Abstract
CRISPR-Cas9 has been adapted as a readily programmable genome manipulation agent, and continuing technological advances rely on an in-depth mechanistic understanding of Cas9 target discrimination. Cas9 interrogates a target by unwinding the DNA duplex to form an R-loop, where the RNA guide hybridizes with one of the DNA strands. It has been shown that RNA guides shorter than the normal length of 20-nucleotide (-nt) support Cas9 cleavage activity by enabling partial unwinding beyond the RNA/DNA hybrid. To investigate whether DNA segment beyond the RNA/DNA hybrid can impact Cas9 target discrimination with truncated guides, Cas9 double-stranded DNA cleavage rates were measured with 16-nt guides on targets with varying sequences at +17 to +20 positions distal to the protospacer-adjacent-motif (PAM). The data reveal a log–linear inverse correlation between and the PAM+(17−20) DNA duplex dissociation free energy , with sequences having smaller showing faster cleavage and a higher degree of unwinding. The results indicate that, with a 16-nt guide, “peripheral” DNA sequences beyond the RNA/DNA hybrid contribute to target discrimination by tuning the cleavage reaction transition state through the modulation of PAM-distal unwinding. The finding provides mechanistic insights for the further development of strategies that use RNA guide truncation to enhance Cas9 specificity.
Graphical Abstract

CRISPR-Cas9 has been a revolutionary tool for genome engineering and manipulation, and its power stems from mechanistic understanding on how Cas9 specifically acquires a target within a complex genome:1–4 (i) base-pairing between a segment (the “guide” or “guide sequence”) of the single-guide RNA (sgRNA) and a segment of DNA designated as the protospacer and (ii) protein–DNA interactions with a short protospacer-adjacent-motif (PAM). However, Cas9 has been shown to bind and cleave genomic targets containing mismatches with the RNA guide, resulting in undesired off-target effects that are detrimental for applications.5–7 Advances in understanding Cas9 targeting mechanisms have led to protein and sgRNA engineering that greatly enhances specificity8–13 and remain the key for further development of Cas9-based applications.
Studies have revealed that Cas9 discriminates a target through unwinding of the DNA duplex to form a three-stranded R-loop, in which the DNA target-strand (TS) forms a hybrid with the RNA guide.14–16 While the guide of an sgRNA is generally 20-nucleotide (nt) long, formation of a stable RNA/DNA hybrid at the PAM-proximal 8–12 base-pair (bp) segment is sufficient to attain complete binding affinity by the Cas9 ribonucleoprotein complex.17,18 Subsequently, the structure and dynamics of the R-loop at the PAM-distal segment coordinates Cas9 nuclease conformational changes, which serve as checkpoint(s) in preventing cleavage of incorrect targets.16,19–22 It has also been shown that RNA guides shorter than 20-nt can support Cas9 cleavage activities.21,23–25 Compared to the 20-nt guide, using truncated guides leads to a shorter RNA/DNA hybrid with a smaller number of RNA/DNA pairings, which would likely amplify the role of RNA-guide/DNA-target mismatch(es), thus enhancing Cas9 specificity.23,24
Recently, we reported that, for RNA guides that are longer than 15-nt, the Cas9 ribonucleoprotein complex partially unwinds PAM-distal DNA beyond the RNA/DNA hybrid, and the rate of double-stranded DNA cleavage correlates with the degree of PAM-distal unwinding.25 This would imply that, with truncated guides, intrinsic properties of the DNA protospacer beyond the RNA/DNA hybrid could modulate Cas9 cleavage rates by tuning the PAM-distal unwinding. Specifically, we hypothesize that the duplex dissociation free energy, which measures the stand-alone DNA duplex stability, can serve as an indicator of the ability of DNA to unwind upon interacting with Cas9, and it would thus correlate with the observed Cas9 double-strand cleavage rate.
In this study, we tested this hypothesis by examining cleavage activities of Streptococcus pyogenes Cas9 (referred to as “Cas9”) ribonucleoprotein complexes containing 16-nt RNA guides on DNA substrates with varying PAM+(17−20) sequences. The measured cleavage rates at saturation show a good log–linear inverse correlation with , the PAM+(17−20) segment DNA dissociation free energy. Sequences with smaller (less stable) were shown to unwind to a larger degree by a 2-amino-purine (2AP) fluorescence assay and indeed yielded larger i.e., faster cleavage. In addition, was found to be most impacted by protospacer base-pairings at PAM+17 and +18. The data demonstrate that with a 16-nt guide, “peripheral” DNA sequences beyond the RNA/DNA hybrid impact target discrimination by tuning the transition state through modulation of PAM-distal unwinding. The finding provides a mechanistic basis for further development of strategies that use RNA guide truncation to reduce “off-target” effects.
MATERIALS AND METHODS
Protein Expression and Purification.
Plasmids encoding Streptococcus pyogenes Cas9 (pMJ806) and catalytically inactive dCas9 (pMJ841, containing D10A/H840A mutations) were obtained from Addgene (http://www.addgene.org/), and the proteins were expressed and purified following previously described procedures.25,26 Purified proteins were stored at −80 °C in a “Storage Buffer” with 20 mM tris, pH 7.5, 250 mM NaCl, 5% glycerol, 5 mM MgCl2, and 0.5 mM tris(2-carboxyethyl)phosphine hydrochloride (TCEP).
sgRNA Preparation.
sgRNAs with the same 80-nt core but varying in guide sequence and length were synthesized by T7 in vitro transcription. The double-stranded DNA templates for transcription were prepared by overlapping PCR as previously described.25 Transcriptions and sgRNA purifications were carried out following previously reported procedures.26 Purified sgRNAs were dissolved in ME buffer (10 mM MOPS, pH 6.5, and 1 mM EDTA) and stored at −20 °C.
DNA Cleavage Assays.
