Abstract
Background:
Multi-arm, multi-stage (MAMS) platform trials can accelerate the identification of disease-modifying treatments for Parkinson’s disease (PD) but there is no current consensus on the optimal outcome measures (OM) for this approach.
Objective:
To provide an up-to-date inventory of OM for disease-modifying PD trials, and a framework for future selection of OM for such trials.
Methods:
As part of the Edmond J Safra Accelerating Clinical Trials in Parkinson Disease (EJS ACT-PD) initiative, an expert group with Patient and Public Involvement and Engagement (PPIE) representatives’ input reviewed and evaluated available evidence on OM for potential use in trials to delay progression of PD. Each OM was ranked based on aspects such as validity, sensitivity to change, participant burden and practicality for a multi-site trial. Review of evidence and expert opinion led to the present inventory.
Results:
An extensive inventory of OM was created, divided into: general, motor and non-motor scales, diaries and fluctuation questionnaires, cognitive, disability and health-related quality of life, capability, quantitative motor, wearable and digital, combined, resource use, imaging and wet biomarkers, and milestone-based. A framework for evaluation of OM is presented to update the inventory in the future. PPIE input highlighted the need for OM which reflect their experience of disease progression and are applicable to diverse populations and disease stages.
Conclusion:
We present a range of OM, classified according to a transparent framework, to aid selection of OM for disease-modifying PD trials, whilst allowing for inclusion or re-classification of relevant OM as new evidence emerges.
Keywords: Parkinson’s disease, neuroprotection, outcome measures, biomarkers, clinical trials, consensus
INTRODUCTION
There is currently no proven intervention to delay the progression of Parkinson’s disease (PD). A number of novel and promising treatment approaches are being developed to address this and need to be tested in clinical trials. Multi-arm, multi-stage (MAMS) platform trials may help accelerate the identification of potentially successful treatments by improving efficiency of the clinical trial process. MAMS trials evaluate multiple agents simultaneously against a shared placebo arm and allow the addition of new arms as well as cessation of ineffective treatments at interim stages. However, there is no current consensus on the most appropriate outcome measures (OM) for disease-modifying trials in PD to be included in such an approach.
The Edmond J Safra Accelerating Clinical Trials in Parkinson Disease (EJS ACT-PD) initiative aims to accelerate the identification of disease-modifying treatments for PD through a MAMS platform trial approach. An important component of this novel approach is the identification and selection of appropriate outcome measures, suitable for inclusion across several different study arms as well as meeting the overarching aim. Here, we present an inventory of outcome measures based on current evidence and make initial recommendations for their potential inclusion as core, supplementary (depending on study arm) or exploratory outcome measures in such trials.
This inventory of potential outcome measures for use in disease-modifying trials is based on a consensus effort by an expert group with strong patient and public engagement input. The group used information from literature reviews, other existing and ongoing efforts, and discussion with regulatory bodies and group discussions. Particular consideration for inclusion in the inventory was given to clinically relevant outcome measures that are meaningful to patients, align with regulatory expectations and provide data to support adoption in larger healthcare systems. For future adaptation according to emerging new evidence, a framework was also created for evaluation and inclusion of outcome measures of potential relevance in the future, including clinical outcome measures, biomarkers, and novel measurement technologies.
METHODS
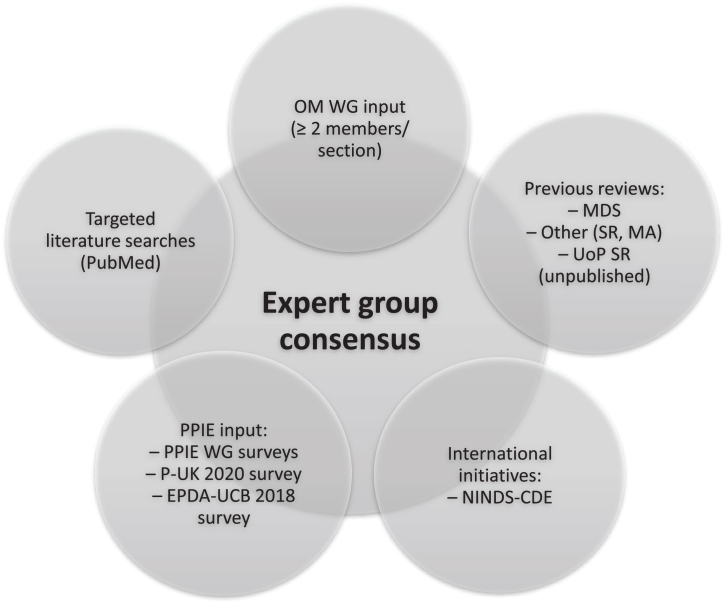
The methodology for this consensus paper is summarized in Fig. 1. A working group (WG) of experts from relevant fields, chaired by AS, was formed to review evidence, and provide expert input in written form and meetings. An initial list of outcome measures for inclusion in the inventory was compiled, based on 1) expert input from the members of the EJS ACT-PD Outcome Measures Working Group (OM WG), 2) the Movement Disorders Society (MDS) critique and recommendation papers, 3) the National Institute of Neurological Disorders and Stroke Common Data Elements (NINDS-CDE) version 2.0, 4) literature searches performed by CGR, 5) patient and public involvement and engagement (PPIE) input, and 6) a systematic review on disease-modifying trials in PD (Dr. Marie-Louise Zeissler, unpublished). Only outcome measures with published data in PD were included.
