Abstract
Purpose
To evaluate patient satisfaction after implantation of the Tecnis Symfony multifocal intraocular lens (MIOL).
Methods
120 eyes of 60 subjects with senile cataract were bilaterally implanted with the Tecnis Symfony IOL. Follow-up examination was performed 6 months postoperatively. Main outcome measures included uncorrected and corrected distance and near visual acuity, manifest refraction, and visual quality metrics. According to their subjective symptoms patient were divided in two groups: satisfied and unsatisfied.
Results
Uncorrected intermediate (0.15 ± 0.11 vs 0.18 ± 0.01, P = 0.04) and near (0.26 ± 0.12 vs 0.31 ± 0.11, P = 0.04) (UIVA, UNVA) log MAR visual acuity was significantly better, cylindrical error less (0.31 ± 0.36 vs 0.67 ± 0.29, P = 0.05), axial length (AL) smaller (23.68 ± 1.3 vs 24.22 ± 1.6, P = 0.05), Strehl ratio higher (0.08 ± 0.08 vs 0.05 ± 0.04, P = 0.03) and mesopic pupil larger (4.3 ± 1.1 vs 3.7 ± 1.05, P = 0.01) among satisfied patients.Residual cylinder, Strehl ratio, halos, mesopic pupil diameter and UNVA were significant predictors of patient satisfaction. Uncorrected distance visual acuity, higher order Strehl ratio and pupil diameter were significant predictors of halos. Near visual acuity significantly correlated (P = 0.018, R = 0.22) with axial length.
Conclusions
Uncorrected cylindrical error, poor reading quality, larger pupil and halos seem to be the most disturbing factors for patients implanted with the Tecnis Symfony IOL.
Keywords: Multifocal IOL, EDOF, Patient satisfaction, Optical quality
Highlights
-
•
Visual quality measures are better indicators of subjective visual dissatisfaction than traditional visual acuity.
-
•
Uncorrected cylindrical error, poor reading quality, larger pupil and halos seem to be the most disturbing factors for patients implanted with the Symfony IOL.
-
•
Patients with shorter axial length had a better uncorrected near visual acuity.
1. Introduction
“The unhappy multifocal lens patient” is a well-known and often very challenging problem for most of the cataract surgeons. The advances in surgical techniques along with the evolution of intraocular lenses (IOLs) over the last decade have raised expectations among patients with cataracts who await almost perfect vision and are less willing to accept spectacles for postoperative vision correction. In an attempt to meet these expectations, established multifocal intraocular lens (MIOL) models have been significantly revised and improved over the years while other models have been newly developed. However, the results were not always as good as expected due to several factors, such as poor refractive outcomes, residual astigmatism and complaints from many patients of poor intermediate vision, decreased contrast sensitivity and the occurrence of disabling photic phenomena (glare and halos) particularly at night.1, 2, 3, 4, 5 The problem worsens when the cause cannot be easily identified and treated by means of capsulotomy or correcting postoperative refractive error with laser enhancement.6 Often these patients complain about poor visual quality under certain circumstances or about fatigue without any objective reason and despite excellent uncorrected visual acuity. Photic phenomena and the frequently used mini-monovision approach by low add multifocal IOLs probably explain part of these problems, while other authors suggest that rather patients' personality is the main cause.7,8 It has been found that patients who are unsatisfied after MIOL implantation often show “neuroticism” as a dominant personality trait compared with satisfied patients with “conscientiousness” and “agreeableness” as dominant personality trait. Therefore, the question arises: how can we objectively asses complaints of poor visual quality and which refractive, optical or biometrical factors contribute to their development? As multifocal IOLs are getting widely used, it seems to be urgent to find answers.
Nowadays, with modern imaging technologies in combination with wavefront aberration measurements it is possible to determine the optical impact of IOL. The HOYA iTrace™ Surgical Workstation (HOYA Surgical Optics GmbH, Germany) based on ray tracing principle is able to measure the retinal image quality objectively.9, 10, 11
Extended depth-of-focus (EDOF) IOLs are popular because of their tolerance to residual refractive errors and lower occurrence of photic phenomena. They also enable an excellent intermediate vision, which is a great advantage compared to the earlier bifocal lenses. The Tecnis Symfony MIOL (Johnson and Johnson Vision, Jacksonville, FL) is a bifocal multifocal IOL made of a diffractive step-like optical profile, intended to extend the range of vision.12, 13, 14, 15
The purpose of our study was to analyse the visual and optical results after implantation of the Symfony MIOL and to find associations between patients' satisfaction and objective outcome measures.
2. Methods
2.1. Patients and implants
In this prospective, case-controlled study 120 eye of 60 patients with age related cataract were included (Table 1). Each patient received, based on the preexisting corneal astigmatism either a toric or a non-toric IOL (55 toric, 65 non toric). The final decision was made after calculating the residual refractive cylinder with the online toric calculator of the lens provider also taking into account the surgically induced astigmatism. The purpose was to achieve the smallest amount of refractive cylinder postoperatively, but to avoid an overcorrection of the cylindrical error. The target refraction of the dominant eye was emmetropia and slight myopia (mini-monovision; −0.5 to −0.75 D) in the non-dominant eye.
Table 1.
Preoperative characteristics. Data are presented as mean ± SD.
| Tecnis Symfony IOL (N = 70) | |
|---|---|
| Patients | 60 |
| Age (year) | 64.7 ± 9.41 |
| Gender (male:female) | 16:19 |
| CDVA (logMAR) | 0.25 ± 0.18 |
| SE (D)Fluorescein tear breakup time, s | −1.28 ± 3.93 |
| AL (mm) | 23.91 ± 1.41 |
| Corneal Astigmatism (D) | 1.45 ± 2.88 |
| Spherical IOL power (D) | 20.65 ± 4.52 |
| Cylindrical IOL power (D) | 0.78 ± 0.98 |
CDVA = corrected distance visual acuity; logMAR, logarithm of the minimum angle of resolution; SE = spherical equivalent; AL = axial length.