Detailed information for Cas9 target sequences is presented in Supporting Information (SI, sect. S1.1). The Cas9 target was embedded in either a ScaI linearized pUC19 plasmid (Figure S1, Table S1) or a fragment (~700 bp) obtained by PCR amplification of exon 3 of the human EMX1 gene. Control experiments showed that differences in the peripheral DNA beyond the 20-nt protospacer had no impact on the measured cleavage rates (SI, sect. S1.2). For a given Cas9 cleavage reaction, the Cas9:sgRNA ribonucleoprotein (RNP) complex at a desired concentration was assembled with a protein:sgRNA molar ratio of 1:1.2 by incubation in the reaction buffer (20 mM Tris, pH 7.5, 100 mM KCl, 5 mM MgCl2) for 10 min at 37 °C. A given DNA substrate was then added to a final concentration of 5 nM. The reaction was allowed to proceed at 37 °C for the desired time and then stopped by addition of denaturing loading dye equal to 1/5th of the reaction sample volume (New England BioLabs cat. no. B7024S supplemented with 0.48% SDS and 60 mM EDTA). The stopped reactions were loaded on a 1% agarose ethidium bromide gel to resolve the uncleaved precursors and cleaved products. Gels were imaged using a Gel Doc XR+ Gel Documentation System (Bio-Rad). DNA bands were selected and quantified by ImageLab 6.0.1, with background correction done by using baseline adjustment. The fraction of products, , at each time point of a reaction was calculated as
| (1) |
with being the uncleaved “precursor” band intensity and and being intensities of cleaved products 1 (top band) and 2 (bottom band), respectively. It has been established that the active Cas9 concentrations used in this study were at saturating conditions.25 The reaction rate constant, which represents , was determined by fitting the time dependence of to a single-exponential using Origin 2018:
| (2) |
with being the active fraction of the target DNA. Multiple measurements were carried out to obtain the average and standard deviation for each reported value.
2-Amino-Purine (2AP) Fluorescence Assay for Assessing Cas9-Induced DNA Unwinding.
DNA strands (unmodified and 2AP-substituted, SI, sect. S3, Table S3) were obtained by solid-phase chemical synthesis from a commercial source (Integrated DNA Technologies, Coralville, IA). To form a target DNA duplex (unmodified or with a 2AP label), the desired target-strand (TS) and nontarget-strand (NTS) were mixed in a 1:1 molar ratio in an “Annealing Buffer” (50 mM Tris, pH 7.5, 100 mM NaCl) and then incubated at room temperature overnight. The mixture was purified by size exclusion chromatography (SEC) using a Superdex 200 increase 10/300 GL column (GE Healthcare) in Annealing Buffer. The purified DNA duplexes were stored at −20 °C for future use.
The catalytically inactive dCas9/sgRNA mixture was first incubated at room temperature for 10 min, followed by addition of the desired DNA duplex in Reaction Buffer, with dCas9:sgRNA:DNA in a molar ratio of 1:1.25:1.25. The ternary mixture was then incubated at 37 °C for 30 min and purified using SEC in a buffer composed of 20 mM Tris, pH 7.5, 5% glycerol, 5 mM MgCl2, and 100 mM KCl. Homogenous complex fractions were pooled (≈2 mL) and used directly for measurements.
For a given 2AP-containing sample, fluorescence emission was measured using a Fluorolog Spectrofluorometer (Horiba Jobin Yvon, Edison, NJ) with excitation set at 320 nm to minimize dCas9 emission interference.25 Absorbance was obtained immediately after the fluorescence measurement on a LAMBDA UV/vis/NIR Spectrophotometer (PerkinElmer). The normalized 2AP quantum yield of a tested sample with respect to the corresponding DNA duplex, , was obtained using the concentration-normalized backgroundcorrected 2AP emission as described in our previous study.25
| (3) |
where and are the extinction coefficients at 260 nm for the sample and the duplex, respectively; is the background-corrected 2AP emission of the sample at 370 nm; is the absorbance of the same sample at 260 nm; is the value for the corresponding duplex.
Multiple Linear Regression Analysis.
To dissect the correlation between Cas9 cleavage rates and the intrinsic dissociation propensity of a given DNA duplex sequence, the dissociation free energy of a designated segment of a DNA/DNA duplex or DNA/RNA hybrid was calculated based on the nearest-neighbor model. Nearest-neighbor parameters reported for DNA/DNA duplex27 and RNA/DNA hybrid28 were used to compute the sequence-dependent standard-state dissociation enthalpy and entropy from which the standard-state dissociation free energy, , was calculated as
| (4) |
where .
Multiple linear regression (MLR) models were trained to predict the measured using the values of various input sequences as features . Training determines the set of parameters that will lead to the best predictions of , as shown below.
| (5) |
Model performance is measured using the adjusted coefficient of determination, , which accounts for the number of features included in modeling to avoid overfitting. can be presented to the model as individual features associated with each dinucleotide step or as a single feature covering multiple steps. For example, if the entire PAM+(17−20) segment is considered as a whole, the sum of of five dinucleotides spanning the segment, , is the only feature:
| (6a) |
and the model is fit to the following:
| (6b) |
Alternatively, a model considering all five dinucleotides separately contains five features:
| (6c) |
The total number of features can then be reduced by removing features that do not appear to be sufficiently informative of as described. For example, a model considering only dinucleotides 17/18 and 18/19 contains two features:
| (6d) |
RESULTS
Cas9 Cleavage with a 16-Nucleotide Guide Is Highly Correlated with DNA Duplex Stability beyond the RNA/DNA Hybrid.
To assess the impact of the DNA segment beyond the RNA/DNA hybrid on Cas9 activity, we first measured double-strand cleavage rates of Cas9 ribonucleoprotein complexes containing a 16-nt guide (Figure 1A, designated as g-16). The DNA substrates have the same PAM+(1−16) sequences that fully match the RNA guide but vary in their PAM+(17−20) segment (Figure 1A, SI, sect. S1). Each DNA substrate was designated as , with the 4-letter subscript indicating PAM+(17−20) nucleotides on the TS in the 5′ to 3′ direction. For example, represents a DNA duplex sequence with the PAM+(17−20) positions being (5′-TATA-3′) on the TS and (3′-atat-5′) on the NTS, while represents a sequence containing TS (5′-GCGG-3′) and NTS (3′-cgcc-5′) at the PAM+(17−20) positions (Figure 1B, Table S1). Note that varying the PAM+(17−20) positions could give rise to ranging from 3.57 to 10.07 kcal/mol. In this work, 26 substrates were studied that uniformly cover the full range of the possible (Table S2).