Fig. 1.
Sources for consensus of outcome measures in disease-modifying trials in Parkinson’s disease. EPDA-UCB, European Parkinson’s Disease Association-UCB Pharma; MA, meta-analysis; MDS, Movement Disorders Society (critique and recommendation papers on different outcome measures); NINDS-CDE, National Institute of Neurological Disorders and Stroke Common Data Elements initiative; OM, Outcome Measures; P-UK, Parkinson’s UK; PPIE, Patient and Public Involvement and Engagement; SR, Systematic review; UoP, University of Plymouth; WG, Working Group.
From this initial list, at least two members of the OM WG with relevant expertise, and supported by CGR, compiled and reviewed the most relevant PD endpoints in each of the following domains: motor, non-motor, disability, health-related quality of life (HR-QoL), resource use, cognitive, digital, quantitative motor, neuroimaging, and wet biomarkers. Where available, MDS critiques and recommendation papers on different measuring instruments for PD in the categories included in this paper were reviewed (https://www.movementdisorders.org/MDS/MDS-Rating-Scales/Rating-Scales-Critiques-and-Recommendations.htm), and the outcome measures classified as “Recommended” were included for consideration. Targeted literature searches in PubMed were then conducted to identify new measures developed since the publication of the MDS recommendations, or new evidence on measures not previously fulfilling the “Recommended” criteria, with a focus on “Suggested” measures. Other recent reviews were considered when writing this consensus paper. For example, for the disability measures, a 2022 systematic review was used to further guide the choice of outcome measures [1]. Similarly, a 2021 systematic review was employed to aid the decision on health-related quality of life outcome measures [2]. Supplementary Table 1 summarizes the “Recommended” instruments according to the corresponding MDS critique and review papers, the Neurological Disorders and Stroke Common Data Elements (http://www.commondataelements.ninds.nih.gov/) [3] in PD version 2.0 recommendations [4], and our selected outcome measures.
This publication also used the National Institute of Neurological Disorders and Stroke Common Data Elements (http://www.commondataelements.ninds.nih.gov/) [3]. More specifically, the NINDS-CDE in PD version 2.0 [4], a NINDS guide to consistently capture and record data across PD studies and to standardize this process to increase comparability of studies, was reviewed. The levels of recommendation for each endpoint (Core, Supplemental – Highly Recommended, Supplemental, Exploratory, Not Recommended), where available, were considered and a modified version was used for classification of outcome measures for disease-modifying trials on the MAMS platform. In short, in the NINDS-CDE classification, “General Core” data elements are required for all NINDS funded studies, “Disease Core” elements collect essential disease-specific (i.e., PD) information and are required for all PD studies, “Disease Supplemental – Highly Recommended” elements have commonly been used and validated in PD and are essential only for some PD studies; “Disease Supplemental” elements are recommended but not required for PD studies and their use varies according to study type, and “Disease Exploratory” elements require further validation but may fill current gaps once validation is complete and can be used as long as their limited or pending validation is acknowledged within the study. We adopted a simplified version of this classification, namely “Core” indicating outcome measure collecting essential PD-specific information to be included in all disease-modifying PD trials (not necessarily as a measure of disease progression); “Supplemental” those that are recommended but not required for all disease-modification studies in PD (i.e., depending on the particular trial); and “Exploratory” those which may fill current gaps once validation is complete but require further validation.
