Abstract
Addressing the treatment and prevention of antibacterial-resistant gram-negative bacterial infections is a priority area of the Antibacterial Resistance Leadership Group (ARLG). The ARLG has conducted a series of observational studies to define the clinical and molecular global epidemiology of carbapenem-resistant and ceftriaxone-resistant Enterobacterales, carbapenem-resistant Pseudomonas aeruginosa, and carbapenem-resistant Acinetobacter baumannii, with the goal of optimizing the design and execution of interventional studies. One ongoing ARLG study aims to better understand the impact of fluoroquinolone-resistant gram-negative gut bacteria in neutropenic patients, which threatens to undermine the effectiveness of fluoroquinolone prophylaxis in these vulnerable patients. The ARLG has conducted pharmacokinetic studies to inform the optimal dosing of antibiotics that are important in the treatment of drug-resistant gram-negative bacteria, including oral fosfomycin, intravenous minocycline, and a combination of intravenous ceftazidime-avibactam and aztreonam. In addition, randomized clinical trials have assessed the safety and efficacy of step-down oral fosfomycin for complicated urinary tract infections and single-dose intravenous phage therapy for adult patients with cystic fibrosis who are chronically colonized with P. aeruginosa in their respiratory tract. Thus, the focus of investigation in the ARLG has evolved from improving understanding of drug-resistant gram-negative bacterial infections to positively affecting clinical care for affected patients through a combination of interventional pharmacokinetic and clinical studies, a focus that will be maintained moving forward.
Keywords: gram-negative, antimicrobial resistance, observational studies, interventional studies, clinical trials
Addressing antibacterial-resistant gram-negative infections is a priority of the Antibacterial Resistance Leadership Group. The focus of investigation has evolved from elucidating the epidemiology of these infections to improving patient care through clinical trials of optimizing pharmacokinetics and of novel therapies.
Antibacterial resistance (AR) in gram-negative bacteria is an increasingly challenging public health issue. Globally, gram-negative bacteria (Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii) account for 4 of the top 6 pathogens in terms of estimated global deaths associated with AR [1]. In the United States, >50 000 hospitalized patients are infected annually by carbapenem-resistant P. aeruginosa (CRPA), carbapenem-resistant Enterobacterales (CRE), or carbapenem-resistant A. baumannii (CRAB), about 8% of whom die from the infection [2]. Extended-spectrum β-lactamase (ESBL)–producing Enterobacterales are now major pathogens in healthcare and community settings, causing approximately 200 000 infections and 9000 deaths annually in the United States.
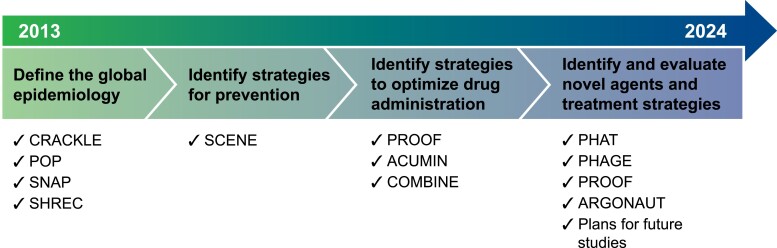
Worryingly, the incidence of these AR gram-negative infections rose significantly during the coronavirus disease 2019 (COVID-19) pandemic owing to increased antimicrobial use and challenges in following infection prevention procedures [3]. Despite the scope of the problem, innovation in therapy has lagged, with no new class of antimicrobial agents with gram-negative activity having been approved in the last 50 years. In addition, significant limitations exist in our knowledge of the global epidemiology of carbapenem-resistant gram-negatives and how to optimize existing agents to improve the outcomes of infected patients and minimize the emergence of AR. Since its inception, the Antibacterial Resistance Leadership Group (ARLG) has identified, designed, and implemented clinical studies that inform the epidemiology, treatment, and prevention of drug-resistant gram-negative bacterial infections and improve outcomes in affected patients (Figure 1). In this article, we review the accomplishments of the ARLG in the area of gram-negative bacterial infections according to each scientific priority (see Table 1 for summaries of studies).
Figure 1.
Antibacterial Resistance Leadership Group (ARLG) clinical studies of drug-resistant gram-negative bacterial infections. Abbreviations: ACUMIN, Acute Care Unit Minocycline; ARGONAUT, ARLG Reference Group for the Testing of Novel Therapeutics; COMBINE, Ceftazidime-Avibactam in Combination With Aztreonam; CRACKLE, Consortium on Resistance Against Carbapenems in Klebsiella and Other Enterobacterales; PHAGE, Study of the Safety and Microbiologic Activity of Bacteriophages; PHAT, Phages That Target MDR Bacteria; POP, Prospective Observational Pseudomonas Study; PROOF, Pharmacokinetics, Pharmacodynamics and Safety/Tolerability of Two Dosing Regimens of Oral Fosfomycin Tromethamine in Healthy Adult Participants; SCENE, Screening for Colonization with Resistant Enterobacterales in Neutropenic Patients with Hematologic Malignancies; SHREC, Study of Highly Resistant Escherichia coli; SNAP, Study Network of Acinetobacter as a Carbapenem-Resistant Pathogen.
Table 1.
