Abstract
Background
Diagnosis and prognostication of severe traumatic brain injury (sTBI) continue to be problematic despite years of research efforts. There are currently no clinically reliable biomarkers, though advances in protein biomarkers are being made. Utilizing Omics technology, particularly metabolomics, may provide new diagnostic biomarkers for sTBI. Several published studies have attempted to determine the specific metabolites and metabolic pathways involved; these studies will be reviewed.
Aims
This scoping review aims to summarize the current literature concerning metabolomics in sTBI, review the comprehensive data, and identify commonalities, if any, to define metabolites with potential clinical use. In addition, we will examine related metabolic pathways through pathway analysis.
Methods
Scoping review methodology was used to examine the current literature published in Embase, Scopus, PubMed, and Medline. An initial 1090 publications were identified and vetted with specific inclusion criteria. Of these, 20 publications were selected for further examination and summary. Metabolic data was classified using the Human Metabolome Database (HMDB) and arranged to determine the ‘recurrent’ metabolites and classes found in sTBI. To help understand potential mechanisms of injury, pathway analysis was performed using these metabolites and the Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Database.
Results
Several metabolites related to sTBI and their effects on biological pathways were identified in this review. Across the literature, proline, citrulline, lactate, alanine, valine, leucine, and serine all decreased in adults post sTBI, whereas both octanoic and decanoic acid increased. Hydroxy acids and organooxygen compounds generally increased following sTBI, while most carboxylic acids decreased. Pathway analysis showed significantly affected glycine and serine metabolism, glycolysis, branched-chain amino acid (BCAA) metabolism, and other amino acid metabolisms. Interestingly, no tricarboxylic acid cycle metabolites were affected.
Conclusion
Aside from a select few metabolites, classification of a metabolic profile proved difficult due to significant ambiguity between study design, sample size, type of sample, metabolomic detection techniques, and other confounding variables found in sTBI literature. Given the trends found in some studies, further metabolomics investigation of sTBI may be useful to identify clinically relevant metabolites.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12868-023-00824-1.
Keywords: Traumatic brain injury (TBI), Severe traumatic brain injury (sTBI), Metabolomics, Scoping review
Introduction
Traumatic brain injury
Traumatic Brain Injury (TBI) is slowly becoming one of the leading causes of death and disability worldwide [1–3]. Currently, about half the world’s population is expected to experience a TBI within their lifetime [4], and by the year 2031, TBI is anticipated to be one of the most common neurological conditions affecting the globe [5, 6]. TBI is defined as a sudden external trauma to the head causing both immediate and delayed alterations to brain function [7]. Following primary injury, secondary injury mechanisms such as cerebral edema, hypoxia, and subarachnoid hemorrhage continue to disrupt the brain’s cells and tissues, causing further damage [8–11]. There are three clinically defined severity levels of TBI based on the Glasgow Coma Scale (GCS): mild traumatic brain injury (mTBI, GCS 13-15), moderate traumatic brain injury (moTBI, GCS 9-12), and severe traumatic brain injury (sTBI, GCS 3-8). sTBI is defined as having a Glasgow Coma Scale (GCS) of 3-8, which is assessed by examining verbal, eye, and motor responses in an individual suspected of having a brain injury [12]. Understandably, sTBI causes the largest economic and societal strains on health care systems as individuals are often unresponsive, or even comatose, require life support, and can have significant residual effects if they survive [13, 14]. A multicenter study by Dawes and colleagues in 2015 found that unadjusted mortality rates varied from 20 to 50% in adults with sTBI (GCS < 9) [15]. Furthermore, the dynamic nature of sTBI pathology can lead to unfortunate misdiagnoses and misinterpretations of severity [16], especially in polytrauma patients. sTBI is a chronic disease process and should be treated as such; the life-long repercussions of these injuries can severely impact an individual's life expectancy and quality of life [17].
Current clinical assessment techniques for sTBI include Computed Tomography (CT) Scans and Magnetic Resonance Imaging (MRI). While these techniques excel in diagnosing the injury, they lack the required specificity to recognize the severity and make accurate outcome predictions for the entire range of sTBI injuries. Additionally, sedation and analgesia can interfere with diagnosis and outcome assessments, especially in patients suffering polytrauma associated with TBI. Therefore, new reliable diagnostic and prognostic methods are needed for more accurate sTBI diagnosis and prognosis.
Metabolomics as a diagnostic and prognostic tool
Metabolomics is a diagnostic tool used to identify metabolites within cells, tissues, and fluids of biological organisms. It is categorized under the ‘Omics’ line of health research technologies along with genomics and proteomics which all focus on identifying, characterizing, and quantifying the biological molecules involved in the structural and functional organization of organisms [18, 19]. Metabolomics focuses on the classification of metabolites within the human metabolome to help gain insight into the pathophysiological processes of various illnesses and diseases. ‘Biomarker’ is the term given to metabolites or characteristics that are recognized indicators of change in biological processes, such as those defining the pathological mechanisms of sTBI [20]. Currently, there are no widely accepted metabolite biomarkers for sTBI. However, the identification of clinically relevant biomarkers could potentially lead to the creation of novel therapeutics, more accurate diagnoses of disease severity, and more reliable prognostication. This could have an immense impact on the treatment protocols for sTBI and support the ongoing research to identify specific metabolites (biomarkers) involved in sTBI pathogenesis.
Metabolic analysis uses a wide array of different sampling methods. Serum and plasma are among the most frequently used sample types, as they are typically the easiest to retrieve from injured individuals. However, collection sites for serum and plasma are generally at least one compartment away from the injured brain, allowing for the interference of confounders, such as compensatory mechanisms and polytrauma injuries, specifically in sTBI patients. Cerebrospinal Fluid (CSF) as well as brain microdialysate can also be used in metabolomics analysis and appears to provide the most precise measurements due to proximity to the injured area. However, CSF retrieval requires invasive methods such as lumbar punctures, and for this reason, they are obtained much less often in TBI. Additionally, sources of metabolomics analysis such as urine, feces, and magnetic resonance imaging spectroscopy (MRIS) have been explored in metabolite determination for TBI in humans [21].
Once samples have been retrieved, several different analytical platforms can be utilized for the identification and quantification of metabolites. Metabolite measurement is divided into two different approaches, targeted and non-targeted. Targeted metabolomics is the identification and quantification of previously defined and chemically characterized metabolites, while untargeted metabolomics is the overall general identification and relative quantification of all measurable analytes and metabolites in a sample, including those that are unknown [22]. The most frequently used analytical approaches for measuring metabolites include gas chromatography-mass spectrometry (GC–MS), liquid chromatography-mass spectrometry (LC–MS), and proton nuclear magnetic resonance (1H-NMR) spectroscopy. MRIS is being used more frequently on the brain as a non-invasive method for identifying metabolites in tissues using sophisticated MRI techniques; however, using current technology, resolution, and metabolite identification are limited [23].
Metabolomics in severe traumatic brain injury
The purpose of this scoping review is to summarize the current literature on metabolomics investigations in sTBI and their metabolic data to determine if any common metabolite patterns exist. Aside from a study published in 2017 by Posti and colleagues [16], a scoping review of this kind, comparing results across a large selection of adult human metabolomic studies with the addition of affected pathways, has yet to be completed, to our knowledge. An updated and comprehensive summary of the current literature on metabolomics in sTBI is needed to determine clinically reliable biomarkers.
This scoping review encompasses most of the recent metabolite literature published for sTBI in adult cohorts. However, there is unfortunately a general lack of clarity between metabolic studies due to differing sampling methods, sample types, analytical techniques, and study designs. This study aims to dissect the differences between the studies and determine if there are any commonalities or overall patterns apparent in metabolites following sTBI. It is hoped that the identification of reliable metabolites as ‘biomarkers’ may be the answer to providing a more precise diagnosis and prognosis for all severities of TBI, especially sTBI. The accumulation of data in this review could aid in the creation of a generalized metabolic profile or provide important clues about potential biomarkers for sTBI in adult cohorts.
Methods
This Scoping review was conducted following a modified version of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) [24]. Four bibliographic databases were explored in this scoping review: CINAHL, Ovid Medline, PubMed, and Scopus. Hand searched articles from select reference lists and the University of Calgary library were also included.