Figure 1.
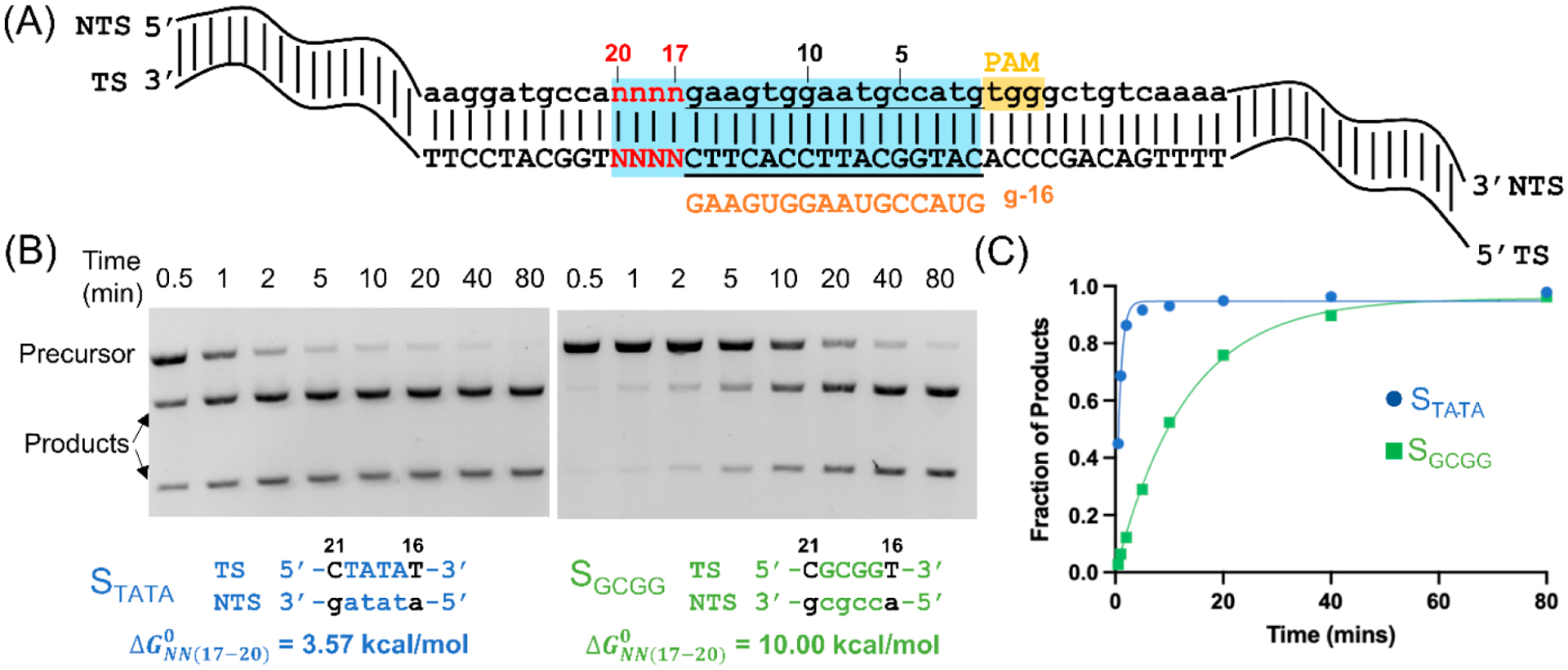
PAM-distal sequence beyond the RNA/DNA hybrid modulates double-stranded DNA cleavage by Cas9 with a 16-nt guide. (A) Schematic representation of DNA substrates. Cas9 target sequence embedded in a linearized pUC19 plasmid was shown by letters, with the PAM highlighted in yellow, the 20-nt protospacer highlighted in blue, the TS sequence represented by uppercase letters, the NTS sequence represented by lowercase letters, and the 16-nt RNA guide indicated with orange uppercase letters. The variable sequence at PAM+(17−20) was indicated by red letters. (B) Double-stranded DNA cleavage results for substrates (left) and (right). Representative electrophoresis images of the time course are shown on top, and the corresponding PAM-distal variable sequences and values are shown at the bottom, with nucleotides varying from positions PAM+17 through +20 (colored) but fixed at +16 and +21 (black). values were calculated based on the nearest-neighbor parameters as described in the Materials and Methods. (C) Single-exponential fit (eq 2) to derived cleavage rate constants for data sets shown in (B). The examples shown here gave and for and and for .
Figure 1 shows representative examples of a time course analysis of cleavage on and . With both substrates, the linearized duplexed precursor was cleaved completely into two shorter fragments (Figure 1B), and the time course of product generation was fit nicely with a single-exponential model (eq 2) to yield the cleavage reaction rate (Figure 1C), which represents of the reaction.25 The measurements yielded for , which is ~16 times slower than that of (Figure 1C, Table S2). Note that is 10.00 kcal/mol for and 3.57 kcal/mol for (Figure 1B, Table S2). As such, the data clearly show that a difference in results in a difference in Cas9 cleavage, with the more stable duplex (i.e., with a higher ) cleaving more slowly.
The correlation between and was found to hold for the 26 substrates studied (Figure 2, Table S2). Linear regression analysis between and (eq 6b, Materials and Methods) yielded a relationship of , with being 0.67 (Figure 2). indicates a high degree of linear dependence of on . The negative slope of −0.52 indicates that a sequence with a higher , which is expected to form a more stable duplex, would have a smaller . Analysis of the 26 substrates clearly supports the notion that the DNA duplex stability at PAM+(17−20) modulates Cas9 cleavage when using a 16-nt guide.
Figure 2.