The Tecnis Symfony ZXR00 (Johnson and Johnson Vision, Jacksonville, FL) is a single-piece, hydrophobic acrylic low-add bifocal lens, with an anterior aspheric surface, and a posterior diffractive surface, which aims to reduce the chromatic aberration of a pseudophakic eye.13, 14, 15
Each patient underwent a complete ophthalmological evaluation. Subjects with previous ocular surgery, trauma, active ocular disease, poorly dilated pupils, or known zonular weakness were excluded from the study.
The study was conducted in compliance with the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH + GCP) of the word health organization (WHO), as well as with applicable country and local requirements regarding ethics committee/institutional review boards. Informed consents were obtained and other statutes or regulations regarding protection of the rights and welfare of human subjects participating in biomedical research were considered.
2.2. Surgery
Five experienced surgeons performed all cataract extractions in local anesthesia. The 2.4 mm, incision, injection of viscoelastic agent, capsulorhexis (with 360° overlapping edges), phacoemulsification and irrigation/aspiration of cortical material were performed as standard procedures. The multifocal IOL was implanted via injector into the capsular bag followed by thorough aspiration of the viscoelastic agent from the eye.
2.3. Preoperative and postoperative examination
Preoperatively all patients underwent an ophthalmic examination including visual acuity assessment, slit lamp biomicroscopy and retinal examination. Retinal optical coherence tomography (OCT) imaging was performed to exclude patients with retinal pathology. Biometry using the IOL Master (Carl Zeiss Meditec AG) and topography with iTrace VFA were performed. The ULIB optimized IOL constants were used to determine IOL power for the MIOLs. The IOL power corresponding to the smallest refractive error, based on the IOL Master measurements, was chosen. Follow up assessments were performed 1 week, 1 and 6 months postoperatively including: autorefractometry, manifest refraction, uncorrected and corrected monocular and binocular distance and near visual acuity and defocus curves. Axial alignment of toric MIOLs was measured using the tilted narrow slit light of the slit lamp, which was adjusted to the marking on the IOL and the degree of axial alignment was read by the investigator. Topographic and aberrometric measurements were performed with dilated pupil. The examiners performing the postoperative examinations were blind to the type of IOL implanted in patient eyes. The surgeons didn't perform the follow-up examinations. To characterize accuracy of the two IOLs, prediction error (PE) was calculated as the arithmetic difference in diopters between the actual and intended refractive outcome.16
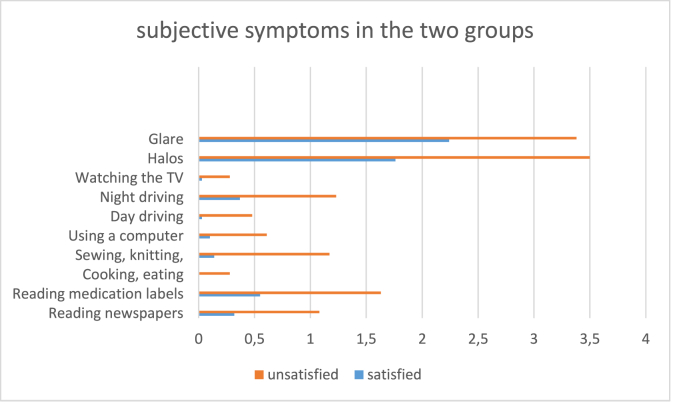
The patients were also asked to complete a previously validated11 questionnaire about their satisfaction with the results of the surgery and their impairment affecting their daily routine, such as: reading, using a computer, driving at day/night and watching TV. Two scores were used to measure the overall satisfaction and disturbance in daily life tasks. The satisfaction-score ranged from 0 to 4 (very satisfied = 0, satisfied = 1, little satisfied = 2, not satisfied = 3, very dissatisfied = 4). Each daily life task was given a disturbance-score from 0 to 2 (not disturbed at all = 0, sometimes disturbed = 1, always disturbed = 2), a higher score showed a lower satisfaction and a higher impairment of the patient. They were also asked to report how often they perceived halos at night or dawn and glare (never = 1, rarely = 2, sometimes = 3, often = 4, always = 5). We have previously shown them animated pictures of halos, starburst and glare for better understanding. See the detailed questions in Fig. 1 and Table 2. Patients were then dichotomized in 2 groups. The points for each answers were summarized and the median value was used to dichotomize. Those whose average was under the entire cohort's median value were classified as the “satisfied” (32 patients) and those whose value was above the median as “unsatisfied” (28 patients).
Fig. 1.
The distribution of subjective complaints among satisfied and unsatisfied patients.
Table 2.
