Abstract
Objective
Determine if targeting higher transcutaneous carbon dioxide improves respiratory stability among very preterm infants on ventilatory support.
Design
Single-centre pilot randomised clinical trial.
Setting
The University of Alabama at Birmingham.
Patients
Very preterm infants on ventilatory support after postnatal day 7.
Interventions
Infants were randomised to two different transcutaneous carbon dioxide levels targeting 5 mm Hg (0.67 kPa) changes with four sessions each lasting 24 hours for 96 hours: baseline-increase-baseline-increase or baseline-decrease-baseline-decrease.
Main outcome measures
We collected cardiorespiratory data evaluating episodes of intermittent hypoxaemia (oxygen saturations (SpO2)<85% for ≥10 s), bradycardia (<100 bpm for ≥10 s), and cerebral and abdominal hypoxaemia on near-infrared spectroscopy.
Results
We enrolled 25 infants with a gestational age of 24 w 6 d±11 d (mean±SD) and birth weight 645±142 g on postnatal day 14±3. Continuous transcutaneous carbon dioxide values (56.8±6.9 in the higher group vs 54.5±7.8 in the lower group; p=0.36) did not differ significantly between groups during the intervention days. There were no differences in intermittent hypoxaemia (126±64 vs 105±61 per 24 hours; p=0.30) or bradycardia (11±16 vs 15±23 per hour; p=0.89) episodes between groups. The proportion of time with SpO2<85%, SpO2<80%, cerebral hypoxaemia or abdominal hypoxaemia did not differ (all p>0.05). There was moderate negative correlation between mean transcutaneous carbon dioxide and bradycardia episodes (r=−0.56; p<0.001).
Conclusion
Targeting 5 mm Hg (0.67 kPa) changes in transcutaneous carbon dioxide did not improve respiratory stability among very preterm infants on ventilatory support but the intended carbon dioxide separation was difficult to achieve and maintain.
Trial registration number
INTRODUCTION
Intentionally reducing the intensity of mechanical ventilation while accepting a higher partial pressure of carbon dioxide (PaCO2) is a commonly used lung-protective strategy in the neonatal intensive care unit.1–3 As well as permitting a lower intensity of mechanical ventilation, permissive hypercapnia may increase respiratory drive.4 It has been demonstrated that CO2 inhalation decreases apnoeic episodes among preterm infants.5 In addition, apnoea may be more likely to occur among infants breathing closer to their apnoeic CO2 threshold, which is higher among preterm infants.6–8 Targeting PaCO2 above the apnoeic threshold among preterm infants on positive pressure respiratory support increases respiratory muscle activity/drive of the diaphragm and accessory respiratory muscles6 and may decrease apnoea and resultant intermittent hypoxaemia.
Alternatively, decreasing respiratory support and alveolar ventilation to achieve hypercapnia may result in respiratory instability among infants with respiratory failure. Apnoea of prematurity is more frequent among infants with altered chemoreceptor responses including those with lower alveolar ventilation, higher alveolar CO2 and a decreased respiratory responsiveness to CO2.7 The objective of this study was to test the hypothesis that targeting a higher transcutaneous CO2 (TcCO2), a surrogate marker of PaCO2, compared with a lower TcCO2 decreases apnoea, bradycardia and intermittent hypoxaemia and improves respiratory stability among very preterm infants on positive pressure respiratory support.
METHODS
Trial design, setting and participants
This single-centre trial was conducted at The University of Alabama at Birmingham. Preterm infants from 22 weeks and 0 days to 28 weeks and 6 days of gestational age and with a birth weight from 401 to 1000 g who were already enrolled in the multicenter Pre-Vent Apnoea observational study ( Clinicaltrials.gov identifier: NCT03174301) were screened for eligibility. We included infants if they remained intubated or on nasal intermittent positive pressure ventilation (NIPPV) after postnatal day 7 with a baseline pH>7.25 and PaCO2>40 mm Hg (5.3 kPa) and had a TcCO2 monitor that was correlated within 5 mm Hg (0.67 kPa) of the PaCO2. We excluded infants judged too unstable by the clinician, infants with major congenital malformations, infants with a terminal illness or decision to withdraw or limit care, and infants whose parents had refused or withdrawn informed consent.
