Abstract
The advancement of infectious disease diagnostics, along with studies devoted to infections caused by gram-negative and gram-positive bacteria, is a top scientific priority of the Antibacterial Resistance Leadership Group (ARLG). Diagnostic tests for infectious diseases are rapidly evolving and improving. However, the availability of rapid tests designed to determine antibacterial resistance or susceptibility directly in clinical specimens remains limited, especially for gram-negative organisms. Additionally, the clinical impact of many new tests, including an understanding of how best to use them to inform optimal antibiotic prescribing, remains to be defined. This review summarizes the recent work of the ARLG toward addressing these unmet needs in the diagnostics field and describes future directions for clinical research aimed at curbing the threat of antibiotic-resistant bacterial infections.
Keywords: diagnostics, bacterial infections, antibacterial resistance
Accurate diagnostics are a critical part of the fight against antibacterial resistance. The Antibacterial Resistance Leadership Group has prioritized support for the development and evaluation of new tests or test strategies designed to optimize use of antibiotics and improve patient outcomes.
Robust diagnostic capabilities are an essential component of effective strategies aimed at preventing, diagnosing, and treating antibiotic-resistant bacterial infections. However, relatively few tests are currently capable of rapidly detecting resistant pathogens directly from clinical specimens. Culture with phenotypic susceptibility testing remains the microbiologic standard for many common bacterial infections, but these methods can be labor intensive and are often hampered by limited sensitivity and slow turn-around-time to results. Thus, the Antibacterial Resistance Leadership Group (ARLG) has identified infectious disease diagnostics as 1 of 3 priority areas within their overarching scientific agenda. Since its inception, the ARLG has fostered advances in the field of infectious disease diagnostics by supporting novel test development, conducting pivotal studies that define optimal diagnostic strategies, and enabling regulatory approval of new tests or test indications that address unmet clinical needs. The priority has been to invest in critical research that would not otherwise be possible or would be unlikely to occur without ARLG involvement.
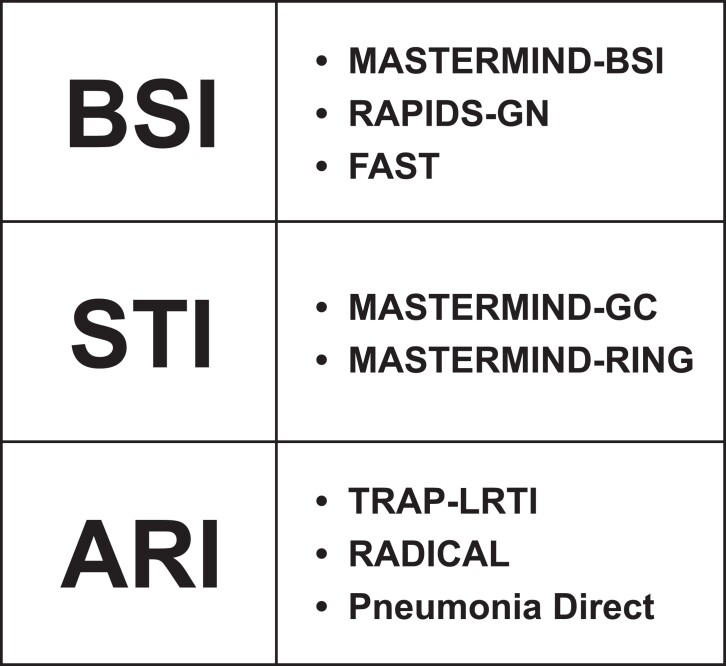
Table 1 summarizes studies that are either new or have been completed since the last ARLG diagnostics update in Clinical Infectious Diseases in 2017 [1]. The current portfolio of diagnostic studies addresses important unmet diagnostic needs for common infectious diseases, including bacterial bloodstream infections (BSIs), sexually transmitted infections, and respiratory tract infections (Figure 1). This article highlights several novel approaches to diagnostics research pioneered by the ARLG, including the development of an adaptable study design for the evaluation of multiple tests in parallel, assessments of the patient-level impact of rapid organism identification with antibacterial resistance (AR) detection, and evaluation of novel pathogen- and host-based diagnostics for which a perfect gold standard for comparison does not exist.
Table 1.
