Abstract
Background
This study compared the role of autophagy regulators Rapamycin and 3-MA in oxidative damage and apoptosis of human lens epithelial cells (HLECs) caused by two doses of Ultraviolet Radiation B (UVB).
Methods
HLECs were irradiated with UVB, and two doses of UVB damage models were constructed. After treatment with autophagy regulators, cell damage tests such as CCK-8, LDH activity, and Ros detection were performed. Western blotting was used to detect the levels of autophagy-related proteins and apoptosis-related proteins. Quantitative real-time PCR (RT-qPCR) was used to detect the mRNA leve of secondary antioxidant enzymes.Flow cytometry was used to examine cell viability and apoptosis. Finally, the proportion of autophagy and apoptosis was observed by electron microscope.
Results
Autophagy inhibitor 3-MA promoted oxidative damage and apoptosis of HLECs at low doses of UVB (5 mJ/cm2), which corresponds to 1.3 h of exposure to sunlight in human eyes. Under the high dose of UVB (50mJ/cm2), which is equivalent to 13 h of exposure to sunlight in human eyes, the autophagy inducer Rapamycin caused more extensive oxidative damage and apoptosis of HLECs. 3-MA was able to reduce this damage, indicating that moderate autophagy is necessary for HLECs to cope with mild oxidative stress. For high dose UVB-induced oxidative stress, the use of 3-MA inhibiting autophagy is more beneficial to reduce cell damage and apoptosis. The mechanisms include degradation of damaged organelles, regulation of the expression of antioxidant enzymes HO-1, NQO1, GCS and regulation of apoptosis-related proteins.
Conclusions
Autophagy played different roles in HLECs oxidative stress induced by two doses of UVB. It provides new ideas for reducing oxidative damage and apoptosis of HLECs to prevent or delay the progression of age-related cataract (ARC).
Keywords: Cataract, UVB, Autophagy, Apoptosis, HLECs, Oxidative stress
1. Introduction
Age-related cataract (ARC) is the most common cause of severe visual impairment and blindness.1 Although the exact mechanism of ARC is not fully understood, it is widely recognized that oxidative stress plays an important role in the pathogenesis of the disease. Ultraviolet(UV)radiation is considered to be the main cause of ARC formation.1, 2, 3, 4
UVA and UVB penetrate the atmosphere. In particular, UVB can cause DNA damage and formation of cyclobutane pyrimidine dimer (Cps), death receptor activation, and reactive oxygen species (ROS) formation. These mechanisms are cross-linked and promote UVB-induced apoptosis.5 Previous studies have shown that the rapid apoptosis of lens epithelial cells induced by UVB initiates the occurrence of cataract.4,6,7
In mammalian cells, autophagy occurs at low levels, thereby preventing the accumulation of damaged and dysfunctional cellular components; this basal level is enhanced during starvation, providing an alternative source of energy. When cells are subjected to metabolic stress, drug therapy, or radiation injury, the levels of intracellular Ca2+, ROS, toxin, NO, growth factors, and hormones are increased, which could induce apoptosis. However, autophagy could reduce oxidative damage and the ROS level by the degradation of abnormal proteins and damaged organelles such as mitochondria.8,9
Paradoxically, autophagy plays a dual role in the regulation of cell death. Whilst mild autophagy protects cells from harmful conditions to promote cell survival, an excess activation of autophagy may accelerate cell injury and apoptotic cell death through unchecked degradative processes.10, 11, 12, 13 Therefore, in lens epithelial cells, the relationship between autophagy, oxidative damage, and apoptosis remains unclear. We hypothesize that autophagy may play different roles at different levels of oxidative stress for HLECs.
In order to induce different degrees of oxidative stress in HLECs, two doses of UVB radiation were selected by experiment in this study: 5 mJ/cm 2 (low dose) and 50 mJ/cm 2 (high dose), which are equivalent to 1.3 h or 13 h of exposure to sunlight in the human eye, respectively. This is based on the maximum solar UVB exposure reaching the human lens epithelium being 3.7 mJ/cm 2 in an hour.14
This study regulated autophagy through pretreatment of HLECs using autophagy regulators Rapamycin (a specific inhibitor of mTOR) and 3-MA (a selective PI3K inhibitor).8 This study explored the role of autophagy in the two levels of oxidative stress in HLECs, providing new ideas for more effective prevention or reduction of oxidative damage and apoptosis in HLECs.
2. Methods
2.1. Cell culture and treatment
Immortalized HLECs (SRA01/04)(Biovector, China) were grown and maintained in 1: 1 Dulbecco's modified Eagle's medium and Ham's F-12 medium (DMEM/F-12) (#10-092-CV, Corning Cellgro, USA), which is supplemented with 10% fetal bovine serum (FBS) (Bioind, Israel), 100 U each of penicillin and streptomycin (Gibco, USA) in a humidified CO2 incubator. HLECs was pretreated with 200 nM Rapamycin or 5 mM 3-MA for 2 h and subsequently irradiated with two doses of UVB.
For UVB irradiation, the cells were treated by the above methodand washed with phosphate buffered saline (PBS). HLECs were then irradiated with a medium-wave ultraviolet lamp for different time periods in PBS (37 °C, air) at an irradiation distance of 0.8 cm. Control cells were treated similarly without irradiation with UVB. The UVB concentration reaching the cells was determined using a radiometer (UVX Digital; San Gabriel, CA) equipped with a 312 nm UVB sensor (UVX-31 type). The maximum exposure time of UVB is limited to 45 min, and the UVB irradiation dose under this condition is 70 mJ/cm2. After incubation with DMEM containing 10% fetal calf serum for 24 h, the cells were washed again with PBS for subsequent processing and detection.