Recruitment and randomisation
After informed consent was obtained, infants were randomised using a 1:1 parallel allocation to one of the two intervention sequences. Randomisation order was computer generated with block sizes of two to four by NA. The allocation was concealed using sequentially numbered opaque sealed envelopes until enrolment by DL.
Interventions
Infants were randomised to two different levels of permissive hypercapnia targeting 5 mm Hg (0.67 kPa) changes from baseline TcCO2 in one of two sequences with four sessions lasting 24 hours each, for a total of 96 hours. The first 24 hours of data collection was used to establish the baseline mean TcCO2 (0–24 hours). Over the next 3 days, we evaluated interventions as follows; either targeted to increase (24–48 hours) followed by return to baseline (48–72 hours), followed by increase (72–96 hours), referred to as the higher group, or decrease-baseline-decrease referred to as the lower group. For example, infants randomised to increase-baseline-increase initially had their TcCO 2 limits (based on correlation with daily capillary blood gas) increased to target 5 mm Hg (0.67 kPa) above baseline by reducing the intensity of mechanical ventilation for 24 hours. This was followed by a decrease in TcCO2 limits to target a 5 mm Hg (0.67 kPa) reduction (back to baseline) by increasing the intensity of mechanical ventilation for the next 24 hours. Then, we increased the TcCO2 limits to target a 5 mm Hg (0.67 kPa) above baseline by reducing the intensity of mechanical ventilation for 24 hours. Ventilation could be adjusted routinely every 4 hours or more frequently if clinically indicated but changes were not mandated. We recommended decreasing pressure/volume before the rate to increase the TcCO2 while the rate was increased before pressure/volume to lower the TcCO2.
These intervention limits were chosen to allow us to determine the effects of altering PaCO2 on respiratory patterns while maintaining the pH at or above 7.20. TcCO2 alarm limits used an upper limit set 15 points above the lower limit during the intervention period. Study team members updated the TcCO2 monitor alarm limits to target the intended PaCO2 and informed members of the clinical care team of the target range for each 24-hour period. Both groups were connected to dedicated study monitors (Philips IntelliVue MP50), which monitored heart rate (HR), respiratory rate (RR) and oxygen saturation (SpO2), and INVOS 5100C Cerebral/Somatic Oximeters, which measured cerebral and abdominal near-infrared spectroscopy (NIRS) using infant/neonatal sensors (Medtronic, Dublin, Ireland). TcCO2 was measured using the SenTec Digital Monitoring System (SenTec Inc, Fenton, Missouri, USA). The study monitors were connected to the central alarm system during the 96-hour study period. Masking of the intervention was not performed as TcCO2 monitors are used in routine clinical care among ventilated infants in our neonatal intensive care unit. However, the outcome assessors (AN and IA) were masked to the study group assignment.
Measures
We collected real-time cardiorespiratory data using ixTrend (ixitos GmbH, Berlin, Germany) from the study monitors.9 The primary outcome measure was the number of episodes of intermittent hypoxaemia (SpO2<85% for ≥10 s) per 24 hours. Secondary outcome measures included the number of episodes of severe intermittent hypoxaemia (SpO2<80% for 10 s) per 24 hours, the number of episodes of bradycardia (HR<100 bpm for ≥10 s) per 24 hours and the proportion of time with cerebral (<55%) and abdominal hypoxaemia (<40%).
Statistical analysis
A sample size of 24 infants was planned a priori for this pilot randomised clinical trial.10 11 We planned to enrol up to 35 patients if needed to achieve the planned sample size to account for attrition due to incomplete data collection. A data and safety monitoring board reviewed all adverse event data from this randomised trial at 6 monthly intervals or sooner for predefined major adverse events.
We used the intention-to-treat principle to compare primary and secondary outcomes. We analysed numeric data using MATLAB (MathWorks, Natick, Massachusetts, USA) and used SAS V.9.4 for all statistical analyses. To compare baseline characteristics between infants on higher TcCO2 and lower TcCO2, we used Wilcoxon test for count and continuous variables and Fisher’s exact test for categorical variables. For data collected by session, we fitted a generalised linear mixed model with random intercept to account for the repeated measures for the same subject over time using gamma distribution for continuous outcomes being modelled, negative binomial for count outcomes and beta distribution for proportions. The main goal was to test for group effects. However, we first checked if there was evidence that the group effects changed over time by fitting models with group, time and group by time interaction. Since the interaction term was not significant (indicating no evidence of group effect, if any, changing over time), the final random intercept models fitted only included group effect. We used Pearson’s correlation to determine the strength and direction of the relationship between outcome measures. A sensitivity analysis was performed removing data from days when infants received non-invasive positive pressure ventilation. No adjustment for multiple testing was done. A p value of <0.05 was considered statistically significant.
