Abstract
Clinical research networks conduct important studies that would not otherwise be performed by other entities. In the case of the Antibacterial Resistance Leadership Group (ARLG), such studies include diagnostic studies using master protocols, controlled phage intervention trials, and studies that evaluate treatment strategies or dynamic interventions, such as sequences of empiric and definitive therapies. However, the value of a clinical research network lies not only in the results from these important studies but in the creation of new approaches derived from collaborative thinking, carefully examining and defining the most important research questions for clinical practice, recognizing and addressing common but suboptimal approaches, and anticipating that the standard approaches of today may be insufficient for tomorrow. This results in the development and implementation of new methodologies and tools for the design, conduct, analyses, and reporting of research studies. These new methodologies directly impact the studies conducted within the network and have a broad and long-lasting impact on the field, enhancing the scientific value and efficiency of generations of research studies. This article describes innovations from the ARLG in diagnostic studies, observational studies, and clinical trials evaluating interventions for the prevention and treatment of antibiotic-resistant bacterial infections.
Keywords: DOOR, MASTERMIND, BED-FRAME, DOOR MAT, SMART COMPASS
Innovations from the Antibacterial Resistance Leadership Group (ARLG) contribute to scientific advancements in antibacterial resistance. These novel methodologies and tools will enhance the scientific value and efficiency of generations of research studies.
Antibacterial resistance (AR) studies pose diverse scientific and operational challenges, varying widely in design, conduct, and analyses. AR studies include diagnostic evaluations, randomized clinical trials for infection treatment and prevention, observational cohorts, pharmacokinetic studies, and surveys of patient perspectives and clinician practices. Studies may evaluate a multitude of pathogens, infection sites, antibiotics, and other interventions. Complex scientific challenges include the delay of important diagnostic information to inform treatment selection, the lack of pragmatism in current approaches, the development of resistance affecting the validity of assay performance and control-group selection, and new interventions, such as phages, for which standard approaches are insufficient. These challenges accentuate innovation imperatives. Collaborative advancements of the Antibacterial Resistance Leadership Group (ARLG) to address these imperatives are described herein.
INNOVATIVE INTERVENTIONS
Interventions to treat or prevent antibiotic-resistant bacterial infections are not limited to drugs. Phage and diagnostic interventions may treat or prevent resistant infections and reduce the development of resistance. Evaluation of sequential treatment strategies, recognizing the dynamic nature of patient management, can help optimize clinical decision making.
Phages
Phage therapy to combat bacterial infections dates back over 100 years, largely centered in the former Soviet Union. Early studies were promising and phage therapy was used in the United States in the 1940s. Phage therapy fell out of favor in the West primarily due to scientific skepticism and the advent of antibiotics [1].
The limited antibacterial pipeline and positive outcomes in anecdotal cases have raised interest in revisiting phages as a treatment option for difficult-to-treat infections. Phages have attractive features, including bactericidal activity, target specificity, avoidance of host tissue damage, preservation of the human microbiome, and synergy with antibiotics. Rigorous scientific investigations are needed to establish phage properties that are routine with conventional antibiotics. For example, evaluating the utility of phage therapy in clinical practice, defining the pharmacokinetic properties of phage products in humans, and defining whether a phage or phage cocktail has effectiveness against a bacteria strain need thorough evaluation. To this end, the ARLG is conducting a randomized, placebo-controlled, double-blind study (PHAGE) of intravenous phage therapy in adult volunteers with cystic fibrosis and Pseudomonas aeruginosa airway colonization [2]. The trial represents one of the first randomized, controlled, blinded trials of phage therapy.
The unprecedented phage approach creates trial design challenges and associated preparations not typically needed with antibiotic trials. Individual phages do not have reliable activity against all strains of bacterial species. Therefore, PHAGE implements a 4-phage cocktail, the efficacy of which is being correlated with the activity of each component phage, and the 4-phage cocktail against individual patient isolates of P. aeruginosa.
Phage susceptibility is assessed by testing phage activity against individual isolates. Unlike antimicrobial susceptibility testing (AST), standardized, accurate, and reproducible phage susceptibility testing (PST) methods, reported with validated interpretive criteria, are lacking. The ARLG's Laboratory Center and Statistics and Data Management Center collaborated to compare agreement between PST results from 3 centers. The results indicated that the reproducibility of PST methods needs further development, which helped inform PHAGE's design.
If phages are used in future clinical practice, PST will likely be performed to inform phage selection, just as AST is used to inform antibiotic selection. The ARLG is developing PST methods, the results of which will be compared with clinical outcomes in PHAGE.
Diagnostics as Interventions
The field of infectious disease diagnostics is in a technological revolution. Novel platforms may provide more or faster diagnostic information than historically done. Whether the tests improve patient care requires scientific evaluation. The ARLG spearheaded the use of randomized controlled trials evaluating the impact of diagnostics on clinical outcomes in patients with serious bacterial infections.
The Blood Culture Identification (BCID) Study demonstrated that a rapid multiplex polymerase chain reaction panel performed on positive blood cultures led to faster appropriate escalation and de-escalation of antibiotics when results were delivered with real-time antimicrobial stewardship. The Rapid Identification and Susceptibility Testing for Gram Negative Bacteremia (RAPIDS-GN) study focused on gram-negative bacillary infections and demonstrated that rapid phenotypic susceptibility testing performed on positive blood cultures delivered with antimicrobial stewardship led to faster times to appropriate antibiotic use than empirically prescribed [3]. These studies provide controlled evidence supporting laboratory adoption of rapid diagnostic methods for microorganism identification and AR detection from positive blood cultures, resulting in uptake in US laboratories, and served as the basis for the ARLG's best-practice guidelines for the use of these rapid diagnostics [4].