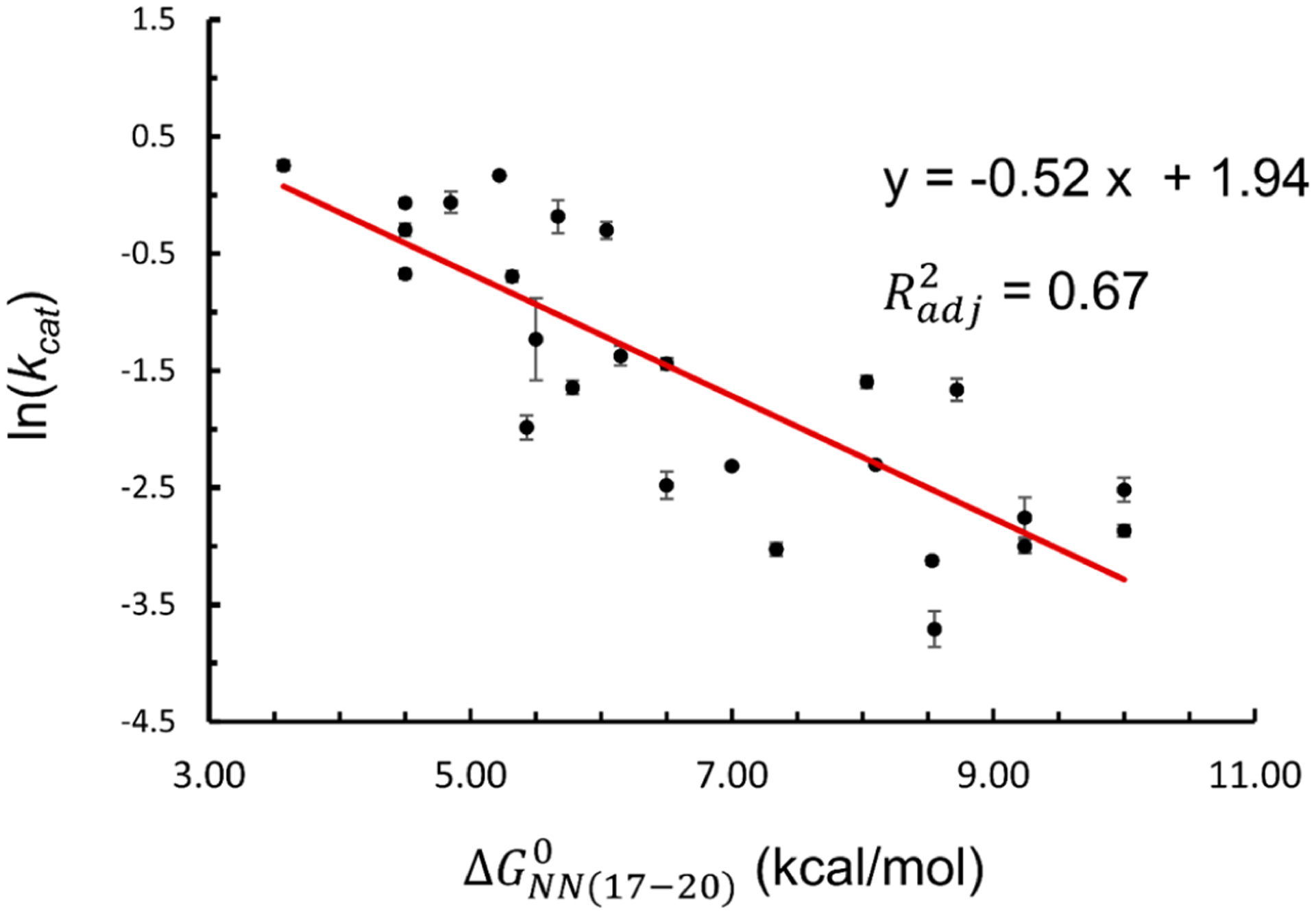
Linear regression analysis of vs . Black dots represent data obtained on the 26 substrates, and the red line represents the best linear fit. See Table S2 for further information.
While of 0.67 indicates a high degree of correlation between cleavage (i.e, ) and duplex stability beyond the RNA/DNA hybrid , we do note that in some cases similar yields quite different . (Figure 2, Table S2). This suggests additional sequence- and/or position-dependent factors may contribute to modulating Cas9 cleavage. Additional analysis indicates that the stacking energy of the PAM-distal NTS also contributes to modulating Cas9 cleavage rate (SI, sect. S2.2). However, more work is needed to fully elucidate the role of these factors (see also Discussion).
PAM-Distal Sequences beyond the RNA/DNA Hybrid Tunes Cleavage Activity by Modulating Cas9-Induced DNA Unwinding.
Our working hypothesis is that the duplex segment beyond the RNA/DNA hybrid tunes cleavage with the 16-nt RNA guide (Figures 1 and 2) by affecting the degree of Cas9-induced PAM-distal unwinding. To test this, a previously developed 2-amino-purine (2AP) assay25 was applied to assess unwinding of two DNA substrates, and (Figure 3, SI, sect. S3). In these measurements, the adenine of the TS PAM+18 position was substituted with 2AP (Figure 3A). The concentration-normalized 2AP fluorescence, which is proportional to the 2AP emission quantum yield, was measured for the DNA duplex and the corresponding ternary complex with the matching g-16 RNA guide. The ratio between the quantum yield of the ternary complex and that of the duplex, (eq 3), reports the degree of DNA unwinding in the Cas9 ternary complex, with higher indicating more unwinding.25 The results show that which has a of 3.57 kcal/mol, gives a of 7.55 ± 0.55, which is significantly higher than that of 4.58 ± 0.53 obtained for , which has a of 5.50 kcal/mol (Figure 3A,B). This indicates that , which has a lower duplex stability, as indicated by the smaller , allows a larger degree of DNA unwinding. Indeed, cleavage of was faster than that of (Figure 3C), consistent with the notion that a larger degree of unwinding allows for higher activity.
Figure 3.
