Abstract
Background:
Despite reports of gross motor problems in mild cognitive impairment (MCI) and Alzheimer’s disease (AD), fine motor function has been relatively understudied.
Objective:
We examined if finger tapping is affected in AD, related to AD biomarkers, and able to classify MCI or AD.
Methods:
Forty-seven cognitively normal, 27 amnestic MCI, and 26 AD subjects completed unimanual and bimanual computerized tapping tests. We tested 1) group differences in tapping with permutation models; 2) associations between tapping and biomarkers (PET amyloid-β, hippocampal volume, and APOE ɛ4 alleles) with linear regression; and 3) the predictive value of tapping for group classification using machine learning.
Results:
AD subjects had slower reaction time and larger speed variability than controls during all tapping conditions, except for dual tapping. MCI subjects performed worse than controls on reaction time and speed variability for dual and non-dominant hand tapping. Tapping speed and variability were related to hippocampal volume, but not to amyloid-β deposition or APOE ɛ4 alleles. Random forest classification (overall accuracy = 70%) discriminated control and AD subjects, but poorly discriminated MCI from controls or AD.
Conclusions:
MCI and AD are linked to more variable finger tapping with slower reaction time. Associations between finger tapping and hippocampal volume, but not amyloidosis, suggest that tapping deficits are related to neuropathology that presents later during the disease. Considering that tapping performance is able to differentiate between control and AD subjects, it can offer a cost-efficient tool for augmenting existing AD biomarkers.
Keywords: Alzheimer’s disease, biomarkers, finger tapping, manual dexterity, motor function
INTRODUCTION
In addition to well-defined cognitive problems, Alzheimer’s disease (AD) and its precursor mild cognitive impairment (MCI) present with problems in a variety of motor domains. Some of the most prominent motor deficits that have been linked to an increased risk for AD include slower walking speed [1], poorer balance [2], larger cognitive-motor dual-tasking cost [3, 4], and weaker muscle strength [5, 6]. Gait dysfunction can already present during the preclinical phase of AD [7], even before the onset of cognitive deficits [8] and can predict incident dementia [9]. Relatively few studies have investigated manual performance in MCI and AD subjects [10], despite evidence that drawing [11] and dexterity as measured by pegboard tests [12–17] are slower, less accurate, and more variable in MCI and AD when compared to control subjects.
Finger tapping is also found to be affected in MCI and AD, with studies generally showing that compared to healthy controls these groups have longer response times (AD [18]), slower tapping speed [19–22] (although one study reported no significant differences in tapping speed between controls and AD subjects [23]), more variable tapping speed [20–22, 24, 25], longer tap intervals [18, 20, 21], longer tap duration [18, 21, 22], weaker tapping force [22], slower finger flexion [22], and less steady hands during tapping [19]. Although some of these results were less pronounced in MCI than in AD [18], index finger tapping speed was not different between individuals with MCI and unspecified dementia in another study [26], which could indicate that tapping is affected early in the disease process, but does not further deteriorate over later stages of AD. While most studies on finger tapping in AD focus on unimanual performance, there have been some reports that bimanual motor function may be more sensitive to the neurodegenerative processes of AD than unimanual function as it requires intact inter-hemispheric communication [15] and because of differences in brain activation underlying these conditions [27]. For instance, Suzumura and colleagues reported that bimanual tasks were more strongly impaired than unimanual tasks in MCI [21] and AD [28] and that contributions of the non-dominant hand more so than the dominant hand are affected during bimanual tasks [18]. Another study revealed that bimanual performance was more often affected than unimanual performance, especially when the bimanual test required the left and right fingers to operate alternating versus synchronous [15]. Changes in asymmetrical motor performance have been reported in MCI and AD before [17, 29–32] and may be related to lateralized differences in neural degeneration [33, 34]. Although there is some indication that deterioration of more complex motor function in AD is partly related to the cognitive dysfunction [35, 36], atrophy of motor brain regions such as the cerebellum suggests that primarily regression of core motor function underlies finger tapping degeneration [37–41]. Moreover, alterations of neurotransmitter systems may also be responsible for changes in tapping performance over the disease course, as AD is associated with a reduction in gamma-aminobutyric acid (GABA) [42], which is involved in motor inhibition [43]. Results from a study that measured primary motor cortex excitability with electromyography in AD during a finger tapping test showed that movement slowness correlated with reduced short-latency afferent inhibition, suggesting cholinergic system degeneration [24]. The cholinergic system is affected early on in MCI [44]. These studies indicate that finger performance, especially bimanual tapping, could be a potential early biomarker for AD. To the best of our knowledge, no studies have compared quantitative tapping measures to established AD biomarkers such as amyloid-β deposition, although one study reported that the Aβ42 levels from cerebrospinal fluid were related to scores on part 3 of the Unified Parkinson Disease Rating Scale, which includes a finger-to-thumb tapping task [45]. Although hippocampal involvement in finger tapping has not been unequivocally determined [46], the limited number of investigations into this area warrant further study. Despite the reported tapping deficits in MCI and AD, only one study has evaluated tapping performance as a classification method [21] and reported that tapping speed yields an area under the curve of 0.79 to distinguish MCI from controls.
In this study, we evaluated whether finger tapping, especially bimanual performance, could be a sensitive measure for the identification of MCI and AD. To this end, we 1) compared unimanual and bimanual motor performance between controls and amnestic MCI and AD subjects; 2) tested the association between finger tapping and well-validated and global biomarkers of AD; and 3) used machine learning to build and evaluate an optimized finger tapping classification model for MCI and AD by using information from multiple tapping measures. Achieving these aims could allow us to examine if finger tapping measures can serve as substitute markers or augment existing AD biomarkers to improve the diagnostic process and ultimately reduce clinical trial costs through enrichment [47]. Finger tapping tests are particularly well-suited to serve as biomarker considering that they are inexpensive, can be administered almost everywhere without the need for highly trained personnel, and have a short administration time. We hypothesized that both unimanual and bimanual performance would be better in controls than in participants with amnestic MCI or AD, and that across all individuals worse tapping performance would significantly correlate with a higher load of existing AD biomarkers. We further expected that by building a prediction model that combines multiple tapping measures, we would be able to accurately classify group membership.
MATERIALS AND METHODS
Participants
Participants were sampled from an ongoing study (R01AG055428; [48–50]) of brain imaging and neuropsychological testing across the dementia spectrum. Cognitively normal subjects were recruited from the community. The majority of amnestic MCI (single or multi-domain) and AD participants were recruited from the University of Utah cognitive disorders’ clinic [51]. Their diagnosis was based on a neurological visit, neuropsychological evaluation, and brain imaging. A minority of amnestic MCI subjects ∼15% came from the community sample who met criteria for amnestic MCI after cognitive evaluation. Confirmation of group assignment was made with the Alzheimer’s Disease Neuroimaging Initiative [52] classification battery, which comprises the Mini-Mental Status Examination [53], the Clinical Dementia Rating Scale [54], and the Wechsler Memory Scale-Revised [55] Logical Memory II Paragraph A. Forty-seven participants were classified as cognitively intact, twenty-seven as amnestic MCI (single or multi-domain), and twenty-six as mild or moderate AD. This study was performed in accordance with the Declaration of Helsinki and was approved by the University of Utah Institutional Review Board. Control subjects and MCI subjects signed informed consent. Individuals with AD signed assent while a Legally Authorized Representative signed informed consent in their name.
Inclusion criteria were 1) age 65 years or older; and 2) availability of a knowledgeable collateral source to comment on their cognition and daily functioning. Exclusion criteria comprised 1) medical comorbidities likely to affect cognition (i.e., neurological conditions, current severe depression, substance abuse, and major psychiatric conditions); 2) inability to complete magnetic resonance imaging (MRI) or positron emission tomography (PET) imaging; 3) inability to complete cognitive assessments due to inadequate vision, hearing, or manual dexterity; 4) being enrolled in a clinical drug trial related to anti-amyloid agents; 5) elevated depression as indicated by a score of greater than 5 on the 15-item Geriatric Depression Scale; and 6) severe dementia as indicated by a Clinical Dementia Rating score of 2 or greater or a Mini-Mental Status Examination score of less than 20.
Complete finger tapping data was available for 100 subjects. Demographic and clinical information is presented in Table 1. The overall group mean age was 74.5±5.9 years of age. Of the participants in our sample, 58.0% were female, and 98.0% were Caucasian or white. Mean premorbid intellectual functioning, as measured by the Reading subtest of the 4th edition of the Wide Range Achievement Test [56], was in the normal range for all three groups. Symptoms of depression, which were assessed using the Geriatric Depression Scale [57], were minimal and below the cut-off score for clinical depression.
Table 1.
Demographics
| Variable | Metric | Controls | MCI | AD | Total | ||
| p | p | ||||||
| Sample size | n | 47 | 27 | 26 | 100 | ||
| Age (y) | m (sd) | 73.7 (5.5) | 74.6 (6.3) | 0.53 | 75.7 (6.1) | 0.17 | 74.5 (5.9) |
| Sex (female) | n (%) | 31 (66) | 11 (40.7) | 0.1 | 16 (61.5) | 0.1 | 58 (58) |
| Right-handed | n (%) | 44 (93.6) | 26 (96.3) | 0.84 | 25 (96.2) | 0.84 | 95 (95) |
| Education | m (sd) | 16.6 (2.3) | 16 (2.7) | 0.31 | 15.2 (2.3) | 0.02 | 16.1 (2.5) |
| WRAT | m (sd) | 112 (7.2) | 108.7 (8.8) | 0.1 | 108 (9.1) | 0.049 | 110 (8.3) |
| GDS | m (sd) | 1 (1.3) | 1.6 (1.3) | 0.09 | 1.5 (1.4) | 0.16 | 1.3 (1.3) |
| MMSE | m (sd) | 28.9 (1.2) | 26.4 (1.9) | <0.001 | 22.8 (2.6) | <0.001 | 26.6 (3.1) |
| Caucasian | n (%) | 47 (100) | 26 (96.3) | 0.4 | 25 (96.2) | 0.4 | 98 (98) |
| AD Biomarkers | |||||||
| SUVR | m (sd) | 0.52 (0.11) | 0.73 (0.15) | <0.001 | 0.77 (0.16) | <0.001 | 0.6 (0.2) |
| Hippocampal Volume | m (sd) | 4.3 (0.48) | 3.66 (0.82) | <0.001 | 3.19 (0.85) | <0.001 | 3.8 (0.8) |
| Number of APOE ɛ4 alleles | <0.001 | <0.001 | |||||
| 0 | n (%) | 35 (76.1) | 7 (28.0) | 9 (34.6) | 51 (52.6) | ||
| 1 | n (%) | 10 (21.7) | 12 (48.0) | 11 (42.3) | 33 (34.0) | ||
| 2 | n (%) | 1 (2.2) | 6 (24.0) | 6 (23.1) | 13 (13.4) | ||
Linear regression analysis was used for continuous variables and Chi-square tests to compare proportions. m, mean; sd, standard deviation; n, number; p, p-value compared to the control group.; Education, years of education completed; WRAT, normative, age corrected standard score of the wide range achievement test-4 reading subtest; GDS, total score on the Geriatric Depression Scale; MMSE, Mini-Mental State Examination; Caucasian, self-reported Caucasian or white race; SUVR, 18F-Flutemetamol PET scan global composite standardized uptake value ratio; Hippocampal volume, bilateral hippocampal volume expressed as per-thousand of the estimated total intracranial volume.
