegrin PG-1 was purchased from SynPep (Dublin, Calif.). Polymyxin B sulfate (lot 25F-0078; 8,156 U/mg) was purchased from Sigma, and its content of polymyxin B base (0.1404 mg/mg of powder) was determined by quantitative analysis of the threonine, leucine, and phenylalanine contents of the powder. We also obtained a similar value (0.1525 of mg polymyxin B base/mg of powder) for the polymyxin B base content when a different polymyxin B preparation (lot 83H1112; 7,800 U/mg) was analyzed in this manner.
Antimicrobial testing. (i) Radial diffusion assays.
The two-stage radial diffusion assay used in these studies has been fully described elsewhere (43). Briefly, the purified peptides were serially diluted in acidified water (0.01% acetic acid) that contained 0.1% human serum albumin (Sigma A-8763). The bacteria listed in Tables 1 and 2 were grown to the mid-logarithmic phase and washed. Approximately 2 × 105 CFU/ml was incorporated into a thin (1.23-mm) agarose underlay gel that contained 1% (wt/vol) agarose (Sigma A-6013), 10 mM sodium phosphate buffer (pH 7.4), and 0.3 mg of Trypticase soy broth powder per ml with or without 100 mM NaCl. A regularly spaced, five-by-five array of wells was made in the underlay gel. The wells, 3.2 mm in diameter, had a 10-μl capacity. Six serially diluted samples of each peptide ranging in concentration from 0.79 to 250 μg/ml were prepared, and 5-μl aliquots were added to the wells. After 3 h, a 10-ml overlay gel composed of 6% Trypticase soy broth powder, 1% agarose, and 10 mM sodium phosphate buffer (pH 7.4) was poured onto the plates, and the plates were incubated overnight to allow the surviving organisms to form microcolonies.
TABLE 1.
Activity against gram-positive bacteria and C. albicansa
| Organism | MIC (μg/ml) of the following compounds in the presence of NaCl at the indicated concn:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| LL-37
|
HNP-1
|
PG-1
|
Vancomycin
|
|||||
| 0 mM | 100 mM | 0 mM | 100 mM | 0 mM | 100 mM | 0 mM | 100 mM | |
| L. monocytogenes EGD | 1.5 | 2.2 | 39.7 | >79.1 | 0.8 | 0.7 | TUb | TU |
| S. epidermidis | 7.6 | 43.9 | 5.2 | >250 | 0.7 | 1.1 | 6.0 | 8.8 |
| S. aureus 930918-3 | 12.5 | 9.2 | 2.5 | 25.0 | 0.7 | 0.7 | 0.3 | 0.4 |
| S. aureus 67395 | 2.9 | 5.4 | 7.9 | >250 | 0.6 | 0.9 | 0.2 | 0.7 |
| S. aureus 68721 | 3.2 | 9.0 | 6.8 | >250 | 0.3 | 0.6 | 0.3 | 0.5 |
| S. aureus 502A | 3.6 | 7.2 | 2.2 | >250 | 0.7 | 0.6 | 0.3 | 0.5 |
| MRSA 1083 | 6.5 | >79.1 | 2.5 | >250 | 0.3 | 0.7 | 0.3 | 0.6 |
| MRSA 30371 | 10.5 | >79.1 | 25.0 | >250 | 0.5 | 0.7 | 0.4 | 0.8 |
| MRSA 54424-1 | 3.0 | 25.0 | 9.6 | >250 | 0.4 | 0.6 | 0.3 | 0.3 |
| MRSA ATCC 33591 | 3.4 | >79.1 | 21.2 | >250 | 0.5 | 0.7 | 0.9 | 1.2 |
| B. subtilis | 2.7 | 0.5 | 6.4 | 1.8 | 0.6 | 0.3 | 0.1 | 0.1 |
| VREFc 94.132 (E. faecium) | 0.7 | 0.6 | 2.7 | 1.0 | 0.2 | 0.5 | >79.1 | >79.1 |
| VREFd CDC 21 (E. faecalis) | 3.5 | 2.0 | 11.9 | >250 | 1.6 | 0.7 | 20.7 | 7.2 |
| C. albicans 820 | >250 | >250 | >250 | >250 | 0.4 | 2.9 | >250 | >250 |
Radial diffusion assays were performed with the standard low-salt underlays and with otherwise identical underlays that also contained 100 mM NaCl. MICs correspond to the x intercepts of plots such as those illustrated in Fig. 2. The molecular mass of vancomycin is 1,448. The masses of the peptides are as follows: LL-37, 4,493; HNP-1, 3,442; PG-1, 2,056.
TU, technically unsatisfactory due to the presence of complex zones of complete and partial clearing.
VREF, vancomycin-resistant E. faecium.
VREF, vancomycin-resistant E. faecalis.
TABLE 2.
Activity against gram-negative bacteriaa
| Organism | MIC (μg/ml) of the following compounds in the presence of NaCl at the indicated concn:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| LL-37
|
HNP-1
|
PG-1
|
Gentamicin
|
|||||
| 0 mM | 100 mM | 0 mM | 100 mM | 0 mM | 100 mM | 0 mM | 100 mM | |
| E. coli ML-35p | 0.6 | 7.6 | 0.7 | 18.4 | 0.5 | 0.7 | 0.02 | 0.08 |
| E. coli ATCC 9637 | 0.1 | 2.1 | 1.8 | 67.1 | 0.2 | 0.5 | >250 | >250 |
| E. coli ATCC 11775 | 1.9 | 2.7 | 2.2 | >250 | 0.2 | 0.6 | 0.03 | 0.09 |
| E. coli MCR 106 | 1.6 | 2.1 | 3.7 | >79.1 | 0.2 | 0.4 | 0.02 | 0.06 |
| S. typhimurium 7953s | 0.4 | 3.6 | 8.4 | >250 | 0.1 | 0.4 | 0.08 | 0.7 |
| S. typhimurium 14028s | 1.2 | 2.9 | >250 | >250 | 1.8 | 0.9 | 0.03 | 0.3 |
| P. aeruginosa MR 3007 | 4.7 | 3.8 | >250 | >250 | 0.8 | 0.7 | 65.6 | >79.1 |
| P. aeruginosa MR 53647-1 CF | 2.5 | 3.7 | >250 | >250 | 0.3 | 0.9 | 4.3 | 5.8 |
| P. aeruginosa AML 654 (mucoid) | 5.7 | 3.6 | >250 | >250 | 0.5 | 0.7 | 6.5 | 25.1 |
| P. aeruginosa CL79 | 1.4 | 0.9 | >250 | >250 | 0.3 | 0.4 | 0.07 | 0.7 |
| P. aeruginosa SBI-N | 1.3 | 4.0 | >250 | >250 | 0.5 | 0.7 | 0.15 | 0.2 |
| B. cepacia 96-11 | >79.1 | >250 | >250 | >250 | 17.0 | 53.9 | 19.4 | >79.1 |
| B. cepacia ATCC 25416 | >79.1 | >250 | >250 | >250 | 7.4 | 22.5 | 25.5 | >79.1 |
| S. maltophilia 411 A-15 | 1.9 | 5.1 | 4.3 | >250 | 0.2 | 0.4 | >79.1 | >250 |
| P. mirabilis ATCC 7002 | 5.7 | >25.0 | >250 | >250 | 0.6 | 1.4 | 0.12 | 0.7 |
| P. vulgaris ATCC 13315 | 2.5 | 5.4 | >79.1 | >250 | 0.2 | 0.6 | 0.07 | 0.08 |
Radial diffusion assays were performed with the standard low-salt underlays and with otherwise identical underlays that also contained 100 mM NaCl. MICs correspond to the x intercepts of plots such as those illustrated in Fig. 2. The molecular mass of gentamicin C1 is 477. The masses of the peptides are as follows: LL-37, 4,493; HNP-1, 3,442; PG-1, 2,056.