RESULTS
We randomised 29 very preterm infants (figure 1). Fifteen infants were randomised to higher TcCO2 and 14 were randomised to lower TcCO2. Three infants randomised to higher TcCO2 and one infant randomised to the lower TcCO2 group were excluded from the analysis as insufficient data were available for comparison (figure 1). Three of these infants were placed on continuous positive airway pressure and one was transferred to another facility during the intervention. We analysed data from the remaining 25 infants with sufficient data for comparison. Infants were studied on postnatal day 14±3 and had a gestational age of 24 weeks and 6±10 days (mean+SD) and birth weight 645±142 g (table 1). There were no differences in clinical characteristics, baseline ventilator parameters or baseline blood gas values.
Figure 1.
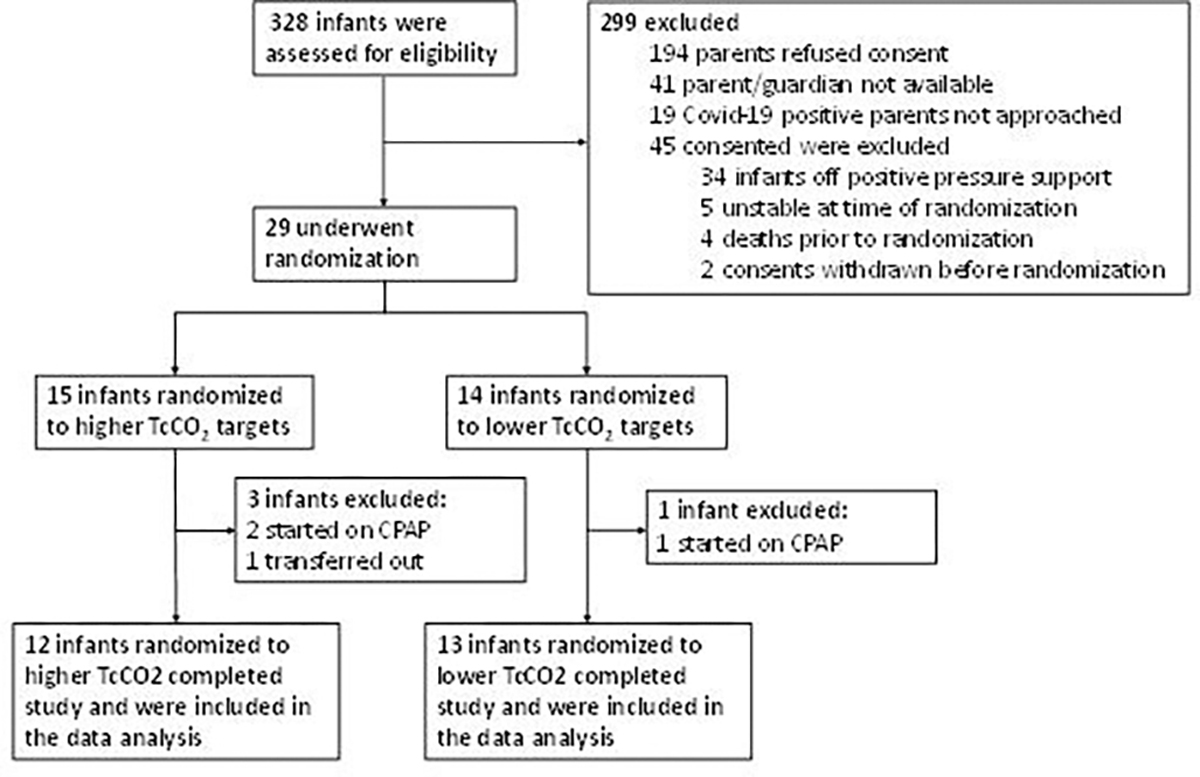
Flow diagram showing the number of infants screened, randomised and analysed. CPAP, continuous positive airway pressure.
Table 1.
