Abstract
Objective
To better understand the epidemiology of preterm birth among Pacific Islanders in the United States and the US-Affiliated Pacific Islands.
Methods
A systematic search of MEDLINE, Embase, CINAHL, PsycINFO, two non-indexed regional journals, and gray literature was conducted. The search was finalized in September 2021. Observational studies published since January 2010 that documented preterm birth outcomes among Pacific Islanders in the United States and the US-Affiliated Pacific Islands were eligible for inclusion. Outcomes of interest included preterm birth prevalence, risk compared to White women, and risk factors for preterm birth among Pacific Islanders.
Results
Fourteen of the 3183 screened articles were included in meta-analyses. Random-effects models were used for pooled estimates with 95% confidence intervals. The pooled prevalence of preterm birth among Pacific Islanders was 11.2%, 95% CI: 9.3%−13.6%. Marshallese women had the highest pooled prevalence (20.7%, 95% CI: 18.6%−23.0%) compared to other Pacific Islander subgroups. Compared to White women, Pacific Islander women had higher odds of experiencing preterm birth (OR=1.40, 95% CI: 1.28–1.53). Four risk factors for preterm birth could be explored with the data available: hypertension, diabetes, smoking, and pre-pregnancy body mass index; hypertension and diabetes significantly increased the odds of preterm birth.
Conclusions
Existing literature suggests that US Pacific Islanders were more likely to experience preterm birth than White women, although the pooled prevalence varied by Pacific Islander subgroup. Data support the need for disaggregation of Pacific Islanders in future research and argue for examination of subgroup-specific outcomes to address perinatal health disparities.
Keywords: preterm birth, Pacific Islander, Marshallese, health disparities, meta-analysis
1 |. INTRODUCTION
The global prevalence of preterm birth (PTB, live birth <37 weeks1) was 10.6% in 20141; in the same year, the prevalence in the United States (US) was 9.6%, placing the US among the ten countries with the highest number of PTBs2. Since PTB contributes significantly to perinatal mortality and morbidity3, understanding risk factors for PTB and reducing its prevalence are national public health priorities.
Racial disparities in the prevalence of PTB are evident in the US4,5. Several factors likely place Pacific Islander women at a higher risk of PTB compared to other populations: a greater burden of obesity-related cardiometabolic diseases prior to conception6,7, lack of access to medical care and social services (depending on citizenship)8–11, and experiences of discrimination12 etc. These risk factors have been shown to be significantly associated with adverse perinatal outcomes in other populations7,13–20, but, despite being among the fastest growing minority groups in the US21, Pacific Islanders are underrepresented in obstetric research22 and their outcomes are frequently aggregated with those of Asian American women23.
In a recent scoping review(24) we qualitatively summarized existing literature on various adverse pregnancy outcomes, including PTB, and described a greater risk of several of those outcomes among Pacific Islanders compared to other groups. To date, there has been no attempt to quantify the pooled prevalence of PTB or to use meta-analyses to describe pooled risk compared to other populations. Our objectives were, therefore, to estimate: (1) the pooled prevalence of PTB among Pacific Islanders in the US and US Affiliated Pacific Islands (USAPI) with meta-analyses stratified by ethnicity subgroup; (2) the pooled odds ratio estimate for PTB among Pacific Islanders compared to non-Hispanic White women; and (3) the impact of the most commonly reported risk factors for PTB (hypertension, diabetes, smoking, and weight status based on body mass index [BMI categories: underweight, overweight, or obesity]) on the odds of PTB among Pacific Islander women.
2 |. METHODS
2.1 |. Search strategy
This systematic review (PROSPERO ID CRD42021281673) was conducted based on papers identified in our previous scoping review(24). The prior review’s search strategy(24) and the protocol(25) for this meta-analysis were deposited on the Open Science Framework and are described briefly here. We followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines24 to present our outcomes. No research ethics board approval was required.
The original scoping review search strategy(24) was developed with two concepts: (1) Pacific Islander and (2) pregnancy and perinatal outcomes. The controlled vocabulary terms and keyword search terms used on MEDLINE (Ovid) are listed in Table S1. Articles without US/USAPI geographic subject indexing were not retrieved; articles that had a US/USAPI subject heading but did not have geographic subject headings were selected for screening.
A search of four databases was completed in August 2020: MEDLINE (Ovid), Embase (Ovid), CINAHL (EBSCO), and PsycINFO (Ovid). Additionally, two regional journals that are not well indexed in major bibliographic databases were hand-searched (the Pacific Journal of Reproductive Health and Pacific Health Dialog). Data reported by national, state and territorial government agencies, for example the Centers for Disease Control, State Departments of Health, and the Pacific Island Health Officers Association were also searched. Citation chaining was conducted on all included articles.
Our study population was Pacific Islander women living in the US and USAPI, including American Samoa, Guam, the Commonwealth of the Northern Mariana Islands (CNMI), the Federated States of Micronesia (FSM) and the Republic of the Marshall Islands (RMI). Studies of New Zealand women with Māori ethnicity (the indigenous Polynesian people of New Zealand) living in the US were included, but data on non-Māori New Zealander women in the US were not included. Articles were excluded if they reported outcomes from Pacific Islander women outside of the US or USAPI, or aggregated Pacific Islander women with other races/ethnicities.
2.2 |. Study selection
To summarize the most recent and relevant literature, articles published between January 2010 and September 2021 were included. Peer-reviewed publications, government reports and PhD dissertations written in the English language met inclusion criteria. Conference abstracts and master’s theses were excluded since they may not have represented final study outcomes. Review articles were also excluded, but their references reviewed for additional primary data sources. Each publication was reviewed by two independent authors. Four team members completed title-abstract and full text screening on Covidence and met to solve vote conflicts.
This review focuses specifically on PTB. Therefore, we selected articles from our prior scoping review that reported PTB outcomes (n=17) for further analysis. Data were collected from the included studies about: (1) the prevalence of PTB; (2) risk of PTB among Pacific Islanders compared to non-Hispanic White women (the most commonly reported reference group in the papers available); and (3) risk factors for PTB. The four most commonly examined risk factors - hypertension, diabetes, smoking during pregnancy, and weight status– are summarized here. To ensure the most up to date data were included in this meta-analysis, the search was repeated in September 2021 and three additional articles identified for inclusion (see Results).
Data extraction, completed by the first author, included participant characteristics and sample size, PTB outcomes, data collection period and data source, study setting and design, and gestational age estimation method. If the study design (i.e. cross-sectional, retrospective, etc.) was not reported by study authors, the first author made a designation according to study methods.