Finger tapping
Collection of finger tapping data
Finger tapping performance was measured using a computerized test that we developed in-house using version 3 of the PsychoPy software suite [58–60]. The task can be downloaded from: https://github.com/vnckppl/FingerTappingTask [61]. We used the same 2017 13” MacBook Pro for task presentation and recording of all the participants’ input. Throughout the data collection period, no software updates (of either the operating system, or the PsychoPy software) were performed. The task that was built to be run independently, without the need for interaction with a study coordinator, has four conditions (left index finger tapping, right index finger tapping, simultaneous index finger tapping, and alternate finger tapping). Each condition consists of three trials which last 10 s. Finger taps are registered by left and right finger presses on the corresponding shift keys on the keyboard. The test starts with on-screen written and video instructions on how to perform each of the four conditions. Next, participants are instructed to place their finger(s) on the correct key(s) and to start the trial by pressing a tapping key. Upon the key press, a ‘Get Ready!’ message appears on the screen for 3 s, immediately followed by ‘Start Pressing!’. During the 10-s trial, a message is displayed on the screen to encourage the participant to press as fast as possible. For example: ‘Press AS FAST AS POSSIBLE with your RIGHT INDEX FINGER’. After 10 s, the screen shows ‘Done!’ for 3 s, immediately followed by information for the next trial. After all conditions are completed once, they are being repeated two more times in the same order. For single finger tapping, participants are instructed to press as fast as possible. For dual finger tapping, participants are instructed to press simultaneously with their left and right index fingers as fast as possible with the goal to complete as many pairs within 10 s. For the alternate tapping test, subjects are instructed to tap using one index finger after the other, as fast as possible. Subjects are allowed to start with either their left or right index finger.
Pre-processing and outcome measures of finger tapping data
Handedness was recorded with the Dutch Handedness Questionnaire [62]. These scores were binarized into left/right handedness and subsequently used to recode left and right hand tapping into dominant and non-dominant tapping scores.
Presence of tapping gaps, defined as periods where subjects paused tapping for 1 s or more were extracted from the raw data for all conditions. An overview of continuous outcome measures is presented in Table 2. Supplementary Figure 1 provides a graphical overview of the outcome measures. Each outcome measure was collected three times. We selected the subject’s median score for each of these outcome measures to yield scores robust against outliers at the subject level.
Table 2.
Overview of Continuous Tapping Metrics. ’All Conditions’ indicates that these measures are collected for unimanual dominant and non-dominant, and bimanual synchronous and alternate tapping. Supplementary Figure 1 provides a graphical overview of these outcome measures
| Condition | Tapping Measure | Description |
| All Conditions | Initial Reaction Time | Time in seconds from the start of the test to the first key press |
| Taps per Second | Rate of finger tapping speed expressed as taps per second | |
| Taps per Second Variance | Variance in rate of finger tapping speed expressed as taps per second | |
| Dual Tapping | Unpaired Taps Rate | Total number of left or right taps that could not be paired with a right or left tap respectively, divided by the total number of taps |
| Tap Pair Duration | Average time between the two taps that make up a pair | |
| Tap Pair Duration Variance | Variance in time between the two taps that make up a pair | |
| Number of Pairs | Total number of left and right tapping pairs | |
| Alternating | Error Rate | The number of incorrect transitions (i.e., left-to-left tap, or right-to-right tap) divided by the total number of taps |
| Alternating Transition Time | Average time between taps in seconds for correct transitions only (i.e., left-to-right tap and right-to-left tap transition) | |
| Alternating Transition Time Variance | Variance in average time between taps for correct transitions only (i.e., left-to-right tap and right-to-left tap transition) | |
| Alternating Transitions | Number of consecutive taps for correct transitions only (i.e., left-to-right tap and right-to-left tap transition) |
By selecting a broad variety of tapping outcome measures, while also statistically adjusting for multiple comparisons, we were able to evaluate the effects of AD pathology on multiple aspects of tapping performance, while also preventing Type I errors.
Alzheimer’s disease biomarkers
The well-established AD biomarkers [63] that we cross-correlate with tapping performance are whole brain amyloid-β deposition, hippocampal volume, and APOE ɛ4 allele status.
Amyloid-β deposition
18F-Flutemetamol, a radioactive diagnostic agent indicated for PET imaging of the brain, was used to estimate amyloid-β neuritic plaque density. The ligand was produced under PET cGMP standards and conducted under an approved FDA Investigational New Drug application (IND). Imaging was performed on a GE Discovery PET/CT 710 (GE Healthcare), which has a full width at half-maximum spatial resolution of 5.0 mm and excellent performance characteristics [64, 65]. Emission imaging took 20 min and was performed 90 min after the injection of approximately 185 mBq (5 mCi) of 18F-Flutemetamol. We used a regional semi-quantitative technique described by [66] and refined by Thurfjell et al. [67] to analyze the 18F-Flutemetamol binding. The CortexID Suite software (GE Healthcare) was used to automatically obtain a composite standardized uptake value ratio (SUVR) in the cerebral cortex which was normalized to the pons [68]. PET imaging was collected on average 27.7±43.2 weeks prior to the motor behavioral assessments.
Hippocampal volume
MRI images were acquired on a 3.0 Tesla Siemens Prisma scanner with a 64-channel head coil. T1-weighted data were acquired using a sagittal MP2RAGE sequence (TR = 5000 ms, TE = 2.93 ms, flip angles = 4° and 5° respectively, acquisition matrix = 256x256, field of view = 256x256 mm, slice thickness 1 mm, resolution = 1x1x1 mm, acquisition time=∼7 min). All scans were examined for the presence of common artifacts, including motion, susceptibility, and distortion, and were determined to be of sufficient quality for quantitative analysis. All data were processed on the same workstation using FreeSurfer image analysis suite v6.0 (https://surfer.nmr.mgh.harvard.edu/) to estimate total intracranial and hippocampal volumes. Technical details have been described previously [69–71]. Hippocampal volumes were expressed as proportion of the estimated total intracranial volume to account for differences in head size [72]. MRI scans were collected on average 32.3±39.9 weeks prior to the motor behavioralassessments.
APOE genotyping
Polymerase chain reaction and fluorescence monitoring using hybridization probes for APOE genotyping was conducted using whole blood samples. Participants were classified into three groups of having either 0, 1, or 2 APOE ɛ4 alleles.
Statistical analysis
Except for classification modeling, all statistical analyses were conducted in R version 4.2.1. The alpha level was set at 0.05 and false discovery rate (FDR) correction was applied to adjust for multiple comparisons.
Demographical information
Group differences in continuous potentially confounding variables were compared using linear regression models. Group differences in categorical potentially confounding variables were compared using χ2 tests using the MASS package (7.3-58).
Group comparisons of motor performance
Logistic regression analysis was used to compare control subjects to the amnestic MCI and AD groups on presence or absence of tapping gaps, defined as periods of 1 s or longer during the 10-s tapping trial where no key was tapped.
Non-parametric tests in the framework of the linear model were conducted using the lmPerm package (2.1.0) to evaluate measures of initial reaction time, tapping rate, tapping variance, and specific measures for alternate and dual tapping (see Table 3). We selected permutation tests in favor of their parametric equivalents for robustness against non-normality of residuals. Permutation tests were set to complete 100,000,000 random permutations. Because we were interested in how (pre)clinical AD differed from cognitively intact individuals with respect to motor function, we tested differences between 1) control subjects and amnestic MCI subjects; and 2) control subjects and AD subjects. The η2 value for the group factor, i.e., the proportion of variance in the model explained by group, is reported as a measure of effect size. All models were adjusted for age and sex.
Table 3.
Group differences in presence of tapping gaps
| aMCI versus Controls | AD versus Controls | |||||
| Condition | OR | SE | p | OR | SE | p |
| Dominant | Inf | Inf | NA | Inf | Inf | NA |
| Non-Dominant | 2.76 | 2.06 | 0.159 | 1.69 | 2.16 | 0.495 |
| Dual Tapping | 20.26 | 3.16 | 0.009 | 25.99 | 3.06 | 0.004 |
| Alternating | Inf | Inf | NA | Inf | Inf | NA |
All metrics are calculated from the maximum values over the subjects’ completed trials; aMCI, amnestic mild cognitive impairment; OR, odds ratio; SE, standard error; Inf, approaches infinity because for these conditions, there were no control subjects with tapping gaps; NA, unable to estimate p-value; bold indicate tests that retain significance after FDR correction.
Multiple comparison correction was applied by running FDR correction on the total, single array of p-values resulting from both the logistic regression analyses and non-parametric linear models for both the comparisons of controls versus amnestic MCI subjects and controls versus AD subjects.
Associations between motor performance and AD biomarkers
We assessed the association between finger tapping performance and our continuous biomarkers (hippocampal volume and amyloid-β) using linear regression models adjusted for age and sex. We report partial correlations (adjusted for age and sex) as a measure of effect size. Variables with a skewness≥1 (i.e., Initial Reaction Time and Taps per Second Variance for all tapping conditions, Tap Pair Duration Variance for dual tapping, and Error Rate, Alternating Transition Time, and Alternating Transition Time Variance for alternating finger tapping) were transformed to ensure assumptions for linear associations were met. This transformation process was a two-step approach: First outliers, defined as values that were outside the range of the mean±2.5 standard deviations, were set to the mean±2.5 standard deviation. Note that applying or not applying this step did not result in significantly different outcomes. Second, a log transformation was applied to the variable. Associations between tapping performance and our categorical biomarker measure (APOE ɛ4 alleles) were tested using Poisson regression models, adjusted for age and sex, with Incidence Rate Ratios as a measure of effect size.