2.2. Cytotoxicity assay
Cytotoxicity assays were performed using a Cell Counting Kit-8 (CCK-8) assay (Dojindo, Japan). Twenty-four hours before the treatment, 1 × 104 cells were seeded in each well of the 96-well plate. Cells were then rinsed with PBS twice and treated with a given dose of UVB. Following UVB-exposure and subsequent culture, the medium was removed, and the cells were washed again with PBS. Each well was subsequently refilled with 90 μL of DMEM/F12 supplemented with 10% FBS and 10 μL of CCK-8 reagents and incubated in 37 °C for 3 h. The cell viability was evaluated with OD450 values using a 96-well microplate reader (Bio-Rad, USA).
2.3. LDH cytotoxicity assay
The membrane integrity of cells was assessed by estimating the amount of LDH present in the culture media (LDH Cytotoxicity Assay Kit (Beyotime Institute of Biotechnology, Jiangsu, China). HLECs were cultured in 96-well plates. Following UVB-exposure and subsequent culture, LDH was measured in medium and cell extracts according to the manufacturer's instruction.
2.4. Transmission Electron Microcopy
The cells were fixed with 7% glutaraldehyde. Paraffin embedding and dehydration were performed subsequently. Ultrathin sections were stained with uranyl acetate and lead citrate. Images were taken with JEM-1200EX Transmission Electron Microscope at 120 V.
2.5. Cell apoptosis assay
HLECs were cultured in 35 mm disks. After treatments, cells were collected by trypsinization and centrifugation at 1500 rpm for 5 min. This was followed by washing cell pellets twice with cold PBS and resuspending in 1∗ Annexin-binding buffer. 5 μL Annexin V and 1 μL 100 μg/ml PI working solution were added in 100 μL cell suspension and incubated in the dark at room temperature for 15 min. After incubation, 400 μL 1∗ Annexin-binding buffer was added. Following gentle mixing, fluorescence intensity was detected with a flow cytometry (CyAn ADP, Beckman Coulter, USA) at emission of 530 nm and 575 nm and excitation at 488 nm. The percentage of cells stained by Annexin V/PI which indicates early apoptosis was shown in bar chart.
2.6. Measurement of intracellular ROS
ROS were measured with the 2′,7′-dichlorofluorescein diacetate (DCFH-DA). Following UVB-exposure and subsequent culture, HLECs were washed three times with PBS. DCFH-DA, diluted to a final concentration of 10 μM, was added to HLECs and these were incubated for 30 min at 37 °C in the dark. After the cells were washed three times with serum-free medium, the fluorescence intensity was detected with a multi-detection microplate reader with excitation at 488 nm and emission at 530 nm within 15 min. Fluorescence signals were captured using a fluorescence micROScope (Leica, Germany). Intracellular levels of ROS were calculated by the average fluorescence intensity as analyzed by Image-Pro Plus software. The measured fluorescence values were expressed as a percentage of the fluorescence in control cells.
2.7. Quantitative real-time PCR (qRT-PCR)
qRT-PCR was performed using StepOnePlus Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA) to study the expression of antioxidant genes. Each sample was run in triplicate. The QuantiTect Primer Assays (Qiagen) and Power SYBR Green PCR Master Mix (Cat. # 4367659, Life Technologies, Grand Island, NY, USA) were used. qRT-PCR data were analyzed using ΔΔCt method. ΔCt was the difference between the Cts (threshold cycles) of the target gene and Cts of the housekeeper gene (reference gene). ΔΔCt was calculated by subtracting ΔCt of the treatment group from ΔCt of the Cont group. Fold change was calculated using the following formula: Fold change = 2ΔΔCt.
The primers used for real-time PCR are followed: GST: 5′-ACAGGGATCATGAAAGACAGTG -3′ and 5′-TCTTCATTCCTTGACCAGACG -3′; NQO1: 5′-TCACCGAGAGCCTAGTTCC-3′and 5′- TCATGGCATAGTTGAAGGACG -3′; HO-1: 5′-CCAGGCAGAGAATGCTGAGTTC-3′ and 5′-AAGACTGGGCTCTCCTTGTTGC-3′, h-GAPDH 5′-GGTGAAGGTCGGAGTCAACG-3′ and 5′-CAAAGTTGTCATGGATGHACC-3′.
2.8. Western blotting analyses
After the indicated treatment, HLECs were lysed and the BCA Protein Assay Kit (Sigma, B9643-1L) was used to determine the total protein concentration. Equal concentrations of total protein samples were loaded into the wells of 4–12% Bolt mini gels (Life Technologies) followed by SDS-PAGE electrophoresis. The gels were then transferred onto PVDF membranes. Following transfer, membranes were blocked with 5% fat-free milk in TBS and then incubated with primary antibodies against Bcl-2 (1: 2000; Cell Signaling Technology, Cat#: 4223), Bax (1: 2000; Cell Signaling Technology, Cat#: 5023), p62 (1: 2000; Cell Signaling Technology, Cat#: 8025), and β-actin (1: 5000; Santa Cruz, Cat#: sc-47778) overnight at 4 °C. Then, appropriate HRP-conjugated secondary antibodies were applied (1: 5000) and signal was detected with the Immobilon method using the enhanced chemiluminescence technique (Pierce, USA). In the end, the data were analyzed using Image J software.
2.9. Statistics
Statistical analysis was performed by using ANOVA, followed by Tukey post-test using Graphpad InStat (version 3.05; GraphPad Software, San Diego, CA, USA). Data are presented as mean ± s.d. P < 0.05 was considered significant.