Questionnaire results. Data are presented as mean ± SD.
| Satisfied patients (N = 32) | Unsatisfied patients (N = 28) | |
|---|---|---|
| General Satisfaction (0–4) | 0.2 ± 0.5 | 0.9 ± 1.1 |
| Reading newspaper (0–2) | 0.3 ± 0.6 | 1.1 ± 0.9 |
| Reading medication label (0–2) | 0.6 ± 0.8 | 1.6 ± 0.6 |
| Using a computer (0–2) | 0.1 ± 0.3 | 0.6 ± 0.7 |
| Driving during the day (0–2) | 0.03 ± 0.18 | 0.48 ± 0.59 |
| Driving during the night (0–2) | 0.37 ± 0.63 | 1.2 ± 0.8 |
| Cooking, eating (0–2) | 0.0 ± 0.0 | 0.3 ± 0.5 |
| Sewing, knitting (0–2) | 0.14 ± 0.4 | 1.2 ± 0.7 |
| Watching TV (0–2) | 0.03 ± 0.19 | 0.3 ± 0.2 |
| Halos (1–5) | 1.8 ± 1.02 | 3.5 ± 1.3 |
| Glare (1–5) | 2.2 ± 1.1 | 2.3 ± 1.1 |
| Total spectacle independence (percent) | 13 | 25 |
2.4. Aberrometry measurements
Corneal topography and ocular quality measurements were performed using the iTrace VFA Visual Function Analyzer. The corneal topography is obtained by Placido disk technology and the ocular wavefront is measured using ray tracing principle.11,12 The iTrace receives signals from the various zones of the MIOL, but selects only the strongest signal for further analysis, which is the far field (distance) of the MIOL. Images were recorded with the patient focusing on a distant target with dilated pupils and a fixed entrance pupil scan size of 4.0 mm. Each measurement was repeated at least 3 times. The best scan (ie, the one with the best quality peaks for individual points) was chosen for the final analysis. Visual quality was described by Strehl ratio and higher order Strehl ratio. The Strehl is the ratio between the point spread function (PSF) of the measured eye and the PSF of an ideal eye where optical quality is limited only by diffraction. It is a number between 0 and 1.17
2.5. Data and statistical analyses
Statistical analyses were performed with SPSS (IBM SPSS Statistics for Windows, version 25,0, IBM, Armonk, NY, USA). All data are presented as means and standard deviations (SD). Patient demographics at baseline were analyzed using Pearson's chi-squared test and Student's t-test. Spearman's rank correlation analysis was used to assess correlations. Generalized estimating equation (GEE) multivariable regression analysis was used to determine significant predictors of patient's satisfaction and halos. In this model, data from the two eyes of the same subject were statistically analyzed as repeated measures. Thus, this analysis takes into account the correlated nature of data from the two eyes of the same patient. The construction of the multivariable logistic regression model has been started with variables that showed the best fit to data in the univariable model, assessed by the QICC (Corrected Quasi Likelihood under Independence Model Criterion) value. The lower the QICC value the better is the model is. In a stepwise approach, new variables were added and the change in the QICC value was calculated. Only variables associated with a P value less than 0.05 were kept in the model.18 A P value of <0.05 was considered significant.
3. Results
The study enrolled a total of 120 eyes of 60 patients with age related cataract. Table 1 shows the patients’ demographics and preoperative data. Initially 67 patients were recruited, but 7 refused follow up visits due concerns related to the Covid pandemic. There were no intraoperative or other complications. None of the IOLs had to be explanted, 5 IOLs needed repositioning within the first two weeks because of significant residual astigmatism due to axial misalignment. The mean postoperative rotation measured on the slit lamp at the 6th month visit was 2.94 ± 3.01°.
The primary endpoint of the study was uncorrected distance visual acuity (UDVA) at 6 months after surgery. According to our hypothesis UDVA of satisfied patients is not inferior to the visual outcome of unsatisfied patients receiving the toric Symfony IOL. A post hoc power analysis was performed. Using two-sided t-test at significance level 0.05 and our values of UDVA (see in Table 2) in the two groups with a sample size of 32 vs 28 patients per group we have 98.9% power to confirm the null hypothesis.
3.1. Visual acuity, refraction and biometrical results
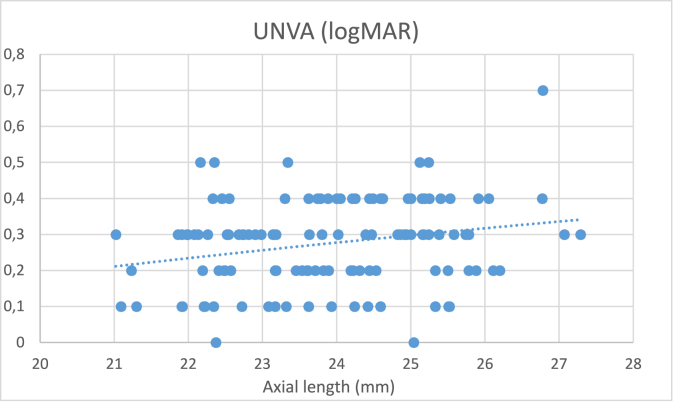
Table 2 shows the detailed patient satisfaction scores. In the satisfied group 13% of patients have achieved spectacle independence, in the unsatisfied group 25% (P > 0.05), the remaining patients needed reading glasses at least occasionally. Fig. 1 depicts the distribution of complaints between the two groups. Table 3 shows the postoperative biometrical, refractive, and visual results in the two study groups. At the 6th month visit no significant difference was found in monocular and binocular uncorrected (UDVA) and corrected (CDVA) distance visual acuity between the groups. Uncorrected intermediate (UIVA) and uncorrected near (UNVA) visual acuity were marginally significantly better among the satisfied patients. Residual refractive cylinder was marginally significantly less in the satisfied group, whereas axial length was shorter and mesopic pupil diameter smaller, although when we applied Bonferroni correction of the P values, these differences have disappeared. As depicted in Fig. 2, uncorrected near visual acuity showed a significant positive correlation with the axial length (P = 0.01; R=0.22). The difference of refractive error between the two eyes (mini-monovision effect) and the predictive error showed similar values in the two groups.
Table 3.