For the PTB prevalence analysis, if a study only reported the prevalence without the specific event number, the number of PTBs was calculated with the formula: N(PTBs)=P*N(total Pacific Islanders) and rounded to the next whole number (N for sample size; P for the prevalence of PTB). For the comparison to non-Hispanic White women, modelling methods and adjusted confounders were recorded. If an article did not report the comparison between Pacific Islanders and White women, odds ratios (OR) were calculated as the odds of having PTB among Pacific Islanders over the odds among White women, based on the proportion of PTB among the two races respectively. For the articles reporting risk factors, modelling methods and adjusted confounders were recorded. Due to the small number of studies available, these risk factors were summarized in one forest plot without pooled estimates.
2.3 |. Quality assessment
We used Joanna Briggs Institute (JBI) critical appraisal tools25 for risk of bias assessment; two authors (BW and KJA) completed the appraisal and met to reach consensus. Articles selected for PTB prevalence meta-analysis were assessed with the JBI checklist for prevalence studies26. Articles used for the comparison between Pacific Islander and White women and the summary for common risk factors were assessed with the JBI checklist for analytical cross-sectional studies27. The total score assigned to each study equals the proportion of “Yes” responses to checklist questions (range: 0%−100%). Since a funnel plot may not accurately assess publication bias for a prevalence meta-analysis28, to be consistent for all the included studies, we used Egger’s test29 to assess publication bias (a test to determine whether the study estimate is related to the size of the study).
2.4 |. Data Synthesis
A total of three meta-analyses were conducted. Along with the overall prevalence meta-analysis, we completed an additional meta-analysis that described prevalence by Pacific Islander sub-group (i.e. Native Hawaiian, Guamanian, Samoan). Subgroups were included if at least two studies reported subgroup-specific outcomes. If an article did not specify which Pacific Islander subgroups were included, or the subgroups were aggregated, it was not included in the subgroup meta-analyses. Meta-analyses were not conducted for the four risk factors due to the limited number of articles reporting these outcomes; they were summarized in one forest plot without pooled estimates.
Since most of the included studies reported their outcomes in the form of ORs, these were used as the measure of association across studies. Hazard ratios (HR) and incidence density ratios (IDR) were considered as relative risks (RR). Where necessary, RRs were transformed into ORs with the formula30 , in which is the prevalence of PTB among non-Hispanic White women. The standard error (SE) of the converted OR was calculated with the formula31 . Since these transformations can overestimate the variance of the ORs derived from the RRs32, we also performed sensitivity analyses that excluded one article with this transformation.
Pooled estimates with 95% confidence intervals are presented with Forest plots sorted by the starting year of data collection. Heterogeneity was assessed with the I2 statistic with low, moderate, and high I2 values of 25%, 50%, and 75%, respectively33. If I2 ≤25% (indicating no evidence of heterogeneity), fixed effects models (Mantel-Haenszel method)34 were used to pool the results. Otherwise, random effects models (DerSimonian and Laird method)35 were used instead. Sources of heterogeneity were explored with meta-regression analysis. All analyses were performed using RStudio (RStudio, Inc., Boston, MA, USA). R package meta36 was used to generate Forest plots and to conduct meta-regression, and package dmetar37 was used to perform Egger’s test for publication bias (P-value <0.05 indicates publication bias).
3 |. RESULTS
Our scoping review(24) identified a total of 3183 articles from four databases. After de-duplication, title-abstract and full text screening, adding seven papers identified through gray literature searching and two from hand-searching journals, 48 articles were included in the scoping review. For the purpose of this analysis focused on PTB, 18 of those articles were selected as potentially relevant for analysis. We later excluded one article38 where RR could not be transformed into an OR since the prevalence of PTB for each race/ethnicity was not provided. In our second search, we identified a total of 617 articles published between January 2020 and September 2021 through the same search strategy. Using the same screening approach, we selected 3 new articles for analysis (Figure 1). After removing potentially overlapping data from different sources (for example, if studies reported data collection using the same data source, with overlapping timelines), a total of 14 studies39–52 were included in the meta-analyses presented here. Two51,52 of the 14 studies were identified through the gray literature search. In both searches, the most common reason for exclusion at the full-text screening stage was the aggregation of Pacific Islanders with Asian American women.
Figure 1.
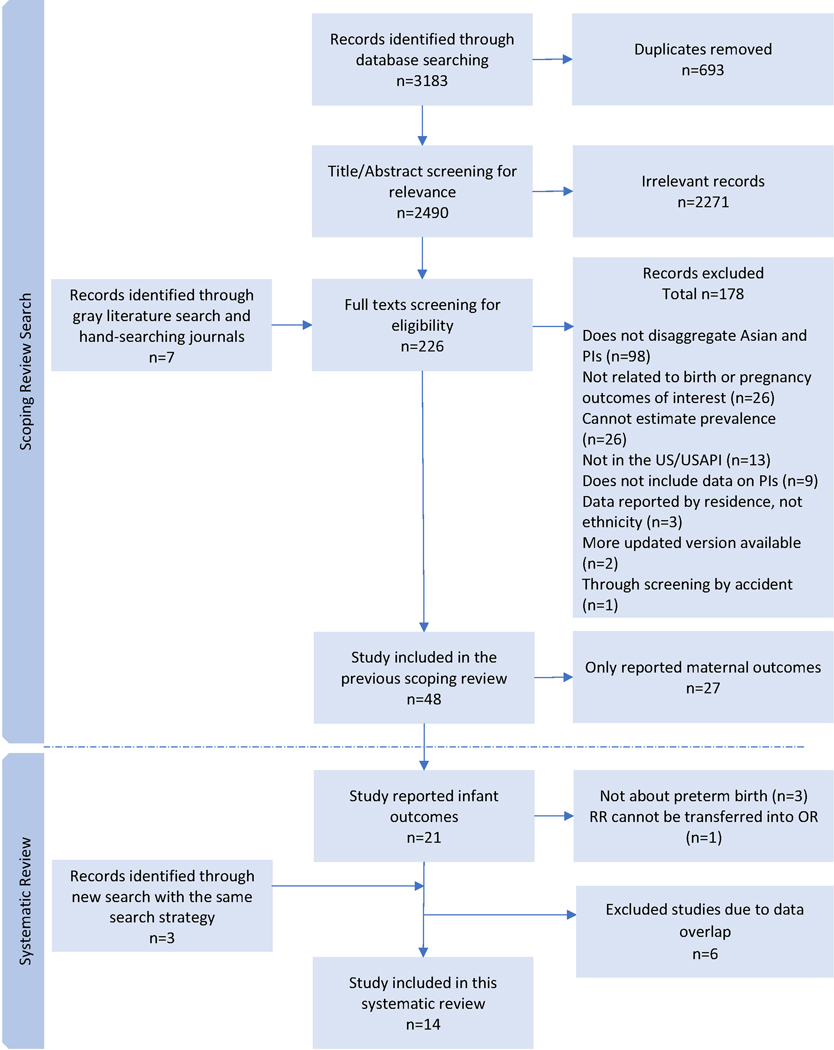
Flow diagram of study selection
3.1 |. Characteristics of the studies
Details of the included studies are presented in Table 1 ordered by the starting year of data collection, which occurred between 1989 to 2018. Of the 14 included studies, 10 (71.4%) studies41,42,44,45,47–52 reported PTB prevalence among Pacific Islanders, 8 (57.1%) studies40,42,44,45,47,48,51,52 were used to estimate risk of PTB compared to White women, and only 2 (14.3%) studies46,50 reported risk factors (hypertension, diabetes, smoking during pregnancy, and weight status [underweight, overweight, or obesity]) for PTB in Pacific Islander-specific analyses. Smoking before pregnancy was not reported46,50.