Classification modeling
We used Balanced Random Forest Classification from the Imbalanced Learn (0.9.1) package, implemented in Python 3.8.10 for classification. Variables considered in the model included 1) the set of 20 continuous tapping performance measures reported in Table 4; 2) a count variable that lists the number of trials with either an onset delay of more than 1 s, a gap of more than 1 s or both; and 3) age and sex. Age and sex were considered to account for any imbalances in these metrics between groups. To reduce the number of variables in the model, we only selected variables that correlated (Spearman) for less than 90% with other variables in the dataset. This resulted in the exclusion of the variable ‘Number of Pairs’ for dual tapping and three alternating tapping variables: 1) ‘Alternating Transition Time’; 2) ‘Alternating Transition Time Variance’; and 3) ‘Alternating Transitions’.
Table 4.
Group differences in continuous tapping metrics
| MCI versus Controls | AD versus Controls | ||||||
| Condition | Tapping Measure | beta | p | η 2 | beta | p | η 2 |
| Dominant | Initial Reaction Time (s) | 0.16 | 0.093 | 0.03 | 0.24 | 0.011 | 0.06 |
| Taps per Second | –0.01 | 0.974 | 0.00 | –0.30 | 0.108 | 0.03 | |
| Taps per Second Variance | 0.00 | 1.00 | 0.02 | 0.01 | 0.003 | 0.08 | |
| Non-Dominant | Initial Reaction Time (s) | 0.46 | 0.006 | 0.07 | 0.57 | 0.001 | 0.11 |
| Taps per Second | –0.14 | 0.523 | 0.00 | –0.42 | 0.047 | 0.04 | |
| Taps per Second Variance | 0.01 | 0.019 | 0.05 | 0.01 | 0.009 | 0.06 | |
| Dual Tapping | Initial Reaction Time (s) | 0.49 | <0.001 | 0.12 | 0.19 | 0.152 | 0.02 |
| Unpaired Taps Ratea | 0.00 | 1.00 | 0.00 | 0.00 | 0.758 | 0.00 | |
| Taps per Second | –0.30 | 0.425 | 0.01 | –0.79 | 0.039 | 0.04 | |
| Taps per Second Variance | 0.01 | 0.002 | 0.09 | 0.01 | 0.014 | 0.05 | |
| Tap Pair Duration (s) | 0.00 | 0.129 | 0.02 | 0.00 | 0.228 | 0.01 | |
| Tap Pair Duration Variance (s) | 0.00 | 0.385 | 0.01 | 0.00 | 0.548 | 0.00 | |
| Number of Pairs | –1.68 | 0.402 | 0.01 | –4.28 | 0.035 | 0.04 | |
| Alternating | Initial Reaction Time (s) | 0.17 | 0.097 | 0.01 | 0.40 | <0.001 | 0.14 |
| Error Rateb | 0.00 | 0.548 | 0.00 | 0.00 | 1.00 | 0.00 | |
| Taps per Second | 0.31 | 0.414 | 0.01 | –0.83 | 0.033 | 0.04 | |
| Taps per Second Variance | 0.00 | 0.14 | 0.02 | 0.01 | <0.001 | 0.15 | |
| Alternating Transition Time (s)c | –0.01 | 0.619 | 0.00 | 0.02 | 0.077 | 0.03 | |
| Alternating Transition Time Variance (s)c | 0.00 | 0.782 | 0.00 | 0.01 | 0.058 | 0.03 | |
| Alternating Transitionsc | 3.61 | 0.35 | 0.01 | –8.31 | 0.033 | 0.04 | |
All metrics are calculated from the median values over the subjects’ completed trials; anumber of unpaired taps divided by total number of taps; bnumber of incorrect transitions (i.e., left-to-left tap, or right-to-right tap) divided by the number of taps; cApplies to correct transitions only (i.e., left-to-right tap and right-to-left tap transitions). Values printed in bold indicate tests that retain significance after FDR correction.
Balanced random forest classification was selected to account for differences in the sample sizes per group, which could otherwise lead to a classification model that has a higher likelihood to predict the largest class [73]. GridSearchCV from the Scikit Learn (1.1.2) package was used to detect the optimal set of hyper parameters with the maximum tree depth ranging from 1-10, 5 levels of number of trees in the forest (5, 10, 25, 50, 100), and either ‘gini’ or ‘entropy’ as the information gain criteria. The data were divided into a training set (70% of the data) and a test set (30% of the data) using the train_test_split algorithm. GridSearchCV was then run on the training set using five-fold cross-validation to obtain a model robust against overfitting. Results indicated that a model with a maximum tree depth of 5, 50 trees in the forest, and ‘gini’ as information gain criteria were optimal given our training data. We finally trained our random forest classification model using these parameters on our training data and applied it to our hold-out testing set.
To investigate the contribution of individual variables to the classification model, we conducted permutation feature importance analysis. This tool, which is part of Scikit learn, recalculates the model accuracy after randomly permuting one individual feature at a time. More specifically, all values of the feature in question are randomly shuffled after which the entire random forest model is being recalculated but now with the reshuffled variable instead of the original data. Changes in the overall model accuracy with the reshuffled values compared to the original data provide an indication how much the particular variable contributes to the overall accuracy of the model. By shuffling the values of the feature multiple times and calculating the new model accuracy each time, a distribution of the feature contribution to the model can be obtained. This process is being repeated for each feature in the model, and each feature was set to be permuted 200 times.
Exploratory analysis of the association between motor brain volume and finger tapping
Frontal regions such as the primary motor cortex (M1) [74–76] and the inferior frontal gyrus (IFG) [77] are involved in rhythm production and perception. Furthermore, degeneration of these areas in MCI and AD has been related to motor dysfunction [10]. Another brain region that regulates finger tapping rhythm [78] and which is affected in AD is the cerebellum [37–39].
In an exploratory analysis, group differences in bilateral volume of the IFG, M1, and the cerebellum were tested. Bilateral motor volumes were obtained in the same FreeSurfer process used to obtain hippocampal volumes and were expressed as percentage of the total intracranial volume to adjust for premorbid brain volume. Group differences were tested using the same linear models as described for testing hippocampal volume. Additionally, motor brain volumes were tested for associations with all tapping outcome measures as described in Table 2, using the same linear regression models as those used to test hippocampal volume and tapping outcome measure associations.
RESULTS
No group differences were observed in mean age or average amount of depressive symptoms as measured with the Geriatric Depression Scale, or the distribution of sex or race. Control subjects had on average completed 1.5 years more education than AD subjects and scored higher on the MMSE than both amnestic MCI and AD subjects. AD subjects had a slightly lower premorbid verbal intelligence as measured with the WRAT.
Group comparisons of motor performance
Binary tapping measures
Logistic regression analysis showed that, compared to control subjects, both amnestic MCI and AD subjects had higher odds of having gaps of 1 second or more during dual tapping, but not for the other tapping conditions (see Table 2). This was potentially related to the rareness of this outcome; for both dominant and alternate tapping none of the control subjects had any gaps.
Continuous tapping measures
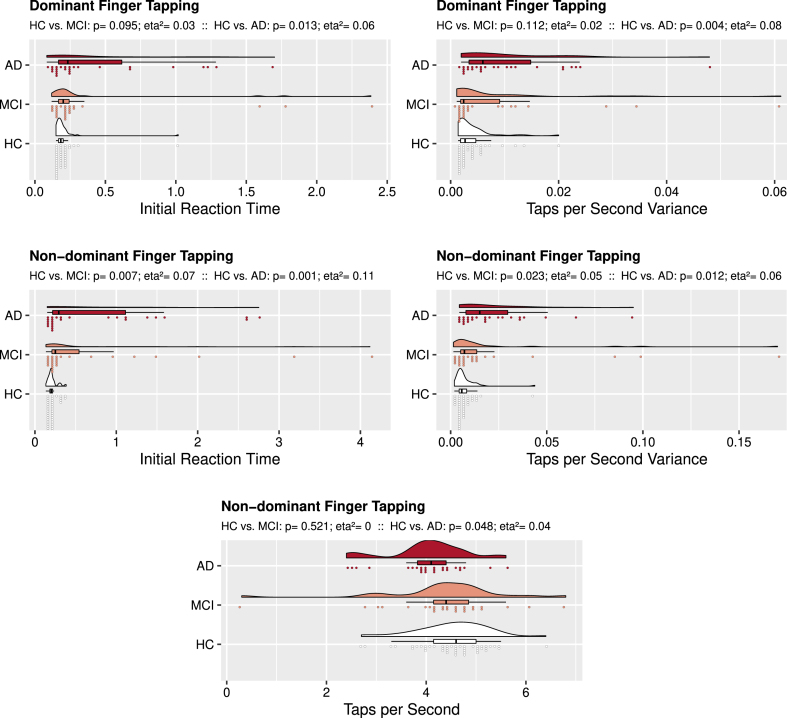
Results from our permutation-based linear models to assess group differences in tapping performance are displayed in Table 4. AD subjects performed worse on all single finger tapping measures than control subjects, except for dominant hand Taps per Second (see Fig. 1). Dominant hand tapping was not different between amnestic MCI and control subjects. For the non-dominant finger, however, those with amnestic MCI had a longer Initial Reaction Time and larger Taps per Second Variance when compared to control subjects.
Fig. 1.
Unimanual Finger Tapping: Group Comparisons. Rain cloud plots with density curves, boxplots, and individual subject scores divided over 75 bins. The top cloud (red) presents data from the AD subject group, the middle cloud (salmon colored) presents data from the amnestic MCI subject group, the bottom cloud (white) presents data from the control group. Above each figure, p-values and eta2 as measure of effect size are reported 1) for the analysis comparing controls to amnestic MCI subjects (’HC vs. MCI’) and 2) for the analysis comparing controls to AD subjects (’HC vs. AD’). For dominant finger tapping AD performed worse than controls on Initial Reaction Time and Taps per Second Variance. For the non-dominant finger tapping, both AD and amnestic MCI performed worse on these measures, while AD also had fewer Taps per Second than controls.
