Abstract
Targeted therapy with Bruton tyrosine kinase (BTK) inhibitors have revolutionized the treatment of patients with various B-cell malignancies. BTK inhibitors such as ibrutinib, zanubrutinib, orelabrutinib, and acalabrutinib have shown good clinical efficacy and better safety profiles than those of traditional chemotherapy and chemoimmunotherapy regimens. Multiple studies on new BTK inhibitors are ongoing, which may provide more therapeutic options for the treatment of B-cell malignancies. Considering the unmet need of evidence on BTK inhibitors in all clinical settings and to standardize the use of BTK inhibitors available in mainland China, Taiwan, Hong Kong, and Macau regions, this consensus has been formulated for the treatment of various B-cell malignancies based on the clinical practice and available evidences on the use of BTK inhibitors. The recommendations of this consensus will provide guidance to physicians and clinical researchers on the effective treatment of B-cell malignancies with BTK inhibitors.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40164-023-00448-5.
Keywords: BTK inhibitors, China, Consensus, Non-Hodgkin lymphoma, Targeted therapy
Background
B-cell malignancies comprise the most common hematologic malignancy, which is categorized as Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) [1]. The GLOBOCAN 2022 estimated 76,510 and 8240 cases of NHL and HL, respectively, in the United States while a much larger proportion of NHL and HL cases (97,788 and 6984, respectively) were estimated in China [2]. The high prevalence of B-cell malignancies demands an effective treatment and management options. With the advancement in tumor biology and modernization of biomedical technology, molecular-targeted therapy has gained attention for the treatment of hematologic malignancies [3]. Bruton's tyrosine kinase (BTK) is a key effector molecule in B-cell development and is expressed in B-cell lymphomas [4]. Hence, targeting BTK to develop new treatment modalities for B-cell malignancies is appealing. Multiple studies have confirmed the therapeutic value of BTK inhibitors for a variety of B-cell malignancies [5]. Based on the clinical study data, the current National Comprehensive Cancer Network (NCCN®) guidelines recommend the use of BTK inhibitors in B-cell malignancies [6]. A Chinese version of the expert consensus on BTK inhibitors for the treatment of B-cell malignant tumors has been published in the Journal of Leukemia & Lymphoma in 2022 [7]. Ibrutinib was the first approved BTK inhibitor in the United States and China [8]. However, the off-target kinase inhibition by ibrutinib is associated with adverse events (AEs), which led to the development of more selective new-generation BTK inhibitors [9], such as, acalabrutinib, zanubrutinib, orelabrutinib, and tirabrutinib. Pirtobrutinib, a non-covalent BTK inhibitor, has also been approved by the Food and Drug Administration (FDA) [10], but it has not been approved in China yet. The approval information is listed in Table 1.
Table 1.
BTK inhibitors for hematologic malignancies
| BTK inhibitor | Indication: FDA approval | Indication: China approval |
|---|---|---|
| Ibrutinib | CLL/SLL, WM, R/R MZL, R/R MCL |
China: CLL/SLL, R/R MCL, WM Hong Kong: CLL/SLL, R/R MCL, WM Taiwan: CLL/SLL, R/R MCL, WM, R/R MZL |
| Acalabrutinib | CLL/SLL, R/R MCL |
China: R/R MCL; R/R CLL/SLL Hong Kong: CLL/SLL, R/R MCL Taiwan: CLL/SLL, R/R MCL |
| Zanubrutinib | CLL/SLL, WM, R/R MCL, R/R MZL |
China: CLL/SLL, WM, R/R MCL Hong Kong: CLL/SLL, WM, R/R MCL Taiwan: CLL/SLL, WM, R/R MCL, R/R MZL |
| Orelabrutinib | None | China: R/R MCL, R/R CLL, R/R MZL |
| Tirabrutinib | R/R PCNSL | Taiwan: R/R PCNSL |
| Pirtobrutinib | R/R MCL | None |
Cutoff date: September 2023
In 2023, ibrutinib voluntarily withdraws the indication for MCL in patients who have received at least 1 prior therapy and for MZL in patients who require systemic therapy and have received at least 1 prior anti-CD20-based therapy, due to requirements related to accelerated approval status granted by the FDA
BTK, Bruton tyrosine kinase; CLL, chronic lymphocytic leukemia; FDA, Food and Drug Administration; MCL, mantle cell lymphoma; MZL, marginal zone B-cell lymphoma; PCNSL, primary central nervous system lymphoma; R/R; relapsed/refractory; SLL, small lymphocytic lymphoma; WM, Waldenström macroglobulinemia
The evidence on the beneficial effects of BTK inhibitors could not address all clinical settings. In order to standardize the use of BTK inhibitors available in mainland China, Taiwan, Hong Kong, and Macau regions and benefit more patients with B-cell malignancies, the specialists of these regions collaborated and formulated the consensus based on the available evidence. This consensus considered the recent evidence as well and provided recommendations based on the level of evidence. The recommendations of this consensus will provide guidance to physicians and clinical researchers on the effective treatment of B-cell malignant tumors with BTK inhibitors.
Methodology
In January 2023, a panel of discussion consisting of medical specialists and experts in clinical research was conducted. The methodology chosen to develop the consensus report was similar to the Nominal Group Technique but not exactly the same. The members in the review committee were from diverse geographic regions in Mainland China, Taiwan, Hong Kong, and Macau and diverse society of hematology. For constituting the committee experts, we approached the hematology societies located in these regions and got recommendations for experts from them. Also, a peer recommendation was taken into consideration while inviting committee panel review members. After finalizing the members of the review committee, we raised the questions that needed to be discussed in the kick-off meeting—to assign recommendations and evidence levels for the use of BTK inhibitors in different B-cell malignancies. The core activity of the review committee was to develop a consensus through systematic review, evidence synthesis, and inputs from practitioners. Evidence for this consensus was selected and reviewed by all committee members of the Chinese Society of Clinical Oncology (CSCO) and hematologists from Taiwan, Hong Kong, and Macau. The existing scientific evidence on BTK inhibitors in the treatment of B-cell malignant tumor was critically reviewed and discussed to formulate recommendations by the consensus members. Based on the quality of existing evidence and the strength of recommendations, the categories of consensus statements were divided in accordance with the NCCN definitions for scientific evidence and recommendations [6] (Table 2). The experts carefully assessed the quality of the cited studies and graded the consensus statements. After three rounds of consensus meetings, the drafted recommendations were rated by the consensus members through an email questionnaire (Additional file 1: Table S1) and then finalized in the summary meeting. The anonymous voting results are also listed followed by each recommendation.
Table 2.
Strength of recommendations
| Category | Description |
|---|---|
| 1 | Based upon high-level evidence, there is uniform consensus that the intervention is appropriate |
| 2A | Based upon low-level evidence, there is uniform consensus that the intervention is appropriate |
| 2B | Based upon low-level evidence, there is consensus that the intervention is appropriate |
| 3 | Based upon any level of evidence, there is a major disagreement that the intervention is appropriate |
For the “uniform consensus” defined in category 1 and category 2A, a majority panel vote of at least 85% is required. For the consensus, defined in category 2B, a panel vote of at least 50% (but < 85%) is required. Lastly, for recommendations where there is a strong panel disagreement regardless of the quality of the evidence, it requires a panel vote of at least 25% to include and designate a recommendation as category 3
Evidence was selected for inclusion if they met the following criteria: clinical trials using BTK inhibitor monotherapy or in combination with chemoimmunotherapy and registered with the Clinialtrial.gov or Chinese Clinical Trial Registry (ChiCTR); inclusion of adult patients with B-cell malignancies of any type, at any stage, and any histology; and the evaluation of survival outcomes, disease control, response rate, quality of life, or toxicity. Retrospective studies were also considered if they fulfilled the abovementioned interventions and outcomes criteria. Studies conducted exclusively on patients with immunological disease or non-hematologic malignancies treated with BTK inhibitors were not considered.
Mechanism of action
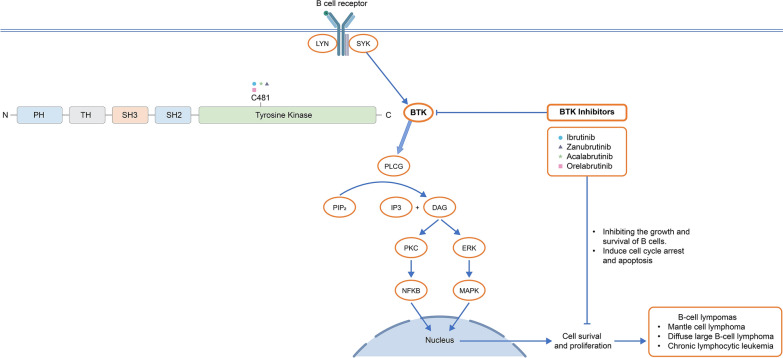
BTK inhibitors target BTK, a protein involved in signaling pathways of various immune cells, including B cells and macrophages. BTK inhibitors covalently bind to a cysteine residue at position 481 in the adenosine triphosphate (ATP) binding site of BTK, irreversibly inhibiting its kinase activity [11, 12]. This binding prevents the transfer of phosphate groups from ATP to downstream signaling proteins, ultimately disrupting B-cell receptor signaling and inducing apoptosis in malignant B cells [13–17]. The mechanism of action of BTK inhibitors is presented in Fig. 1. BTK inhibitors represent an important and effective class of drugs for the treatment of B-cell malignancies [13].
Fig. 1.
Mechanism of action of BTK inhibitors
Pharmacokinetics and pharmacodynamics
BTK inhibitors are rapidly absorbed after oral administration with a median time to the peak concentration (Tmax) of 1–2 h [18]. Zanubrutinib and acalabrutinib can be administered without food, as food did not result in significant effects on the area under the curve (AUC) for these drugs [19, 20], whereas administration with food significantly increased the AUC of ibrutinib by twofold, compared with administration following an overnight fast [21]. BTK inhibitors undergo extensive metabolism, primarily via a CYP3A-mediated pathway [19–21]. It is suggested to avoid acalabrutinib and ibrutinib in patients with severe hepatic impairment, whereas zanubrutinib requires dosage modification in this patient population [22]. Moreover, co-administration of acalabrutinib with proton pump inhibitors should be avoided [23].
BTK inhibitors have the ability to cross the blood–brain barrier (BBB); therefore, these can be considered as a treatment option for patients with primary central nervous system lymphoma (CNSL) [24]. A proof-of-concept phase Ib study demonstrated a low cerebrospinal fluid (CSF) penetration of ibrutinib, as the median (range) AUC0–24 nM.h in plasma was 977 (327–1562), whereas it was only 7.7 (2.21–16.5) in the CSF (CSF to plasma ratio: 0.78%). Furthermore, at a dose of 840 mg, the median (range) of 4 (0–24) h of the time above its enzymatic half-maximal inhibitory concentration (IC50; 0.5 nM) was observed in the CSF [25]. In a case series, the assessment of CSF distribution of zanubrutinib showed an excellent capability of zanubrutinib to cross the BBB. The mean plasma and CSF concentration of zanubrutinib was 143,190.6 ± 93,302.7 and 2941.1 ± 2382.01 pg/mL, respectively, with a median CSF/plasma ratio of 2.39% ± 1.71%, and the corrected CSF/plasma ratio after considering the high protein binding rate (94%) was 42.7% ± 27.7% (range, 8.6%–106.3%). Moreover, 95.7% of the samples had peak CSF concentrations of zanubrutinib above the enzymatic IC50 values [26].
Zanubrutinib and acalabrutinib are more selective than ibrutinib against off-target kinases [27, 28]. The results from kinase inhibition and cell-based assays demonstrated that zanubrutinib exhibited higher selectivity than ibrutinib, acalabrutinib, and acalabrutinib and its major metabolite (M27), with a selectivity comparable with orelabrutinib, by kinase profiling [29].
BTK resynthesis was faster in patients with chronic lymphocytic leukemia (CLL) than in healthy volunteers; therefore, it is hypothesized that complete/sustained BTK occupancy may improve the efficacy outcomes [27]. Zanubrutinib has exposure coverage above its IC50 during the entire dose interval for both twice-daily and once-daily dosing schedules with a high ratio of Ctrough/IC50, resulting in significantly higher concentrations than IC50 during the entire 24 h dosing period for both dosing schedules [30]. However, the corresponding ratios of Ctrough/IC50 for ibrutinib and acalabrutinib were estimated to be consistently lower than 1, even after considering the active metabolites [11, 31].
Clinical recommendations for BTK inhibitors in the treatment of malignant lymphoma
Chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL)
CLL/SLL is characterized by the presence of monoclonal B-cell population in the peripheral blood [32]. The B-cell receptor activation plays an important role in the pathogenesis of CLL [33]. Therefore, the targeted inhibition of BTK can effectively treat CLL. With the advent of BTK inhibitors, the treatment armamentarium of CLL has expanded, offering an effective and well-tolerated therapeutic option in both treatment-naïve (TN) and relapsed/refractory (R/R) settings [34–37]. BTK inhibitors are recommended for the treatment of patients with CLL/SLL regardless of age, fitness, deletion of chromosome 17 (del [17p]) and/or TP53 mutation, and immunoglobulin heavy-chain variable-region (IGHV) gene mutational status. Multiple phase III studies have compared the efficacy and safety profile of new-generation BTK inhibitors (zanubrutinib and acalabrutinib) with the first-generation BTK inhibitor, ibrutinib. However, till date, head-to-head comparison of orelabrutinib with first-generation BTK inhibitor is not available and further research is warranted to demonstrate the benefit of orelabrutinib over the first-generation BTK inhibitor.
Treatment-naïve patients with CLL/SLL
Previously untreated patients with CLL/SLL have shown superior efficacy with BTK inhibitors than chemoimmunotherapy regimens [38]. TN patients with or without del(17p) and/or TP53 mutation have benefited from BTK inhibitors. Moreover, patients with and without IGHV mutation have also shown efficacy with BTK inhibitors over chemoimmunotherapy regimens. Currently, targeted therapy with BTK inhibitors has gained popularity as the most efficacious and safe treatment option for patients with CLL/SLL [39]. Few studies have demonstrated efficacy and tolerable safety profile of BTK inhibitors even in the elderly population [40]. The evidence of studies on BTK inhibitors in patients with CLL/SLL has been summarized in Table 3.
Table 3.