Research Priorities for the Gram-negative Committee in the Antibacterial Resistance Leadership Group
| Research Priority/Study Title | Study Description | Status |
|---|---|---|
| 1. Define the global epidemiology of multidrug-resistant gram-negative bacterial infections to optimize the design and execution of interventional studies | ||
| Multi-Drug Resistant Organism (MDRO) Network | Observational cohort studies of hospitalized patients with resistant gram-negative bacteria | … |
| CRACKLE-2 (Consortium on Resistance Against Carbapenems in Klebsiella and Other Enterobacterales) | International cohort of patients with carbapenem-resistant Enterobacterales | Analysis partially completed [4–7] |
| POP (Prospective Observational Pseudomonas Study) | International cohort of patients with carbapenem-resistant Pseudomonas aeruginosa | Analysis partially completed [8] |
| SNAP (Study Network of Acinetobacter as a Carbapenem-Resistant Pathogen) | International cohort of patients with carbapenem-resistant Acinetobacter baumannii | Analysis partially completed [9] |
| SHREC (Study of Highly Resistant Escherichia coli) | Domestic cohort of patients with ceftriaxone-resistant and ceftriaxone-susceptible E. coli bloodstream infections | Analysis partially completed [10] |
| 2. Evaluate novel strategies to prevent multidrug-resistant gram-negative bacterial infections in immunocompromised hosts | ||
| SCENE (Screening for Colonization with Resistant Enterobacterales in Neutropenic Patients with Hematologic Malignancies) | Multicenter cohort study to assess the prevalence and clinical impact of colonization with fluoroquinolone-resistant Enterobacterales in neutropenic patients with hematologic cancers | Ongoing |
| 3. Identify strategies to optimize the administration of antibiotics for the treatment of gram-negative bacterial infections | ||
| PROOF (Pharmacokinetics, Pharmacodynamics and Safety/Tolerability of Two Dosing Regimens of Oral Fosfomycin Tromethamine in Healthy Adult Participants) | Phase 1 clinical trial to evaluate the pharmacokinetics, pharmacodynamics, and safety/tolerability of different dosing regimens of oral fosfomycin | Completed [11, 12] |
| FOCUS (The Fosfomycin Oral for Complicated Urinary Syndromes Study) | Randomized clinical trial to assess the safety and efficacy of fosfomycin versus levofloxacin oral step-down therapy for complicated urinary tract infections | Data analysis underway |
| ACUMIN (Acute Care Unit Minocycline) | Phase 4 clinical trial to evaluate the pharmacokinetics of intravenous minocycline in critically ill patients | Completed [13] |
| COMBINE (Ceftazidime-Avibactam in Combination with Aztreonam) | Phase 1 clinical trial to evaluate the safety and pharmacokinetics of ceftazidime-avibactam in combination with aztreonam | Completed [14, 15] |
| 4. Identify and evaluate novel antimicrobial agents or treatment strategies for multidrug-resistant gram-negative bacterial infections | ||
| ARGONAUT III, IV, and V (ARLG Reference Group for the Testing of Novel Therapeutics) | Characterization of the in vitro activity of novel agents against genetically defined clinical isolates of carbapenem-resistant gram-negative bacteria | Analysis partially completed |
| PHAT (Phages That Target MDR Bacteria) | Isolation and characterization of lytic bacteriophages that target P. aeruginosa and Enterobacter species | Completed [16, 17] |
| PHAGE (Study of the Safety and Microbiologic Activity of Bacteriophages) | Phase 1b/2 randomized, placebo-controlled trial of the safety and microbiologic activity of a single intravenous dose of bacteriophage therapy in patients with cystic fibrosis who are colonized with P. aeruginosa | Ongoing [18] |
Abbreviations: ARLG, Antibacterial Resistance Leadership Group; MDR, multidrug-resistant.
RESEARCH PRIORITY 1: DEFINE THE GLOBAL EPIDEMIOLOGY OF MULTIDRUG-RESISTANT GRAM-NEGATIVE BACTERIAL INFECTIONS TO OPTIMIZE THE DESIGN AND EXECUTION OF INTERVENTIONAL STUDIES
Multidrug-Resistant Organism Network Studies
CRACKLE was initially launched as a multicenter, observational study at hospitals in the Midwest United States to better understand the emerging public health problem of CRE [19]. Under ARLG leadership, CRACKLE evolved into a global network, the Multi-Drug Resistant Organism (MDRO) Network to comprehensively study clinically important gram-negative pathogens and infections from 5 regions (the United States, South/Central America, China, the Middle East, and Australia/Singapore). Four prospective, observational studies of hospitalized patients have been completed to address specific pathogens: CRACKLE-2 for CRE, POP for CRPA, Study Network of Acinetobacter as a Carbapenem-Resistant Pathogen (SNAP) for CRAB, and SHREC for ceftriaxone-resistant E. coli (see Table 1 for full names of all studies mentioned in the text). MDRO Network studies combine detailed clinical data with in-depth phenotypic and genotypic characterization of isolates. In addition, these studies assess the feasibility of, and build infrastructure for, future interventional trials that will address these key antimicrobial-resistant gram-negative infections. Table 2 summarizes the key features and findings of each study.
Table 2.