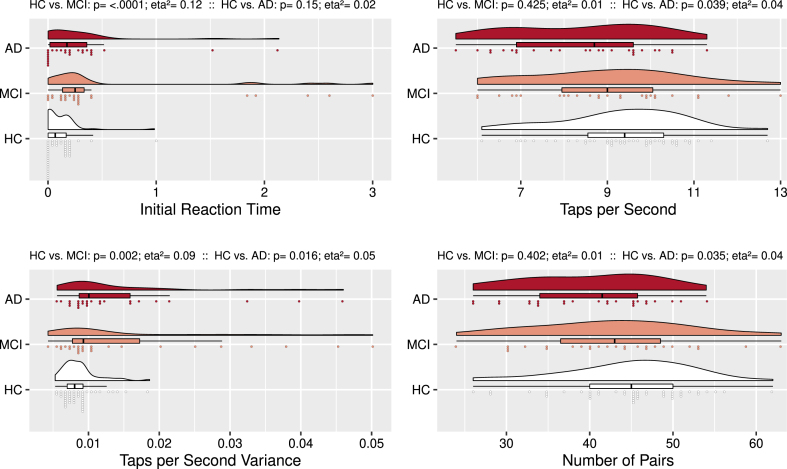
Amnestic MCI subjects had a longer Initial Reaction Time and larger Taps per Second Variance in the dual tapping condition (see Fig. 2) than control subjects. AD subjects also had larger Taps per Second Variance than controls, but also fewer Taps per Second during dual tapping. They also had fewer Number of Pairs than control subjects.
Fig. 2.
Synchronous Dual Finger Tapping: Group Comparisons. Rain cloud plots with density curves, boxplots, and individual subject scores divided over 75 bins. Compared to controls, amnestic MCI subjects had a longer Initial Reaction Time and larger Taps per Second Variance. Compared to controls, AD subjects had fewer Taps per Second, larger Taps per Second Variance, and fewer Number of Pairs.
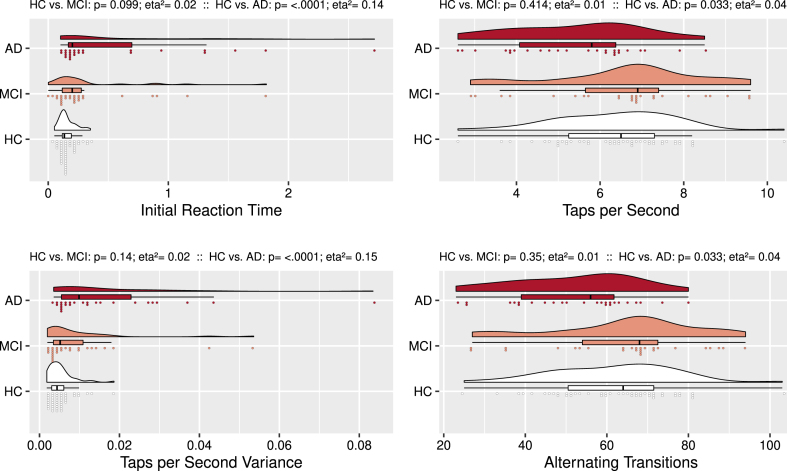
AD subjects performed worse than control subjects (see Fig. 3) on the alternate tapping condition. Specifically, they showed a longer Initial Reaction Time, fewer Taps per Second, larger Taps per Second Variance, and completed fewer Alternating Transitions. Alternating tapping performance was not statistically different between control and amnestic MCI subjects.
Fig. 3.
Alternate Finger Tapping: Group Comparisons. Rain cloud plots with density curves, boxplots, and individual subject scores divided over 75 bins. Compared to controls, AD subjects had a longer Initial Reaction Time, fewer Taps per Second, larger Taps per Second Variance, and fewer Consecutive Taps.
Associations between motor performance and AD biomarkers
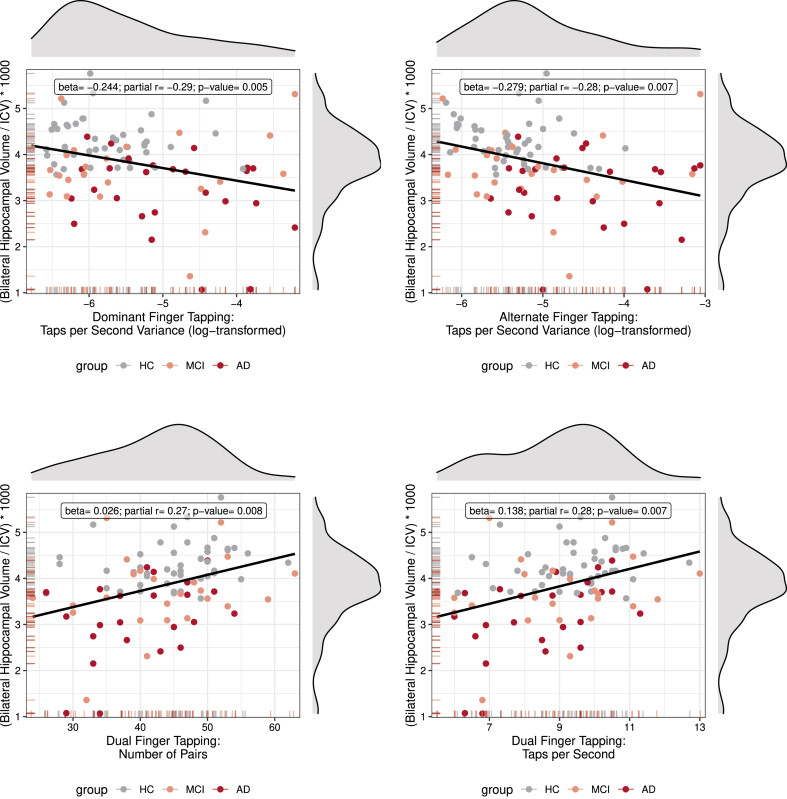
For the entire sample, hippocampal volume was positively related to 1) Taps per Second (Cohen’s d = 0.53, p = 0.014) and Taps per Second Variance (Cohen’s d = 0.60, p = 0.005) for dominant finger tapping; 2) Taps per Second (Cohen’s d = 0.58, p = 0.007), Taps per Second Variance (Cohen’s d = 0.50, p = 0.019), and Number of Pairs (Cohen’s d = 0.57, p = 0.008) for synchronous dual finger tapping; and 3) Taps per Second Variance (Cohen’s d = 0.58, p = 0.007), Alternating Transition Time (Cohen’s d = 0.44, p = 0.038), and Alternating Transition Time Variance (Cohen’s d = 0.44, p = 0.037) for alternate finger tapping. The four significant associations with a Cohen’s d≥0.57, i.e., medium to large effect size [79], survived FDR correction for multiple comparisons. Correlation plots of these outcomes are displayed in Fig. 4. Only one association between finger tapping performance and APOE ɛ4 allele status was observed (non-dominant hand Initial Reaction Time, Incidence Rate Ratio = 1.35, p = 0.033) and one association between finger tapping performance and amyloid-β was observed (dual synchronous finger tapping Initial Reaction Time, Cohen’s d = 0.54, p = 0.010). These two observations did not survive FDR correction.
Fig. 4.
Correlations between Hippocampal Volume and Tapping Performance. Scatter plots with density curves (on top and on the right side of each graph) and carpet plots (on the left and bottom side of axis) for both variables. Each graph shows the individual observations color coded for group, and a regression line. Inside the graph is a text box with summary statistics of the partial correlation (adjusted for age and sex) and regression, as well as the p-value for the analysis. Note that, even though individual values are color coded for group, the statistics were calculated for the entire collapsed sample.
Tapping performance as classifier of group
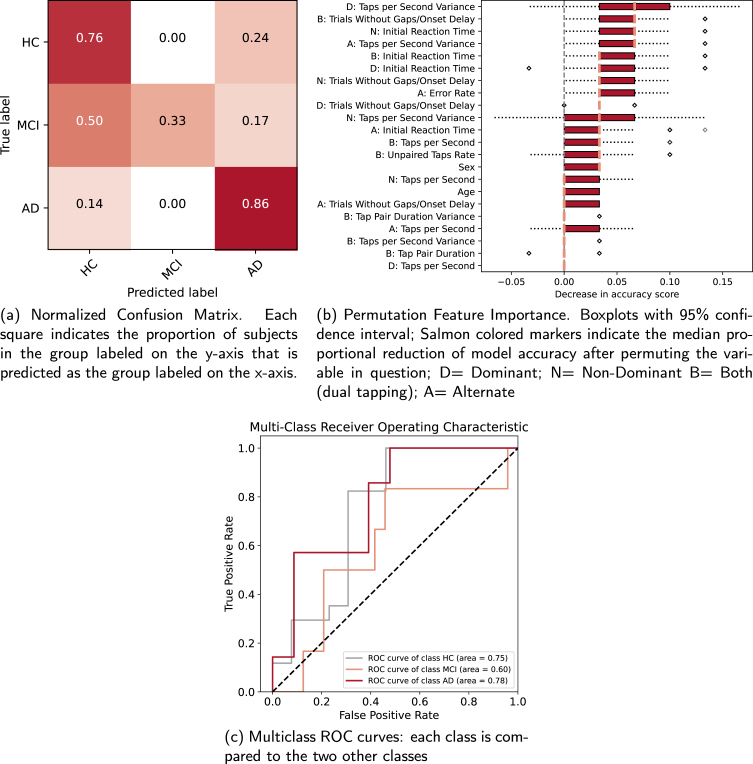
The overall classification accuracy of the training data was 56%, while the overall classification accuracy of the test data was 70%. This indicates that 70% of the subjects of an independent data set were accurately labeled by our classification model. This is a 22% increase over a null model that would simply predict the most frequent class for all observations (i.e., 47 controls would be accurately predicted out of 98 participants).