Postoperative biometrical, refractive, and visual results in the two study groups. Data are presented as mean ± SD.
| Satisfied patients (N = 32) | Unsatisfied patients (N = 28) | P | Bonferroni adjusted P v | |
|---|---|---|---|---|
| Spherical error (D) | −0.54 ± 0.51 | −0.53 ± 0.46 | 0.91 | 1.0 |
| Cylindric error (D) | 0.31 ± 0.36 | 0.67 ± 0.29 | 0.05∗ | 0.9 |
| Corneal Astigmatism (D) | 0.85 ± 0.61 | 0.96 ± 0.5 | 0.45 | 1.0 |
| Monocular UDVA (logMAR) | 0.12 ± 0.19 | 0.06 ± 0.09 | 0.36 | 1.0 |
| Binocular UDVA (logMAR) | 0.05 ± 0.07 | 0.01 ± 0.06 | 0.75 | 1.0 |
| 0.06 | ||||
| Monocular CDVA (logMAR) | −0.01 ± 0.05 | 0.008 ± 0.04 | 0.68 | 1.0 |
| Monocular UIVA (logMAR)Fluorescein tear breakup time, s | 0.15 ± 0.11 | 0.18 ± 0.07 | 0.04∗ | 0.72 |
| Binocular UIVA (logMAR) | 0.11 ± 0.07 | 0.13 ± 0.06 | 0.34 | 1.0 |
| 0.07 | ||||
| Monocular CIVA (logMAR) | 0.1 ± 0.06 | 0.12 ± 0.05 | 0.24 | 1.0 |
| v± | ||||
| Monocular UNVA (logMAR) | 0.26 ± 0.12 | 0.31 ± 0.11 | 0.04∗ | 0.72 |
| Binocular UNVA (logMAR) | 0.19 ± 0.11 | 0.22 ± 0.12 | 0.25 | 1.0 |
| Monocular CNVA (logMAR) | 0.12 ± 0.05 | 0.12 ± 0.05 | 0.45 | 1.0 |
| CDVA improvement | 0.23 ± 0.29 | 0.24 ± 0.25 | 0.84 | 1.0 |
| Axial length (mm) | 23.68 ± 1.25 | 24.22 ± 1.61 | 0.05∗ | 0.9 |
| Refractive error difference (D) | 0.58 ± 0.74 | 0.46 ± 0.52 | 0.5 | 1.0 |
| ± | ||||
| PE (D) | −0.19 ± 0.71 | −0.14 ± 0.62 | 0.76 | 1.0 |
| ± | ||||
| Mesopic pupil diameter (mm) | 3.7 ± 1.05 | 4.26 ± 1.1 | 0.01∗ | 0.18 |
| Photopic pupil diameter (mm) | 2.86 ± 1.17 | 3.28 ± 1.01 | 0.68 | 1.0 |
SE = spherical equivalent; UDVA = uncorrected distance visual acuity, CDVA = corrected distance visual acuity; UIVA = uncorrected intermediate visual acuity; CIVA = corrected intermediate visual acuity; UNVA: uncorrected near visual acuity; CNVA = corrected near visual acuity; Refractive error difference = the difference of refractive error (D) between the two eyes; PE = Predictive Error; P: difference between the two groups using students t-test.
Fig. 2.
Correlation between postoperative uncorrected near visual acuity and axial length.
3.2. Aberrations and visual quality
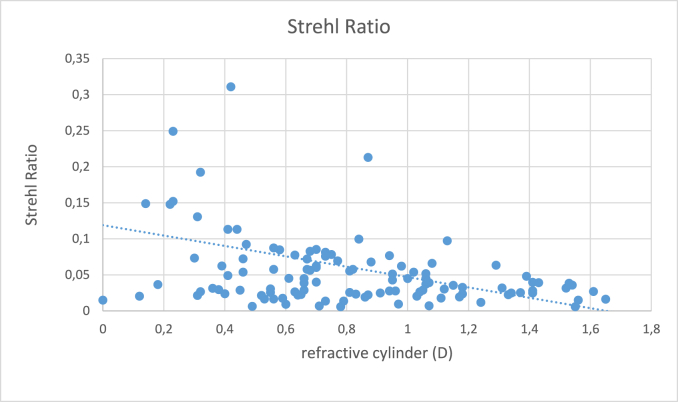
Table 4 shows the postoperative lower and higher order aberrometric (RMS) and visual quality (Strehl ratio and MTF) results in the two groups. The total Strehl ratio of the eye was significantly higher among the satisfied patients indicating better visual quality. There was no significant difference in angle kappa or angle alpha distance across the groups. As depicted on Fig. 3, Strehl ratio showed a significant negative correlation with the residual refractive cylinder (P < 0.001; r: 0.4). Interestingly uncorrected visual acuity showed no significant correlation with residual refractive cylinder (P = 0.74).
Table 4.
Postoperative Aberrations, Visual Quality and Angle Alppha and Kappa results in the two study groups for 4 mm pupil. Data are presented as mean ± SD.
| Satisfied patients (N = 32) | Unsatisfied patients (N = 28) | P | Bonferroni adjusted P | |
|---|---|---|---|---|
| RMS (μm) | 0.64 ± 0.68 | 0.6 ± 0.29 | 0.77 | 1.0 |
| Strehl | 0.08 ± 0.08 | 0.05 ± 0.04 | 0.03∗ | 0.24 |
| MTF | 0.24 ± 0.07 | 0.25 ± 0.1 | 0.31 | 1.0 |
| Higher order terms | ||||
| HORMS (μm) | 0.32 ± 0.37 | 0.22 ± 0.13 | 0.09 | 0.72 |
| HO Strehl | 0.19 ± 0.15 | 0.11 ± 0.14 | 0.14 | 1.0 |
| HO MTF | 0.39 ± 0.11 | 0.4 ± 0.11 | 0.66 | 1.0 |
| Angle Alpha distance (mm) | 0.38 ± 0.16 | 0.39 ± 0.16 | 0.59 | 1.0 |
| Angle Kappa distance (mm) | 1.35 ± 1.34 | 1.55 ± 1.46 | 0.47 | 1.0 |
RMS = root mean square; HORMS = higher order root mean square; MTF = modulation transfer function; SA = spherical aberration; ∗statistically significant difference between preoperative and postoperative values at a = 0.05 level; P: difference between the two groups using the Mann- Whitney U test.