Strategies and Dynamic Interventions
Patient management is a dynamic sequence of decisions, with therapeutic adjustments made over time. Adjustments are tailored to the unique circumstances encountered with individual patients as new information becomes available.
Clinicians face 2 major decision points regarding treatment selection for serious bacterial infections: empiric and definitive therapy. Empiric therapy is based on clinicians’ immediate best judgment as to which treatment will provide the best patient outcome given the as-yet-unknown pathogen and AST results. Definitive therapy selection occurs 24–72 hours later and benefits from knowledge of the organism, AST results, early empiric therapy tolerability, and the patient's clinical course. Traditional antibiotic trials are often nonpragmatic, comparing drugs for definitive therapy when susceptibilities are known. However, clinical decision making is better informed by understanding which therapeutic strategy—that is, a sequence of decision rules guiding empiric and definitive therapies—optimizes patient outcomes.
Sequential, Multiple-Assignment, Randomized Trials for Comparing Personalized Antibiotic Strategies
Comparing Personalized Antibiotic Strategies (COMPASS) is a trial design that compares strategies for antibiotic decision making [5]. COMPASS was applied in a randomized pragmatic clinical trial Fosfomycin Oral for Complicated Urinary Syndromes (FOCUS) evaluating 2 strategies, oral fosfomycin versus oral levofloxacin, for initial or step-down therapy for complicated urinary tract infection (cUTI) without bacteremia. The strategies allow for therapeutic adjustment if resistance or intolerability is observed.
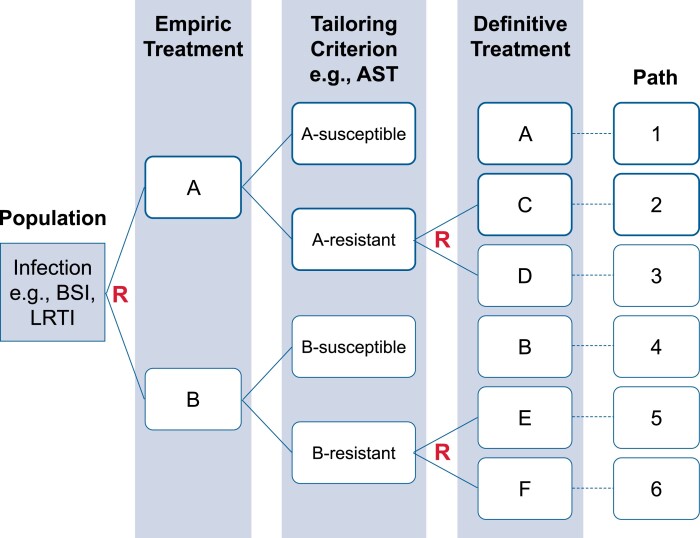
Sequential, Multiple-Assignment, Randomized Trials for Comparing Personalized Antibiotic Strategies (SMART COMPASS) allows evaluation of multiple, definitive therapy options. This pragmatic design mirrors clinical antibiotic treatment decision making and identifies the patient-management strategy that optimizes outcomes. The design can be implemented as a master protocol and used to compare definitive therapies with conditioning upon empiric therapy. SMART COMPASS is valuable in the setting of antibiotic resistance when therapeutic adjustments may be necessary due to resistance [5]. Figure 1 displays a schema for the SMART COMPASS trial design, comparing 4 treatment strategies.
Figure 1.
Schema for a SMART COMPASS comparing 4 strategies. A patient is initially randomized to empiric therapy A or B. For definitive therapy, if AST indicates susceptibility to empiric therapy then they remain on the empiric therapy. If AST indicates resistance, then there are therapeutic adjustments and the patient is randomized a second time to empiric therapy options. Four strategies are compared. Begin with empiric therapy A. If susceptible, then remain on A. If resistant, then change to C. Begin with empiric therapy A. If susceptible, then remain on A. If resistant, then change to D. Begin with empiric therapy B. If susceptible, then remain on B. If resistant, then change to E. Begin with empiric therapy B. If susceptible, then remain on B. If resistant, then change to F. The design is efficient in that some patients contribute information to more than 1 strategy. There are 4 strategies but 6 paths. Patients on the same strategy can receive different treatments depending on AST results. Abbreviations: AST, antimicrobial susceptibility testing; BSI, bloodstream infection; LRTI, lower respiratory tract infection; R, Randomization; SMART COMPASS, Sequential, Multiple-Assignment, Randomized Trials for Comparing Personalized Antibiotic Strategies.
TOOLS TO EVALUATE THE BENEFITS AND HARMS OF INTERVENTIONS
New methodologies provide researchers with more informative and efficient tools for clinical trials and other studies evaluating the benefits and harms of interventions.
Desirability of Outcome Ranking
Randomized clinical trials are the gold standard for evaluating the benefits and harms of interventions but often fail to provide the necessary evidence to inform medical decision making. The primary reasons are failure to (1) recognize the most important questions for treating patients in clinical practice and the inability of traditional approaches to directly address these questions and (2) utilize recognition of these flaws as the motivation for the design, monitoring, analysis, and reporting of clinical trials. Matching questions of a pragmatic origin with their clinical importance is a promising opportunity [6, 7].