The group-specific precision, defined as the proportion of a group that was classified correctly (i.e., ‘true positives’) was 76% for controls, 100% for amnestic MCI, and 45% for AD. The confusion matrix (see Fig. 5a) shows in each cell the proportion of the class in the row that is predicted as the class in the column. The diagonal represents the group-wise ‘recall’ of the classification model. It shows that 76% of the healthy controls among all control subjects and 86% of AD subjects among all AD subjects were accurately classified, while 33% of amnestic MCI subjects among all amnestic MCI subjects were correctly labeled. In 50% of the cases amnestic MCI subjects were classified as control subjects, and in 17% of the cases as AD subjects. Precision and recall can be combined into a single ‘F1’ score [80], which is defined as:
Fig. 5.
Random forest classification metrics for the classification of group.
F1 is less biased against disproportional sample size of classes and ranges from 0-1 with higher scores indicating more specific and sensitive models. F1 scores for control, amnestic MCI, and AD subjects were 0.76, 0.50, and 0.67 respectively. The overall multi-class area under the curve (AUC) score for the classification model was 0.76. Figure 5c shows receiver-operator curves and corresponding AUC scores for each group in comparison to the two other groups. AUC scores for the control group, amnestic MCI group, and AD group were 0.75, 0.60, and 0.78 respectively. While the AUC for the control and AD groups indicates acceptable, bordering excellent, discrimination, the AUC for the amnestic MCI group equals poor discrimination [81].
An overview of the permutation feature importance is displayed in Fig. 5b. Values indicate the decrease in model accuracy after random permutation of its values. The five most important features that were used for classification of the test hold-out data were 1) variance in tapping speed during dominant hand finger tapping; 2) the number of dual tapping trials without gaps or onset delays; 3) initial reaction time for non-dominant hand finger tapping; 4) variance in tapping speed during alternate finger tapping; and 5) initial reaction time during dual finger tapping.
Exploratory analysis of the association between motor brain volume and finger tapping
No significant between-group differences in the volume of the bilateral IFG, M1, and the cerebellum were observed between controls and aMCI subjects (all p > 0.26), or controls and AD subjects (all p > 0.82). Dominant hand Taps per Second was significantly associated with volume of the bilateral inferior frontal gyri (beta=-0.05, p = 0.005, Cohen’s d = 0.61), but none of the brain-behavioral associations survived FDR correction for multiple comparisons.
DISCUSSION
Finger tapping performance in AD
We studied the association between different stages of AD and uni/bimanual finger tapping performance, as significant associations could point towards this motor task as a quick and affordable screening measure for AD. Our results show that both amnestic MCI and AD are associated with slower initial reaction time, regardless of tapping condition or hand side. Relative to control subjects, amnestic MCI subjects performed worse on 4/20 tapping outcome measures, while AD subjects performed worse on 12/20 outcome measures. Finger tapping variability of the non-dominant hand and during dual tapping were affected in both amnestic MCI and AD. The AD group additionally showed more variability in dominant and alternate finger tapping, slower non-dominant, dual, and alternate finger tapping, as well as fewer dual tapping pair construction, and alternate tapping correct consecutive taps.
While AD subjects performed worse on both the dominant and the non-dominant hand tapping tests, amnestic MCI subjects only performed worse than controls when using their non-dominant hand. This gradual loss of asymmetry has been observed in MCI and AD previously and is independent of aging in general [29–31]. For example, compared to control subjects, MCI participants displayed more symmetric grasping and inserting movements on the Purdue Pegboard test [17], and AD subjects exhibited more similar finger tapping pace for both hands [32]. Considering handedness-related cortical morphological asymmetry (e.g., deeper central sulcal depth of the hemisphere controlling the dominant hand [33]), our observed pattern could potentially be explained by accelerated asymmetric cortical thinning that has been observed in AD [34]. Alternatively, between-group differences in cognitive function required for learning a novel motor task [82] could explain why AD subjects performed worse than amnestic MCI subjects [83]. Yet another potential explanation is that non-dominant hand performance is more susceptible to pathological changes related to AD, because the non-dominant hand is less trained than the dominant hand. As such, there is less motor reserve [84] of non-dominant hand performance, and deterioration of non-dominant performance will therefore show up earlier in the course of the disease than dominant-hand performance. Our results show that in comparison with controls, alternate tapping was worse in AD subjects but not in amnestic MCI subjects. Gamma-aminobutyric acid (GABA) is an amino acid that is involved in cerebral inhibition of motor function required for alternating bilateral movements [43]. Reductions of GABA levels that have been observed in AD, but not in MCI, could explain the discrepancy in alternate tapping performance of these two groups [42]. Changes in the cholinergic system may also be underlying the performance deficits in AD, as previous research has shown that tapping slowness in AD correlated with reduced short-latency afferent inhibition measured with transcranial magnetic stimulation that could be explained by reduced cholinergic interneuron excitability [24].
Of all measures affected in amnestic MCI and AD participants, Initial Reaction Time and Taps per Second Variance had the largest effect sizes for all tapping conditions that survived multiple comparisons correction. Taps per Second was significant for all but the dominant hand condition, but these outcomes did not survive multiple comparison correction. A meta-analysis on simple reaction time in MCI corroborates our findings and suggests that slower response time in this population is related to attention as well as motor processes at the neural level [35]. Previous research has shown that tapping variability, but not tapping speed in older adults is affected when adding a secondary task [36], thus suggesting a cognitive component to performance. Variability in tapping speed [40] as well as bimanual coordination [41] have been linked to cerebellar functioning, a region that until recently was thought to be relatively unaffected by AD because it exhibits much less amyloid deposition than supratentorial regions [85]. Current research, however, has indicated that the cerebellum is in fact affected by AD pathology and could therefore be a region of interest when studying motor dysfunction in AD [37–39].
It should be noted that while our tapping speed and pair-forming outcome measures did not exhibit significant deviations from normality, the distributions of the initial reaction time and variance measures were highly skewed. The long tail of the skewed distributions suggests that only a small subset of individuals with amnestic MCI and AD performed extremely poorly, while the majority performs more similar to control subjects. Potentially, tapping performance deficits and motor problems in general define a subgroup of AD patients [86]. Alternatively, subjects with substantially poorer motor performance may have other neurological comorbidities that commonly present with AD, that we were unable to detect during subject screening such as cerebrovascular disease including atherosclerosis, white matter pathology, and infarctions, but also Lewy bodies [87, 88]. Lewy body disease in particular is known to be associated with motor dysfunction, including slower tapping speed [89]. Ideally, future studies would evaluate such comorbidities to statistically control for them.
Associations between motor performance and AD biomarkers
Hippocampal volume was related to finger tapping speed and variability under both unimanual and bimanual conditions. These observations remained significant after multiple comparisons correction. Although we found some indications that finger tapping performance was related to global amyloid-β deposition and the number of APOE ɛ4 alleles, these results did not survive multiple comparison correction. The pattern of associations of finger tapping with individual AD biomarkers indicates that tapping performance is more affected by structural brain changes that generally occur later in the disease process [90], and less by amyloidosis which starts earlier in the disease process [91, 92]. Surprisingly, volume of brain regions that had previously been implicated in finger tapping performance such as the cerebellum, primary motor cortex, and IFG were not associated with tapping performance in our sample. Potentially, functional compensation through increased brain activation may have attenuated the volumetric associations [93]. A previous study reported a positive association in AD patients between Aβ42 levels from cerebrospinal fluid and scores on part 3 of the Unified Parkinson Disease Rating Scale, which measures various motoric functions including gait, balance, finger-to-thumb tapping, tremor, and speech [45]. Potentially, the gait and balance measures may have been driving this association, explaining the discrepancy between these results and those reported here. The lack of a statistically significant association between finger tapping performance and well-validated, global amyloid-β depositions resembles reports that show amyloid-β deposits in cognitively normal individuals [94], underlining that not all brain pathology directly affects behavior, potentially due to motor reserve [95]. Alternatively, despite our observation that a global amyloid-β measure may be relatively insensitive to motor function, regional measures of amyloid-β could be predictive, the same way different cognitive functions display region-specific associations with amyloid-β deposition [96]. Future studies should estimate regional depositions of amyloid-β, for example of the primary motor cortex, to assess if they are predictive of finger tapping performance.
Although there is limited evidence that the hippocampus plays a role in simple finger tapping [46], we did observe an association between several tapping measures and hippocampal volume. It is possible that, in this case, hippocampal volume reflects general brain atrophy or even that of motor brain regions, which has resulted in the observed brain-behavioral association. To the best of our knowledge, few studies have explicitly looked at the neural underpinnings of finger tapping performance in AD [10]. A recent study however has revealed that reduced short-latency afferent inhibition partially explains slow movement in AD [24]. To better understand if motor dysfunction in AD is related to general neurodegeneration, or linked to specific brain regions, whole-brain functional and structural imaging mechanistic studies are warranted. More specifically, morphological and brain activation studies of the primary motor cortex could explain variation in our observed tapping deficits in aMCI and AD, as previous work indicates that tapping performance largely depends on primary motor cortex activation [97], bradykinesia in AD is related to motor cortex dysfunction [24, 45], and primary motor cortex plasticity and excitability are affected already in MCI [25, 98, 99].
The range of Cohen’s d coefficients for the four observed significant associations between hippocampal volume and finger tapping performance that survived multiple comparisons correction was 0.57-0.60. This equates to medium-to-large effect sizes [79]. Taps per Second Variance for alternating tapping was the measure that most strongly differed between controls and AD subjects (η2 = 0.15) and also significantly correlated with hippocampal volume, while surviving multiple comparisons correction for both analyses. All finger tapping outcome measures that significantly related to hippocampal volume also showed significant differences between controls and either amnestic MCI or AD subjects. Based on these results, finger tapping speed may substitute hippocampal volume as AD biomarker but is not suited to substitute amyloid-β or APOE ɛ4 allele status. It may also provide non-redundant information, considering that even these existing biomarkers are limited in predicting AD development [100]. Tapping measures could also be a cost-efficient tool for augmenting existing biomarkers [47] or they can form part of a set of motor measures that together make up a sensitive biomarker for AD [101, 102].
Tapping performance as classifier of group
Our random forest classifier that used finger tapping performance measures to predict group membership had an overall accuracy of 70%, outperforming the null model. Permutation feature analysis revealed that dominant hand Taps per Second Variance contributed most strongly to the classification model. This is in line with the results of our regression models that show that dominant hand Taps per Second Variance is moderately to strongly affected in AD, but not significantly in amnestic MCI.
