Abstract
Purpose
To evaluate the preclinical in vivo therapeutic response of Lenvatinib-eluting microspheres (LEN-EM) transcatheter arterial chemoembolization (LEN-TACE) in an hepatocellular carcinoma (HCC) rat model.
Methods
Magnetic resonance imaging (MRI) visible LEN-EM was fabricated with poly(lactide-co-glycolide) and iron oxide nanoparticles by a double-emulsion method. The morphology, LEN loading/release kinetics, and MRI contrast effect of LEN-EM were evaluated. For in vivo study, N1S1 HCC rats were treated with LEN-TACE (LEN: 2.4 mg/kg, n = 5) using LEN-EM, systemic LEN (LEN: 0.4 mg/kg, oral gavage daily for 7 days, n = 5), control (intra-arterial (IA) saline infusion, n = 5), and non-tumor control (n = 3). Tumor size changes were measured for 2 weeks. Histology, comparative LEN plasma concentration, hematologic markers, liver profile, and serum chemistry among the groups were measured.
Results
LEN-EM with 33 μm in average size was prepared in an optimized emulsion process. LEN loading efficiency was 58.7%. LEN was continuously released for 500 h. LEN-TACE showed the delivered LEN-EM surrounding tumor tissue in MRI-T2* images. The LEN-TACE group demonstrated a statistically significant larger tumor volume reduction compared to the other groups at 2 weeks post-procedure. Quantification data of Terminal deoxynucleotidyl transferase dUTP nick end labeling positive cells confirmed increased cancer cell death in the LEN-TACE group compared to control groups. Additional histology, hematologic markers, and liver profiles showed minimal side effects of LEN-TACE.
Conclusion
LEN-TACE using IA delivery of LEN-EM demonstrated an effective therapeutic efficacy in an HCC rat animal model.
Keywords: Transcatheter arterial chemoembolization, Hepatocellular carcinoma, Lenvatinib, Drug eluting microspheres, Local drug delivery
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cause of cancer morbidity and 4th leading cause of cancer mortality in the world [1]. Since HCC tumors can be insidious and often remain asymptomatic until they reach inoperable late stages, most systemic and regional therapies offer palliation rather than a cure. Transcatheter arterial chemoembolization (TACE) as a mainstay of the treatment of HCC (intermediate stage) has produced survival advantages by achieving higher concentrations of cytotoxic drugs within targeted liver tumors and minimizing systemic exposure compared to systemic chemotherapy. However, the activation of tumorigenic growth factors such as hypoxia-inducible factor-1a (HIF-1a) and vascular endothelial growth factor (VEGF) following TACE has been reported [2, 3], which may ultimately lead to the recurrence of malignancy [4]. Countering the stimulation of these growth factors may be critical to maximize the longitudinal efficacy of TACE therapies for HCC [5]. Food and Drug Administration (FDA) approved that Lenvatinib (LEN) with TACE combination could be a promising approach to maximize the efficacy of both LEN and TACE for the treatment of HCC. Clinical trials have shown positive synergistic effects of systemic sorafenib plus TACE [6–17]. LEN uniquely inhibits vascular endothelial growth factor receptor (VEGFR) and fibroblast growth factor receptor (FGFR). This co-inhibition of VEGFR and FGFR of LEN will allow an enhanced therapeutic efficacy in the combination with TACE [18, 19]. REFLECT phase 3, the multi-centered clinical trial, reported increased-progression-free survival and objective response rate of LEN compared to sorafenib [20]. However, the safety issues related to the toxicity of systemic delivered LEN remain problematic [21]. In this preclinical study, LEN-TACE was performed by transcatheter-directed hepatic intra-arterial delivery of MRI visible LEN-eluting microspheres (LEN-EM) in an established N1S1 HCC rat model. The potential therapeutic efficacy of LEN-TACE was evaluated in an animal model.
Materials and Methods
Lenvatinib-Eluting Microspheres (LEN-EM) Fabrication
As-synthesized iron oxide nanoparticles (IONP) [22, 23] and LEN were co-encapsulated in poly(lactide-co-glycolide) (PLGA) microspheres using the double emulsion solvent evaporation method [23]. The morphology of LEN-EM was characterized with an optical microscope and scanning electron microscope (SEM). The average size of fabricated LEN-EM (1 wt.% IONP and 5.9 wt.% LEN) was 36.2 μm ± 5.7 μm. Further details are provided in the online supplementary information.
Cells
N1S1 Hepatoma cell line was acquired from American Type Culture Collection (CRL-1604, Manassas, VA, USA), which were kept at 37 °C in a humidified 5% CO2 atmosphere, and cultured in Dulbecco’s Modified Eagle Media (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA) and 1% penicillin/streptomycin (Gibco, Grand Island, NY, USA).