Table 1.
Details of included studies and risk of bias assessment method
| Study | Data collection | Study design | State/Setting | Pacific Islanders | GA measurement method | PTB definition |
|---|---|---|---|---|---|---|
| Korinek et al., 202145 a, c | 1989–2015 | Cross-sectional studye | Utah (Utah Population Database) | Native Hawaiian or Other Pacific Islanders (n=10,438). | Not reported | GA <37 weeks |
| Nembhard et al., 201942 a, b, c | 1997–2013 | Cross-sectional study | Arkansas (Birth records) | Marshallese (n=2,488, missing value n=270) †. | Ultrasound (74%) | GA <37 weeks |
| Delara et al., 201848 a, c | 1999–2005 | Retrospective cohort study | California (Birth records) | Pacific Islander (n=840). | Best clinical estimate on the birth certificate | GA <37 weeks |
| Ju et al., 201846 d | 2000–2011 | Retrospective cohort study | Hawaii (Hawaii’s Pregnancy Risk Assessment Monitoring System) | Native Hawaiian or Pacific Islander (n=7,657). | Last menstrual period, or algorithm calculation based on other similar records. | GA <37 weeks |
| Hirai et al., 201347 a, b, c | 2002–2009 | Cross-sectional studye | Hawaii (Hawai’i State Linked Birth/Infant Death Cohort Files) | Native Hawaiian (n=40,917) †. | Clinical estimate (99.8%) or last menstrual period (0.2%) | GA <37 weeks |
| Schempf et al., 201039 b | 2003–2005 | Cross-sectional studye | California and Hawaii (Birth certificate data) | Native Hawaiian (n=16,805) †; Guamanian (n=1,406) †; Marshallese (n=938) f; Samoan (n=4,820) †; Tongan (n=1,594) | Last menstrual period (CA); last menstrual period and clinical estimate (HA) | GA <37 weeks |
| Washington State Department of Health, 201552,58 a, c | 2004–2015 | Descriptive studye | Washington (Washington State Birth Certificate Data) | Pacific Islander (n=10,900). | Not reported | GA <37 weeks |
| Altman et al., 201950 a b, d | 2007–2012 | Retrospective cohort study | California (California Office of Statewide Health Planning and Development birth cohort database) | Hawaiian (n=756) †; Guamanian (n=844) †; Samoan (n=2852) †; Other Pacific Islander (n=5422); More than one (n=596). | Not reported | GA <37 weeks |
| Dela Cruz et al., 201849 a | 2007–2014 | Retrospective cohort study cohort study | Commonwealth of the Northern Northern Mariana Islands (Hospital records) | Chamorro/Carolinian (n=2,799); Other Pacific Islander (unspecified, n=785). | Obstetric estimate | GA <37 weeks |
| Ratnasiri et al., 201840 c | 2007–2016 | Retrospective cohort study | California (Birth Statistical Master Files) | Guamanian, Hawaiian, Samoan, and other Pacific Islander (n not reported). | Obstetric estimate | GA <37 weeks |
| Morisaki et al., 201743 b | 2009–2012 | Descriptive studye | United States (The National Natality File) | Hawaiian (n=152) †; Guamanian (n=904) †; Samoan (n=2,481) †. | Not reported | Not reported |
| Hawaii State Department of Health, 201951 a, b, c | 2012–2015 | Descriptive studye | Hawaii (The Pregnancy Risk Assessment Monitoring System) | Native/Part Hawaiian (n=1,693) †; Samoan (n=67) †; Other Pacific Islander (Guamanian or Other, n=270). | Clinical estimate | GA <37 weeks |
| Quan et al., 202141 a, b | 2015–2017 | Cross-sectional studye | United States (The National Natality File) | Hawaiian (n=2,423) †; Guamanian (n=3,081) †; Samoan (n=6,362) †. | Last menstrual period | GA <37 weeks |
| Martin et al., 201844 a c | 2018 | Descriptive studye | United States (The National Natality File) | Native Hawaiian or Other Pacific Islander (n=9,476) | Obstetric estimate | GA <37 weeks |
GA, gestational age; PTB, preterm birth; CA, California; HA, Hawaii.
Labeled studies were included in general prevalence meta-analysis.
Labeled studies were included in subgroup ethnicity prevalence meta-analysis.
Labeled studies were included in risk comparison meta-analysis.
Labeled studies were included in the summary of risk factors for preterm birth.
Labeled study design was not reported and was decided by the first author’s judgement.
Since diagnosing PTB relies on accurate gestational age assessment, we summarized the gestational age (GA) estimation method in each included study (Table 1). Most of the included studies39,40,44,47–49,51 used clinical or obstetric estimates of GA53 which were calculated from a combination of ultrasound and last menstrual period (LMP). One study42 assessed GA with ultrasound only for the most of enrolled women, and three studies39,41,46 used LMP. One study54 used participant reported PTB (the symptom of PTB, yes/no), and the rest of studies43,45,50,52 did not report how GA was assessed. Except for one study43, where the definition of PTB was not reported, all included studies used the standard definition of live birth at <37 weeks gestation.
Outcomes of the risk of bias assessment are presented in Table S2-Table S4. Scores ranged from 37.5% to 100.0%, with higher scores indicating a lower risk of bias. Modeling methods and adjusted confounders in each included studies in the meta-analyses (Figure 4-Figure 5) are summarized in Table S5-Table S6. Among the 8 articles40,42,44,45,47,48,51,52 used in the analysis that compared PTB risk for Pacific Islander and White women, three40,45,48 (43.8%) included race/ethnicity as an adjusted confounder, while the rest of the comparison estimates were calculated from PTB prevalence in Pacific Islanders and White women.