Inspection of individual classes indicates that prediction model accuracy is driven by the classification of control and AD subjects. Our model predicted control subjects with both good precision (i.e., ‘most subjects classified as controls were truly controls in the sample’) and recall (i.e., ‘most true control subjects in the sample were classified as controls’). For AD subjects, precision was lower (i.e., not everybody who was classified as AD was actually an AD subject, sometimes they were control subjects), but recall was even higher. For amnestic MCI subjects, however, the model precision was high (i.e., if a subject was classified as amnestic MCI they were always truly amnestic MCI), but the recall was at chance level: amnestic MCI subjects had an equal chance to be classified as controls, amnestic MCI and AD. The AUC metrics further demonstrate the discrepancy in the predictive ability of our model for controls and AD versus amnestic MCI. The overall AUC value of 0.76 and those for the control and AD groups are comparable to a classification study that used random forest classification on speech and eye-tracking data to distinguish control subjects from a mixed sample of subjects with MCI, subjective memory complaints, and AD [103]. A meta-analysis on machine learning methods employing neuropsychological tests to discriminate controls from MCI and AD participants showed that cognitive measures had similar sensitivity to distinguish controls from AD as we report here, but that cognitive measures are better at telling apart controls from MCI [104] than our tapping measures. Although our outcomes thus suggest that finger tapping performance may not be suited as an early detection tool, it is a simple, inexpensive, 10-min test that requires little training and for which the scoring is fully automated that is able to accurately distinguish individuals with and without AD, at 86% accuracy. As such, it could be used in conjunction with other measures and biomarkers as combined outcome measure for clinical trials aiming to prevent development of MCI or AD. Decreases in tapping performance could contribute to this joint outcome measure, as an indicator of disease progression.
Strengths and limitations
This is one of few studies applying an elaborate assessment of unimanual and bimanual finger tapping function in a well-defined sample along the AD continuum. Our study is unique in that it directly relates motor performance measures to three established AD biomarkers: hippocampal volume, brain amyloid-β deposition, and APOE ɛ4 allele status. By combining frequentist statistical methods and machine learning modeling we were able to yield an understanding of how AD pathology affects motor function and how it can be used as a disease classifier. Although we collect a variety of validated, global biomarkers, we did not collect tau, which plays a major role in AD neuropathology [105]. Because tau deposits present later in the AD trajectory than amyloid-β, but before hippocampal atrophy [90], finger tapping may be more sensitive to this biomarker.
Our sample included almost exclusively white, Caucasian subjects. Although, to the best of our knowledge, there are no studies showing racial differences in finger tapping performance, we cannot rule out that they exist. It is thus not possible to extrapolate our results to other populations, and we should therefore aim to include diverse populations in studies on tapping performance and dementia.
AD has been investigated thoroughly from a neural and cognitive perspective and large datasets with such features are readily available in the public domain. Motor measures have been studied much less in AD, and large datasets of motor behavior in MCI and AD are not available. Though our moderate sample size allowed us to gauge tapping performance in AD, it was less optimal for classification model construction. Combining datasets or adding motor measures to ongoing large studies will help identifying if motor measures could be viable predictors for preclinical AD.
Our finger tapping test is light-weight and can be freely installed on any operating system. It also does not require special hardware to run. However, this test has not been validated, although it has been modeled after existing tapping tests in terms of the information that is recorded. Additionally, because it only uses a keyboard as input device, it is not able to collect certain information, such as pressure of the key press, that may be informative. To collect such information, additional hardware and alterations of the software are required.
Although we excluded individuals with neurological disorders and those who were using antipsychotic or anticonvulsant medications that could affect the motor system, we did not exclude individuals taking medications that might have adversely affected motor function. Future studies should record medications affecting the motor system to allow adjustment or stratification to explore such potential effects.
The time difference between collection of our MRI and PET imaging biomarkers and our tapping data was approximately 30 weeks on average. Although this amount of time is relatively short for considerable neurodegeneration to accumulate [106], we acknowledge that not collecting the behavioral and biomarker data at the exact same time may have led to weaker correlations. Future studies should aim to collect motor and AD imaging biomarkers closer in time to reduce the chance of Type II errors.
Conclusions and future directions
This study indicates that unimanual and bimanual finger tapping performance are affected in amnestic MCI and more so in AD. Especially initial reaction time and tapping speed variability are affected, though it seems that these differences are driven by a select group of amnestic MCI and AD participants that perform particularly poor, even compared to other amnestic MCI and AD subjects. In AD subjects, both dominant and non-dominant unimanual finger tapping and alternate and simultaneous tapping were significantly affected, while in amnestic MCI subjects only unimanual non-dominant hand and synchronous bimanual tapping were affected. Finger tapping speed and variance in speed were predictive of hippocampal volume, but tapping measures were not significantly related to other conventional AD biomarkers such as amyloid-β deposition and APOE ɛ4 allele status. The link between tapping performance and hippocampal volume could reflect general neurodegeneration rather than hippocampal atrophy in particular. A machine learning classification model was well able to discriminate control subjects from AD subjects but did poorly when trying to distinguish MCI from the other two groups. Our findings indicate that AD is linked to poorer finger tapping, but that this may not be used to identify patients early in the AD disease process but may be better suited for later in the course of the disease. In their current form, finger tapping tests could be a cost-efficient tool for augmenting existing AD biomarkers.
Future research should focus on the specificity of unimanual and bimanual finger tapping dysfunction for amnestic MCI and AD. Comparing finger tapping performance between individuals with amnestic MCI, AD, dementia with Lewy bodies [89] and Parkinson’s disease [20] can help identify if there are differences in patterns of tapping performance that are unique to these groups. This in turn could facilitate the development of prediction and classification models. Combining motor measures with assessments of activities of daily living (see for example [107]) in individuals with amnestic MCI and AD can provide information on the clinical relevance of motor measures assessed in a lab environment.
Supplementary Material
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-221297.
FUNDING
This work was supported by NIH/NIA grants R01AG055428 awarded to Kevin Duff, K01AG073578 awarded to Vincent Koppelmans, and K01AG075166 awarded to Jace King.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available on request from the corresponding author.
REFERENCES
- [1]. Beauchet O, Annweiler C, Callisaya ML, De Cock AM, Helbostad JL, Kressig RW, Srikanth V, Steinmetz JP, Blumen HM, Verghese J, Allali G (2016) Poor gait performance and prediction of dementia: Results from a meta-analysis. J Am Med Dir Assoc 17, 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Bahureksa L, Najafi B, Saleh A, Sabbagh M, Coon D, Mohler MJ, Schwenk M (2016) The impact of mild cognitive impairment on gait and balance: A systematic review and meta-analysis of studies using instrumented assessment. Gerontology 63, 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Bishnoi A, Hernandez ME (2020) Dual task walking costs in older adults with mild cognitive impairment: A systematic review and meta-analysis. Aging Ment Health 25, 1618–1629. [DOI] [PubMed] [Google Scholar]
- [4]. Ceïde ME, Ayers EI, Lipton R, Verghese J (2018) Walking while talking and risk of incident dementia. Am J Geriatr Psychiatry 26, 580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA (2007) Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology 29, 66–73. [DOI] [PubMed] [Google Scholar]
- [6]. Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA (2009) Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol 66, 1339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Buchman AS, Bennett DA (2011) Loss of motor function in preclinical Alzheimer’s disease. Expert Rev Neurother 11, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J (2010) The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol 67, 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Kueper JK, Speechley M, Lingum NR, Montero-Odasso M (2017) Motor function and incident dementia: A systematic review and meta-analysis. Age Ageing 46, 729–738. [DOI] [PubMed] [Google Scholar]
- [10]. Koppelmans V, Silvester B, Duff K (2022) Neural mechanisms of motor dysfunction in mild cognitive impairment and Alzheimer’s disease: A systematic review. J Alzheimers Dis Rep 6, 307–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Yu NY, Chang SH (2016) Kinematic analyses of graphomotor functions in individuals with Alzheimer’s disease and amnestic mild cognitive impairment. J Med Biol Eng 36, 334–343. [Google Scholar]
- [12]. Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA (2006) Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch Neurol 63, 1763. [DOI] [PubMed] [Google Scholar]
- [13]. Bailon O, Roussel M, Boucart M, Krystkowiak P, Godefroy O (2010) Psychomotor slowing in mild cognitive impairment, Alzheimer’s disease and Lewy body dementia: Mechanisms and diagnostic value. Dement Geriatr Cogn Disord 29, 388–396. [DOI] [PubMed] [Google Scholar]