Typical analyses for anti-infective clinical trials involve intervention comparisons for each efficacy and safety outcome. Estimated outcome-specific effects are potentially combined in benefit–risk analyses. However, summing marginal analyses of each outcome does not characterize the patient experience. The approach does not incorporate associations between outcomes or recognize the cumulative nature of various outcomes on patients and suffers from competing risk challenges when interpreting outcome-specific results. Since efficacy and safety analyses are conducted on different analysis populations, the population to which these analyses generalize is unclear. Treatment effect heterogeneity is typically naively evaluated based on a single efficacy or safety endpoint rather than benefit–risk.
Desirability of Outcome Ranking (DOOR) is a paradigm for the design, analysis, and interpretation of clinical trials and other research studies based on patient-centric benefit–risk evaluation [6–9]. DOOR increases pragmatism and addresses the most important “real world” question for clinical decision making: How does the desirability of the resulting patient experiences compare between therapeutic alternatives when comprehensively considering the full scope of benefits and harms? DOOR “uses outcomes to analyze patients rather than patients to analyze outcomes.”
The first step in implementation is defining a DOOR outcome that represents a patient-centric response, combining benefits and harms. This requires evaluating the tradeoffs among outcomes and the cumulative nature of benefits and harms on patients. A goal is to define gradations of patient response, enabling recognition of important differences in ultimate patient responses.
The ARLG Innovations Working Group is a National Institutes of Health (NIH)–US Food and Drug Administration (FDA) collaboration with patient representatives developing publicly available tools to support researcher and FDA evaluations and package-insert language for anti-infective drugs. One goal of the group is to create standardized, validated DOOR outcomes for use in registrational trials for 4 common licensing indications in infectious diseases: cUTI, complicated intra-abdominal infection (cIAI), hospital-acquired/ventilator-associated bacterial pneumonia (HABP/VABP), and acute bacterial skin and skin structure infection (ABSSSI). A DOOR infographic facilitating communication and understanding is shown in Figure 2.
Figure 2.
DOOR infographic [10]. Credit: Kim Best and Kerry Stenke, Duke Clinical Research Institute. Figure used with permission. Abbreviation: DOOR, Desirability of Outcome Ranking.
The Oak Ridge Institute for Science and Education (ORISE) DOOR fellowship provides an opportunity to leverage vast registrational trial data from the FDA Division of Anti-infective Products to advance DOOR outcome development and trial design while developing the next generation of researchers. Representing an FDA-ARLG collaboration, this fellowship provides a platform for scientific interaction among varied stakeholders engaged in combating AR, offering a unique means of collaborating to advance trial design. DOOR outcomes have been developed and applied to cIAI through the ORISE DOOR fellowship (Table 1) and cUTI [11, 12]. Studies developing DOOR outcomes in HABP/VABP and ABSSI trials are ongoing.
Table 1.
Complicated Intra-abdominal Infection–Specific Desirability of Outcome Ranking Endpoint Resulting from the FDA ORISE Fellowshipa
| DOOR Ranka | Alive? | Count of Eventsb |
|---|---|---|
| 0 (most desirable) | Yes | 0 of 7 |
| 1 | Yes | 1 of 7 |
| 2 | Yes | 2 of 7 |
| 3 | Yes | 3 of 7 |
| 4 | Yes | 4 of 7 |
| 5 | Yes | 5 of 7 |
| 6 | Yes | 6 of 7 |
| 7 | Yes | 7 of 7 |
| 8 (least desirable) | No (death) | Any |
Abbreviations: DOOR, Desirability of Outcome Ranking; FDA, US Food and Drug Administration; ORISE, Oak Ridge Institute for Science and Education.
Each participant was assigned a DOOR between 0 and 8 by counting the number of events a participant experienced within each component category. Study participants classified as having the outcomes of “indeterminate” or “clinical failure” were counted as having 1 event in the “absence of clinical response” category. Up to 2 events were counted in the categories of infectious complications, surgical/percutaneous procedures, and serious adverse events. These events comprised the final DOOR.
Sum of: Absence of clinical response: 0 or 1 event; infectious complications: 0, 1, or 2 events; surgical percutaneous interventions: 0, 1, or 2 events; serious adverse events: 0, 1, or 2 events.
DOOR has been applied in several studies [13–19]. Researchers have proposed a DOOR approach that integrates patient preferences of outcome importance, concluding that it can be used in pivotal trials or comparative effectiveness trials for a patient-centered evaluation of a therapeutic intervention [20]. DOOR is being used in the ARLG's PHAGE trial and the Dalbavancin as an Option for Treatment of S. aureus Bacteremia (DOTS) trial [2, 21]. DOOR has been recommended and applied to Data and Safety Monitoring Board (DSMB) evaluations [22, 23]. The Council for International Organizations of Medical Sciences is releasing a report in which DOOR principles are an integral part of benefit–risk recommendations.
The recommended statistical analysis plan for DOOR and an online application implementing the analyses are freely available (https://methods.bsc.gwu.edu/) [24]. Ongoing work with DOOR includes the following: (1) analyses in which subgroups are identified and evaluated based on patient-centric benefit–risk rather than naïve evaluation of a single variable; (2) longitudinal evaluation of DOOR as a dynamic patient state, realizing the importance of knowing whether events occurred, when, for how long, and whether they resolve or relapse; and (3) meta-analyses of multiple studies using DOOR to aid regulatory evaluation of multiple studies.