List of Recently Completed and New Antibiotic Leadership Group Diagnostic Studies
| Study | Primary Objective | Design | Status |
|---|---|---|---|
| MASTERMIND-GC | Determine test characteristics of multiple NAATs for the diagnosis of extragenital gonorrhea and chlamydia to enable FDA clearance for a new testing indication (ie, new specimen type) | Observational | Complete |
| MASTERMIND-RING | Determine test characteristics of multiple NAATs for the detection of ciprofloxacin-resistant versus -sensitive gonorrhea directly in clinical specimens to enable FDA clearance of new tests | Observational | Design phase |
| MASTERMIND-BSI | Determine test characteristics of multiple tests for the direct detection of bacterial bloodstream infection to enable FDA clearance of new tests | Observational | Study set-up |
| RAPIDS-GN | Assess the impact of a rapid organism identification, with or without rapid phenotypic AST, from positive BCs on antibiotic prescribing and patient outcomes | Interventional | Complete |
| FAST | Assess clinical outcomes among patients with GN bloodstream infections who have rapid phenotypic AST versus standard culture and AST from BCs in areas with a high prevalence of AR | Interventional | Study set-up |
| TRAP-LRTI | Compare outcomes for patients with signs of non-pneumonia LRTI and a low PCT level who are treated with azithromycin or placebo | Interventional | Complete |
| RADICAL-2 | Determine the test characteristics of a novel host gene expression profile differentiating bacterial from viral ARIs | Observational | Complete |
| RADICAL-3 | Determine the clinical impact of the rapid host-based diagnostic on antibacterial use and clinical outcomes among patients with ARI | Interventional | Design phase |
| RADICAL-510(k) | Determine the test characteristics of the host gene expression profile for differentiating bacterial from viral ARIs in an FDA registrational trial | Observational | Design phase |
| PDP | Assess the accuracy and predictive of pathogen- and host-directed tests for an adjudicated diagnosis of VAP | Observational | Study set-up |
| REPORT-ABC | Evaluate community and academic hospital reporting practices for AR markers included in rapid molecular BC identification panels | Survey | Complete |
Abbreviations: AR, antimicrobial resistance; ARI, acute respiratory illness; AST, antibiotic susceptibility testing; BC, blood culture; FAST, Fast Antibiotic Susceptibility Testing for Gram-negative Bacteremia; FDA, US Food and Drug Administration; GN, gram-negative; LRTI, lower respiratory tract infection; MASTERMIND-BSI, Master Protocol for Evaluating Multiple Infection Diagnostics for Rapid Detection of Bloodstream Infection; MASTERMIND-GC, Master Protocol for Evaluating Multiple Infection Diagnostics–Gonorrhoeae and Chlamydia Testing of Extragenital Specimens; MASTERMIND-RING, Master Protocol for Evaluating Multiple Infection Diagnostics–Resistant Neisseria gonorrhoeae; NAAT, nucleic acid amplification test; PCT, procalcitonin; PDP, Pneumonia Direct Pilot; RADICAL, Rapid Diagnostics in Categorizing Acute Lung Infections; RAPIDS-GN, Rapid Identification and Phenotypic Susceptibility Testing for Gram-negative Bacteremia; REPORT-ABC, Reporting of Antimicrobial Resistance from Blood Cultures; TRAP-LRTI, Targeted Reduction of Antibiotics using Procalcitonin in Lower Respiratory Tract Infection; VAP, ventilator-associated pneumonia.
Figure 1.
ARLG diagnostic studies focused on the detection of bloodstream, sexually transmitted, and respiratory tract infections. Abbreviations: ARI, acute respiratory illness; ARLG, Antibacterial Resistance Leadership Group; BSI, bloodstream infection; FAST, Fast Antibiotic Susceptibility Testing for Gram-negative Bacteremia; MASTERMIND-BSI, Master Protocol for Evaluating Multiple Infection Diagnostics for Rapid Detection of Bloodstream Infection; MASTERMIND-GC, Master Protocol for Evaluating Multiple Infection Diagnostics–Gonorrhoeae and Chlamydia Testing of Extragenital Specimens; MASTERMIND-RING, Master Protocol for Evaluating Multiple Infection Diagnostics–Resistant Neisseria gonorrhoeae; RADICAL, Rapid Diagnostics in Categorizing Acute Lung Infections; RAPIDS-GN, Rapid Identification and Phenotypic Susceptibility Testing for Gram-negative Bacteremia; STI, sexually transmitted infection; TRAP-LRTI, Targeted Reduction of Antibiotics using Procalcitonin in Lower Respiratory Tract Infection.
MASTER PROTOCOL FOR EVALUATING MULTIPLE INFECTION DIAGNOSTICS (MASTERMIND)
MASTERMIND is an adaptable platform created by the ARLG to facilitate regulatory approvals for new diagnostic tests. The MASTERMIND approach uses a single patient's sample(s) to simultaneously evaluate the performance of multiple different assays. This unique strategy helps overcome many of the obstacles that diagnostic companies face when seeking device clearance from the US Food and Drug Administration (FDA) by providing logistical coordination of specimen collection and testing with economies of scale. In addition, the new tests can be compared with one another to assess overall agreement and diagnostic accuracy.