Search strategy
The research goal was to comprehensively gather published literature since the year 2000 on metabolomics in severe traumatic brain injury among adults and create a profile of recurrent metabolites and related pathways that could support clinical applications. A key concept chart was created including sets of search terms to be explored, such as “metabolomics in sTBI”, “data analysis” and, “biomarkers in sTBI”, as well as potential free text terms like “metabolites”, “severe traumatic brain injury” and “adult cohort”. Search phrases were then created using free text terms and Boolean operators such as OR/AND where appropriate. The main phrase chosen to search the databases was “metabolomics” AND “severe traumatic brain injury”. Additional phrases, including “biomarkers” AND "severe traumatic brain injury,” were also applied in the primary search. Further hand searching revealed several more articles which were also included in the study.
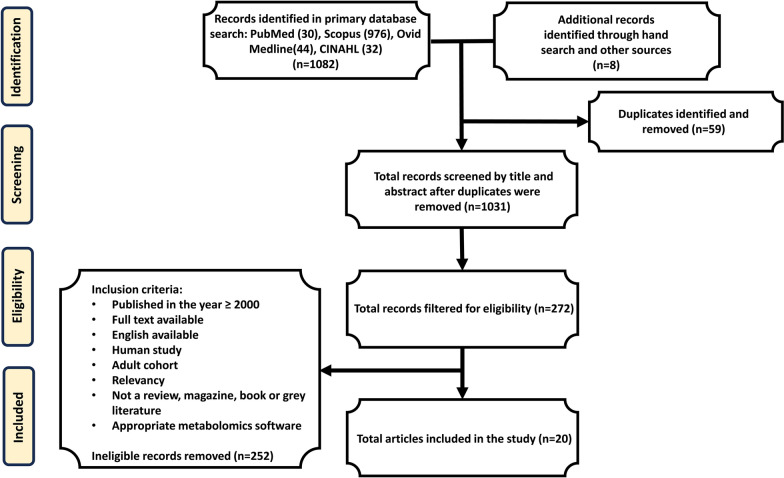
The database search retrieved 1082 publications in total; PubMed (n = 30), Scopus (n = 976), Medline Ovid (n = 44), and CINAHL (n=32), and 8 additional hand searched references (see Fig. 1, consort diagram). The publications were combined in Clarivate Analytics: EndNote, a referencing software, and 59 duplicates were removed, resulting in 1031 publications qualifying for the screening process. Based on the primary search, abstracts, and titles were screened and eliminated if deemed irrelevant. Screening greatly reduced the number of publications to 272. Eligibility was then decided using previously established inclusion criteria for the remaining publications. The included articles were published in or after the year 2000—with an emphasis on publications within the last decade, full text, and availability in English. Results were then filtered for human studies with adult cohorts and appropriate metabolomics software analysis. Literature was not included if classified as a review, grey literature, magazine, or book. Additional studies were later removed for irrelevancy, other types of TBI not including sTBI, and lack of clarity in results. The eligibility process yielded 20 suitable publications for metabolomics investigations in sTBI to be summarized in this scoping review.
Fig. 1.
Consort Diagram for the publication selection process
Data collection and classification
The included publications were reviewed in full and comprehensive summary tables were produced using Microsoft Excel. Publications were summarized using headings such as year, author, sample type and size, detection technique, major metabolite findings, and other varying statistics. A subsequent table was generated using the major metabolite findings and incorporated a more thorough breakdown of the exact metabolites found in each study and whether they increased, decreased, or were unchanged. In this analysis, ‘recurrent’ metabolites were defined as any metabolite that was found either increasing or decreasing in two or more of the included studies and was therefore determined to have a stronger connection to sTBI in adults. All metabolites were then further analyzed through organization by class using the Human Metabolome Database (HMDB). The Class, Subclass, Super Class, and HMDB code of each metabolite were included in the initial summary table. The format of these tables allowed for the direct comparison and visualization of specific metabolites and patterns between studies.
Pathway analysis
A pathway analysis diagram was then manually generated using the major metabolite findings and the Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Database. The diagram was produced to demonstrate the major pathways affected by sTBI pathogenesis. Once specific pathways were identified, further literature analysis was conducted to expand on the results. Emphasis was placed on those showing the same metabolites affected in more than one study.
Results
The present scoping review analyzed 20 publications with established investigations of metabolomics in severe traumatic brain injury among adults. A summary (Additional file 1: Table S1) is provided in the Additional file for the context and setting of the examined publications.
Many of the explored studies undertook broad metabolic analysis in adult cohorts searching for overall metabolite alterations post TBI, while a select few specified on individual metabolites. For example, a study by Marino and colleagues used proton nuclear magnetic resonance imaging (1H-MRIS) to examine metabolic levels of N-acetylaspartate (NAA), choline, creatine, and lactate following brain injury [25], whereas Jeter and colleagues utilized LC–MS and GC–MS to measure L-arginine levels and branched chain amino acids (BCAA) in patients post TBI [26, 27]. Another study examined extracellular NAA in microdialysate using high-performance liquid chromatography (HPLC) [28]. Methionine alteration post TBI was investigated by Dash and colleagues in 2016 using LC–MS and GS-MS [29]. More recent literature includes work by Bykowski and coworkers who utilized 1H-NMR technology in urine to determine changes in metabolites during TBI recovery and the correlation to injury severity [30]. Earlier this year, Mondello and his team published a study that used LC–MS to investigate serum glycome patterns following TBI [31] and another study used Center-TBI data to describe the human metabolome associated with TBI [32]. A more detailed breakdown of the reviewed studies can be found in Table A1.
Table 1.
15 recurrent metabolites (bolded) found indicative of stronger connection to severe traumatic brain injury pathology
| Metabolite | Sample type | Change identified | Supplemental | Refs. |
|---|---|---|---|---|
| Octanoic acid | ||||
| Serum/Plasma | Increase | Increased as TBI severity increased | Thomas et al. [32] | |
| Serum/Microdialysate | Increase | Found upregulated in sTBI patients and remained high in most patients | Oresic et al. [33] | |
| Decanoic acid | ||||
| Serum/Plasma | Increase | Increased as TBI severity increased | Thomas et al. [32] | |
| Serum/Microdialysate | Increase | Found upregulated in sTBI patients and remained high in most patients | Oresic et al. [33] | |
| Serine | ||||
| Serum/Microdialysate | Decrease | Found downregulated in all TBIs and more strongly in sTBI | Oresic et al. [33] | |
| Serum/Plasma | Decrease | Found decreased in all TBI patients overall | Thomas et al. [32] | |
| Serum/Plasma | Decrease | ≤ 60% the concentration in jugular blood compared to HC | Wolahan et al. [34] | |
| Inositol/Myo-inositol | ||||
| Inositol | Serum/Microdialysate | Increase | Found to increase in patients with detectable pathology on a CT scan or the presence of a mass lesion following TBI | Dickens et al. [36] |
| Myo-inositol | Serum/Plasma | Increase | Found elevated in TBI patients and proportional to differing severity | Thomas et al. [32] |
| Alanine | ||||
| Serum/Microdialysate | Decrease | Found downregulated in all TBIs and more strongly in sTBI | Oresic et al. [33] | |
| Serum/Plasma | Decrease | Found decreased in TBI patients overall | Thomas et al. [32] | |
| Choline | ||||
| Serum/Plasma | Decrease | Significantly reduced in sTBI compared to mTBI and healthy volunteers | Dash et al. [29] | |
| Serum/Plasma | Increase | Net cerebral release or increase in jugular venous blood | Wolahan et al. [34] | |
| MR imaging | Increase | Decrease in patients with differing degrees of TBI, including sTBI | Marino et al. [25] | |
| Lactate | ||||
| CSF | Increase | A slight statistically increasing trend in sTBI patients compared to non-injured controls | Glenn et al. [37] | |
| MR Imaging | Increase | Diffusely high signal of lactate resonance intensity in patients with acute TBI including sTBI | Marino et al. [25] | |
| CSF | Increase | Significantly increased compared to control and survival groups | Stefani et al. [38] | |
| Citrulline | ||||
| Serum/Plasma | Decrease | ≤ 60% the concentration in jugular blood compared to HC | Wolahan et al. [34] | |
| Serum/Plasma | Decrease | Significantly reduced in the plasma of sTBI patients | Jeter et al. [26] | |
| Proline and derivatives | ||||