Figure 4.
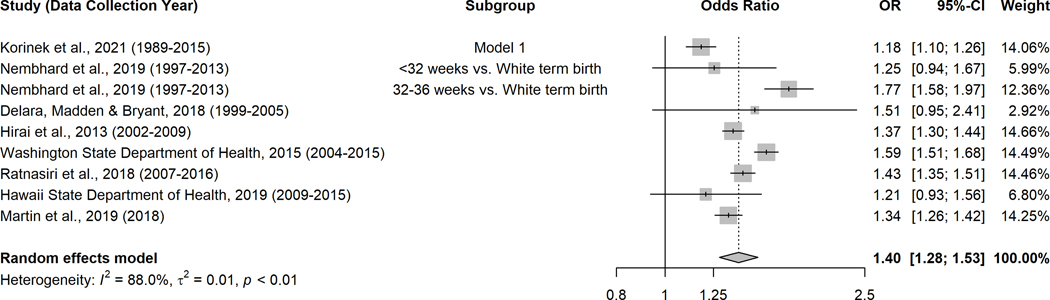
Forest plot of risk of preterm birth among Pacific Islanders compared to White women
Figure 5.
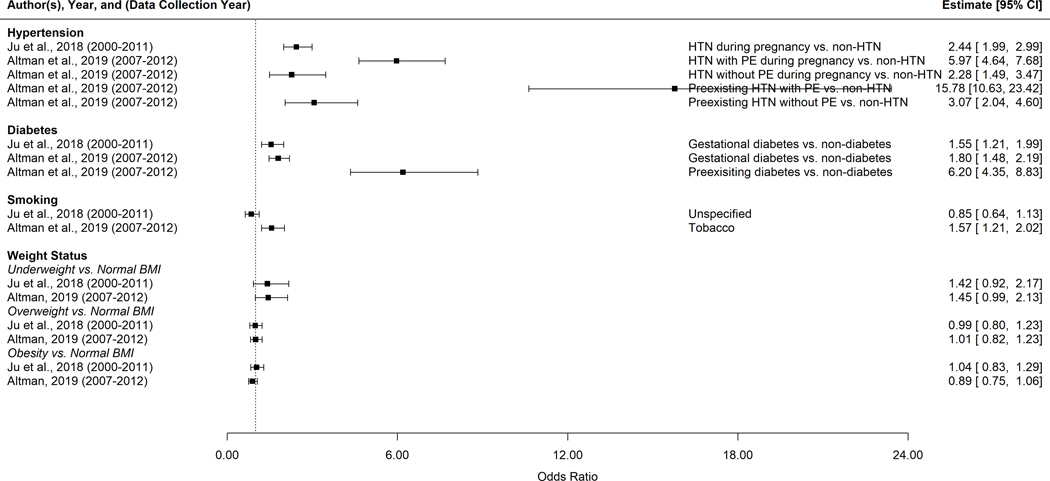
Summary of reported risk factors for preterm birth among Pacific Islanders in the US and the USAPI*
* HTN, hypertension; PE, pre-eclampsia; BMI, body mass index, (underweight: <18.5 kg/m2, normal BMI: 18.5–24.9 kg/m2, overweight: 25–29.9 kg/m2, obesity: >= 30 kg/m2).
3.2 |. Prevalence of PTB
Ten studies41,42,44,45,47–52 reported prevalence of PTB among Pacific Islanders (Figure 2). The overall random-effects pooled prevalence was 11.2% (95% CI: 9.3%−13.6%). The outcome of PTB prevalence meta-analyses by ethnicity subgroup is presented in Figure 3. Among Guamanian, Hawaiian41,47,50,51 and Samoan39,41,50,51 women, the prevalence of PTB in the past 25 years was similar to the pooled estimate, yet the prevalence among Marshallese39,42 was twofold higher (20.7%, 95% CI: 18.6%−23.0%) than the other Pacific Islanders subgroups (Figure 3).
Figure 2.
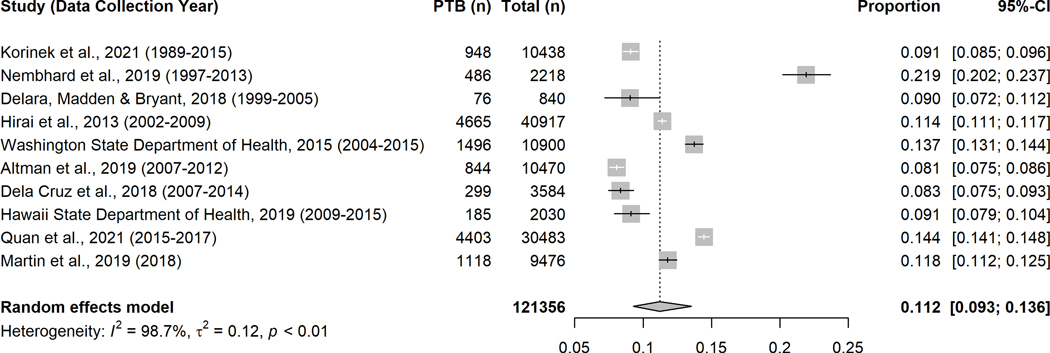
Forest plot of preterm birth prevalence among Pacific Islanders
Figure 3.