Baseline characteristics and clinical demographics of study participants
| Higher TcCO2 (N=12) | Lower TcCO2 (N=13) | |
|---|---|---|
| Gestational age, weeks and days, mean±SD (days) | 25 0/7±14/7 | 24 5/7±8/7 |
| Birth weight, grams, mean±SD | 654±167 | 637±120 |
| Days after birth at study entry, days±SD | 14±3 | 14±3 |
| Male sex, no (%) | 8 (67) | 6 (46) |
| Race | ||
| Black, no (%) | 10 (83) | 7 (54) |
| White, no (%) | 2 (17) | 6 (46) |
| Antenatal steroids, no (%) | 12 (100) | 13 (100) |
| Histological chorioamnionitis, no (%) | 6 (50) | 7 (54) |
| Caesarean section delivery, no (%) | 10 ((83) | 10 (77) |
| Surfactant, no (%) | 11 (92) | 13 (100) |
| Days ventilated before study entry, days, median (range) | 12 (6–15) | 13 (5–17) |
| Mean airway pressure (cmH2O), mean±SD | 8±1 | 8±1 |
| Positive end-expiratory pressure (cmH2O), mean±SD | 5±1 | 5±1 |
| Ventilator rate (breaths per minute),* mean±SD | 42±23 | 31±13 |
| FiO2, mean±SD | 49±16 | 38±13 |
| pH, mean (SD) | 7.31±0.07 | 7.30±0.05 |
| PaCO2 (mm Hg), mean (SD) | 55±8 | 53±7 |
| HCO3 (mmol/L), mean (SD) | 27±4 | 26±4 |
Excluded high-frequency ventilation rate.
The number of days on invasive mechanical ventilation or high-frequency ventilation during the intervention did not differ (table 2). There was a difference in the change in level of support (changing between NIPPV, invasive mechanical ventilation and high-frequency ventilation) between groups with four infants in the higher TcCO2 group escalating support and two infants in the lower TcCO2 group deescalating support during the study period.
Table 2.
Respiratory support during the study period and blood gas data by permissive hypercapnia target group*
| Higher TcCO2 (N=12) | Lower TcCO2 (N=13) | P value | |
|---|---|---|---|
| Number of days on Invasive ventilation, n (%) | 12 (48) | 13 (52) | 0.99† |
| 0 | 2 (17) | 2 (15) | |
| 1 | 1 (8) | 1 (8) | |
| 2 | 9 (75) | 10 (77) | |
| Number of days on high frequency, days (%) | 12 (48) | 13 (52) | 0.220† |
| 0 | 10 (83) | 13 (100) | |
| 1 | 1 (8) | 0 (0) | |
| 2 | 1 (8) | 0 (0) | |
| Change in support during study period, n (%) | 0.03† | ||
| Escalated support | 4 (33) | 0 (0) | |
| Deescalated support | 0 (0) | 2 (15) | |
| No change | 8 (67) | 11 (85) | |
| Mean airway pressure (cmH2O), mean±SD | 9±2 | 8±2 | 0.29‡ |
| Positive end-expiratory pressure (cmH2O), mean±SD | 5±1 | 5±1 | 0.93‡ |
| Ventilator rate (breaths per minute),§ mean±SD | 45±21 | 37±21 | 0.40‡ |
| FiO2, mean±SD | 48±24 | 44±21 | 0.65‡ |
| pH, mean±SD | 7.29±0.08 | 7.30±0.07 | 0.51‡ |
| PaCO2 (mm Hg), mean±SD | 54±7 | 52±9 | 0.49‡ |
| HCO3 (mmol/L), mean±SD | 26±4 | 26±4 | 0.74‡ |
Observed mean and SD.
Fisher’s exact test.
P values based on random effects mixed model with only group effect based on gamma distribution.
Excluded high-frequency ventilation rate.
Based on the repeated measure models, ventilator and blood gas parameters did not differ between groups during the intervention period (table 2). There were no differences in episodes of intermittent hypoxaemia (126±64 vs 105±61 per 24 hours; p=0.30), severe intermittent hypoxaemia (42±34 vs 37±25 per 24 hours; p=0.74) or bradycardia (11±16 vs 15±23 per 24 hours; p=0.89) between higher and lower TcCO2 target groups during the intervention days (table 3). The proportion of time with SpO2 <85% (8%±7% vs 7%±7%; p=0.47), SpO2 <80% (3%±3% vs 2%±2%; p=0.65), cerebral hypoxaemia (25%±28% vs 21%±30%; p=0.61) or abdominal hypoxaemia (16%±21% vs 15%±24%; p=0.93) did not differ between groups during the intervention days. Continuous TcCO2 values (56.8±6.9 in the higher group vs 54.5±7.8 in the lower group; p=0.36) did not differ significantly between groups during the intervention days.