Fig. 3.
Correlation between postoperative Strehl ratio and residual refractive cylinder.
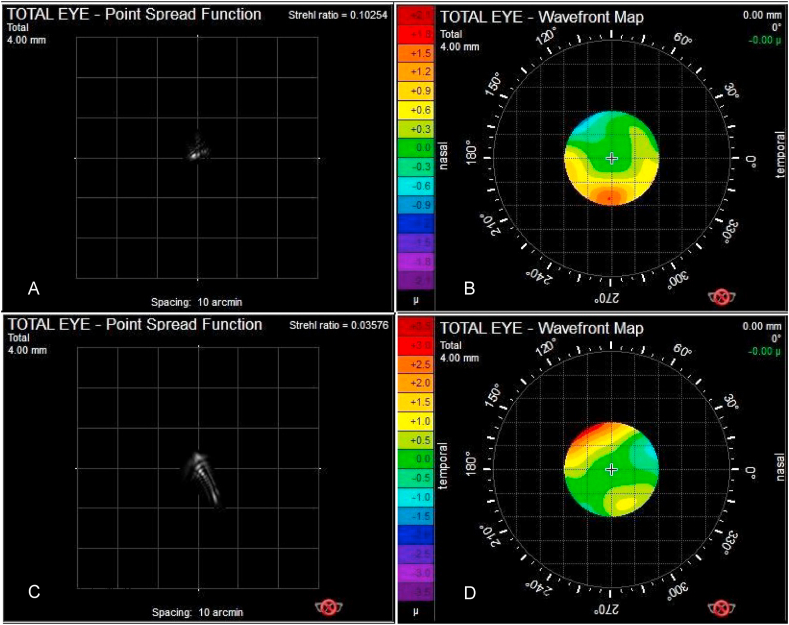
Fig. 4A–D depict the postoperative PSF (point spread function) and wavefront images of satisfied (4A, B) and an unsatisfied (4B,C) patient measured at a 4 mm entrance pupil. See a decrease in the Strehl Ratio and a degradation of the PSF image by the unsatisfied patient.
Fig. 4.
The postoperative PSF (point spread function) and wavefront images of patients
A. The point spread picture of a satisfied patient. B. The wavefront picture of a satisfied patient.
C. The point spread picture of an unsatisfied patient. D. The wavefront picture of an unsatisfied patient.
3.3. Risk factors
To determine significant predictors of patients' dissatisfaction, multivariable regression analyses (GEE) was performed. In this model, the most important confounders (see Table 5) were included. In the best fit model (QICC: 34.76) postoperative residual refractive cylinder, total Strehl ratio, mesopic pupil diameter, halo perception and uncorrected near visual acuity had a significant influence on patients' satisfaction. With regard to halos, higher order Strehl Ratio and mesopic pupil diameter were significant predictors (Table 6).
Table 5.
GEE logistic regression analysis of factors independently associated with dissatisfaction.
| Variables | Coefficient β | P | 95% CI |
|---|---|---|---|
| refractive cylinder (D) | 0.361 | 0.02 ∗ | 0.042–0.68 |
| Strehl Ratio | −6.12 | 0.019 ∗ | −11.23–1.01 |
| mesopic pupil diameter (mm) | 0.267 | <0.001 ∗ | 0.129–0.405 |
| Halos | 0.646 | <0.001 ∗ | 0.034–1.259 |
| UNVA | 6.47 | 0.017 ∗ | 1.17–11.77 |
HO = higher order; UNVA: uncorrected near visual acuity.
QIC: 34.309; QICC: 34.755
Table 6.
GEE logistic regression analysis of factors independently associated with halos.
| Variables | Coefficient β | P | 95% CI |
|---|---|---|---|
| HO Strehl Ratio | −3.48 | 0.04 ∗ | −6.77–−0.19 |
| mesopic pupil diameter (mm) | 0.65 | 0.001 ∗ | −0.26–1.03 |
HO = higher order; UDVA = uncorrected distance visual acuity.
QIC: 55.622; QICC: 61.316.
4. Discussion
Multifocal IOL patients often complain about visual disturbances like fluctuating, blurred vision, eye fatigue, reduced contrasts, halos and glare especially at night, symptoms that are impossible to verify with standard visual acuity tests. For physicians it can be quite time consuming to determine the origin of these problems. Quality of life questionnaires can be a useful tool for clinicians to better differentiate the symptoms.19
The aim of the current study was to evaluate the clinical outcomes, and the biometrical data of subjects, who underwent implantation of the Tecnis Symfony IOL and to correlate these data with the patients' complaints. Furthermore, we intended to identify prognostic factors which can predict patients‘ satisfaction.