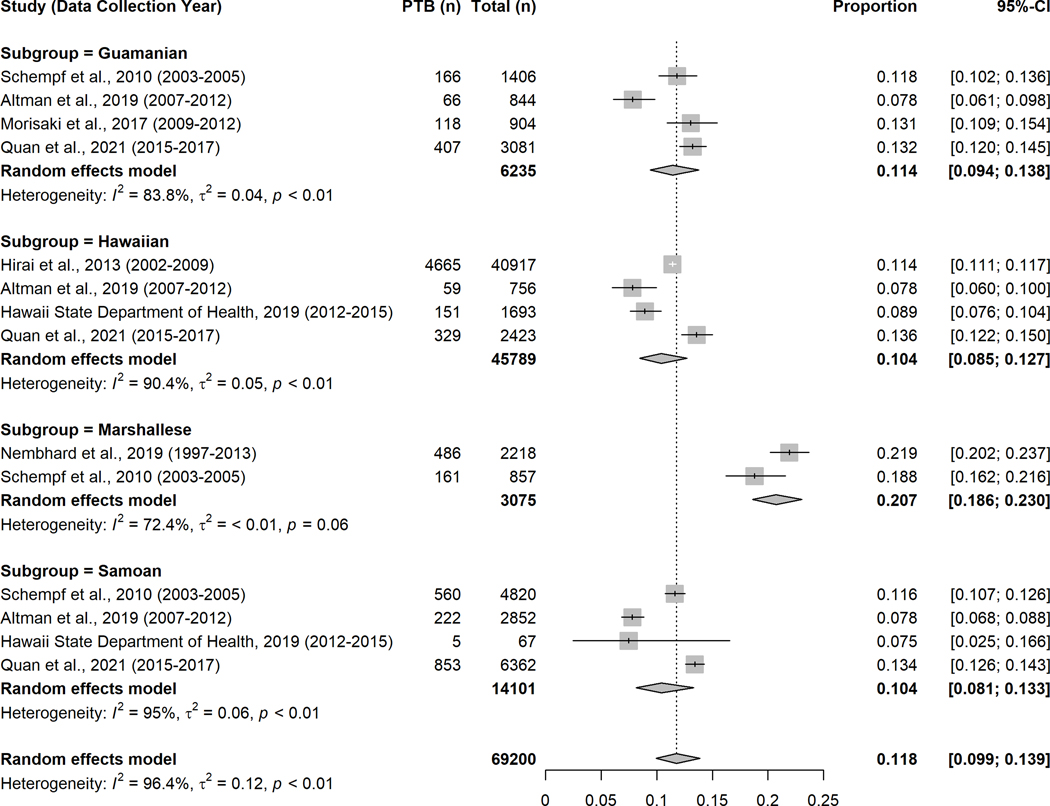
Forest plot of preterm birth prevalence among Pacific Islanders by four ethnicity subgroups*
* Please note, the overall estimate presented here is not expected to match that in Figure 2 since different studies reported with subgroup ethnicity outcomes were included in these analyses.
3.3 |. Risk of PTB compared to White women
Eight studies39,40,42–48,51,52,54–59 were used to compare risk of PTB among Pacific Islanders and White women (Figure 4). Compared to White women, Pacific Islanders had 1.40 times the odds of having a PTB (random-effects pooled OR=1.40, 95% CI: 1.28–1.53). To ensure the accuracy of this finding, we also conducted a sensitivity analysis in which we excluded the article42 that reported with RR rather than OR, and found the association was still significant with the same direction (random-effects pooled OR=1.37, 95% CI: 1.26–1.48).
3.4 |. Summary of risk factors for PTB among Pacific Islanders in the US and USAPI
We only identified two articles46,50 that reported risk factors for PTB in this population (Figure 5). Pacific women with any type of hypertension or diabetes tended to have higher odds of PTB compared to Pacific women without hypertension or diabetes. The observed associations between smoking and PTB were not consistent. Furthermore, we did not observe differences in the odds of PTB among Pacific Islander based on weight status.
3.5 |. Sources of heterogeneity
We checked four characteristics of each article to identify potential sources of heterogeneity: starting year of data collection, study duration, publication year, and study quality based on the JBI checklist score. None of these characteristics explained the observed heterogeneity in either the prevalence of PTB estimate (starting year of data collection, P=0.64; study duration length, P=0.53; publication year, P=0.78; study quality, P=0.80) or risk of PTB compared to White women (starting year of data collection, P=0.81; study duration length, P=0.80; publication year, P=0.51; study quality, P=0.62).
3.6 |. Publication bias
We did not identify any publication bias in our meta-analyses: for the PTB prevalence estimate, P=0.44; for the risk of PTB compared with White women, P=0.95
4 |. DISCUSSION
This is the first meta-analyses of data related to PTB among women of Pacific Islander ethnicity in the US and USAPI and these findings move significantly beyond the current status quo by providing pooled estimate of PTB prevalence among Pacific Islanders, as well as ethnicity-specific subgroup estimates, and by quantifying PTB-related health inequity among this group compared to White women.
Racial and ethnic disparities in PTB have been well documented in the US. Non-Hispanic Black women (13.6%) are almost 1.5 times more likely than non-Hispanic White women (9.5%) to experience PTB60. Our findings place most Pacific Islander women at relatively lower risk compared to non-Hispanic Black women, but at higher risk than White women and markedly higher risk than has been reported for Asian/Pacific Islanders combined (8.9% based on data from 2017–201961), highlighting the importance of disaggregating these groups. The increased risk of PTB among Pacific Islander women compared to non-Hispanic White women may be due to poorer antenatal care uptake, pre-pregnancy or maternal obesity, limited healthcare access, or experience of discrimination12,49,50,62–65, although further research is needed.
The particularly high prevalence of PTB reported among Marshallese women deserves further attention, even though they comprised a relatively small proportion of all women included in our meta-analyses. Much of the existing data on the perinatal health of Marshallese women comes from residents of Arkansas (as did two-thirds of the Marshallese sample included in our meta-analyses). Prior studies among Marshallese women resident in Arkansas have described delayed and underutilized prenatal care compared to non-Hispanic White women42. Cited barriers, which may be specific to the Arkansas setting, include delaying care until health crises62–64, language barriers38, lower educational attainment39, and distrust of Western medicine or health professionals8–11,66. Importantly, at the time of data collection by two of the studies (1997–201342, 2003–200539), Marshallese migrants were not eligible for most Federal benefits, including Medicaid, despite having legal residence and rights to work in the US63. Recent restoration of Medicaid eligibility for Compact of Free Association (COFA) migrants (December 2020)67,68 may positively impact health care utilization among Marshallese women in coming years, although the perinatal health of this at-risk group should be closely monitored. Meanwhile, further programs like the ongoing community-based participatory research in Arkansas should be promoted to better understand the cultural and environmental context for Marshallese women in the state and to address the noted disparities in perinatal outcomes69.
Few studies conducted Pacific Islander-specific analyses of risk factors for PTB. While smoking is a well-known risk factor for PTB among other ethnic groups70,71, we did not observe this consistently among the studies included in our review. Previous studies suggest that the association between smoking and PTB may be modified by factors such as trimester-specific smoking patterns70 and passive or initiative smoking72,73, which we were unable to explore given the paucity of studies. Hypertension and diabetes are also important risk factors for PTB74–77, and may be of particular relevance to Pacific Islander women given their increased risk of pre-pregnancy obesity78,79 and excessive gestational weight gain80. It is unclear whether the significance of the observed associations resulted from the chronic conditions alone or were modified by higher body mass index (BMI), which is known to independently increase the odds of PTB7. Although the combination of a high prevalence of overweight or obesity among the general Pacific Islander population and prior studies in other race/ethnic groups suggesting increased risk of PTB among women with this risk factor7,81 provided some of the motivation for this work, we only identified two studies46,50 that examined the association between BMI and PTB (neither of which showed an association). Both included speculated as to why they did not observe this expected association, but more research is needed to examine whether PTB risk is modified by maternal BMI in this population as it appears to be in others.