Summary of Key Features and Findings of the Multi-Drug Resistant Organism (MDRO) Network Studies
| Study | Isolates | Setting and Years | No. of Patients | Carbapenemase Prevalence | 30-d Mortality Rate in Infected Patients | Summary of Key Findings |
|---|---|---|---|---|---|---|
| CRACKLE- 2 [4–6] |
CRE (all body sites) |
49 US hospitals; 2016–2017 |
1040 | 59% (92% of CP-CRE had KPC) |
Overall: 24% (107/449) |
|
| CRKP (all body sites) |
71 Hospitals in South America, Australia, China, Lebanon, Singapore, and the US; 2017–2018 |
991 | 90% (91% of CP-CRE had KPC); Australia, Lebanon, and Singapore: 75%; China: 98%; South America: 75%; US: 88% |
Overall: 19% (93/502); BSI: 34% (44/130) |
|
|
| CP Escherichia coli (all body sites) |
26 Hospitals in Australia, China, Colombia, Lebanon, Singapore, and the US; 2017–2018 |
114 | 57% Serine carbapenemase, 43% MBLs (96% of MBLs had NDM) | Overall: 16% (18/114) |
|
|
| POP [8] | CRPA (bloodstream, respiratory, urine, and wound isolates) |
44 Hospitals in South/Central America, Australia, China, Lebanon, Saudi Arabia, Singapore, and the US; 2018–2019 |
972 | 22% (49% of CP-CRPA had KPC-2% and 36% had VIM-2); Australia and Singapore: 57%; China: 32%; Middle East: 30%; South/Central America: 69%; US: 2% |
Overall: 18% (105/581); BSI: 30% (21/69); pneumonia: 19% (69/358) |
|
| SNAP | CRAB (all sites) |
46 Hospitals in South/Central America, Australia, China, Lebanon, Saudi Arabia, Singapore, and the US; 2017–2019 |
842 | 91% (88% of CP-CRAB had OXA-23); Australia and Singapore: 100%; China: 99%; Middle East: 100%; South/Central America: 92%; US: 83% | Overall: 24% (128/536); BSI: 42% |
|
| SHREC [10] |
E. coli
(bloodstream isolates) |
14 US hospitals; 2020–2021 |
300 | NA (150 CRO-resistant, 150 CRO-susceptible) | CRO-resistant: 13% (20/150); CRO-susceptible: 8% (12/150) |
|
Abbreviations: BSI, bloodstream infection; CG, clonal group; CP, carbapenemase-producing; CRAB, carbapenem-resistant Acinetobacter baumannii; CRACKLE-2, Consortium on Resistance Against Carbapenems in Klebsiella and Other Enterobacterales; CRE, carbapenem-resistant Enterobacterales; CRKP, carbapenem-resistant Klebsiella pneumoniae; CRO, ceftriaxone; CRPA, carbapenem-resistant Pseudomonas aeruginosa; DOOR, desirability of outcome ranking; KPC, Klebsiella pneumoniae carbapenemase; MBL, metallo-β-lactamase; NA, not applicable; NDM, New Delhi metallo-β-lactamase; POP, Prospective Observational Pseudomonas Study; SHREC, Study of Highly Resistant Escherichia coli; SNAP, Study Network of Acinetobacter as a Carbapenem-Resistant Pathogen; VIM-2, Verona integron-encoded metallo-β-lactamase 2.
CRE (CRACKLE-2)
Patients were enrolled into CRACKLE-2 based on antimicrobial susceptibility testing at participating hospitals using the Centers for Disease Control and Prevention's CRE definition [20]. Carbapenemase genes were present in 59% of CRE isolates from 1040 patients at US hospitals, with 92% of carbapenemase-producing (CP) CRE isolates harboring a K. pneumoniae carbapenemase gene [4]. Some isolates without a carbapenemase gene were susceptible to all carbapenems when tested in a central laboratory. However, clinical outcomes did not differ significantly among patients infected with CP-CRE, non-CP-CRE, or isolates that tested carbapenem susceptible in the central laboratory. In an international cohort of 991 patients with carbapenem-resistant K. pneumoniae, 90% of carbapenem-resistant K. pneumoniae isolates harbored a carbapenemase gene, blaKPC in 91% [5]. Important differences in patient and bacterial characteristics and clinical outcomes between global regions were identified. A bacterial genetic marker that encodes a bacterial lipopolysaccharide, O2 variant 2, was associated with lower mortality rate, highlighting the need for additional research on bacterial biomarker–based risk stratification.
In addition, CRACKLE-2 enrolled 114 patients with CP E. coli, of whom 43% had metallo-β-lactamases (MBLs) [6]. Patients infected with MBL-producing E. coli had a higher probability of a favorable desirability of outcome ranking (DOOR) and lower 30-day mortality rate than patients infected with E. coli that harbored serine carbapenemases.
CRPA (POP)
In the first analysis of 972 patients with CRPA enrolled in POP, the 30-day mortality rate was 18% among infected patients [8]. This rate was highest with bacteremia (30%), but substantial differences in mortality rates were observed among geographic regions. In addition, the prevalence of carbapenemases varied by geographic region: carbapenemase genes were present in 2% of US isolates but in 30%, 32%, 57%, and 69% of isolates from the Middle East, China, Australia/Singapore, and South/Central America, respectively. K. pneumoniae carbapenemase 2 and Verona integron-encoded metallo-β-lactamase 2 were the most common carbapenemases. Compared with CRPA isolates without a carbapenemase, CP-CRPA isolates were more likely to exhibit high-level meropenem resistance and less likely to be susceptible to other antipseudomonal agents. Infections caused by CP-CRPA were associated with increased mortality rates compared with those caused by CRPA without a carbapenemase. These findings highlight the global geographic variability in CRPA, with implications for the optimal diagnosis and treatment of CRPA infections in each region and for the design of interventional trials of therapeutics against CRPA.