3. Results
3.1. The role of autophagy in different degrees of UVB irradiation damage in LECs
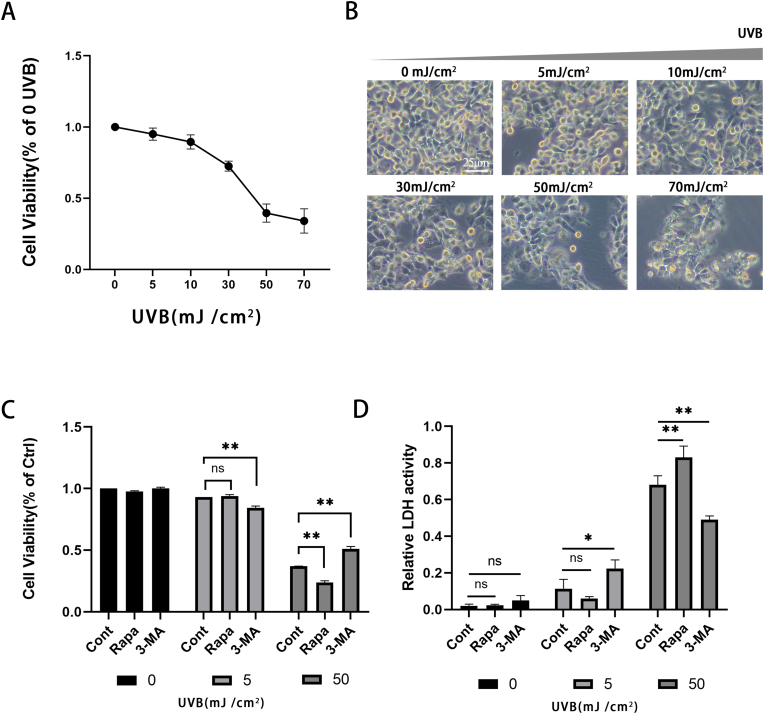
UVB can induce apoptosis and even necrosis of lens epithelial cells. Based on previous studies, we set up UVB dose gradients (0, 5, 10, 30, 50, 70 mJ/cm2) to detect the cell viability of HLECs (Fig. 1A). It was found that at 24 h after UVB irradiation in the 50 mJ/cm2 group, the colony density of the cells was decreased, the cell morphology was irregular, and more vacuoles appeared in the cytoplasm. However, was no significant difference between the 5 mJ/cm2 group and the control group (Fig. 1B). At the dose of 5 mJ/cm2 (Fig. 1C), UVB had little effect on cell viability. At doses above 50 mJ/cm2, cell viability decreased by more than half compared with the control group, and oxidative damage of HLECs induced by UVB was significant. Therefore, we classified the cells irradiated with UVB at the doses of 5 mJ/cm2 as the low does group and 50 mJ/cm2 as the high dose group.
Fig. 1.
The role of autophagy in the survival of HLECs under different degrees of UVB irradiation damage
(A) Detecting cell viability of HLECs treated with vary doses of UVB (0, 5, 10, 30, 50, 70 mJ/cm2) (B) The difference between the 5 and 50mJ/cm2 group and the control group was observed under the optical microscope.(C) Detecting cell viability of Rapamycin or 3-MA pretreated HLECs irradiated with low or high doses of UVB (D) LDH release of HLECS pretreated with Rapamycin or 3-MA and irradiated with UVB at low or high does. LDH release was determined using a commercial kit (mean ± s.d.; n = 3 ∗∗P < 0.01.)..
We subsequently pretreated HLECs with Rapamycin or 3-MA for 2 h and then irradiated the cells with low or high doses of UVB radiation. In the low dose group, the effect of Rapamycin pretreatment on cell viability was not significantly different from that of the control group, but the pretreatment of 3-MA resulted in a significant loss of cell viability. In the high dose group, the pretreatment of Rapamycin led to a more significant decrease in cell viability, while the pretreatment with 3-MA showed higher cell viability than that of the control group and we discovered the same trend in the LDH release assay (Fig. 1D). To conclude, we found that the autophagy regulators Rapamycin and 3-MA may play different roles in the different degrees of oxidative stress in HLECs.