In vivo study in N1S1 HCC Rat Model
All procedures involving experimental animals were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Northwestern University. A total of 18 Sprague–Dawley (SD) rats (Charles River Laboratories, Wilmington, MA, USA) weighing between 250 and 330 g were used for this study. N1S1 tumor implantation and transcatheter intrahepatic arterial injection (IA) procedure methods with a 1.5-Fr microcatheter were previously described in earlier publications [23–25]. N1S1 HCC rats were divided into 3 groups. Group 1 (n = 5) was treated with IA-delivery of LEN-EM (LEN: 2.4 mg/kg). The tumor size was measured with MR images for 2 weeks. Group 2 (n = 5) was treated with systemic administration of LEN. 0.4 mg/kg of LEN was administered daily to each rat for 7 consecutive days via oral gavage. LEN was diluted in DMSO to 10 mg/ml concentration and then added to PBS to the concentration of 0.4 mg/kg daily based on each rat’s body weight. IA saline infusion group was assigned as the control group (Group 3, n = 5). Additional non-tumor group (n = 3) was used for the hematologic toxicity study only.
In Vivo MRI
T2*-weighted images were collected at pre- and post-LEN-TACE. MR scans were performed with 7 T MRI scanner (BioSpec, Bruker, Billerica, MA) in both coronal and axial orientations using a turbo spin-echo sequence with the following parameters: TR/TE = 2000/30 ms, 1 mm slice thickness, FOV 71 × 85 mm, 216 × 256 matrices, and respiratory triggering with an MRI-compatible small animal gating system (Model 1025, SA Instruments, Stony Brook, NY). MRI measurement was then repeated 7 and 14 days post-treatment.
Tumor Size Measurement
The width (a) and length (b) of the tumors were measured using MRI at baseline, 7, and 14 days post-treatment. The tumor volume (V) was calculated using the formula V = a2 × b/2, where a is the width, b is the length, and a≦b [26]. The tumor volume was normalized by its initial volume: Vt/V0 (Vt is the tumor volume on the day of post-therapy and V0 is the initial tumor volume).
Histology and Immunohistochemistry
To confirm anti-tumor therapeutic efficacy, the livers were harvested from the rats at 2 weeks post-infusion, and the tumor-containing segments were resected for microtome sectioning. Five-micrometer slices through the center of each tumor were used for TUNEL staining as following previous reports [26, 27]. To evaluate the possible systemic toxicity of each treatment, H&E staining of the harvested lung, heart, and kidney tissues was performed. All slides were analyzed using a TissueFAXS microscope (TissueGnostics GmbH, Vienna, Austria). Quantitative analysis of tumor cell death was performed using ImageJ (NIH, Bethesda, MD, USA).
Measurement of Plasma LEN Using High-Performance Liquid Chromatography (HPLC)
0.15 mL of blood was collected from the tail vein with a heparinized 24 G catheter at pre-treatment, 6 h post-treatment, and 3 days post-treatment. Blood samples were centrifuged at 4000 g at 4 °C for 15 min in a heparinized plasma separation tube. Plasma supernatants were separated, and blood cells were discarded. Plasma was treated with methanol, purified using a 45 μm filter, and diluted in additional methanol before being analyzed by HPLC.
Blood Test
Blood was destined for complete metabolic profile and serum chemistry analysis were placed in heparinized plasma separation tubes, inverted 5 times, centrifuged at 4000 g at 4 °C for 15 min to separate the plasma whole cells, and placed on ice. Complete blood count with differential blood samples was placed in EDTA-containing tubes and placed on ice. Samples were sent to IDEXX Laboratories Inc. for machine analysis.
Statistical Analysis
Statistical significance of differences between two groups was determined using the Student’s unpaired t test. P < 0.05 was considered to indicate a significant difference. All statistical analyses were performed using the GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Fabrication of MRI Visible Lenvatinib-Eluting Microspheres
MRI visible LEN-eluting microspheres (LEN-EM) were fabricated with 3 components, including LEN, cube-shaped superparamagnetic IONP, and poly(lactide-co-glycolide) (PLGA) through an emulsion method (Fig. 1A). 20 nm IONP, as shown in the transmission electron microscope (TEM) image, was synthesized for MRI contrast effect and sustained LEN release in the LEN-EM. For efficient transcatheter-directed IA injection in HCC rats, the size was tailored to 30–50 um (Fig. 1B). LEN-EM displayed spherical and smooth surface characteristics on scanning electron microscope images (Fig. 1B). Spherical LEN-EM was readily transferrable through a 1.5 Fr catheter. The amount of loaded LEN in LEN-EM was determined to be 5.87 ± 0.03%, and the loading efficiency was 58.7%. During the first 6 h of physiological condition (phosphate-buffered solution (PBS), 37 °C), LEN was eluted approximately 7% with a burst manner, and then the LEN release rate was stabilized (Fig. 1C). The sustained LEN release was maintained for 500 h. The cumulative LEN release amount was 22% for 500 h (Fig. 2A). In vitro agarose gel phantom experiments revealed strong negative MRI T2* contrast effects of LEN-EM with increasing decay rates from the signal phasing effect of IONP. T2* signal values were linearly decreased from 527 to 193 ms at increasing LEN-EM concentrations ranging from 0 mg/mL to 2 mg/mL (Fig. 1D).