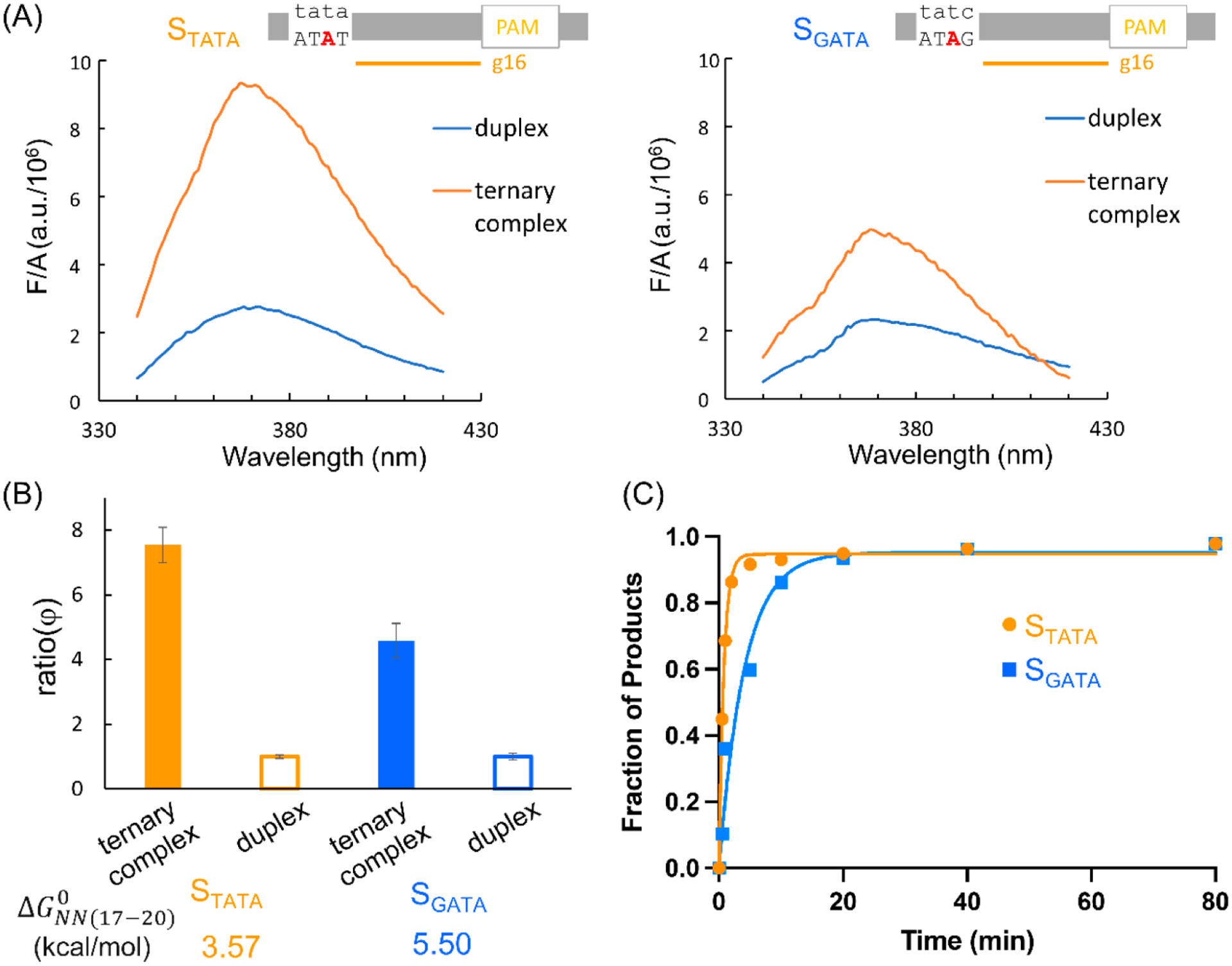
Sequence-dependent PAM-distal unwinding assessed by the 2AP fluorescence assay. (A) Representative concentration-normalized 2AP emission spectra for substrates (left) and . (B) Measured average values for substrates (left) and . See SI,sect. S3 for more details. (C) measurements for and . The examples shown here gave and for and and for .
Furthermore, with the 16-nt guide, studies show that the measured has a measurable dependence on the concentration of KCl in the reaction buffer, with clearly decreasing with increasing [KCl] (SI, sect. S4). This is consistent with the notion that increasing KCl concentration enhances DNA duplex stability, subsequently reducing DNA unwinding and impeding Cas9 cleavage. Together, the data strongly support the hypothesis that PAM-distal DNA sequences modulate unwinding and, in turn, tune Cas9 cleavage activity.
PAM+(17–20) DNA Tunes in Vitro Cleavage Activity with Multiple 16-nt RNA Guide Sequences.
To evaluate if the guide sequence also plays a role in the modulation of Cas9 cleavage rates by the PAM+(17−20) positions, studies were carried out on samples containing different 16-nt RNA guide sequences, which vary in the stability between the TS and NTS strands as well as that between the RNA-guide and the DNA (Figure 4). Analysis showed that between and as well as were negative and that between and was 0.14 (Figure 4B). These results indicate that there is no strong correlation between the observed in vitro cleavage activity and the varying sequences at the PAM+(1−16) segment involved in RNA/DNA interactions. On the other hand, an of 0.85 was obtained between the measured values and the corresponding , the DNA duplex dissociation free energy for the PAM+(17−20) segment beyond the RNA/DNA hybrid (Figure 4B). Furthermore, the additional data points fit nicely to the linear regression model originally obtained from analyzing the 26 sequences using the original 16-nt RNA guide (SI, sect. S5). Together, the results indicate that PAM+(17−20) positions are predictive of Cas9 activity with multiple 16-nt RNA guides.
Figure 4.

Sequences of the 16-nt RNA guide do not significantly influence cleavage activity. (A) Detailed sequence of four samples with three different 16-nt guide sequences. (B) Analysis of the corresponding linear correlation between and different aspects of the thermodynamic stability of the samples.