3.2. Mechanism of autophagy in oxidative stress
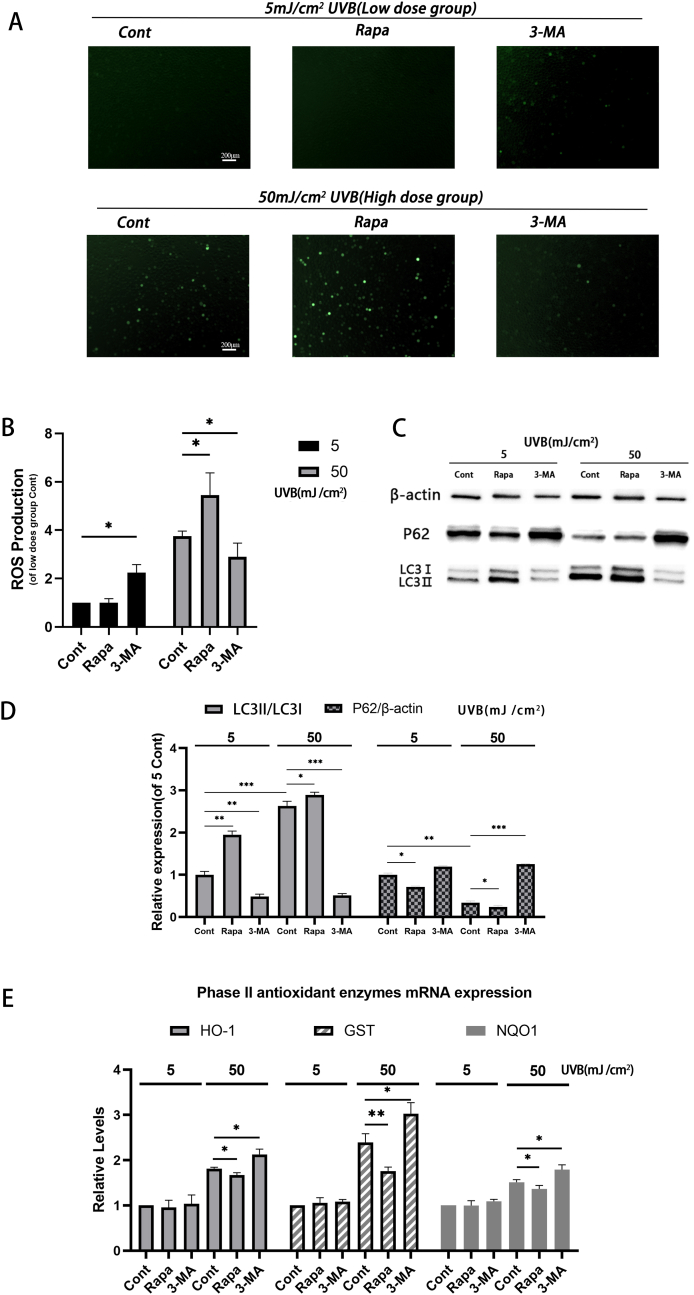
In order to further study the different roles of autophagy under oxidative stress, we explored the relationship between autophagy and intracellular ROS level, which is the most important damage factor of oxidative stress. We detected the ROS in the labeled cells by commercial kit and observed by fluorescence microscope (Fig. 2A).
Fig. 2.
Effect of autophagy on antioxidase system under oxidative stress
(A-B) ROS determined by DCFH-DA. Two hours after pretreatment with Rapamycin or 3-MA, HLECs were irradiated with two doses of UVB. After 24 h, intracellular ROS levels were determined using a commercial kit. (A) Observation under a fluorescence microscope, and (B) detection by a fluorescence microplate reader. (C) Western blot was used to detect the levels of p62, LC3I, and LC3II protein 24 h after two does of UVB irradiation to HLECs pretreated with Rapamycin or 3-MA. (D) p62, LC3I and LC3II protein level. (E) Expression level of HO-1, GST and NQO1 expression level detected by qPCR (mean ± s.d.; n = 3, ∗P < 0.05, ∗∗P < 0.01) 24 h after two doses of UVB irradiation to HLECs pretreated with Rapamycin or 3-MA.
In HLEC cells treated with low does UVB, the level of ROS was significantly increased only in the cells pretreated with 3-MA (Fig. 2B, P < 0.05), but there was no significant difference between the cells pretreated with Rapamycin and the control group. However, in the high dose group, the intracellular ROS levels of both groups and the control increased. HLECs pretreated with Rapamycin had significantly higher ROS levels than those of the control group (P < 0.05), while 3-MA pretreatment was significantly lower than that of the control group (P < 0.05).
To detect the actual effect of autophagy regulators on autophagy regulation, we also detected the levels of autophagy-related proteins p62, LC3I, and LC3II by Western blot (Fig. 2C). Our results were the same as expected. Rapamycin enhanced autophagy in both low dose and high dose groups, while 3-MA had the opposite effect (Fig. 2D).
In our study, autophagy promoted the accumulation of intracellular ROS in the high-dose group, which is different from previous understanding. We also detected the expression of three phase II antioxidant enzymes that play a major role in the antioxidant system (Fig. 2E). We found that the regulation of autophagy had little effect on the expression of antioxidant enzymes HO-1, GST, and NQO1 in low-dose group. However, in the high-dose group, the enhancement of autophagy inhibited the expression of these three antioxidant enzymes, while the effect of 3-MA was on the contrary. This may indicate that autophagy promotes the accumulation of ROS in cells by regulating the expression of antioxidant enzymes under strong oxidative stress.