Table 3.
Outcome measures by permissive hypercapnia target group during intervention days
| Higher TcCO2 target | Lower TcCO2 target | P value | |
|---|---|---|---|
| No of episodes of intermittent hypoxaemia per 24 hours, mean±SD | 126±64 | 105±61 | 0.30* |
| Percentage of time SpO2<85%, mean±SD | 8±7 | 7±7 | 0.47* |
| No of episodes of severe intermittent hypoxaemia per 24 hours, mean±SD | 42±34 | 37±25 | 0.74† |
| Percentage of time SpO2<80%, mean±SD | 3±3 | 2±2 | 0.65‡ |
| No of episodes of bradycardia per 24 hours, mean±SD | 11±16 | 15±23 | 0.89† |
| Percentage of time cerebral near-infrared spectroscopy <55%, mean±SD | 25±28 | 21±30 | 0.61‡ |
| Percentage of time abdominal near-infrared spectroscopy <40%, mean±SD | 16±21 | 15±24 | 0.93‡ |
| TcCO2 (mm Hg), mean±SD | 56.8±6.9 | 54.5±7.8 | 0.36* |
P values based on random effects mixed model with only group effect based on gamma distribution.
P values based on random effects mixed model with only group effect based on negative binomial distribution.
P values based on random effects mixed model with only group effect based on beta distribution.
Overall, there was moderate negative correlation between mean TcCO2 and episodes of bradycardia (r=−0.56; p<0.001) (figure 2), but no correlation was noted between TcCO2 and intermittent hypoxaemia or severe intermittent hypoxaemia (online supplemental figure S1). There was weak positive correlation between mean TcCO2 and the percentage of time with cerebral (r=0.26; p=0.009) and abdominal hypoxaemia (r=0.27; p=0.009) (online supplemental figure S1). There was also a positive correlation between mean TcCO2 and FiO2 (r=0.36; p<0.001), mean TcCO2 and mean airway pressure (r=0.48; p<0.001), and mean TcCO2 and bicarbonate (r=0.47; p<0.001) (online supplemental figure S2). In addition, there was moderate positive correlation between mean daily TcCO2 and once per day PaCO2 (r=0.60; p<0.001) (online supplemental figure S2).
Figure 2.
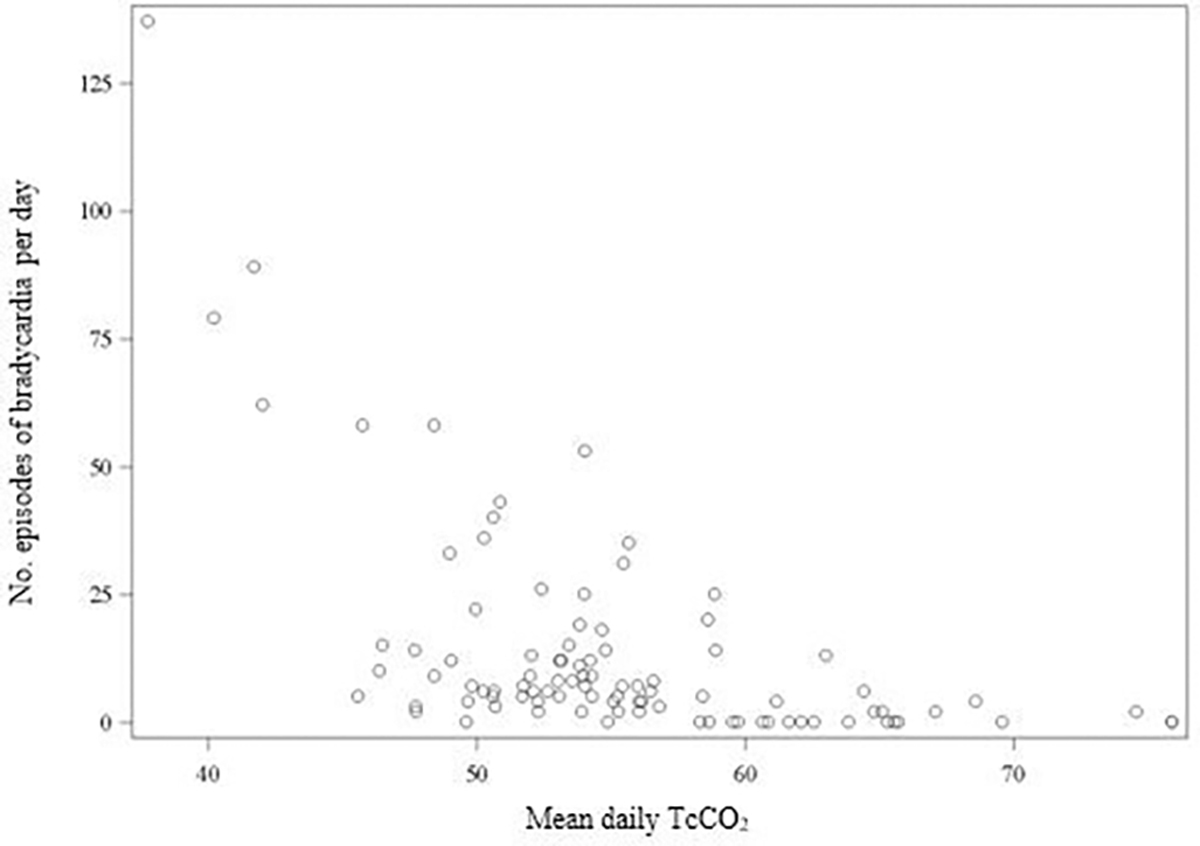
Correlation between episodes of bradycardia (<100 beats per minute for ≥10 s) per day and mean daily transcutaneous carbon dioxide (TcCO2). There was moderate negative correlation between mean TcCO2 and episodes of bradycardia (r=−0.56; p<0.001).
In a sensitivity analysis excluding infants who were not on invasive mechanical ventilation, there remained no difference in episodes of intermittent hypoxaemia, severe intermittent hypoxaemia, the proportion of time with low SpO2, cerebral hypoxaemia, abdominal hypoxaemia or episodes of bradycardia (results not shown). In this sensitivity analysis, the ventilator settings, the mean TcCO2, PaCO2, pH and bicarbonate also did not differ.