MASTERMIND-GC
The MASTERMIND approach was used to evaluate the performance of assays designed to detect Neisseria gonorrhoeae (NG) and Chlamydia trachomatis (MASTERMIND-GC) in extragenital specimens [1, 2]. The original MASTERMIND-GC study evaluated 3 nucleic acid amplification tests (NAATs) in a cross-sectional study of 2598 participants [3]. Extragenital gonococcal infections are important contributors to disease transmission and the spread of AR [4–8]. Prior to MASTERMIND-GC, however, no NAATs had clearance from the FDA for use with noncervical or urethral specimens. The lack of FDA-cleared tests limited access to testing despite public health guidelines recommending NAAT-based pharyngeal and rectal screening for at-risk patients [9, 10]. MASTERMIND-GC generated key clinical data to support FDA clearance of 2 commercially available NAATs with indications for use with extragenital specimens [11]. Beyond fulfilling a critical public health and clinical need, the MASTERMIND program brought together multiple stakeholders, including industry partners, governmental agencies, and academic study sites, in a productive and efficient alliance.
MASTERMIND–Ciprofloxacin Resistance in Neisseria gonorrhoeae (MASTERMIND-RING)
Building on the success of MASTERMIND-GC, the ARLG is currently in the planning phases of MASTERMIND-RING, a master protocol designed to evaluate multiple molecular tests to predict ciprofloxacin resistance or susceptibility in NG. Gonorrhea is the second most reported bacterial communicable disease [9], and drug-resistant NG is an urgent AR threat, yet no FDA-cleared tests are currently available that predict drug resistance directly from clinical specimens. This has led the Centers for Disease Control and Prevention to recommend only parenteral ceftriaxone for gonorrhea therapy [9]. However, ceftriaxone is a large intramuscular injection not suitable for expedited partner therapy or for those with cephalosporin allergies. Moreover, ceftriaxone-nonsusceptible NG strains have now been reported [12], threatening this mainstay of therapy.
An FDA-cleared diagnostic test that quickly rules out ciprofloxacin-resistant NG directly from clinical specimens would provide clinicians with an oral therapeutic alternative to parenteral ceftriaxone. Ciprofloxacin resistance and susceptibility in NG can be determined by identifying a mutation in gyrA [13]. The presence of a wildtype gyrA gene predicts ciprofloxacin susceptibility and identifies patients who will achieve clinical cure with a single dose of oral ciprofloxacin [14]. In MASTERMIND-RING, multiple investigational NAATs will be compared with a reference standard for gyrA sequencing. The goal is to generate clinical data that will lead to successful FDA 510(k) submissions for device clearance.
MASTERMIND-BSI
The ARLG has developed a master protocol for the evaluation of rapid diagnostics that target bacteria directly in blood (MASTERMIND-BSI). Bloodstream infections pose a major threat to short- and long-term mortality and early appropriate antibiotic therapy confers a survival benefit [15–18]. To ensure adequate coverage given the possibility of AR, providers often select broad empiric antibiotics until standard organism detection and corresponding antibiotic susceptibility testing (AST) are reported, a process that can take 48–120 hours. Conversely, other patients may be on antibiotics that are too narrow, placing them at risk for worsening clinical status and death.
A potential solution to address the tension between appropriate therapy and adverse consequences of unnecessarily broad antibiotics is the development of methods for rapid microorganism identification and AR determination without the need for culture [19]. Previous studies have demonstrated decreased time to appropriate antibiotics, lower mortality, shorter durations of hospital and intensive care unit (ICU) stay, and reduced costs when rapid identification methods are paired with antimicrobial stewardship (AS) [20–23]. However, most prior generations of rapid detection tests require pre-amplification of pathogens in culture. The next-generation assays under development are entirely culture independent.
MASTERMIND-BSI will be a prospective, observational study to determine the performance of multiple investigational tests for pathogen identification directly from blood of participants with suspected BSI. This study is expected to generate clinical data to support FDA applications for clearance of multiple investigational tests using different technologies for organism identification. Future directions will include the evaluation of rapid diagnostics that can predict AR directly from clinical specimens and studies to understand the anticipated clinical benefits of such culture-independent tests.
STUDIES ASSESSING THE IMPACT OF RAPID ORGANISM IDENTIFICATION WITH RESISTANCE DETERMINATION FROM POSITIVE BLOOD CULTURES
Relatively few interventional studies have assessed the impact of infectious disease diagnostics on patient-important outcomes, yet this level of evidence is ideally required to inform best practice. The ARLG conducted the first randomized controlled study of a rapid molecular Blood Culture-based IDentification (BCID) test to evaluate the effect of test implementation strategies on clinical outcomes [24]. In this study, faster antibiotic de-escalation occurred only in the group with rapid testing plus AS and this was driven by participants with gram-positive bacteremia.