Evidence of BTK inhibitors in patients with CLL/SLL
| Patients type | Treatment regimen | Efficacy | Study | Phase | Patients (N) |
|---|---|---|---|---|---|
| Patients with treatment naïve CLL/SLL | |||||
| Without del(17p) and TP53 mutation | IR vs. FCR (fludarabine 25 mg/m2, cyclophosphamide 50 mg/m2, rituximab 50 mg/m2) |
Statistically significant improvement in PFS and OS in the IR group compared with the FCR group (HR: 0.35; P < 0.001 and HR: 0.17; P < 0.001, respectively) |
E1912 [41] | 3 | 354 vs. 175 |
| Ibrutinib vs. chlorambucil |
PFS: median-NR vs. 15 months; 59% vs. 9% (HR: 0.154; 95% CI: 0.108–0.220) 7-year OS: median NR vs. 89 months; 78% in ibrutinib (HR: 0.453; 95% CI: 0.276–0.743) ORR: 92% vs. 37% |
RESONATE-2 [42] | 3 | 136 vs. 133 | |
| Zanubrutinib vs. BR | Better PFS improvement with zanubrutinib than BR regimen; 2-year PFS rate: 85.5% (HR: 0.42; 95% CI: 0·28–0·63; P < 0.0001) | SEQUOIA [38] | 3 | 241 vs. 238 | |
| BR vs. ibrutinib monotherapy vs. IR | 2-year PFS rate: 74% vs. 87% (HR: 0.39; 95% CI: 0.26–0.58; P < 0.001) vs. 88% (HR: 0.38; 95% CI, 0.25–0.59; P < 0.001) | Alliance [43] | 3 | 182 vs. 182 vs. 183 | |
| Ibrutinib plus obinutuzumab vs chlorambucil (0.5 mg/kg bodyweight on days 1 and 15 of each 28-day cycle) plus obinutuzumab (100 mg on day 1, 900 mg on day 2, 1000 mg on day 8, and 1000 mg on day 15 of cycle 1, then 1000 mg on day 1 of each 28-day cycle for cycles 2–6) |
30 months PFS: 79% vs. 31% Median PFS: HR: 0·23; 95% CI: 0·15–0·37; P < 0.0001 |
iLLUMINATE [44] | 3 | 113 vs. 116 | |
| Acalabrutinib + obinutuzumab vs. acalabrutinib monotherapy vs. obinutuzumab (day 1: 100 mg, 2: 900 mg, 8: 1000 mg, and 15: 1000 mg of cycle 1 and on day 1: 1000 mg of cycles 2–6) + chlorambucil (0.5 mg/kg on days 1 and 15 of each cycle) |
Median PFS at 48-month follow-up: NR vs. NR vs. 17.5 months; PFS rate: 87% vs. 77.9% vs. 25.1%; P < 0.0001, P < 0.0001, and P = 0.0296 OS rate: 92.9% vs. 87.6% vs. 88.0%; P = 0.064, P = 0.9164, P = 0.0836 |
ELEVATE-TN [51] | 3 | 179 vs. 179 vs. 177 | |
| With del(17p) and TP53 mutation | IR vs. Ibrutinib monotherapy vs. BR | Median PFS: NR vs. NR vs. 7 months | Alliance [43] | 3 | 11 vs. 9 vs. 14 |
| Zanubrutinib | ORR: 94.5%; 18-month PFS rate: 90.6%; 18-month OS rate: 95.1% | SEQUOIA [38] | 3 | 109 | |
| Acalabrutinib | 48-month PFS rate: 74.8% | ELEVATE-TN [51] | 3 | 25 | |
| IGHV unmutated | IR vs. FCR | 5-year PFS rates: 75% vs. 33% | E1912 [41] | 3 | 210 vs. 71 |
| Acalabrutinib + obinutuzumab vs. acalabrutinib monotherapy vs. obinutuzumab + chlorambucil |
48-month PFS rates: 77.1% vs. 85.7% (P < 0.0001 for A + O vs. O + C, P < 0.0001 for A vs. O + C) |
ELEVATE-TN [51] | 3 | 103 vs. 119 vs. 116 | |
| Zanubrutinib vs. BR | Longer PFS with zanubrutinib than with BR (HR: 0.24; 95% CI: 0.13–0.43) | SEQUOIA [38] | 3 | 125 vs. 121 | |
| IGHV mutation | IR vs. FCR | 5-year PFS rates: 83% vs. 68% | E1912 [172] | 3 | 70 vs. 44 |
| Acalabrutinib + obinutuzumab vs. acalabrutinib monotherapy vs. obinutuzumab + chlorambucil | 48-month PFS rates: 87% vs. 77.9% vs. 25.1% (P = 0.0012 for A + O vs. O + C, P = 0.0551 for A vs. O + C) | ELEVATE-TN [51] | 3 | 58 vs. 74 | |
| Zanubrutinib vs. BR | Longer PFS with zanubrutinib than with BR (HR: 0.35; 95% CI: 0.19–0.64) | SEQUOIA [38] | 3 | 109 | |
| Patients with R/R CLL/SLL | |||||
| Without del(17p) and TP53 mutation | Acalabrutinib vs. idelalisib + rituximab or BR | 42-month PFS rate: 63% vs. 21%; HR: 0.30; 95% CI: 0.20–0.44; P < 0.0001 | ASCEND [58] | 3 | 155 vs. 155 |
|
Zanubrutinib vs ibrutinib |
24-month PFS rate: 78.4% vs. 65.9%; HR: 0.65; 95% CI: 0.49–0.86; P = 0.002 | ALPINE [55] | 3 | 207 vs. 208 | |
| With del(17p) and TP53 mutation | Acalabrutinib vs. ibrutinib | Median PFS of 38.4 months in both the groups | ELEVATE-RR [54] | 3 | 268 vs. 265 |
| Zanubrutinib vs. ibrutinib | 24-month PFS: 72.6% vs. 54.6%; HR: 0.53; 95% CI: 0.31–0.88 | ALPINE [55] | 3 | 75 vs. 75 | |
| Acalabrutinib vs. idelalisib + rituximab or BR | 42-month PFS: 57% vs. 10%; HR: 0.22; 95% CI: 0.12–0.39; P < 0.001 | ASCEND [58] | 3 | 22 vs. 13 | |
| Ibrutinib vs. ofatumumab | HR: 0.175; 95% CI: 0.115–0.258 | RESONATE [53] | 3 | 147 | |
| IGHV mutated | Acalabrutinib vs. idelalisib + rituximab or BR | 68% vs. 36%, HR: 0.34; 95% CI: 0.13–0.93; P = 0.027 | ASCEND [58] | 3 | 21 |
| Ibrutinib vs. ofatumumab | HR: 0.103; 95% CI: 0.067–0.159 | RESONATE [53] | 3 | 181 | |
| IGHV unmutated | Acalabrutinib vs. idelalisib + rituximab or BR | 59% vs. 17%, HR: 0.29; 95% CI: 0.20–0.41; P < 0.001 | ASCEND [58] | 3 | 109 vs. 119 |
| Zanubrutinib vs. ibrutinib | HR: 0.64; 95% CI: 0.47–0.87 | ALPINE [55] | 3 | 72 vs. 98 | |
| Ibrutinib vs. ofatumumab | HR: 0.200; 95% CI: 0.113–0.353 | RESONATE [53] | 3 | 85 | |
A, acalabrutinib; BR, bendamustine plus rituximab; BTK, Bruton tyrosine kinase; C, chlorambucil; CI, confidence interval; CLL, chronic lymphocytic leukemia; FCR, fludarabine, cyclophosphamide, rituximab; IR, ibrutinib + rituximab; HR, hazard ratio; IGHV, immunoglobulin heavy-chain variable-region; MZL, marginal zone B-cell lymphoma; NR, not reached; PFS, progression-free survival; SLL, small lymphocytic lymphoma; TN, treatment naïve; O, obinutuzumab; ORR, overall response rate; OS, overall survival
Treatment-naïve patients with CLL/SLL with or without del(17p) and/or TP53 mutation
Patients without del(17p) have shown superior efficacy with BTK inhibitors than with chemoimmunotherapy in various studies. In the phase III ECOG-ACRIN E1912 study, younger patients without del(17p) received either ibrutinib plus rituximab (50 mg/m2 of body surface area on day 1 of cycle 2; 325 mg/m2 on day 2 of cycle 2; and 500 mg/m2 on day 1 of cycles 3 through 7) or chemoimmunotherapy with rituximab (50 mg/m2), fludarabine (25 mg/m2), and cyclophosphamide (50 mg/m2) (FCR). After a median follow-up of 34 months, the improvement in progression-free survival (PFS) and overall survival (OS) of the ibrutinib plus rituximab was statistically significant (P < 0.001) when compared with the FCR regimen [41]. In the RESONATE-2 study involving patients aged > 65 years, comparison of ibrutinib with chlorambucil showed significantly better PFS, OS, and overall response rate (ORR) in TN patients with CLL without del(17p) after a long-term follow-up [42]. Similarly, in the SEQUOIA study, zanubrutinib was associated with a significantly better PFS than bendamustine plus rituximab (BR) regimen (hazard ratio [HR]: 0.42, 95% confidence interval [CI]: 0.28–0.63, P < 0.0001) [38]. The results of the Alliance study showed that the ibrutinib monotherapy and ibrutinib plus rituximab had significantly better PFS than the BR regimen [43]. Moreover, the iLLUMINATE study observed that patients could benefit by the addition of BTK inhibitors in the monoclonal antibody regimen, as significantly longer PFS was obtained with ibrutinib plus obinutuzumab than chlorambucil (0.5 mg /kg on days 1 and 15 of each 28-day cycle) plus obinutuzumab (100 mg on day 1, 900 mg on day 2, 1000 mg on day 8, and 1000 mg on day 15 of cycle 1, then 1000 mg on day 1 of each 28-day cycle for cycles 2–6) [44]. Furthermore, in the ELEVATE‑TN study, elderly patients randomly received acalabrutinib and obinutuzumab, acalabrutinib monotherapy, or obinutuzumab (100 mg on day 1, 900 mg on day 2, 1000 mg on day 8, and 1000 mg on day 15 of cycle 2 and on day 1 [1000 mg] of cycles 2–6) and chlorambucil (0.5 mg/kg on days 1 and 15 of each cycle) and showed that acalabrutinib as monotherapy or in combination with obinutuzumab was associated with significantly longer PFS [45].
The patients with mutation of the TP53 tumor suppressor gene or the deletion of chromosome 17p (del[17p]) where TP53 is encoded, are high-risk patients with TP53 mutation and del(17p) influencing the prognosis of patients with CLL [46]. Studies have shown that patients with del(17p) or a mutation of TP53 have poor response to initial chemoimmunotherapy or are prone to relapse after remission, and BTK inhibitors are considered as the first choice of treatment for these patient populations [47]. For patients with del(17p) in the Alliance study, PFS was longer in patients receiving ibrutinib when compared with those in the BR group [43]. The SEQUOIA study included patients with del(17p) who were treated with zanubrutinib monotherapy, and the observed ORR, PFS, and OS rates were 94.5%, 90.6%, and 95.1%, respectively [38]. In the ELEVATE-TN study, patients with del(17p) and/or TP53 mutation treated with acalabrutinib monotherapy or in combination with obinutuzumab for 48 months had significantly higher PFS rates compared with those treated with chlorambucil combined with obinutuzumab [45].
Treatment-naïve patients with CLL/SLL with or without IGHV mutations
Multiple studies have demonstrated the efficacy of BTK inhibitors over chemoimmunotherapy in patients with and without IGHV mutation. IGHV mutational status is considered to be one of the prognostic markers for disease progression with unmutated IGHV as an unfavorable prognostic marker [48]. Patients with unmutated IGHV genes are known to have worse outcomes following chemotherapy or chemoimmunotherapy [49]. However, the advent of BTK inhibitors has changed the course of action with improved responses in patients with IGHV-unmutated CLL [50].
The phase III ECOG-ACRIN E1912 study showed that ibrutinib in combination with rituximab showed better PFS than the FCR regimen in patients without IGHV mutation (HR: 0.26; 95% CI: 0.14–0.50) [41]. In the SEQUOIA study, a consistently longer PFS was observed with zanubrutinib monotherapy compared with BR regimen even in patients with unmutated IGHV [38]. Similarly, in the ELEVATE-TN study, acalabrutinib either as monotherapy or in combination with obinutuzumab showed effectiveness in patients with unmutated IGHV (48-month PFS rates: 77.1% in the acalabrutinib monotherapy group and 85.7%, in the acalabrutinib plus obinutuzumab group) [51].
Although BTK inhibitor therapy looks superior to chemoimmunotherapy in most patient populations in TN setting, conclusion cannot be drawn on the most effective frontline therapy in young and fit patients without del(17p) and/or TP53 mutation and with mutated IGHV because of the limited course of chemoimmunotherapy and the PFS plateau observed with long-term follow-ups.
Patients with R/R CLL/SLL
The advent of BTK inhibitors has revolutionized the treatment of patients with R/R CLL/SLL. Table 3 summarizes the major clinical studies on BTK inhibitors for R/R CLL/SLL. BTK inhibitors have shown better efficacy than chemoimmunotherapy in patients with R/R CLL [52]. A 6-year follow-up of the RESONATE study in patients with R/R CLL receiving ibrutinib showed efficacy irrespective of high‐risk clinical or genomic features with a tolerable safety profile [53]. Patients receiving ibrutinib showed a significantly longer PFS than those receiving ofatumumab (44.1 months vs. 8.1 months; HR: 0.148; 95% CI: 0.113–0.196; P < 0.001). No new safety concerns were observed with ibrutinib confirming the long-term efficacy and safety of ibrutinib in previously treated patients with CLL [53]. However, the head-to-head comparison of BTK inhibitors showed zanubrutinib to be superior to ibrutinib in efficacy (HR: 0.65; 95% CI: 0.49–0.86; P = 0.002). Moreover, the new-generation BTK inhibitors showed better safety profiles than ibrutinib especially in terms of cardiovascular events [54, 55].
Patients with R/R CLL/SLL with or without del(17p) and/or TP53 mutation
In patients with CLL/SLL, the del(17p) or TP53 gene mutation is the most important single poor prognostic factor [56]. Previously, patients with R/R CLL/SLL with or without del(17p) and/or TP53 mutation were heavily treated; however, they were associated with poor response to classical chemoimmunotherapy [57]. In the ASCEND study, R/R CLL patients with or without del(17P) and/or TP53 mutation treated with acalabrutinib monotherapy had a significantly longer PFS than those receiving investigator’s choice (idelalisib plus rituximab [IR] or BR) with an acceptable safety profile [52]. Two randomized studies in patients with R/R CLL provided a direct comparison of first- and new-generation BTK inhibitors (ELEVATE-RR: ibrutinib vs. acalabrutinib; ALPINE: ibrutinib vs. zanubrutinib) [54, 55]. In the head-to-head, phase III, ELEVATE‑RR study of acalabrutinib and ibrutinib, the median PFS in both the groups was 38.4 months (95% CI: 33.0–38.6 and 95% CI: 33.0–41.6 in acalabrutinib and ibrutinib, respectively) with no statistically significant difference observed between acalabrutinib and ibrutinib [54]. However, zanubrutinib demonstrated a statistically significant improvement in PFS when compared with ibrutinib in high-risk patients with del(17p) and/or TP53 mutation (72.6% vs. 54.6%, HR: 0.53; 95% CI: 0.31–0.88). At 24 months, the PFS rate was 78.4% in the zanubrutinib group and 65.9% in the ibrutinib group. Moreover, zanubrutinib showed a safety profile better than that of ibrutinib with fewer cardiac disorders compared with the ibrutinib group, and none of the patients in the zanubrutinib group had cardiac disorder–related death, whereas six patients who received ibrutinib had fatal cardiac disorders [55].
Patients with R/R CLL/SLL with or without IGHV mutation
IGHV mutated or unmutated patients with R/R CLL/SLL have benefited from BTK inhibitors. In a phase III, ASCEND study, better PFS benefit was observed with acalabrutinib monotherapy than IR or BR regimen in patients with unmutated IGHV (P < 0.001) and mutated IGHV (P = 0.027) [58]. Similarly, the RESONATE study demonstrated that the efficacy of ibrutinib was better than that of ofatumumab in both IGHV mutated (HR: 0.103; 95% CI: 0.067–0.159) and unmutated (HR: 0.200; 95% CI: 0.113–0.353) patients. The direct comparison of ibrutinib with zanubrutinib in the ALPINE study showed that the efficacy of zanubrutinib was superior to ibrutinib in IGHV unmutated patients (HR: 0.64; 95% CI: 0.47–0.87) [55].
Recommendations
BTK inhibitors are recommended in patients with CLL/SLL regardless of age, fitness, del(17p) and/or TP53 mutation, IGHV mutational status, and therapeutic settings (frontline or salvage; category 1; Agree: 100%, 18/18).
Despite no apparent impact on decision-making regarding BTK inhibitor utility, tests for common prognosis biomarkers, such as del(17p) and/or TP53 mutation and IGHV mutational status, are still recommended for both scientific interests and long-term therapeutic planning after failure of BTK inhibitor (category 2A; Agree: 94%, 17/18).
Acalabrutinib and zanubrutinib have a more favorable safety profile than ibrutinib, especially in terms of cardiovascular events. Furthermore, zanubrutinib has also shown a superior efficacy profile than ibrutinib. Based on the results of head-head comparison studies, zanubrutinib could be recommended as the most preferred treatment regimen for patients with CLL/SLL (category 1; Agree: 94%, 17/18).
Mantle cell lymphoma
Mantle cell lymphoma (MCL) is a distinct subtype of NHL with highly heterogenous presentation and aggressive clinical course [59, 60]. Although chemoimmunotherapy and stem cell transplantation have improved the outcome of patients with MCL, most of them had a relapse and are subjected to treatment-emergent AEs [61].
Patients with TN MCL
BTK inhibitors can be considered in previously untreated patients with MCL, if conventional chemotherapy cannot be tolerated. Table 4 presents the vital studies of BTK inhibitors in the treatment of patients with MCL. The indolent form of MCL requires individualized management. The IMCL-2015 study showed the efficacy of ibrutinib in combination with rituximab with a high rate of complete response (CR) and undetectable minimal residual disease (MRD) observed in indolent clinical forms of MCL [62]. BTK inhibitors are the preferred choice of treatment for patients with MCL considered to be unsuitable candidates for autologous stem cell transplantation (ASCT). The SHINE study involving elderly patients with previously untreated MCL evidenced the benefit of BTK inhibitors as a significantly prolonged PFS was observed with ibrutinib plus standard chemoimmunotherapy consisting of bendamustine at a dose of 90 mg/m2 of body surface area and rituximab at a dose of 375 mg/m2 (median PFS: 80.6 months in the ibrutinib group and 52.9 months in the placebo group) [63]. Several exploratory studies using BTK inhibitors in MCL are ongoing to evaluate the efficacy of BTK inhibitors in patients with MCL [64, 65]. The results from the ongoing BGB‑3111‑306 study (MANGROVE) in TN patients with MCL who are ineligible for ASCT comparing the efficacy of zanubrutinib plus rituximab with BR may provide further insight into the efficacy of BTK inhibitors for the treatment of patients with MCL [65].
Table 4.