| Proline | Serum/Plasma | Decrease | ≤ 60% the concentration in arterial plasma compared to HC | Wolahan et al. [34] |
| Proline | Serum/Plasma | Decrease | Significantly reduced in the plasma of sTBI patients compared to healthy volunteers, orthopedic controls, and mTBI patients | Jeter et al. [26] |
| 5-oxoproline | Serum/Plasma | Decrease | Significantly reduced in sTBI patients compared to mTBI and healthy volunteers | Dash et al. [29] |
| Hydroxyproline | Serum/Plasma | Decrease | Significantly reduced in the plasma of sTBI patients compared to healthy volunteers, orthopedic controls, and mTBI patients | Jeter et al. [26] |
| Hydroxyproline | Serum/Plasma | Decrease | ≤ 60% the concentration in jugular blood compared to HC | Wolahan et al. [34] |
| Methionine | ||||
| Urine | Increase | Significant negative correlation (increase) to patients decreasing GCS scores | Bykowski et al. [30] | |
| Serum/Plasma | Decrease | Significant reduction in plasma relative to healthy volunteers | Dash et al. [29] | |
| Xanthine | ||||
| Serum/Plasma | Increase | Significant net cerebral release/increase in jugular venous blood | Wolahan et al. [34] | |
| Urine | Increase | Increased levels following recovery | Bykowski et al. [30] | |
| N-acetylaspartate | ||||
| Microdialysate | Decrease | Steep decline of extracellular NAA seen in 8 patients early on | Shannon et al. [28] | |
| MR Imaging | Decrease | Decrease in patients with differing degrees of TBI, including sTBI | Marino et al. [25] | |
| 2/3-hydroxybutryic acid | ||||
| 2-hydroxybutyrate | Serum/Plasma | Increase | Found significantly increased in sTBI patients | Dash et al. [29] |
| 2-hydroxybutyric acid | Serum/Microdialysate | Increase | Found upregulated in sTBI patients | Oresic et al. [33] |
| 3-hydroxybutyric acid | Serum/Microdialysate | Increase | Found upregulated in sTBI patients | Oresic et al. [33] |
| Valine | ||||
| Serum/Plasma | Decrease | Reduction in levels detected in the plasma of sTBI patients compared to all other groups | Jeter et al. [27] | |
| Serum/Plasma | Decrease | Significantly decreased during the first week post Stbi compared to controls | Vuille-Dit-Bille [35] | |
| Leucine | ||||
| Serum/Plasma | Decrease | Decrease in patients with sTBI compared to healthy volunteers and mTBI patients | Jeter et al. [27] | |
| Serum/Plasma | Decrease | Significantly decreased during the first week post sTBI compared to controls | Vuille-Dit-Bille [35] | |
CT computed tomography; GCS Glasgow Coma Scale; HC healthy controls; mTBI minor traumatic brain injury; NAA N-acetylaspartate; sTBI severe traumatic brain injury; TBI traumatic brain injury
Fifteen ‘recurrent’ metabolites were identified across publications
In this review, a metabolite was defined as ‘recurrent’ if it was found either increasing or decreasing in two or more of the included studies and was therefore determined to have a stronger connection to sTBI. Fifteen ‘recurrent’ metabolites were identified in this analysis and are displayed below [Table 1], along with their respective references, methods for sample collection, and supplemental information. The collected data shows that two medium-chain fatty acids, octanoic and decanoic acid, both increase in adults after sTBI [32, 33]. The data also displays an increase in 2- and 3-hydroxybutyric acids following sTBI [29, 33].
Furthermore, this review found that after sTBI, serine [32–34], alanine [32, 33], proline [26, 29, 34], valine [27, 35], and leucine [27, 35] all decreased, while conflicting results were found for methionine [29, 30]. Choline presented conflicting results as well, but more studies declared an increase in choline following sTBI [25, 29, 34]. Inositol [36] and myo-inositol [32] were also found to increase after sTBI, while n-acetylaspartate [25, 28] and citrulline [26, 34] were found to decrease. Finally, an increase in both xanthine [30, 34] and lactate [25, 37, 38] was found following sTBI in adults. These metabolites represent findings supported by more than one study and thus may serve as a preliminary metabolic profile of sTBI in adults. However, it is important to consider the limitations of combining primary data from several studies to reach conclusions, which will be addressed in the discussion below.
Classification of all metabolites by human metabolome database identified clear alterations in metabolite groups
To further analyze and specify metabolic alterations following sTBI in adults, the collected metabolite data was categorized using the Human Metabolome Database (HMDB). For this analysis, all metabolite data was included, together with the ‘recurrent’ metabolites. Thirteen different metabolite classes were identified from the collection of data. Table 2 presents the metabolites arranged by class along with their respective references, sample collection method, and supplemental information. Summarization of the literature displayed that after sTBI, carboxylic acids and derivatives primarily decreased. However, several exceptions existed, including creatine, glutamine, phenylalanine, methionine, glutamate, and tyrosine, which all increased. Following sTBI, a consistent increase was seen in hydroxy acids and derivatives. Aside from an elevation in octanoic and decanoic acids, fatty acyls were found to primarily decrease. Organooxygen compounds mostly increased following sTBI, aside from a select few glucose derivatives which decreased. Both purine nucleosides and imidazopyrimidines exhibited increasing trends. Adversely, keto acids and derivatives and organonitrogens both presented inconsistent results between classified metabolites, rendering conclusions difficult. Other classes with trivial amounts of metabolites were also identified but were not deemed significant enough to warrant any major conclusions.
Table 2.
Human metabolome database (HMDB) classification of collected metabolites by class (bolded)
| HMDB class | Metabolite | Sample type | Change identified | Supplemental | Refs. |
|---|---|---|---|---|---|
| Fatty acyls | |||||
| Isobutyrylcarnitine | Serum/Plasma | Decrease | Significant decrease in sTBI patients compared to healthy volunteers, orthopedic patients and mTBI | Jeter et al. [27] | |
| Isoleucine | Serum/Plasma | Decrease | Significant decrease in sTBI patients compared to healthy volunteers, orthopedic patients and mTBI | Jeter et al. [27] | |
| Propionylcarnitine | Serum/Plasma | Decrease | Decent reduction in sTBI patients compared to healthy volunteers | Jeter et al. [27] | |
| Succinylcarnitine | Serum/Plasma | Decrease | Only differed by a decrease in sTBI patients compared to orthopedic injury patients | Jeter et al. [27] | |
| Hydroxyisovaleryl carnitine | Serum/Plasma | Decrease | Found decreased exclusively in sTBI patients compared to healthy volunteers | Jeter et al. [27] | |
| Octanoic acid | Serum/Microdialysate | Increase | Found upregulated in sTBI patients and remained high in most patients | Oresic et al. [33] | |
| Decanoic acid | Serum/Microdialysate | Increase | Found upregulated in sTBI patients and remained high in most patients | Oresic et al. [33] | |
| Octanoic acid | Serum/Plasma | Increase | As TBI severity increased, octanoic acid increased | Thomas et al. [32] | |
| Decanoic acid | Serum/Plasma | Increase | As TBI severity increased, decanoic acid increased | Thomas et al. [32] | |
| 2-methylbutyrylcarnitine | Serum/Plasma | Decrease | Significant decrease in sTBI patients compared to healthy volunteers and mTBI | Jeter et al. [27] | |
| Isovalerylcarnitine | Serum/Plasma | Decrease | Significant decrease in sTBI patients compared to healthy volunteers, orthopedic controls, and mTBI | Jeter et al. [27] | |
| Keto acids and derivatives | |||||
| α-ketobutyrate | Serum/Plasma | Increase | Found significantly increased in the plasma of sTBI patients | Dash et al. [29] | |
| Methylglutarylcarnitine | Serum/Plasma | Increase | Plasma levels were found significantly increased in sTBI patients compared to healthy volunteers, orthopedic controls, and mTBI | Jeter et al. [27] | |
| 4-methyl-2-oxopentanoate | Serum/Plasma | Decrease | Decreased in sTBI patients compared to healthy volunteers | Jeter et al. [27] | |