Building on these results, the ARLG supported the Rapid Identification and Phenotypic Susceptibility testing for Gram negative bacteremia (RAPIDS GN) trial, which was conducted in 2 US academic medical centers [21]. In this study, participants with gram-negative bacilli identified on staining of positive blood cultures (BCs) were randomized to either (1) standard culture and AST of positive BC bottles (control) or (2) rapid organism identification and phenotypic AST using the Accelerate PhenoTest BC kit (Accelerate Diagnostics, Tucson, AZ) directly from the positive BC bottles. Both groups had AS support. Compared with the control group, the rapid testing group had earlier modifications in antibiotic treatment. Changes in antibiotic prescribing varied by resistance mechanism. Specifically, among patients with multidrug-resistant organisms, time from randomization to first antibiotic escalation was faster with the intervention, and among patients with antimicrobial-susceptible organisms, time to first antibiotic de-escalation was also faster. The groups did not differ in clinical outcomes. These results suggest that rapid phenotypic AST methods implemented together with AS can lead to an earlier switch from empirical to pathogen-directed antibiotic therapy during management of gram-negative bacteremia. Important limitations of the BCID and RAPIDS GN trials were the lack of power to detect differences in clinical outcomes and the low rates of multidrug-resistant gram-negative infections. Thus, empirical antibiotic therapy was effective in most patients, potentially limiting the ability to detect differences in mortality or other clinical outcomes.
To address these limitations, the ARLG is developing a third study, the Fast Antibiotic Susceptibility Testing for Gram negative bacteremia (FAST) trial. FAST is a multicenter, multinational, randomized controlled trial comparing clinical outcomes among patients with gram-negative BSIs who undergo rapid phenotypic AST versus standard culture and AST, all under the direction of AS. The FAST trial will utilize the REVEAL platform (bioMerieux), a metabolomic-based AST system, as the rapid testing method and will enroll in southern Europe, the Middle East, and Asia, all areas with high gram-negative bacteremia resistance rates.
Reporting of Antimicrobial Resistance From Blood Cultures (REPORT-ABC)
As demonstrated in the BCID study [24], implementation of a rapid, molecular BC identification panel in combination with AS benefits patient care. As a result, these tests are increasingly viewed as the diagnostic standard of care for BSIs [22], but there is little guidance on how results should be reported to best guide treatment. Certain reporting approaches, such as requiring providers to access separate documents outside of the report for interpretive guidance, may undermine the value of the test [25]. As rapid diagnostics from positive BCs become more common, and panels continue to grow in complexity, understanding best reporting practices is critical to ensure timely treatment. It is imperative that patient-facing clinicians can easily interpret results to positively impact care.
REPORT-ABC was a survey-based study to assess how clinical laboratories implement and report rapid BC results, with a focus on AR markers from molecular panels [26]. Significant heterogeneity in molecular platforms and workflows were found across community and academic laboratories serving hospitals of various sizes. In addition, AR reporting varied from simply listing targets as “Detected” or “Not Detected” to interpreting results with or without therapeutic guidance.
Based on these results, combined with available literature, the ARLG assembled best-practice recommendations for implementing, testing, and reporting rapid molecular BC panels. As next steps, the ARLG plans to create additional guidance to help standardize AR testing and reporting to optimally align with current treatment guidelines and ultimately help clinicians interpret and act on results.
RESPIRATORY DIAGNOSTIC STUDIES INCORPORATING HOST-DERIVED BIOMARKERS OF INFECTION
Acute respiratory illness (ARI) is the most common reason for seeking medical care and an important driver of inappropriate antibacterial use [27, 28]. Bacterial and viral ARIs present with similar signs and symptoms. Additionally, multiple noninfectious processes can mimic bacterial pneumonia. Strategies to identify patients with symptoms of a lower respiratory tract infection (LRTI) unlikely to benefit from antibiotics have the potential to reduce unnecessary antibiotic toxicities and mitigate the development and transmission of AR. While pathogen-directed diagnostics, such as culture, antigen testing, and NAATs, remain the mainstay of ARI diagnosis, these methods have important limitations. As a complement to pathogen-directed testing, the host's immune response to infection has been leveraged as an alternative method to differentiate colonization from infection and bacterial from viral ARI [29].
The ARLG has been actively involved in studying diagnostic strategies for ARI that incorporate host biomarkers of infection. In addition, the ARLG has been integral to the development and evaluation of novel host-response signatures that are predictive of bacterial infection, with an eye toward curbing AR.