Evidence of BTK inhibitors in patients with MCL
| Patients type | Treatment regimen | Efficacy | Study | Phase | Patients (N) |
|---|---|---|---|---|---|
| Patients with treatment naïve MCL | |||||
| Indolent clinical forms | Ibrutinib + rituximab | ORR: 84%; CR rate: 80%; estimated PFS at 36 months: 93% | IMCL-2015 [62] | 2 | 50 |
| Untreated MCL | Zanubrutinib + rituximab followed by rituximab (375 mg/m2), dexamethasone (20 mg), cytarabine (2000 mg/m2), and oxaliplatin (130 mg/m2) (R-DHAOx) then zanubrutinib maintenance |
CR rate: 88.2% (15/17); MRD negative rate: 100% Study ongoing |
NCT04624958 [173] | 2 | 17 |
| ASCT eligible | Ibrutinib + R-CHOP followed by w/wo ASCT + ibrutinib maintenance | ORR: 98%; CR rate: 45%; 3-year FFS rate: 86% | TRIANGLE [66] | 3 | 807 |
| Zanubrutinib + rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2, prednisone 100 mg, (R-CHOP)/ dexamethasone 40 mg, rituximab 375 mg/m2, cytarabine 2 × 2 g/m2, cisplatin 100 mg/m2 (R-DHAOx) ± ASCT |
CR: 85.7% (6/7); MRD negative rate: 100% Study ongoing |
NCT04736914 [64] | 2 | 47 | |
| ASCT ineligible | Ibrutinib + BR vs. placebo + BR | Median PFS: 80.6 vs. 52.9 months; CR rate: 65.5% vs. 65.5% | SHINE [63] | 3 | 523 |
| Zanubrutinib + rituximab vs. BR | Ongoing | MANGROVE [65] | 3 | 500 | |
| Patients with R/R MCL | |||||
| Overall | Zanubrutinib | ORR: 83.7%; CR rate: 77.9%; median PFS: 33 months | BGB-3111–206 [72] | 2 | 86 |
| Acalabrutinib | ORR: 81%; CR rate: 40%; 12-month PFS rate: 67%, 12-month; OS rate: 87% | ACE-LY-004 [14] | 2 | 124 | |
| Ibrutinib | ORR: 68%; CR rate: 21%; median PFS: 13.9 months | PCYC-1104 [8] | 2 | 111 | |
| Orelabrutinib | ORR: 82.5%; CR rate: 24.7% | ICP-022-MCL [73] | 2 | 97 | |
ASCT, autologous stem cell transplantation; BR, bendamustine plus rituximab; BTK, Bruton tyrosine kinase; CR, complete response; FFS, failure-free survival; MCL, mantle cell lymphoma; MRD, minimal residual disease; PFS, progression-free survival; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R/R, relapsed/refractory; SLL, small lymphocytic lymphoma; ORR, overall response rate; OS, overall survival
Patients suitable for ASCT received induction with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab, dexamethasone, cytarabine, and cisplatin (R-DHAP) and were randomly assigned to the control arm (R-CHOP or R-DHAP induction followed by ASCT and observation), ASCT-ibrutinib arm (R-CHOP plus ibrutinib or R-DHAP induction followed by ASCT and 2 years of ibrutinib maintenance), and ibrutinib monotherapy arm (R-CHOP plus ibrutinib or R-DHAP and 2 years of ibrutinib maintenance) in the phase III, TRIANLGE study [66]. After a median follow-up of 31 months, the addition of ibrutinib during induction and as maintenance with or without ASCT evidenced the efficacy of BTK inhibitor [67].
Patients with R/R MCL
Before the advent of BTK inhibitors, patients with R/R MCL had a limited therapeutic option with generally poor outcomes [68, 69]. Both national and international guidelines recommend the use of BTK inhibitors for the treatment of patients with R/R MCL [70, 71]. Table 4 presents the details of vital studies on BTK inhibitors used for the treatment of patients with R/R MCL. A long-term follow-up of a phase II study showed durable responses and tolerable safety profile of zanubrutinib in patients with R/R MCL [72]. Similarly, acalabrutinib demonstrated clinically meaningful survival benefits and a favorable safety profile in the treatment of patients with R/R MCL [14]. Ibrutinib as a monotherapy in patients with R/R MCL also showed durable response and favorable toxicity profile [8]. Furthermore, Chinese patients with R/R MCL have demonstrated sustained efficacy and long-term safety with orelabrutinib in a phase II study [73]. In April 2023, ibrutinib was voluntarily withdrawn from the US market as the treatment option for patients with R/R MCL, but the decision has no impact on ibrutinib in China so far [74]. For the treatment of R/R MCL, initiating BTK inhibitors at the earliest can provide better therapeutic efficacy to the patients. A meta-analysis of zanubrutinib in R/R MCL showed that patients who received zanubrutinib as the second-line therapy was associated with better survival outcomes than those who received it as later-line therapy [75]. The ORR and CR rates of the zanubrutinib monotherapy for MCL are generally higher than those of the first-generation BTK inhibitor monotherapy. This may be due to the structural optimization of the new-generation BTK inhibitors, resulting in higher target occupancy and longer inhibition time. Recently, non-covalent BTK inhibitor, pirtobrutinib has demonstrated the effectiveness as monotherapy in patients with R/R MCL with an ORR of 58% (95% CI: 46.9–68.1) [76]; however, it has not yet been approved in China, Hong Kong, Taiwan, and Macau.
Recommendations
For TN patients
BTK inhibitors combined with chemoimmunotherapy are recommended for the treatment of patients with MCL aged ≥ 65 years or frail patients (category 2B; agree: 83%, 15/18).
BTK inhibitors are recommended for the treatment of patients both suitable and unsuitable for ASCT during induction and as a maintenance therapy (category 2B; Agree: 78%, 14/18).
For R/R patients
BTK inhibitors are the preferred treatment of choice for patients with R/R MCL (category 2A) and are recommended to start treatment with BTK inhibitors as early as possible for better outcomes (category 2A; Agree: 94%, 17/18).
Acalabrutinib and zanubrutinib have more favorable safety profiles than ibrutinib, especially in terms of cardiovascular events. Zanubrutinib further demonstrated a superior efficacy profile compared with ibrutinib. Based on the results of head-to-head comparison studies, zanubrutinib is recommended as the most preferred treatment regimen for patients with MCL (category 2B; Agree: 72%, 13/18).
Diffuse large B-cell lymphoma
Diffuse Large B-Cell Lymphoma (DLBCL) is the most common aggressive NHL with an incidence of 7 cases per 100,000 people per year [77]. DLBCL can be divided into germinal center B cell (GCB), activated B cell (ABC), or non-GCB type and unclassified type by genotyping. Molecular classification of DLBCL helps to personalize the therapy for DLBCL [78].
Currently, there is no standard BTK inhibitor-based regimen for the treatment of patients with newly diagnosed DLBCL. In addition to BTK inhibitor monotherapy, the combination of BTK inhibitors and R-CHOP regimen is also used. The results of the PHOENIX study showed that in previously untreated patients with ABC DLBCL, ibrutinib in combination with R-CHOP regimen did not meet the primary endpoint of the study as the event-free survival (EFS) was not improved with the combination therapy [79]. Moreover, the addition of ibrutinib did not have significant difference in the PFS (70.8% vs. 68.1%), OS (82.8% vs. 81.4%), and CR (67.3% vs. 68.0%) rates. The ESCALADE study of acalabrutinib in combination with R-CHOP is currently ongoing in patients with TN non-GCB aged ≤ 65 years. The results of the study will provide evidence on the beneficial effects of acalabrutinib addition to R-CHOP regimen in patients aged ≤ 65 years with untreated non-GCB DLBCL [80]. Multiple studies have evidenced that patients with R/R DLBCL can be effectively treated with BTK inhibitors [70]. The latest CSCO guidelines added zanubrutinib, a new generation of BTK inhibitor for the treatment of patients with R/R DLBCL [71]. The evidence of studies is presented in Table 5.
Table 5.
Evidence of BTK inhibitors in patients with DLBCL
| Patients type | Treatment regimen | Efficacy | Study | Phase | N |
|---|---|---|---|---|---|
| Non-GCB | |||||
| TN | Ibrutinib + R-CHOP (rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2, and oral prednisone [or equivalent] 100 mg) vs. placebo + R-CHOP | 36-month PFS rate: 70.8% vs. 68.1%; 36-month OS rate: 82.8% vs. 81.4%; CR rate: 67.3% v 68.0% | PHOENIX [79] | 3 | 419 vs. 419 |
| TN, ≤ 65 years | Acalabrutinib + R-CHOP | Ongoing | ESCALADE [80] | 3 | 600 |
| R/R | Zanubrutinib | ORR, non-GCB: 36%, GCB: 25% | BGB-3111–207 [83] | 2 | 29 |
| R/R, ASCT ineligible | Zanubrutinib + lenalidomide |
Best ORR: 90.9%, CR: 36.4% Study ongoing |
BGB-3111–110 [84] | 1 | 27 |
| R/R | Ibrutinib | ORR, non-GCB: 37%, GCB: 5% | NCT00849654 NCT01325701 [85] | 2 | 80 |
| R/R | Acalabrutinib | ORR, non-GCB: 24% (5/21); 4 CR and 1 PR | NCT02112526 [86] | 1b | 21 |
| BCL2/MYC expression | |||||
| TN | Ibrutinib + R-CHOP | DE: CR: 67.5%; PR 22.8% | PHOENIX, post hoc [174] | 3 | 200 |
| R/R | Ibrutinib | DE: ORR 47%; CR 37% | Landsburg et al. [90] | Case-series | 25 |
| R/R | Zanubrutinib | DE: ORR 61%; NDE: ORR 29% | BGB-3111–207 [83] | Post-hoc | 121 |
| TN | Zanubrutinib + R-CHOP | Ongoing | NCT05189197 [92] | 2 | 41 |
| CD79B, MYD88 (MCD) | |||||
| TN | Ibrutinib + R-CHOP | MCD and aged ≤ 60 years: 3-year EFS and OS: 100% | PHOENIX, post hoc [94] | 3 | 31 |
| TN | Orelabrutinib + R-CHOP | Ongoing | NCT05234684 [95] | 3 | 150 |
| R/R | Zanubrutinib | CD79B mutation: ORR 46%; MYD88 mutation: ORR 40%; MYD88 + CD79B (MCD) mutation: ORR 50% | BGB-3111–207 [83] | 2 | 41 |
| Elderly and unfit/frail | |||||
| TN | Ibrutinib + rituximab + lenalidomide | ORR 66.7%; CR rate: 56.7%; 2-year PFS rate: 53·3%; 2-year OS rate: 66·7% | NCT03949062 [99] | 2 | 30 |
| TN | Zanubrutinib + rituximab + lenalidomide vs. R-mini-CHOP (rituximab 375 mg/m2 on day 1, cyclophosphamide 400 mg/m2, doxorubicin 25 mg/m2, and vincristine 1 mg on day 2, and prednisone 40 mg/m2 on days 2–6, every 21 days) | Ongoing | NCT05179733 [100] | 3 | 280 |
ASCT, autologous stem cell transplantation; BCL2, B-cell lymphoma 2; BTK, Bruton tyrosine kinase; CR, complete response; DE, double expressor, DLBCL, diffuse large B-cell lymphoma; EFS, event-free survival; GCB, germinal center B cell; MYC, myelocytomatosis oncogene; NDE, non-double expressor; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R/R, relapsed/refractory; TN, treatment naïve
Non-GCB/ABC DLBCL
B-cell receptor (BCR) signaling can be activated via two different pathways: antigen-dependent signaling and antigen-independent tonic signaling. In non-GCB DLBCL, the constitutive activation of BCR and NF-κB signaling was associated with lymphomagenesis and cancer cell survival, which may explain the relationship between BTK inhibitor treatment and the response of patients with non-GCB DLBCL [81, 82].
The BGB-3111-207 study showed the efficacy of zanubrutinib in patients with R/R DLBCL. Furthermore, patients with non-GCB showed better ORR than patients with GCB DLBCL (36% vs. 25%) [83]. Zanubrutinib in combination with lenalidomide has shown efficacy even in patients with R/R DLBCL who are not eligible for ASCT [84]. A phase I/II clinical trial in patients with R/R DLBCL showed better survival outcomes in those with ABC DLBCL than those with GCB DLBCL (ORR: 37% vs. 5%) supporting the use of the ibrutinib-based therapy in patients with ABC DLBCL [85]. Acalabrutinib monotherapy has also demonstrated efficacy in patients with non-GCB R/R DLBCL (ORR: 24%) [86].
Patients with BCL2/MYC expression, CD79B/MYD88 mutation
Patients with DLBCL having translocation of both myelocytomatosis oncogene (MYC) and B-cell lymphoma 2 (BCL2) are known to have an aggressive clinical course and poor outcome [87, 88]. In a post hoc subgroup analysis of TN patients in the phase III, PHOENIX trial, a numerical trend was observed toward improved EFS and PFS with ibrutinib in combination with R‐CHOP when compared with R-CHOP alone in patients with high MYC/BCL2 co‐expression [89]. Ibrutinib as monotherapy also demonstrated the efficacy with an ORR of 47% and a CR rate of 37% in patients with R/R non-CGB DLBCL with co-expression of MYC and BCL2 protein in a case series [90]. Zanubrutinib also showed beneficial effects in patients with R/R DLBCL with MYC/BCL2 co‐expression. Patients with MYC and BCL2 double-expressor DLBCL showed a higher ORR (61% vs. 29%), longer PFS (5.4 months vs. 3.6 months), and OS (10 months vs. 7 months) than non-double expressors [91]. Currently, a phase II study evaluating the efficacy of zanubrutinib plus R-CHOP is ongoing in patients with DLBCL with co-expression of BCL2 and MYC, which may provide the evidence on the efficacy of zanubrutinib addition in TN patients [92].
MYD88/CD79B (MCD) mutation is the most common mutation associated with ABC subtype DLBCL arising in immune-privileged sites, enriched with MYD88 L265P and/or CD79B gain-of-function mutations. MYD88 is a key molecule mediating Toll-like receptor signaling, whereas CD79B is part of the B-cell receptor complex that plays a role in maintaining the cell surface expression of the receptor [93].
The survival benefit of addition of ibrutinib to R-CHOP chemotherapy was observed in younger patients with MCD subtype of DLBCL. Patients who received ibrutinib in combination with R-CHOP showed a 3-year EFS and OS of 100%, compared with a significantly lower EFS and OS of 48% and 69.6%, respectively, with R-CHOP monotherapy [94]. An ongoing phase III BELIEVE-01 study of orelabrutinib plus R-CHOP in TN DLBCL with MCD will provide evidence on the efficacy of orelabrutinib in this patient population [95]. Zanubrutinib also showed efficacy in patients with non-GCB DLBCL and CD79B mutations, where patients with CD79B mutations showed a significantly higher ORR than those without CD79B mutations (60% vs. 26.9%; P = 0.005) [91].
Elderly and unfit/frail patients
The incidence of DLBCL increases with age, especially for those aged > 75 years [96]. Treatment of DLBCL in elderly patients poses a challenge due to high chances of remission failure associated with comorbidities and standard immunochemotherapy intolerance [97]. Thus, the reduced-intensity regimens (R-miniCHOP) are a useful option for treating elderly or unfit patients with DLBCL. However, the benefit of R-miniCHOP in unfit population remains uncertain as a relatively high proportion of drug discontinuation occurs due to toxicity [98].
Ibrutinib, rituximab, and lenalidomide in TN unfit or frail patients with DLBCL aged ≥ 75 years showed CR, ORR, 2-year PFS, and 2-year OS of 56.7%, 66.7%, 53.3%, and 66.7%, respectively, suggesting clinical effectiveness and safety of ibrutinib in combination with rituximab and lenalidomide as the first-line treatment in older patients with DLBCL [99]. An ongoing phase III study comparing the efficacy of zanubrutinib in combination with rituximab and lenalidomide with R-mini-CHOP will provide evidence on the efficacy of zanubrutinib in TN, unfit or frail elderly patients with DLBCL [100].
Recommendations
BTK inhibitors are recommended as an optional treatment regimen in patients with non-GCB DLBCL (category 2B; Agree: 67%, 12/18) and in patients with DLBCL having specific subtypes (correlation of BCL2/MYC expression, MCD mutation; category 2A; Agree: 94%, 17/18).
For patients with poor response or unfit (elderly or frail) for standard chemotherapy (i.e., R-CHOP), BTK inhibitors with less intensity chemotherapy (i.e., R-mini-CHOP) or chemo-free regimen (BTK inhibitors with rituximab and/or lenalidomide) could be recommended (category 2B; Agree: 83%, 15/18).
Central nervous system lymphoma
Central nervous system lymphoma (CNSL) is an uncommon type of NHL that originates within the central nervous system (CNS). It is considered an extranodal lymphoma and primarily affects the brain, spinal cord, and leptomeninges [101]. The diagnosis and treatment of CNSL can be challenging due to the unique anatomical location of the tumor and the limited number of effective therapies. However, advancements in the understanding of the biology of CNSL and the development of novel treatment strategies have improved outcomes for patients with this disease [102]. In 2022, a Chinese expert consensus for the management of primary CNSL has been published, which suggested that patients with R/R primary CNSL can be treated with BTK inhibitors with or without high-dose chemotherapy as re-induction therapy [103]. The evidence of studies on BTK inhibitors in patients with CNSL is summarized in Table 6.
Table 6.