CRAB (SNAP)
CRAB has emerged as a critical priority antibiotic-resistant pathogen [2]. SNAP enrolled 990 patients with CRAB, and clinical and genome sequencing data are available for 842 of them. Marked geographic differences in clinical presentations were evident. The 30-day mortality rate was 24% for infected patients, and bloodstream infection and higher age-adjusted Charlson comorbidity index were independently associated with the 30-day mortality rate. Clonal group 2 strains predominated in all regions except South/Central America, representing 59%–97% of strains in other regions. These findings highlight differences in CRAB infection types and clinical outcomes across regions and will inform the design and execution of future interventional studies of this increasingly important pathogen.
Ceftriaxone-Resistant E. coli (SHREC)
The incidence of ceftriaxone-resistant E. coli has increased owing to increasing numbers of isolates that produce ESBLs [2]. SHREC compared the clinical outcomes of 300 patients with bloodstream infections due to ceftriaxone-resistant (n = 150) and ceftriaxone-susceptible E. coli (n = 150) [10]. Patients infected with ceftriaxone-resistant E. coli were more likely than those infected with ceftriaxone-susceptible E. coli to have a high acuity of illness and longer delays until receipt of active antibiotic therapy (12 vs 1 hour, respectively). Unadjusted DOOR analyses indicated a significantly higher probability of a worse clinical outcome in the ceftriaxone-resistant group. In addition, these patients had a longer median postinfection length of stay (8 vs 6 days, respectively) and were more likely to be admitted to a long-term care facility (22% vs 12%). However, no differences were observed in the DOOR probability or 30-day mortality rate between groups after adjustments for confounding. Thus, patients with ceftriaxone-resistant E. coli bloodstream infection generally had worse clinical outcomes than those with ceftriaxone-susceptible E. coli bloodstream infection, with differences driven primarily by host factors.
RESEARCH PRIORITY 2: EVALUATE NOVEL STRATEGIES TO PREVENT MULTIDRUG-RESISTANT GRAM-NEGATIVE INFECTIONS IN IMMUNOCOMPROMISED HOSTS
Screening for Colonization With Fluoroquinolone-Resistant Enterobacterales in Neutropenic Patients (SCENE)
Patients with hematologic cancers and hematopoietic cell transplant (HCT) recipients who receive chemotherapy frequently experience severe neutropenia and gastrointestinal mucositis, placing them at high risk for life-threatening bacteremia from Enterobacterales [21]. Fluoroquinolone (FQ) prophylaxis is recommended to prevent bacterial infections in patients expected to have prolonged neutropenia [22]. However, FQ resistance has become increasingly common, and many FQ-resistant Enterobacterales (FQRE) that cause breakthrough infection also produce ESBLs that confer resistance to therapies for fever and neutropenia [23].
SCENE is an observational study to determine whether screening for FQRE colonization before neutropenia can identify patients who are at high risk of gram-negative bacteremia despite FQ prophylaxis. A single-center study found that 31% of HCT recipients colonized with FQRE before transplantation developed gram-negative bacteremia during posttransplant neutropenia, compared with only 1% of noncolonized patients [24]. SCENE expands on these findings by enrolling 820 patients who have acute leukemia or are undergoing HCT at 10 oncology centers. Participants receive FQ prophylaxis per the standard of care, are screened for colonization with FQRE before neutropenia, and are followed up for episodes of bacteremia. The incidence of gram-negative bacteremia during neutropenia will be compared between FQRE-colonized and noncolonized patients, and bloodstream isolates will be compared with colonizing strains to determine whether participants develop bacteremia from their colonizing strain.
RESEARCH PRIORITY 3: IDENTIFY STRATEGIES TO OPTIMIZE THE ADMINISTRATION OF ANTIBIOTICS FOR THE TREATMENT OF GRAM-NEGATIVE INFECTIONS
Pharmacokinetics, Pharmacodynamics, and Safety/Tolerability of Oral Fosfomycin (PROOF)
E. coli is the most common urinary pathogen and has increasingly become resistant to oral therapies, such as FQs, trimethoprim-sulfamethoxazole, and oral β-lactam agents. Thus, there is a critical need for alternative oral antibiotics to treat complicated urinary tract infections (cUTIs) [2]. Fosfomycin tromethamine is an old oral agent that remains active against most E. coli strains, including ESBL-producing E. coli. It is approved in the United States as a single-dose treatment of uncomplicated urinary tract infection, but the pharmacokinetic-pharmacodynamic (PK/PD) profiles and safety of multiple-dose regimens for cUTI are unknown. The PROOF study randomized 18 healthy adult participants to receive oral fosfomycin 3 g every other day for 3 doses or 3 g daily for 7 days, followed by a crossover period when participants received the opposite regimen [11]. Systemic plasma PK parameters on days 1 and 5 and cumulative urinary excretion of fosfomycin were similar after every-other-day or daily dosing. The most common fosfomycin-related treatment-emergent adverse events were gastrointestinal. Daily dosing of fosfomycin was associated with significantly more diarrhea than the every-other-day regimen (diarrhea-free days, 61% [daily] vs 77% [every other day]; P < .001).
In addition, PROOF evaluated the ex vivo urinary bactericidal activity and PD profiles of fosfomycin against strains of E. coli, K. pneumoniae, and Proteus mirabilis [12]. Urinary antibacterial activity of fosfomycin was similar for the daily and every-other-day dosing regimens. Fosfomycin had reliable urinary bactericidal activity against E. coli, but not against the other organisms.