PAM + 17 and 18 Nucleotides Are Most Influential in Determining when Using g-16 RNA Guides.
The analysis reported in Figure 2 considered the stability over the entire segment of PAM+(17−20) (eqs 6a and 6b, Materials and Methods). However, the five dinucleotides within this segment are not equal in their positioning within the Cas9 RNP, particularly with respect to the RNA/DNA hybrid. To examine whether the contribution to Cas9 activation from individual positions varies, we further carried out MLR analysis on models predicting of 26 sequences, considering the stability of every dinucleotide step along the PAM+(17−20) DNA as individual features (e.g., eq 6c, Materials and Methods).
Table 1 summarizes the values that are obtained for a given model, with the row number indicating the starting position of the first dinucleotide and the column number indicating the final position of the last dinucleotide considered. For example, the cell corresponding to row 17 column 18 indicates that a model with one feature, the dissociation free energy of dinucleotide 17/18 (i.e., ), gave an value of 0.73 (Table 1, row 17, column 18). This is higher than that obtained with the PAM+(17−20) segment considered as a whole (, Figure 2), indicating that positions PAM+(17−18) alone can better predict the measured activities. Expanding to a two-feature model by adding dinucleotide 18/19 (i.e., eq 6d) returned an even higher of 0.78 (Table 1, row 17, column 19), while further including 19/20 and 20/21 dinucleotides did not improve (Table 1, row 17, columns 20 and 21).
Table 1.
Values Obtained from MLR Analysis of vs of All Possible Contiguous Stretches of the PAM+(17−20) DNA
| start/end | 17 | 18 | 19 | 20 | 21 |
|---|---|---|---|---|---|
| 16 | 0.29 | 0.77 | 0.80 | 0.79 | 0.78 |
| 17 | 0.73 | 0.78 | 0.78 | 0.77 | |
| 18 | 0.55 | 0.54 | 0.54 | ||
| 19 | 0.26 | 0.26 | |||
| 20 | 0.17 |
The conclusion that positions PAM+(17−18) are the most influential on the measured Cas9 activity can be drawn similarly from data shown in row 16 of Table 1, as models including positions PAM+(17−18) gave high values (0.77 and 0.80, Table 1, row 16, columns 18 and 19), while including additional positions did not result in model improvement. Furthermore, models including only the PAM+(19−20) positions gave (Table 1, rows 19 and row 20), indicating these positions have little influence on Cas9 activity.
Taken together, the analysis shows that, with the 16-nt guide, the contribution to Cas9 activation from individual positions varies, with the PAM+(17−18) positions playing the dominant role.
DISCUSSION
Data presented clearly demonstrate that, for Cas9 enzymes containing 16-nt RNA guides, DNA protospacer sequences beyond the RNA/DNA hybrid tune Cas9 cleavage rates (i.e., ) (Figures 1 and 2). This is linked to variable capacities for DNA unwinding (Figure 3), which can be predicted to a high degree by the computed intrinsic duplex stability at the DNA protospacer segment beyond the RNA/DNA hybrid (i.e., ). As the transition state free energy barrier of Cas9 cleavage is proportional to , the observed linear correlation between and (Figure 2) therefore indicates that is proportional to . This reveals that DNA unwinding at the PAM+(17−20) positions is the major factor that tunes the transition state for the Cas9 enzyme with the 16-nt guide. This further expands the prior finding that truncated guides support partial protospacer unwinding beyond the RNA/DNA hybrid, and the degree of unwinding is correlated with Cas9 cleavage activity.25
Our analysis also revealed that the PAM+(17−18) positions are the most influential in determining , while positions PAM+(19−20) show little impact (Table 1). An atomic force microscopy study has indicated that PAM+(14−17) RNA/DNA hybrid stability, which is directly linked to unwinding of DNA, serves as a gate to control Cas9 activation.19 A recent study reported that Cas9 twists and bends the PAM-proximal DNA duplex to enable RNA/DNA hybrid formation and propagation.29 We have previously proposed that PAM-distal unwinding beyond RNA/DNA hybrid occurs as part of the sequential mechanism for R-loop formation and that, in order to propagate the R-loop, Cas9 is able to “pre-unwind” PAM-distal DNA base-pair(s) ahead of the RNA/DNA hybrid to expose the target strand and assess pairing with the RNA guide.25 With the 16-nt guide, the RNA/DNA hybrid formally terminates at PAM+16, and Cas9’s “pre-unwinding” ability is likely responsible for unwinding the PAM+(17−20) segment. Given that PAM+(17−18) is adjacent to the terminus of the RNA/DNA hybrid, their DNA stability (i.e., high and/or ) likely impacts the ability of Cas9 to “pre-unwind”, in particular unwinding at PAM+17/18 that influences the “gate”, thus accounting for their significant roles in tuning cleavage rates (Table 1).
Note that duplex stability is one aspect of DNA shape, which is defined as the intrinsic physical properties of a DNA duplex that are collectively determined by its sequence.30 Our results expand on prior reports that DNA shape contributes to target discrimination in Cas931 and Cas12a.32 While Cas9 activity significantly correlates with intrinsic DNA duplex stability (Figures 1 and 2), additional DNA shape or sequence-dependent features may influence Cas9 function with 16-nt guides. One aspect may be intrinsic single-stranded properties of the DNA (SI, sect. S2.2), although much more work is needed in this area. Furthermore, variations in protein–DNA interactions may play a role. With 20-nt guides, contacts between the protein and the PAM-distal segment of the RNA/DNA hybrid play a key role in facilitating conformational changes to achieve the catalytically competent state.16,20–22,33 With 16-nt guides, RNA nucleotides at PAM+(17−20) are completely missing. The resulting loss of RNA/protein and RNA/DNA interactions may render the protein to directly interrogate the DNA duplex, thus elevating the role of intrinsic shape features of the DNA duplex, including the PAM+(17−20) stability, as reported here (Figures 1 and 2). On the other hand, the lack of PAM+(17−20) nucleotides in the RNA guide results in loss of negative charges, steric bulkiness, and functional groups for hydrogen bonding and ion coordination, all of which may impact the protein–RNA–DNA interactions that drive the system to the catalytically active conformation.16,20–22,33 As such, it remains to be learned whether intrinsic DNA duplex stability plays a similar role between the 16-nt guides studied here and other ones, such as 20-nt guides with PAM+(17−20) mismatches to target DNA or longer truncated guides (i.e., 17-nt or 18-nt guides) that extend the RNA/DNA hybrid.