Health-Related Quality of Life
The ARLG's health-related quality of life (HRQoL) task force elevates the patient perspective on how they feel, function, and survive in registrational trials of antimicrobials for resistant infections. Alignment with the Combating Antibiotic-resistant Bacteria National Action Plan was ensured by including patient representatives and members with expertise in study design and methods, and implementation and measurement science. Novel approaches are used to reduce gaps in understanding patient experiences and to design and execute research studies with health consumers in mind. The goals of the HRQoL task force include the following:
Assess the HRQoL of patients with infections
Establish whether HRQoL measures are “fit-for-purpose” in bacterial syndromes
Incorporate HRQoL in antimicrobial registrational trials
Initial work by the task force included the study of HRQoL in patients with Staphylococcus aureus or gram-negative bloodstream infections and the development of an HRQoL survey for use in the DOTS trial [21, 25].
DESIGNING TRIALS WITH DIAGNOSTIC INTERVENTIONS: EVALUATING CLINICAL AND ANTIBIOTIC USE OUTCOMES
Rapid diagnostic tests are needed to ensure timely administration of appropriate treatment, prevent toxicity from unnecessary treatments, and prevent resistance from overprescribing antibiotics. Clinical and stewardship outcomes must be considered during evaluation of rapid diagnostic testing strategies.
The Rapid Diagnostic Test for the Categorization of Acute Respiratory Illness (RADICAL III) trial is evaluating the effects of a rapid diagnostic test in patients with suspected acute respiratory infection on (1) a DOOR clinical outcome and (2) antibacterial exposure using a co-primary endpoint (CPE) design allowing simultaneous evaluation of clinical and stewardship effects of the diagnostic intervention. CPE trials are challenging to design [26]. ARLG investigators extended their methods to develop an efficient RADICAL III design and provide the DSMB with a tool for informed evaluation of the advantages and disadvantages of trial continuation [27].
TOOLS TO EVALUATE THE ACCURACY AND UTILITY OF DIAGNOSTICS
New methodologies provide researchers with more informative and efficient tools for diagnostic research studies.
Master Protocol for Evaluating Multiple Infection Diagnostics
The lack of accurate rapid diagnostics assessing the presence of bacterial infection results in the overuse of antimicrobial agents, the emergence of AR, and suboptimal patient care. Accurate rapid diagnostics are needed to address these challenges.
The Master Protocol for Evaluating Multiple Infection Diagnostics (MASTERMIND) was developed to evaluate multiple diagnostics simultaneously within a single study, providing efficiencies of specimen collection and characterization [28]. MASTERMIND offers central trial organization, standardization of methods and definitions, and common reference standards.
MASTERMIND was utilized to evaluate simultaneously the performance of nucleic acid amplification tests for the detection of Neisseria gonorrhoeae and Chlamydia trachomatis in extragenital sites [29]. The study resulted in FDA clearance of the first 2 diagnostic tests for extragenital testing for these pathogens, demonstrating the value of MASTERMIND for scientific evaluation. The study serves as a model of collaboration between academic partners, diagnostics companies, and government agencies, which are necessary for these studies. MASTERMIND designs are in development to evaluate diagnostics for the identification of bacteria directly from blood (MASTERMIND-BSI), ciprofloxacin-resistant N. gonorrhoeae (MASTERMIND-RING), and pneumonia.
Benefit–Risk Evaluation of Diagnostics: A Framework and Average Weighted Accuracy
Traditional diagnostic evaluation includes estimation of sensitivity, specificity, and predictive values. Although useful, these measures are insufficient for guiding medical decision making regarding diagnostic alternatives. The results of the clinical application of a diagnostic can be measured by diagnostic yield (ie, the distribution of true positives, true negatives, false positives, and false negatives), a function of the prevalence. The value of the yield depends on the relative importance of false-positive versus false-negative errors as different errors carry different consequences. However, prevalence and relative importance vary geographically and temporally.
Benefit–Risk Evaluation of Diagnostics: A Framework (BED-FRAME) and Average Weighted Accuracy (AWA) address these challenges by providing a systematic and pragmatic approach to evaluate and compare diagnostic alternatives to aid in clinical decision making [30, 31]. Results are presented as the prevalence and relative importance vary, allowing tailored decision making depending on local, temporal, and treatment availability context.
BED-FRAME and AWA methods were applied to design the RADICAL study [31] that evaluated the utility of a host-response–based diagnostic test in categorizing acute respiratory tract illness into bacterial or viral or neither etiology. These methods were used to evaluate the ability of rapid molecular diagnostics to detect resistance associated with 3 “critical” priority pathogens identified by the World Health Organization: carbapenem-resistant (CR) Enterobacterales [32], CR P. aeruginosa [33], and CR Acinetobacter baumannii [34]. An online app for implementing BED-FRAME and AWA analyses will be freely available. Figure 3 displays example output from the tool for a study comparing rapid molecular diagnostics platforms for the detection of resistance to imipenem in Acinetobacter species [30, 34].
Figure 3.
