Abstract
Provided herein are novel pyrazolopyrazine compounds as SHP2 inhibitors, pharmaceutical compositions, use of such compounds in treating glioblastoma, and processes for preparing such compounds.
Important Compound Classes
Title
Pyrazolopyrazine Compounds as SHP2 Inhibitors
Patent Publication Number
WO 2023/114954 A1
Publication Date
June 22, 2023
Priority Applications
US 63/291,012 and US 63/431,260
Priority Dates
December 17, 2021, and December 8, 2022
Inventors
Begis, G.; Bianciotto, M.; Devillers, I.; Foricher, Y.; Genevois-Borella, A.; Gill, A. L.; Karlsson, A.; Koltun, E. S.
Assignee Company
Genzyme Corporation, USA, and Revolution Medicines Inc., USA
Disease Area
Glioblastoma
Biological Target
SHP2
Summary
Src homology region 2 (SH2)-containing protein tyrosine phosphatase 2 (SHP2) is a ubiquitously expressed protein tyrosine phosphatase encoded by the PTPN11 gene that contributes to multiple cellular functions, including proliferation, differentiation, cell cycle maintenance, and migration. SHP2 is involved in signaling through the Ras-mitogen-activated protein kinase, the JAK-STAT, or the phosphoinositol-3-kinase-AKT pathways.
Mutations in the PTPN11 gene and subsequently in SHP2 have been identified in several diseases, such as Noonan syndrome, Leopard syndrome, juvenile myelomonocytic leukemias, melanoma, neuroblastoma, acute myeloid leukemia, and cancers of the breast, lung, colon, and brain, including glioblastoma. As such, SHP2 represents a highly attractive target for the development of novel therapies for the treatment of various diseases, including cancer. For treating or preventing cancers associated with the brain, an SHP2 inhibitor with brain penetration capability is particularly attractive.
The present application describes a series of novel pyrazolopyrazine compounds as SHP2 inhibitors for the treatment of glioblastoma. Further, the application discloses compounds, their preparation, use, and pharmaceutical composition, and treatment.
Definitions
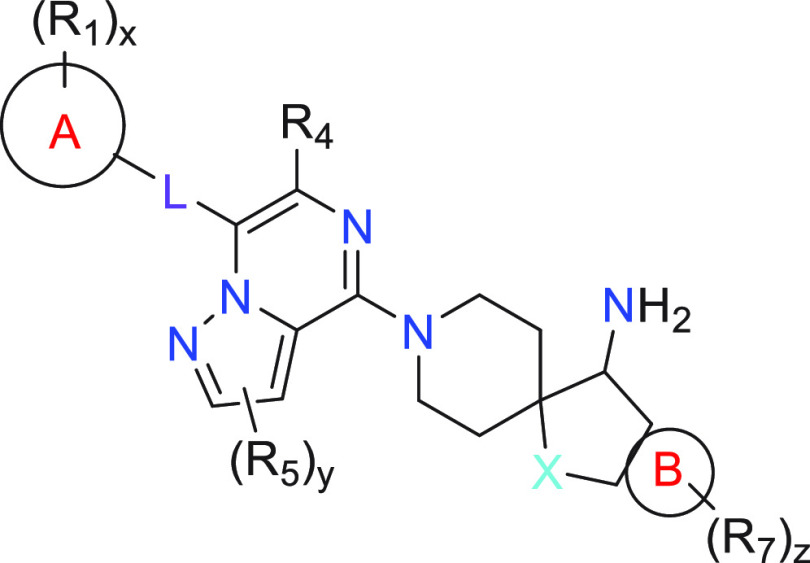
Ring A = C3–C6 cycloalkyl, phenyl, 5- to 6-membered heterocycloalkyl, 5- to 6-membered heteroaryl, wherein heterocycloalkyl and heteroaryl contain 1–3 heteroatoms selected from N, O, and S;
R1 = halo, cyano, -NR2aR2b, C1–C6 alkyl, oxo, hydroxy, C1–C6 haloalkyl, C1–C6 alkoxy, C1–C6 alkyl-OH, C1–C6 alkyl-CN, -C(O)NR2aR2b, -C(O)(C1–C6 alkyl), -COOH, -COO(C1–C6 alkyl), -Si(Ra)(Rb)(Rc), -P(O)(Ra)(Rb), -OP(O)(Ra)(Rb), C3–C6 cycloalkyl, 5- to 6-membered heterocycloalkyl, 5- to 6-membered heteroaryl, wherein heterocycloalkyl and heteroaryl contain 1–3 heteroatoms selected from N, O, and S; L = bond, S, O, C(O), or N(Rd);
X = CR3aR3b, NR3a, or O;
R4 = H, C1–C6 alkyl, C1–C6 alkyl-OH, C1–C6 haloalkyl, or -NH2;
R5 = halo, C1–C6 alkyl, C1–C6 haloalkyl, -(C1–C6 alkylene)(C1–C6 alkoxy), or C1–C6 alkyl-OH;
Ring B = fused phenyl or 5- to 6-membered heteroaryl containing 1–3 heteroatoms selected from N, O, and S;
R7 = C1–C6 alkyl, halo, C1–C6 alkoxy, C1–C6 alkyl-OH, OH, CN, Si(Ra)(Rb)(Rc), -P(O)(Ra)(Rb), -OP(O)(Ra)(Rb), -NR2aR2b, or C1–C6 haloalkyl;
x = 0–5; y = 0–2; and z = 0–4.