Procalcitonin-Directed Treatment of Lower Respiratory Tract Infection
Procalcitonin (PCT) is a host-derived inflammatory marker that correlates with the presence and severity of bacterial infection. Previous meta-analyses have shown that PCT-based management algorithms can safely promote reductions in antibiotic exposure for patients with ARI [30, 31], and the FDA subsequently cleared PCT as an aid to antibiotic decision making for selected patients with suspected pneumonia. However, clinical trials of PCT algorithms have been plagued by variable protocol adherence [30] and some well-conducted randomized trials have shown no measurable impact on antibiotic use [32].
To answer lingering questions about the utility of PCT-driven management strategies, the ARLG completed a randomized, double-blind, placebo-controlled trial comparing the safety and efficacy of azithromycin versus placebo to treat suspected LRTI in outpatient participants with low PCT levels [33]. The Targeted Reduction of Antibiotics using Procalcitonin in Lower Respiratory Tract Infection (TRAP-LRTI) study enrolled 499 adult participants with acute LRTI and a serum PCT concentration of 0.25 ng/mL or less. Individuals with known or suspected pneumonia based on chest imaging and clinical diagnosis and immunocompromised hosts were excluded. Participants were randomized 1:1 to receive oral azithromycin for 5 days or matching placebo. The primary outcome, clinical improvement at day 5 in the intention-to-treat analysis, was not significantly different between groups. Thus, TRAP-LRTI did not establish that placebo was noninferior to azithromycin for individuals with low PCT levels. However, the per-protocol analysis at days 11 and 28, when recovery from a viral illness would have been expected, did demonstrate that azithromycin was noninferior to placebo. Furthermore, the prespecified Desirability of Outcome Ranking (DOOR) showed no significant difference between groups, except for an increased rate of abdominal pain as a side effect of azithromycin.
Host Gene Expression Profiles for Acute Respiratory Illness
Over the past 15 years, the ARLG has supported the Rapid Diagnostics in Categorizing Acute Lung Infections (RADICAL) program to develop and validate host-response–based assays that measure gene expression to differentiate bacterial, viral, and noninfectious ARI [34–37]. Host-response gene profiling is a potential alternative to common protein-based biomarkers, such as PCT and C-reactive protein, and represents a novel strategy that is pathogen agnostic. To develop gene expression tests, differential expression patterns are identified and selected for inclusion into a host classifier based on machine-learning techniques, such as sparse logistic regression. The classifier is then validated in various populations to assess test performance and predictive value.
The RADICAL program has made considerable progress moving from preclinical development into validation of a diagnostic tool that could realistically be implemented in the clinic [38, 39]. In the pivotal RADICAL-2 study of 755 participants, test performance showed an overall sensitivity of 89.8%, a specificity of 82.1%, and a negative-predictive value (NPV) of 97.9% for bacterial infection. In contrast, PCT had a sensitivity of 28.6%, a specificity of 87.0%, and an NPV of 87.6% for bacterial infection.
Given the favorable performance of the host classifier in RADICAL-2, plans are underway for an FDA 510(k) registrational trial slated to begin within the next year. In parallel, an additional clinical study (RADICAL-3) is in the design phase, with the primary objective of determining the impact of the rapid host-based diagnostic on antibacterial use and clinical outcomes among patients with ARI.
Diagnostics for Ventilator-Associated Pneumonia
Ventilator-associated pneumonia (VAP) is one of the most common nosocomial infections complicating critical care medicine, but a definitive diagnosis is difficult because the signs and symptoms overlap with multiple noninfectious processes. Additionally, diagnostic uncertainty results in unnecessary antibiotic use in the ICU [40, 41]. Selecting effective empiric therapy for VAP is complicated because multidrug-resistant pathogens may be isolated early- and in late-onset cases.
New approaches for a more accurate VAP diagnosis are urgently needed. One strategy that has yet to be explored involves combining pathogen- and host-directed testing. The ARLG is currently designing a proof-of-concept study to assess whether combining molecular diagnostics for bacteria and AR with host gene expression profiling improves the diagnosis of VAP. Pneumonia Direct Pilot (PDP) is a prospective, observational, multicenter, diagnostic feasibility study that will enroll intubated adult patients to determine the accuracy of pathogen- and host-directed testing for the diagnosis of VAP versus an adjudicated reference standard. If a test, or a test combination, can be identified that improves the predictive value of VAP beyond current approaches, a follow-on interventional trial of the new strategy will be considered. The eventual goal is to devise a more impactful diagnostic approach for VAP, where AR is detected quickly and patients who do not need antibiotics are rapidly and confidently identified.