Fosfomycin for Complicated Urinary Tract Infection (FOCUS)
FOCUS was a multicenter, randomized, open-label pragmatic superiority trial that evaluated the efficacy of oral fosfomycin versus oral levofloxacin in cUTIs, including pyelonephritis. The trial compared 2 strategies for initial or step-down oral therapy for cUTI without bacteremia after 0–48 hours of parenteral antibiotic therapy. Participants received 3 g of fosfomycin or 750 mg (or dose adjusted for kidney function) of levofloxacin daily for 5–7 days. Clinical and microbiological cures were assessed at the end of therapy and test of cure (approximately 21 days from the start of antibiotics). The study used a unique pragmatic design, Comparing Personalized Antibiotic Strategies (COMPASS), valuable in the AR setting because the management of patients with cUTI is dynamic. Treatment was tailored based on AR patterns and participants’ ability to tolerate the prescribed antibiotic. This approach was different from conventional cUTI trials that compare drugs rather than treatment strategies. Analysis of the trial data is ongoing.
Minocycline Pharmacokinetics in Critically Ill Patients (ACUMIN)
ACUMIN was a phase IV PK study that evaluated intravenous minocycline concentrations in critically ill patients [13]. Approved for intravenous use by the US Food and Drug Administration (FDA) in 2015, minocycline is increasingly used to treat infections caused by multidrug-resistant (MDR) A. baumannii [25]. Before this study, little was known about the PK profile of intravenous minocycline, because prior PK data were only from uninfected participants who were not critically ill [26]. Furthermore, it was unclear whether intravenous minocycline required dosage adjustments for renal impairment or whether FDA-approved dosages in critically ill patients were sufficient to reliably achieve PK/PD exposure targets associated with the killing of A. baumannii [27, 28].
ACUMIN found no relationship between minocycline clearance and creatinine clearance, indicating that the dosage should not be adjusted for renal impairment. In addition, the study found that dose adjustments are not required for weight, sex, or albumin concentrations. The PK/PD target attainment analyses suggested that even the highest FDA-approved intravenous minocycline dosage (200 mg every 12 hours) confers a suboptimal probability of target attainment in plasma for a substantial proportion of critically ill patients with A. baumannii infections. These analyses suggest that combination antibiotic therapy should be considered in patients with Acinetobacter infections when intravenous minocycline is used and that the highest FDA-approved dosage of 200 mg every 12 hours should be used to improve the probability of target attainment. These data cast uncertainty on the appropriateness of the current FDA and the Clinical and Laboratory Standards Institute minocycline susceptibility breakpoint of ≤4 µg/mL.
Ceftazidime-Avibactam in Combination With Aztreonam (COMBINE)
Treatment of infections caused by MBL-producing gram-negative bacteria is challenging because MBLs hydrolyze all β-lactams except aztreonam and are not inhibited by available β-lactamase inhibitors [29]. Although MBLs do not directly hydrolyze aztreonam, these bacteria are frequently resistant to aztreonam because they produce other β-lactamases that hydrolyze aztreonam. Since avibactam inhibits most β-lactamases except MBLs, the combination of ceftazidime-avibactam and aztreonam effectively kills MBL-producing bacteria in vitro [30]. COMBINE was a phase 1 clinical trial to assess the safety and PK of this combination in healthy volunteers. Participants received ceftazidime-avibactam or aztreonam by continuous or intermittent infusion or ceftazidime-avibactam combined with aztreonam by intermittent infusion for 7 days [14].
Overall, 19 (40%) of 48 participants had alanine aminotransferase/aspartate aminotransferase (ALT/AST) elevations, and 2 participants who received 8 g daily aztreonam by continuous infusion experienced severe ALT/AST elevations. All participants with ALT/AST elevations were asymptomatic, with no other findings suggestive of liver injury, and the addition of ceftazidime-avibactam to aztreonam did not increase the risk of ALT/AST elevation. In the population PK analyses, administration of the combination reduced aztreonam clearance by 16% but had a negligible effect on ceftazidime clearance [15]. These results suggest that the combination of ceftazidime-avibactam and aztreonam is safe when administered as 2-hour intermittent infusions, but close monitoring of liver function is prudent when using high-dose aztreonam by continuous infusion.
RESEARCH PRIORITY 4: IDENTIFY AND EVALUATE NOVEL ANTIMICROBIAL AGENTS OR TREATMENT STRATEGIES FOR MDR GRAM-NEGATIVE BACTERIAL INFECTIONS
In Vitro Activity of New Agents vs Carbapenem-Resistant Gram-negatives (ARGONAUT)
The development of novel treatments for carbapenem-resistant gram-negative pathogens remains a critical priority, and a comprehensive understanding of the spectrum of activity of new antimicrobial agents is essential. To address this need, ARGONAUT studies examined the in vitro activity of novel therapeutics against genetically characterized carbapenem-resistant gram-negative isolates. ARGONAUT-III, IV, and V examined the susceptibility of K. pneumoniae and P. aeruginosa isolates to cefepime-taniborbactam and ceftibuten–VNRX-5236. Taniborbactam is a novel boronic acid transition state inhibitor that inhibits both serine carbapenemases and MBLs [31]. Partnered with cefepime, this combination had positive results in a phase 3 clinical trial for cUTIs. VNRX-5236 is another boronic acid transition state inhibitor that inhibits ESBLs and serine carbapenemases and is being developed as an orally bioavailable prodrug, VNRX-7145 [32]. The combination of VNRX-7145 with ceftibuten (an oral cephalosporin) is currently in phase 1 clinical trials.