There are several considerations that should be made in interpreting the findings presented here. First, while we made efforts to reduce the potential overlap in study participants represented by the studies included, we may not have been able to account for all overlap. We suspect that there may be a very small degree of overlap between a Hawaii State Department of Health report51 and Quan et al41 based on both studies including data from 2015. We conducted a sensitivity analysis excluding the Hawaii report51 (due to its smaller sample size compared to Quan et al41) and did not find an obvious change (11.5%, 95% CI 9.3%−14.1%). Second, it should be noted that transforming RRs into ORs may overestimate association (i.e. Altman et al, preexisting hypertension with pre-eclampsia vs. non-hypertension, RR=8.0, 95% CI 5.9–10.7, transferred OR=15.78, 95% CI 10.63–23.42). Third, we observed high heterogeneity in our analyses. Recent methodological studies indicate that this is common in proportional meta-analyses due to the nature of the data82,83 and should not necessarily be of concern. We attempted to identify the source by including publication characteristics in meta-regression analyses and did not find an immediate explanation so exploring the heterogeneity in these studies should be the focus of future research efforts. Fourth, methods for estimating GA varied. Studies reported with obstetric estimates of GA may underestimate the PTB prevalence53,84. Concerns have been raised in similar studies about the self-reported nature of ethnicity. While ethnicity was likely self-reported by most participants included here, we do not consider this a weakness since PTB has a number of social as well as biological risk factors that may be best captured through group identity. Similarly, White women were selected as the reference group for the risk comparison of PTB since this comparison was reported the most in relevant studies, although best practice may have been to select the comparison group with the lowest PTB prevalence. Finally, by design, we were limited in this analysis to examining only those risk factors reported in the existing literature. Beyond these individual characteristics, there are many social determinants of health that are as yet unexplored and which may be of equal or greater importance in explaining risk of PTB among this group.
Study strengths include the use of MOOSE reporting guidelines24, strict article screening criteria, and a comprehensive search of multiple databases. We also recorded the adjustment methods and confounding factors in each study, and conducted sensitivity analyses to verify our meta-analysis outcomes.
Based on our meta-analysis, PTB prevalence among Pacific Islanders in the US and USAPI was 11.2%, and they were more likely than non-Hispanic White women to experience PTB. Marshallese women had a markedly higher pooled prevalence 20.7% than the average for Pacific Islanders and should be a target for prevention efforts. Our findings support ongoing calls to disaggregate the health outcomes of Pacific Islander women from those of Asian American ethnicity and argue for further disaggregation of data to represent the many Pacific Islander subgroups that make up this larger group in order to reveal the true burden of adverse pregnancy outcomes.
Supplementary Material
Funding:
This work was supported by US National Institutes of Health (PI: Hawley, NLH, grant number R03HD093993). The funders had no role in the design, analysis, or compiling of this manuscript.
Footnotes
PROSPERO ID: CRD42021281673
Disclosure statement: The authors report no conflict of interest.
Contributorship Statement: BW and NLH conceived the study, with the help from KA, KN, RS, and MM. KA, KN, RS, MM, and NLH developed the search strategy. KA, RS, MM, and NLH completed the study screening. BW and NLH extracted data from included studies. BW, KA, and NLH wrote the initial draft of the manuscript. All authors provided read, provided feedback on, and approved the final manuscript.
REFERENCES
- 1.World Health Organization. Preterm birth. Accessed September 11, 2020. https://www.who.int/en/news-room/fact-sheets/detail/preterm-birth
- 2.Chawanpaiboon S, Vogel JP, Moller A-B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ely DM, Driscoll AK. Infant Mortality in the United States, 2018: Data From the Period Linked Birth/Infant Death File. Natl Vital Stat Rep. 2020;69(7):1–18. [PubMed] [Google Scholar]
- 4.Osypuk TL, Acevedo-Garcia D. Are racial disparities in preterm birth larger in hypersegregated areas? Am J Epidemiol. 2008;167(11):1295–1304. [DOI] [PubMed] [Google Scholar]
- 5.Burris HH, Lorch SA, Kirpalani H, Pursley DM, Elovitz MA, Clougherty JE. Racial disparities in preterm birth in USA: a biosensor of physical and social environmental exposures. Arch Dis Child. 2019;104(10):931–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawley NL, McGarvey ST. Obesity and diabetes in Pacific Islanders: the current burden and the need for urgent action. Curr Diab Rep. 2015;15(5):29. [DOI] [PubMed] [Google Scholar]
- 7.McDonald SD, Han Z, Mulla S, Beyene J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. Br Med J. 2010;341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayers BL, Hawley NL, Purvis RS, Moore SJ, McElfish PA. Providers’ perspectives of barriers experienced in maternal health care among Marshallese women. Women Birth. 2018;31(5):e294–e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104(2):e16–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McElfish PA, Ayers BL, Purvis RS, Long CR, Esquivel M, Steelman SC. Best practices for community-engaged participatory research with Pacific Islander communities in the USA and USAPI: protocol for a scoping review. BMJ Open. 2018;8(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McElfish PA, Narcisse M-R, Long CR, et al. Leveraging community-based participatory research capacity to recruit Pacific Islanders into a genetics study. J Community Genet. 2017;8(4):283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorkin DH, Ngo-Metzger Q, De Alba I. Racial/ethnic discrimination in health care: impact on perceived quality of care. J Gen Intern Med. 2010;25(5):390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braveman P, Heck K, Egerter S, et al. Worry about racial discrimination: A missing piece of the puzzle of Black-White disparities in preterm birth? PLoS One. 2017;12(10):e0186151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franck LS, McLemore MR, Williams S, et al. Research priorities of women at risk for preterm birth: findings and a call to action. BMC Pregnancy Childbirth. 2020;20(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giurgescu C, Zenk SN, Dancy BL, Park CG, Dieber W, Block R. Relationships among neighborhood environment, racial discrimination, psychological distress, and preterm birth in African American women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E51–E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray R, Bonellie S, Chalmers J, Greer I, Jarvis S, Williams C. Social inequalities in preterm birth in Scotland 1980–2003: findings from an area-based measure of deprivation. BJOG. 2008;115(1):82–90. [DOI] [PubMed] [Google Scholar]