The resulting total zone diameters were measured to the nearest 0.1 mm and, after subtracting the diameter of the well, were expressed in units (1 unit = 0.1 mm). A linear relationship was obtained between the zone diameter and the log10 peptide concentration. The minimal effective concentration was determined by performing a least-mean-squares fit and solving for the x intercept with a Hewlett-Packard 20S Scientific Calculator (or equivalent).
(ii) Broth microdilution assays.
Broth microdilution assays were performed according to the guidelines of the National Committee for Clinical Laboratory Standards (NCCLS), except that our 10× stock solutions of the peptides and control antibiotics were prepared in sterile 0.01% acetic acid with 0.2% bovine serum albumin instead of in Mueller-Hinton broth (MHB), because standard MHB formed a precipitate with PG-1 and LL-37 (see below). Bacterial inocula were prepared according to NCCLS guidelines (33a) and were adjusted appropriately by spectrophotometry at 620 nm to provide 2 × 105 CFU ml−1 in the microplate wells. We tested two concentration series for each organism, such that the final antibiotic concentrations used for series 2 were 50% higher than those used for series 1.
We prepared two forms of MHB, designated “standard MHB” and “refined MHB,” in the study described in this report. Standard MHB (BBL 4311443; Becton Dickinson, Cockeysville, Md.) was prepared precisely according to the manufacturer’s instructions and contained 128 mM sodium, <2 mM potassium, 108 mM chloride, 0.95 mM phosphate, 0.40 mM calcium, and 0.15 mM magnesium (pH 7.3). Refined MHB was prepared by pumping 20 ml of standard MHB through a series of three Sep-Pak Plus NH2 anion-exchange filters (part no. 20535; Waters, Milford, Mass.). A flow rate of 1 ml per min was maintained with a Sage model 355 Syringe pump (Orion, Cambridge, Mass.). Refined MHB contained 138 mM sodium, <2 mM potassium, 112 mM chloride, 0.13 mM phosphate, 0.40 mM calcium, and 0.15 mM magnesium, as determined by the Clinical Pathology Laboratory, University of California, Los Angeles. Standard and refined MHB contained similar concentrations of aspartate (8.0 and 8.1 mM, respectively) and glutamate (11.9 and 11.2 mM, respectively), as determined by direct (i.e., without further sample hydrolysis) amino acid analysis. Refined MHB did not form a precipitate in the presence of 500 μg of PG-1 per ml, whereas standard MHB formed a copious precipitate under such conditions (data not shown) and also formed a precipitate with 128 μg of LL-37 per ml. Both standard and refined MHB supported luxuriant growth of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis.
(iii) Colony counting assays.
Mid-logarithmic-phase E. coli ML-35p, grown as described above for the radial diffusion assays, was washed twice in 10 mM sodium phosphate buffer (pH 7.4) and was used at a final concentration of 2 × 106 CFU/ml. These bacteria were exposed to a range of LL-37 concentrations in 100 μl of one of the following three media: (i) 10 mM sodium phosphate buffer (pH 7.4) containing 3 mg of Trypticase soy broth powder ml−1 with 100 mM sodium chloride, (ii) standard MHB, or (iii) refined MHB. After a 2-h incubation, 10-μl aliquots were removed, diluted 1:100 with the appropriate assay medium, and transferred to Trypticase soy agar plates with a SprialSpreader (SpiralTech, Rockville, Md.). Colonies were counted after incubation for 24 h at 37°C.
Membrane permeabilization.
To examine the ability of antimicrobial peptides to permeabilize the inner and outer membranes of gram-negative bacteria, we simplified a previously described procedure that uses E. coli ML-35p (27, 28). As originally described, the assay was performed in a single cuvette that contained two substrates, PADAC (a cephalosporin) and o-nitrophenyl-β-d-galactose (ONPG; a β-galactosidase substrate). The cuvette was monitored at multiple wavelengths, and the respective β-lactamase and β-galactosidase hydrolysis reactions were deconvoluted algebraically. In the present version, the assays were performed in 96-well microtiter plates (Corning, Corning, N.Y.) and were followed at a single wavelength (420 nm) that was sensitive to the hydrolysis both of ONPG and of PADAC. The reactions were monitored with a SpectraMax 250 Microplate Spectrophotometer (Molecular Devices, Sunnyvale, Calif.) with SOFTmaxPRO software supplied by the manufacturer. Both substrates were purchased from Calbiochem (La Jolla, Calif.).
Briefly, PADAC stock solutions (approximately 91 μM) were prepared in 10 mM sodium phosphate buffer (pH 7.4), and their concentrations were determined spectrophotometrically by using a molar extinction coefficient of 43,802 at 570 nm. The ONPG stock solution was also prepared in 10 mM buffer. The incubation wells contained sodium phosphate buffer (pH 7.4) with or without 100 mM NaCl, 1.5 × 106 CFU of washed, stationary-phase E. coli ML-35p, and either 2.5 mM ONPG or 20 μM PADAC. The reactions were started by adding the peptides of interest (either LL-37 or melittin) or an equivalent volume of acidified water (negative controls). The final incubation volumes were 150 μl. Assays were run at 37°C, with shaking performed for 10 s every minute.
LPS binding.