RNA guide truncation has been used as one of the strategies to enhance Cas9 specificity, and it has been reported that 18-nt and 17-nt guides can significantly reduce off-target effects in human genome editing.23,24 However, guides shorter than 17-nt do not appear to exhibit cleavage activity in cells.23,24 Data presented here indicate that, with a 16-nt guide, Cas9 cleavage could occur in vitro and could differ by more than 50-fold depending on the PAM+(17−20) DNA sequence (Figure 2, Table S2). This suggests that, while selecting genomic targets for 16-nt guides, consideration of the “peripheral” PAM+(17−20) positions may provide additional information that promotes successful editing. In addition, we note that rules for defining an optimal target matching a 16-nt guide may differ from those matching a 20-nt guide or other truncated guides (e.g., 17-nt or 18-nt guides), especially considering the role of the PAM-distal RNA/DNA hybrid in enabling Cas9 activation.16,20–22,33 Much remains to be learned about Cas9 activation with 16-nt and other truncated guides.
CONCLUSION
In conclusion, the data presented show that, with 16-nt guides, intrinsic DNA duplex stability at the PAM+(17−20) protospacer segment modulates Cas9-induced PAM-distal unwinding, which in turn tunes the transition state of the cleavage reaction, thus impacting Cas9 activity. This clearly demonstrates that, with a 16-nt guide, “peripheral” DNA sequences beyond the DNA/RNA hybrid play a role in Cas9 target discrimination. The finding provides a mechanistic understanding for further developing strategies based on RNA guide truncation to enhance Cas9 specificity and reduce “off-target” effects.
Supplementary Material
ACKNOWLEDGMENTS
This work has been supported by the National Science Foundation (MCB-1818107 to P.Z.Q.) and the National Institute of Health (R35GM145341 to P.Z.Q. and R35GM130376 to R.R.). The authors would like to thank Yun Fang (Department of Chemistry, University of Southern California) for assistance on protein purification and performing gel electrophoresis.
Footnotes
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.biochem.3c00250
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.biochem.3c00250.
Additional information on DNA substrates, thermodynamic parameters of DNA duplex segment, 2-amino-purine fluorescence data, dependence of activities on KCl concentration, and additional information on impact of varying sequence of the 16-nt RNA guide (PDF)
Accession Codes
UniProt protein ID: Q99ZW2.
Contributor Information
Yue Li, Department of Chemistry, University of Southern California, Los Angeles, California 90089, United States;.
Brendon H. Cooper, Department of Quantitative and Computational Biology, University of Southern California, Los Angeles, California 90089, United States
Yukang Liu, Department of Chemistry, University of Southern California, Los Angeles, California 90089, United States.
Difei Wu, Department of Chemistry, University of Southern California, Los Angeles, California 90089, United States.
Xiaojun Zhang, Department of Chemistry, University of Southern California, Los Angeles, California 90089, United States.
Remo Rohs, Department of Chemistry and Department of Quantitative and Computational Biology, University of Southern California, Los Angeles, California 90089, United States;.
Peter Z. Qin, Department of Chemistry, University of Southern California, Los Angeles, California 90089, United States;
REFERENCES
- (1).Jinek M; Chylinski K; Fonfara I; Hauer M; Doudna JA; Charpentier E A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337 (6096), 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Gasiunas G; Barrangou R; Horvath P; Siksnys V Cas9-CrRNA Ribonucleoprotein Complex Mediates Specific DNA Cleavage for Adaptive Immunity in Bacteria. P Natl. Acad. Sci. USA 2012, 109 (39), E2579–E2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Cong L; Ran FA; Cox D; Lin SL; Barretto R; Habib N; Hsu PD; Wu XB; Jiang WY; Marraffini LA; Zhang F Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339 (6121), 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Mali P; Yang L; Esvelt KM; Aach J; Guell M; DiCarlo JE; Norville JE; Church GM RNA-Guided Human Genome Engineering via Cas9. Science 2013, 339 (6121), 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Fu Y; Foden JA; Khayter C; Maeder ML; Reyon D; Joung JK; Sander JD High-Frequency off-Target Mutagenesis Induced by CRISPR-Cas Nucleases in Human Cells. Nat. Biotechnol 2013, 31 (9), 822–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lin YN; Cradick TJ; Brown MT; Deshmukh H; Ranjan P; Sarode N; Wile BM; Vertino PM; Stewart FJ; Bao G CRISPR/Cas9 Systems Have off-Target Activity with Insertions or Deletions between Target DNA and Guide RNA Sequences. Nucleic Acids Res. 2014, 42 (11), 7473–7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Yang LH; Grishin D; Wang G; Aach J; Zhang CZ; Chari R; Homsy J; Cai XY; Zhao Y; Fan JB; Seidman C; Seidman J; Pu W; Church G Targeted and Genome-Wide Sequencing Reveal Single Nucleotide Variations Impacting Specificity of Cas9 in Human Stem Cells. Nat. Commun 2014, 5, 5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kim D; Luk K; Wolfe SA; Kim J-S Evaluating and Enhancing Target Specificity of Gene-Editing Nucleases and Deaminases. Annu. Rev. Biochem 2019, 88, 191–220. [DOI] [PubMed] [Google Scholar]