| 3-methyl-2-oxovalerate | Serum/Plasma | Decrease | Significant decrease in sTBI patients compared to healthy volunteers, orthopedic patients, and mTBI | Jeter et al. [27] | |
| Carboxylic acids and derivatives | |||||
| Methionine | Serum/Plasma | Decrease | Significant reduction in plasma relative to healthy volunteers | Dash et al. [29] | |
| Betaine | Serum/Plasma | Decrease | Significantly reduced compared to mTBI group and healthy volunteers | Dash et al. [29] | |
| Dimethylglycine | Serum/Plasma | Decrease | Significant decrease in sTBI patients relative to healthy volunteers | Dash et al. [29] | |
| Cysteine | Serum/Plasma | Decrease | Showed a significant reduction in sTBI patients | Dash et al. [29] | |
| Glycine | Serum/Plasma | Decrease | Significantly reduced in sTBI and mTBI | Dash et al. [29] | |
| Gamma-glutamylvaline | Serum/Plasma | Decrease | Relative levels were found significantly decreased in sTBI patients | Dash et al. [29] | |
| Gamma-glutamylleucine | Serum/Plasma | Decrease | Relative levels were found significantly decreased in sTBI patients | Dash et al. [29] | |
| Gamma-glutamylisoleucine | Serum/Plasma | Decrease | Relative levels were found significantly decreased in sTBI patients | Dash et al. [29] | |
| Gamma-glutamyltyrosine | Serum/Plasma | Decrease | Relative levels were found significantly decreased in sTBI patients | Dash et al. [29] | |
| Gamma-glutamylphenylalanine | Serum/Plasma | Decrease | Relative levels were found significantly decreased in sTBI patients | Dash et al. [29] | |
| 5-oxoproline | Serum/Plasma | Decrease | Significantly reduced in sTBI patients compared to mTBI and healthy volunteers | Dash et al. [29] | |
| 2-Aminobutyric acid | Serum/Plasma/Microdialysate | Decrease | Found lower concentrations in patients with detectable CT features following TBI | Dickens et al. [36] | |
| Citrulline | Serum/Plasma | Decrease | Significantly reduced in the plasma of sTBI patients | Jeter et al. [26] | |
| Ornithine | Serum/Plasma | Decrease | Significant decrease in the plasma of sTBI patients compared to healthy volunteers, orthopedic controls, and mTBI patients | Jeter et al. [26] | |
| Proline | Serum/Plasma | Decrease | Significantly reduced in the plasma of sTBI patients compared to healthy volunteers, orthopedic controls, and mTBI patients | Jeter et al. [26] | |
| 4-Hydroxyproline | Serum/Plasma | Decrease | Significantly reduced in the plasma of sTBI patients compared to healthy volunteers, orthopedic controls, and mTBI patients | Jeter et al. [26] | |
| Creatine | Serum/Plasma | Increase | Significantly increased in sTBI patients compared to HC and orthopedic controls | Jeter et al. [26] | |
| Valine | Serum/Plasma | Decrease | Significantly decreased during the first week post sTBI compared to controls | Vuille-Dit-Bille [35] | |
| Valine | Serum/Plasma | Decrease | Reduction in valine levels detected in the plasma of sTBI patients compared to all other groups | Jeter et al. [27] | |
| Serine | Serum/Microdialysate | Decrease | Found downregulated in all TBIs, more strongly in sTBI | Oresic et al. [33] | |
| Leucine | Serum/Plasma | Decrease | Significantly decreased during the first week post sTBI compared to controls | Vuille-Dit-Bille [35] | |
| Isoleucine | Serum/Plasma | Decrease | Significantly decreased during the first week post sTBI compared to controls | Vuille-Dit-Bille [35] | |
| Leucine | Serum/Plasma | Decrease | Decrease in patients with sTBI compared to healthy volunteers and mTBI patients | Jeter et al. [27] | |
| Alanine | Serum/Microdialysate | Decrease | Found downregulated in all TBIs, more strongly in sTBI | Oresic et al. [33] | |
| Glutamine | CSF | Increase | Statistically increasing trend in moTBI and sTBI patients compared to non-injured controls | Glenn et al. [37] | |
| Creatinine | CSF | Decrease | Significantly decreased concentrations of total creatinine in moTBI and sTBI patients | Glenn et al. [37] | |
| Proline | Serum/Plasma | Decrease | ≤ 60% the conc. in arterial plasma and jugular blood compared to HC | Wolahan et al. [34] | |
| Hydroxyproline | Serum/Plasma | Decrease | ≤ 60% the conc. in jugular blood compared to HC | Wolahan et al. [34] | |
| Serine | Serum/Plasma | Decrease | ≤ 60% the conc. in jugular blood compared to HC | Wolahan et al. [34] | |
| Citrulline | Serum/Plasma | Decrease | ≤ 60% the conc. in jugular blood compared to HC | Wolahan et al. [34] | |
| eNAA | Microdialysate | Decrease | Steep decline of extracellular NAA seen in 8 patients early on | Shannon et al. [28] | |
| Phenylalanine | Serum/Plasma | Increase | Significantly increased during the first posttraumatic week following TBI | Vuille-Dit-Bille [35] | |
| Methionine | Urine | Increase | Significant negative correlation (increase) to patients decreasing GCS scores | Bykowski et al. [30] | |
| NAA | MR Imaging | Decrease | Decrease in patients with differing degrees of TBI, including sTBI | Marino et al. [25] | |
| L-arginine | Serum/Plasma | Decrease | Significant reduction compared to healthy volunteers, orthopedic injury patients, and mTBI | Jeter et al. [26] | |
| Serine | Serum/Plasma | Decrease | Found decreased in TBI patients overall | Thomas et al. [32] | |
| Alanine | Serum/Plasma | Decrease | Found decreased in TBI patients overall | Thomas et al. [32] | |
| Glutamate | CSF | Increase | Significantly increased compared to control and survival groups | Stefani et al. [38] | |
| Tyrosine | Serum/Plasma | Increase | Significantly increased for both posttraumatic weeks following TBI | Vuille-Dit-Bille [35] | |
| Asparagine | Serum/Plasma | Decrease | TBI blood had less than or equal to 60% the concentration of HC in jugular blood | Wolahan et al. [34] | |
| Threonine | Serum/Plasma | Decrease | Found decreased in TBI patients overall | Thomas et al. [32] | |
| Hydroxy acids and derivatives | |||||
| 2-hydroxybutyrate | Serum/Plasma | Increase | Found significantly increased in sTBI | Dash et al. [29] | |
| 2-hydroxybutyric acid | Serum/Microdialysate | Increase | Found upregulated in sTBI patients | Oresic et al. [33] | |
| 3-hydroxybutyric acid | Serum/Microdialysate | Increase | Found upregulated in sTBI patients | Oresic et al. [33] | |
| Lactate | CSF | Increase | A slight increase compared to non-injured controls in moTBI and sTBI patients | Glenn et al. [37] | |
| Lactate | CSF | Increase | Significantly increased compared to control and survival groups | Stefani et al. [38] | |
| Lactate | MR Images | Increase | Diffusely high signal of lactate resonance intensity in patients with acute TBI | Marino et al. [25] | |
| Organooxygen compounds | |||||
| Inositol | Serum/Plasma/Microdialysate | Increase | Found to increase in patients with detectable pathology on a CT scan or the presence of a mass lesion | Dickens et al. [36] | |
| (2,3-BPG) | Serum/Microdialia | Increase | Found upregulated in sTBI patients and a strong association with TBI severity, roughly a 100-fold upregulation compared to controls | Oresic et al. [33] | |
| Propylene glycol | CSF | Increase | Significantly higher concentrations in moTBI and sTBI patients | Glenn et al. [37] | |
| Gluconate | Serum/Plasma | Decrease | TBI blood had less than or equal to 60% the concentration of HC in arterial plasma | Wolahan et al. [34] | |
| Glucose-6-phosphate | Serum/Plasma | Decrease | Significant decrease in jugular venous blood from arterial levels | Wolahan et al. [34] | |
| Glycerol | Microdialysis | Increase | Significant positive conc. correlations between extracellular NAA and glycerol of 8 patients | Shannon et al. [28] | |
| Ribonic acid | Serum/Plasma | Increase | Found to increase in patients with detectable pathology on a CT scan or the presence of a mass lesion following TBI | Dickens et al. [36] | |
| Kynurenine | CSF | Increase | Displayed median levels similar to control days 0–3, but at days 4 and 5 showed significant elevation over controls post sTBI | Yan et al. [62] | |
| Myo-inositol | Serum/Plasma | Increase | Found elevated in TBI patients and proportional to differing severity | Thomas et al. [32] | |
| Organic carbonic acids and derivatives | |||||
| Urea | Serum/Plasma | Decrease | Significant decrease in plasma of sTBI patients compared to healthy volunteers, orthopedic controls, and mTBI patients | Jeter et al. [26] | |