A quantitative chromogenic Limulus amoebocyte assay was performed with the reagents contained in a QCL-1000 kit (BioWhittaker, Walkersville, Md.). Incubations were performed in flat-bottom, nonpyrogenic 96-well tissue culture plates (catalog no. 3596; Costar, Cambridge, Mass.). Stock solutions of LL-37 and polymyxin B (7,600 U/mg; Sigma) were prepared in endotoxin-free acidified water (0.01% acetic acid) at 40 μg/ml and were serially diluted in this solution. In step 1, 25 μl of the peptide solution and 25 μl of an E. coli O111:B4 lipopolysaccharide (LPS), containing 1 endotoxin U/ml, were mixed in a well, and the plate was incubated for 30 min at 37°C to permit peptide and LPS binding to occur. In step 2, 50 μl of the amoebocyte lysate reagent was added. In step 3, performed exactly 10 min later, 100 μl of chromogenic substrate (acetyl–Ile–Glu–Ala–Arg–p-nitroanilide) was introduced. Thereafter, the incubation was continued at 37°C for 20 min, while the liberation of p-nitroaniline was monitored every 60 s at 405 nm with a SpectraMax 250 Kinetic Microplate Spectrophotometer (Molecular Devices). The change in optical density (ΔOD) between 10 and 16 min was calculated for the control sample (which contained peptide but no LPS), and this value was subtracted from the ΔOD between 10 and 16 min for the experimental samples, which contained peptide plus LPS. The difference provided an index of LPS-mediated Limulus procoagulant activation during step 1 of the incubation. Since the assays were done kinetically, we could also monitor spontaneous procoagulant activation to ensure that LL-37 and melittin did not inhibit the procoagulant enzymes directly (neither did).
Hill plots were performed by graphing log10 peptide (LL-37) or lipopeptide (polymyxin B) concentration against log10 [(FI)/(1.0 − FI)], where FI was the fractional inhibition of procoagulant activity observed in the chromogenic assay. FI equaled the percent inhibition divided by 100. Thus, an FI of 0.75 corresponded to 75% inhibition of procoagulant activity.
CD spectroscopy.
Circular dichroism (CD) measurements were performed with an AVIV 62DS spectropolarimeter (AVIV Associates, Lakewood, N.J.) that was routinely calibrated with (+)-10-camphorsulfonic acid (1 mg/ml) in a 1-mm-path-length cell (19). The sample compartment was fitted with a customized cell holder to position the sample cuvette near the photomultiplier tube, thereby minimizing light-scattering artifacts induced by peptide-liposome dispersions (48). Peptide-solvent solutions were measured in 0.1- to 0.5-mm-light-path demountable cells that were scanned from 260 to 190 nm at a rate of 10 nm/min, with 0.2-nm intervals. The results were expressed as the mean residue ellipticity (MRE), [θ]MRE (degree centimeter2 decimoles−1), and the percentage of α-helical conformation was estimated by the following equation (4): % α-helix = [θ]MRE222/{−39,500 [1 − (2.57/n)]} deg cm2 dmol−1.
CD spectral deconvolution was performed with the NCOMP program (2), based on polylysine reference spectra (11). Peptide sample concentrations were determined by quantitative amino acid analysis, performed at the Protein Microsequencing Facility, University of California, Los Angeles.
Preparation of liposomes.
Palmitoyl-oleoyl-phosphatidylglycerol (POPG) was purchased from Avanti Polar Lipids (Alabaster, Ala.). The large unilamellar vesicles used in liposome lysis and CD measurements were formed by freezing-thawing followed by extrusion through an extrusion device (Lipex, Ottawa, Ontario, Canada), as described previously (17, 48). Briefly, liposomes were prepared by hydrating 2.5 mg of lipid with 1 ml of 10 mM phosphate buffer (pH 7.5), followed by seven freeze-thaw cycles and five extrusions through 100-nm-pore-size polycarbonate membranes. Diphosphoryl lipid A, prepared from E. coli F-583 (an Rd mutant), was purchased from Sigma, and dispersions were prepared as described by Chen et al. (3).
Secondary structural predictions.
Neural network-based secondary structural predictions for the helical peptides were carried out on-line (http://www.embl-heidelberg.de) with the PHD Predict Protein program (37). Hydrophobic moments were calculated with the MOMENT computer program (6). An 11-residue amino acid sequence window, corresponding to the length of an average helical segment in a protein, was used to identify amphipathic helical motifs.
RESULTS
Antimicrobial activity.
We tested LL-37, HNP-1, and PG-1 against a group of gram-positive bacteria, including Listeria monocytogenes, Staphylococcus epidermidis, S. aureus (four strains), methicillin-resistant S. aureus (MRSA; four strains), B. subtilis, and vancomycin-resistant strains of Enterococcus faecalis and Enterococcus faecium (Table 1). HNP-1 was broadly effective only when assayed in underlay gels that contained 10 mM phosphate buffer. When the underlay gels were supplemented with 100 mM NaCl, HNP-1 was active only against B. subtilis and vancomycin-resistant E. faecium. LL-37 was generally at least as potent as HNP-1 in low-salt underlays, and except against MRSA, it retained its activity in underlay gels containing 100 mM NaCl. PG-1 worked well against the entire panel under both the low- and high-salt conditions. Table 1 also indicates the sensitivities of these organisms to vancomycin, a conventional glycopeptide antibiotic that was used as a control. These data also show that only PG-1 killed Candida albicans under these experimental conditions.
Table 2 shows the activities of the peptides against gram-negative bacteria. LL-37 was highly effective against all of the gram-negative bacteria in the panel except for Proteus mirabilis under high-salt conditions and Burkholderia cepacia under low- or high-salt conditions. Protegrin PG-1 had somewhat greater overall potency than LL-37 and was unaffected by 100 mM NaCl. Like LL-37, PG-1 was also relatively ineffective against B. cepacia. Under low-salt conditions, HNP-1 showed good activity against E. coli (four strains), Stenotrophomonas maltophilia, and a phoP strain of Salmonella typhimurium (12, 14), but it was inactive against the wild-type strain S. typhimurium 14028S. Under high-salt conditions, HNP-1 retained slight activity against two of the four E. coli strains tested but it was otherwise inactive. Even under low-salt conditions, HNP-1 had little activity (MIC, >80 μg/ml) against P. aeruginosa, B. cepacia, Proteus vulgaris, or P. mirabilis. Table 2 also indicates the sensitivities of these organisms to gentamicin, a conventional aminoglycoside antibiotic that was used as a control.
Effect of divalent cations.
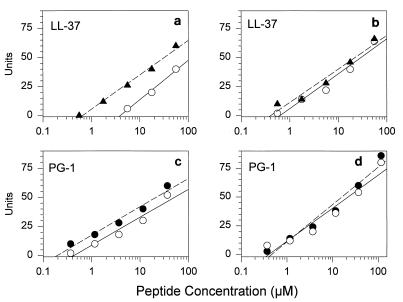
To see if divalent cations affected the antibacterial effects of LL-37 and PG-1, we used E. coli ML-35p and L. monocytogenes EGD as targets. For E. coli, the addition 1 mM Ca2+ to the underlay gels increased the MIC of LL-37 substantially and the MIC of PG-1 slightly (Fig. 2). In contrast, 1 mM Ca2+ did not decrease either peptide’s activity against L. monocytogenes. The addition of Mg2+ (1 mM) was not inhibitory for either peptide with either organism, and 1 mM Ca2+ plus 1 mM Mg2+ was no more inhibitory than Ca2+ alone for E. coli (data not shown).