Fig. 1.
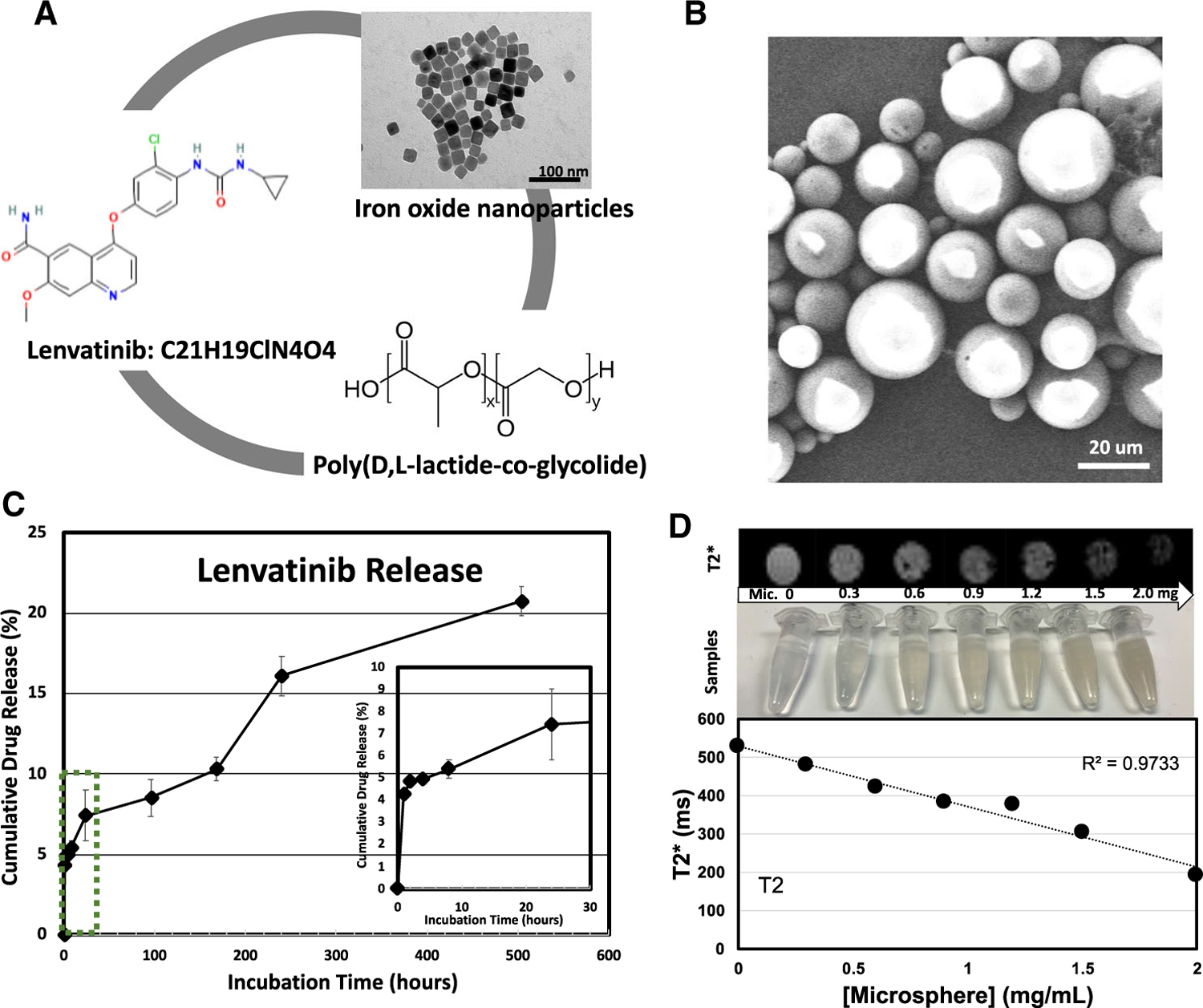
A Components of Lenvatinib-eluting microspheres (LEN-EM) including LEN, PLGA matrix and cube-shaped iron oxide nanoparticles. B Scanning electron microscope (SEM) images of fabricated LEN-EM. C Time-dependent LEN drug release profiles. (inset) zoomed drug release profiles in early time point until 30 h. D T2 weighted images of LEN-EM with various concentration in 1% agarose gel. (bottom) LEN-EM concentration-dependent T2* signal change
Fig. 2.