| Quinolines and derivatives | |||||
| Kynurenic acid | CSF | Increase | Increased post sTBI compared to controls and reached a plateau after day 2 which lasted until day 5 | Yan et al. [62] | |
| Pyridines and derivatives | |||||
| Niacinamide | Serum/Plasma | Decrease | TBI blood had less than or equal to 60% the concentration of HC in jugular blood | Wolahan et al. [34] | |
| Quinolinic acid | CSF | Increase | Concentration significantly increased between days 1 and 5 compared to controls | Yan et al. [62] | |
| Imidazopyrimidines | |||||
| Xanthine | Serum/Plasma | Increase | Significant net cerebral release or increase in jugular venous blood | Wolahan et al. [34] | |
| Xanthine | Urine | Increase | Increased levels following recovery | Bykowski et al. [30] | |
| Hypoxanthine | Urine | Increase | Found increased following recovery | Bykowski et al. [30] | |
| Diazines | |||||
| Thymine | Urine | Increase | Significant negative correlation (increase) to patients decreasing GCS scores | Bykowski et al. [30] | |
| Purine nucleosides | |||||
| Adenosine | Urine | Increase | Found to be significantly upregulated over recovery | Bykowski et al. [30] | |
| Inosine | Urine | Increase | Found upregulated in urine | Bykowski et al. [30] | |
| Deoxyinosine | Urine | Increase | Found upregulated after recovery | Bykowski et al. [30] | |
| Guanosine | Urine | Increase | Found upregulated in urine | Bykowski et al. [30] | |
| Indoles and derivatives | |||||
| Indole-3-propionic acid | Serum/Microdialysis | Decrease | Found downregulated in all TBIs, more strongly in sTBI | Oresic et al. [33] | |
| Tryptophan | CSF | Increase | Increased in CSF compared to controls between days 0 to 5 in sTBI, however, median concentrations did not change much | Yan et al. [62] | |
| Tryptophan | Plasma/Serum | Decrease | Decreased post sTBI in serum from days 0 to 4 | Yan et al. [62] | |
| Organonitrogen compounds | |||||
| Choline | Serum/Plasma | Decrease | Significantly reduced compared to mTBI and healthy volunteers | Dash et al. [29] | |
| Choline | Serum/Plasma | Increase | Net cerebral release or increase in jugular venous blood | Wolahan et al. [34] | |
| Choline | MR Imaging | Increase | From patients with differing degrees of TBI including sTBI | Marino et al. [25] | |
| Spermidine | Serum/Plasma | Decrease | Significantly lower levels post moTBI and sTBI | Huang et al. [65] | |
CSF cerebrospinal fluid; CT computed tomography; GCS Glasgow Coma Scale; HC healthy controls; HMDB Human Metabolome Database; mTBI minor traumatic brain injury; moTBI moderate traumatic brain injury; NAA N-acetylaspartate; sTBI severe traumatic brain injury; TBI traumatic brain injury
Pathway analysis revealed affected glycine and serine metabolism, branched chain amino acid metabolism, glycolysis, and several other amino acids metabolisms
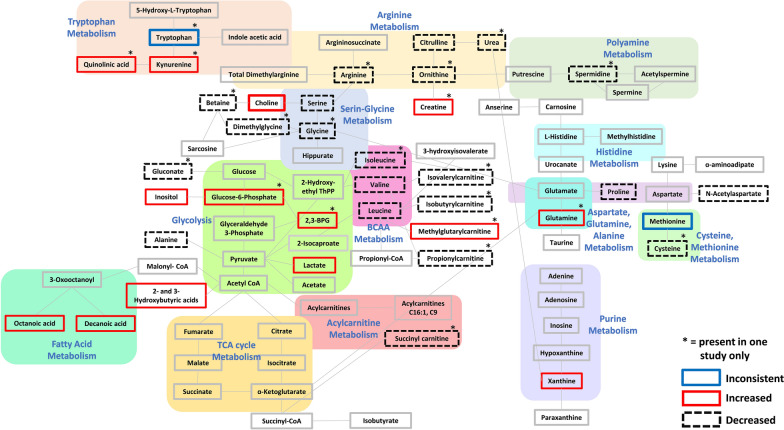
Further exploration of the collected metabolic data led to the manual generation of a metabolic pathway analysis diagram using the KEGG Pathway Database (Fig. 2). The pathway analysis diagram demonstrated significantly affected glycine and serine metabolism, with most metabolites displaying a decrease in concentration following sTBI. Furthermore, BCAA metabolism was significantly affected, attributable to a decrease in most metabolites involved. Glycolysis was also significantly affected, displaying an increase in most metabolites. Fatty acid metabolism was also affected due to an increase in octanoic and decanoic acids. The amino acids with the most altered metabolic pathways appeared to be tryptophan, which exhibited an increase in most of the related metabolites, and arginine, which exhibited a decrease in most of the related metabolites. Cysteine and methionine metabolism were also affected, displaying a general decrease in most metabolites. Only a few metabolites were identified for polyamine metabolism, acylcarnitine metabolism, and purine metabolism. TCA cycle metabolism displayed no affected metabolites following sTBI in the collected data.
Fig. 2.
Pathway analysis diagram generated manually using metabolites identified in the reviewed literature and the KEGG Pathway Database. Pale colored boxes represent metabolic pathways. Pathways are simplified for summary purposes. Increased metabolites found in reviewed studies are indicated by a thick red outline and decreased metabolites are indicated by a thick black dashed outline. Metabolites with conflicting results are indicated by a dark blue outline. An asterisk (*) was placed on each metabolite that was found in only one of the reviewed studies. All other highlighted metabolites without an asterisk were present in more than one reviewed study and encompass the fifteen ‘recurrent’ metabolites identified in this review
Discussion
The results of this scoping review suggest several metabolites with biomarker potential for sTBI in adults discovered through a review of the current primary literature. However, it is important to consider the role that sample origin may play in the significance of metabolite data. This review included publications with several sample origins since different biological sources can be applied for metabolic analysis. Sample origin should be considered when interpreting the data, as biological sources more closely related to the site of injury (the brain) give more precise measurements of local metabolic changes. It is also important to consider which sample type is most accessible for each severity of TBI. Serum and plasma are typically the easiest to collect from all TBI patients, while the methods of retrieving CSF and microdialysate are better suited for sTBI patients, who may already be sedated and instrumented. Due to sampling location, CSF and microdialysate also provide a more accurate depiction of how metabolites surrounding the brain are affected. Despite this, serum and plasma were the most common biological sources used by the publications in this review, followed by CSF and microdialysate. Urine and MRIS were also utilized in some of the publications, however, conflicting results have been found for urine in the past, and MRIS can only provide a very limited resolution or area of brain assessment to date [23].
To understand TBI pathophysiology, it is important to consider the mechanisms by which the brain injury occurs and how metabolite alteration could affect the progression and severity of sTBI. TBI is widely understood as a consequence of both primary injuries; damage occurring at the moment of injury, such as an impact or penetration, and secondary injuries; widespread damage produced hours or days after injury due to a cascade of cellular and inflammatory processes [39, 40]. Familiar secondary injury mechanisms include hypotension [11], hypoxemia [10, 11], ischemia potentially caused by a hypermetabolic surge [8], excitotoxicity [29], and oxidative stress [29, 41]. These secondary injuries could be caused by the metabolic and biochemical changes that occur in the brain following a primary insult, such as those identified in this review.
In the brain, amino acids play an important role in the synthesis of small-molecule neurotransmitters. Figure 2 displays the significant changes in glycine and serine metabolism attributable to sTBI. Glycine is the main inhibitory neurotransmitter in the brainstem and spinal cord and is metabolized from L-serine, which binds to glycine receptors. Serine also plays a significant role in the brain, as it acts as a neuromodulator and neurotransmitter under different conditions. This study identified a recurrent decrease in serine following sTBI, but only one study reported a decrease in glycine [25]. Serine plays a neuroprotective role in the CNS by decreasing neurotoxicity through the activation of glycine receptors and reducing inflammation by lowering proinflammatory factors [42]. Therefore, a decrease in serine could lead to enhanced inflammatory responses and cerebral ischemia following a head injury [43]. A recent study injecting L-serine into rats sustaining a TBI found decreased neurological deficit scores, decreased neuron loss, and overall greater neuroprotection following L-serine injection [44].