FIG. 2.
Effect of calcium on antimicrobial activity. Solid symbols show the zone diameters from radial diffusion assays that were performed in underlay gels containing 10 mM phosphate buffer (pH 7.4), 100 mM NaCl, 0.3 mg of Trypticase soy broth powder per ml, 1% agarose, and either E. coli ML-35p (a and c) or L. monocytogenes (b and d). The open symbols indicate the results obtained when these underlay gels were supplemented with 1 mM calcium chloride. The intersections of the continuous or interrupted regression lines with the x axis define the respective MICs. The concentrations on the x axis refer to the concentration of peptide in the 5-μl volume that was initially added to the well. Note that the x axis (concentration) is drawn with a logarithmic scale.
Effect of sample volume.
To minimize the amount of peptide consumed, all of the radial diffusion assays described in this report were performed with 5-μl samples that filled the sample wells to only half their height. Consequently, the introduced peptides were free to diffuse upward, as well as outward (radially), once they entered the underlay gels. Such upward diffusion would, in effect, allow up to a twofold additional dilution of the peptide concentration initially placed into the well. We therefore examined the effect of sample volume experimentally and found that the use of 10-μl sample volumes yielded MICs that were approximately 30 to 40% lower than those obtained with 5-μl samples (data not shown).
Broth microdilution assays.
We also tested the activities of LL-37 and PG-1 against four organisms in broth microdilution assays that were set up according to NCCLS guidelines. In these assays, we used two types of MHB. One of these (standard MHB) was prepared in the customary manner, and the other (refined MHB) was subjected to anion-exchange chromatography in order to deplete it of (poly)anionic inhibitors, as discussed later. Table 3 shows the results obtained by these broth microdilution assays and compares them with the results obtained by radial diffusion assays. Note that the MIC determinations performed with standard MHB yielded values that were 3- to >20-fold higher, depending on the organism, than those obtained with refined MHB. When LL-37 was tested in refined MHB, two of the MICs were higher and two were lower than the values returned by radial diffusion assays. When LL-37 was tested in standard MHB, all of the values were at least threefold higher than those obtained in our radial diffusion assays.
TABLE 3.
Comparison of radial diffusion and broth microdilution assaysa
| Organism | MIC (μg/ml) by the following assay with the indicated medium:
|
|||||
|---|---|---|---|---|---|---|
| LL-37
|
PG-1
|
|||||
| Radial diffusion assay with 100 mM NaCl | Broth microdilution with refined MHB | Broth microdilution with standard MHB | Radial diffusion assay with 100 mM NaCl | Broth microdilution with refined MHB | Broth microdilution with standard MHB | |
| E. coli ML-35p | 7.6 | 6.0–8.0 | 24–32 | 0.7 | 0.75–1.0 | 2.0–3.0 |
| P. aeruginosa MR 3007 | 3.8 | 8.0–12.0 | >32 | 0.7 | 1.5–2.0 | 6.0–8.0 |
| B. subtilis | 0.5 | 0.18–0.25 | 2.0–3.0 | 0.3 | 0.023–0.03 | 0.5–0.75 |
| S. aureus 930918-3 | 9.2 | 2.0–3.0 | >64 | 0.7 | 0.375–0.5 | 4.0–6.0 |
Broth microdilution assays were performed according to NCCLS guidelines with standard MHB or MHB that had been refined by anion-exchange chromatography, as described in the text. Two concentrations are given for broth microdilution results. The lower one is the highest concentration that allowed growth, and the higher one is the lowest concentration that prevented it, i.e., the MIC. By subculturing the MIC wells, we determined that these MICs corresponded to minimal bactericidal concentrations. Radial diffusion assays were performed with 5-μl samples.
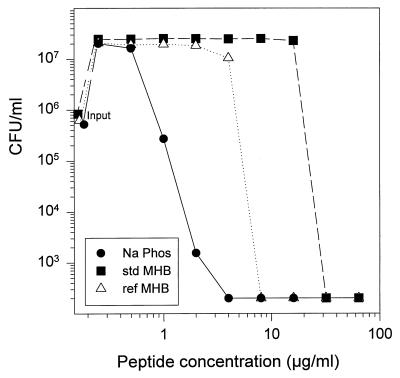
We also demonstrated this phenomenon by performing a colony count assay wherein various concentrations of LL-37 were incubated with E. coli ML-35p in three different media: standard MHB, refined MHB, and the medium added to the agarose underlays (10 mM sodium phosphate plus 100 mM NaCl and 1% [vol/vol] Trypticase soy broth powder). Figure 3 shows that standard MHB required about 10 times more LL-37 than refined MHB to reduce the E. coli colony count to or below 200 colonies/ml, which was our limit of detection. The sodium phosphate buffer formulation that we used to prepare our high-salt underlays was more supportive of bactericidal activity than refined MHB, and the concentration-dependent fall in colony count occurred more gradually in this medium instead of with the abruptness noted in either MHB formulation. Overall, these results indicate that standard MHB is suboptimal for the testing of antimicrobial peptides and that its utility can be improved substantially by subjecting it to anion-exchange chromatography prior to use.
FIG. 3.
Effects of various media on the activity of LL-37. Mid-logarithmic-phase E. coli ML-35p, approximately 2 × 106 CFU/ml, was incubated for 2 h with various concentrations of LL-37 in three different media: 10 mM sodium phosphate buffer plus 100 mM NaCl and dilute Trypticase soy broth (•), standard (std) MHB (■), and refined (ref) MHB (▵).
Membrane permeabilization.
We previously described the use of E. coli ML-35p to examine the ability of antimicrobial peptides to permeabilize the membranes of a gram-negative organism by real-time spectrophotometry (27, 28). Because this bacterium constitutively expresses cytoplasmic β-galactosidase but lacks the membrane permease that transports β-galactosides across its inner (plasma) membrane, it cannot hydrolyze ONPG until its inner membrane undergoes permeabilization. E. coli ML-35p also expresses a periplasmic β-lactamase, so that monitoring of the hydrolysis of a bulky and poorly penetrating cephalosporin (PADAC) also allows outer membrane permeabilization to be followed spectrophotometrically.
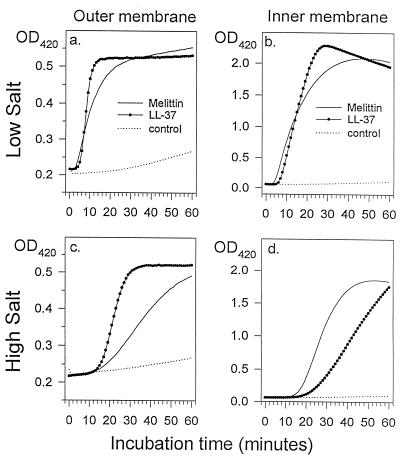
Figure 4 shows the effects of 5 μg of LL-37 per ml on the membranes of E. coli ML-35p. As a control, we used 20 μg of melittin per ml. Melittin is a strongly hemolytic, cytotoxic, and antimicrobial peptide found in bee venom. LL-37 required about 4 min to permeabilize the organism’s outer membrane under low-salt conditions and required about 14 min when the medium contained 100 mM NaCl. The inner membrane of organisms exposed to 5 μg of LL-37 per ml became permeable in approximately 6 min under low-salt conditions and after 18 min when 100 mM NaCl was present. Thus, the outer and inner membrane permeabilizations mediated by LL-37 were closely coupled in time.