DISCUSSION
This trial of late permissive hypercapnia targeted different ranges of TcCO2 among very preterm infants on positive pressure respiratory support. Targeting a slightly higher TcCO2 did not alter measures of respiratory stability. However, the intended TcCO2 separation was not realised as achieved TcCO2 regressed to the baseline and did not differ between groups. Infants in both groups had frequent episodes of intermittent hypoxaemia, cerebral hypoxaemia and abdominal hypoxaemia. The positive correlations between mean TcCO2 and FiO2, mean airway pressure, and cerebral and abdominal hypoxaemia indicate that higher TcCO2 was a marker for increased respiratory illness severity. Bradycardia events occurred relatively frequently in many infants, and higher achieved levels of TcCO2 were associated with fewer episodes of bradycardia.
Limitations
This pilot trial of 25 very preterm infants did not use a cross-over design and was not powered to detect potentially important clinical differences related to levels of permissive hypercapnia.1 12–15 Enrolled infants had two periods of intervention in order to assess the feasibility of making relatively small intentional alterations in TcCO2 targets and also to increase the study power. It is possible that the 5 mm Hg (0.67 kPa) intended change may have been difficult to achieve because of the inherent random variation in respiratory drive and expected fluctuations in carbon dioxide due to compensatory changes in spontaneous ventilation, episodes of cessation of spontaneous ventilation or changes unrelated to intervention such as endotracheal tube secretions or shifting atelectasis. In addition, the small sample size increases the risk of confounding and the inclusion of infants on NIPPV may have impacted the ability to target TcCO2 as it may be difficult to influence the carbon dioxide levels because the mechanisms causing hypoxaemia and bradycardia, such as apnoea, asynchrony or forced expirations, may differ between intubated and non-intubated infants. 16 17 Future physiologic studies targeting late permissive hypercapnia to assess control of breathing may limit recruitment to infants on invasive mechanical ventilation, require tighter TcCO2 ranges, the use of different absolute pCO2 ranges rather than changes from baseline, mandated ventilator changes to achieve the target range and/or the use of a no-man’s-land to achieve separation.