Evidence of BTK inhibitors in patients with CNSL
| Patients type | BTK inhibitor regimens | Efficacy | Study | Phase | N |
|---|---|---|---|---|---|
| TN, R/R CNSL | TN: zanubrutinib + rituximab R/R: zanubrutinib + HD-MTX (methotrexate at 3.5–5.0 g/m2 d1 and cytarabine at 2.0 g every 12 h on d2 and d3, every 21 days per cycle) + rituximab |
TN: ORR CR 100% (5/5) R/R: ORR, CR 60% (3/5) |
Zhang et al. [26] | Retrospective | 10 |
| Orelabrutinib + immunotherapy + chemotherapy + radiotherapy |
TN: ORR 100% (4/4); CR 25% (1/4) R/R: ORR 60% (9/15); CR 26.6% (4/15) |
Wu et al. [104] | Retrospective | 19 | |
| R/R CNSL | Tirabrutinib | ORR: 64%; CR/CRu 34%; PFS: 2.9 months; OS: NR | ONO-4059 [105] | 2 | 44 |
| Ibrutinib | ORR: 59%; CR: 23%; PFS: 4.8 months; OS: 19.2 months | NCT02542514 [107] | 2 | 52 | |
| Zanubrutinib + HD-MTX + rituximab | Achieved CR after adding zanubrutinib for 3 cycles | Cheng et al. [110] | Case report | 1 | |
| R/R CNSL/SCNSL | Ibrutinib + HD-MTX + rituximab | ORR: 80%; CR: 53.3% | NCT02315326 [108] | 1b | 15 |
| R/R CNSL/PVRL | Ibrutinib + DA-TEDDi-R | ORR: 93%; CR: 86% | Lionakis et al. [25] | 1b | 16 |
| R/R CNSL | Orelabrutinib + lenalidomide + rituximab + HD-MTX + TMZ | ORR: 86.7% (13/15); CR: 73.7% (11/13) | Yang et al. [109] | Retrospective | 15 |
BTK, Bruton tyrosine kinase; CNSL, central nervous system lymphoma; SCNSL, secondary CNSL; CR, complete response; DA-TEDDi-R, rituximab, liposomal doxorubicin, temozolomide, etoposide and dexamethasone; HD-MTX, high-dose methotrexate; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PVRL, primary vitreoretinal lymphoma; R/R, relapsed/refractory; TN, treatment naïve
Patients with TN CNSL
A retrospective evaluation of primary CNSL revealed that all patients (100%) achieved a CR. Out of five patients, four patients were treated with zanubrutinib + rituximab whereas one patient was treated with only zanubrutinib [26]. In a retrospective study of patients receiving an orelabrutinib-based regimen, 4 patients with TN CNSL achieved an ORR of 100%, with one patient achieving CR rate of 25%. The study also found that both the 6-month PFS and OS rates were 100% [104].
Patients with R/R CNSL
In 2022, tirabrutinib (ONO-4059) was approved for the treatment of R/R CNSL in Taiwan. In a phase I/II study involving 44 patients with R/R primary CNSL treated with tirabrutinib, ORR was observed in 64% of patients, with 34% achieving CR/unconfirmed CR (CRu), which indicated favorable efficacy of tirabrutinib in patients with R/R primary CNSL [105]. Currently, an open-label, phase II PROSPECT study (NCT04947319) is evaluating the safety and efficacy of tirabrutinib for patients with newly diagnosed or R/R primary CNSL that may provide further insight into tirabrutinib for the treatment of patients with primary CNSL [106].
A retrospective study analyzed the outcomes of five patients with R/R CNSL who received zanubrutinib-containing regimens. Of these patients, 60% (3 out of 5) achieved CR [26]. Similarly, in another study consisting of patients with R/R CNSL who received an orelabrutinib-based regimen, 60% of patients achieved an ORR, with 26.6% (4 patients) achieving CR [104]. Several phase Ib/II studies have demonstrated the effectiveness of ibrutinib-based regimens for the treatment of CNSL. In a phase II study, ibrutinib monotherapy was shown to have a disease control rate of 70%, with 19% of patients achieving a CR. The median PFS and OS were 4.8 months (95% CI: 2.8–12.7) and 19.2 months (95% CI: 7.2–NR), respectively [107]. A phase Ib trail demonstrated 80% ORR when patients were treated with ibrutinib-based regimens. The median PFS for all patients was 9.2 months, and the 1-year OS rate was 71.1% [108]. In a retrospective study, patients with primary CNSL (PCNSL) received orelabrutinib-based regimens and showed that the ORR was 86.7%, with 73.3% of patients achieving CR. Additionally, the study found that circulating tumor DNA (ctDNA) levels in both blood and CSF were closely associated with tumor recurrence and treatment response [109]. A case report described a 53-year-old man with R/R PCNSL who was treated with zanubrutinib and achieved CR [110].
Recommendations
It is recommended that patients with CNSL be treated with BTK inhibitor-based regimens, either alone or in combination with chemotherapy, as a treatment approach for induction/re-induction and the maintenance therapy in both TN and R/R patients (category 2B; Agree: 72%, 13/18).
Waldenström macroglobulinemia
Waldenström macroglobulinemia (WM) is a type of rare lymphoproliferative disorder that is characterized by the abnormal production of monoclonal immunoglobulin M (IgM) protein and the infiltration of lymphoplasmacytic cells into the bone marrow. The B-cell receptor signaling pathway is an important factor in the development of WM, and, hence, BTK inhibitors are a promising therapeutic option for this disease [111]. Overall, BTK inhibitors have expanded the range of treatment options available for patients with WM, making them a well-tolerated and effective treatment option for both newly diagnosed and R/R cases. A phase III trial compared the efficacy of new generation BTK inhibitors, zanubrutinib with ibrutinib, and demonstrated the effectiveness of both for WM with a trend toward better response quality and less toxicity, particularly cardiovascular toxicity associated with zanubrutinib [112]. For acalabrutinib and orelabrutinib, a lack of data exist from head-to-head studies to compare the efficacy or safety profile with the first-generation BTK inhibitor ibrutinib. Further exploration is needed to demonstrate the benefit of acalabrutinib and orelabrutinib. The evidence of studies on BTK inhibitors in patients with WM is summarized in Table 7.
Table 7.
Evidence of BTK inhibitors in patients with WM
| Patients type | Treatment regimen | Efficacy | Study | Phase | N |
|---|---|---|---|---|---|
| Patients with WM (Overall) | |||||
| TN | Ibrutinib + rituximab | PFS: NR (rate 68%); RR: 76%, PR: 45%; VGPR: 29%; CR: 1%; TTNT: NR | iNNOVATE [113] | 3 | 75 |
| R/R | Ibrutinib | RR: 77%; VGPR: 29%; PR: 48% | iNNOVATE [114] | 3 | 31 |
| R/R | Ibrutinib | ORR: 90.5%, MRR: 79.4%, 5-year OS: 87% | NCT01614821 [115] | 2 | 63 |
| TN | Zanubrutinib | MR: 87.5%; VGPR: 33.3%; PR: 54.2% | BGB-3111-AU003 [175] | 1/2 | 24 |
| R/R | Zanubrutinib | MR: 79.6%; VGPR: 49%; PR: 28.6% | BGB-3111-AU003 [175] | 1/2 | 49 |
| TN | Zanubrutinib | MR: 64%; VGPR: 27%; PR: 36% | NCT04052854 [176] | 2 | 11 |
| R/R | Zanubrutinib | MR 90%; VGPR: 43%; PR: 33% | NCT04052854 [176] | 2 | 30 |
| R/R | Zanubrutinib | MR: 69.8%; VGPR: 32.6% | BGB-3111–210 [177] | 2 | 44 |
| TN | Zanubrutinib | MR: 21%; PR: 47%; VGPR: 26% | ASPEN [112] | 3 | 19 |
| R/R | Zanubrutinib | MR: 16%; PR: 49%; VGPR: 29% | ASPEN [112] | 3 | 83 |
| TN | Acalabrutinib | ORR: 93%; MR: 14%; PR: 71%; VGPR: 7% | NCT02180724 [178] | 2 | 14 |
| R/R | Acalabrutinib | ORR: 94%; MR: 15%; PR 47%; VGPR: 32% | NCT02180724 [178] | 2 | 92 |
| R/R | Orelabrutinib | MR: 80.9%; VGPR: 21.3%; PR: 59.6% | ICP-CL-00105 [179] | 2 | 47 |
| WM patients with MYD88 mutation | |||||
| MYD88L265PCXCR4WT | Ibrutinib | ORR: 100%; MRR: 91.2% | NCT01614821 [119] | 2 | 34 |
| R/R: 34; TN: 2 | Acalabrutinib | ORR: 94%; MRR: 78% | NCT02180724 [178] | 2 | 36 |
| MYD88L265PCXCR4WT | Orelabrutinib | MRR: 84.6% | ICP-CL-00105 [179] | 2 | – |
| MYD88 mutation | Zanubrutinib vs. ibrutinib | CR + VGPR: 36.3% vs. 25.3% | ASPEN [180] | 3 | 201 |
| WM patients with MYD88 wild type | |||||
| MYD88WTCXCR4WT | Ibrutinib | ORR: 71.4%; MRR: 28.6% | NCT01614821 [119] | 2 | 7 |
| R/R: 13; TN: 1 | Acalabrutinib | ORR: 79%; MRR: 57% | NCT02180724 [178] | 2 | 14 |
| MYD88WTCXCR4WT | Orelabrutinib | ORR: 25% | ICP-CL-00105 [179] | 2 | – |
| MYD88WT | Zanubrutinib | MRR: 65% (including 34% CR + VGPR) | ASPEN [120] | 3 | 28 |
| WM patients with CXCR4 mutation | |||||
| MYD88MutCXCR4Mut | Ibrutinib | MRR: 68.2%; VGPR: 9.1%; median PFS: 38% | NCT01614821 [115] | 2 | 2 |
| CXCR4 mutation | Zanubrutinib vs. ibrutinib |
VGPR: 21% vs. 10% MRR: 79% vs. 65% |
ASPEN [120] | 3 | 201 |
BTK, Bruton tyrosine kinase; CR, complete response; ORR, overall response rate; OS, overall survival; MR, minor response; MRR, major response rate; NR, not reached; PFS, progression-free survival; PR, partial response; R/R, relapsed/refractory; TN, treatment naïve; TTNT, time to next line of therapy; VGPR, very good partial response; WM, Waldenström macroglobulinemia
Overall patients
Based on the results of several clinical trials, BTK inhibitors have emerged as an effective and well-tolerated treatment option for WM. The iNNOVATE trial, a randomized, double-blind, placebo-controlled study, showed that ibrutinib plus rituximab significantly improved PFS compared with placebo plus rituximab, with a 54-month PFS rate of 68% versus 25%, respectively, in patients with TN WM. Higher response rates (RRs; 76% vs. 31%) were obtained in ibrutinib plus rituximab versus placebo plus rituximab [113]. Similarly, the effectiveness of ibrutinib monotherapy in rituximab-refractory patients was also observed (60 months of PFS: 40%, ORR: 87%, RR: 77%) [114]. A long-term follow-up of ibrutinib in previously treated patients with WM also demonstrated the efficacy of ibrutinib with an ORR of 90.5%, a major response rate (MRR) of 79.4%, and a 5-year OS rate of 87% for all patients [115]. In the ASPEN trial, zanubrutinib demonstrated non-inferiority to ibrutinib with a very good partial response (VGPR) in both TN (26% vs. 17%) and R/R patients (29% vs. 20%) with WM. As zanubrutinib has a higher degree of selectivity, the safety profile also showed differences. Atrial fibrillation and hypertension were reported at greater frequencies with ibrutinib compared with zanubrutinib. As atrial fibrillation is a well-recognized complication of ibrutinib therapy and is relative to an age-matched controlled population, patients appear to be at a continuously increased risk for the development of atrial fibrillation over the course of therapy [112]. Additionally, a multicenter, phase II trial evaluated the activity and safety of acalabrutinib as a single agent and reported an ORR of 93% in both TN and R/R patients with 7% and 33% of TN and R/R patients, respectively achieving a VGPR [116]. These findings suggest that BTK inhibitors, including ibrutinib, zanubrutinib, and acalabrutinib, are highly effective and well-tolerated options for the treatment of patients with WM. So far, only phase II studies have evaluated the efficacy of acalabrutinib in patients with WM; hence, conclusion cannot be drawn on its efficacy in this patient population. Several phase III studies of acalabrutinib in patients with WM are warranted.
Patients with WM having MYD88 mutation
Activating somatic mutation of myeloid differentiation factor 88 (MYD88) is common and well investigated in WM. Mutation of MYD88 might lead to a BTK-mediated activation of NFκB resulting in nuclear translocation and malignant cell growth [117]. The presence of such mutations affects the prognosis and response to targeted therapies, in particular, BTK inhibitors [118].
Patients with R/R WM having MYD88 mutations with wild-type CXCR4 have shown better outcomes associated with ibrutinib monotherapy (ORR: 100%; MRR: 91.2%) [119]. In a phase II trial, out of 36 patients with MYD88L265P mutation, the overall response and major response were reported in 34 (94%) and 28 (78%) patients, respectively, with acalabrutinib monotherapy [116]. The ASPEN, the largest phase III trial with a head-to-head comparison of zanubrutinib and ibrutinib showed higher CR + VGPR rate associated with zanubrutinib than ibrutinib (36.3% vs. 25.3%), demonstrating a long-term safety and better tolerability [120].
Patients with WM having wild-type MYD88
The prognosis of wild-type MYD88 (MYD88WT) tumors is poor [121]. Patients with MYD88WT are known to have a lower response rate (none > 50%) to ibrutinib [122]. In contrast to the favorable outcomes with ibrutinib in patients with MYD88 mutation, the results for ibrutinib-treated patients with MYD88WT tumors were poor (ORR: 71.4%; MRR: 28.6% for patients with MYD88WT and CXCR4WT) [119]. In patients with MYD88WT WM, treatment with orelabrutinib showed an ORR of only 25%, suggesting a poor response even with orelabrutinib in this patient population. Patients with MYD88WT are less likely to benefit from BTK inhibitors than the mutation counterparts. A phase II trial reported an ORR and MRR of 79% and 57%, respectively, with acalabrutinib, which is much lower than those with MYD88 mutation subtypes [116]. Patients with MYD88WT in the ASPEN trial who received zanubrutinib 160 mg twice a day demonstrated a CR with a MRR of 65% (including 34% CR + VGPR), suggesting that zanubrutinib can achieve a high response rate even in patients with MYD88WT [120].
Patients with WM having CXCR4 mutation
Somatic mutations in the C-terminal domain of CXCR4 lead to CXCR4 signaling and are present in 30% to 35% of patients with WM [119]. CXCR4 mutation mostly occur in those with MYD88 mutations but some patients with MYD88WT also express CXCR4 mutations [123]. Patients with WM having CXCR4 mutation is usually associated with a delayed response, fewer major responses, and shorter PFS to BTK inhibitors, particularly ibrutinib [115]. In a head-to-head comparison of zanubrutinib and ibrutinib in the ASPEN trial, zanubrutinib demonstrated better VGPR (21% vs. 10%) and MRR (79% vs. 65%) than ibrutinib in patients with CXCR4 mutation. Similar efficacy in VGPR (45% vs. 31%) was observed in patients with CXCR4WT mutation [120].
Recommendations
BTK inhibitors as a monotherapy or in combination with rituximab are recommended for the treatment of WM (category 1; Agree: 100%, 18/18).
Zanubrutinib is one of the treatment options for patients with MYD88WT (category 2A; Agree: 100%, 18/18).
Zanubrutinib is the preferred treatment option for patients with CXCR4 mutation (category 1; Agree: 89%, 16/18).
Zanubrutinib is recommended as the preferred treatment regimen rather than ibrutinib considering the balance of efficacy and safety, especially for CV events in a head-to-head study (category 1; Agree: 100%, 18/18).
Marginal zone B-cell lymphoma
Marginal zone B-cell lymphoma (MZL) is a subtype of NHL that originates from memory B cells in the marginal zone of lymphoid tissues [124]. It accounts for about 7% of all NHL cases and can affect different organs such as the spleen, lymph nodes, and mucosa-associated lymphoid tissue (MALT). Standard treatments for MZL include chemotherapy, immunotherapy, and radiation therapy, but R/R MZL remains challenging to treat, and there is a need for new treatment options [125]. The evidence of studies on BTK inhibitors in patients with MZL is summarized in Table 8.
Table 8.
Evidence of BTK inhibitors in patients with MZL
| Patients type | BTK inhibitor regimens | Efficacy | Study | Phase | N |
|---|---|---|---|---|---|
| Patients with R/R MZL | Zanubrutinib | ORR: 68.2%; CR rate: 25.8%; median PFS: NR | MAGNOLIA [126] | 2 | 68 |
| Acalabrutinib | ORR: 53%; CR rate: 13%; median PFS: 27.4 months | ACE-LY-003 [127] | 1/2 | 43 | |
| Ibrutinib | ORR: 58%; CR rate: 10%; median PFS: 15.7 months | PCYC-1121 [128] | 2 | 63 |
BTK, Bruton tyrosine kinase; CR, complete response; R/R, relapsed/refractory; MZL, marginal zone B-cell lymphoma; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; R/R, relapsed/refractory
Patients with R/R MZL
The clinical trials, namely MAGNOLIA, ACE-LY-003, and PCYC-1121, have investigated the efficacy of BTK inhibitors in patients with R/R MZL. The MAGNOLIA trial found that the single agent zanubrutinib resulted in a high ORR of 68.2%, with a CR of 25.8 [126]. The ACE-LY-003 trial demonstrated efficacy of acalabrutinib where patients had a median PFS of 27.4 months with achieving 67% of PFS rate at 12 months [127]. Finally, the PCYC-1121 trial showed that single agent ibrutinib had an ORR of 58%, with a median PFS of 15.7 months (95% CI: 12.2–30.4) with better outcomes in patients previously treated with rituximab (ORR: 81%; median PFS: 30.4 months) [128]. These results suggest that BTK inhibitors may be an effective treatment for R/R MZL, and further studies are needed to determine the optimal use of these drugs. In April 2023, ibrutinib was voluntarily withdrawn from the US market as the treatment option for patients with MZL who require systemic therapy and have received at least 1 prior anti-CD20-based therapy [74]. Till date, only zanubrutinib has been approved by the FDA for the treatment of patients with R/R MZL [129, 130].