Example output from BED-FRAME analyses. Output from the R shiny app displaying contours of the between-platform difference (molecular beacons [MB] vs polymerase chain reaction/electrospray ionization mass spectrometry [PCR/ESI-MS]) in weighted accuracy (ie, overall percentage correctly classified, adjusted for the relative importance of a false-resistant test result relative to a false-susceptible test result) for various combinations of relative importance and imipenem susceptibility rates. Gray indicates combinations of relative importance and imipenem susceptibility rates for which MB would provide superior results. Yellow indicates combinations of relative importance and imipenem susceptibility rates for which PCR/ESI-MS would provide superior results. Black indicates the border where accuracy is equivalent. A point is shown for a resistance rate = 0.25 and relative importance = 0.25 (a false-resistant test result, which would result in avoidance of treatment with a covering drug, is 25% as important as a false-susceptible test result, which would result in treatment with a non-covering drug) where there is a weighted accuracy advantage of 5.9% for PCR/ESI-MS. Abbreviations: BED-FRAME, Benefit–Risk Evaluation of Diagnostics: A Framework; WA, weighted accuracy.
Desirability of Outcome Ranking for the Management of Antimicrobial Therapy
Antibiotics are classified by spectrum, narrow to broad. Resistance to broad spectrums generally implies resistance to narrower spectrums. It is desirable to select an antibiotic from the narrowest spectrum for which the target infection is susceptible. This minimizes toxicity, cost, and the development of resistance due to selective pressure.
The desirability of treatment selection based on diagnostic guidance can be classified using Desirability of Outcome Ranking for the Management of Antimicrobial Therapy (DOOR MAT), a framework for assessing antibiotic selection strategies in the presence of drug resistance (Figure 4) [6, 7, 35]. DOOR MAT has been used in several studies to evaluate the clinical utility of rapid diagnostics for the treatment of bloodstream infections.
Figure 4.
Schematic for DOOR MAT. Red indicates selection of an antibiotic for which the target infection is deemed resistant (most undesirable). Green indicates selection of an antibiotic from the narrowest spectrum for which the target infection is deemed susceptible (most desirable). The shade of gray indicates selection of an antibiotic from spectrums for which the target infection is deemed susceptible but not the narrowest. Lighter shades of gray indicate more desirability—that is, closer to the most-desirable narrowest spectrum for which the target infection is deemed susceptible. Abbreviations: AST, antimicrobial susceptibility testing; DOOR MAT, Desirability of Outcome Ranking for the Management of Antimicrobial Therapy, R, Resistant; S, Susceptible.
DEVELOPING IDEAS
Platform Trials
Pseudomonas aeruginosa is known for its intrinsic resistance to most antibiotics and its propensity to become resistant during therapy. The prevalence of multidrug-resistant P. aeruginosa is 15–30% in regions worldwide. Outcomes associated with P. aeruginosa infections can be poor, with a mortality of approximately 30% in P. aeruginosa bacteremia, higher than other bacteria causing bloodstream infections. Randomized trial evidence to inform treatment is limited. Many important questions remain unanswered, such as whether single or combination antibiotic therapy for empiric and definitive treatment is optimal.
Platform trials are randomized multi-arm trials that allow adding and removing new interventions during the trial. Many trends labelled as innovations are compromises of robustness and rigor, lowering the usual evidentiary standard and introducing greater uncertainty [6, 7]. These compromises often plague platform trials, reducing integrity, replicability, and applicability [36–39]. Commitment to scientific objectivity and robustness is critical for optimizing patient care. The ARLG is developing a platform trial to inform treatment selection for P. aeruginosa in bloodstream infections/sepsis, HABP/VABP, and cUTI.
Real-World Data
Completion of randomized trials that evaluate interventions to treat resistant infections is challenging. Such trials often require enrollment at many international sites to identify enough eligible trial participants for sufficient power. The evolving availability of real-world data (RWD) provides the opportunity to obtain evidence to inform clinical decision making until randomized evidence is obtained.
A collaboration between the ARLG and NIH Clinical Center investigators will use RWD to pursue answers to important practical questions for clinical practice that may not be soon answered in clinical trials. A developing project will compare therapeutic options for the treatment of Acinetobacter infections. Statistical methods to control for confounding due to the observational nature of the data are in development. These data will be helpful for planning eligibility criteria and enhancing recruitment in future clinical trials [40].
CONCLUSIONS
New ideas are needed to address the evolving challenges associated with AR research. Essential advances are unlikely to be realized exclusively through industry initiatives. The advancements utilized across the ARLG program have resulted in a portfolio of first-in-kind studies (Table 2). These innovations have helped fill this important void in AR research and advanced the AR field in unprecedented ways.
Table 2.