In this study, we measured cerebral oxygenation but did not assess cerebral perfusion. Large fluctuations in carbon dioxide, whether higher or lower, are associated with brain injury among extremely preterm infants, possibly due to impaired cerebral autoregulation in this population.15 Higher thresholds of carbon dioxide with lower limits of pH than we used have been trialled previously within the first 2 weeks after birth but were associated with an increased risk of necrotising enterocolitis.14 Our study used transient mild changes in targeted carbon dioxide levels after 7 days of age, which is beyond the highest risk period of intraventricular haemorrhage. In addition, we used a pH control lower limit of 7.20 that has been tested in a large randomised clinical trial and did not result in adverse effects.18 We avoided hypocapnia, which may diminish cerebral blood flow by using a lower TcCO2 limit of 40 mm Hg.
Permissive hypercapnia is widely used in neonatology,18 19 but the effects on respiratory stability have not been studied. We used continuous TcCO2 monitors that correlated with daily blood gas measurements alongside detailed continuous physiologic data to assess respiratory control and oxygenation. It is known that preterm infants on supplemental oxygen therapy have frequent episodes of hypoxaemia.9 In the current study, despite being on positive pressure respiratory support, infants in both groups had frequent episodes of intermittent hypoxaemia, severe intermittent hypoxaemia, cerebral hypoxaemia and abdominal hypoxaemia. Previous studies have found that intermittently measured PaCO2 levels above 55 mm Hg were associated with modestly higher cerebral saturations on NIRS in very preterm infants.20 It is also known that small changes in FiO2 modulate NIRS measures,21 but in our study the difference in FiO2 and the proportion of time with hypoxaemia was not significant.
We found a moderate negative correlation between TcCO2 and episodes of bradycardia. It is possible that achieving carbon dioxide levels above the apnoeic threshold8 decreases episodes of apnoea and associated bradycardia among ventilated very preterm infants. Carbon dioxide administration has been shown to reduce apnoea among spontaneously breathing preterm infants.5 22 As most infants were intubated in the current study, it is likely that centrally mediated responses to CO2 rather than known differences in CO2 thresholds of upper airway muscles6 were responsible. However, future randomised controlled trials of late permissive hypercapnia among intubated preterm infants focused on bradycardia are needed to determine causality. Finally, we noted a high rate of refusal of informed consent possibly due to the lack of anticipated benefit for participants.
CONCLUSION
Targeting higher carbon dioxide levels did not improve respiratory stability among very preterm infants on positive pressure respiratory support although the intended TcCO2 separation was not achieved. Future studies of permissive hypercapnia may consider stricter mandated interventions and target ranges to increase the TcCO2 separation.
Supplementary Material
WHAT IS ALREADY KNOWN ON THIS TOPIC
Permissive hypercapnia is a commonly used lung-protective strategy in the neonatal intensive care unit.
The effect of altering carbon dioxide levels on respiratory stability among ventilated very preterm infants is not known.
WHAT THIS STUDY ADDS
Targeting higher carbon dioxide levels did not improve respiratory stability among very preterm infants on positive pressure respiratory support but the intended change in carbon dioxide levels was not achieved.
There was moderate negative correlation between higher levels of transcutaneous carbon dioxide and fewer episodes of bradycardia.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Randomised controlled trials of late permissive hypercapnia focused on bradycardia, and associated outcomes among intubated preterm infants may require stricter mandated interventions and TcCO2 target ranges.
Funding
This study was supported by grants from the National Institute of Health (NHLBI U01HL133536, U01HL133708 and K23HL157618).
Footnotes
Competing interests WAC is on the board of MEDNAX, Inc; NA is on the advisory board for Radiometer, Shire and Resbiotic.
Patient consent for publication Consent obtained from parent(s)/guardian(s).