THE PROBLEM OF IMPERFECT REFERENCE STANDARDS
As new testing modalities emerge, the limitations of existing diagnostic reference standards have become increasingly evident. This is particularly relevant in situations where a new test may be more sensitive and/or specific than the current standard-of-care test or when a clinical syndrome is difficult to diagnose using a combination of clinical assessments and laboratory testing.
The lack of a reliable “gold” standard has been an important consideration in the design of MASTERMIND-GC, MASTERMIND-BSI, RADICAL, and PDP. For the MASTERMIND projects, molecular diagnostics for NG and C. trachomatis and BSIs may be much more sensitive than a traditional culture comparator. Therefore, MASTERMIND-GC adopted a novel approach of combining the investigational tests into a multi-test comparator, with utilization of an additional tie-breaking test as needed [3]. For MASTERMIND-BSI, predefining a test or series of tests as a comparator proves challenging given the wide variety of invasive infections that can lead to bacteremia. For this study, a key secondary endpoint will be a clinically adjudicated comparator, which will incorporate expert review of standard-of-care testing and medical records. The RADICAL and PDP teams have also developed adjudicated reference standards for ARI and VAP, which, coupled with expert panel review, will allow classification of the type of infection, the likely etiologic agent, and levels of confidence associated with the classification.
With accumulating experience in clinical trial design for diagnostics, an ARLG working group recently reviewed and summarized potential solutions for an imperfect reference standard that researchers can utilize when designing their own studies [42]. Investigators facing this problem have multiple options to consider, including creating multi-test comparators (comparators incorporating combinations of the new tests under evaluation), using latent class modeling with statistical algorithms to identify homogeneous subgroups within heterogeneous populations, and creating clinically adjudicated case definitions.
CONCLUSIONS AND FUTURE DIRECTIONS
The ARLG has established a research program in infectious disease diagnostics that spans the continuum of support for new assay development, analytical and clinical test validation, outcomes studies, and registrational trials. A common thread is the focus on innovative tests or testing strategies with the potential to help combat AR. For new or improved tests to be widely adopted, test procedures need to be affordable and practical for the targeted clinical setting and ideally have evidence supporting improved outcomes through testing. Additional studies assessing the clinical impact and cost-effectiveness of new diagnostics are needed. Furthermore, corresponding implementation science is required to determine how best to incorporate new tests into clinical practice, including in resource-constrained settings. As thought leaders in the development and execution of diagnostics trials, the ARLG is poised to continue to advance this science.
Contributor Information
Kimberly E Hanson, Division of Infectious Diseases, Department of Medicine, University of Utah, Salt Lake City, Utah, USA; Division of Clinical Microbiology, Department of Pathology, University of Utah, Salt Lake City, Utah, USA.
Ritu Banerjee, Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Sarah B Doernberg, Division of Infectious Diseases, Department of Medicine, University of California, San Francisco, San Francisco, California, USA.
Scott R Evans, Department of Biostatistics, George Washington University, Washington, DC, USA.
Lauren Komarow, George Washington University Biostatistics Center, Rockville, Maryland, USA.
Michael J Satlin, Division of Infectious Diseases, Department of Medicine, Weill Cornell Medicine, New York, New York, USA.
Nyssa Schwager, Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina, USA.
Patricia J Simner, Division of Medical Microbiology, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
L Gayani Tillekeratne, Division of Infectious Diseases, Department of Medicine, Duke University School of Medicine, Durham, North Carolina, USA; Duke Global Health Institute, Duke University, Durham, North Carolina, USA.
Robin Patel, Division of Clinical Microbiology, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota, USA; Division of Public Health, Infectious Diseases, and Occupational Medicine, Department of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
for the Antibacterial Resistance Leadership Group:
Ephraim Tsalik, Erin Abbenante, Keri Baum, Maria Souli, Elizabeth Mocka, Deborah Hopkins, Abhigya Giri, Lijuan Zeng, Kerryl Greenwood-Quaintance, Andrew Dodd, Grant Booth, Yixuan Li, Jason Waller, Praneeta Raza, Zoe Sund, Cathy Wickward, Lijuan Zeng, Weixiao Dai, Toshimitsu Hamasaki, Varduhi Ghazaryan, Erica Raterman, Tamika Samuel, and Marina Lee
Notes
Author contributions. K. E. H. and R. P. were responsible for the content and design of the paper. K. E. H., R. B., S. B. D., M. J. S., P. J. S., and L. G. T. participated in the drafting and critical revision of the manuscript. R. P., S. R. E., and L. K. also provided critical revisions for important intellectual content. N. S. participated in the concept and design, facilitated acquisition of data, provided administrative and technical support, and provided supervision of the studies reported.