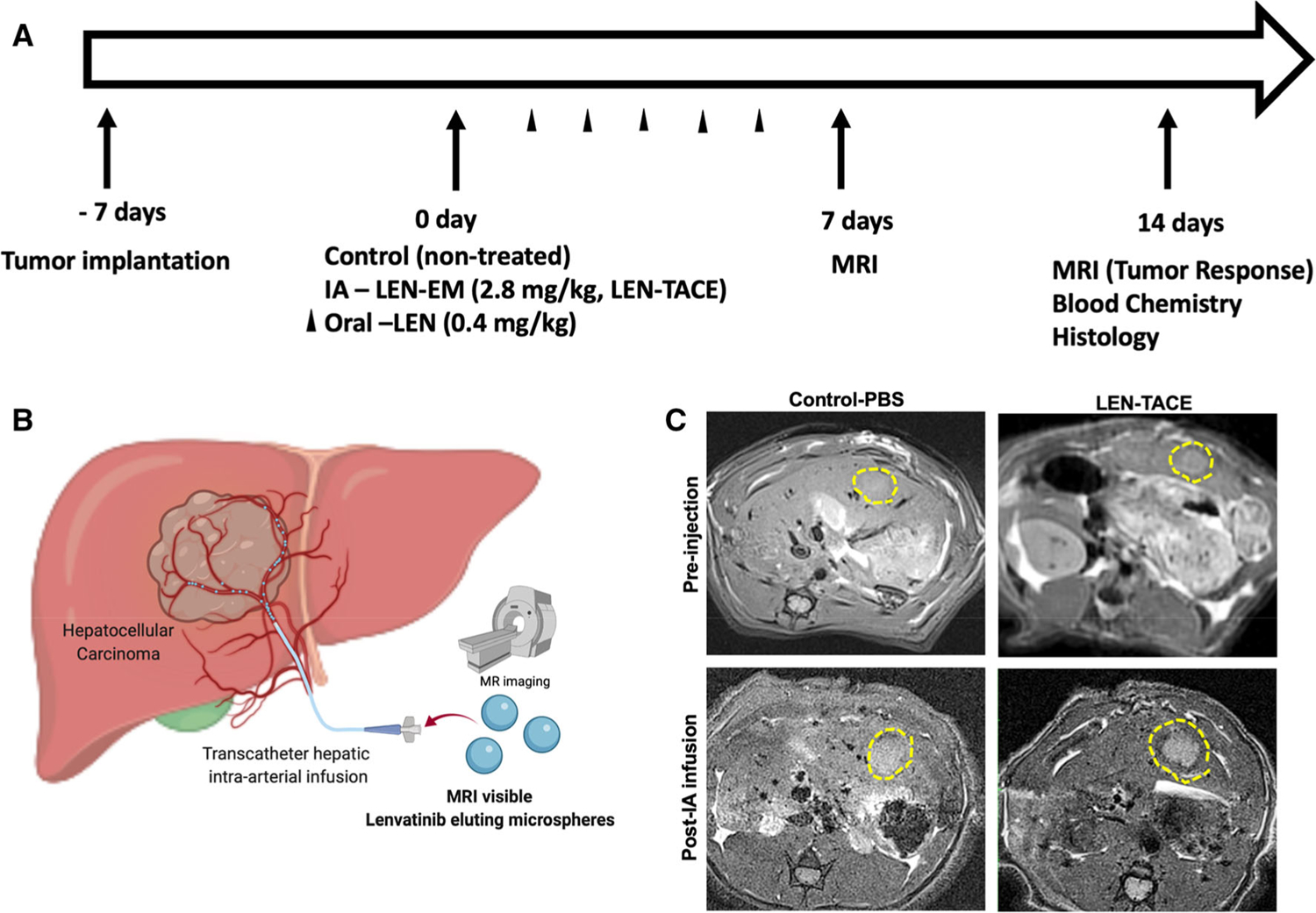
In vivo HCC tumor growth inhibition after LEN-TACE. A Treatment schedule of each group (non-treated HCC tumor, IA infusion of LEN-EM (LEN-TACE), and systemic LEN treatment). B Schematics of IA infusion of drug-eluting microspheres (LEN-TACE). C MRI T2* weighted images of rats treated with pre-injection (PBS or LEN-EM) and post-IA infusion (PBS or LEN-EM). Yellow lines depict tumor regions
In Vivo Evaluation of the Comparative Therapeutic Response of Transcatheter Intra-arterial Infused LEN-EM with Systemic Lenvatinib Treatment
Tumor responses and safety of each treatment were evaluated with MRI monitoring of tumors and blood chemistry (Fig. 2A). Pre-procedural MR T2*-weighted images at 2 weeks post-tumor implantation showed oval-shaped tumors of volume between 0.6 to 0.9 cm3 in the left lobe of the liver (Fig. 2B). LEN-TACE showed the delivered LEN-EM with the hypo-MRI T2* signal at the tumor region (Fig. 2B). The group treated with LEN-TACE demonstrated significant tumor growth suppression while control and systemic groups showed progressively enlarging hyperdense tumor regions (Fig. 3A). The relative tumor volume change (Vt/V0) at 2 weeks post-procedure was 0.43 in LEN-TACE groups, which significantly suppressed the tumor growth compared to the control (2.53) and systemic LEN (2.25) groups (Fig. 3B). All rats in the LEN-TACE showed a complete tumor regression in the 7 T MRI scanning after 2 weeks. However, no significant tumor growth changes were noted between N1S1 tumor controls and systemic LEN. Both control groups grew tumors at a similar rate; the size of tumor doubled in each week. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) immunohistology showing apoptotic cell death demonstrated that LEN-TACE effectively induced HCC cell death (Fig. 3C). The TUNEL positive area around the HCC tumor rim was much larger in the group of LEN-TACE than those in control and systemic LEN groups (Fig. 3C). Quantification data of TUNEL confirmed significantly increased TUNEL positive cells in LEN-TACE (Fig. 3D). 444 TUNEL positive cells/cm2 were observed in the LEN-TACE group. Each control and systemic LEN group showed 102 and 185 TUNEL positive cells/cm2, respectively. The LEN concentration in blood plasma showed the sustained LEN-release in the LEN-TACE group. LEN concentrations of LEN-TACE at 6 h post-IA infusion reached a 38 ng/ml maximum, and the concentration was maintained at a similar level (30 ng/ml) at 3 days post-IA infusion (Supplementary information Fig. 1). However, the systemic LEN administration group showed ~ 59 ng/ml LEN concentration in the plasma at 6 h post-administration, but the LEN concentration in the plasma was not detected at 3 days post-treatment. These results indicate that LEN-TACE could offer extended LEN exposure to the local HCC tumor area.