Recommendations
BTK inhibitor is recommended as one of the treatment options for patients with R/R MZL (category 2A; Agree: 94%, 17/18).
Zanubrutinib is highly recommended considering a better safety profile than ibrutinib, especially in terms of cardiovascular events (category 1; Agree: 89%, 16/18).
Follicular lymphoma
Follicular lymphoma (FL) is the second most common type of indolent NHL [131]. In patients with previously untreated FL, ibrutinib in combination with once-weekly rituximab for 4 weeks demonstrated clinical activity and durable responses with tolerable safety profile. At a median study follow‐up of 34 months in the combination therapy, the ORR was 85% in the first-line FL treatment [132]. An international phase III study (PERSPECTIVE) evaluating the efficacy of ibrutinib plus rituximab versus rituximab plus placebo in TN elderly and/or unfit patients with FL is ongoing [133].
However, unlike other B-cell malignancies, the studies supporting the use of BTK inhibitors for R/R FL is insubstantial, especially as a monotherapy. A phase II study that assessed the efficacy and safety of ibrutinib monotherapy in patients with R/R FL (N = 110) showed ORR of only 20.9%, failing to meet the primary efficacy endpoint for the study [134]. In a phase II ROSEWOOD study, zanubrutinib plus obinutuzumab demonstrated superior PFS over obinutuzumab monotherapy (68.3% vs. 45.8%) in patients with R/R FL, suggesting a beneficial effect associated with BTK inhibitors when given as a combination therapy [135].
Recommendations
Zanubrutinib with obinutuzumab is recommended as one of the treatment options for patients with R/R FL (category 2B; Agree: 83%, 15/18).
Clinical applications of BTK inhibitors and management of AEs
Different lymphoid malignancies have been extensively treated using BTK inhibitors owing to their positive clinical response, improved efficacy, and the ease of administration. However, there are some important factors that need further consideration before starting patients on BTK inhibitor therapy. The presence of comorbidities in patients including bleeding diathesis, the management of surgical procedures as well as incidence of AEs (hypertension and other cardiovascular diseases, liver and kidney dysfunctions) need to be carefully evaluated. Drug–drug and drug–food interactions as well as the presence of infection and vaccination in the patients need to be considered before starting them on BTK inhibitor therapy. It is also necessary to assess the presence of autoimmune diseases in patients before starting BTK inhibitor therapy, because the incidence of autoimmune complications is very common in lymphoid malignancies [136].
Drug–drug interactions
BTK inhibitors have a range of drug–drug interactions with agents that are mainly metabolized by cytochrome P450 3A (CYP3A) pathway; hence, the co-administration of CYP3A inhibitors or inducers with BTK inhibitors should be used with caution. From the information listed in package inserts, the co-administration of ibrutinib, acalabrutinib, and zanubrutinib with a strong or moderate CYP3A inhibitor may increase the plasma concentration of BTK inhibitors. It is recommended to interrupt BTK inhibitor treatment when taking strong CYP3A inhibitors for a short term (such as anti-infectives for ≤ 7 days), and in case of moderate CYP3A inhibitors, patients need to be closely monitored for adverse reactions.
It is recommended to make dietary adjustments in patients who consume grapefruit juice and other foods that interact with the CYP3A enzyme system and might interfere with BTK inhibitor therapy. Thus, a collaborative monitoring of treatment course by the pharmacist and the medical team is required for optimal results during the BTK inhibitor treatment [137].
Acalabrutinib is absorbed less efficiently in patients receiving gastric acid–reducing agents leading to a decrease in the plasma concentration of acalabrutinib [138]. Therefore, it is recommended to avoid a concomitant use of proton pump inhibitors and acalabrutinib [23]. In addition, when using an H2-receptor antagonist (H2RA), acalabrutinib should be taken 2 h before (or 10 h after) receiving H2RA, with an interval of 2 h between antacids and acalabrutinib consumption is suggested [139]. It is notable that many patients with B-cell malignancies remain on gastric acid—reducing agents for a prolonged period of time; hence, there is a possibility of clinically relevant interaction between BTK inhibitors and gastric acid—reducing regimens [20].
Investigation of genetic mutations
For successful treatment of patients with B-cell malignancies, the detection of the mutated or abnormal molecules or genes is necessary to guide the individualized treatment. Gene mutation detection plays an important role in the classification of hematologic tumors and the determination of the etiology and pathogenesis of hematologic diseases. Patients with B-cell malignancies are recommended to undergo genetic examinations for following mutations before the initiation of treatment or in cases of poor prognosis: (1) MCL: detection of TP53 mutation; (2) CLL/SLL: detection of del(11q), del(17p)/TP53 deletion or mutation and IGHV mutation status, and so on; (3) WM: detection of MYD88 L265P and CXCR4 WHIM mutation; (4) DLBCL: detection of MYD88 L265P, CD79b, bcl-6, bcl-2, Notch1/2 and myc mutations [140].
Safety and AEs management
Although BTK inhibitors are generally safe and well tolerated, several studies have shown incidences of unique toxicities that require monitoring for their optimal management in order to achieve the best possible outcomes in patients being treated with BTK inhibitors [141, 142]. Majority of the AEs were of grades 1 to 2. As the incidence of ≥ grade 3 was very low, which could be managed by prolonging the treatment time, the rate of patients discontinuing BTK inhibitor treatment due to AEs was very low. The first-generation BTK inhibitor ibrutinib showed off-target effects due to low specificity. The most common reason for discontinuing ibrutinib involved the incidence of AEs such as atrial fibrillation, bleeding events, arthralgias, rash, diarrhea, and cytopenia with discontinuation or dosage reduction in 12% of patients.
As compared with ibrutinib, new-generation covalent BTK inhibitors such as acalabrutinib and zanubrutinib are more selective in nature, with less off-target activity and better tolerability with lesser AEs. Discontinuation rates were only 9%–11% in patients treated with acalabrutinib. In the ALPINE study, the discontinuation rate due to AEs in the zanubrutinib arm was 7.8%, whereas it was 13% in the ibrutinib arm. The most common AEs after treatment with new-generation covalent BTK inhibitors are neutropenia, thrombocytopenia, rash, bruises, leukopenia, and so on, which can be managed according to the instructions and related guidelines [35, 45]. Headache is the most frequently occurring AE linked with acalabrutinib. Several phase III studies have shown that patients treated with ibrutinib had a higher incidence of atrial fibrillation than those treated with zanubrutinib or acalabrutinib [54, 112]. Neutropenia was found to be the most common AE in patients treated with zanubrutinib, but its occurrence did not lead to a significant increase in infection [34, 143]. The primary AEs associated with BTK inhibitors are included in the following section.
Hemorrhage
Hemorrhage is a commonly occurring AE in patients treated with ibrutinib, whereas its incidence is relatively less in patients treated with the new-generation BTK inhibitors. Grade 3 or higher bleeding events were observed in 2.3% and 1.3% of patients with MCL treated with zanubrutinib and acalabrutinib, respectively, whereas ibrutinib showed a slightly higher incidence rate of 6% [14, 72, 144]. Patients with grade 3 or higher hemorrhage should be permanently discontinued from BTK inhibitors unless the disorder is curable and the risk of rebleeding is acceptable. The increased risk of hemorrhage upon administration of BTK inhibitors may be due to impairment in collagen-induced platelet activation, similar to the effects of aspirin. In ibrutinib-treated and acalabrutinib-treated patients, BTK and TEC kinases are both irreversibly inhibited, and, hence, both are at equal risks of bleeding events [145, 146]. In the ASPEN study, the incidence of grade 3 or higher hemorrhage was 8.9% in the zanubrutinib arm and 10.2% in the ibrutinib arm in patients with WM.
Studies have suggested that co-administration of BTK inhibitors with direct oral anticoagulants, such as rivaroxaban, dabigatran etexilate, and apixaban, and antiplatelet agents may increase the risk of hemorrhage [147]. The assessment of effect of anti-coagulant or antiplatelet agent in addition to ibrutinib in the PCYC-1102 study recorded a major bleeding event in 9% and 4% of patients, respectively [148]. Therefore, a case-to-case risk-versus-benefit profile should be considered while co-administering BTK inhibitor with antiplatelet or anticoagulant therapy with close monitoring of any hemorrhage events. In addition, it is recommended to withhold BTK inhibitor administration for 3–7 days before and after surgery, depending on the type of surgery and the potential risk of a bleeding event.
Thrombocytopenia and neutropenia
Thrombocytopenia and neutropenia were commonly observed in patients with CLL on BTK inhibitor treatment [149]. In the ALPINE study, patients with R/R CLL showed the incidence of grade 3 or higher in 3.4% of patients in the zanubrutinib arm and 5.2% in the ibrutinib arm. Among the patients, 21% in the zanubrutinib arm and 18.2% in the ibrutinib arm experienced grade 3 or higher neutropenia, which may be due to on-target toxicity [34, 143]. Dose interruptions are recommended for first to third occurrences of grade 3 or 4 neutropenia and thrombocytopenia, and dose discontinuation is recommended after the fourth occurrence. The occurrences of neutropenia and thrombocytopenia are caused by various complex mechanisms of immune dysregulation that are a consequence of CLL disease [9].
Infection
Infections were common in patients treated with BTK inhibitors because patients with B-cell malignancy are immunocompromised and at a highly increased risk of infections despite receiving effective therapy. The incidence of infection (of any grade) is observed in > 50% of patients receiving BTK inhibitor treatment [9]. However, most of these infections are grade 1 or 2 and are easily managed without any dose adjustment. In the ALPINE study, the zanubrutinib arm showed 26.5% of grade 3 or higher infections in patients with CLL, whereas the ibrutinib arm showed a slightly higher incidence of 28.1% [150]. Similarly, in the ASPEN study, the zanubrutinib arm demonstrated a 21.8% incidence of grade 3 or higher infections in patients with WM, whereas the ibrutinib arm showed a higher incidence of 27.6% [112]. In the ACE-CL-006 study, acalabrutinib and ibrutinib-treated patients with CLL had comparable incidence rates of grade 3 or higher infections at 30.8% and 30.0%, respectively [54].
The prevalence of opportunistic infections further increases in patients with grade 3 or higher infection; the risk of opportunistic infections such as Aspergillus fumigatus, Pneumocystis jirovecii, and other infections is increased. In case of fever and other symptoms related to infection, the etiology and pathogenic microorganisms should be determined with the help of complete medical examination. Prophylactic treatment should be considered in case of high-risk patients, and these patients should be continuously monitored for infection and treated immediately [136, 151].
Hypertension
Hypertension is a common AE observed in patients treated with BTK inhibitors. In a study, among 562 patients with lymphoid malignancies receiving ibrutinib treatment, 78.3% of patients developed new or worsened hypertension, of which 17.7% were of 3 grade or higher, thereby suggesting ibrutinib treatment to be associated with a substantial increase in the incidence and severity of hypertension [152]. Furthermore, the ACE-CL-001 phase II study reported the long-term follow-up (41 months) results in which 18% patients had hypertension (all grades), 10% of which were of grades 1–2, and 7% were of grade 3 or higher [17]. Thus, the incidence of hypertension in patients treated with ibrutinib was significantly higher as compared with those treated with acalabrutinib. In the ASPEN study, the incidence of any grade and grade 3 or higher hypertension in patients treated with zanubrutinib was significantly lower than those treated with ibrutinib (14.9% vs. 25.5% and 9.9% vs. 24.4%) [112]. Similarly, in the ACE-CL-006 study, the incidence of hypertension in the acalabrutinib arm was lower than that in the ibrutinib arm (8.6% vs. 20.2% and 4.1% vs. 8.7%) [54].
The mechanism responsible for hypertension is proposed to be PI3k/Akt inhibition, thereby downregulating PI3K p110-alpha as well as nitrous oxide synthesis [152, 153]. Patients should be monitored for treatment-emergent hypertension and managed by judicious optimization of baseline hypertension before treatment initiation, require regular monitoring of blood pressure during clinic visits, and need appropriate medical therapy for hypertension. Antihypertensive medications may require dose modification following discontinuation of BTK inhibitor therapy [9, 154].
Atrial fibrillation
Atrial fibrillation has been reported in 6%–10% of untreated patients with CLL, which has been observed to increase with patients’ age [155, 156]. Clinical studies in patients under ibrutinib treatment showed the 2-year incidence rate between 10 and 16% [157]. Unlike first-generation BTK inhibitors, the incidence of atrial fibrillation is negligible in patients treated with the new-generation BTK inhibitors. When patients with WM treated with zanubrutinib and ibrutinib were compared for the safety results in the ASPEN study, the incidence of atrial fibrillation was 2.0% and 15.3%, respectively [112]. Another clinical study, the ALPINE study conducted in patients with CLL/SLL evaluated atrial fibrillation as the secondary endpoint. The results showed that the incidence of atrial fibrillation was 5.2% and 13.3% in patients treated with zanubrutinib and ibrutinib, respectively, thereby underlining a significantly lower atrial fibrillation in the zanubrutinib group [150]. In the ACE-CL-006 study, the incidence of atrial fibrillation was 9.4% in the acalabrutinib arm and 16% in the ibrutinib arm. These results from head-to-head comparison studies demonstrated the superiority of new-generation BTK inhibitors in terms of safety, especially in CV events [54].
Although the mechanism of BTK inhibitors related to atrial fibrillation is still unclear, it is thought that the inhibition of PI3K signaling, which is crucial for cardiac protection under stress that is regulated by BTK and TEC, may play a role in the incidence of atrial fibrillation [153].
Patients with cardiac risk factors, hypertension, and acute infection may have an increased risk of arrhythmia [158], so such patients should be treated with zanubrutinib to reduce the chances of atrial fibrillation. Patients should be monitored regularly for arrhythmias during treatment, and ibrutinib therapy should be withheld in patients with symptoms and/or signs of ventricular tachycardia. Patients presenting with symptoms of arrhythmia (such as palpitations, dizziness, fainting, chest discomfort, or new-onset dyspnea) should be clinically evaluated and should undergo an electrocardiogram as indicated. When atrial fibrillation occurs, the treatment should be adjusted in time. For patients with atrial fibrillation having symptoms that cannot be completely controlled, the drug can be restarted at the initial dose or half the dose after atrial fibrillation is fully controlled, according to the physician’s assessment.
Headache
Headache is one of the most frequently occurring AEs observed in 22%–51% of patients treated with acalabrutinib therapy [17, 45, 159]. The incidence of acalabrutinib treatment-related headaches is usually observed during the initial phase of treatment, which is mild in nature and occurs for a limited duration [160]. In pivotal studies, about 70% of patients showed grade 1 or 2 headaches, during initial cycles (particularly weeks 1–3) [17, 45]. In the ACE-CL-006 study, the incidence of any grade and grade 3 or higher headaches was significantly higher in the acalabrutinib arm than in the ibrutinib arm, at 34.6% versus 20.2% and 1.5% versus 0%, respectively [54]. These headaches can be managed mostly without any medical interventions, or can be effectively treated using acetaminophen or caffeine, while avoiding the use of nonsteroidal anti-inflammatory drugs, if possible. Only 1% of headaches lead to treatment discontinuation [159]. Although mechanism(s) for these headaches is still unclear, it could be caused by calcitonin gene-related peptide (CGRP) agonism, which is of interest, given the new class of migraine medications designed to work by antagonizing CGRP [161].
BTK inhibitor intolerance and resistance
Although treatment with first- and new-generation BTK inhibitors has extensively showed positive clinical response in patients with B-cell malignancies, patients have developed primary and secondary resistance due to drug intolerance leading to treatment discontinuation [162, 163]. The preliminary results of the single-arm, open-label, multicenter, phase II BGB‑3111‑215 study conducted in patients with R/R B-cell malignancies (CLL/SLL, MCL, MZL, or WM; N = 64) who were intolerant to previous BTK inhibitors (ibrutinib and acalabrutinib) showed good efficacy and tolerability to treatment with zanubrutinib. There was no recurrence of AEs in 75% of patients with ibrutinib and acalabrutinib intolerance after receiving zanubrutinib treatment. After zanubrutinib treatment, the incidence of lower grade AEs was observed in 90% and 33% of patients with ibrutinib and acalabrutinib intolerance, respectively. All grade 4 intolerance events and 68.3% of grade 3 intolerance events did not recur after zanubrutinib treatment [164, 165].
In another phase II clinical study, 60 patients with CLL having ibrutinib tolerance were treated with acalabrutinib and tolerance levels of patients during acalabrutinib and ibrutinib treatments were compared. About 40% of patients reported similar AEs in both treatments, whereas 67% of AEs were of lower grade with acalabrutinib than ibrutinib treatment. Further, 57% of AEs observed with ibrutinib treatment did not recur upon treatment with acalabrutinib [166]. Multiple mechanisms responsible for the resistance of BTK inhibitors include mutations in BTK and downstream signaling molecules, such as PLCγ2, CARD11, and BCL10 leading to prolonged and BTK-independent NF-κB activation resulting in tumor cell growth. Further, overactivation of PI3K/Akt/mTOR and the non-canonical NF-κB pathways can also inhibit cancer cell apoptosis. Additionally, overexpression of integrin β1 in the microenvironment in association with activated PI3K pathway also facilitate tumor growth. All these mechanisms cumulatively contribute to the resistance against BTK inhibitors [5].