ARGONAUT-III and IV evaluated the in vitro activity of cefepime-taniborbactam and cefibuten–VNRX-5236, respectively, against 200 CP K. pneumoniae isolates from CRACKLE. The addition of taniborbactam increased cefepime susceptibility (minimum inhibitory concentration, ≤8 µg/mL) from 13.5% to 99.0%, a susceptibility percentage higher than that of meropenem-vaborbactam (95.5%) or ceftazidime-avibactam (98.0%) [33]. Using a provisional ceftibuten susceptibility breakpoint of ≤1 µg/mL, the addition of VNRX-5236 increased ceftibuten susceptibility from 4.5% to 92.5%, highlighting the potential for the combination to be an effective oral agent for carbapenem-resistant K. pneumoniae infections.
ARGONAUT-V evaluated the in vitro activity of cefepime-taniborbactam against 197 P. aeruginosa isolates previously characterized [34]. Taniborbactam increased cefepime susceptibility (minimum inhibitory concentration, ≤8 µg/mL) from 67.0% to 82.7%, edging out ceftazidime-avibactam (79.7%) and ceftolozane-tazobactam (77.7%).
Isolation and Characterization of Bacteriophages Against MDR Gram-negatives (PHAT)
Phages (viruses of bacteria) were historically used to treat bacterial infections before the availability of antibiotics and have gained renewed interest as potential therapies for AR infections [35]. Phages are attractive alternatives to broad-spectrum antibacterials because they are specific for target pathogens, leaving other members of the microbiome intact, and their effect is self-limited because they replicate only in the presence of a susceptible bacterial host. In the PHAT study, phages that target P. aeruginosa and Enterobacter spp. were isolated, characterized, and assembled into species-specific libraries [16, 17]. These libraries were then made available for screening against clinical isolates, and several of these phages were used to treat patients with antibiotic-resistant infections under expanded access and compassionate use protocols.
Phage Therapy for Patients With Cystic Fibrosis Colonized With Pseudomonas Aeruginosa (PHAGE)
Patients with cystic fibrosis (CF) are prone to lower respiratory tract infections caused by MDR gram-negative pathogens, such as P. aeruginosa. These infections are increasingly challenging to treat because resident bacteria become increasingly resistant and are protected in biofilms, further compromising antibiotic effectiveness [36]. Thus, novel treatment approaches are needed for MDR organisms in persons with CF. Bacteriophages are particularly attractive therapies for gram-negative bacteria in patients with CF because they penetrate biofilms, avoid host tissue damage, and are synergistic with antibiotics [37].
PHAGE is a phase 1b/2 multicenter, randomized, placebo-controlled, clinical trial investigating the safety and efficacy of a single dose of intravenous phage therapy in adult volunteers with CF who have chronic respiratory colonization with P. aeruginosa [18]. The intravenous phage consists of a mixture of 4 antipseudomonal phages selected based on their broad range of activity against P. aeruginosa isolates. The primary study outcomes include the safety and microbiological activity of intravenous phage therapy. This trial will provide important insights into the safety and efficacy of phage therapy and establish a foundation for future larger, multidose phage trials.
ARLG FUTURE DIRECTIONS AND UNMET NEEDS IN GRAM-NEGATIVE BACTERIAL INFECTIONS
The overarching priority of the ARLG is to improve the diagnosis and treatment of infections caused by antimicrobial-resistant bacteria. For gram-negatives, our effort has focused mainly on understanding and improving therapy for ESBL-producing and carbapenem-resistant bacteria. The ARLG has designed and implemented natural history, PK, and interventional studies to address relevant knowledge gaps for the treatment of these organisms. Future directions will be guided by a survey conducted by the ARLG in collaboration with the Infectious Diseases Society of America, in which the top outstanding scientific questions endorsed by the infectious diseases community were “Are there safe and effective carbapenem-sparing regimens for ESBL-positive bloodstream infections?” and “What is the role of combination therapy versus monotherapy for the treatment of drug-resistant gram-negatives?” The ARLG will continue to work on identifying study opportunities that are feasible and will help answer these important study questions to improve care for patients affected by these pathogens.
Contributor Information
Michael J Satlin, Department of Pathology and Laboratory Medicine, Weill Cornell Medicine, New York, New York, USA; Division of Infectious Diseases, Department of Medicine, Weill Cornell Medicine, New York, New York, USA.
David van Duin, Division of Infectious Diseases, Department of Medicine, University of North Carolina School of Medicine, Chapel Hill, North Carolina, USA.
Pranita D Tamma, Division of Pediatric Infectious Diseases, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Thomas P Lodise, Department of Pharmacy Practice, Albany College of Pharmacy and Health Sciences, Albany, New York, USA.
Daria Van Tyne, Division of Infectious Diseases, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Keith A Rodvold, Department of Pharmacy Practice, College of Pharmacy, University of Illinois at Chicago, Chicago, Illinois, USA.
Nadine Rouphael, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Scott R Evans, Department of Biostatistics, George Washington University, Washington, DC, USA.
Vance G Fowler, Division of Infectious Diseases, Department of Medicine, Duke University School of Medicine, Durham, North Carolina, USA; Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina, USA.
Toshimitsu Hamasaki, George Washington University Biostatistics Center, Rockville, Maryland, USA.
Robin Patel, Division of Clinical Microbiology and Division of Public Health, Infectious Diseases, and Occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Lauren Komarow, George Washington University Biostatistics Center, Rockville, Maryland, USA.
Keri Baum, Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina, USA.
Maria Souli, Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina, USA.
Nyssa Schwager, Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina, USA.
Robert A Bonomo, Research Service, Louis Stokes Cleveland Veterans Affairs Medical Center, Cleveland, Ohio, USA.