Fig. 3.
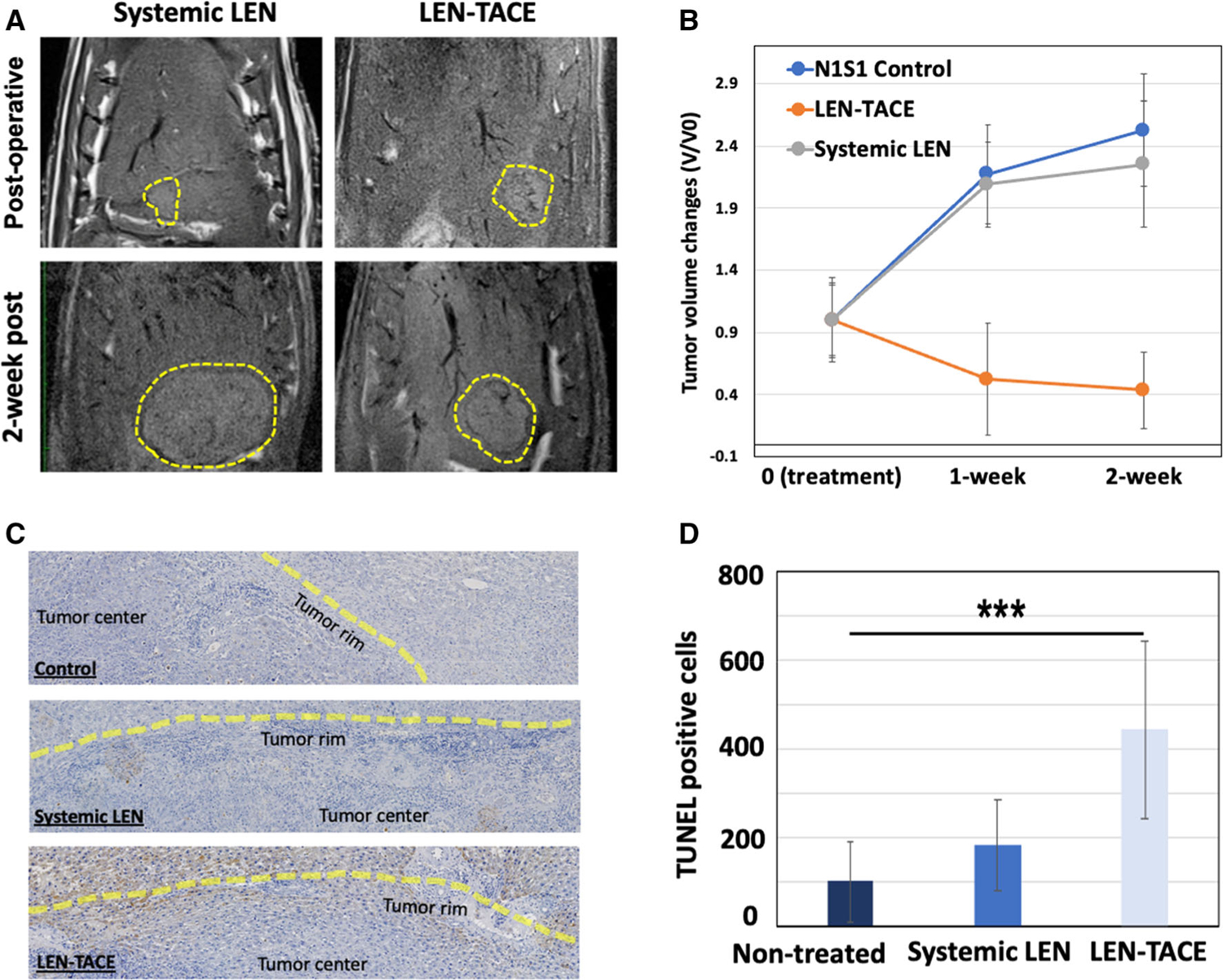
A MR T2-weighted images of HCC rats treated with control, LEN-TACE, and systemic LEN treatment. Yellow dotted outlines in MR images indicate tumors at 1 h and 3 weeks post treatment. B Tumor growth graph shows the regression of tumor volume after LEN-TACE (5 rats per group, three treatment groups: ***p < 0.001). C Immunohistochemical analyses of tumor tissue after 3 weeks of LEN-TACE treatments. Tumor tissue specimens stained with TUNEL. The brown color on slide section images indicates TUNEL-positive apoptotic cells (scale bar, 25 mm). Yellow lines are corresponding to the tumor rim region. D Quantitative analysis of TUNEL-positive apoptotic cells in 1 cm2 (***P < 0.001)
Safety of LEN-TACE
Hematologic liver function and metabolism markers after LEN-TACE treatment showed no significant difference between a non-tumor and non-treated tumor control group (Table 1). Serum symmetric dimethylarginine (SDMA) measurement showing renal toxicity was also similar level with control groups.