- [14]. Fritz NE, Kegelmeyer DA, Kloos AD, Linder S, Park A, Kataki M, Adeli A, Agrawal P, Scharre DW, Kostyk SK (2016) Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture 50, 1–7. [DOI] [PubMed] [Google Scholar]
- [15]. Martin E, Blais M, Albaret JM, Pariente J, Tallet J (2017) Alteration of rhythmic unimanual tapping and anti-phase bimanual coordination in Alzheimer’s disease: A sign of inter-hemispheric disconnection? Hum Mov Sci 55, 43–53. [DOI] [PubMed] [Google Scholar]
- [16]. de Paula JJ, Albuquerque MR, Lage GM, Bicalho MA, Romano-Silva MA, Malloy-Diniz LF (2016) Impairment of fine motor dexterity in mild cognitive impairment and Alzheimer’s disease dementia: Association with activities of daily living. Braz J Psychiatry 38, 235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Vasylenko O, Gorecka MM, Waterloo K, Rodríguez-Aranda C (2022) Reduction in manual asymmetry and decline in fine manual dexterity in right-handed older adults with mild cognitive impairment. Laterality 27, 581–604. [DOI] [PubMed] [Google Scholar]
- [18]. Suzumura S, Osawa A, Nagahama T, Kondo I, Sano Y, Kandori A (2016) Assessment of finger motor skills in individuals with mild cognitive impairment and patients with Alzheimer’s disease: Relationship between finger-to-thumb tapping and cognitive function. Japan J Compr Rehabil Sci 7, 19–28. [Google Scholar]
- [19]. Kluger A, Gianutsos JG, Golomb J, Ferris SH, George AE, Franssen E, Reisberg B (1997) Patterns of motor impairment in normal aging, mild cognitive decline, and early Alzheimer’s disease. J Gerontol B Psychol Sci Soc Sci 52B, P28–P39. [DOI] [PubMed] [Google Scholar]
- [20]. Roalf DR, Rupert P, Mechanic-Hamilton D, Brennan L, Duda JE, Weintraub D, Trojanowski JQ, Wolk D, Moberg PJ (2018) Quantitative assessment of finger tapping characteristics in mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease. J Neurol 265, 1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Suzumura S, Osawa A, Kanada Y, Keisuke M, Takano E, Sugioka J, Natsumi M, Nagahama T, Shiramoto K, Kuno K, Kizuka S, Satoh K, Sakurai H, Sano Y, Mizuguchi T, Kandori A, Kondo I (2022) Finger tapping test for assessing the risk of mild cognitive impairment. Hong Kong J Occup Ther 35, 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Kutz DF, Fröhlich S, Rudisch J, Müller K, Voelcker-Rehage C (2022) Finger tapping as a biomarker to classify cognitive status in 80+-year-olds. J Pers Med 12, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Kragh FJ, Bruun M, Budtz-Jørgensen E, Hjermind LE, Schubert R, Reilmann R, Nielsen JE, Hasselbalch SG (2018) Quantitative measurements of motor function in Alzheimer’s disease, frontotemporal dementia, and dementia with Lewy bodies: A proof-of-concept study. Dement Geriatr Cogn Disord 46, 168–179. [DOI] [PubMed] [Google Scholar]
- [24]. Bologna M, Guerra A, Colella D, Cioffi E, Paparella G, Vita AD, D’Antonio F, Trebbastoni A, Berardelli A (2020) Bradykinesia in Alzheimer’s disease and its neurophysiological substrates. Clin Neurophysiol 131, 850–858. [DOI] [PubMed] [Google Scholar]
- [25]. Colella D, Guerra A, Paparella G, Cioffi E, Vita AD, Trebbastoni A, Berardelli A, Bologna M (2021) Motor dysfunction in mild cognitive impairment as tested by kinematic analysis and transcranial magnetic stimulation. Clin Neurophysiol 132, 315–322. [DOI] [PubMed] [Google Scholar]
- [26]. Hesseberg K, Tangen GG, Pripp AH, Bergland A (2020) Associations between cognition and hand function in older people diagnosed with mild cognitive impairment or dementia. Dement Geriatr Cogn Dis Extra 10, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Koeneke S, Lutz K, Wüstenberg T, Jäncke L (2004) Bimanual versus unimanual coordination: What makes the difference? Neuroimage 22, 1336–1350. [DOI] [PubMed] [Google Scholar]
- [28]. Suzumura S, Osawa A, Maeda N, Sano Y, Kandori A, Mizuguchi T, Yin Y, Kondo I (2018) Differences among patients with Alzheimer’s disease, older adults with mild cognitive impairment and healthy older adults in finger dexterity. Geriatr Gerontol Int 18, 907–914. [DOI] [PubMed] [Google Scholar]
- [29]. Francis KL, Spirduso WW (2000) Age differences in the expression of manual asymmetry. Exp Aging Res 26, 169–180. [DOI] [PubMed] [Google Scholar]
- [30]. Teixeira LA (2008) Categories of manual asymmetry and their variation with advancing age. Cortex 44, 707–716. [DOI] [PubMed] [Google Scholar]
- [31]. Sebastjan A, Skrzek A, Ignasiak Z, Sławińska T (2017) Age-related changes in hand dominance and functional asymmetry in older adults. PLoS One 12, e0177845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Massman PJ, Doody RS (1996) Hemispheric asymmetry in Alzheimer’s disease is apparent in motor functioning. J Clin Exp Neuropsychol 18, 110–121. [DOI] [PubMed] [Google Scholar]
- [33]. Hammond G (2002) Correlates of human handedness in primary motor cortex: A review and hypothesis. Neurosci Biobehav Rev 26, 285–292. [DOI] [PubMed] [Google Scholar]
- [34]. Roe JM, Vidal-Piñeiro D, Sørensen Ø, Brandmaier AM, Düzel S, Gonzalez HA, Kievit RA, Knights E, Kühn S, Lindenberger U, Mowinckel AM, Nyberg L, Park DC, Pudas S, Rundle MM, Walhovd KB, Fjell AM, Westerhausen R; Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing (2021) Asymmetric thinning of the cerebral cortex across the adult lifespan is accelerated in Alzheimer’s disease. Nat Commun 12, 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Andriuta D, Diouf M, Roussel M, Godefroy O (2019) Is reaction time slowing an early sign of Alzheimer’s disease? A meta-analysis. Dement Geriatr Cogn Disord 47, 281–288. [DOI] [PubMed] [Google Scholar]
- [36]. Sommervoll Y, Ettema G, Vereijken B (2011) Effects of age, task, and frequency on variability of finger tapping. Percept Mot Skills 113, 647–661. [DOI] [PubMed] [Google Scholar]
- [37]. Schmahmann JD (2016) Cerebellum in Alzheimer’s disease and frontotemporal dementia: Not a silent bystander. Brain 139, 1314–1318. [DOI] [PubMed] [Google Scholar]
- [38]. Tabatabaei-Jafari H, Walsh E, Shaw ME, Cherbuin N (2017) The cerebellum shrinks faster than normal ageing in Alzheimer’s disease but not in mild cognitive impairment. Hum Brain Mapp 38, 3141–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Jacobs HIL, Hopkins DA, Mayrhofer HC, Bruner E, van Leeuwen FW, Raaijmakers W, Schmahmann JD (2018) The cerebellum in Alzheimer’s disease: Evaluating its role in cognitive decline. Brain 141, 37–47. [DOI] [PubMed] [Google Scholar]
- [40]. Théoret H, Haque J, Pascual-Leone A (2001) Increased variability of paced finger tapping accuracy following repetitive magnetic stimulation of the cerebellum in humans. Neurosci Lett 306, 29–32. [DOI] [PubMed] [Google Scholar]
- [41]. Boisgontier MP, Cheval B, van Ruitenbeek P, Cuypers K, Leunissen I, Sunaert S, Meesen R, Adab HZ, Renaud O, Swinnen SP (2018) Cerebellar gray matter explains bimanual coordination performance in children and older adults. Neurobiol Aging 65, 109–120. [DOI] [PubMed] [Google Scholar]
- [42]. Huang D, Liu D, Yin J, Qian T, Shrestha S, Ni H (2016) Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur Radiol 27, 2698–2705. [DOI] [PubMed] [Google Scholar]
- [43]. Mooney RA, Cirillo J, Byblow WD (2017) GABA and primary motor cortex inhibition in young and older adults: A multimodal reliability study. J Neurophysiol 118, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo E, Snyder PJ, Khachaturian ZS (2018) The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 141, 1917–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Schirinzi T, Lorenzo FD, Sancesario GM, Lazzaro GD, Ponzo V, Pisani A, Mercuri NB, Koch G, Martorana A (2018) Amyloid-mediated cholinergic dysfunction in motor impairment related to Alzheimer’s disease. J Alzheimers Dis 64, 525–532. [DOI] [PubMed] [Google Scholar]
- [46]. Burman DD (2019) Hippocampal connectivity with sensorimotor cortex during volitional finger movements: Laterality and relationship to motor learning. PLoS One 14, e0222064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Ballard C, Atri A, Boneva N, Cummings JL, Frölich L, Molinuevo JL, Tariot PN, Raket LL (2019) Enrichment factors for clinical trials in mild-to-moderate Alzheimer’s disease. Alzheimers Dement (N Y) 5, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Duff K, Atkinson TJ, Suhrie KR, Dalley BCA, Schaefer SY, Hammers DB (2017) Short-term practice effects in mild cognitive impairment: Evaluating different methods of change. J Clin Exp Neuropsychol 39, 396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Duff K, Anderson JS, Mallik AK, Suhrie KR, Atkinson TJ, Dalley BC, Morimoto SS, Hoffman JM (2018) Short-term repeat cognitive testing and its relationship to hippocampal volumes in older adults. J Clin Neurosci 57, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Duff K, Horn KP, Hoffman JM (2019) Long-term changes in 18F-Flutemetamol uptake in nondemented older adults. Alzheimer Dis Assoc Disord 33, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Duff K, Suhrie KR, Hammers DB, Dixon AM, King JB, Koppelmans V, Hoffman JM (2021) Repeatable battery for the assessment of neuropsychological status and its relationship to biomarkers of Alzheimer’s disease. Clin Neuropsychol 37, 157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Alzheimer’s Disease Neuroimaging Initiative (2008) Alzheimer’s Disease Neuroimaging Initiative: ADNI2 Procedures Manual.
- [53]. Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [54]. Morris JC (1993) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- [55]. Wechsler D (1987) WMS-R: Wechsler Memory Scale–Revised: Manual, Psychological Corporation, New York. [Google Scholar]
- [56]. Berg JL, Durant J, Banks SJ, Miller JB (2016) Estimates of premorbid ability in a neurodegenerative disease clinic population: Comparing the test of premorbid functioning and the wide range achievement test, 4th edition. Clin Neuropsychol 30, 547–557. [DOI] [PubMed] [Google Scholar]
- [57]. Yesavage JA, Brink T, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982) Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17, 37–49. [DOI] [PubMed] [Google Scholar]
- [58]. Peirce J, Gray JR, Simpson S, MacAskill M, Höchenberger R, Sogo H, Kastman E, Lindeløv JK (2019) PsychoPy2: Experiments in behavior made easy. Behav Res Methods 51, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Peirce JW (2008) Generating stimuli for neuroscience using psychopy. Front Neuroinform 2, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Peirce JW (2007) Psychopy-psychophysics software in python. J Neurosci Methods 162, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Koppelmans V (2023) vnckppl/FingerTappingTask: InitialRelease, Zenodo.
- [62]. van Strien JW (1988), The Dutch Handedness Questionnaire.