Future perspectives
The emergence of resistance and toxicity are the major limitations that lead to treatment discontinuation. To improve survival outcomes in patient with BTK inhibitor intolerance, several approaches are being explored. The use of non-covalent reversible BTK inhibitors is currently being investigated. Some representative examples of these third-generation reversible BTK inhibitors include pirtobrutinib (LOXO-305), nemtabrutinib, vecabrutinib, and HMPL-760, which are effective against wild-type and mutant Cys481 malignancies [5]. In a phase I/II BRUIN trial, 276 patients with R/R CLL/SLL received pirtobrutinib and 75% of the patients discontinued prior to BTK inhibitor therapy due to resistance; the ORR was 74%, whereas the median PFS was 19.4 months. Nemtabrutinib also showed similar trend in the BELLWAVE-001 trial, with ORR of 56% and median PFS of 26.3 months among responders, despite a smaller sample size including 57 patients. Additionally, reversible BTK inhibitors such as HBW-3‑10 and HBW-3‑20 are still under development in China. Targeted therapies are being used for preventing the activation of bypassing signaling pathways, such as PI3K inhibitors, BCL-2 inhibitors, SYK and LYN inhibitors, and HSP90 inhibitors [167].
Moreover, combination of existing treatment options might also be a novel treatment option for B-cell malignancies. In the CAPTIVATE-FD, CAPTIVATE-MRD, and GLOW studies, the combination of ibrutinib plus venetoclax was adopted in a fixed treatment duration, MRD patients, and elderly or patients with comorbidities, respectively. In CAPTIVATE-FD, ORR was 97% and CR was 56%. However, in the CAPTIVATE-MRD study, the 3-year DFS was similar in the placebo arm and the ibrutinib combination arm. The efficacy and safety from these trials showed that such all-oral, chemotherapy-free regimen can provide a synergistic and comprehensive disease control.
Recently, the progress in the development of BTK-targeted proteolysis targeting chimeras (PROTACs) has gained interest as an effective and alternate strategy to inhibit BTK activity. PROTACs are structurally composed of three elements, one end is a warhead ligand that binds to the target protein, the other end is a ligand that binds to the E3 ubiquitin ligase, and a linker that couples the two ligands [168]. A preclinical study with UBX-382 exhibited outstanding degradation potency against various mutant BTKs, suggesting the use in patients with recurrent cancers, specifically ABC DLBCL, who have received prior treatment with BTK inhibitors [169]. Four BTK degraders, such as NX-2127 (NCT04830137) and NX-5948 (NCT05131022) from Nurix Therapeutics, HSK-29116 (NCT04861779) and BGB-16673 (NCT05006716) small molecule drugs from Haisco and BeiGene, respectively, have entered clinical trials. PROTACs have great therapeutic potential with their unique advantages, and with the development of technology and in-depth research, PROTACs are expected to provide clinical therapeutic benefits in the near future.
Conclusion
BTK inhibitors such as ibrutinib, zanubrutinib, orelabrutinib, and acalabrutinib, have shown good clinical efficacy in the treatment of various B-cell malignancies with better safety profiles compared with traditional chemotherapy and chemoimmunotherapy regimens, especially for elderly patients or patients who cannot tolerate conventional chemotherapy. The comparison of different BTK inhibitors has gained interest in the recent years. The head-to-head comparison of BTK inhibitors in the ALPINE and ASPEN studies provided guidance for the optimal selection of BTK inhibitors to a certain extent. More studies such as head-to-head comparisons are warranted to guide on the optimal treatment option. The outcomes of patients who progress following BTK inhibitors, especially those with primary resistant disease, are dismal [170, 171]. There are several therapies in trials that will provide valuable information in the future. Patients that are intolerant to the first-generation BTK inhibitors may switch to new-generation BTK inhibitors to improve efficacy and safety. Multiple studies on new BTK inhibitors are ongoing, which may bring more therapeutic options for patients with B-cell malignancies. PROTACs are likely to overcome the resistance and toxicity associated with BTK inhibitors, and future research on the use of PROTACs may provide some evidence for patients with B-cell malignancies. Further progress on the research to provide more therapeutic options are anticipated and the breakthrough progress will be updated.
Supplementary Information
Additional file 1. Table S1. Recommendations questionnaire
Acknowledgements
Not applicable.
Abbreviations
- ABC
Activated B cell
- AE
Adverse event
- ASCT
Autologous stem-cell transplantation
- ATP
Adenosine triphosphate
- AUC
Area under the curve
- BBB
Blood–brain barrier
- BCL-2
B-cell lymphoma 2
- BCR
B-cell receptor
- BR
Bendamustine plus rituximab
- BTK
Bruton tyrosine kinase
- CGRP
Calcitonin gene-related peptide
- CLL
Chronic lymphocytic leukemia
- CNS
Central nervous system
- CNSL
Central nervous system lymphoma
- CR
Complete response
- CRu
Unconfirmed CR
- CSCO
Chinese Society of Clinical Oncology
- CSF
Cerebrospinal fluid
- ctDNA
Circulating tumor DNA
- CYP3A
Cytochrome P450 3A
- DLBCL
Diffuse large B-cell lymphoma
- EFS
Event-free survival
- FCR
Rituximab, fludarabine, and cyclophosphamide
- FDA
Food and Drug Administration
- FL
Follicular lymphoma
- GCB
Germinal center B cell
- H2RA
H2-receptor antagonist
- HL
Hodgkin lymphoma
- HR
Hazard ratio
- IC50
Enzymatic half-maximal inhibitory concentration
- IGHV
And immunoglobulin heavy-chain variable-region gene
- IgM
Immunoglobulin M
- MALT
Mucosa-associated lymphoid tissue
- MCL
Mantle cell lymphoma
- MRD
Minimal residual disease
- MYC
Myelocytomatosis oncogene
- MZL
Marginal zone B-cell lymphoma
- NCCN
National Comprehensive Cancer Network
- NHL
Non-Hodgkin lymphoma
- ORR
Overall response rate
- OS
Overall survival
- PFS
Progression-free survival
- PROTACs
BTK-targeted proteolysis targeting chimeras
- R-CHOP
Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
- R-DHAP
Rituximab, dexamethasone, cytarabine, and cisplatin
- R/R
Relapsed/refractory
- RR
Response rate
- SLL
Small lymphocytic lymphoma
- Tmax
Median time to the peak concentration
- TN
Treatment naïve
- VGPR
Very good partial response
- WM
Waldenström macroglobulinemia
Author contributions
YS, SJW, ZS, DZ, TSYC, HH, LQ, JL, TT, JZ, YPS, WHH, WZ, HSYL, WX, NC, JM, CSC, and EWCT contributed to the conceptualization and formulation of the recommendations and have reviewed and approved the final manuscript.
Funding
The study did not receive any funding support.
Availability of data and materials
All information in this consensus can be found in the additional files and references.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All the authors and members declare that they have no competing interests.
Footnotes
Yuqin Song and Shang-Ju Wu are co-first authors.
The original online version of this article was revised: Country name in affiliations are corrected.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jun Ma, Email: majun0322@126.com.
Cheng-Shyong Chang, Email: cs4816@gmail.com.
Eric Wai Choi Tse, Email: ewctse@hku.hk.
References
- 1.Meng X, Min Q, Wang J-Y. B cell lymphoma. Adv Exp Med Biol. 2020;1254:161–181. doi: 10.1007/978-981-15-3532-1_12. [DOI] [PubMed] [Google Scholar]
- 2.Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135:584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu T, Liu T. Advances in the target therapy of hematological malignancies. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45:642–646. [PubMed] [Google Scholar]
- 4.da Cunha-Bang C, Niemann CU. Targeting Bruton’s tyrosine kinase across B-cell malignancies. Drugs. 2018;78:1653–1663. doi: 10.1007/s40265-018-1003-6. [DOI] [PubMed] [Google Scholar]
- 5.Alu A, Lei H, Han X, Wei Y, Wei X. BTK inhibitors in the treatment of hematological malignancies and inflammatory diseases: mechanisms and clinical studies. J Hematol Oncol. 2022;15:138. doi: 10.1186/s13045-022-01353-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. Guidelines Detail [Internet]. NCCN® Guidelines for B-cell lymphomas. [cited 2023 Aug 31]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1480
- 7.Lymphoma Expert Committee of Chinese Society of Clinical Oncology (CSCO). Consensus of Chinese experts on Bruton tyrosine kinase inhibitors in treatment of B cell malignant tumors. J Leukemia Lymphoma. 2022;31(9):513–526. doi: 10.3760/cma.j.cn115356-20220718-00211. [DOI] [Google Scholar]
- 8.Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipsky A, Lamanna N. Managing toxicities of Bruton tyrosine kinase inhibitors. Hematol Am Soc Hematol Educ Program. 2020;2020:336–345. doi: 10.1182/hematology.2020000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Research C for DE and. FDA grants accelerated approval to pirtobrutinib for relapsed or refractory mantle cell lymphoma. FDA [Internet]. 2023 [cited 2023 May 16]; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pirtobrutinib-relapsed-or-refractory-mantle-cell-lymphoma
- 11.Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:323–332. doi: 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burger JA, Keating MJ, Wierda WG, Hartmann E, Hoellenriegel J, Rosin NY, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15:1090–1099. doi: 10.1016/S1470-2045(14)70335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391:659–667. doi: 10.1016/S0140-6736(17)33108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134:851–859. doi: 10.1182/blood.2019001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu W, Zhou K, Wang T, Yang S, Liu L, Hu Y, et al. Orelabrutinib in relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma patients: Multi-center, single-arm, open-label, phase 2 study. Am J Hematol. 2023;98(4):571–579. doi: 10.1002/ajh.26826. [DOI] [PubMed] [Google Scholar]
- 17.Byrd JC, Wierda WG, Schuh A, Devereux S, Chaves JM, Brown JR, et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: updated phase 2 results. Blood. 2020;135:1204–1213. doi: 10.1182/blood.2018884940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parmar S, Patel K, Pinilla-Ibarz J. Ibrutinib (Imbruvica): a novel targeted therapy for chronic lymphocytic leukemia. P T. 2014;39:483–519. [PMC free article] [PubMed] [Google Scholar]
- 19.Brukinsa® [package insert]. Beijing China: BeiGene Co. Ltd; 2019 November.
- 20.Calquence® [package insert]. AstraZeneca Gaithersburg MD. November 2019.
- 21.Imbruvica [package insert] Sunnyvale, California: Pharmacyclics, Inc. 2014.
- 22.Lovell AR, Jammal N, Bose P. Selecting the optimal BTK inhibitor therapy in CLL: rationale and practical considerations. Ther Adv Hematol. 2022;13:20406207221116576. doi: 10.1177/20406207221116577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CALQUENCE® (acalabrutinib) capsules, for oral use. 2017; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/210259s000lbl.pdf
- 24.Shen J, Liu J. Bruton’s tyrosine kinase inhibitors in the treatment of primary central nervous system lymphoma: a mini-review. Front Oncol. 2022;12:1034668. doi: 10.3389/fonc.2022.1034668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31:833–843.e5. doi: 10.1016/j.ccell.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Li Y, Zhuang Z, Wang W, Wei C, Zhao D, et al. Preliminary evaluation of zanubrutinib-containing regimens in dlbcl and the cerebrospinal fluid distribution of Zanubrutinib: a 13-Case Series. Frontiers in Oncology [Internet]. 2021 [cited 2023 Mar 11];11. Available from: 10.3389/fonc.2021.760405 [DOI] [PMC free article] [PubMed]
- 27.Barf T, Covey T, Izumi R, van de Kar B, Gulrajani M, van Lith B, et al. Acalabrutinib (ACP-196): a covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363:240–252. doi: 10.1124/jpet.117.242909. [DOI] [PubMed] [Google Scholar]
- 28.Guo Y, Liu Y, Hu N, Yu D, Zhou C, Shi G, et al. Discovery of Zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton’s tyrosine kinase. J Med Chem. 2019;62:7923–7940. doi: 10.1021/acs.jmedchem.9b00687. [DOI] [PubMed] [Google Scholar]
- 29.Shadman M, Flinn IW, Levy MY, Porter R, Burke JM, Cultrera JL, et al. Phase 2 study of Zanubrutinib in BTK inhibitor-intolerant patients (Pts) with relapsed/refractory B-cell malignancies. Blood. 2021;138:1410. doi: 10.1182/blood-2021-148544. [DOI] [Google Scholar]
- 30.Tam CS, Ou YC, Trotman J, Opat S. Clinical pharmacology and PK/PD translation of the second-generation Bruton’s tyrosine kinase inhibitor, zanubrutinib. Expert Rev Clin Pharmacol. 2021;14:1329–1344. doi: 10.1080/17512433.2021.1978288. [DOI] [PubMed] [Google Scholar]
- 31.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kay NE, Hampel PJ, Van Dyke DL, Parikh SA. CLL update 2022: a continuing evolution in care. Blood Rev. 2022;54:100930. doi: 10.1016/j.blre.2022.100930. [DOI] [PubMed] [Google Scholar]
- 33.Messmer BT, Albesiano E, Efremov DG, Ghiotto F, Allen SL, Kolitz J, et al. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J Exp Med. 2004;200:519–525. doi: 10.1084/jem.20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alshemmari SH, Siddiqui MA, Pandita R, Osman HY, Cherif H, O’Brien S, et al. Evidence-based management of chronic lymphocytic leukemia: consensus statements from the Gulf region. Acta Haematol. 2023 doi: 10.1159/000531675. [DOI] [PubMed] [Google Scholar]
- 37.Molica S, Rossi M, Allsup D. Consensus statements highlight the need of harmonizing CLL management worldwide. Acta Haematol. 2023 doi: 10.1159/000533349. [DOI] [PubMed] [Google Scholar]
- 38.Tam CS, Brown JR, Kahl BS, Ghia P, Giannopoulos K, Jurczak W, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 2022;23:1031–1043. doi: 10.1016/S1470-2045(22)00293-5. [DOI] [PubMed] [Google Scholar]
- 39.Small S, Ma S. Frontline treatment for chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): targeted therapy vs. chemoimmunotherapy. Curr Hematol Malig Rep. 2021;16:325–335. doi: 10.1007/s11899-021-00637-1. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15:48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shanafelt TD, Wang XV, Kay NE, Hanson CA, O’Brien S, Barrientos J, et al. Ibrutinib–rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381:432–443. doi: 10.1056/NEJMoa1817073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barr PM, Owen C, Robak T, Tedeschi A, Bairey O, Burger JA, et al. Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:3440–3450. doi: 10.1182/bloodadvances.2021006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517–2528. doi: 10.1056/NEJMoa1812836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:43–56. doi: 10.1016/S1470-2045(18)30788-5. [DOI] [PubMed] [Google Scholar]
- 45.Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395:1278–1291. doi: 10.1016/S0140-6736(20)30262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Döhner H, Stilgenbauer S, Benner A, Leupolt E, Kröber A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 47.Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:23–33. doi: 10.1016/j.annonc.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Crombie J, Davids MS. IGHV mutational status testing in chronic lymphocytic leukemia. Am J Hematol. 2017;92:1393–1397. doi: 10.1002/ajh.24808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. doi: 10.1182/blood.V94.6.1848. [DOI] [PubMed] [Google Scholar]
- 50.Guo A, Lu P, Galanina N, Nabhan C, Smith SM, Coleman M, et al. Heightened BTK-dependent cell proliferation in unmutated chronic lymphocytic leukemia confers increased sensitivity to ibrutinib. Oncotarget. 2015;7:4598–4610. doi: 10.18632/oncotarget.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia. 2022;36:1171–1175. doi: 10.1038/s41375-021-01485-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, et al. ASCEND: Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38:2849–2861. doi: 10.1200/JCO.19.03355. [DOI] [PubMed] [Google Scholar]
- 53.Munir T, Brown JR, O’Brien S, Barrientos JC, Barr PM, Reddy NM, et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94:1353–1363. doi: 10.1002/ajh.25638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Byrd JC, Hillmen P, Ghia P, Kater AP, Chanan-Khan A, Furman RR, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39:3441–3452. doi: 10.1200/JCO.21.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown JR, Eichhorst B, Hillmen P, Jurczak W, Kaźmierczak M, Lamanna N, et al. Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2023;388:319–332. doi: 10.1056/NEJMoa2211582. [DOI] [PubMed] [Google Scholar]
- 56.Eichhorst B, Hallek M. Prognostication of chronic lymphocytic leukemia in the era of new agents. Hematol Am Soc Hematol Educ Program. 2016;2016:149–155. doi: 10.1182/asheducation-2016.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edelmann J, Gribben JG. Managing patients with TP53-deficient chronic lymphocytic leukemia. J Oncol Pract. 2017;13:371–377. doi: 10.1200/JOP.2017.023291. [DOI] [PubMed] [Google Scholar]
- 58.Ghia P, Pluta A, Wach M, Lysak D, Šimkovič M, Kriachok I, et al. Acalabrutinib versus investigator’s choice in relapsed/refractory chronic lymphocytic leukemia: final ASCEND trial results. Hemasphere. 2022;6:e801. doi: 10.1097/HS9.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vose JM. Mantle cell lymphoma: 2015 update on diagnosis, risk-stratification, and clinical management. Am J Hematol. 2015;90:739–745. doi: 10.1002/ajh.24094. [DOI] [PubMed] [Google Scholar]
- 60.Li G, Liu X, Chen X. Simultaneous development of zanubrutinib in the USA and China. Nat Rev Clin Oncol. 2020;17:589–590. doi: 10.1038/s41571-020-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romaguera JE, Fayad LE, Feng L, Hartig K, Weaver P, Rodriguez MA, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150:200–208. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- 62.Giné E, de la Cruz F, Jiménez Ubieto A, López Jimenez J, Martín García-Sancho A, Terol MJ, et al. Ibrutinib in combination with rituximab for indolent clinical forms of mantle cell lymphoma (IMCL-2015): a multicenter, open-label, single-arm, Phase II trial. J Clin Oncol. 2022;40:1196–1205. doi: 10.1200/JCO.21.02321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang ML, Jurczak W, Jerkeman M, Trotman J, Zinzani PL, Belada D, et al. Ibrutinib plus bendamustine and rituximab in untreated mantle-cell lymphoma. N Engl J Med. 2022;386:2482–2494. doi: 10.1056/NEJMoa2201817. [DOI] [PubMed] [Google Scholar]