A four-fold strategy was used to include PPIE input. First, data from a recent survey from Parkinson’s UK [5] was reviewed, in which 790 participants (people with PD (PwP) in different stages, partners, carers or family members) reported the symptoms they would most like to see improved. Furthermore, the 2018 European Parkinson’s Disease Association-UCB (EPDA-UCB) survey results were reviewed to extract the most challenging symptoms according to 984 respondents (PwP and families/carers) [6]. In addition to that, a questionnaire was completed by members of the PPIE Working Group of the EJS ACT-PD Consortium about their judgement on the most bothersome symptoms of PD. Measures for the highest-ranking motor and non-motor symptoms in both surveys were included in this list. Whilst this information is primarily relevant to symptomatic treatments, the aim of any disease-modifying treatment would be to reduce functional disability relevant to patients. The results of these surveys on the highest-ranking symptoms for patients inform about the critical aspects of the condition that disease-modifying therapies should aim to delay or ideally avoid. Further input was sought from two PPIE groups (total n = 22) about the maximal acceptable duration and frequency of study assessments, either remote or in-person. This approach aimed to guide the maximal number of outcome measures/visit to be included in a disease-modifying PD trial.
All outcome measures identified using the information from the above-mentioned strategies were then evaluated for their potential use in disease-modifying PD trials based on: feasibility, clinical meaningfulness for PwP and clinicians, acceptability to regulators, burden on PwP and clinician, reliability, validity, existence of a suggested clinically meaningful cut-off in PD, sensitivity to change, relevance for specific PD subgroups, interpretability, and current NINDS-CDE classification. When several outcome measures for the same feature arose from the search strategies with comparable properties, the frequency of use of each in clinical trials (obtained via a search in clinicaltrials.gov of the considered outcome measure) and its use in disease-modifying trials (identified in the systematic review (Dr. Marie-Louise Zeissler, unpublished)) together with expert opinion and PPIE input was considered for selection to inclusion.
Compilation of information from all sources was combined in a final report by CGR and AS and reviewed by the expert group. The expert group discussed the information in several meetings, and reviewed several drafts of the list. This methodology is intended to guide future evaluation of evidence on outcome measures for inclusion in future trials in the MAMS platform.
RESULTS
Triangulation of the above methods resulted in a range of outcome measures on global impression, motor, non-motor features of PD, overall progression, disability, health-related quality of life, resource use, digital and quantitative outcome measures and neuroimaging and wet biomarkers. The full list and classification based on our criteria is shown in Table 1. Further details on the Core outcome measures are included in Table 2, and information on each individual outcome measures included in the above table can be found in the Supplementary Material.
Table 1.
Selected outcome measures for consideration and level of recommendation
| Category | Instrument/Test | NINDS-CDE v2.0 classification | Proposed classification for disease-modifying trials |
| Global – Generic | CGI-I | Supplemental-HR | Core |
| Global – PD-specific | MDS-UPDRS (and UPDRS, and remote versions) | Core | Core |
| LEDD | NI | Core* | |
| Motor – General | MDS-UPDRS Gait-axial score | NI | Supplemental |
| Hoehn &Yahr scale | Core | Core | |
| UDysRS | Supplemental-HR | Supplemental | |
| Gait, balance, and falls | Question about falls (e.g., ProFaNE falls definition) | NI | Core |
| Mini-BESTest | NI | Supplemental | |
| Berg Balance Scale | NI | Supplemental | |
| FES-I | NI | Supplemental | |
| ABC Scale | NI | Supplemental | |
| Speech and swallowing | SWAL-QOL | Supplemental | Supplemental |