Studies and Associated Innovations Across the Antibacterial Resistance Leadership Group (ARLG) Program
| Study | Associated Innovation |
|---|---|
| BCID, RAPIDS-GN [3], FAST: Blood Culture Identification, Rapid Identification and Susceptibility Testing for Gram Negative Bacteremia, and Fast Antibiotic Susceptibility Testing for Gram Negative Bacteremia | Evaluation of optimal clinical use of novel rapid diagnostics. Randomization at local laboratory level. |
| COMBINE: Phase 1 clinical trial to assess the safety and pharmacokinetics of ceftazidime-avibactam in combination with aztreonam in healthy volunteers | Pharmacokinetics of a novel combination of 2 antibiotics. Dose selection using Hollow Fiber Infection Method. |
| DOTS: Dalbavancin as an Option for Treatment of Complicated Staphylococcus aureus Bacteremia [19] | Prospective utilization of DOOR. Incorporates ARLG health-related quality of life measures. |
| FOCUS: Fosfomycin Oral for Complicated Urinary Syndromes | First clinical trial to prospectively utilize COMPASS. |
| GENO-STELLAR: Genomics, Sequencing-based Typing, Epidemiology, Linkage, and Antimicrobial Resistance Tool (https://genostellar.net) | Application utilizing whole-genome sequencing and clinical data from ARLG studies to predict phenotypic susceptibility of a patient's bacterial strain. |
| PROVIDE: Evaluation of the association between initial vancomycin exposure and failure rates in hospitalized patients with methicillin-resistant S. aureus bloodstream infections [14] | Utilization of DOOR to evaluate dosing based on benefits and harms. |
| MASTER-GC [31], MASTERMIND-BSI and -RING: Master protocol for evaluating multiple infection diagnostics for Neisseria gonorrhoeae and Chlamydia trachomatis, bloodstream infections, and ciprofloxacin-resistant N. gonorrhoeae | Utilized MASTERMIND—multiple diagnostics assessed in a single study. |
| OPTIMIZE-GNI: Optimization of Beta-lactam Dosing in Critically Ill Patients with Suspected or Documented Antimicrobial Resistant Gram-negative Infections with Cystatin C | Evaluates novel cystatin C methodology to inform antibiotic dosing in critically ill patients. |
| PHAGE: Phase 1b/2 trial of intravenously administered phage to determine safety and microbiological activity in persons with cystic fibrosis chronically colonized with Pseudomonas aeruginosa [2] | Novel therapeutic. Development of phage susceptibility testing methodology. |
| Pneumonia Direct Pilot: Diagnostic feasibility study to determine the accuracy of pathogen- and host-directed testing for the diagnosis of ventilator-associated pneumonia (VAP) | Utilizes novel method combining molecular diagnostics for bacteria and resistance with host gene expression profiling. |
| RADICAL series [33]: Rapid Diagnostics in Categorizing Acute Lung Infections | Utilizes novel host response bacterial vs viral technology. Uses BED-FRAME and AWA. |
| SNAP, CRACKLE II [19, 20], POP [18], SHREC [41]: Multi-Drug Resistant Organism Clinical Trials Network (MDRO-CTN) studies focusing on carbapenem-resistant Acinetobacter baumannii, Enterobacterales, P. aeruginosa, and ceftriaxone-resistant Escherichia coli | Utilizes a unique global clinical trial network specifically designed by ARLG to address resistant gram-negative infections. DOOR analyses in gram-negative disease. |
Abbreviations: AWA, Average Weighted Accuracy; BED-FRAME, Benefit–Risk Evaluation for Diagnostics: A Framework; COMBINE, AVYCAZ® in Combination with Aztreonam; COMPASS, Comparing Personalized Antibiotic Strategies; CRACKLE II, Consortium on Resistance Against Carbapenems in Klebsiella pneumoniae and other Enterobacteriaceae; DOOR, Desirability of Outcome Ranking; MASTER-GC, Master Protocol - Gonorrhoeae and Chlamydia Testing of Extragenital Specimens; MASTERMIND, Master Protocol for Evaluating Multiple Infection Diagnostics; MASTERMIND-BSI, Master Protocol for Evaluating Multiple Infection Diagnostics for Rapid Detection of Bloodstream Infection; MASTERMIND-RING, Master Protocol for Evaluating Multiple Infection Diagnostics-Resistant Neisseria gonorrhoeae; POP, Prospective Observational Pseudomonas Study; PROVIDE, Prospective Observational Study to Validate the Pharmacodynamic Index for Vancomycin Among Patients with Methicillin-Resistant Staphylococcus aureus Bloodstream Infections; SHREC, Study of Highly Resistant Escherichia coli.
Contributor Information
Scott R Evans, George Washington University Biostatistics Center, Rockville, Maryland, USA.
Robin Patel, Division of Clinical Microbiology and Division of Public Health, Infectious Diseases, and Occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Toshimitsu Hamasaki, George Washington University Biostatistics Center, Rockville, Maryland, USA.
Jessica Howard-Anderson, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Tori Kinamon, Duke University School of Medicine, Durham, North Carolina, USA.
Heather A King, Department of Population Health Sciences, Duke University School of Medicine, Durham, North Carolina, USA; Division of General Internal Medicine, Department of Medicine, Duke University School of Medicine, Durham, North Carolina, USA; Center of Innovation to Accelerate Discovery and Practice Transformation, Health Services Research and Development, Durham Veterans Affairs Health Care System, Durham, North Carolina, USA.
Deborah Collyar, Patient Advocates in Research, Danville, California, USA.
Heather R Cross, Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina, USA.
Henry F Chambers, Division of Infectious Diseases, Department of Medicine, University of California, San Francisco, San Francisco, California, USA.
Vance G Fowler, Jr, Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina, USA; Department of Medicine, Duke University School of Medicine, Durham, North Carolina, USA.