- 64.Huang H. A perspective study of zanubrutinib-based induction and maintenance therapy in young and fit patients with untreated mantle cell lymphoma (BRIDGE) [Internet]. clinicaltrials.gov; 2021 Aug. Report No.: NCT04736914. Available from: https://clinicaltrials.gov/ct2/show/NCT04736914
- 65.BeiGene. A phase 3 randomized, open-label, multicenter study comparing zanubrutinib (BGB-3111) plus rituximab versus bendamustine plus rituximab in patients with previously untreated mantle cell lymphoma who are ineligible for stem cell transplantation [Internet]. clinicaltrials.gov; 2022 Sep. Report No.: NCT04002297. Available from: https://clinicaltrials.gov/ct2/show/NCT04002297
- 66.Dreyling M, Ladetto M, Doorduijn JK, Gine E, Jerkeman M, Mey U, et al. Triangle: autologous transplantation after a Rituximab/Ibrutinib/ara-c containing induction in generalized mantle cell lymphoma—a randomized European MCL Network trial. Blood. 2019;134:2816. doi: 10.1182/blood-2019-127863. [DOI] [Google Scholar]
- 67.Dreyling M, Doorduijn JK, Gine E, Jerkeman M, Walewski J, Hutchings M, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: results from the randomized triangle trial by the European MCL network. Blood. 2022;140:1–3. doi: 10.1182/blood-2022-163018. [DOI] [Google Scholar]
- 68.Goy A, Sinha R, Williams ME, Kalayoglu Besisik S, Drach J, Ramchandren R, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013;31:3688–3695. doi: 10.1200/JCO.2013.49.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goy A, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20:520–525. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.NCCN. NCCN clinical practice guidelines in oncology: B—cell lymphomas. version 1. 2020[EB/OL] . 2021; Available from: https://www.nccn.org/professionals/physician_gls/pdf/b‑cell.pdf
- 71.Zhu J, Ma J. Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for malignant lymphoma 2021 (English version) Chin J Cancer Res. 2021;33:289–301. doi: 10.21147/j.issn.1000-9604.2021.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song Y, Zhou K, Zou D, Zhou J, Hu J, Yang H, et al. Zanubrutinib in relapsed/refractory mantle cell lymphoma: long-term efficacy and safety results from a phase 2 study. Blood. 2022;139:3148–3158. doi: 10.1182/blood.2021014162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song Y, Song Y, Liu L, Zhang M, Li Z, Ji C, et al. Long-term safety and efficacy of orelabrutinib monotherapy in chinese patients with relapsed or refractory mantle cell lymphoma: a multicenter, open-label, phase II study. Blood. 2020;136:1. doi: 10.1182/blood-2020-141781. [DOI] [Google Scholar]
- 74.Update on IMBRUVICA® (ibrutinib) U.S. Accelerated Approvals for Mantle Cell Lymphoma and Marginal Zone Lymphoma Indications. 2023; Available from: https://news.abbvie.com/news/press-releases/
- 75.Zhou K, Zou D, Zhou J, Hu J, Yang H, Zhang H, et al. Zanubrutinib monotherapy in relapsed/refractory mantle cell lymphoma: a pooled analysis of two clinical trials. J Hematol Oncol. 2021;14:167. doi: 10.1186/s13045-021-01174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loxo@Lilly Presents Updated Pirtobrutinib Data from the Phase 1/2 BRUIN Clinical Trial at the 2022 American Society of Hematology Annual Meeting | Eli Lilly and Company [Internet]. [cited 2023 May 16]. Available from: https://investor.lilly.com/news-releases/news-release-details/loxolilly-presents-updated-pirtobrutinib-data-phase-12-bruin
- 77.Stegemann M, Denker S, Schmitt CA. DLBCL 1L—what to expect beyond R-CHOP? Cancers (Basel) 2022;14:1453. doi: 10.3390/cancers14061453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nowakowski GS, Czuczman MS. ABC, GCB, and double-hit diffuse large b-cell lymphoma: does subtype make a difference in therapy selection? Am Soc Clin Oncol Educ Book. 2015;10.14694/EdBook_AM.2015.35.e449. [DOI] [PubMed]
- 79.Younes A, Sehn LH, Johnson P, Zinzani PL, Hong X, Zhu J, et al. Randomized phase iii trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37:1285–1295. doi: 10.1200/JCO.18.02403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sehn LH, Kahl BS, Matasar MJ, Lenz G, Izutsu K, Zhao W, et al. ESCALADE: A phase 3 study of acalabrutinib in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for patients ≤65y with untreated non-germinal center B-cell–like (non-GCB) diffuse large B-cell lymphoma (DLBCL). JCO. 2021;39:TPS7572–TPS7572.
- 81.Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang H, Xiang B, Song Y, Zhang H, Zhao W, Zou D, et al. Zanubrutinib monotherapy for relapsed or refractory non-germinal center diffuse large B-cell lymphoma. Blood Adv. 2022;6:1629–1636. doi: 10.1182/bloodadvances.2020003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang H, Zhou K, Cheng Y, Zhang L, Yang H, Zou L, et al. preliminary safety and efficacy of zanubrutinib in combination with lenalidomide in patients with relapsed/refractory diffuse large B-cell lymphoma. Blood. 2022;140:3750–3752. doi: 10.1182/blood-2022-155979. [DOI] [Google Scholar]
- 85.Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21:922–926. doi: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dyer MJ, De Vos S, Ruan J, Flowers C, Maddocks KJ, Rule S, et al. Acalabrutinib monotherapy in patients (pts) with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) JCO. 2018;36:7547–7547. doi: 10.1200/JCO.2018.36.15_suppl.7547. [DOI] [Google Scholar]
- 87.Aukema SM, Siebert R, Schuuring E, van Imhoff GW, Kluin-Nelemans HC, Boerma E-J, et al. Double-hit B-cell lymphomas. Blood. 2011;117:2319–2331. doi: 10.1182/blood-2010-09-297879. [DOI] [PubMed] [Google Scholar]
- 88.Li S, Lin P, Fayad LE, Lennon PA, Miranda RN, Yin CC, et al. B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t(14;18)(q32;q21): an aggressive disease with heterogeneous histology, germinal center B-cell immunophenotype and poor outcome. Mod Pathol. 2012;25:145–156. doi: 10.1038/modpathol.2011.147. [DOI] [PubMed] [Google Scholar]
- 89.Zhu J, Hong X, Song YQ, Hodkinson B, Balasubramanian S, Wang S, et al. Ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone in patients with previously untreated non-germinal centre B-cell-like diffuse large B-cell lymphoma: A Chinese subgroup analysis of the phase III PHOENIX trial. EJHaem. 2022;3:1154–1164. doi: 10.1002/jha2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Landsburg DJ, Hughes ME, Koike A, Bond D, Maddocks KJ, Guo L, et al. Outcomes of patients with relapsed/refractory double-expressor B-cell lymphoma treated with ibrutinib monotherapy. Blood Adv. 2019;3:132–135. doi: 10.1182/bloodadvances.2018026401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haiyan Yang, Yufu Li, Sung Yong Oh, Jianfeng Zhou, Constantine S. Tam, Yiling Yu, et al. Biomarker Identification in Relapsed/Refractory Non-germinal Center B-cell like Diffuse Large B-cell Lymphoma Treated With Zanubrutinib. Available from: https://library.ehaweb.org/eha/2020/eha25th/293735/haiyan.yang.biomarker.identification.in.relapsed.refractory.non-germinal.html
- 92.MD JC. Efficacy and Safety of Zanubrutinib in combination with R-CHOP in newly diagnosed non-GCB DLBCL patients with double expression: a single-arm, open-label, phase II study [Internet]. clinicaltrials.gov; 2022 Feb. Report No.: NCT05189197. Available from: https://clinicaltrials.gov/ct2/show/NCT05189197
- 93.Bonzheim I, Sander P, Salmerón-Villalobos J, Süsskind D, Szurman P, Gekeler F, et al. The molecular hallmarks of primary and secondary vitreoretinal lymphoma. Blood Adv. 2022;6:1598–1607. doi: 10.1182/bloodadvances.2021004212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilson WH, Wright G, Huang DW, Hodkinson B, Balasubramanian S, Fan Y, et al. Effect of Ibrutinib with R-CHOP chemotherapy in genetic subtypes of DLBCL. Cancer Cell. 2021;39:1643–1653.e3. doi: 10.1016/j.ccell.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beijing InnoCare Pharma Tech Co., Ltd. A Phase III, Randomized, Double-blind, Placebo-controlled, Multicenter Study Evaluating the Efficacy and Safety of Orelabrutinib Plus R-CHOP Versus Placebo Plus R-CHOP in Treatment-naïve Patients With MCD Subtype DLBCL [Internet]. clinicaltrials.gov; 2022 Nov. Report No.: NCT05234684. Available from: https://clinicaltrials.gov/ct2/show/NCT05234684
- 96.Krok-Schoen JL, Fisher JL, Stephens JA, Mims A, Ayyappan S, Woyach JA, et al. Incidence and survival of hematological cancers among adults ages ≥75 years. Cancer Med. 2018;7:3425–3433. doi: 10.1002/cam4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chiappella A, Castellino A, Nicolosi M, Santambrogio E, Vitolo U. Diffuse large B-cell Lymphoma in the elderly: standard treatment and new perspectives. Expert Rev Hematol. 2017;10:289–297. doi: 10.1080/17474086.2017.1305264. [DOI] [PubMed] [Google Scholar]
- 98.Verner E, Johnston A, Pati N, Hawkes E, Lee H-P, Cochrane T, et al. Efficacy of ibrutinib, rituximab and mini-CHOP in very elderly patients with newly diagnosed diffuse large B cell lymphoma: primary analysis of the Australasian Leukaemia & Lymphoma Group NHL29 Study. Blood. 2021;138:304. doi: 10.1182/blood-2021-149019. [DOI] [Google Scholar]
- 99.Xu P-P, Shi Z-Y, Qian Y, Cheng S, Zhu Y, Jiang L, et al. Ibrutinib, rituximab, and lenalidomide in unfit or frail patients aged 75 years or older with de novo diffuse large B-cell lymphoma: a phase 2, single-arm study. Lancet Healthy Longev. 2022;3:e481–e490. doi: 10.1016/S2666-7568(22)00123-4. [DOI] [PubMed] [Google Scholar]
- 100.Weili Z. The Efficacy and Safety of Zanubrutinib, Rituximab and Lenalidomide (ZR2) Versus Rituximab Combined With Low-dose CHOP (R-miniCHOP) in the Treatment of Unfit or Frail de Novo Diffuse Large B-cell Lymphoma Patients Aged Older Than or Equal to 70 Years: A Multicenter, Prospective, Randomized, Open-label, Controlled Trial [Internet]. clinicaltrials.gov; 2022 Dec. Report No.: NCT05179733. Available from: https://clinicaltrials.gov/ct2/show/NCT05179733
- 101.Hoang-Xuan K, Bessell E, Bromberg J, Hottinger AF, Preusser M, Rudà R, et al. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015;16:e322–332. doi: 10.1016/S1470-2045(15)00076-5. [DOI] [PubMed] [Google Scholar]
- 102.Batchelor T, Loeffler JS. Primary CNS lymphoma. J Clin Oncol. 2006;24:1281–1288. doi: 10.1200/JCO.2005.04.8819. [DOI] [PubMed] [Google Scholar]
- 103.Chen T, Liu Y, Wang Y, Chang Q, Wu J, Wang Z, et al. Evidence-based expert consensus on the management of primary central nervous system lymphoma in China. J Hematol Oncol. 2022;15:136. doi: 10.1186/s13045-022-01356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Orelabrutinib-bruton tyrosine kinase inhibitor-based regimens in the treatment of central nervous system lymphoma: a retrospective study | SpringerLink [Internet]. [cited 2023 Mar 11]. Available from: 10.1007/s10637-022-01219-5
- 105.Narita Y, Nagane M, Mishima K, Terui Y, Arakawa Y, Yonezawa H, et al. Phase I/II study of tirabrutinib, a second-generation Bruton’s tyrosine kinase inhibitor, in relapsed/refractory primary central nervous system lymphoma. Neuro Oncol. 2020;23:122–133. doi: 10.1093/neuonc/noaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Study of Tirabrutinib (ONO-4059) in Patients With Primary Central Nervous System Lymphoma (PROSPECT Study). Available from: https://clinicaltrials.gov/ct2/show/NCT04947319
- 107.Soussain C, Choquet S, Blonski M, Leclercq D, Houillier C, Rezai K, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II “proof-of-concept” iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121–130. doi: 10.1016/j.ejca.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 108.Grommes C, Tang SS, Wolfe J, Kaley TJ, Daras M, Pentsova EI, et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood. 2019;133:436–445. doi: 10.1182/blood-2018-09-875732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang C, Cui Y, Ren X, Li M, Yu K, Shen S, et al. Orelabrutinib Combined With Lenalidomide and Immunochemotherapy for Relapsed/Refractory Primary Central Nervous System Lymphoma: A Retrospective Analysis of Case Series. Frontiers in Oncology [Internet]. 2022 [cited 2023 Mar 11];12. Available from: 10.3389/fonc.2022.901797 [DOI] [PMC free article] [PubMed]
- 110.Cheng Q, Wang J, Lv C, Xu J. Successful management of a patient with refractory primary central nervous system lymphoma by zanubrutinib. OTT. 2021;14:3367–3372. doi: 10.2147/OTT.S309408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.How I treat Waldenström macroglobulinemia | Blood | American Society of Hematology [Internet]. [cited 2023 Mar 9]. Available from: https://ashpublications.org/blood/article/126/6/721/34613/How-I-treat-Waldenstrom-macroglobulinemia
- 112.Tam CS, Opat S, D’Sa S, Jurczak W, Lee H-P, Cull G, et al. A randomized phase 3 trial of zanubrutinib vs. ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020;136:2038–2050. doi: 10.1182/blood.2020006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Buske C, Tedeschi A, Trotman J, García-Sanz R, MacDonald D, Leblond V, et al. Ibrutinib plus rituximab versus placebo plus rituximab for Waldenström’s macroglobulinemia: final analysis from the randomized Phase III iNNOVATE Study. J Clin Oncol. 2022;40:52–62. doi: 10.1200/JCO.21.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trotman J, Buske C, Tedeschi A, Matous JV, MacDonald D, Tam CS, et al. Single-agent ibrutinib for rituximab-refractory waldenström macroglobulinemia: final analysis of the substudy of the phase III InnovateTM trial. Clin Cancer Res. 2021;27:5793–5800. doi: 10.1158/1078-0432.CCR-21-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Treon SP, Meid K, Gustine J, Yang G, Xu L, Liu X, et al. Long-term follow-up of Ibrutinib monotherapy in symptomatic, previously treated patients with Waldenström Macroglobulinemia. J Clin Oncol. 2021;39:565–575. doi: 10.1200/JCO.20.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Owen RG, McCarthy H, Rule S, D’Sa S, Thomas SK, Tournilhac O, et al. Acalabrutinib monotherapy in patients with Waldenström macroglobulinemia: a single-arm, multicentre, phase 2 study. Lancet Haematol. 2020;7:e112–e121. doi: 10.1016/S2352-3026(19)30210-8. [DOI] [PubMed] [Google Scholar]
- 117.Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012;367:826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 118.Treon SP, Xu L, Guerrera ML, Jimenez C, Hunter ZR, Liu X, et al. Genomic landscape of Waldenström Macroglobulinemia and its impact on treatment strategies. J Clin Oncol. 2020;38:1198–1208. doi: 10.1200/JCO.19.02314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372:1430–1440. doi: 10.1056/NEJMoa1501548. [DOI] [PubMed] [Google Scholar]
- 120.Tam CSL, Garcia-Sanz R, Opat S, D’Sa S, Jurczak W, Lee H-P, et al. ASPEN: Long-term follow-up results of a phase 3 randomized trial of zanubrutinib (ZANU) versus ibrutinib (IBR) in patients with Waldenström macroglobulinemia (WM) JCO. 2022;40:7521–7521. doi: 10.1200/JCO.2022.40.16_suppl.7521. [DOI] [Google Scholar]
- 121.Treon SP, Gustine J, Xu L, Manning RJ, Tsakmaklis N, Demos M, et al. MYD88 wild-type Waldenstrom macroglobulinaemia: differential diagnosis, risk of histological transformation, and overall survival. Br J Haematol. 2018;180:374–380. doi: 10.1111/bjh.15049. [DOI] [PubMed] [Google Scholar]
- 122.Treon SP, Xu L, Hunter Z. MYD88 mutations and response to ibrutinib in Waldenström’s Macroglobulinemia. N Engl J Med. 2015;373:584–586. doi: 10.1056/NEJMc1506192. [DOI] [PubMed] [Google Scholar]
- 123.Xu L, Hunter ZR, Tsakmaklis N, Cao Y, Yang G, Chen J, et al. Clonal architecture of CXCR4 WHIM-like mutations in Waldenström macroglobulinaemia. Br J Haematol. 2016;172:735–744. doi: 10.1111/bjh.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.MALT lymphoma: from morphology to molecules | Nature Reviews Cancer [Internet]. [cited 2023 Mar 9]. Available from: https://www.nature.com/articles/nrc1409 [DOI] [PubMed]
- 125.Jp A, Bs K. Current Treatments in Marginal Zone Lymphoma. Oncology (Williston Park, NY) [Internet]. 2022 [cited 2023 Mar 9];36. Available from: https://pubmed.ncbi.nlm.nih.gov/35436062/ [DOI] [PubMed]
- 126.Opat S, Tedeschi A, Linton K, McKay P, Hu B, Chan H, et al. The MAGNOLIA trial: zanubrutinib, a next-generation bruton tyrosine kinase inhibitor, demonstrates safety and efficacy in relapsed/refractory marginal zone lymphoma. Clin Cancer Res. 2021;27:6323–6332. doi: 10.1158/1078-0432.CCR-21-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Strati P, Coleman M, Champion R, Ma S, Patti C, Levy MY, et al. A phase 2, multicentre, open-label trial (ACE-LY-003) of acalabrutinib in patients with relapsed or refractory marginal zone lymphoma. Br J Haematol. 2022;199:76–85. doi: 10.1111/bjh.18368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Noy A, de Vos S, Coleman M, Martin P, Flowers CR, Thieblemont C, et al. Durable ibrutinib responses in relapsed/refractory marginal zone lymphoma: long-term follow-up and biomarker analysis. Blood Adv. 2020;4:5773–5784. doi: 10.1182/bloodadvances.2020003121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Update on IMBRUVICA® (ibrutinib) U.S. Accelerated Approvals for Mantle Cell Lymphoma and Marginal Zone Lymphoma Indications | Johnson & Johnson [Internet]. Content Lab U.S. [cited 2023 Apr 12]. Available from: https://www.jnj.com/update-on-imbruvica-ibrutinib-u-s-accelerated-approvals-for-mantle-cell-lymphoma-and-marginal-zone-lymphoma-indications
- 130.Research C for DE and. FDA D.I.S.C.O. Burst Edition: FDA approvals of Brukinsa (zanubrutinib), for adult patients with relapsed or refractory marginal zone lymphoma, and Exkivity (mobocertinib) for adult patients with locally advanced or metastatic non-small cell lung cancer with epidermal growth factor receptor exon 20 insertion mutations. FDA [Internet]. 2022 [cited 2023 Apr 12]; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approvals-brukinsa-zanubrutinib-adult-patients-relapsed-or-refractory
- 131.Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90:790–795. doi: 10.1002/ajh.24086. [DOI] [PubMed] [Google Scholar]
- 132.Fowler NH, Nastoupil L, De Vos S, Knapp M, Flinn IW, Chen R, et al. The combination of ibrutinib and rituximab demonstrates activity in first-line follicular lymphoma. Br J Haematol. 2020;189:650–660. doi: 10.1111/bjh.16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fowler NH, Flinn I, Rule S, Chen RW, Kwei L, Beaupre DM, et al. A multicenter, randomized, double-blind, placebo-controlled phase III study of the Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib, in combination with rituximab versus placebo in combination with rituximab in patients with treatment-naive follicular lymphoma (PERSPECTIVE). JCO. 2017;35:TPS7576–TPS7576.