| SDQ | Supplemental | Supplemental | |
| ROMP | Supplemental | Supplemental | |
| Fluctuations | PD Home Diary (Hauser Diary) | Supplemental-HR | Supplemental |
| CAPSIT-PD On/Off Diary | Supplemental-HR | Supplemental | |
| WOQ-9 and WOQ-19 | Supplemental-HR | Supplemental | |
| Non-motor – General | NMSQ | Supplemental | Supplemental |
| NMSS | NI | Supplemental | |
| MDS-NMS | Supplemental-HR | Supplemental | |
| Fatigue | FSS | Supplemental | Supplemental |
| Pain | KPPS | Supplemental | Supplemental |
| Sleep | PDSS-2 | Supplemental-HR | Supplemental |
| ESS | Supplemental-HR | Supplemental | |
| Depression | PHQ-9 | Supplemental | Core |
| GDS-15 | Supplemental-HR | Supplemental | |
| C-SSRS | Supplemental-HR | Supplemental** | |
| Apathy | AS | Supplemental-HR | Supplemental |
| AES | NI | Supplemental | |
| LARS | Supplemental-HR | Supplemental | |
| Psychosis | SAPS-PD | Supplemental-HR | Supplemental |
| eSAPS-PD | Supplemental-HR | Supplemental | |
| Autonomic dysfunction | SCOPA-AUT | Supplemental | Supplemental |
| Cognitive measures | MoCA | Core | Core |
| DRS-2 | Supplemental (MDRS) | Supplemental | |
| PD-CRS | Supplemental | Supplemental | |
| ACE-III | Supplemental | Supplemental | |
| ADAS-Cog | Supplemental | Supplemental | |
| MMSE | Supplemental | Supplemental | |
| MMP | NI | Supplemental | |
| SCOPA-COG | Supplemental | Supplemental | |
| Disability | S&E ADL | Supplemental-HR | Core |
| FSQ | NI | Supplemental | |
| Capability | ICECAP | NI | Core |
| Carer measures | PQoL Carers | NI | Supplemental |
| PDQ-Carer | NI | Supplemental | |
| Zarit Burden Interview | NI | Supplemental | |
| HR-QoL – Generic | EQ-5D-5L | Supplemental-HR (EQ-5D) | Core |
| SF-36 | Supplemental-HR | Supplemental | |
| SF-12 | NI | Supplemental | |
| PROMIS/Neuro-QoL | Supplemental-HR (Neuro-QoL) | Supplemental | |
| HUI | NI | Supplemental | |
| HR-QoL – PD-specific | PDQ-8 | NI | Core |
| PDQ-39 | Supplemental-HR | Supplemental | |
| Resource use | CSRI in combination with EHR | NI | Core |
| EHR in combination with CSRI | NI | Core | |
| Milestone-based OM | To be determined (see Supplement) | NI | Exploratory |
| Digital measures – Active only | OPDC Smartphone app | Exploratory | Exploratory |
| CloudUPDRS smartphone-based measures of limb-specific tremor/bradykinesia | Exploratory | Exploratory | |
| Mobility lab system (APDM)-measures acquired typically in controlled settings | Exploratory | Exploratory | |
| mPower smartphone-derived composite (dominantly motor) impairment score | Exploratory | Exploratory | |
| Digital measures – Passive only | PKG-based proxy measures of whole-body tremor/bradykinesia/dyskinesia | Exploratory | Exploratory |
| MM4D-based proxy measure of whole-body tremor/dyskinesia | Exploratory | Exploratory | |
| Axivity (AX3 &AX6) gait accelerometer | Exploratory | Exploratory | |
| Digital measures – Active and passive | Roche smartphone app | Exploratory | Exploratory |
| Other digital/timed motor measures | Exploratory | Exploratory | |
| Quantitative motor measures | TUG 3 meter | NI | Supplemental |
| Purdue Pegboard test | NI | Supplemental | |
| Alternate tap test | NI | Supplemental | |
| BRAIN tap test | NI | Supplemental | |
| 9-hole peg test | NI | Supplemental | |
| Composite quantitative motor measures | OPDC composite clinical score | NI | Exploratory |
| Molecular neuroimaging | Dopaminergic SPECT | NI (PET-SPECT Localization: Supplemental – HR; Supplemental) | Exploratory |
| Dopaminergic PET | See above | Exploratory | |
| Non-dopaminergic SPECT | See above | Exploratory | |
| Non-dopaminergic PET | See above | Exploratory | |
| Magnetic Resonance Spectroscopy | Supplemental-HR | Exploratory | |
| Structural neuroimaging | T1 Structural sequence | Supplemental-HR | Exploratory |
| Diffusion imaging | NI | Exploratory | |
| Multiple Parametric Mapping Protocol | NI | Exploratory | |
| Neuromelanin | NI | Exploratory | |
| Iron-sensitive sequences | NI | Exploratory | |
| Wet biomarkers | Plasma/serum NfL | NI | Exploratory |
| Plasma tau | NI | Exploratory | |