The Tecnis Symfony has a diffractive step-like optical profile, intended to extend the range of vision.12,13 Earlier studies have evaluated the visual outcome of this multifocal IOL and have reported an improvement in the visual acuity at far and intermediate distances after cataract surgery.20, 21, 22 For individuals who wish for a continuous range of functional vision, extended depth of focus (EDOF) IOLs have been developed in which incoming waves of light are focused in an extended longitudinal plane.23, 24, 25 The drawback of the Symfony IOL is its high spectral dependency, which can also affect visual acuity and contrast sensitivity.26,27 In the current study, we found similar results to those previously reported by other authors. The main drawback of this type of MIOL is the often insufficient uncorrected near visual acuity. In order to achieve a better near functional vision, a slightly myopic correction (mini-monovision) in the non-dominant eye has been recommended by the experts.8,14,28 Although most patients in our study had a good distance and intermediate functional visual acuity using this approach, only 18% have respectively achieved complete spectacle independence. This percentage might seem very low, but it has to be considered, that we decidedly encouraged our patients to use reading glasses for long-term reading to avoid fatigue, even when they had a good functional reading vison in one eye. Our multivariable regression model has also confirmed this observation, that uncorrected near visual acuity has a significant influence on patients' dissatisfaction. Interestingly, the amount of “monovision”(difference in spherical diopters between the two eyes) did not have a positive effect on patients' contentment. Hence, increasing near visual acuity only in the non-dominant eye, did not result in higher satisfaction in our cohort. This is probably the result of the combination of suboptimal far and insufficient near vision due to the low-add character of the Symfony lens.
Another interesting finding of the present study is, that uncorrected near visual acuity showed a significant positive correlation with the axial length (AL) (Fig. 2). The shorter the eye was, the better the near vision was. This explains why dissatisfaction was more common among patients with longer AL (Table 3). Our finding is in accordance with the theoretical calculations of Savini et al.,29 having described, that the near focal distance in case of MIOLs increases with increasing values of AL. Eyes with a longer axial length have generally an increased effective lens position (ELP), which increases the needed near add power. With the same add power, the near distance was closer in hyperopic eyes and farther in myopic eyes; According to this theory a hyperopic eye would require a lower add, and a myopic eye a higher add. In case of the Symfony, which is a low add MIOL, this means that the near focal distance is too large in the longer eyes to allow a sufficient reading ability. To our knowledge this relation hasn't been proven in a clinical setting before.
EDOF MIOLs have gained popularity in the recent years because of their tolerance to residual astigmatism. In preoperative planning, we were careful to correct corneal astigmatism. Whenever a toric IOL was suggested by the online calculator, we implanted one, otherwise we performed the incision in the steep meridian and only patients with regular corneal astigmatism were included. Earlier studies30,31 have already proven the good rotational stability of the Symfony MIOL, in our dataset the average rotation was 2.94 ± 3.01°. We rigorously controlled our patients during the early postoperative period and repositioned the IOL after performing a vector analysis by refractively significant residual astigmatism. Still, even with state-of-the-art measurement and calculation methods, it is unrealistic to achieve perfect postoperative emmetropia, because of several sources of error like posterior corneal astigmatism, IOL misalignment, surgically induced astigmatism, effective lens position, decentration and toric markers.32,33 The postoperative cylindrical error was significantly less in our satisfied patient cohort (0.31 ± 0.36 vs 0.67 ± 0.29 D), which was certainly the reason why the Strehl ratio was slightly higher (0.08 ± 0.08 vs 0.05 ± 0.04) among them as well. Interestingly when we correlated the uncorrected visual acuity with the residual refractive cylinder, it did not reach the level of significance, quite contrary to Strehl ratio, which showed a significant negative correlation with the residual refractive cylinder (P < 0.001; R = 0.4), see also Fig. 3. Our multivariable regression model has also identified postoperative residual refractive cylinder and total Strehl ratio as significant predictors of patients' satisfaction. We are confident that Strehl is a better indicator to describe patients' symptoms and visual quality as just conventional visual acuity. With the means of ray tracing aberrometry and the measures of optical quality it is possible to interpret these sometimes very vague complaints.34,35
The performance of wavefront aberrometry in case of multifocal IOLs have been widely discussed, but there are several publications addressing this issue4,34,35 and recently a study of Jun et al. has shown that the aberration measurements performed by the iTrace of a multifocal IOL are comparable to those of a monofocal IOL.36 Another study from Palomino-Bautista et al. has also confirmed that measuring aberrations and Strehl Ratio on multifocal IOLs (Symfony inclusively) has a clinical relevance.37 Anyhow these measurements have a limitation, the aberrrometry is performed at the wavelength of 632 nm, which can't fully represent the behavior of the MIOL at the whole light spectrum.
However, as visible in Fig. 1, the most considerable differentiating symptom between “happy” and “unhappy” patients were the presence of halos and glare, which are well known side effects of multifocal IOLs.38 Halos together with mesopic pupil diameter were also the strongest confounders in the regression model to determine patients' dissatisfaction (Table 5). With regard to halos, higher order Strehl Ratio and mesopic pupil diameter were significant predictors (Table 6). This finding is also in accordance with the publication of de Vries et al.,39 who showed that astigmatism, posterior capsule opacification, and a large pupil were the most significant etiologies causing dissatisfaction after implantation of MIOLs.
Our study has indeed some limitations, we haven't performed conventional contrast sensitivity measurement, which is also a possible explanation for poorer results in case of MIOLs. Furthermore, we did not measure the IOL decentration which could also have an impact on optical quality, although in a previous publication of ours,34 we have found this effect very low and we haven't used a control group.
In conclusion, we can say, that insufficient reading ability, uncorrected cylindrical error, a worse Strehl ratio and halos seem to be the most disturbing factors for patients implanted with the Symfony IOL. Halos are associated with larger mesopic pupil diameter and higher order aberrations (HO Strehl ratio). Objective visual quality measures are better indicators of subjective visual dissatisfaction than traditional visual acuity alone. Patients with shorter axial length seem to achieve a better uncorrected near visual acuity.
Study Approval
The authors confirm that any aspect of the work covered in this manuscript that involved human patients or animals was conducted with the ethical approval of all relevant bodies and the study was performed in accordance with the Declaration of Helsinkiand the protocol was approved by the local Ethics Committee of the City of Vienna (Ethikkommission der Stadt Wien; approval number: EK 17-241-0118). An informed consent has been signed by each patient.