FIG. 4.
Membrane permeabilization. We tested the ability of LL-37 (5 μg/ml) to permeabilize the outer (a and c) and inner (b and d) membranes of E. coli ML-35p. Experiments were carried out either in 10 mM sodium phosphate buffer (a and b) or in 10 mM phosphate buffer plus 100 mM NaCl (panels c and d). Melittin (20 μg/ml) was used as a positive (lytic) standard, and acidified water was used as the negative control. OD420, optical density at 420 nm.
LPS binding.
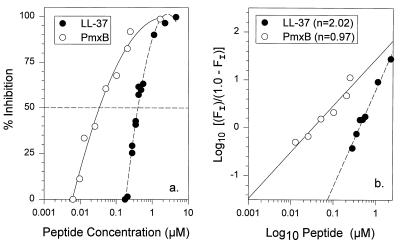
We used a sensitive and precise Limulus chromogenic assay to examine the ability of LL-37 to bind LPS from E. coli O111:B4 and to compare its binding to that of polymyxin B. As shown in Fig. 5a, approximately 0.36 μM LL-37 or 30 nM polymyxin B bound half of the E. coli LPS under the conditions used in our assay. Thus, on a molar basis, LL-37 was approximately 10% as potent as polymyxin B in binding LPS. Figure 5a also shows that the LPS-binding curves for polymyxin B and LL-37 differ in shape. Whereas the LL-37-binding isotherm is distinctly sigmoidal, the polymyxin B curve is not. Because sigmoidal curves suggest cooperativity, we also graphed the data as a Hill plot (Fig. 5b). By Hill plot analysis, the LL-37 binding data were linear (r = 0.992) and had a slope of 2.02, suggesting positive cooperativity between two ligand molecules and the LPS. In contrast, the polymyxin B line had a slope of 0.97, suggesting that polymyxin-LPS-binding events occurred independently rather than cooperatively.
FIG. 5.
LPS binding. (a) Binding of polymyxin B (PmxB) and LL-37 to LPS from E. coli O1111:B4, as determined by chromogenic Limulus assays. (b) The same data from panel a but as a Hill plot. The term log10 [(FI)/(1.0 − FI)] on the ordinate is described in the Materials and Methods section. The Hill coefficient (n) shown in the figure corresponds to the slope of the continuous or interrupted regression lines.
Conformational studies.
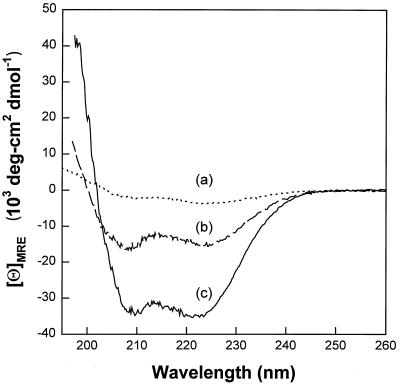
In CD spectroscopy measurements, α-helical secondary structures are revealed by double dichroic minima at 208 and 222 nm (2, 50). Our CD studies revealed a largely random coil conformation with only 9.6 to 9.9% α helix in phosphate buffer with or without 100 mM NaCl unless structure-promoting exogenous elements were also present. These could be relatively simple, such as either 30% trifluoroethanol (48% α-helical conformation) or 20 mM sodium dodecyl sulfate micelles (87.1% α-helical conformation). They could also be more complex, such as POPG liposomes (94.6% α-helical conformation), membranes whose anionicity simulates that present in bacterial membranes. As was reported for the antimicrobial peptide derived from rabbit CAP-18 (3), LL-37 also showed enhanced helical conformation (40.2% α helix) in the presence of diphosphoryl lipid A purified from an Rd E. coli mutant, strain F-583. Several examples of our CD findings can be seen in Fig. 6, including measurements taken in phosphate buffer (curve a), in the presence of diphosphoryl lipid A (curve b), and with POPG liposomes (curve c).
FIG. 6.
CD spectrometry. The spectra were measured in phosphate buffer (curve a), in the presence of diphosphoryl lipid A (curve b), and with POPG liposomes (curve c) and are described in the text.
DISCUSSION
Although our principal goals in these studies were to examine the properties of LL-37, we also performed NCCLS-type broth microdilution assays to substantiate the results of our radial diffusion assays. We found that the use of conventional MHB in a standard NCCLS broth microdilution assay vastly underestimates the activity of LL-37 and that this problem could be remedied by passing the MHB through an anion-exchange column before using it.
Since this point has important practical consequences for the testing of other polycationic antimicrobial molecules, this use of anion-exchange chromatography merits discussion and explanation. The major component of MHB is an acid hydrolysate of casein (17.5 g/liter), and its only other constituents are beef extract (3 g/liter) and starch (1.5 g/liter). Although bovine casein is a cheap and dependable nutrient source, it has an atypical amino acid composition. Glutamic acid and glutamine constitute 18.8% (39 of 208) of its residues, and it also contains 8 aspartic acid plus asparagine residues and 5 phosphorylated serines. Consequently, 25.0% (52 of 208) of the amino acids released by its total hydrolysis would be dicarboxylic or phosphorocarboxylic acids. Moreover, since the remarkable ESLSSSEE sequence (residues 14 to 20) of casein contains seven clustered, negatively charged glutamate and phosphoserine residues, incomplete hydrolysis could leave residual polyanionic peptides. Because most antimicrobial peptides are polycations, it should not be surprising that many are incompatible with conventional MHB, which would complex or even precipitate them. Subjecting MHB to simple anion-exchange chromatography to remove anionic inhibitors, as illustrated by these studies, enhanced the utility of MHB for broth microdilution studies for LL-37, a cationic antimicrobial molecule. Although ion-exchange cartridge columns were convenient for our small-scale experiments, one would probably use a batch process for larger-scale production of refined MHB.
Whereas multiple antimicrobial peptide precursors of the cathelicidin family have been described in cattle and pigs (51), hCAP-18 is believed to be the only human cathelicidin (1). Although it was discovered relatively recently, its concentration in the human neutrophil (0.63 mg of hCAP-18/109 cells) makes hCAP-18 as abundant as lactoferrin, on a molar basis (41). Normal plasma contains 1.18 μg of hCAP-18/ml, which circulates in high-molecular-weight complexes (41). This concentration of circulating hCAP-18 might suffice to detoxify low concentrations of LPS that enter the concentration (Fig. 5a and b).