| Plasma α-syn | NI | Exploratory | |
| CSF NfL | NI | Exploratory | |
| CSF tau | NI | Exploratory | |
| CSF α-syn | NI | Exploratory | |
| CSF α-syn aggregation | NI | Exploratory | |
| CSF Aβ | NI | Exploratory | |
| Salivary markers (e.g., salivary α-syn) | NI | Exploratory |
*Change in LEDD is recommended as Core in trials including PD patients taking symptomatic medication (i.e., not drug-naïve). **Administration of the C-SSRS is recommended if screening question on the PHQ-9 is >0. Core, to be included in all disease-modifying PD trials; Supplemental, recommended but not required for all disease-modification studies in PD depending on the particular trial; Exploratory, may fill current gaps once validation is complete but require further validation; ABC Scale, Activities-Specific Balance Confidence Scale; ACE-III, Addenbrooke’s Cognitive Examination; ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive Subscale; AES, Apathy Evaluation Scale; AS, Apathy Scale; BRAIN, Bradykinesia-Akinesia Incoordination; C-SSRS, Columbia Suicide Severity Rating Scale; CAPSIT-PD, Core assessment program for surgical interventional therapies in Parkinson’s disease; CGI-I, Clinical Global Impression Scale-Improvement; CSF, Cerebrospinal fluid; CSRI, Client Service Receipt Inventory; DRS-2, Mattis Dementia Rating Scale Second Edition; EHR, Electronic health records; EHR, Electronic Health Records; EQ-5D-5L, EuroQoL 5-dimension 5-level questionnaire; eSAPS-PD, Scale for the Assessment of Positive Symptoms in Parkinson’s Disease, enhanced version; ESS, Epworth Sleepiness Scale; FES-I, Falls Efficacy Scale International; FSQ, Functional Status Questionnaire; FSS, Fatigue Severity Scale; GDS-15, 15-item Geriatric Depression Scale; HUI, Health Utility Index; ICECAP, ICEpop CAPability measures; KPPS, King’s Parkinson’s Disease Pain Scale; LARS, Lille Apathy Rating Scale; LEDD, Levodopa-Equivalent Daily Dose; MDRS, Mattis Dementia Rating Scale; MDS-NMS, Movement Disorder Society-sponsored Non-motor Rating Scale; MDS-UPDRS, Movement Disorders Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale; Mini-BESTest, Mini-Balance Evaluation Systems Test; MM4D, Motor fluctuations Monitor for Parkinson’s Disease; MMP, Mini-Mental Parkinson; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; NfL, neurofilament light chain; NI, not included; NINDS-CDE v2.0, National Institute of Neurological Disorders and Stroke Common Data Elements version 2.0; NMSQ, Non-motor Symptoms Questionnaire; NMSS, Non-motor Symptoms Scale; OPDC, Oxford Parkinson’s Disease Centre; PD-CRS, Parkinson’s Disease-Cognitive Rating Scale; PDQ-8, 8-item version of the Parkinson’s Disease Questionnaire; PDQ-39, 39-tem version of the Parkinson’s Disease Questionnaire; PDQ-Carer, 29-item Parkinson Disease Questionnaire for Carers; PDSS-2, Parkinson’s Disease Sleep Scale-2; PET, positron emission tomography; PHQ-9, 9-item Patient Health Questionnaire; PKG, Parkinson’s Personal KinetiGraph® (formerly Parkinson’s KinetiGraph®); PQoL carers, carers quality-of-life questionnaire for parkinsonism; ProFaNE, Prevention of Falls Network Earth; PROMIS/Neuro-QoL, Patient-Reported Outcomes Measurement Information System/Quality of Life in Neurological Disorders; ROMP, Radboud Oral Motor Inventory for Parkinson’s Disease; S&E ADL SCALE, Schwab and England Activities of Daily Living Scale; SAPS-PD, Scale for the Assessment of Positive Symptoms in Parkinson’s Disease; SCOPA-AUT, Scales for Outcomes in Parkinson’s disease-AUTonomic symptoms; SCOPA-COG, Scales for Outcomes in Parkinson’s disease- COGnitive symptoms; SDQ, Swallowing Disturbance Questionnaire; SF-12, 12-Item Short Form Survey; SF-36, 36-Item Short Form Survey; SPECT, single-photon emission computerized tomography; Supplemental-HR, Supplemental-Highly Recommended; SWAL-QOL, Generic Scale for Dysphagia-Related Outcomes (Quality of Life); TUG, Timed Up and Go; UPDRS, Unified Parkinson’s Disease Rating Scale; UDysRS, Unified Dyskinesia Rating Scale; WOQ-9, 9-item Wearing Off Questionnaire; WOQ-19, 19-item Wearing Off Questionnaire; α-syn, alpha-synuclein; Aβ, amyloid beta
Table 2.