Key Structures
Biological Assay
The SHP2 phosphatase assay was performed. The compounds described in this application were tested for their ability to inhibit SHP2. The SHP2 IC50 values (nM) are shown in the table below.
Biological Data
The following table shows representative
compounds that were tested for SHP2 inhibition and the biological
data obtained from testing representative examples.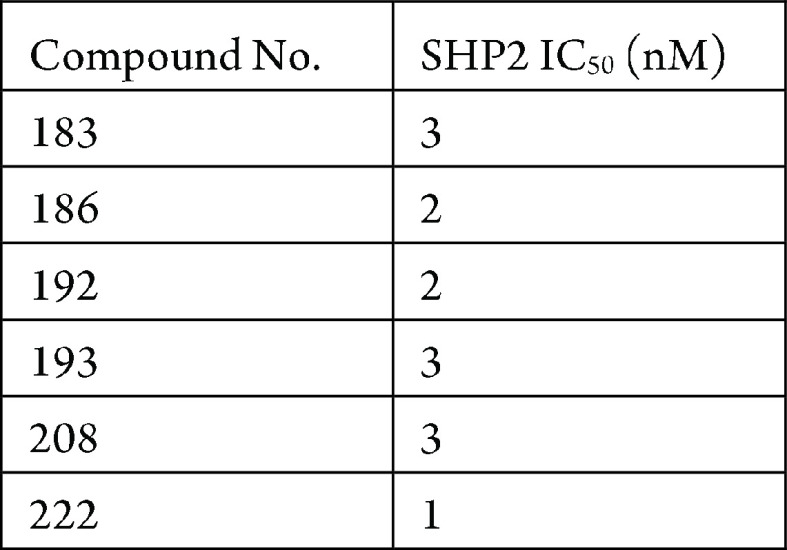
Claims
Total claims: 29
Compound claims: 25
Pharmaceutical composition claims: 1
Method of treatment claims: 2
Method of inhibition claims: 1
Recent Review Articles
The author declares no competing financial interest.
References
- Awadasseid A.; Zhou Y.; Zhang K.; Tian K.; Wu Y.; Zhang W. Current studies and future promises of PD-1 signal inhibitors in cervical cancer therapy. Biomed. Pharmacother. 2023, 157, 114057. 10.1016/j.biopha.2022.114057. [DOI] [PubMed] [Google Scholar]
- Meng Y.; Bai R.; Cui J. Precision targeted therapy for EGFR mutation-positive NSCLC : Dilemmas and coping strategies. Thorac. Cancer 2023, 14, 1121–1134. 10.1111/1759-7714.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; Zhou X.; Fu D.; Le C.; Wang J.; Zhou Q.; Liu X.; Yuan Y.; Ding K.; Xiao Q. Targeting RAS mutants in malignancies: successes, failures, and reasons for hope. Cancer Commun. 2023, 43, 42–74. 10.1002/cac2.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H.-C.; Yu J. Targeted therapy for pancreatic ductal adenocarcinoma: Mechanisms and clinical study. MedComm 2023, 4, e216. 10.1002/mco2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y.; Gotoh N. Inflammatory cytokine-enriched microenvironment plays key roles in the development of breast cancers. Cancer Sci. 2023, 114, 1792–1799. 10.1111/cas.15734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.; Zhao M.; Zhang H.; Yu B. Double-edged roles of protein tyrosine phosphatase SHP2 in cancer and its inhibitors in clinical trials. Pharmacol. Ther. 2022, 230, 107966. 10.1016/j.pharmthera.2021.107966. [DOI] [PubMed] [Google Scholar]