Our binding studies with LL-37 showed a Hill coefficient of 2.02, indicative of positive cooperativity between two molecules of the ligand (LL-37) and the receptor molecule (LPS). Polymyxin B showed a more typical hyperbolic binding curve, and the Hill coefficient of 1.08 suggested that its binding to LPS was simple and noncooperative. Although polymyxin B can bind a variety of anionic phospholipids, including phosphatidyl glycerol and cardiolipin (45), its interactions with the glucosamine phosphates and 2-keto-3-deoxyoctulosonic acid carboxylates found in bacterial LPS (33) have received considerably more scrutiny.
Previous studies of LPS binding have typically used radiolabelled or dansylated peptides or similarly modified LPS (33, 38). Binding of dansyl-polymyxin to unmodified P. aeruginosa LPS (33) was noted to be cooperative and of high affinity (Hill coefficient, 1.98; S0.5 [an estimate of affinity] = 0.38 μM). The binding of polymyxin to dansylated LPS from Rc and Re mutants of S. typhimurium LT2 had a Kd of 0.3 to 0.5 μM and occurred without evident cooperativity (38). The use of a highly precise and sensitive version of the chromogenic Limulus assay allowed us to examine binding without structurally modifying either the peptides or the LPS. Further studies to define the precise portions of LPS that bind LL-37 could be of considerable interest.
Since the residues of LL-37 constitute approximately one-quarter of the hCAP-18 propeptide, conversion of circulating hCAP to LL-37 would liberate about 0.25 μg of LL-37 per ml. Although this concentration appears to be too low to exert microbicidal actions alone (Tables 1 and 2), leukocyte-derived antimicrobial proteins can also work synergistically, as recently demonstrated for rabbit neutrophil defensins BPI and “p15s” (30, 31). The possibility that LL-37 acts synergistically with other host-defense molecules also remains to be explored.
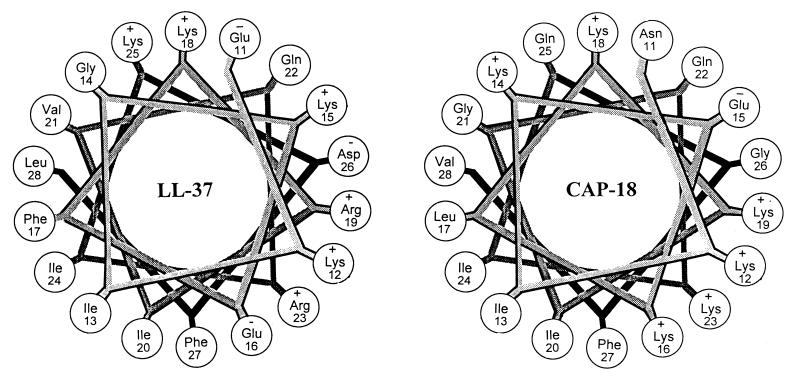
Rabbit CAP-18 (26), the homolog of human hCAP-18 found in rabbit granulocytes, has received extensive study because its C-terminal domain can bind and neutralize LPS and can prevent potentially deleterious consequences of LPS release in vitro (18, 25) and in vivo (44, 47). Whereas the signal sequence and cathelin domains of CAP-18 show marked primary sequence homology to hCAP-18 and other cathelicidins, primary structural homology between its C-terminal region and the corresponding domain of hCAP-18 is considerably less marked (Fig. 1). Nevertheless, their functions and secondary structures appear to be similar (Fig. 7).
FIG. 7.
Helical wheel. Residues 11 to 28 of the mature LL-37 peptide and the corresponding residues of rabbit CAP-18 (Fig. 1) are shown. Note the sequestration of polar and apolar residues (all of the polar residues of LL-37 are clustered between 11:30 and 5:30 when the figure is visualized as a clock face). CAP-18 shows a very similar pattern.
Two-dimensional nuclear magnetic resonance (2D-NMR) and CD measurements have defined the solution structure (3) of CAP-18106–137, the 32-residue portion of CAP-18 that constitutes its LPS-binding domain (25). Consistent with our findings for LL-37 (Fig. 6), this CAP-18 peptide fragment (GLRKRLRKFRNKIKEKLKKI GQKIQGLLPKLA) adopts an unordered, random coil conformation in aqueous solution and forms a long, straight, stable amphipathic α helix in 30% trifluoroethanol or in the presence of lipid A (3).
Neural network predictions suggest that LL-37 has a long α-helical segment, similar to the residue specific structure of CAP-18 assumed by 2D-NMR in a membrane-like environment (3). The high α-helical hydrophobic moments (μα) of LL-37 residues 11 to 22 (μα = 0.90) and CAP-18 residues 11 to 22 (μα = 0.67) indicate similar sequence segment amphipathicity. The helical wheel axial projections shown in Fig. 7 indicate that the antimicrobial domains of CAP-18 and LL-37 demonstrate strikingly similar segregation of polar and strong nonpolar residues.
On the basis of structural predictions, a 20-residue peptide corresponding to CAP18106–125 (GLRKRLRKFRNKIKEKKLKKI) was synthesized (46) and tested for its antimicrobial activity against E. coli, S. typhimurium, P. aeruginosa, Bacillus megaterium, and S. aureus. Although nonhemolytic for human erythrocytes, even at 50 mM, 1 mM peptide concentrations were reported to permeabilize the inner membrane of E. coli, and the peptides at concentrations of 0.4 to 4.0 μM killed all of the test organisms. The 32- and 37-residue peptides corresponding to CAP-18106–137 and CAP-18106–142 also were active against several additional bacteria but lacked activity against C. albicans (24). Recently, a covalent immunoglobulin G–CAP-18106–138 conjugate was reported to bind to LPS, protect sensitized mice from LPS-induced mortality (7), and kill gram-negative bacteria (7).
The noteworthy resistance of B. cepacia to each of the antimicrobial peptides examined in our studies is consistent with observations showing the primacy of oxidative mechanisms in leukocyte-mediated host defenses against this opportunistic pathogen in both humans (42) and mice (32). Whereas the present studies with S. typhimurium 14028S (wild type) and 7953S (its phoP derivative) confirmed a previously reported correlation between phoP and resistance to human defensins (12), the wild-type and phoP strains showed similar susceptibilities to LL-37 and PG-1. Thus, the bacterial responses regulated by phoP (12, 14, 20) do not provide S. typhimurium with global immunity to leukocyte-derived antimicrobial peptides. The mechanisms responsible for the resistance of B. cepacia to LL-37 and PG-1 merit further investigation.