Yohei Doi, Division of Infectious Diseases, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Departments of Microbiology and Infectious Diseases, Fujita Health University School of Medicine, Aichi, Japan.
for the Antibacterial Resistance Leadership Group:
Minggui Wang, Eric Wenzler, Robert Schooley, Beth Evans, Deborah Hopkins, Felecia Cathcart, Elizabeth Mocka, Carl Schuler, Zoe Sund, Smitha Zaharoff, Cathy Wickward, Leslie Estes, Donald Mau, Grant Booth, Erin Abbenante, Minal Bhojani, Hirra Zahir, Lizhao Ge, Abhigya Giri, Weixiao Dai, Guoqing Diao, Tamara Fidler, Wanying Shao, Yixuan Li, Lijuan Zeng, Shanshan Zhang, Kerryl Greenwood-Quaintance, Suzannah Schmidt Malan, Krupa Mukesh Parmar, Scott Cunningham, Varduhi Ghazaryan, Erica Raterman, Tamika Samuel, Marina Lee, and Seema Nayak
Notes
Acknowledgments. The authors thank the following study team members for their contributions, making studies and results possible: Minggui Wang, Eric Wenzler, Robert Schooley, Beth Evans, Deborah Hopkins, Felecia Cathcart, Elizabeth Mocka, Carl Schuler, Zoe Sund, Smitha Zaharoff, Cathy Wickward, Leslie Estes, Donald Mau, Grant Booth, Erin Abbenante, Minal Bhojani, Hirra Zahir, Lizhao Ge, Abhigya Giri, Weixiao Dai, Guoqing Diao, Tamara Fidler, Wanying Shao, Yixuan Li, Lijuan Zeng, Shanshan Zhang, Kerryl Greenwood-Quaintance, Suzannah Schmidt Malan, Krupa Mukesh Parmar, Scott Cunningham, Varduhi Ghazaryan, Erica Raterman, Tamika Samuel, Marina Lee, Seema Nayak, other Division of Microbiology and Infectious Diseases (DMID) team members, and the members of the EMMES and Clinical Research Operations and Management Support (CROMS) teams. The authors also thank all study sites and study participants, without whom this work would not be possible.
Author Contributions. M. J. S. and Y. D. conceptualized the manuscript and drafted the overall manuscript. S. R. E., V. G. F., T. H., R. P., K. B., M. S., and N. S. were responsible for the acquisition, analysis, or interpretation of data. L. K. provided statistical analysis. D. v. D., P. D. T., T. P. L., D. V. T., K. A. R., N. R., and R. A. B. drafted sections of the manuscript. M. J. S. and Y. D. finalized the manuscript. V. G. F. obtained funding. K. B., M. S., and N. S. provided administrative, technical, or material support. V. G. F., K. B., M. S., and N. S. provided supervision. All authors were involved with the scientific review and editing of the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant UM1AI104681).
Supplement sponsorship. This article appears as part of the supplement “The Antibacterial Resistance Leadership Group (ARLG): Innovation and Evolution,” sponsored by the Antibacterial Resistance Leadership Group.
References
- 1. Antimicrobial Resistance Collaborators . Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399:629–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Antibiotic resistance threats in the United States, 2019. Available at:http://cdc.gov/drugresistance/biggest-threates.html. Accessed 22 May 2023.
- 3. Centers for Disease Control and Prevention . COVID-19: U.S. impact of antimicrobial resistance: 2022 special report. Available at:http://cdc.gov/drugresistance/pdf/covid19-impact-report-508.pdf. Accessed 22 May 2023.
- 4. van Duin D, Arias CA, Komarow L, et al. . Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): a prospective cohort study. Lancet Infect Dis 2020; 20:731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang M, Earley M, Chen L, et al. . Clinical outcomes and bacterial characteristics of carbapenem-resistant Klebsiella pneumoniae complex among patients from different global regions (CRACKLE-2): a prospective, multicentre cohort study. Lancet Infect Dis 2022; 22:401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boutzoukas AE, Komarow L, Chen L, et al. . International epidemiology of carbapenemase-producing Escherichia coli. Clin Infect Dis 2023; 77:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shrestha R, Luterbach CL, Dai W, et al. . Characteristics of community-acquired carbapenem-resistant Enterobacterales. J Antimicrob Chemother 2022; 77:2763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reyes J, Komarow L, Chen L, et al. . Global epidemiology and clinical outcomes of carbapenem-resistant Pseudomonas aeruginosa and associated carbapenemases (POP): a prospective cohort study. Lancet Microbe 2023; 4:e159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iovleva A, Mustapha MM, Griffith MP, et al. . Carbapenem-resistant Acinetobacter baumannii in U.S. hospitals: diversification of circulating lineages and antimicrobial resistance. mBio 2022; 13:e0275921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tamma PD, Komarow L, Ge L, et al. . Clinical impact of ceftriaxone resistance in Escherichia coli bloodstream infections: a multicenter prospective cohort study. Open Forum Infect Dis 2022; 9:ofac572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wenzler E, Bleasdale SC, Sikka M, et al. . Phase I study to evaluate the pharmacokinetics, safety, and tolerability of two dosing regimens of oral fosfomycin tromethamine in healthy adult participants. Antimicrob Agents Chemother 2018; 62:e00464-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wenzler E, Meyer KM, Bleasdale SC, et al. . Ex vivo urinary bactericidal activity and urinary pharmacodynamics of fosfomycin after two repeated dosing regimens of oral fosfomycin tromethamine in healthy adult subjects. Antimicrob Agents Chemother 2020; 64:e02102-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lodise TP, Van Wart S, Sund ZM, et al. . Pharmacokinetic and pharmacodynamic profiling of minocycline for injection following a single infusion in critically