- 17.Newnham JP, White SW, Meharry S, et al. Reducing preterm birth by a statewide multifaceted program: an implementation study. Am J Obstet Gynecol. 2017;216(5):434–442. [DOI] [PubMed] [Google Scholar]
- 18.Nkansah-Amankra S, Dhawain A, Hussey JR, Luchok KJ. Maternal social support and neighborhood income inequality as predictors of low birth weight and preterm birth outcome disparities: analysis of South Carolina Pregnancy Risk Assessment and Monitoring System survey, 2000–2003. Matern Child Health J. 2010;14(5):774–785. [DOI] [PubMed] [Google Scholar]
- 19.Smith CJ, Baer RJ, Oltman SP, et al. Maternal dyslipidemia and risk for preterm birth. PLoS One. 2018;13(12):e0209579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith LK, Draper ES, Manktelow BN, Dorling JS, Field DJ. Socioeconomic inequalities in very preterm birth rates. Arch Dis Child Fetal Neonatal Ed. 2007;92(1):F11–F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindsay Hixson BBH, Myoung Ouk kim. The Native Hawaiian and Other Pacific Islander Population: 2010. U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau. Accessed October 7, 2020. https://www.census.gov/prod/cen2010/briefs/c2010br-12.pdf [Google Scholar]
- 22.Yamasato K, Chern I, Lee M-J. Racial/Ethnic Representation in United States and Australian Obstetric Research. Matern Child Health J. 2021;25(5):841–848. [DOI] [PubMed] [Google Scholar]
- 23.Kaholokula JKa, Okamoto SK, Yee BW. Special issue introduction: Advancing Native Hawaiian and other Pacific Islander health. Asian Am J Psychol. 2019;10(3):197. [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 25.Joanna Briggs Institute. Critical Appraisal Tools Accessed May 5, 2021. https://jbi.global/critical-appraisal-tools
- 26.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–153. [DOI] [PubMed] [Google Scholar]
- 27.Moola S, Munn Z, Tufanaru C, et al. Chapter 7: Systematic reviews of etiology and risk. Accessed May 5, 2021. https://synthesismanual.jbi.global
- 28.Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67(8):897–903. [DOI] [PubMed] [Google Scholar]
- 29.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jun Z, Yu Kai F. What’s the Relative Risk. JAMA. 1998;280(19):1690. [DOI] [PubMed] [Google Scholar]
- 31.Zeng X-T, Deng A-P, Li C, Xia L-Y, Niu Y-M, Leng W-D. Periodontal disease and risk of head and neck cancer: a meta-analysis of observational studies. PLoS One. 2013;8(10):e79017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160(4):301–305. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 35.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 36.Schwarzer G, Schwarzer MG. Package ‘meta’. Accessed April 10, 2021. https://mirror-hk.koddos.net/CRAN/web/packages/meta/meta.pdf
- 37.Harrer M, Cuijpers P, Furukawa T, Ebert DD. dmetar: Companion R Package For The Guide ‘Doing Meta-Analysis in R’. Accessed April 10, 2021. http://dmetar.protectlab.org/
- 38.Sentell T, Chang A, Ahn HJ, Miyamura J. Maternal language and adverse birth outcomes in a statewide analysis. Women Health. 2016;56(3):257–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schempf AH, Mendola P, Hamilton BE, Hayes DK, Makuc DM. Perinatal outcomes for Asian, Native Hawaiian, and other Pacific Islander mothers of single and multiple race/ethnicity: California and Hawaii, 2003–2005. Am J Public Health. 2010;100(5):877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratnasiri AW, Parry SS, Arief VN, et al. Temporal trends, patterns, and predictors of preterm birth in California from 2007 to 2016, based on the obstetric estimate of gestational age. Matern Health Neonatol Perinatol. 2018;4(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quan NS, Kramer MR. Revealing the variations in impact of economic segregation on preterm birth among disaggregated Asian ethnicities across MSAs in the United States: 2015–2017. SSM Popul Health. 2021;14:100813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nembhard WN, Ayers BL, Collins RT, et al. Adverse pregnancy and neonatal outcomes among Marshallese women living in the United States. Matern Child Health J. 2019;23(11):1525–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morisaki N, Kawachi I, Oken E, Fujiwara T. Social and anthropometric factors explaining racial/ethnical differences in birth weight in the United States. Sci Rep. 2017;7(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK. Births: final data for 2018. Natl Vital Stat Rep. 2019;68(13):1–47. [PubMed] [Google Scholar]
- 45.Korinek K, Ahmmad Z. The Racial Configuration of Parent Couples and Premature Birth: an Analysis of the Utah Population Database. J Racial Ethn Health Disparities. 2021:1–15. [DOI] [PubMed] [Google Scholar]
- 46.Ju AC, Heyman MB, Garber AK, Wojcicki JM. Maternal obesity and risk of preterm birth and low birthweight in Hawaii PRAMS, 2000–2011. Matern Child Health J. 2018;22(6):893–902. [DOI] [PubMed] [Google Scholar]
- 47.Hirai AH, Hayes DK, Taualii MM, Singh GK, Fuddy A, Loretta J. Excess infant mortality among Native Hawaiians: identifying determinants for preventive action. Am J Public Health. 2013;103(11):e88–e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delara RMM, Madden E, Bryant AS. Short interpregnancy intervals and associated risk of preterm birth in Asians and Pacific Islanders. J Matern Fetal Neonatal Med. 2018;31(14):1894–1899. [DOI] [PubMed] [Google Scholar]
- 49.Cruz RD, Grant J, Heck JE, Cash HL. Peer Reviewed: Disparities in Adverse Perinatal Outcomes Among Pacific Islanders in the Commonwealth of the Northern Mariana Islands. Prev Chronic Dis. 2018;15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altman MR, Baer RJ, Jelliffe-Pawlowski LL. Patterns of preterm birth among women of native Hawaiian and Pacific Islander Descent. Am J Perinatol. 2019;36(12):1256–1263. [DOI] [PubMed] [Google Scholar]
- 51.Hawaii State Department of Health. Hawaii Pregnancy Risk Assessment Monitoring System (PRAMS). Hawaii State Department of Health. Accessed May 17, 2021. https://health.hawaii.gov/fhsd/files/2019/07/PRAMS-Trend-Report-ALL-FINAL6-2019-LR.pdf [Google Scholar]