Table 1.
Changes of hematological and metabolic markers in each treatment group of the control group (non-treated N1S1 tumor), systemic-LEN, LEN-TACE, and non-tumor rats
| Bloodwork | N1S1 Tumor | Systemic LEN | LEN-TACE | Non-tumor CTRL |
|---|---|---|---|---|
| AST (U/L) | 161 | 141 | 80 | 82 |
| ALT (U/L) | 76 | 49 | 58 | 48 |
| ALP (U/L) | 287 | 173 | 215 | 224 |
| Albumin:Globulin | 0.9 | 0.9 | 0.7 | 1.1 |
| Hb (g/dL) | 16.8 | 14.3 | 13.5 | 14.1 |
| WBC (K/μL) | 11.2 | 12.3 | 23 | 9 |
| % Lymphocyte | 69 | 68 | 76 | 73 |
| Lymphocytes/μL | 7728 | 8364 | 17,480 | 6570 |
| Bilirubin (mg/dL) | 0.1 | 0.1 | 0.2 | 0.1 |
| Cholesterol (mg/dL) | 74 | 78 | 71 | 95 |
| SDMA (μg/dL) | 5 | 5 | 5 | 6 |
Discussion
For more than a decade, sorafenib has been a standard of care systemic agent for the first-line treatment of advanced or unresectable HCC patients. Recently, LEN (Lenvima; Eisai and Merck & Co.) was approved as a first-line treatment in Japan in March 2018 and in the US and Europe in August 2018 [20, 28, 29]. LEN showed 5-month longer tumor response rate and 2-folds higher objective response rate than sorafenib [20, 30]. As the efficacy of antiangiogenic sorafenib and TACE sequential therapy for HCC is proved, it has suggested that the normalization of tumor vasculature with LEN therapy may improve the therapeutic outcomes in a combination with TACE [18, 19]. However, the systemic administration of LEN in monotherapy or combination with TACE commonly induces patients’ intolerance due to the adverse effects, and limiting LEN’s therapeutic potential. Although local delivery of LEN should be advantageous in enhancing the therapeutic outcomes of LEN therapy, there is a paucity of studies evaluating the benefit of locally delivered LEN.
In this study, superparamagnetic IONC-loaded PLGA microspheres [31–33] were used for local delivery of LEN to HCC via transcatheter-directed hepatic intra-arterial infusion (LEN-TACE). TACE relies upon differences in the blood supply between HCC and normal liver tissues. While hepatic arteries supply ~ 90% of blood flow to HCC, it only supplies ~ 25% of the flow to the normal liver [34]. Thus, TACE involves with the local infusion of chemotherapeutic drugs and drug-eluting microspheres [24, 35–37]. Here, magnetic PLGA microsphere drug delivery platform was suited to encapsulate and solubilize hydrophobic LEN. The sustained LEN elution and MR T2* contrast effects of LEN-EM allowed an MR monitored LEN delivery and enhanced therapeutic response. Postop-erative MR imaging can assure the deposition of LEN-EM surrounding the tumor region. It is known that IA drug delivery via the hepatic artery has shown an approximately 85% of tumor localization rate. The tumoral drug release of LEN-TACE minimized the systemic drug exposure. The sustained LEN release in LEN-TACE can maintain the effective dosage level of LEN for the therapeutic efficacy (Supplementary information Fig. 1) [19]. Subsequently, significant tumor regression was demonstrated in the HCC rat animal model. LEN-TACE induced a higher tumoricidal effect compared to systemic LEN administration. TUNEL histological analysis represented the effective tumor cell killing of LEN-TACE. Locally delivered LEN-TACE also showed comparable safety with non-treated control rats based on blood tests. However, there are some limitations of the study with the small sample size and lack of dosage variation of LEN-TACE and systemic LEN treatment. Future studies would require extensive tumor response, pharmacokinetics, and safety data to validate the enhanced therapeutic outcomes of LEN-TACE. Further, based on recent findings about LEN-mediated immune modulation [20], combining LEN-TACE with various immunotherapies needs to be investigated for the treatment of HCC.