Helen W Boucher, Tufts University School of Medicine and Tufts Medicine, Boston, Massachusetts, USA.
for the Antibacterial Resistance Leadership Group:
Pranita Tamma, Robert Schooley, Ritu Banerjee, Maria Souli, Zoe Sund, Beth Evans, Grant Booth, Leslie Estes, Kerryl Greenwood-Quaintance, Krupa Mukesh Parmar, Scott Cunningham, Nyssa Schwager, Cathy Wickward, Holly Geres, Weixiao Dai, Yijie He, Sarah Doernberg, Michael Satlin, Nadine Rouphael, Gayani Tillekeratne, Keri Baum, Praneeta Raza, Lauren Komarow, Andrew Dodd, Deborah Hopkins, Yixuan Li, Ephraim Tsalik, Thomas Holland, and Shanshan Zhang
Notes
Author Contributions. Concept and design: S. R. E. Acquisition, analysis, or interpretation of data: S. R. E., R. P., T. H., J. H.-A., T. K., H. K., H. F. C., H. C., and H. B. Drafting of the manuscript: S. R. E., R. P., and H. B. Critical revision of the manuscript for important intellectual content: S. R. E., R. P., D. C., H. C., and V. G. F. Statistical analysis: S. R. E. and T. H. Obtained funding: H. C., H. F. C., and V. G. F. Administrative, technical, or material support: H. C. Supervision: S. R. E. and H. C.
Acknowledgments. The authors thank the following study team members for their contribution in making studies and results possible: Pranita Tamma, Robert Schooley, Ritu Banerjee, Maria Souli, Zoe Sund, Beth Evans, Grant Booth, Leslie Estes, Kerryl Greenwood-Quaintance, Krupa Mukesh Parmar, Scott Cunningham, Nyssa Schwager, Cathy Wickward, Holly Geres, Weixiao Dai, Yijie He, Sarah Doernberg, Michael Satlin, Nadine Rouphael, Gayani Tillekeratne, Keri Baum, Praneeta Raza, Lauren Komarow, Andrew Dodd, Deborah Hopkins, Yixuan Li, Ephraim Tsalik, Thomas Holland, Shanshan Zhang, and the ARLG Innovations Working Group.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the position or policy of Duke University, the U.S. Department of Veterans Affairs, or the U.S. government.
Financial support. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number UM1AI104681.
Supplement sponsorship. This article appears as part of the supplement “The Antibacterial Resistance Leadership Group (ARLG): Innovation and Evolution,” sponsored by the Antibacterial Resistance Leadership Group.
References
- 1. American Society for Microbiology . Phage therapy: past, present and future. Available at: https://asm.org/Articles/2022/August/Phage-Therapy-Past,-Present-and-Future. Accessed 16 August 2023.
- 2. Tamma PD, Souli M, Billard M, et al. Safety and microbiological activity of phage therapy in persons with cystic fibrosis colonized with Pseudomonas aeruginosa: study protocol for a phase 1b/2, multicenter, randomized, double-blind, placebo-controlled trial. Trials 2022; 23:1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banerjee R, Komarow L, Virk A, et al. Randomized trial evaluating clinical impact of RAPid IDentification and susceptibility testing for gram-negative bacteremia: RAPIDS-GN. Clin Infect Dis 2021; 73:e39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simner PJ, Dien Bard J, Doern C, et al. Reporting of antimicrobial resistance from blood cultures, an Antibacterial Resistance Leadership Group survey summary: resistance marker reporting practices from positive blood cultures. Clin Infect Dis 2023; 76:1550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evans SR, Follmann D, Liu Y, et al. Sequential, multiple-assignment, randomized trials for COMparing personalized antibiotic StrategieS (SMART-COMPASS). Clin Infect Dis 2019; 68:1961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evans SR. Radical thinking: scientific rigor and pragmatism. Stat Biopharm Res 2022; 14:140–52. [Google Scholar]
- 7. Evans SR. Our most important discovery: the question. Stat Biopharm Res 2022; 14:398–407. [Google Scholar]
- 8. Evans SR, Rubin D, Follmann D, et al. Desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic risk (RADAR). Clin Infect Dis 2015; 61:800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans SR, Follmann D. Using outcomes to analyze patients rather than patients to analyze outcomes: a step toward pragmatism in benefit:risk evaluation. Stat Biopharm Res 2016; 8:386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Antibacterial Resistance Leadership Group. Summary of Results. Available at https://arlg.org/summary-of-results. Accessed September 11, 2023. [Google Scholar]
- 11. Kinamon T, Gopinath R, Waack U, et al. Exploration of a potential desirability of outcome ranking endpoint for complicated intra-abdominal infections using 9 registrational trials for antibacterial drugs. Clin Infect Dis 2023; 77;649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howard-Anderson J, Hamasaki T, Dai W, et al. Improving traditional registrational trial end points: development and application of a desirability of outcome ranking end point for complicated urinary tract infection clinical trials. Clin Infect Dis 2023; 76:e1157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Duin D, Lok JJ, Earley M, et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 2018; 66:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor's new clothes: pRospective observational evaluation of the association between initial VancomycIn exposure and failure rates among ADult HospitalizEd patients with methicillin-resistant Staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis 2020; 70:1536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams DJ, Creech CB, Walter EB, et al. Short- vs standard-course outpatient antibiotic therapy for community-acquired pneumonia in children: the SCOUT-CAP randomized clinical trial. JAMA Pediatr 2022; 176:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsalik EL, Rouphael NG, Sadikot RT, et al. Efficacy and safety of azithromycin versus placebo to treat lower respiratory tract infections associated with low procalcitonin: a randomised, placebo-controlled, double-blind, non-inferiority trial. Lancet Infect Dis 2023; 23:484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reyes J, Komarow L, Chen L, et al. Global epidemiology and clinical outcomes of carbapenem-resistant Pseudomonas aeruginosa and associated carbapenemases (POP): a prospective cohort study. Lancet Microbe 2023; 4:e159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang M, Earley M, Chen L, et al. Clinical outcomes and bacterial characteristics of carbapenem-resistant Klebsiella pneumoniae complex among patients from different global regions (CRACKLE-2): a prospective, multicentre, cohort study. Lancet Infect Dis 2022; 22:401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Duin D, Arias CA, Komarow L, et al. Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): a prospective cohort study. Lancet Infect Dis 2020; 20:731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu Y, Zhao Q, Zou J, et al. A composite endpoint for treatment benefit according to patient preference. Stat Biopharm Res 2022; 14:408–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turner NA, Zaharoff S, King H, et al. Dalbavancin as an Option for Treatment of S. aureus bacteremia (DOTS): study protocol for a phase 2b, multicenter, randomized, open-label clinical trial. Trials 2022; 23:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evans SR, Zeng L, Dai W. The data and safety monitoring board: the toughest job in clinical trials. NEJM Evid 2023; 2. [DOI] [PubMed] [Google Scholar]
- 23. Evans SR, Bigelow R, Chuang-Stein C, et al. Presenting risks and benefits: helping the data monitoring committee do its job. Ann Intern Med 2020; 172:119–25. [DOI] [PubMed] [Google Scholar]
- 24.The GWU Biostatistics Center DOOR Methodology Research Group. DOOR analyses: standard edition. Available at: https://methods.bsc.gwu.edu/. Accessed 13 June 2023.
- 25. King HA, Doernberg SB, Miller J, et al. Patients’ experiences with Staphylococcus aureus and gram-negative bacterial bloodstream infections: a qualitative descriptive study and concept elicitation phase to inform measurement of patient-reported quality of life. Clin Infect Dis 2021; 73:237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamasaki T, Asakura K, Evans SR, Ochiai T. Group-sequential clinical trials with multiple co-objectives. Cham/Heidelberg/New York: Springer, 2016. [Google Scholar]
- 27. Evans SR, Li L, Wei LJ. Data monitoring in clinical trials using prediction. Drug Inf J 2007; 41:733–42. [Google Scholar]
- 28. Patel R, Tsalik EL, Petzold E, et al. MASTERMIND: bringing microbial diagnostics to the clinic. Clin Infect Dis 2017; 64:355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Doernberg SB, Komarow L, Tran TTT, et al. Simultaneous evaluation of diagnostic assays for pharyngeal and rectal Neisseria gonorrhoeae and Chlamydia trachomatis using a master protocol. Clin Infect Dis 2020; 71:2314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Evans SR, Pennello G, Pantoja-Galicia N, et al. Benefit-risk evaluation for diagnostics: a framework (BED-FRAME). Clin Infect Dis 2016; 63:812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Y, Tsalik EL, Jiang Y, et al. Average weighted accuracy: pragmatic analysis for a rapid diagnostics in categorizing acute lung infections (RADICAL) study. Clin Infect Dis 2020; 70:2736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Evans SR, Hujer AM, Jiang H, et al. Rapid molecular diagnostics, antibiotic treatment decisions, and developing approaches to inform empiric therapy: pRIMERS I and II. Clin Infect Dis 2016; 62:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Evans SR, Tran TTT, Hujer AM, et al. Rapid molecular diagnostics to inform empiric use of ceftazidime/avibactam and ceftolozane/tazobactam against Pseudomonas aeruginosa: PRIMERS IV. Clin Infect Dis 2019; 68:1823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Evans SR, Hujer AM, Jiang H, et al. Informing antibiotic treatment decisions: evaluating rapid molecular diagnostics to identify susceptibility and resistance to carbapenems against Acinetobacter spp. in PRIMERS III. J Clin Microbiol 2016; 55:134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wilson BM, Jiang Y, Jump RLP, et al. Desirability of outcome ranking for the management of antimicrobial therapy (DOOR MAT): a framework for assessing antibiotic selection strategies in the presence of drug resistance. Clin Infect Dis 2021; 73:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dodd LE, Freidlin B, Korn EL. Platform trials—beware the noncomparable control group. N Engl J Med 2021; 384:1572–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Korn EL, Freidlin B. Outcome–adaptive randomization: is it useful? J Clin Oncol 2011; 29:771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Emerson SS, Fleming TR. Adaptive methods: telling “the rest of the story”. J Biopharm Stat 2010; 20:1150–65. [DOI] [PubMed] [Google Scholar]
- 39. Huskins WC, Fowler VG Jr, Evans S. Adaptive designs for clinical trials: application to healthcare epidemiology research. Clin Infect Dis 2018; 66:1140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Evans SR, Paraoan D, Perlmutter J, Raman SR, Sheehan JJ, Hallinan ZP. Real-world data for planning eligibility criteria and enhancing recruitment: recommendations from the clinical trials transformation initiative. Ther Innov Regul Sci 2021; 55:545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tamma PD, Komarow L, Ge L, et al. Clinical impact of ceftriaxone resistance in Escherichia coli bloodstream infections: a multicenter prospective cohort study. Open Forum Infect Dis 2022; 9:ofac572. [DOI] [PMC free article] [PubMed] [Google Scholar]