Author Contributions
The authors confirm contribution to the paper as follows: Conception and design of study: KM, PVVM; Data collection: KM, SZS, JS; Analysis and interpretation of results: KM, SZS, JS; Drafting the manuscript: KM, SSZ, JS; All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We'd like to thank for providing language help and proof reading the article to Christina Vecsei.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
- PE
Prediction error
- MIOL
Multifocal intraocular lens
- IOLs
Intraocular lens
- EDOF
Extended depth-of-focus
- OCT
Optical coherence tomography
- PSF
Point spread function
- SD
Standard deviations
- GEE
Generalized estimating equation
- UDVA
Uncorrected distance visual acuity
- CDVA
Corrected distance visual acuity
- UIVA
Uncorrected intermediate visual acuity
- UNVA
Uncorrected near visual acuity
- AL
Axial length
- ELP
Effective lens position
References
- 1.Alio J.L., Plaza-Puche A.B., Férnandez-Buenaga R., et al. Multifocal intraocular lenses: an overview. Surv Ophthalmol. 2017;62:611–634. doi: 10.1016/j.survophthal.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Ortiz D., Alió J.L., Bernabéu G., et al. Optical performance of monofocal and multifocal intraocular lenses in the human eye. J Cataract Refract Surg. 2008;34:755–762. doi: 10.1016/j.jcrs.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 3.Grzybowski A., Kanclerz P., Tuuminen R. Multifocal intraocular lenses and retinal diseases. Graefes Arch Clin Exp Ophthalmol. 2020;258:805–813. doi: 10.1007/s00417-020-04603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santhiago M.R., Wilson S.E., Netto M.V., et al. Modulation transfer function and optical quality after bilateral implantation of a +3.00 D versus a +4.00 D multifocal intraocular lens. J Cataract Refract Surg. 2012;38:215–220. doi: 10.1016/j.jcrs.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 5.Braga-Mele R., Chang D., Dewey S., et al. Multifocal intraocular lenses: relative indications and contraindications for implantation. J Cataract Refract Surg. 2014;40:313–322. doi: 10.1016/j.jcrs.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Piñero D.R., Ayala Espinosa M.J., Alió J.L. LASIK outcomes following multifocal and monofocal intraocular lens implantation. J Refract Surg. 2010;26:569–577. doi: 10.3928/1081597X-20091030-02. [DOI] [PubMed] [Google Scholar]
- 7.Rudalevicius P., Lekaviciene R., Auffarth G.U., et al. Relations between patient personality and patients’ dissatisfaction after multifocal intraocular lens implantation: clinical study based on the five factor inventory personality evaluation. Eye. 2020;34:717–724. doi: 10.1038/s41433-019-0585-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandoval H.P., Lane S., Slade S.G., et al. Defocus curve and patient satisfaction with a new extended depth of focus toric intraocular lens targeted for binocular emmetropia or slight myopia in the non-dominant eye. Clin Ophthalmol. 2020;26:1791–1798. doi: 10.2147/OPTH.S247333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piñero D.P., Sánchez-Pérez P.J., Alió J.L. Repeatability of measurements obtained with a ray tracing aberrometer. Optom Vis Sci. 2011;88:1099–1105. doi: 10.1097/OPX.0b013e3182223788. [DOI] [PubMed] [Google Scholar]
- 10.Visser N., Berendschot T.T., Verbakel F., et al. Evaluation of the comparability and repeatability of four wavefront aberrometers. Invest Ophthalmol Vis Sci. 2011;52:1302–1311. doi: 10.1167/iovs.10-5841. [DOI] [PubMed] [Google Scholar]
- 11.Miháltz K., Vécsei-Marlovits P.V. The impact of visual axis position on the optical quality after implantation of multifocal intraocular lenses with different asphericity values. Graefes Arch Clin Exp Ophthalmol. 2021;259:673–683. doi: 10.1007/s00417-020-05052-5. [DOI] [PubMed] [Google Scholar]
- 12.Gatinel D., Loicq J. Clinically relevant optical properties of bifocal, trifocal, and extended depth of focus intraocular lenses. J Refract Surg. 2016;32:273–280. doi: 10.3928/1081597X-20160121-07. [DOI] [PubMed] [Google Scholar]
- 13.Kanclerz P., Toto F., Grzybowski A., et al. Extended depth-of-field intraocular lenses: an update. Asia Pac J Ophthalmol. 2020;9:194–202. doi: 10.1097/APO.0000000000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandoval H.P., Lane S., Slade S., et al. Extended depth-of-focus toric intraocular lens targeted for binocular emmetropia or slight myopia in the nondominant eye: visual and refractive clinical outcomes. J Cataract Refract Surg. 2019;45:1398–1403. doi: 10.1016/j.jcrs.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Weeber H.A., Meijer S.T., Piers P.A. Extending the range of vision using diffractive intraocular lens technology. J Cataract Refract Surg. 2015;41:2746–2754. doi: 10.1016/j.jcrs.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 16.Savini G., Barboni P., Ducoli P., et al. Influence of intraocular lens haptic design on refractive error. J Cataract Refract Surg. 2014;40:1473–1478. doi: 10.1016/j.jcrs.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Lombardo M., Lombardo G. Wave aberration of human eyes and new descriptors of image optical quality and visual performance. J Cataract Refract Surg. 2010;36:313–331. doi: 10.1016/j.jcrs.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Hanley J.A., Negassa A., Edwardes M.D.B., et al. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 19.Grzybowski A., Kanclerz P., Muzyka-Woźniak M. Methods for evaluating quality of life and vision in patients undergoing lens refractive surgery. Graefes Arch Clin Exp Ophthalmol. 2019;257:1091–1099. doi: 10.1007/s00417-019-04270-w. [DOI] [PubMed] [Google Scholar]