- [63]. Veitch DP, Weiner MW, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR Jr, Jagust W, Morris JC, Petersen RC, Saykin AJ, Shaw LM, Toga AW, Trojanowski JQ; Alzheimer’s Disease Neuroimaging Initiative (2018) Understanding disease progression and improving Alzheimer’s disease clinical trials: Recent highlights from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement 15, 106–152. [DOI] [PubMed] [Google Scholar]
- [64]. Sunderland JJ, Christian PE (2014) Quantitative PET/CT scanner performance characterization based upon the society of nuclear medicine and molecular imaging clinical trials network oncology clinical simulator phantom. J Nucl Med 56, 145–152. [DOI] [PubMed] [Google Scholar]
- [65]. Yester M, Al-Senan R, White S (2014) NEMA testing of GE Discovery 710 PET scanner compared to a simplified protocol for routine testing of PET scanners. J Nucl Med 55, 2157. [Google Scholar]
- [66]. Vandenberghe R, Laere KV, Ivanoiu A, Salmon E, Bastin C, Triau E, Hasselbalch S, Law I, Andersen A, Korner A, Minthon L, Garraux G, Nelissen N, Bormans G, Buckley C, Owenius R, Thurfjell L, Farrar G, Brooks DJ (2010) 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: A phase 2 trial. Ann Neurol 68, 319–329. [DOI] [PubMed] [Google Scholar]
- [67]. Thurfjell L, Lilja J, Lundqvist R, Buckley C, Smith A, Vandenberghe R, Sherwin P (2014) Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: Concordance with visual image reads. J Nucl Med 55, 1623–1628. [DOI] [PubMed] [Google Scholar]
- [68]. Lundqvist R, Lilja J, Thomas BA, Lötjönen J, Villemagne VL, Rowe CC, Thurfjell L (2013) Implementation and validation of an adaptive template registration method for 18F-flutemetamol imaging data. J Nucl Med 54, 1472–1478. [DOI] [PubMed] [Google Scholar]
- [69]. Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97, 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation. Neuron 33, 341–355. [DOI] [PubMed] [Google Scholar]
- [71]. Fischl B (2004) Automatically parcellating the human cerebral cortex. Cereb Cortex 14, 11–22. [DOI] [PubMed] [Google Scholar]
- [72]. Jäncke L, Mérillat S, Liem F, Hänggi J (2015) Brain size, sex, and the aging brain. Hum Brain Mapp 36, 150–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Agusta ZP, Adiwijaya A (2018) Modified balanced random forest for improving imbalanced data prediction. Int J Adv Intelligent Inform 5, 58. [Google Scholar]
- [74]. Chan AM, Baker JM, Eskandar E, Schomer D, Ulbert I, Marinkovic K, Cash SS, Halgren E (2011) First-pass selectivity for semantic categories in human anteroventral temporal lobe. J Neurosci 31, 18119–18129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Grahn JA, Rowe JB (2009) Feeling the beat: Premotor and striatal interactions in musicians and nonmusicians during beat perception. J Neurosci 29, 7540–7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Proksch S, Comstock DC, Médé B, Pabst A, Balasubramaniam R (2020) Motor and predictive processes in auditory beat and rhythm perception. Front Hum Neurosci 14, 578546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77]. Konoike N, Kotozaki Y, Miyachi S, Miyauchi CM, Yomogida Y, Akimoto Y, Kuraoka K, Sugiura M, Kawashima R, Nakamura K (2012) Rhythm information represented in the fronto-parieto-cerebellar motor system. Neuroimage 63, 328–338. [DOI] [PubMed] [Google Scholar]
- [78]. Witt ST, Laird AR, Meyerand ME (2008) Functional neuroimaging correlates of finger-tapping task variations: An ALE meta-analysis. Neuroimage 42, 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79]. Cohen J (1988), Statistical power analysis for the behavioral sciences, Routledge. [Google Scholar]
- [80]. Takahashi K, Yamamoto K, Kuchiba A, Koyama T (2021) Confidence interval for micro-averaged F1 and macro-averaged F1 scores. Appl Intell (Dordr) 52, 4961–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Hosmer DW, Lemeshow S, Sturdivant RX (2013) Applied Logistic Regression, Third Edition.. John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- [82]. Grill F, Johansson J, Axelsson J, Brynolfsson P, Nyberg L, Rieckmann A (2021) Dissecting motor and cognitive component processes of a finger-tapping task with hybrid dopamine positron emission tomography and functional magnetic resonance imaging. Front Hum Neurosci 15, 733091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83]. Uemura K, Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, Anan Y, Suzuki T (2013) Cognitive function affects trainability for physical performance in exercise intervention among older adults with mild cognitive impairment. Clin Interv Aging 8, 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84]. Bastos P, Barbosa R (2022) Motor reserve: How to build neuronal resilience against ageing and neurodegeneration? Rev Neurol (Paris) 178, 845–854. [DOI] [PubMed] [Google Scholar]
- [85]. Shahnur A, Nakano M, Ishihara S, Kakuda N, Miyasaka T, Uchiyama H, Shirai Y, Moniruzzaman M, Saito T, Saido TC, Nishimura M, Funamoto S (2021) A potential defense mechanism against amyloid deposition in cerebellum. Biochem Biophys Res Commun 535, 25–32. [DOI] [PubMed] [Google Scholar]
- [86]. Mukherjee S, Mez J, Trittschuh EH, Saykin AJ, Gibbons LE, Fardo DW, Wessels M, Bauman J, Moore M, Choi SE, Gross AL, Rich J, Louden DKN, Sanders RE, Grabowski TJ, Bird TD, McCurry SM, Snitz BE, Kamboh MI, Lopez OL, De Jager PL, Bennett DA, Keene CD, Larson EB; EPAD Study Group; Investigators from ACT; Investigators from ROS; Investigators from MAP; Investigators from ADNI; Investigators from the University of Pittsburgh ADRC; Crane PK (2018) Genetic data and cognitively defined late-onset Alzheimer’s disease subgroups. Mol Psychiatry 25, 2942–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87]. Attems J, Jellinger KA (2014) The overlap between vascular disease and Alzheimer’s disease - lessons from pathology. BMC Med 12, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. Beach TG, Malek-Ahmadi M (2021) Alzheimer’s disease neuropathological comorbidities are common in the younger-old. J Alzheimers Dis 79, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89]. Gnanalingham KK, Byrne EJ, Thornton A, Sambrook MA, Bannister P (1997) Motor and cognitive function in Lewy body dementia: Comparison with Alzheimer’s and Parkinson’s diseases. J Neurol Neurosurg Psychiatry 62, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ (2013) Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol 12, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91]. Liu CC, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat Rev Neurol 9, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92]. Hampel H, Hardy J, Blennow K, Chen C, Perry G, Kim SH, Villemagne VL, Aisen P, Vendruscolo M, Iwatsubo T, Masters CL, Cho M, Lannfelt L, Cummings JL, Vergallo A (2021) The amyloid-beta pathway in Alzheimer’s disease. Mol Psychiatry 26, 5481–5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93]. Zapparoli L, Mariano M, Paulesu E (2022) How the motor system copes with aging: A quantitative meta-analysis of the effect of aging on motor function control. Commun Biol 5, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94]. Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE (2008) Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 65, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. Elbaz A, Vicente-Vytopilova P, Tavernier B, Sabia S, Dumurgier J, Mazoyer B, Singh-Manoux A, Tzourio C (2013) Motor function in the elderly: Evidence for the reserve hypothesis. Neurology 81, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96]. Stevens DA, Workman CI, Kuwabara H, Butters MA, Savonenko A, Nassery N, Gould N, Kraut M, Joo JH, Kilgore J, Kamath V, Holt DP, Dannals RF, Nandi A, Onyike CU, Smith GS (2022) Regional amyloid correlates of cognitive performance in ageing and mild cognitive impairment. Brain Commun 4, fcac016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97]. Bologna M, Guerra A, Paparella G, Giordo L, Fegatelli DA, Vestri AR, Rothwell JC, Berardelli A (2018) Neurophysiological correlates of bradykinesia in Parkinson’s disease. Brain 141, 2432–2444. [DOI] [PubMed] [Google Scholar]
- [98]. Lorenzo FD, Bonnì S, Picazio S, Motta C, Caltagirone C, Martorana A, Koch G (2020) Effects of cerebellar theta burst stimulation on contralateral motor cortex excitability in patients with Alzheimer’s disease. Brain Topogr 33, 613–617. [DOI] [PubMed] [Google Scholar]
- [99]. Ferreri F, Guerra A, Vollero L, Ponzo D, Määtta S, Könönen M, Vecchio F, Pasqualetti P, Miraglia F, Simonelli I, Corbetta M, Rossini PM (2021) TMS-EEG biomarkers of amnestic mild cognitive impairment due to Alzheimer’s disease: A proof-of-concept six years prospective study. Front Aging Neurosci 13, 737281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100]. Ezzati A, Abdulkadir A, Jack CR Jr, Thompson PM, Harvey DJ, Truelove-Hill M, Sreepada LP, Davatzikos C; Alzheimer’s Disease Neuroimaging Initiative; Lipton RB (2021) Predictive value of ATN biomarker profiles in estimating disease progression in Alzheimer’s disease dementia. Alzheimers Dement 17, 1855–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101]. Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA (2011) Combinations of motor measures more strongly predict adverse health outcomes in old age: The rush memory and aging project, a community-based cohort study. BMC Med 9, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102]. Yamada Y, Shinkawa K, Kobayashi M, Caggiano V, Nemoto M, Nemoto K, Arai T (2021) Combining multimodal behavioral data of gait, speech, and drawing for classification of Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis 84, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103]. Jang H, Soroski T, Rizzo M, Barral O, Harisinghani A, Newton-Mason S, Granby S, da Cunha Vasco TM, Lewis C, Tutt P, Carenini G, Conati C, Field TS (2021) Classification of Alzheimer’s disease leveraging multi-task machine learning analysis of speech and eye-movement data. Front Hum Neurosci 15, 716670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104]. Battista P, Salvatore C, Berlingeri M, Cerasa A, Castiglioni I (2020) Artificial intelligence and neuropsychological measures: The case of Alzheimer’s disease. Neurosci Biobehav Rev 114, 211–228. [DOI] [PubMed] [Google Scholar]
- [105]. Medeiros R, Baglietto-Vargas D, LaFerla FM (2010) The role of Tau in Alzheimer’s disease and related disorders. CNS Neurosci Ther 17, 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106]. Jagust WJ, Landau SM; Alzheimer’s Disease Neuroimaging Initiative (2021) Temporal dynamics of beta-amyloid accumulation in aging and Alzheimer disease. Neurology 96, e1347–e1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107]. Miyawaki T, Kumamoto K, Shimoda K, Tozato F, Iwaya T (2017) Relationship among motor function, ADL disability, and psychological concerns in elderly people with locomotive disorders. J Orthop Sci 22, 339–344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available on request from the corresponding author.