3.3. Effect of autophagy on oxidative stress-induced apoptosis in two degrees of HLECs
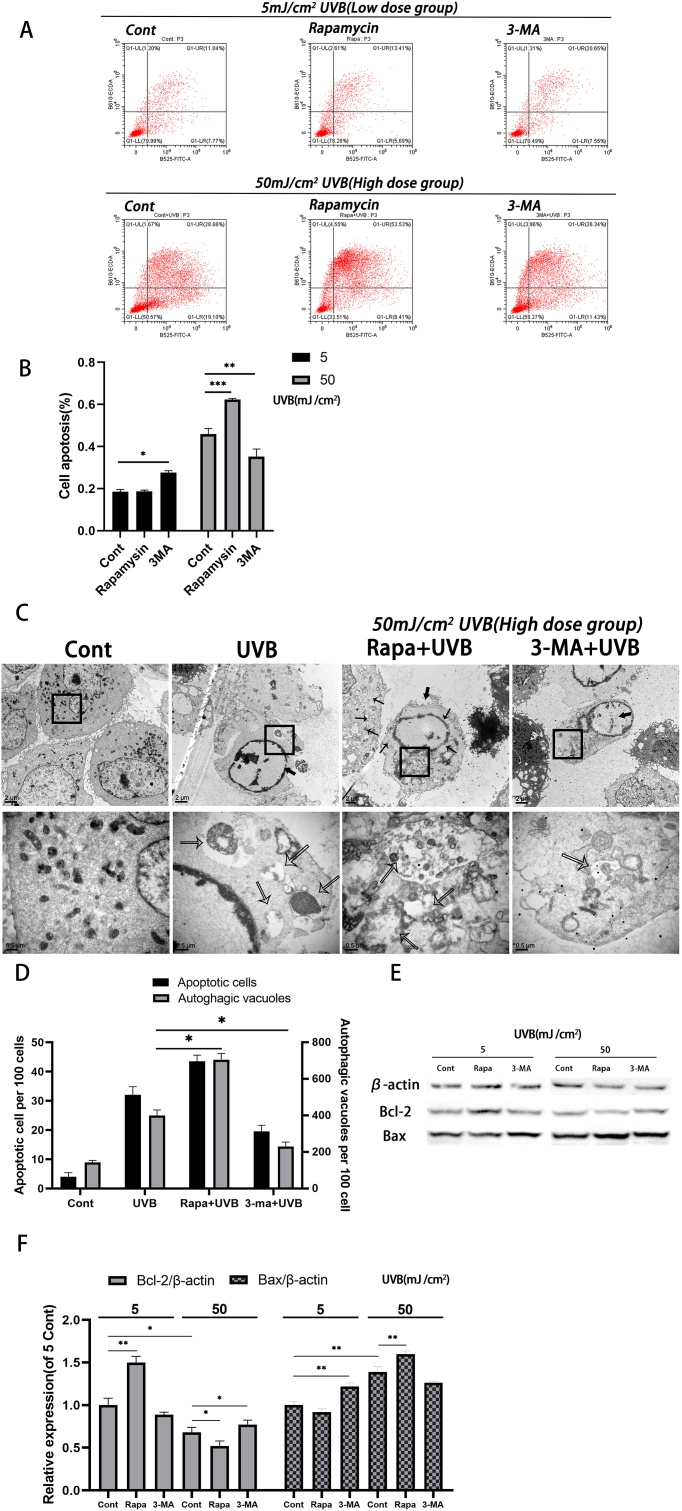
To further investigate the role of Rapamycin and 3-MA in different degrees of HLEC oxidative stress, we stained the cells with AnnexinV-FITC/PI and analyzed the apoptosis by flow cytometry (FCM) (Fig. 3A). It was found that in the low dose group, the cells pretreated with 3-MA had significant apoptosis compared with the control group (P < 0.05). In the high dose group, the apoptosis rate of 3-MA pretreated cells was significantly lower than that of the control group (P < 0.01), while Rapamycin pretreated cells led to more significant apoptosis (P < 0.001, Fig. 3B).
Fig. 3.
Apoptosis assay of differently treated HLECs under two doses of UVB irradiation
(A-B) Annexin V/PI staining detected by flow cytometry. (C–D) Counting apoptotic cells and autophagosomes by Transmission Electron Microcopy. 100 cells were randomly selected and the apoptotic cells (thick arrows) and the number of autophagosomes per cell (thin arrows) were counted. (E) Two hours after pretreatment with Rapamycin or 3-MA, HLECs was irradiated with two doses of UVB. After 24 h, Western blot was used to detect the levels of Bcl-2 and Bax protein. (F) Bcl-2 and Bax protein level (mean ± s.d.; n = 3, ∗P < 0.05,∗∗P < 0.01,∗∗∗P < 0.001).
Autophagy and apoptosis in HLECs were subsequently observed by transmission electron microscope (TEM) (Fig. 3C). We found that UVB at the dose of 50 mJ/cm2 induced apoptosis, which was characterized by nuclear chromatin aggregation and edge aggregation. More apoptosis and intracellular autophages were observed in the Rapamycin pretreatment group than in the UVB control group, while the 3-MA pretreatment group showed less apoptosis and intracellular autophages compared with the UVB control group (Fig. 3D). In the cells pretreated with Rapamycin, we also observed a significant increase in vacuoles in dying cells; a large number of organelles were degraded by autophagy lysosomes and some cells had no typical characteristics of apoptosis (Fig. 3C).
Western blot was carried out to detect the levels of apoptosis-related proteins Bcl-2 and Bax. Bcl-2 is an important anti-apoptotic protein and Bax is a marker molecule of apoptosis that is important for the process’ occurrence. Consistent with the previous results, we found that autophagy significantly increased the expression of Bcl-2 in the low dose group. In the high dose group, the further enhancement of autophagy down-regulated the expression of Bcl-2 and promoted the expression of Bax, while inhibition of autophagy promoted the expression of Bcl-2. These results indicate that 3-MA pretreatment inhibits autophagy in HLECs and can reduce apoptosis of HLECs compared to the control group under high dose of UVB irradiation. Rapamycin pretreatment can enhance HLEC autophagy. Although the level of apoptosis was not significantly different from that of the control group under low doses of UVB, it was more pronounced under higher doses of UVB.