- 20.Black S. A clinical assessment of visual performance of combining the TECNIS( ) Symfony Extended Range of Vision IOL (ZXR00) with the +3.25 D TECNIS Multifocal 1-piece IOL (ZLB00) in subjects undergoing bilateral cataract extraction. Clin Ophthalmol. 2018;23:2129–2136. doi: 10.2147/OPTH.S175901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cochener B., Boutillier G., Lamard M., et al. A comparative evaluation of a new generation of diffractive trifocal and extended depth of focus intraocular lenses. J Refract Surg. 2018;34:507–514. doi: 10.3928/1081597X-20180530-02. [DOI] [PubMed] [Google Scholar]
- 22.Schojai M., Schultz T., Jerke C., et al. Visual performance comparison of 2 extended depth-of-focus intraocular lenses. J Cataract Refract Surg. 2020;46:388–393. doi: 10.1097/j.jcrs.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 23.Barnett B.P. FOCUSED (femtosecond optimized continuous uncorrected sight with EDOF and diffractive multifocal IOLs) - a review. Curr Opin Ophthalmol. 2021;32:3–12. doi: 10.1097/ICU.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 24.Breyer D.R.H., Beckers L., Ax T., et al. Current review: multifocal intraocular lenses and extended depth of focus intraocular lenses. Klin Monbl Augenheilkd. 2020;237:943–957. doi: 10.1055/a-1111-9380. [DOI] [PubMed] [Google Scholar]
- 25.Reinhard T., Maier P., Böhringer D., et al. Comparison of two extended depth of focus intraocular lenses with a monofocal lens: a multi-centre randomised trial. Graefes Arch Clin Exp Ophthalmol. 2021;259:431–442. doi: 10.1007/s00417-020-04868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Łabuz G., Auffarth G.U., Özen A., et al. The effect of a spectral filter on visual quality in patients with an extended-depth-of-focus intraocular lens. Am J Ophthalmol. 2019;208:56–63. doi: 10.1016/j.ajo.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Millán M.S., Vega F. Extended depth of focus intraocular lens: chromatic performance. Biomed Opt Express. 2017;8:4294–4309. doi: 10.1364/BOE.8.004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson M.A., Edmiston A.M., Bedi R. Optimum refractive target in patients with bilateral implantation of extended depth of focus intraocular lenses. Clin Ophthalmol. 2020;18:455–462. doi: 10.2147/OPTH.S237457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savini G., Hoffer K.J., Lombardo M., et al. Influence of the effective lens position, as predicted by axial length and keratometry, on the near add power of multifocal intraocular lenses. J Cataract Refract Surg. 2016;42:44–49. doi: 10.1016/j.jcrs.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 30.Gundersen K.G. Rotational stability and visual performance 3 months after bilateral implantation of a new toric extended range of vision intraocular lens. Clin Ophthalmol. 2018;18:1269–1278. doi: 10.2147/OPTH.S173120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee B.S., Onishi A.C., Chang D.F. Comparison of rotational stability and repositioning rates of 2 presbyopia-correcting and 2 monofocal toric intraocular lenses. J Cataract Refract Surg. 2021;47:622–626. doi: 10.1097/j.jcrs.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 32.Hirnschall N., Findl O., Bayer N., et al. Sources of error in toric intraocular lens power calculation. J Refract Surg. 2020;36(10):646–652. doi: 10.3928/1081597X-20200729-03. [DOI] [PubMed] [Google Scholar]
- 33.Lipsky L., Barrett G. Comparison of toric intraocular lens alignment error with different toric markers. J Cataract Refract Surg. 2019;45:1597–1601. doi: 10.1016/j.jcrs.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Miháltz K., Vécsei-Marlovits P.V. The impact of visual axis position on the optical quality after implantation of multifocal intraocular lenses with different asphericity values. Graefes Arch Clin Exp Ophthalmol. 2021;259:673–683. doi: 10.1007/s00417-020-05052-5. [DOI] [PubMed] [Google Scholar]
- 35.Alió J.L., Plaza-Puche A.B., Javaloy J., et al. Comparison of the visual and intraocular optical performance of a refractive multifocal IOL with rotational asymmetry and an apodized diffractive multifocal IOL. J Refract Surg. 2012;28:100–105. doi: 10.3928/1081597X-20120110-01. [DOI] [PubMed] [Google Scholar]
- 36.Jun I., Choi Y.J., Kim E.K., et al. Internal spherical aberration by ray tracing-type aberrometry in multifocal pseudophakic eyes. Eye. 2012;26:1243–1248. doi: 10.1038/eye.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palomino-Bautista C., Sánchez-Jean R., Carmona-Gonzalez D., et al. Depth of field measures in pseudophakic eyes implanted with different type of presbyopia-correcting IOLS. Sci Rep. 2021;11:12081. doi: 10.1038/s41598-021-91654-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao K., Friedman D.S., Jin S., et al. Multifocal versus monofocal intraocular lenses for age-related cataract patients: a system review and meta-analysis based on randomized controlled trials. Surv Ophthalmol. 2019;64:647–658. doi: 10.1016/j.survophthal.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 39.de Vries N.E., Webers C.A., Touwslager W.R., et al. Nuijts RM Dissatisfaction after implantation of multifocal intraocular lenses. J Cataract Refract Surg. 2011;37:859–865. doi: 10.1016/j.jcrs.2010.11.032. [DOI] [PubMed] [Google Scholar]