- 134.Gopal AK, Schuster SJ, Fowler NH, Trotman J, Hess G, Hou J-Z, et al. Ibrutinib as treatment for patients with relapsed/refractory follicular lymphoma: results from the open-label, multicenter, phase II DAWN study. J Clin Oncol. 2018;36:2405–2412. doi: 10.1200/JCO.2017.76.8853. [DOI] [PubMed] [Google Scholar]
- 135.Zinzani PL, Mayer J, Auer R, Bijou F, de Oliveira AC, Flowers C, et al. Zanubrutinib plus obinutuzumab (ZO) versus obinutuzumab (O) monotherapy in patients (pts) with relapsed or refractory (R/R) follicular lymphoma (FL): Primary analysis of the phase 2 randomized ROSEWOOD trial. JCO. 2022;40:7510–7510. doi: 10.1200/JCO.2022.40.16_suppl.7510. [DOI] [Google Scholar]
- 136.Brown JR. How I treat CLL patients with ibrutinib. Blood. 2018;131:379–386. doi: 10.1182/blood-2017-08-764712. [DOI] [PubMed] [Google Scholar]
- 137.Fancher KM, Pappacena JJ. Drug interactions with Bruton’s tyrosine kinase inhibitors: clinical implications and management. Cancer Chemother Pharmacol. 2020;86:507–515. doi: 10.1007/s00280-020-04137-6. [DOI] [PubMed] [Google Scholar]
- 138.Sharma S, Pepin X, Burri H, Zheng L, Kuptsova-Clarkson N, de Jong A, et al. Bioequivalence and relative bioavailability studies to assess a new acalabrutinib formulation that enables coadministration with proton-pump inhibitors. Clin Pharmacol Drug Dev. 2022;11:1294–1307. doi: 10.1002/cpdd.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patel D, Bertz R, Ren S, Boulton DW, Någård M. A systematic review of gastric acid-reducing agent-mediated drug-drug interactions with orally administered medications. Clin Pharmacokinet. 2020;59:447–462. doi: 10.1007/s40262-019-00844-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378:1396–1407. doi: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smolewski P, Robak T. Current treatment of refractory/relapsed chronic lymphocytic leukemia: a focus on novel drugs. AHA. 2021;144:365–379. doi: 10.1159/000510768. [DOI] [PubMed] [Google Scholar]
- 142.Robak T, Witkowska M, Smolewski P. The role of Bruton’s kinase inhibitors in chronic lymphocytic leukemia: current status and future directions. Cancers. 2022;14:771. doi: 10.3390/cancers14030771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu W, Yang S, Zhou K, Pan L, Li Z, Zhou J, et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. J Hematol Oncol. 2020;13:48. doi: 10.1186/s13045-020-00884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rule S, Jurczak W, Jerkeman M, Rusconi C, Trneny M, Offner F, et al. Ibrutinib versus temsirolimus: 3-year follow-up of patients with previously treated mantle cell lymphoma from the phase 3, international, randomized, open-label RAY study. Leukemia. 2018;32:1799–1803. doi: 10.1038/s41375-018-0023-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Series J, Garcia C, Levade M, Viaud J, Sié P, Ysebaert L, et al. Differences and similarities in the effects of ibrutinib and acalabrutinib on platelet functions. Haematologica. 2019;104:2292–2299. doi: 10.3324/haematol.2018.207183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Goldmann L, Duan R, Kragh T, Wittmann G, Weber C, Lorenz R, et al. Oral Bruton tyrosine kinase inhibitors block activation of the platelet Fc receptor CD32a (FcγRIIA): a new option in HIT? Blood Adv. 2019;3:4021–4033. doi: 10.1182/bloodadvances.2019000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chai-Adisaksopha C, Crowther M, Isayama T, Lim W. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: a systematic review and meta-analysis. Blood. 2014;124:2450–2458. doi: 10.1182/blood-2014-07-590323. [DOI] [PubMed] [Google Scholar]
- 148.Jones JA, Hillmen P, Coutre S, Tam C, Furman RR, Barr PM, et al. Use of anticoagulants and antiplatelet in patients with chronic lymphocytic leukaemia treated with single-agent ibrutinib. Br J Haematol. 2017;178:286–291. doi: 10.1111/bjh.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moreno C, Hodgson K, Ferrer G, Elena M, Filella X, Pereira A, et al. Autoimmune cytopenia in chronic lymphocytic leukemia: prevalence, clinical associations, and prognostic significance. Blood. 2010;116:4771–4776. doi: 10.1182/blood-2010-05-286500. [DOI] [PubMed] [Google Scholar]
- 150.Hillmen P, Eichhorst B, Brown JR, Lamanna N, O’Brien SM, Tam CS, et al. Zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma: interim analysis of a randomized phase III trial. J Clin Oncol. 2023;41:1035–1045. doi: 10.1200/JCO.22.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rogers KA, Mousa L, Zhao Q, Bhat SA, Byrd JC, El Boghdadly Z, et al. Incidence of opportunistic infections during ibrutinib treatment for B-cell malignancies. Leukemia. 2019;33:2527–2530. doi: 10.1038/s41375-019-0481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dickerson T, Wiczer T, Waller A, Philippon J, Porter K, Haddad D, et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;134:1919–1928. doi: 10.1182/blood.2019000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McMullen JR, Boey EJH, Ooi JYY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124:3829–3830. doi: 10.1182/blood-2014-10-604272. [DOI] [PubMed] [Google Scholar]
- 154.O’Brien SM, Brown JR, Byrd JC, Furman RR, Ghia P, Sharman JP, et al. Monitoring and managing BTK inhibitor treatment-related adverse events in clinical practice. Frontiers in Oncology [Internet]. 2021 [cited 2023 Mar 10];11. Available from: 10.3389/fonc.2021.720704 [DOI] [PMC free article] [PubMed]
- 155.Aronow WS, Banach M. Atrial fibrillation: the new epidemic of the ageing world. J Atr Fibrillation. 2009;1:154. doi: 10.4022/jafib.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shanafelt TD, Parikh SA, Noseworthy PA, Goede V, Chaffee KG, Bahlo J, et al. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL) Leuk Lymphoma. 2017;58:1630–1639. doi: 10.1080/10428194.2016.1257795. [DOI] [PubMed] [Google Scholar]
- 157.Ganatra S, Sharma A, Shah S, Chaudhry GM, Martin DT, Neilan TG, et al. Ibrutinib-Associated Atrial Fibrillation. JACC: Clin Electrophysiol. 2018;4:1491–1500. doi: 10.1016/j.jacep.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 158.Kong KA, Jung S, Yu M, Park J, Kang IS. Association between cardiovascular risk factors and the severity of coronavirus disease 2019: Nationwide Epidemiological Study in Korea. Front Cardiovasc Med. 2021;8:732518. doi: 10.3389/fcvm.2021.732518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. JCO. 2020;38:2849–2861. doi: 10.1200/JCO.19.03355. [DOI] [PubMed] [Google Scholar]
- 160.Dolan S, Christofides A, Doucette S, Shafey M. Highlights from ASCO 2020: updates on the treatment of chronic lymphocytic leukemia. Curr Oncol. 2020;27:420–432. doi: 10.3747/co.27.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Carmine Belin A, Ran C, Edvinsson L. Calcitonin gene-related peptide (CGRP) and cluster headache. Brain Sci. 2020;10:30. doi: 10.3390/brainsci10010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burger JA, Barr PM, Robak T, Owen C, Ghia P, Tedeschi A, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34:787–798. doi: 10.1038/s41375-019-0602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Epperla N, Shana’ah AY, Jones D, Christian BA, Ayyappan S, Maddocks K, et al. Resistance mechanism for ibrutinib in marginal zone lymphoma. Blood Adv. 2019;3:500–2. [DOI] [PMC free article] [PubMed]
- 164.Shadman M, Sharman JP, Levy MY, Misleh J, Zafar SF, Freeman BB, et al. Phase 2 study of zanubrutinib in patients with relapsed/refractory B-cell malignancies intolerant to ibrutinib/acalabrutinib. Blood. 2020;136:51–52. doi: 10.1182/blood-2020-134621. [DOI] [Google Scholar]
- 165.Shadman M, Sharman JP, Levy MY, Porter R, Zafar SF, Burke JM, et al. Preliminary results of the phase 2 study of zanubrutinib in patients with previously treated B-cell malignancies intolerant to ibrutinib and/or acalabrutinib. JCO. 2021;39:e19506–e19506. doi: 10.1200/JCO.2021.39.15_suppl.e19506. [DOI] [Google Scholar]
- 166.Rogers KA, Thompson PA, Allan JN, Coleman M, Sharman JP, Cheson BD, et al. Phase II study of acalabrutinib in ibrutinib-intolerant patients with relapsed/refractory chronic lymphocytic leukemia. Haematologica. 2021;106:2364–2373. doi: 10.3324/haematol.2020.272500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang H, Zhang W, Yang J, Zhou K. The resistance mechanisms and treatment strategies of BTK inhibitors in B-cell lymphoma. Hematol Oncol. 2021;39:605–615. doi: 10.1002/hon.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gadd MS, Testa A, Lucas X, Chan K-H, Chen W, Lamont DJ, et al. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol. 2017;13:514–521. doi: 10.1038/nchembio.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lim YS, Yoo S-M, Patil V, Kim HW, Kim H-H, Suh B, et al. Orally bioavailable BTK PROTAC active against wild-type and C481 mutant BTKs in human lymphoma CDX mouse models. Blood Adv. 2023;7:92–105. doi: 10.1182/bloodadvances.2022008121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Epperla N, Zhao Q, Chowdhury SM, Shea L, Moyo TK, Reddy N, et al. Predictive factors and outcomes for ibrutinib in relapsed/refractory marginal zone lymphoma: a multicenter cohort study. J Hematol Oncol. 2022;15:96. doi: 10.1186/s13045-022-01316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Epperla N, Hamadani M, Cashen AF, Ahn KW, Oak E, Kanate AS, et al. Predictive factors and outcomes for ibrutinib therapy in relapsed/refractory mantle cell lymphoma-a “real world” study. Hematol Oncol. 2017;35:528–535. doi: 10.1002/hon.2380. [DOI] [PubMed] [Google Scholar]
- 172.Bennett R, Anderson MA, Seymour JF. Unresolved questions in selection of therapies for treatment-naïve chronic lymphocytic leukemia. J Hematol Oncol. 2023;16:72. doi: 10.1186/s13045-023-01469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cai Q, Xia Y, Liu P, Zhang Y, Huang H, Gao Y, et al. Frontline treatment with zanubrutinib plus rituximab (ZR) followed by short course R-Dhaox shows potent efficacy in patients with mantle cell lymphoma (MCL) - preliminary results of phase II CHESS clinical trial. Blood. 2022;140:9356–9357. doi: 10.1182/blood-2022-166237. [DOI] [Google Scholar]
- 174.Johnson PWM, Balasubramanian S, Hodkinson B, Shreeve SM, Sun S, Srinivasan S, et al. Clinical impact of ibrutinib plus R-CHOP in untreated DLBCL coexpressing BCL2 and MYC in the phase 3 PHOENIX trial. Blood Adv. 2023;7:2008–2017. doi: 10.1182/bloodadvances.2022009389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Trotman J, Opat S, Gottlieb D, Simpson D, Marlton P, Cull G, et al. Zanubrutinib for the treatment of patients with Waldenström macroglobulinemia: 3 years of follow-up. Blood. 2020;136:2027–2037. doi: 10.1182/blood.2020006449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Castillo JJ, Kingsley EC, Narang M, Yimer HA, Dasanu CA, Melear JM, et al. Zanubrutinib in patients with treatment-naïve or relapsed/refractory Waldenström macroglobulinemia: An expanded-access study of 50 patients in the United States. EJHaem. 2023;4:301–304. doi: 10.1002/jha2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.An G, Zhou D, Zhao W, Zhou K, Li J, Zhou J, et al. Safety and efficacy of the Bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) in patients with WaldenstrC6m macroglobulinemia from a phase 2 trial. Blood. 2020;136:42–43. doi: 10.1182/blood-2020-136783. [DOI] [Google Scholar]
- 178.Owen R, McCarthy H, Rule S, D’Sa S, Thomas SK, Forconi F, et al. Acalabrutinib in patients (pts) with Waldenström macroglobulinemia (WM) JCO. 2018;36:7501–7501. doi: 10.1200/JCO.2018.36.15_suppl.7501. [DOI] [Google Scholar]
- 179.Cao X-X, Jin J, Fu C-C, Yi S-H, Zhao W-L, Sun Z-M, et al. Evaluation of orelabrutinib monotherapy in patients with relapsed or refractory Waldenström’s macroglobulinemia in a single-arm, multicenter, open-label, phase 2 study. EClinicalMedicine. 2022;52:101682. doi: 10.1016/j.eclinm.2022.101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dimopoulos MA, Opat S, D’Sa S, Jurczak W, Lee H-P, Cull G, et al. Zanubrutinib versus ibrutinib in symptomatic Waldenström macroglobulinemia: final analysis from the randomized phase III ASPEN study. J Clin Oncol. 2023;JCO2202830. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Recommendations questionnaire
Data Availability Statement
All information in this consensus can be found in the additional files and references.