The metabolism of arginine, another essential amino acid, was also affected following sTBI (Fig. 2). One biological function of arginine is to serve as a precursor of nitric oxide, which has many physiological functions in the brain, such as protection against further brain injury [45, 46]. Therefore, a reduction in arginine and related metabolites is likely unfavorable to sTBI patients. Arginine participates in the synthesis of creatine, and a deficiency of creatine is associated with many neurological conditions including speech impairments [45, 47]. Valine, leucine, and isoleucine are all BCAAs in a close relationship with aromatic amino acid catabolism, which produces brain neurotransmitters such as serotonin, dopamine, and norepinephrine [48]. In this review, two common BCAAs, valine and leucine, were found to decrease following sTBI. Valine and leucine are both important amino acids in the compartmentalization of glutamate [48], which is a primary excitatory neurotransmitter commonly found in excess after TBI and can cause a secondary injury known as glutamate excitotoxicity [49]. Glutamate excitotoxicity leads to cell apoptosis and neuronal death, which could be responsible for reduced cognitive function [50]. Figure 2 shows significantly affected BCAA metabolism, with nearly all metabolites showing a reduction following sTBI. Alanine is synthesized by BCAAs (valine, leucine, and isoleucine) and was also found in this review to be decreased. Experimentally, BCAAs are known to carry a neuroprotective role and contain neurorestorative properties when supplemented post injury [27, 51, 52]. Therefore, a reduction in BCAAs may contribute to diminished neuroprotection and a high susceptibility to glutamate excitotoxicity.
Glycolysis is a fundamental process by which the body produces energy, both aerobically and anaerobically. Disruptions in cerebral oxidative metabolism can have significant impacts on the recovering brain and have been correlated with poor long-term outcomes such as vegetative state and death [53, 54]. Several of the primary metabolites involved in glycolysis metabolism (see Fig. 2), such as lactate and inositol, were found to be increased following sTBI in this review. An increase in anaerobic glycolysis suggests a higher rate of glucose being metabolized per mole of oxygen through anaerobic mechanisms, which could be considered as the injured brain entering a state of ‘hyperglycolysis’ [54, 55]. A surge in lactate among sTBI patients, as seen in this review, supports the notion of increased anaerobic glycolysis energy metabolism [56]. Lactate has been interpreted in TBI as both (1) a therapeutic option to compensate for decreased cerebral metabolic rate [57] and cognitive impairment [58] and (2) as potentially harmful in having associations with hypoxia and mitochondrial dysfunction [59].
A secondary component of this study included the classification of metabolites designated by the Human Metabolome Database. Exceptions found within some of the categories indicate the large lack of uniformity across current metabolomic studies. The most significant findings from this section of the analysis were an increase in hydroxy acids and a general decrease in carboxylic acids following sTBI. Carboxylic acids and their derivatives encompass numerous amino acids, including serine, alanine, tyrosine, asparagine, threonine, the BCAAs and other closely related derivatives such as gamma-glutamylvaline and citrulline. The overarching similarity between many of the carboxylic acids is the role they may play in excitotoxicity and biochemical alterations which cause a change in regular homeostatic levels of the brain. The altered metabolites in the hydroxy acids and derivatives class included increased 2/3-hydroxybutyric acid and lactate. Increased 2/3-hydroxybutyric acid is associated with poor outcomes and could be connected to ketogenic metabolism, where the increased presence of ketone bodies may be responsible for increasing blood–brain barrier permeability [60]. Deeper understanding of the affected classes involved in sTBI pathology could aid in the potential creation of specialized therapeutics, and thus require further research.
Methionine is an important precursor for glutathione, an antioxidant molecule that works to reduce the stress caused by oxidative damage, therefore, a decrease in methionine could allow for elevated oxidative damage [29]. Contradicting results were found for methionine levels post sTBI in this review. Increases in urine methionine levels were only found in one study [30], however, urine is not as reliable as other biological sources of metabolites, and supporting literature primarily points towards a decrease in methionine leading to poorer clinical outcomes. The tricarboxylic acid (TCA) cycle is an ascertained affected pathway in the progression of secondary injury in sTBI, however, surprisingly, no significant recurrent changes were identified in the TCA cycle metabolites from the reviewed literature (Fig. 2). This could be due in part to the time of sample collection after the head injury, as it is unclear if cerebral TCA metabolism changes over time following sTBI. Interestingly, other omics-type studies show a reduction in TCA protein gene expression, and alterations in TCA enzymes have been seen in closed-head impact mouse models post sTBI [61].
Limitations
The complex nature of TBI, distinguishing between primary and secondary brain injury, the confounding effects of polytrauma, and the potential effects of medications and feeding (nutrition) on metabolomics in patients with sTBI interfere with determining a precise metabolomic profile for sTBI using metabolomics, especially when examining metabolite changes from the reviewed literature. While this study produced several sufficiently supported metabolites changes prevalent in sTBI research, it is too early to determine whether these metabolites can be used clinically as biomarkers for sTBI until controlled validation studies are completed. Comparing clinical findings across a multitude of studies evokes numerous potential confounders and difficulties. As mentioned, the differences in sampling methods—CSF in comparison to serum/plasma and urine, and even venous blood compared to arterial blood—can account for large ambiguities in metabolite concentrations across the datasets. Another large confounder is the time a sample was collected (2 h. post sTBI vs. 6d post sTBI) which affects metabolite concentration and cannot be easily accounted for in this study. Further, there is no reason to assume that metabolomic changes in mTBI are like those seen in sTBI. While this study aimed to specify sTBI data collected from adults, several studies included patients just over the age of 16 or combined varying degrees of TBI in their data. Furthermore, data that was not verifiable or entirely discernible was not included, potentially leading to the dismissal of important metabolite changes and publications. Thus, many confounders, including those mentioned above, undoubtedly had an affect on the results presented here.
Conclusion
This scoping review sought to identify commonalities between the published primary literature investigating metabolomics in sTBI to determine if a reliable set of metabolites (‘biomarkers’) or a metabolic profile could be determined that may be of clinical use. This study adds to the current knowledge on metabolomics in sTBI by summarizing and compiling the recent literature to determine potentially clinically relevant biomarkers. To our knowledge, a scoping review as comprehensive as that presented here, has not been completed to date, likely due to the large variability between metabolomics studies in sTBI. While identifying key metabolites between studies proved challenging and potentially problematic, fifteen ‘recurrent’ metabolites, several HMDB classes, and their affected pathways were identified in this review. Furthermore, these metabolites and their pathways were supported by suggesting potential secondary mechanisms of injury, such as oxidative damage and excitotoxicity caused by the alterations in metabolite concentrations following sTBI. This study recognizes several metabolites with biomarker potential; however, it is clear that further studies are needed to determine the significance and useability of these findings. Once that is achieved, more specialized therapeutics could be designed to slow or alter the mechanisms by which sTBI causes injury, potentially decreasing the detrimental effects of sTBI overall.
Supplementary Information
Additional file 1: Table S1. Summary table of eligible publications exploring metabolomics in severe traumatic brain injury following selection process.
Acknowledgements
We acknowledge the Critical Care Strategic Clinical Network (CC SCN) for funding a Summer Studentship to R.P.F.
Abbreviations
- 1H-MRIS
Proton nuclear magnetic resonance imaging
- 1H-NMR
Proton nuclear magnetic resonance
- 31P-MRS
Phosphorous magnetic resonance spectroscopy
- BCAA
Branched chain amino acid
- BDNF
Brain-derived neurotrophic factor
- CSF
Cerebrospinal fluid
- CT
Computed tomography
- GC–MS
Gas chromatography-mass spectrometry
- GC-QTOFMS
Gas chromatography coupled to quadrupole time-of-flight mass spectrometry
- GCxGC-MS
2D gas chromatography coupled to mass spectrometry
- GCxGC-TOFMS
2D Gas Chromatography coupled to Time-Of-Flight Mass Spectrometry
- GDNF
Glial cell-derived neurotrophic factor
- HMBD
Human metabolome database
- HPLC
High-performance liquid chromatography
- KEGG
Kyoto encyclopedia of genes and genomes
- LC–MS
Liquid chromatography-mass spectrometry
- LC-QTOFMS
Liquid Chromatography Coupled to Quadrupole Time-Of-Flight Mass Spectrometry
- moTBI
Moderate traumatic brain injury
- MRI
Magnetic resonance imaging
- MRIS
Magnetic resonance imaging spectroscopy
- mTBI
Minor traumatic brain injury
- NAA
N-acetylaspartate
- sTBI
Severe traumatic brain injury
- TBI
traumatic brain injury
- UV
Ultraviolet
Author contributions
RPF conducted the literature search and review, as well as writing the initial draft. MMB and RPF created the pathway analysis diagram. CHL and MMB participated in the writing, review, and editing. BWW was involved in all parts of this project. All authors have read and agree to submitting the manuscript for publication.