Ethics approval This study involves human participants and was approved by The University of Alabama at Birmingham Institutional Review Board (IRB-160519008). Participants gave informed consent to participate in the study before taking part.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
REFERENCES
- 1.Finer NN, Carlo WA, Walsh MC, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med 2010;362:1970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn MS, Kaempf J, de Klerk A, et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 2011;128:e1069–76. [DOI] [PubMed] [Google Scholar]
- 3.Rojas-Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2012:CD000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambalavanan N, Carlo WA, Wrage LA, et al. Paco2 in surfactant, positive pressure, and oxygenation randomised trial (support). Arch Dis Child Fetal Neonatal Ed 2015;100:F145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvaro RE, Khalil M, Qurashi M, et al. Co (2) inhalation as a treatment for apnea of prematurity: a randomized double-blind controlled trial. J Pediatr 2012;160:252–257. [DOI] [PubMed] [Google Scholar]
- 6.Carlo WA, Martin RJ, Difiore JM. Differences in CO2 threshold of respiratory muscles in preterm infants. J Appl Physiol (1985) 1988;65:2434–9. [DOI] [PubMed] [Google Scholar]
- 7.Gerhardt T, Bancalari E. Apnea of prematurity: I. Lung function and regulation of breathing. Pediatrics 1984;74:58–62. [PubMed] [Google Scholar]
- 8.Khan A, Qurashi M, Kwiatkowski K, et al. Measurement of the CO2 apneic threshold in newborn infants: possible relevance for periodic breathing and apnea. J Appl Physiol (1985) 2005;98:1171–6. [DOI] [PubMed] [Google Scholar]
- 9.Travers CP, Carlo WA, Nakhmani A, et al. Environmental or nasal cannula supplemental oxygen for preterm infants: a randomized cross-over trial. J Pediatr 2018;200:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machin D, Campbell MJ, Tan SB, et al. Chapter 16: feasibility and pilot studies. In: Sample sizes for clinical, laboratory and epidemiology studies. 2018: 251–67. [Google Scholar]
- 11.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355:i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlo WA, Stark AR, Wright LL, et al. Minimal ventilation to prevent bronchopulmonary dysplasia in extremely-low-birth-weight infants. J Pediatr 2002;141:370–4. [DOI] [PubMed] [Google Scholar]
- 13.Mariani G, Cifuentes J, Carlo WA. Randomized trial of permissive hypercapnia in preterm infants. Pediatrics 1999;104:1082–8. [DOI] [PubMed] [Google Scholar]
- 14.Thome UH, Genzel-Boroviczeny O, Bohnhorst B, et al. Permissive hypercapnia in extremely low birthweight infants (PHELBI): a randomised controlled multicentre trial. Lancet Respir Med 2015;3:534–43. [DOI] [PubMed] [Google Scholar]
- 15.Fabres J, Carlo WA, Phillips V, et al. Both extremes of arterial carbon dioxide pressure and the magnitude of fluctuations in arterial carbon dioxide pressure are associated with severe intraventricular hemorrhage in preterm infants. Pediatrics 2007;119:299–305. [DOI] [PubMed] [Google Scholar]
- 16.Bolivar JM, Gerhardt T, Gonzalez A, et al. Mechanisms for episodes of hypoxemia in preterm infants undergoing mechanical ventilation. J Pediatr 1995;127:767–73. [DOI] [PubMed] [Google Scholar]
- 17.Esquer C, Claure N, D’Ugard C, et al. Mechanisms of hypoxemia episodes in spontaneously breathing preterm infants after mechanical ventilation. Neonatology 2008;94:100–4. [DOI] [PubMed] [Google Scholar]
- 18.Payne NR, LaCorte M, Sun S, et al. Evaluation and development of potentially better practices to reduce bronchopulmonary dysplasia in very low birth weight infants. Pediatrics 2006;118 Suppl 2:S65–72. [DOI] [PubMed] [Google Scholar]
- 19.van Kaam AH, De Jaegere AP, Rimensberger PC, et al. Incidence of hypo-and hyper-capnia in a cross-sectional European cohort of ventilated newborn infants. Arch Dis Child Fetal Neonatal Ed 2013;98:F323–6. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman SB, Lakhani A, Viscardi RM. The association between carbon dioxide, cerebral blood flow, and autoregulation in the premature infant. J Perinatol 2021;41:324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riera J, Hyttel-Sorensen S, Bravo MC, et al. The safeboosc phase II clinical trial: an analysis of the interventions related with the oximeter readings. Arch Dis Child Fetal Neonatal Ed 2016;101:F333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Saif S, Alvaro R, Manfreda J, et al. A randomized controlled trial of theophylline versus CO2 inhalation for treating apnea of prematurity. J Pediatr 2008;153:513–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.