Supplementary Material
Acknowledgements
This work was mainly supported by grants R01CA218659 and R01EB026207 from the National Cancer Institute and National Institute of Biomedical Imaging and Bioengineering. Also, this work was supported by the Center for Translational Imaging and Mouse Histology and Phenotyping Laboratory at Northwestern University. Illustrations were originally created by authors through Biorender.
Funding
This study was funded by grants R01CA218659 and R01EB026207 from the National Cancer Institute and National Institute of Biomedical Imaging and Bioengineering.
Abbreviations
- HCC
Hepatocellular carcinoma
- IA
Intra-artery
- LEN
Lenvatinib
- LEN-Ems
Lenvatinib-eluting microspheres
- TACE
Transcatheter arterial chemoembolization
- HIF-1a
Hypoxia-inducible factor-1a
- VEGF
Vascular endothelial growth factor
- FDA
Food and Drug Administration
- VEGFR
Vascular endothelial growth factor receptor
- FGFR
Fibroblast growth factor receptor
- IONP
Iron oxide nanoparticles
- PLGA
Poly(lactide-co-glycolide)
- PBS
Phosphate-buffered saline
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- SDMA
Serum symmetric dimethylarginine
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00270-022-03242-8.
Conflict of interest The authors declare that they have no competing interests.
Ethical Approval All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
- 1.Kim E, Viatour P. Hepatocellular carcinoma: old friends and new tricks. Exp Mol Med. 2020;52(12):1898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, Farinati F. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103(4):914–21. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Hu DY, Chu Q, Wu JH, Gao C, Zhang YQ, Huang YR. Cell apoptosis and regeneration of hepatocellular carcinoma after transarterial chemoembolization. World J Gastroenterol: WJG. 2004;10(13):1876–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han G, Yang J, Shao G, Teng G, Wang M, Yang J, Liu Z, Feng G, Yang R, Lu L, Chao Y, Wang J. Sorafenib in combination with transarterial chemoembolization in Chinese patients with hepatocellular carcinoma: a subgroup interim analysis of the START trial. Future Oncol. 2013;9(3):403–10. [DOI] [PubMed] [Google Scholar]
- 5.Geschwind JF, Chapiro J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2016;14(8):585–7. [PubMed] [Google Scholar]
- 6.Chao Y, Chung YÄ, Han G, Yoon JÄ, Yang J, Wang J, Shao GÄ, Kim BI, Lee TÄ. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer. 2015;136(6):1458–67. [DOI] [PubMed] [Google Scholar]
- 7.Geschwind JF. Chemoembolization for hepatocellular carcinoma: where does the truth lie? J Vasc Intervent Radiol: JVIR. 2002;13(10):991–4. [DOI] [PubMed] [Google Scholar]
- 8.Geschwind JF, Ramsey DE, Choti MA, Thuluvath PJ, Huncharek MS. Chemoembolization of hepatocellular carcinoma: results of a metaanalysis. Am J Clin Oncol. 2003;26(4):344–9. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–42. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain MN, Gray BN, Heggie JC, Chmiel RL, Bennett RC. Hepatic metastases: a physiological approach to treatment. Br J Surg. 1983;70(10):596–8. [DOI] [PubMed] [Google Scholar]
- 11.Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, Kohara K, Shigenobu S, Ishibashi K, Arima T. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97(5):1253–62. [DOI] [PubMed] [Google Scholar]
- 12.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–71. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10(19):2878–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8(5):292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4(6):423–36. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Waxman DJ. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol Cancer Ther. 2008;7(12):3670–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teoh D, Secord AA. Antiangiogenic agents in combination with chemotherapy for the treatment of epithelial ovarian cancer. Int J Gynecol Cancer. 2012;22(3):348–59. [DOI] [PubMed] [Google Scholar]
- 18.Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Okubo S, Tominaga L, Kajiwara A, Kasuya K, Iritani S, Fujiyama S, Hosaka T, Saitoh S, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Ikeda K, Arase Y, Hashimoto M, Kozuka T, Kumada H. Lenvatinib-transarterial chemoembolization sequential therapy as an effective treatment at progression during lenvatinib therapy for advanced hepatocellular carcinoma. Liver Cancer. 2020;9(6):756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ando Y, Kawaoka T, Amioka K, Naruto K, Ogawa Y, Yoshikawa Y, Kikukawa C, Kosaka Y, Uchikawa S, Morio K, Fujino H, Nakahara T, Murakami E, Yamauchi M, Tsuge M, Hiramatsu A, Fukuhara T, Mori N, Takaki S, Tsuji K, Nonaka M, Hyogo H, Aisaka Y, Masaki K, Honda Y, Moriya T, Naeshiro N, Takahashi S, Imamura M, Chayama K, Aikata H. Efficacy and safety of lenvatinib-transcatheter arterial chemoembolization sequential therapy for patients with intermediate-stage hepatocellular carcinoma. Oncology. 2021;99(8):507–17. [DOI] [PubMed] [Google Scholar]