LL-37 and defensins are located in neutrophil granule populations that differ in content and in behavior. The placement of defensins in azurophil granules enables them to enter a locale, the phagosome, wherein the potentially inhibitory effects of NaCl could be modified by ion-transporting membrane pumps or overcome by high local concentrations of defensins. In contrast, LL-37 is stored as a propeptide (hCAP-18) and is placed in a secretory organelle, the secondary granule. As a result, extracellular bacteria (or free LPS) may encounter LL-37 primarily in its cathelin-containing precursor form (hCAP-18), whereas ingested bacteria may encounter LL-37, the microbicidal domain of hCAP-18, after proteolytic processing by enzymes of the neutrophil (or perhaps the microbial target). While much remains to be learned about both LL-37 and hCAP-18, the activity of LL-37 against P. aeruginosa, including mucoid and antibiotic-resistant strains, suggests that it could provide a suitable template for designing topical bronchopulmonary microbicides for use in conditions such as cystic fibrosis.
ACKNOWLEDGMENTS
This work was supported by U.S. Public Health Service grants AI 22839, AI 37945, and HL 46809 and by a grant from the Cystic Fibrosis Foundation.
We thank Kym Faull for performing the electrospray ionization-mass spectrometry, Ken Miyasaki for help with the capillary zone electrophoresis, and Audree Fowler for performing the amino acid analyses. We also thank Fred Heffron, Ian Holder, Elizabeth Wagar, and Spencer A. Benson for contributing bacterial strains. Special thanks go to James Bowie for use of the AVIV CD spectropolarimeter and David Eisenberg for the hydrophobic moment computer program.
REFERENCES
- 1.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman H G, Gudmundsson G H. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruch M D, Dhingra M M, Gierasch L M. Side chain-backbone hydrogen bonding contributes to helix stability in peptides derived from an α-helical region of carboxypeptidase A. Proteins Struct Funct Genet. 1991;10:130–139. doi: 10.1002/prot.340100206. [DOI] [PubMed] [Google Scholar]
- 3.Chen C, Brock R, Luh F, Chou P-J, Larrick J W, Huang R-F, Huang T-H. The solution structure of the active domain of CAP18—a lipopolysaccharide binding protein from rabbit leukocytes. FEBS Lett. 1995;370:46–52. doi: 10.1016/0014-5793(95)00792-8. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y H, Yang J T, Chau K H. Determination of the helix and β form of proteins in aqueous by circular dichroism. Biochemistry. 1974;13:3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- 5.DeLeo F R, Quinn M T. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J Leukocyte Biol. 1996;60:677–691. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg D, Weiss R M, Terwillinger T C, Wilcox W. Hydrophobic moments and protein structure. Faraday Symp Chem Soc. 1982;17:109–116. [Google Scholar]
- 7.Fletcher M A, Kloczewiak M A, Loiselle P M, Ogata M, Vermeulen M V, Zanzot E M, Warren H S. A novel peptide-IgG conjugate, CAP18106–138–IgG, that binds and neutralizes endotoxin and kills gram-negative bacteria. J Infect Dis. 1997;175:621–632. doi: 10.1093/infdis/175.3.621. [DOI] [PubMed] [Google Scholar]
- 8.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, Gudmundsson G H. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 9.Gallo R L, Kim K J, Bernfield M, Kozak C A, Zanetti M, Merluzzi L, Gennaro R. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272:13088–13093. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 10.Ganz T, Lehrer R I. Antimicrobial peptides of leukocytes. Curr Opin Hematol. 1997;4:53–58. doi: 10.1097/00062752-199704010-00009. [DOI] [PubMed] [Google Scholar]
- 11.Greenfield N, Fasman G. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1967;8:4108–4115. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- 12.Groisman E A, Heffron F, Solomon F. Molecular genetic analysis of the Escherichia coli phoP locus. J Bacteriol. 1992;174:486–491. doi: 10.1128/jb.174.2.486-491.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gudmundsson G H, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996;238:325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 14.Guo L, Lim K B, Gunn J S, Bainbridge B, Darveau R P, Hackett M, Miller S I. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276:250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 15.Hampton M B, Kettle A J, Winterbourn C C. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect Immun. 1996;64:3512–3517. doi: 10.1128/iai.64.9.3512-3517.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harwig S S L, Ganz T, Lehrer R I. Neutrophil defensins: purification, characterization and antimicrobial testing. Methods Enzymol. 1994;236:163–172. doi: 10.1016/0076-6879(94)36015-4. [DOI] [PubMed] [Google Scholar]
- 17.Harwig S S L, Waring A J, Yang H J, Cho Y, Tan L, Lehrer R I. Intramolecular disulfide bonds enhance the antimicrobial and lytic activities of protegrins at physiological sodium chloride concentrations. Eur J Biochem. 1996;240:352–357. doi: 10.1111/j.1432-1033.1996.0352h.x. [DOI] [PubMed] [Google Scholar]
- 18.Hirata M, Shimomura Y, Yoshida M, Morgan J G, Palings L I, Wilson D, Yen M H, Wright S C, Larrick J W. Characterization of a rabbit cationic protein (CAP18) with lipopolysaccharide-inhibitory activity. Infect Immun. 1994;62:1421–1426. doi: 10.1128/iai.62.4.1421-1426.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson W C., Jr Protein secondary structure and circular dichroism: a practical guide. Proteins Struct Funct Genet. 1990;7:205–214. doi: 10.1002/prot.340070302. [DOI] [PubMed] [Google Scholar]
- 20.Johnston C, Pegues D A, Hueck C J, Lee A, Miller S I. Transcriptional activation of Salmonella typhimurium invasion genes by a member of the phosphorylated response-regulator superfamily. Mol Microbiol. 1996;22:715–727. doi: 10.1046/j.1365-2958.1996.d01-1719.x. [DOI] [PubMed] [Google Scholar]
- 21.Kokryakov V N, Harwig S S L, Panyutich E A, Shevchenko A A, Aleshina G M, Shamova O V, Korneva H A, Lehrer R I. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993;327:231–236. doi: 10.1016/0014-5793(93)80175-t. [DOI] [PubMed] [Google Scholar]
- 22.Kopitar M, Ritonja A, Popovic T, Gabrijelcic D, Krizaj I, Turk V. A new type of low-molecular mass cysteine proteinase inhibitor from pig leukocytes. Hoppe-Seyler’s Biol Chem. 1989;370:1145–1151. doi: 10.1515/bchm3.1989.370.2.1145. [DOI] [PubMed] [Google Scholar]