4. Discussion
The relationship between autophagy and apoptosis is complex and there is a high degree of cROSstalk. Previous studies have shown that regulatory factors such as Beclin-1,Bcl-2,ATG5,ATG3,ATG12,caspase and p53 play an important role in these two pathways.15 Although a large number of previous studies have shown that autophagy plays a cellular protective role under oxidative stress,16, 17, 18 recent studies have shown that autophagy may also regulate cell survival by inducing apoptosis or autophagic cell death.17, 18, 19 Different from previous studies, we explored the relationship between autophagy and cell survival under different degrees of oxidative stress.By comparing the differences of cell viability, cytotoxicity, endogenous ROS level, expression of antioxidant enzymes and apoptosis between two doses of UVB, Rapamycin and 3-MA, we found that low dose (5 mJ/cm2) or high dose (50 mJ/cm2) of UVB induced mild or strong oxidative stress in HLEC, and autophagy may play different roles in these two levels of oxidative stress. That is, under mild oxidative stress, autophagy helps to reduce the oxidative damage of HLEC; under strong oxidative stress, the inhibition of autophagy contributes to the better survival of HLEC, and this effect may be related to the regulation of the expression of antioxidant enzymes (GST, NQO1,NO-1). This indicates that there is a more complex role between autophagy and cell survival in lens epithelial cells. This study provides a new insight for the prevention of HLEC oxidative damage induced by UVB from the perspective of autophagy and is of great significance for the prevention and delay of ARC.
Oxidative stress refers to the imbalance of intracellular oxidation and anti-oxidation defense mechanisms in the state of elevated ROS levels. There are abundant antioxidant enzymes in cells to prevent the accumulation of ROS in cells. In addition, the increase of ROS, the key damage factor of intracellular oxidative damage, can also induce autophagy to clear damaged mitochondria and reduce the production of ROS.20, 21, 22, 23, 24 Autophagy, which is strictly regulated during development and disease, participates in this negative feedback regulation and plays a key role in maintaining homeostasis in cells and tissues.10In many specific cells and conditions, autophagy can reduce the sensitivity of cells to oxidative stress and keep them alive. In our experiment, we observed that HLECs up-regulated autophagy in response to different doses of UVB irradiation, which was characterized by regulation of autophagy-related proteins and an increase in cytoplasmic autophagy bodies.In low-dose UVB-treated HLECs, oxidative stress increases the level of intracellular ROS, which stimulates autophagy and up-regulates the expression of intracellular antioxidant enzymes, and finally leads to the decrease of ROS to the physiological level. Pretreatment with the autophagy inducer Rapamycin did not further reduce cell damage and improve its survival rate, but cells pretreated with 3-MA showed significant cytotoxicity and higher apoptosis. This may be due to the fact that under moderate oxidative stress, the up-regulation of autophagy and antioxidant enzymes can effectively and timely reduce the level of intracellular ROS.25,26 When autophagy is inhibited, this synergy is affected, resulting in the continuous accumulation of endogenous ROS, which may lead to further cellular oxidative damage and apoptosis. We observed that compared with the low dose group, high dose of UVB significantly increased the level of intracellular ROS and stimulated autophagy. The difference is that although Rapamycin pretreatment further increased the level of autophagy, it also down-regulated the expression of antioxidant enzyme GST, NQO1,NO-1 and caused a higher level of intracellular ROS. However, by inhibiting strong autophagy, 3-MA up-regulated the expression of second-stage antioxidant enzymes and promoted the decrease of ROS level. Previous studies have shown that p62 can activate the Nrf2 antioxidant pathway by promoting Keap1 degradation and induce the expression of downstream phase II antioxidant enzymes such as HO-1, NQO1, GCS.27, 28, 29, 30, 31 Autophagy disorders lead to prolonged activation time of p62-dependent Nrf2 and up-regulated expression of antioxidant enzymes.32, 33, 34 Activating the p62/Keap1/Nrf2 pathway can up-regulate antioxidant enzyme systems such as NQO1, NO-1, and GPX4, and help prevent ferroptosis caused by accumulation of iron-dependent lipid reactive oxygen species in various tissues and cells such as hepatocellular carcinoma cells (HCC) and dopaminergic cells. ferroptosis caused by accumulation of reactive oxygen species, in which high levels of p62 expression are necessary for the antioxidant system to exert cytoprotective effects.32, 33, 34We detected the level of p62 protein and found that Rapamycin and 3-MA can regulate the expression of p62 protein, but the expression of p62 protein affects the expression of antioxidant enzymes only under strong oxidative stress, suggesting that p62/keap1/Nrf2, a non-classical pathway, may play an important role under strong oxidative stress. We speculate that Rapamycin may induce cell death by inhibiting the activation of the p62/Keap1/Nrf2 pathway, down-regulating the expression of antioxidant enzymes and promoting the accumulation of ROS in HLECs. This hypothesis needs to be verified by further experiments.
Our results have confirmed that autophagy regulates intracellular ROS levels, which may affect the occurrence of apoptosis[8]. In addition, it has been suggested that autophagy-associated protein 12 (Atg12) binds and inhibits Bcl-2, to invalidate an important anti-apoptotic member of the Bcl-2 family.35Similarly, we also found that the effect of autophagy on apoptosis was consistent with the level of Bcl-2 protein. But the causal relationship between them needs to be further verified. Apoptotic cells and autophagic vesicles in the high-dose group were observed and counted by TEM. It was found that the number of autophagic vesicles appeared to be positively correlated with the number of apoptotic cells. In addition, there is growing evidence that autophagy may also promote cell death through excessive autodegradation and degradation of essential cellular components.36,37We also observed by TEM that there were a large number of vacuoles in the cells treated with Rapamycin and a large number of organelles surrounded by autophagy lysosomes. these dying cells did not show typical characteristics of apoptosis, which may be described as autophagic cell death or cell death accompanied by autophagy.17,18In summary, our research shows that autophagy regulates cell survival through multiple mechanisms, and that this effect is different under different degrees of oxidative stress.