Acknowledgments. The authors thank the members of the Antibacterial Resistance Leadership Group (ARLG) Diagnostics Subcommittee for contributing to the development of the diagnostics priorities of the ARLG scientific agenda, and for their time spent reviewing and discussing study proposals. The authors also thank the following study team members for their contribution making studies and results possible: Ephraim Tsalik, Erin Abbenante, Keri Baum, Maria Souli, Elizabeth Mocka, Deborah Hopkins, Abhigya Giri, Lijuan Zeng, Kerryl Greenwood-Quaintance, Andrew Dodd, Grant Booth, Yixuan Li, Jason Waller, Praneeta Raza, Zoe Sund, Cathy Wickward, Lijuan Zeng, Weixiao Dai, Toshimitsu Hamasaki, Varduhi Ghazaryan, Erica Raterman, Tamika Samuel, Marina Lee, other Division of Microbiology and Infectious Diseases (DMID) team members, and team members from the Emmes Company, LLC and DMID Clinical Research Operations and Management Support. The authors also thank all study sites and study participants, without whom this work would not be possible.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number UM1AI104681.
Supplement sponsorship. This article appears as part of the supplement “The Antibacterial Resistance Leadership Group (ARLG): Innovation and Evolution,” sponsored by the Antibacterial Resistance Leadership Group.
References
- 1. Tsalik EL, Petzold E, Kreiswirth BN, et al. Advancing diagnostics to address antibacterial resistance: the diagnostics and devices committee of the Antibacterial Resistance Leadership Group. Clin Infect Dis 2017; 64(Suppl 1):S41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel R, Tsalik EL, Petzold E, Fowler VG, Klausner JD, Evans S. MASTERMIND: bringing microbial diagnostics to the clinic. Clin Infect Dis 2017; 64:355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doernberg SB, Komarow L, Tran TTT, et al. Simultaneous evaluation of diagnostic assays for pharyngeal and rectal Neisseria gonorrhoeae and Chlamydia trachomatis using a master protocol. Clin Infect Dis 2020; 71:2314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernstein KT, Stephens SC, Barry PM, et al. Chlamydia trachomatis and Neisseria gonorrhoeae transmission from the oropharynx to the urethra among men who have sex with men. Clin Infect Dis 2009; 49:1793–7. [DOI] [PubMed] [Google Scholar]
- 5. Jones RB, Rabinovitch RA, Katz BP, et al. Chlamydia trachomatis in the pharynx and rectum of heterosexual patients at risk for genital infection. Ann Intern Med 1985; 102:757–62. [DOI] [PubMed] [Google Scholar]
- 6. Lewis DA. Will targeting oropharyngeal gonorrhoea delay the further emergence of drug-resistant Neisseria gonorrhoeae strains? Sex Transm Infect 2015; 91:234–7. [DOI] [PubMed] [Google Scholar]
- 7. Weinstock H, Workowski KA. Pharyngeal gonorrhea: an important reservoir of infection? Clin Infect Dis 2009; 49:1798–800. [DOI] [PubMed] [Google Scholar]
- 8. Whittles LK, Didelot X, Grad YH, White PJ. Testing for gonorrhoea should routinely include the pharynx. Lancet Infect Dis 2018; 18:716–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 2021; 70:1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Workowski KA, Bolan GA; Centers for Disease Control and Prevention . Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 11. US Food and Drug Administration . FDA clears first diagnostic tests for extragenital testing for chlamydia and gonorrhea. May 23, 2019. Available at: https://www.fda.gov/news-events/press-announcements/fda-clears-first-diagnostic-tests-extragenital-testing-chlamydia-and-gonorrhea. Accessed 5 July 2023.
- 12. The Commonwealth of Massachusetts . Department of Public Health. Clinical Alert: January 19, 2023: Multidrug non-susceptible gonorrhea in Massachusetts. January 19, 2023. Available at: https://www.mass.gov/doc/clinical-alert-on-non-susceptible-gonorrhea-january-19-2023/download. Accessed 5 July 2023.