- 23.Larrick J W, Hirata M, Balint R F, Lee J, Zhong J, Wright S C. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larrick J W, Hirata M, Shimomoura Y, Yoshida M, Zheng H, Zhong J, Wright S C. Antimicrobial activity of rabbit CAP18-derived peptides. Antimicrob Agents Chemother. 1993;37:2534–2539. doi: 10.1128/aac.37.12.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larrick J W, Hirata M, Zheng H, Zhong J, Bolin D, Cavaillon J-M, Warren H S, Wright S C. A novel granulocyte-derived peptide with lipopolysaccharide-neutralizing activity. J Immunol. 1994;152:231–240. [PubMed] [Google Scholar]
- 26.Larrick J W, Morgan J G, Palings I, Hirata M, Yen M H. Complementary DNA sequence of rabbit CAP-18. A unique lipopolysaccharide binding protein. Biochem Biophys Res Commun. 1991;179:170–175. doi: 10.1016/0006-291x(91)91350-l. [DOI] [PubMed] [Google Scholar]
- 27.Lehrer R I, Barton A, Daher K A, Harwig S S L, Ganz T, Selsted M E. Interaction of human defensins with Escherichia coli: mechanism of bactericidal activity. J Clin Invest. 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehrer R I, Barton A, Ganz T. Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J Immunol Methods. 1988;108:153–158. doi: 10.1016/0022-1759(88)90414-0. [DOI] [PubMed] [Google Scholar]
- 29.Levy O. Antibiotic proteins of polymorphonuclear leukocytes. Eur J Haematol. 1996;56:263–277. doi: 10.1111/j.1600-0609.1996.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 30.Levy O, Ooi C E, Elsbach P, Doerfler M E, Lehrer R I, Weiss J. Antibacterial proteins of granulocytes differ in interaction with endotoxin. Comparison of bactericidal/permeability-increasing protein, p15s, and defensins. J Immunol. 1995;154:5403–5410. [PubMed] [Google Scholar]
- 31.Levy O, Ooi C E, Weiss J, Lehrer R I, Elsbach P. Individual and synergistic effects of rabbit granulocyte proteins on Escherichia coli. J Clin Invest. 1994;94:672–682. doi: 10.1172/JCI117384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mardiney M, III, Jackson S H, Spratt S K, Li F, Holland S M, Malech H L. Enhanced host defense after gene transfer in the murine p47phox-deficient model of chronic granulomatous disease. Blood. 1997;89:2268–2275. [PubMed] [Google Scholar]
- 33.Moore R A, Bates N C, Hancock R E W. Interaction of polycationic antibiotics with Pseudomonas aeruginosa lipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob Agents Chemother. 1986;29:496–500. doi: 10.1128/aac.29.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.National Committee for Clinical Laboratory Standards. Document M7-A3. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 34.Panyutich A, Shi J, Boutz P L, Zhao C, Ganz T. Porcine polymorphonuclear leukocytes generate extracellular microbicidal activity by elastase-mediated activation of secreted proprotegrins. Infect Immun. 1997;65:978–985. doi: 10.1128/iai.65.3.978-985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rice W G, Ganz T, Kinkade J M, Jr, Selsted M E, Lehrer R I, Parmley R T. Defensin-rich granules of human neutrophils. Blood. 1987;70:757–765. [PubMed] [Google Scholar]
- 36.Ritonja A, Kopitar M, Jerala R, Turk V. Primary structure of a new cysteine proteinase inhibitor from pig leukocytes. FEBS Lett. 1989;255:211–214. doi: 10.1016/0014-5793(89)81093-2. [DOI] [PubMed] [Google Scholar]
- 37.Rost B, Sander C. Prediction of protein secondary structure at better the 70% accuracy. J Mol Biol. 1993;232:584–599. doi: 10.1006/jmbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- 38.Schindler M, Osborn M J. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry. 1979;18:4425–4430. doi: 10.1021/bi00587a024. [DOI] [PubMed] [Google Scholar]
- 39.Scocchi M, Skerlavaj B, Romeo D, Gennaro R. Proteolytic cleavage by neutrophil elastase converts inactive storage proforms to antibacterial bactenecins. Eur J Biochem. 1992;209:589–595. doi: 10.1111/j.1432-1033.1992.tb17324.x. [DOI] [PubMed] [Google Scholar]
- 40.Sorensen O, Arnljots K, Cowland J B, Bainton D F, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 41.Sorensøn O, Cowland J B, Askaa J, Borregaard N. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods. 1997;206:53–59. doi: 10.1016/s0022-1759(97)00084-7. [DOI] [PubMed] [Google Scholar]
- 42.Speert D P, Bond M, Woodman R C, Curnutte J T. Infection with Pseudomonas cepacia in chronic granulomatous disease: role of nonoxidative killing by neutrophils in host defense. J Infect Dis. 1994;170:1524–1531. doi: 10.1093/infdis/170.6.1524. [DOI] [PubMed] [Google Scholar]
- 43.Steinberg D, Lehrer R I. Designer assays for antimicrobial peptides. Methods Mol Biol. 1997;78:169–186. doi: 10.1385/0-89603-408-9:169. [DOI] [PubMed] [Google Scholar]
- 44.Tasaka S, Ishizaka A, Urano T, Sayama K, Sakamaki F, Nakamura H, Terashima T, Waki Y, Soejima K, Nakamura M, Matsubara H, Fujishima S, Kanazawa M, Larrick J W. A derivative of cationic antimicrobial protein attenuates lung injury by suppressing cell adhesion. Am J Respir Cell Mol Biol. 1996;15:738–744. doi: 10.1165/ajrcmb.15.6.8969268. [DOI] [PubMed] [Google Scholar]
- 45.Teuber M, Miller I R. Selective binding of polymyxin B to negatively charged lipid monolayers. Biochim Biophys Acta. 1977;467:280–289. doi: 10.1016/0005-2736(77)90305-4. [DOI] [PubMed] [Google Scholar]
- 46.Tossi A, Scocchi M, Skerlavaj B, Gennaro R. Identification and characterization of a primary antibacterial domain in CAP18, a lipopolysaccharide binding protein from rabbit leukocytes. FEBS Lett. 1994;339:108–112. doi: 10.1016/0014-5793(94)80395-1. [DOI] [PubMed] [Google Scholar]
- 47.VanderMeer T J, Menconi M J, Zhuang J, Wang H, Murtaugh R, Bouza C, Stevens P, Fink M P. Protective effects of a novel 32-amino acid C-terminal fragment of CAP18 in endotoxemic pigs. Surgery. 1995;117:656–662. doi: 10.1016/s0039-6060(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 48.Waring A J, Harwig S S L, Lehrer R I. Structure and activity of protegrin-1 in model lipid membranes. Protein Peptide Lett. 1996;3:177–184. [Google Scholar]
- 49.White S H, Wimley W C, Selsted M E. Structure, function, and membrane integration of defensins. Curr Opin Struct Biol. 1995;5:521–527. doi: 10.1016/0959-440x(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 50.Woody R W. Circular dichroism of peptides. In: Udenfriend S, Meienhofer J, Hruby V J, editors. The peptides: synthesis and biology. Vol. 7. New York, N.Y: Academic Press, Inc.; 1985. pp. 15–114. [Google Scholar]
- 51.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 52.Zhao C, Ganz T, Lehrer R I. The structure of porcine protegrin genes. FEBS Lett. 1995;368:197–202. doi: 10.1016/0014-5793(95)00633-k. [DOI] [PubMed] [Google Scholar]