Many evidences show that the ocular UV exposure accumulation (COUV) is an important risk factor for cataract development, and that HLEC apoptosis is an important pathological mechanism for cataract.2,5,7,38, 39, 40 Although in the past few decades, ARC can be effectively treated by surgery, it is still the most common cause of blindness in the world, and there is still no affordable effective drug that can inhibit or reverse the progress of cataract.41 In view of the fact that oxidative stress plays an important role in the pathogenesis of cataract, reducing and preventing oxidative stress has become a reasonable potential therapeutic target for cataract,39 while for senile cataracts, which account for the majority of cataract, there may be long-term low-and medium-dose UVB exposure, and autophagy flux decreases with age. This aggravates the progression of cataract, and the use of autophagy inducers may help to restore autophagy flux and reduce oxidative damage in HLECs to prevent and improve cataract progression.
Given that autophagy may play different roles in oxidative stress of HLECs induced by two doses of UVB, at least by degrading damaged organelles, regulating the expression of antioxidant enzymes, and regulating the levels of apoptosis-related proteins, it can provide us with better cell protection. These results provide important evidence for further expanding the different roles of autophagy in HLECs under oxidative stress, and also provide new ideas for preventing and delaying the progress of ARC by reducing oxidative damage and apoptosis in HLECs.
Study approval
The authors confirm that any aspect of the work covered in this manuscript that involved human patients or animals was conducted with the ethical approval of all relevant bodies and the study was performed in accordance with the Declaration of Helsinki.
Author contributions
The authors confirm contribution to the paper as follows: Conception and design of study: HY, XS; Performed the experiments: HY; Analysis and interpretation of results: XP, YC, SZ; Drafting the manuscript: HY, XS; All authors reviewed the results and approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant no. 81970781); the Natural Science Foundation of Zhejiang Province (no. LY17H090004); the National Natural Science Foundation of China (Grant no. 81800807).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thanks are due to Professor Hangping Yao for assistance with the experiments and valuable discussion. We would like to thank the personnel of the Institute of Infectious Diseases, the first affiliated Hospital of Zhejiang University for excellent technical assistance.
Abbreviations
- 3-MA
a selective PI3K inhibitor
- ARC
age-related cataract
- Atg12
autophagy-associated protein 12
- CCK-8
cell counting Kit-8
- COUV
the ocular UV exposure accumulation
- Cps
cyclobutane pyrimidine dimer
- DCFH-DA
2′,7′-dichlorofluorescein diacetate
- FBS
fetal bovine serum
- FCM
flow cytometry
- HCC
hepatocellular carcinoma cells
- HLECs
human lens epithelial cells
- LDH
Lactate Dehydragenase
- PBS
phosphate buffered saline
- qRT-PCR
quantitative real-time PCR
- Rapamycin
a specific inhibitor of mTOR
- TEM
transmission electron microscope
- UV
ultraviolet
- UVB
ultraviolet radiation B
References
- 1.Asbell PA, Dualan I, Mindel J, et al. Age-related cataract. Lancet (London, England) 2005;365(9459):599–609. doi: 10.1016/S0140-6736(05)17911-2. [DOI] [PubMed] [Google Scholar]
- 2.Lofgren S. Solar ultraviolet radiation cataract. Exp Eye Res. 2017;156:112–116. doi: 10.1016/j.exer.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 3.West SK, Longstreth JD, Munoz BE, et al. Model of risk of cortical cataract in the US population with exposure toincreased ultraviolet radiation due to stratospheric ozone depletion. Am J Epidemiol. 2005;162(11):1080–1088. doi: 10.1093/aje/kwi329. [DOI] [PubMed] [Google Scholar]
- 4.Dillon J. Sunlight exposure and cataract. JAMA. 1999;281:230. doi: 10.1001/jama.281.3.229. PMID: 9918472. [DOI] [PubMed] [Google Scholar]
- 5.Ji Y, Cai L, Zheng T, et al. The mechanism of UVB irradiation induced-apoptosis in cataract. Mol Cell Biochem. 2015;401(1):87–95. doi: 10.1007/s11010-014-2294-x. [DOI] [PubMed] [Google Scholar]
- 6.Sreekumar PG, Ishikawa K, Spee C, et al. The mitochondrial-derived peptide humanin protects RPE cells from oxidative stress, senescence, and mitochondrial dysfunction. Invest Ophthalmol Vis Sci. 2016 2016-03-01;57(3):1238–1253. doi: 10.1167/iovs.15-17053. PMID: 26990160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts JE. Ultraviolet radiation as a risk factor for cataract and macular degeneration. Eye Contact Lens. 2011;37(4):246–249. doi: 10.1097/ICL.0b013e31821cbcc9. [DOI] [PubMed] [Google Scholar]
- 8.Ravanan P, Srikumar IF, Talwar P. Autophagy: the spotlight for cellular stress responses. Life Sci. 2017;188:53–67. doi: 10.1016/j.lfs.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 9.Rubinstein AD, Kimchi A. Life in the balance - a mechanistic view of the crosstalk between autophagy andapoptosis. J Cell Sci. 2012;125(Pt 22):5259–5268. doi: 10.1242/jcs.115865. [DOI] [PubMed] [Google Scholar]
- 10.Gao M, Monian P, Pan Q, et al. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26(9):1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Levine B. Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ. 2015;22(3):367–376. doi: 10.1038/cdd.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galluzzi L, Vicencio JM, Kepp O, et al. To die or not to die: that is the autophagic question. Curr Mol Med. 2008;8(2):78–91. doi: 10.2174/156652408783769616. [DOI] [PubMed] [Google Scholar]