Funding
R.P.F. (B.W.W. and C.H.L. as supervisors) received funding from the Critical Care Strategic Clinical Network (CC SCN) of the Alberta Health Services to help fund this work.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention: Centers for Disease Control and Prevention. https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html. Accessed 17 Oct 2022.
- 2.International Brain Injury Association. https://www.internationalbrain.org/resources/brain-injury-facts. Accessed 17 Oct 2022.
- 3.Northern Brain Injury Association. https://www.nbia.ca/brain-injury-statistics/. Accessed 17 Oct 2022.
- 4.Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 5.Mapping connections: an understanding of neurological conditions in Canada. Ottawa, ON: Public Health Agency of Canada = Agence de la santé publique du Canada; 2014.
- 6.Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menon DK, Schwab K, Wright DW, Maas AI. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1637–1640. doi: 10.1016/j.apmr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Yoshino A, Hovda DA, Kawamata T, Katayama Y, Becker DP. Dynamic changes in local cerebral glucose utilization following cerebral concussion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561(1):106–119. doi: 10.1016/0006-8993(91)90755-K. [DOI] [PubMed] [Google Scholar]
- 9.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 10.Clark RSB, Kochanek PM, Dixon CE, Chen M, Marion DW, Heineman S, et al. Early neuropathologic effects of mild or moderate hypoxemia after controlled cortical impact injury in rats. J Neurotrauma. 1997;14(4):179–189. doi: 10.1089/neu.1997.14.179. [DOI] [PubMed] [Google Scholar]
- 11.Andrews PJD, Piper IR, Dearden NM, Miller JD. Secondary insults during intrahospital transport of head-injured patients. The Lancet. 1990;335(8685):327–330. doi: 10.1016/0140-6736(90)90614-B. [DOI] [PubMed] [Google Scholar]
- 12.Royal College of Physicians and Surgeons of Glasgow. The Glasgow Structured Approach to Assessment of the Glasgow Coma Scale. https://www.glasgowcomascale.org/. Accesssed 17 Oct 2022.
- 13.McGarry LJ, Thompson D, Millham FH, Cowell L, Snyder PJ, Lenderking WR, et al. Outcomes and Costs of Acute Treatment of Traumatic Brain Injury. J Trauma Inj Infect Crit Care. 2002;53(6):1152–1159. doi: 10.1097/00005373-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 14.The Multi-Society Task Force on PVS Medical Aspects of the Persistent Vegetative State. N Engl J Med. 1994;330(22):1572–1579. doi: 10.1056/NEJM199406023302206. [DOI] [PubMed] [Google Scholar]
- 15.Dawes AJ, Sacks GD, Cryer HG, Gruen JP, Preston C, Gorospe D, et al. Compliance with evidence-based guidelines and interhospital variation in mortality for patients with severe traumatic brain injury. JAMA Surg. 2015;150(10):965. doi: 10.1001/jamasurg.2015.1678. [DOI] [PubMed] [Google Scholar]
- 16.Posti JP, Dickens AM, Orešič M, Hyötyläinen T, Tenovuo O. Metabolomics profiling as a diagnostic tool in severe traumatic brain injury. Front Neurol. 2017;18(8):398. doi: 10.3389/fneur.2017.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27(8):1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 18.Vailati-Riboni M, Palombo V, Loor JJ. What are omics sciences? In: Ametaj BN, editor. Periparturient diseases of dairy cows. Cham: Springer International Publishing; 2017. [Google Scholar]
- 19.Roessner U, Bowne J. What is metabolomics all about? Biotechniques. 2009;46(5):363–365. doi: 10.2144/000113133. [DOI] [PubMed] [Google Scholar]
- 20.Mayeux R. Biomarkers: potential uses and limitations. NeuroRx. 2004;1(2):182–188. doi: 10.1602/neurorx.1.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banoei MM, Casault C, Metwaly SM, Winston BW. Metabolomics and biomarker discovery in traumatic brain injury. J Neurotrauma. 2018;35(16):1831–1848. doi: 10.1089/neu.2017.5326. [DOI] [PubMed] [Google Scholar]
- 22.Roberts LD, Souza AL, Gerszten RE, Clish CB. Targeted Metabolomics. Curr Protoc Mol Biol. 2012 doi: 10.1002/0471142727.mb3002s98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klemm A, Rzanny R, Funfstuck R, Werner W, Schubert J, Kaiser W, et al. 31P-magnetic resonance spectroscopy (31P-MRS) of human allografts after renal transplantation. Nephrol Dial Transplant. 1998;13(12):3147–3152. doi: 10.1093/ndt/13.12.3147. [DOI] [PubMed] [Google Scholar]
- 24.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 25.Marino S, Zei E, Battaglini M, Vittori C, Buscalferri A, Bramanti P, et al. Acute metabolic brain changes following traumatic brain injury and their relevance to clinical severity and outcome. J Neurol Neurosurg Psychiatry. 2006;78(5):501–507. doi: 10.1136/jnnp.2006.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeter CB, Hergenroeder GW, Ward NH, Moore AN, Dash PK. Human traumatic brain injury alters circulating l-arginine and its metabolite levels: possible link to cerebral blood flow, extracellular matrix remodeling, and energy status. J Neurotrauma. 2012;29(1):119–127. doi: 10.1089/neu.2011.2029. [DOI] [PubMed] [Google Scholar]
- 27.Jeter CB, Hergenroeder GW, Ward NH, Moore AN, Dash PK. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30(8):671–679. doi: 10.1089/neu.2012.2491. [DOI] [PubMed] [Google Scholar]
- 28.Shannon RJ, van der Heide S, Carter EL, Jalloh I, Menon DK, Hutchinson PJ, et al. Extracellular N -acetylaspartate in human traumatic brain injury. J Neurotrauma. 2016;33(4):319–329. doi: 10.1089/neu.2015.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dash PK, Hergenroeder GW, Jeter CB, Choi HA, Kobori N, Moore AN. Traumatic brain injury alters methionine metabolism: implications for pathophysiology. Front Syst Neurosci. 2016 doi: 10.3389/fnsys.2016.00036/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bykowski EA, Petersson JN, Dukelow S, Ho C, Debert CT, Montina T, et al. Urinary metabolomic signatures as indicators of injury severity following traumatic brain injury: a pilot study. IBRO Neurosci Rep. 2021;11:200–206. doi: 10.1016/j.ibneur.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mondello S, Sandner V, Goli M, Czeiter E, Amrein K, Kochanek PM, et al. Exploring serum glycome patterns after moderate to severe traumatic brain injury: A prospective pilot study. ClinicalMedicine. 2022;50:101494. doi: 10.1016/j.eclinm.2022.101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas I, Dickens AM, Posti JP, Czeiter E, Duberg D, Sinioja T, et al. Serum metabolome associated with severity of acute traumatic brain injury. Nat Commun. 2022;13(1):2545. doi: 10.1038/s41467-022-30227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orešič M, Posti JP, Kamstrup-Nielsen MH, Takala RSK, Lingsma HF, Mattila I, et al. Human serum metabolites associate with severity and patient outcomes in traumatic brain injury. EBioMedicine. 2016;12:118–126. doi: 10.1016/j.ebiom.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolahan SM, Lebby E, Mao HC, McArthur D, Real C, Vespa P, et al. Novel metabolomic comparison of arterial and jugular venous blood in severe adult traumatic brain injury patients and the impact of pentobarbital infusion. J Neurotrauma. 2019;36(2):212–221. doi: 10.1089/neu.2018.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vuille-Dit-Bille RN, Ha-Huy R, Stover JF. Changes in plasma phenylalanine, isoleucine, leucine, and valine are associated with significant changes in intracranial pressure and jugular venous oxygen saturation in patients with severe traumatic brain injury. Amino Acids. 2012;43(3):1287–1296. doi: 10.1007/s00726-011-1202-x. [DOI] [PubMed] [Google Scholar]
- 36.Dickens AM, Posti JP, Takala RSK, Ala-Seppälä H, Mattila I, Coles JP, et al. Serum metabolites associated with computed tomography findings after traumatic brain injury. J Neurotrauma. 2018;35(22):2673–2683. doi: 10.1089/neu.2017.5272. [DOI] [PubMed] [Google Scholar]