- 20.Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F, Baron A, Park J-W, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng A-L. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, Aikata H, Kawaguchi Y, Wada Y, Numata K, Inaba Y, Kuromatsu R, Kobayashi M, Okusaka T, Tamai T, Kitamura C, Saito K, Haruna K, Okita K, Kumada H. REFLECT: a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol. 2020;55(1):113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Choi H, Yu B, Kim D-H. Synthesis of iron oxide nanocube patched Janus magnetic nanocarriers for cancer therapeutic applications. Chem Commun. 2020;56(62):8810–3. [DOI] [PubMed] [Google Scholar]
- 23.Park W, Gordon AC, Cho S, Huang X, Harris KR, Larson AC, Kim D-H. Immunomodulatory magnetic microspheres for augmenting tumor-specific infiltration of natural killer (NK) cells. ACS Appl Mater Interfaces. 2017;9(16):13819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park W, Cho S, Ji J, Lewandowski RJ, Larson AC, Kim D-H. Development and validation of sorafenib-eluting microspheres to enhance therapeutic efficacy of transcatheter arterial chemoembolization in a rat model of hepatocellular carcinoma. Radiol: Imaging Cancer. 2021;3(1):e200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D-H, Li W, Chen J, Zhang Z, Green RM, Huang S, Larson AC. Multimodal imaging of nanocomposite microspheres for transcatheter intra-arterial drug delivery to liver tumors. Sci Rep. 2016;6(1):29653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park W, Chen J, Cho S, Park S-J, Larson AC, Na K, Kim D-H. Acidic pH-triggered drug-eluting nanocomposites for magnetic resonance imaging-monitored intra-arterial drug delivery to hepatocellular carcinoma. ACS Appl Mater Interfaces. 2016;8(20):12711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, White SB, Harris KR, Li W, Yap JW, Kim D-H, Lewandowski RJ, Shea LD, Larson AC. Poly (lactide-co-glycolide) microspheres for MRI-monitored delivery of sorafenib in a rabbit VX2 model. Biomaterials. 2015;61:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiraoka A, Kumada T, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Tada T, Toyoda H, Nouso K, Tsutsui A, Nagano T, Itokawa N, Hayama K, Imai M, Joko K, Tanaka H, Tamai T, Koizumi Y, Hiasa Y, Michitaka K, Kudo M, Real-life Practice Experts for, H. C. C. S. G., Group, H. C. C. Important clinical factors in sequential therapy including lenvatinib against unresectable hepatocellular carcinoma. Oncology 2019;97(5), 277–85. [DOI] [PubMed] [Google Scholar]
- 29.Dawkins J, Webster RM. The hepatocellular carcinoma market. Nat Rev Drug Discov. 2019;18(1):13–4. [DOI] [PubMed] [Google Scholar]
- 30.Llovet JM, Montal R, Villanueva A. Randomized trials and endpoints in advanced HCC: role of PFS as a surrogate of survival. J Hepatol. 2019;70(6):1262–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park W, Cho S, Ji J, Lewandowski RJ, Larson AC, Kim DH. Development and validation of sorafenib-eluting microspheres to enhance therapeutic efficacy of transcatheter arterial chemoembolization in a rat model of hepatocellular carcinoma. Radiol Imaging Cancer. 2021;3(1): e200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Sheu AY, Li W, Zhang Z, Kim DH, Lewandowski RJ, Omary RA, Shea LD, Larson AC. Poly(lactide-co-glycolide) microspheres for MRI-monitored transcatheter delivery of sorafenib to liver tumors. J Control Release. 2014;184:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, White SB, Harris KR, Li W, Yap JW, Kim DH, Lewandowski RJ, Shea LD, Larson AC. Poly(lactide-co-glycolide) microspheres for MRI-monitored delivery of sorafenib in a rabbit VX2 model. Biomaterials. 2015;61:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ackerman NB. Experimental studies on the circulation dynamics of intrahepatic tumor blood supply. Cancer. 1972;29(2):435–9. [DOI] [PubMed] [Google Scholar]
- 35.Kim D-H, Chen J, Omary RA, Larson AC. MRI visible drug eluting magnetic microspheres for transcatheter intra-arterial delivery to liver tumors. Theranostics. 2015;5(5):477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim DH, Choy T, Huang S, Green RM, Omary RA, Larson AC. Microfluidic fabrication of 6-methoxyethylamino numonafide-eluting magnetic microspheres. Acta Biomater. 2014;10(2): 742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho S, Min NG, Park W, Kim S-H, Kim D-H. Janus Microcar-riers for magnetic field-controlled combination chemotherapy of hepatocellular carcinoma. Adv Func Mater. 2019;29(26):1901384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