- 13.Wang K. Autophagy and apoptosis in liver injury. Cell Cycle. 2015;14(11):1631–1642. doi: 10.1080/15384101.2015.1038685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cencer CS, Chintala SK, Townsend TJ, et al. PARP-1/PAR activity in cultured human lens epithelial cells exposed to two levelsof UVB light. Photochem Photobiol. 2018;94(1):126–138. doi: 10.1111/php.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukhopadhyay S, Panda PK, Sinha N, et al. Autophagy and apoptosis: where do they meet? Apoptosis. 2014 2014-04-01;19(4):555–566. doi: 10.1007/s10495-014-0967-2. PMID: 24415198. [DOI] [PubMed] [Google Scholar]
- 16.Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22(3):377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper KF. Till death do us part: the marriage of autophagy and apoptosis. Oxid Med Cell Longev. 2018 doi: 10.1155/2018/4701275. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doherty J, Baehrecke EH. Life, death and autophagy. Nat Cell Biol. 2018;20(10):1110–1117. doi: 10.1038/s41556-018-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Zhou Y, Xia Q, et al. All-trans-retinal induces autophagic cell death via oxidative stress and theendoplasmic reticulum stress pathway in human retinal pigment epithelial cells. Toxicol Lett. 2020 doi: 10.1016/j.toxlet.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Berthoud VM, Beyer EC. Oxidative stress, lens gap junctions, and cataracts. Antioxid redox sign. 2009;11(2):339–353. doi: 10.1089/ars.2008.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shefa U, Jeong NY, Song IO, et al. Mitophagy links oxidative stress conditions and neurodegenerative diseases. Neural Regen Res. 2019;14(5):749–756. doi: 10.4103/1673-5374.249218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94(3):909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. CELL. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Gupta SK, Trivedi D, Srivastava S, et al. Lycopene attenuates oxidative stress induced experimental cataract development:an in vitro and in vivo study. Nutrition. 2003;19(9):794–799. doi: 10.1016/s0899-9007(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 25.Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22(3):377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vikram A, Anish R, Kumar A, et al. Oxidative stress and autophagy in metabolism and longevity. Oxid Med Cell Longev. 2017;281:230. doi: 10.1155/2017/3451528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ichimura Y, Waguri S, Sou Y, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selectiveautophagy. Mol Cell. 2013;51(5):618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Shah SZA, Zhao D, Hussain T, et al. p62-Keap1-NRF2-ARE pathway: a contentious player for selective targeting ofAutophagy, oxidative stress and mitochondrial dysfunction in prion diseases. Front Mol Neurosci. 2018;11:310. doi: 10.3389/fnmol.2018.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang T, Harder B, Rojo de la Vega M, et al. p62 links autophagy and Nrf2 signaling. Free Radic Biol Med. 2015;88(Pt B):199–204. doi: 10.1016/j.freeradbiomed.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartolini D, Dallaglio K, Torquato P, et al. Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellularcarcinoma. Transl Res : J Lab Clin Med. 2018;193:54–71. doi: 10.1016/j.trsl.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Kageyama S, Saito T, Obata M, et al. Negative regulation of the keap1-Nrf2 pathway by a p62/sqstm1 splicing variant. Mol Cell Biol. 2018;38(7) doi: 10.1128/MCB.00642-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inami Y, Waguri S, Sakamoto A, et al. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol. 2011;193(2):275–284. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni H, Boggess N, McGill MR, et al. Liver-specific loss of Atg5 causes persistent activation of Nrf2 and protectsagainst acetaminophen-induced liver injury. Toxicol Sci : an off J Soc Toxicol. 2012;127(2):438–450. doi: 10.1093/toxsci/kfs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni H, Woolbright BL, Williams J, et al. Nrf2 promotes the development of fibrosis and tumorigenesis in mice withdefective hepatic autophagy. J Hepatol. 2014;61(3):617–625. doi: 10.1016/j.jhep.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubinstein AD, Eisenstein M, Ber Y, et al. The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members topromote mitochondrial apoptosis. Mol Cell. 2011;44(5):698–709. doi: 10.1016/j.molcel.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Jung S, Choe S, Woo H, et al. Autophagic death of neural stem cells mediates chronic stress-induced decline of adult hippocampal neurogenesis and cognitive deficits. Autophagy. 2020;16(3):512–530. doi: 10.1080/15548627.2019.1630222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer N, Zielke S, Michaelis JB, et al. AT 101 induces early mitochondrial dysfunction and HMOX1 (heme oxygenase 1) totrigger mitophagic cell death in glioma cells. Autophagy. 2018;14(10):1693–1709. doi: 10.1080/15548627.2018.1476812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rwei P, Alex Gong C, Luo L, et al. In vitro investigation of ultrasound-induced oxidative stress on human lensepithelial cells. Biochem. Bioph. Res. CO. 2017;482(4):954–960. doi: 10.1016/j.bbrc.2016.11.139. [DOI] [PubMed] [Google Scholar]
- 39.Yu Y, Jiang H, Li H, et al. Alpha-A-crystallin protects lens epithelial cell-derived iPSC-like cells against apoptosis induced by oxidative stress. Cell Reprogram. 2016;18(5):327–332. doi: 10.1089/cell.2016.0017. [DOI] [PubMed] [Google Scholar]
- 40.Liu L, Yu R, Shi Y, et al. Transduced protein transduction domain linked HSP27 protected LECs against UVB radiation-induced damage. Exp Eye Res. 2014;120:36–42. doi: 10.1016/j.exer.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Skinner C, Miraldi Utz V. Pharmacological approaches to restoring lens transparency: real worldapplications. Ophthalmic Genet. 2017;38(3):201–205. doi: 10.1080/13816810.2016.1214971. [DOI] [PubMed] [Google Scholar]