ill adults in a phase IV open-label multicenter study (ACUMIN). Antimicrob Agents Chemother 2021; 65:e01809-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lodise TP, O'Donnell JN, Raja S, et al. . Safety of ceftazidime-avibactam in combination with aztreonam (COMBINE) in a phase I, open-label study in healthy adult volunteers. Antimicrob Agents Chemother 2022; 66:e0093522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lodise TP, O'Donnell JN, Balevic S, et al. . Pharmacokinetics of ceftazidime-avibactam in combination with aztreonam (COMBINE) in a phase 1, open-label study of healthy adults. Antimicrob Agents Chemother 2022; 66:e0093622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finney AG, Perry JM, Evans DR, et al. . Isolation and characterization of lytic bacteriophage targeting diverse Enterobacter spp. clinical isolates. Phage (New Rochelle) 2022; 3:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nordstrom HR, Evans DR, Finney AG, et al. . Genomic characterization of lytic bacteriophages targeting genetically diverse Pseudomonas aeruginosa clinical isolates. iScience 2022; 25:104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tamma PD, Souli M, Billard M, et al. . Safety and microbiologic activity of phage therapy in persons with cystic fibrosis colonized with Pseudomonas aeruginosa: study protocol for a phase 1b/2, multicenter, randomized, double-blind, placebo-controlled trial. Trials 2022; 23:1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Duin D, Perez F, Rudin SD, et al. . Surveillance of carbapenem-resistant Klebsiella pneumoniae: tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother 2014; 58:4035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention . Healthcare-associated infections (HAI): CRE technical information. Available at:https://www.cdc.gov/hai/organisms/cre/technical-info.html#Definition. Accessed 24 May 2023.
- 21. Freifeld AG, Bow EJ, Sepkowitz KA, et al. . Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52:427–31. [DOI] [PubMed] [Google Scholar]
- 22. Taplitz RA, Kennedy EB, Bow EJ, et al. . Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J Clin Oncol 2018; 36:3043–54. [DOI] [PubMed] [Google Scholar]
- 23. Satlin MJ, Chavda KD, Baker TM, et al. . Colonization with levofloxacin-resistant extended-spectrum β-lactamase-producing Enterobacteriaceae and risk of bacteremia in hematopoietic stem cell transplant recipients. Clin Infect Dis 2018; 67:1720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Satlin MJ, Chen L, Douglass C, et al. . Colonization with fluoroquinolone-resistant Enterobacterales decreases the effectiveness of fluoroquinolone prophylaxis in hematopoietic cell transplant recipients. Clin Infect Dis 2021; 73:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goff DA, Kaye KS. Minocycline: an old drug for a new bug: multidrug-resistant Acinetobacter baumannii. Clin Infect Dis 2014; 59(suppl 6):S365–6. [DOI] [PubMed] [Google Scholar]
- 26. Welling PG, Shaw WR, Uman SJ, Tse FL, Craig WA. Pharmacokinetics of minocycline in renal failure. Antimicrob Agents Chemother 1975; 8:532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ritchie DJ, Garavaglia-Wilson A. A review of intravenous minocycline for treatment of multidrug-resistant Acinetobacter infections. Clin Infect Dis 2014; 59(suppl 6):S347–80. [DOI] [PubMed] [Google Scholar]
- 28. United States Food and Drug Administration: MINOCIN®: minocycline for injection. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050444s049lbl.pdf. Accessed 23 May 2023.
- 29. Yang Y, Yan YH, Schofield CJ, McNally A, Zong Z, Li GB. Metallo-β-lactamase-mediated antimicrobial resistance and progress in inhibitor discovery. Trends Microbiol 2023; 37:735–48. [DOI] [PubMed] [Google Scholar]
- 30. Lodise TP, Smith NM, O'Donnell N, et al. . Determining the optimal dosing of a novel combination regimen of ceftazidime/avibactam with aztreonam against NDM-1-producing Enterobacteriaceae using a hollow-fibre infection model. J Antimicrob Chemother 2020; 75:2522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hamrick JC, Docquier JD, Uehara T, et al. . VNRX-5133 (taniborbactam), a broad-spectrum inhibitor of serine- and metallo-β-lactamases, restores activity of cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2020; 64:e01963-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chatwin CL, Hamrick JC, Trout REL, et al. . Microbiological characterization of VNRX-5236, a broad-spectrum β-lactamase inhibitor for rescue of the orally bioavailable cephalosporin cefibuten as a carbapenem-sparing agent against strains of Enterobacterales expressing extended-spectrum β-lactamases and serine carbapenemases. Antimicrob Agents Chemother 2021; 65:e0055221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clinical and Laboratory Standards Institute (CLSI) . Performance standards for antimicrobial susceptibility testing. 30th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute, 2020. [Google Scholar]
- 34. Evans SR, Tran TTT, Hujer AM, et al. . Rapid molecular diagnostics to inform empiric use of ceftazidime/avibactam and ceftolozane/tazobactam against Pseudomonas aeruginosa: PRIMERS IV. Clin Infect Dis 2019; 68:1823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Domingo-Calap P, Delgado-Martínez J. Bacteriophages: protagonists of a post-antibiotic era. Antibiotics (Basel) 2018; 7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev 2022; 15:194–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suh GA, Lodise TP, Tamma PD, et al. . Considerations for the use of phage therapy in clinical practice. Antimicrob Agents Chemother 2022; 66:e0207121. [DOI] [PMC free article] [PubMed] [Google Scholar]