- (9).Moon SB; Kim DY; Ko J-H; Kim J-S; Kim Y-S Improving CRISPR Genome Editing by Engineering Guide RNAs. Trends Biotechnol 2019, 37 (8), 870–881. [DOI] [PubMed] [Google Scholar]
- (10).Galizi R; Jaramillo A Engineering CRISPR Guide RNA Riboswitches for in Vivo Applications. Curr. Opin Biotechnol 2019, 55, 103–113. [DOI] [PubMed] [Google Scholar]
- (11).Anzalone AV; Koblan LW; Liu DR Genome Editing with CRISPR–Cas Nucleases, Base Editors, Transposases and Prime Editors. Nat. Biotechnol 2020, 38 (7), 824–844. [DOI] [PubMed] [Google Scholar]
- (12).Doudna JA The Promise and Challenge of Therapeutic Genome Editing. Nature 2020, 578 (7794), 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Huang X; Yang D; Zhang J; Xu J; Chen YE Recent Advances in Improving Gene-Editing Specificity through CRISPR–Cas9 Nuclease Engineering. Cells 2022, 11 (14), 2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Jiang F; Doudna JA CRISPR–Cas9 Structures and Mechanisms. Annu. Rev. Biophys 2017, 46 (1), 505–529. [DOI] [PubMed] [Google Scholar]
- (15).Zuo Z; Liu J Allosteric Regulation of CRISPR-Cas9 for DNA-Targeting and Cleavage. Curr. Opin. Struct. Biol 2020, 62, 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Pacesa M; Loeff L; Querques I; Muckenfuss LM; Sawicka M; Jinek M R-Loop Formation and Conformational Activation Mechanisms of Cas9. Nature 2022, 609 (7925), 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Sternberg SH; Redding S; Jinek M; Greene EC; Doudna JA DNA Interrogation by the CRISPR RNA-Guided Endonuclease Cas9. Nature 2014, 507 (7490), 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Boyle EA; Andreasson JOL; Chircus LM; Sternberg SH; Wu MJ; Guegler CK; Doudna JA; Greenleaf WJ High-Throughput Biochemical Profiling Reveals Sequence Determinants of DCas9 off-Target Binding and Unbinding. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (21), 5461–5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Josephs EA; Kocak DD; Fitzgibbon CJ; McMenemy J; Gersbach CA; Marszalek PE Structure and Specificity of the RNA-Guided Endonuclease Cas9 during DNA Interrogation, Target Binding and Cleavage. Nucleic Acids Res. 2015, 43 (18), 8924–8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Chen JS; Dagdas YS; Kleinstiver BP; Welch MM; Sousa AA; Harrington LB; Sternberg SH; Joung JK; Yildiz A; Doudna JA Enhanced Proofreading Governs CRISPR–Cas9 Targeting Accuracy. Nature 2017, 550 (7676), 407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Dagdas YS; Chen JS; Sternberg SH; Doudna JA; Yildiz A A Conformational Checkpoint between DNA Binding and Cleavage by CRISPR-Cas9. Science Advances 2017, 3 (8), No. eaao0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Pacesa M; Lin C-H; Cléry A; Saha A; Arantes PR; Bargsten K; Irby MJ; Allain FH-T; Palermo G; Cameron P; Donohoue PD; Jinek M Structural Basis for Cas9 Off-Target Activity. Cell 2022, 185 (22), 4067–4081.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Fu YF; Sander JD; Reyon D; Cascio VM; Joung JK Improving CRISPR-Cas Nuclease Specificity Using Truncated Guide RNAs. Nat. Biotechnol 2014, 32 (3), 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Zhang J-P; Li X-L; Neises A; Chen W; Hu L-P; Ji G-Z; Yu J-Y; Xu J; Yuan W-P; Cheng T; Zhang X-B Different Effects of SgRNA Length on CRISPR-Mediated Gene Knockout Efficiency. Sci. Rep 2016, 6 (1), 28566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Li Y; Liu Y; Singh J; Tangprasertchai NS; Trivedi R; Fang Y; Qin PZ Site-Specific Labeling Reveals Cas9 Induces Partial Unwinding Without RNA/DNA Pairing in Sequences Distal to the PAM. CRISPR J. 2022, 5 (2), 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Tangprasertchai NS; Di Felice R; Zhang X; Slaymaker IM; Vazquez Reyes C; Jiang W; Rohs R; Qin PZ CRISPR–Cas9 Mediated DNA Unwinding Detected Using Site-Directed Spin Labeling. ACS Chem. Biol 2017, 12 (6), 1489–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).SantaLucia J Jr.; Allawi HT; Seneviratne PA Improved Nearest-Neighbor Parameters for Predicting DNA Duplex Stability. Biochemistry 1996, 35 (11), 3555–3562. [DOI] [PubMed] [Google Scholar]
- (28).Banerjee D; Tateishi-Karimata H; Ohyama T; Ghosh S; Endoh T; Takahashi S; Sugimoto N Improved Nearest-Neighbor Parameters for the Stability of RNA/DNA Hybrids under a Physiological Condition. Nucleic Acids Res. 2020, 48 (21), 12042–12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Cofsky JC; Soczek KM; Knott GJ; Nogales E; Doudna JA CRISPR–Cas9 Bends and Twists DNA to Read Its Sequence. Nat. Struct Mol. Biol 2022, 29 (4), 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Rohs R; Jin X; West SM; Joshi R; Honig B; Mann RS Origins of Specificity in Protein-DNA Recognition. Annu. Rev. Biochem 2010, 79, 233–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Newton MD; Taylor BJ; Driessen RPC; Roos L; Cvetesi N; Allyjaun S; Lenhard B; Cuomo ME; Rueda DS DNA Stretching Induces Cas9 Off-Target Activity. Nat. Struct Mol. Biol 2019, 26 (3), 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Jiang W; Singh J; Allen A; Li Y; Kathiresan V; Qureshi O; Tangprasertchai N; Zhang X; Parameshwaran HP; Rajan R; Qin PZ CRISPR-Cas12a Nucleases Bind Flexible DNA Duplexes without RNA/DNA Complementarity. ACS Omega 2019, 4 (17), 17140–17147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Bravo JPK; Liu M-S; Hibshman GN; Dangerfield TL; Jung K; McCool RS; Johnson KA; Taylor DW Structural Basis for Mismatch Surveillance by CRISPR-Cas9. Nature 2022, 603 (7900), 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