- 13. Bristow CC, Mortimer TD, Morris S, et al. Whole-genome sequencing to predict antimicrobial susceptibility profiles in Neisseria gonorrhoeae. J Infect Dis 2023; 227:917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klausner JD, Bristow CC, Soge OO, et al. Resistance-guided treatment of gonorrhea: a prospective clinical study. Clin Infect Dis 2021; 73:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garnacho-Montero J, Ortiz-Leyba C, Herrera-Melero I, et al. Mortality and morbidity attributable to inadequate empirical antimicrobial therapy in patients admitted to the ICU with sepsis: a matched cohort study. J Antimicrob Chemother 2008; 61:436–41. [DOI] [PubMed] [Google Scholar]
- 16. Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 2010; 54:4851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tacconelli E, Göpel S, Gladstone BP, et al. Development and validation of BLOOMY prediction scores for 14-day and 6-month mortality in hospitalised adults with bloodstream infections: a multicentre, prospective, cohort study. Lancet Infect Dis 2022; 22:731–41. [DOI] [PubMed] [Google Scholar]
- 18. McNamara JF, Righi E, Wright H, Hartel GF, Harris PNA, Paterson DL. Long-term morbidity and mortality following bloodstream infection: a systematic literature review. J Infect 2018; 77:1–8. [DOI] [PubMed] [Google Scholar]
- 19. Spellberg B, Srinivasan A, Chambers HF. New societal approaches to empowering antibiotic stewardship. JAMA 2016; 315:1229–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant gram-negative bacteremia. J Infect 2014; 69:216–25. [DOI] [PubMed] [Google Scholar]
- 21. Banerjee R, Komarow L, Virk A, et al. Randomized trial evaluating clinical impact of RAPid IDentification and susceptibility testing for gram-negative bacteremia: RAPIDS-GN. Clin Infect Dis 2021; 73:e39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Messacar K, Parker SK, Todd JK, Dominguez SR. Implementation of rapid molecular infectious disease diagnostics: the role of diagnostic and antimicrobial stewardship. J Clin Microbiol 2017; 55:715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang AM, Newton D, Kunapuli A, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 2013; 57:1237–45. [DOI] [PubMed] [Google Scholar]
- 24. Banerjee R, Teng CB, Cunningham SA, et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 2015; 61:1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Donner LM, Campbell WS, Lyden E, Van Schooneveld TC. Assessment of rapid-blood-culture-identification result interpretation and antibiotic prescribing practices. J Clin Microbiol 2017; 55:1496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simner PJ, Dien Bard J, Doern C, et al. Reporting of antimicrobial resistance from blood cultures, an antibacterial resistance leadership group survey summary: resistance marker reporting practices from positive blood cultures. Clin Infect Dis 2023; 76:1550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee GC, Reveles KR, Attridge RT, et al. Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med 2014; 12:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315:1864–73. [DOI] [PubMed] [Google Scholar]
- 29. Tsalik EL, McClain M, Zaas AK. Moving toward prime time: host signatures for diagnosis of respiratory infections. J Infect Dis 2015; 212:173–5. [DOI] [PubMed] [Google Scholar]
- 30. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2017; 10:CD007498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018; 18:95–107. [DOI] [PubMed] [Google Scholar]
- 32. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med 2018; 379:236–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsalik EL, Rouphael NG, Sadikot RT, et al. Efficacy and safety of azithromycin versus placebo to treat lower respiratory tract infections associated with low procalcitonin: a randomised, placebo-controlled, double-blind, non-inferiority trial. Lancet Infect Dis 2023; 23:484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zaas AK, Burke T, Chen M, et al. A host-based RT-PCR gene expression signature to identify acute respiratory viral infection. Sci Transl Med 2013; 5:203ra126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zaas AK, Chen M, Varkey J, et al. Gene expression signatures diagnose influenza and other symptomatic respiratory viral infections in humans. Cell Host Microbe 2009; 6:207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woods CW, McClain MT, Chen M, et al. A host transcriptional signature for presymptomatic detection of infection in humans exposed to influenza H1N1 or H3N2. PLoS One 2013; 8:e52198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsalik EL, Henao R, Nichols M, et al. Host gene expression classifiers diagnose acute respiratory illness etiology. Sci Transl Med 2016; 8:322ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsalik EL, Khine A, Talebpour A, et al. Rapid, sample-to-answer host gene expression test to diagnose viral infection. Open Forum Infect Dis 2019; 6:ofz466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ko ER, Henao R, Frankey K, et al. Prospective validation of a rapid host gene expression test to discriminate bacterial from viral respiratory infection. JAMA Netw Open 2022; 5:e227299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nussenblatt V, Avdic E, Berenholtz S, et al. Ventilator-associated pneumonia: overdiagnosis and treatment are common in medical and surgical intensive care units. Infect Control Hosp Epidemiol 2014; 35:278–84. [DOI] [PubMed] [Google Scholar]
- 41. Klompas M. Does this patient have ventilator-associated pneumonia? JAMA 2007; 297:1583–93. [DOI] [PubMed] [Google Scholar]
- 42. Patel R, Tsalik EL, Evans S, Fowler VG, Doernberg SB; Antibacterial Resistance Leadership Group . Clinically adjudicated reference standards for evaluation of infectious diseases diagnostics. Clin Infect Dis 2023; 76:938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]