- 37.Glenn TC, Hirt D, Mendez G, McArthur DL, Sturtevant R, Wolahan S, et al. Metabolomic analysis of cerebral spinal fluid from patients with severe brain injury. In: Katayama Y, Maeda T, Kuroiwa T, et al., editors. Brain edema XV. Vienna: Springer Vienna; 2013. [DOI] [PubMed] [Google Scholar]
- 38.Stefani MA, Modkovski R, Hansel G, Zimmer ER, Kopczynski A, Muller AP, et al. Elevated glutamate and lactate predict brain death after severe head trauma. Ann Clin Transl Neurol. 2017;4(6):392–402. doi: 10.1002/acn3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mt Sinai J Med J Transl Pers Med. 2009;76(2):97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- 40.Davis AE. Mechanisms of Traumatic Brain Injury: Biomechanical, Structural and Cellular Considerations. Crit Care Nurs Q. 2000;23(3):1–13. doi: 10.1097/00002727-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Bayır H, Kagan VE, Borisenko GG, Tyurina YY, Janesko KL, Vagni VA, et al. Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. J Cereb Blood Flow Metab. 2005;25(6):673–684. doi: 10.1038/sj.jcbfm.9600068. [DOI] [PubMed] [Google Scholar]
- 42.Sun L, Qiang R, Yang Y, Jiang ZL, Wang GH, Zhao GW, et al. L-serine treatment may improve neurorestoration of rats after permanent focal cerebral ischemia potentially through improvement of neurorepair. PLoS ONE. 2014;9(3):e93405. doi: 10.1371/journal.pone.0093405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang GH, Jiang ZL, Chen ZQ, Li X, Peng LL. Neuroprotective effect of L-serine against temporary cerebral ischemia in rats. J Neurosci Res. 2010 doi: 10.1002/jnr.22365. [DOI] [PubMed] [Google Scholar]
- 44.Zhai PP, Xu LH, Yang JJ, Jiang ZL, Zhao GW, Sun L, et al. Reduction of inflammatory responses by l-serine treatment leads to neuroprotection in mice after traumatic brain injury. Neuropharmacology. 2015;95:1–11. doi: 10.1016/j.neuropharm.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 45.Dalangin R, Kim A, Campbell RE. The role of amino acids in neurotransmission and fluorescent tools for their detection. Int J Mol Sci. 2020;21(17):6197. doi: 10.3390/ijms21176197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cherian L, Hlatky R, Robertson CS. Nitric oxide in traumatic brain injury. Brain Pathol. 2004;14(2):195–201. doi: 10.1111/j.1750-3639.2004.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulze A. Creatine deficiency syndromes. Mol Cell Biochem. 2003;244(1–2):143–150. doi: 10.1023/A:1022443503883. [DOI] [PubMed] [Google Scholar]
- 48.Fernstrom JD. Branched-chain amino acids and brain function. J Nutr. 2005;135(6):1539S–1546S. doi: 10.1093/jn/135.6.1539S. [DOI] [PubMed] [Google Scholar]
- 49.Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48(5):394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Sowers JL, Sowers ML, Shavkunov AS, Hawkins BE, Wu P, DeWitt DS, et al. Traumatic brain injury induces region-specific glutamate metabolism changes as measured by multiple mass spectrometry methods. iScience. 2021;24(10):103108. doi: 10.1016/j.isci.2021.103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dickerman RD, Williamson J, Mathew E, Butt CM, Bird CW, Hood LE, et al. Branched-chain amino acids are neuroprotective against traumatic brain injury and enhance rate of recovery: prophylactic role for contact sports and emergent use. Neurotrauma Rep. 2022;3(1):321–332. doi: 10.1089/neur.2022.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aquilani R, Boselli M, Boschi F, Viglio S, Iadarola P, Dossena M, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2008;89(9):1642–1647. doi: 10.1016/j.apmr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 53.Glenn TC, Kelly DF, Boscardin WJ, McArthur DL, Vespa P, Oertel M, et al. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J Cereb Blood Flow Metab. 2003;23(10):1239–1250. doi: 10.1097/01.WCB.0000089833.23606.7F. [DOI] [PubMed] [Google Scholar]
- 54.Jaggi JL, Obrist WD, Gennarelli TA, Langfitt TW. Relationship of early cerebral blood flow and metabolism to outcome in acute head injury. J Neurosurg. 1990;72(2):176–182. doi: 10.3171/jns.1990.72.2.0176. [DOI] [PubMed] [Google Scholar]
- 55.Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg. 1997;86(2):241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- 56.Brooks GA, Martin NA. Cerebral metabolism following traumatic brain injury: new discoveries with implications for treatment. Front Neurosci. 2015 doi: 10.3389/fnins.2014.00408/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glenn TC, Martin NA, Horning MA, McArthur DL, Hovda DA, Vespa P, et al. Lactate: brain fuel in human traumatic brain injury: a comparison with normal healthy control subjects. J Neurotrauma. 2015;32(11):820–832. doi: 10.1089/neu.2014.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holloway R, Zhou Z, Harvey HB, Levasseur JE, Rice AC, Sun D, et al. Effect of lactate therapy upon cognitive deficits after traumatic brain injury in the rat. Acta Neurochir. 2007;149(9):919–927. doi: 10.1007/s00701-007-1241-y. [DOI] [PubMed] [Google Scholar]
- 59.Carpenter KLH, Jalloh I, Hutchinson PJ. Glycolysis and the significance of lactate in traumatic brain injury. Front Neurosci. 2015 doi: 10.3389/fnins.2015.00112/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vavilala MS, Richards TL, Roberts JS, Chiu H, Pihoker C, Bradford H, et al. Change in blood–brain barrier permeability during pediatric diabetic ketoacidosis treatment*. Pediatr Crit Care Med. 2009 doi: 10.1097/PCC.0b013e3181c013f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lazzarino G, Amorini AM, Signoretti S, Musumeci G, Lazzarino G, Caruso G, et al. Pyruvate dehydrogenase and tricarboxylic acid cycle enzymes are sensitive targets of traumatic brain injury induced metabolic derangement. Int J Mol Sci. 2019;20(22):5774. doi: 10.3390/ijms20225774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan EB, Frugier T, Lim CK, Heng B, Sundaram G, Tan M, et al. Activation of the kynurenine pathway and increased production of the excitotoxin quinolinic acid following traumatic brain injury in humans. J Neuroinflammation. 2015;12(1):110. doi: 10.1186/s12974-015-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eiden M, Christinat N, Chakrabarti A, Sonnay S, Miroz JP, Cuenoud B, et al. Discovery and validation of temporal patterns involved in human brain ketometabolism in cerebral microdialysis fluids of traumatic brain injury patients. EBioMedicine. 2019;44:607–617. doi: 10.1016/j.ebiom.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas I, Dickens AM, Posti JP, Mohammadian M, Ledig C, Takala RSK, et al. Integrative analysis of circulating metabolite profiles and magnetic resonance imaging metrics in patients with traumatic brain injury. Int J Mol Sci. 2020;21(4):1395. doi: 10.3390/ijms21041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang J, Zhang H, Zhang J, Yu H, Lin Z, Cai Y. spermidine exhibits protective effects against traumatic brain injury. Cell Mol Neurobiol. 2020;40(6):927–937. doi: 10.1007/s10571-019-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pinggera D, Steiger R, Bauer M, Kerschbaumer J, Luger M, Beer R, et al. Cerebral energy status and altered metabolism in early severe TBI: first results of a prospective 31P-MRS feasibility study. Neurocrit Care. 2021;34(2):432–440. doi: 10.1007/s12028-020-01042-x. [DOI] [PubMed] [Google Scholar]
- 67.Pinggera D, Steiger R, Bauer M, Kerschbaumer J, Beer R, Rietzler A, et al. Repeated 31 P-magnetic resonance spectroscopy in severe traumatic brain injury: insights into cerebral energy status and altered metabolism. J Neurotrauma. 2021;38(20):2822–2830. doi: 10.1089/neu.2021.0143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Summary table of eligible publications exploring metabolomics in severe traumatic brain injury following selection process.
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].