- 52.Washington State Department of Health. Preterm Delivery for Singleton Births. Accessed May 17, 2021. https://www.doh.wa.gov/Portals/1/Documents/Pubs/160-015-MCHDataRptPrenatalDeliv.pdf
- 53.Vogel JP, Chawanpaiboon S, Moller A-B, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3–12. [DOI] [PubMed] [Google Scholar]
- 54.Mattheus D, Shannon M, Lim E, Gandhi K. The Association Between Socio-demographic Factors, Dental Problems, and Preterm Labor for Pregnant Women Residing in Hawai ‘i. Hawaii J Med Public Health. 2016;75(8):219. [PMC free article] [PubMed] [Google Scholar]
- 55.Kim S, Choi S, Chung-Do JJ, Fan VY. Comparing Birth Outcomes in Hawai ‘i between US-and Foreign-Born Women. Hawaii J Med Public Health. 2018;77(8):188. [PMC free article] [PubMed] [Google Scholar]
- 56.Crowell DH, Rudoy R, Nigg CR, Sharma S, Baruffi G. Perspective on Racial-Ethnic Birth Weight. Hawaii Med J. 2010;69(9):216. [PMC free article] [PubMed] [Google Scholar]
- 57.Seattle & King County Public Health. Health of Mothers and Infants by Race/Ethnicity. Accessed May 17, 2021. https://www.kingcounty.gov/depts/health/data/~/media/depts/health/data/documents/Health-of-Mothers-and-Infants-by-Race-Ethnicity.ashx
- 58.Washington State Department of Health. Birth Risk Factors by Maternal Race Dashboards. Accessed May 18, 2021. https://www.doh.wa.gov/DataandStatisticalReports/HealthDataVisualization/BirthDashboards/BirthRiskFactorsRace
- 59.Tiwari R, Enquobahrie DA, Wander PL, Painter I, Souter V. A retrospective cohort study of race/ethnicity, pre-pregnancy weight, and pregnancy complications. J Matern Fetal Neonatal Med. 2021:1–8. [DOI] [PubMed] [Google Scholar]
- 60.Martin J, Osterman M. Describing the increase in preterm births in the United States, 2014–2016. NCHS Data Brief. 2018;(312):1–8. [PubMed] [Google Scholar]
- 61.March of Dimes Foundation. Preterm by race: United States, 2017–2019 Average. Accessed August 2, 2021. https://www.marchofdimes.org/Peristats/ViewSubtopic.aspx?reg=99&top=3&stop=62&lev=1&slev=1&obj=1
- 62.Choi JY. Seeking health care: Marshallese migrants in Hawai ‘i. Ethn Health. 2008;13(1):73–92. [DOI] [PubMed] [Google Scholar]
- 63.McElfish PA, Hallgren E, Yamada S. Effect of US health policies on health care access for Marshallese migrants. Am J Public Health. 2015;105(4):637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams DP, Hampton A. Barriers to health services perceived by Marshallese immigrants. J Immigr Minor Health. 2005;7(4):317–326. [DOI] [PubMed] [Google Scholar]
- 65.LeFevre ML. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(12):902–910. [DOI] [PubMed] [Google Scholar]
- 66.Ayers BL, Purvis RS, Bing WI, et al. Structural and socio-cultural barriers to prenatal care in a US Marshallese community. Matern Child Health J. 2018;22(7):1067–1076. [DOI] [PubMed] [Google Scholar]
- 67.Asian & Pacific Islander American Health Forum. Health care restored for COFA citizens in december 2020 Omnibus COVID relief bill. Accessed August 17, 2021. https://www.apiahf.org/wp-content/uploads/2020/12/Dec-2020-COFA-Factsheet_Once-Enacted5.pdf
- 68.Congress. Consolidated Appropriations Act, 2021. Accessed August 17, 2021. https://rules.house.gov/sites/democrats.rules.house.gov/files/BILLS-116HR133SA-RCP-116-68.pdf
- 69.McElfish PA, Moore R, Laelan M, Ayers BL. Using CBPR to address health disparities with the Marshallese community in Arkansas. Annals of Human Biology. 2018;45(3):264–271. [DOI] [PubMed] [Google Scholar]
- 70.Moore E, Blatt K, Chen A, Van Hook J, DeFranco EA. Relationship of trimester-specific smoking patterns and risk of preterm birth. Am J Obstet Gynecol. 2016;215(1):109. e1–109. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ion R, Bernal AL. Smoking and preterm birth. Reprod Sci. 2015;22(8):918–926. [DOI] [PubMed] [Google Scholar]
- 72.Cui H, Gong T-T, Liu C-X, Wu Q-J. Associations between passive maternal smoking during pregnancy and preterm birth: evidence from a meta-analysis of observational studies. PLoS One. 2016;11(1):e0147848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qiu J, He X, Cui H, et al. Passive smoking and preterm birth in urban China. Am J Epidemiol. 2014;180(1):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Köck K, Köck F, Klein K, Bancher-Todesca D, Helmer H. Diabetes mellitus and the risk of preterm birth with regard to the risk of spontaneous preterm birth. J Matern Fetal Neonatal Med. 2010;23(9):1004–1008. [DOI] [PubMed] [Google Scholar]
- 76.Mann JR, McDermott S, Griffith MI, Hardin J, Gregg A. Uncovering the complex relationship between pre-eclampsia, preterm birth and cerebral palsy. Paediatr Perinat Epidemiol. 2011;25(2):100–110. [DOI] [PubMed] [Google Scholar]
- 77.Sibai BM, Caritis SN, Hauth JC, et al. Preterm delivery in women with pregestational diabetes mellitus or chronic hypertension relative to women with uncomplicated pregnancies. Am J Obstet Gynecol. 2000;183(6):1520–1524. [DOI] [PubMed] [Google Scholar]
- 78.Singh GK, DiBari JN. Marked disparities in pre-pregnancy obesity and overweight prevalence among US women by race/ethnicity, nativity/immigrant status, and sociodemographic characteristics, 2012–2014. J Obes. 2019;2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeng X HD, Shor R, Feigal D, Roberson E, Fuddy L. “Preconception Overweight/Obesity and Pregnancy Fact Sheet. Hawai’i Department of Health, Family Health Services Division. Accessed August 18, 2021. https://health.hawaii.gov/mchb/files/2013/05/obesity.pdf [Google Scholar]
- 80.Hawley NL, Johnson W, Hart CN, et al. Gestational weight gain among American Samoan women and its impact on delivery and infant outcomes. BMC Pregnancy Childbirth. 2015;15(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Torloni MR, Betran AP, Daher S, et al. Maternal BMI and preterm birth: a systematic review of the literature with meta-analysis. The Journal of Maternal-Fetal & Neonatal Medicine. 2009;22(11):957–970. [DOI] [PubMed] [Google Scholar]
- 82.von Hippel PT. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC medical research methodology. 2015;15(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barker TH, Migliavaca CB, Stein C, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Medical Research Methodology. 2021;21(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barradas DT, Dietz PM, Pearl M, England LJ, Callaghan WM, Kharrazi M. Validation of Obstetric Estimate Using Early Ultrasound: 2007 C alifornia Birth Certificates. Paediatr Perinat Epidemiol. 2014;28(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