Brief overview of suggested Core OM in disease-modifying PD trials
| Category | Instrument/Test | Brief description | Rater | Delivery | Length (min) | Strengths | Limitations |
| Global – Generic | CGI-I | 7-point categorical scale (level of improvement/worsening) to determine the progress and treatment response of patients | Clinician | In-person or remote | <5 | BriefOverall assessmentBroad use in clinical trials | No clinimetric data outside PsychiatrySubjectiveNot PD-specific |
| Global – PD-related | MDS-UPDRS | PD-specific scale with 4 parts: I: non-motor experiences of daily living (IA and IB) II: motor experiences of daily living III: motor examinationIV: motor complications | IB, II: PatientIA, III, IV: Clinician | In-personDeliverable remotely except for part III (Rigidity and Postural stability items) | 30– 40 (whole) | Gold standard OM in most PD trialsComprehensive (motor, non-motor, medication-related complications) Widely used in trialsGood clinimetric propertiesClinically meaningful cut-offs availablePD-specific | LengthyRequires trainingAssociated costsNeeds in-person assessment (part III)Part I: screening of NMSPart III: excessive weight on tremor |
| LEDD* | Summary of total daily antiparkinsonian medications | Clinician | In-person or remote | <5 | PD-specific Widely used in PD (including disease-modifying trials) Potential indirect measure of efficacy | Different methods for calculation, although standard formulae suggested | |
| Motor | Hoehn &Yahr scale | 5-stage categorization of PD according to functional disability | Clinician | In-person | <5 | PD-specific Brief Excellent clinimetric properties Wide experience in PD clinical trials | Non-granular – less responsive to change than other OMs No minimal clinically important difference |
| Falls | Question about falls (such as the International ProFaNE falls definition)** | One question: In the past n months, have you had any fall including a slip or trip in which you lost your balance and landed on the floor or ground or lower level? | Clinician or patient | In-person or remote | <5 | Very brief Administrable remotely International definition | Less detailed than other falls scales Not PD-specific |
| Cognition | MoCA | 30-point test assessing different cognitive domains, namely: short-term memory, visuospatial abilities, executive functions, attention, concentration, working memory, language, and orientation to time and place | Clinician | In-person, but deliverable remotely | 10 (20 if remote) | Brief Used in PD (including disease-modifying trials) Sensitive to change, less ceiling effect than MMSE Excellent clinimetric properties Clinically meaningful cut-offs defined for PD-MCI and PDD | Requires training Limited sensitivity for specific cognitive domains Low variability of scores (limited sensitivity to change) Not PD-specific |
| Depression | PHQ-9 | Depression module from the PRIME-MD diagnostic instrument for common mental disorders, scores each of the 9 DSM-IV depression criteria from 0 to 3 according to frequency | Patient | In-person or remote | 3 | BriefUsed in PD (including disease-modifying trials) | Less sensitive to change than others (e.g., GDS-15)Not PD-specific |
| Disability | S&E ADL | Scale measuring the level of functional independence in 10 levels of ability to perform various chores, distributed in 10% intervals from 0% (“Bedridden”) to 100% (“Completely independent”) | Patient or clinician | In-person or remote | <5 | BriefWidely availableUsed in PD (including disease-modifying trials) Good clinimetric propertiesResponsive to change | Not PD-specific |
| Capability | ICECAP | Scale measuring wellbeing beyond HR-QoL for a more meaningful economic assessment of interventions ICECAP-A (adults) has 5 questions on: stability, attachment, achievement, autonomy, and enjoyment; ICECAP-O (older people) covers: attachment, security, role, enjoyment, and control. | Patient | In-person or remote | <5 | BriefEasy to completePreviously used in similar patient populationsFree to useIf collected at repeated timepoints then it allows calculation of CALYs | Not PD-specificCannot be used in standard cost-utility analysis as it does not return QALYs that are required for cost-utility analysis |
| HR-QoL – Generic | EQ-5D-5L | Measure of perceived health, constituted by 5 items with 5 response options and a VAS on the health status on the day of questionnaire completion, as perceived by the patient, from 0 to 100 | Patient or clinician | In-person or remote | <5 | BriefWidely used, including in PDGood clinimetric propertiesIf collected at repeated timepoints then it allows calculation of QALYs that can be used in cost-utility analysis, which is commonly used in health technology assessment | Not PD-specificNot as granular as other OMNo clinically meaningful cut-off available |
| Category | Instrument/Test | Brief description | Rater | Delivery | Length (min) | Strengths | Limitations |
| HR-QoL – PD-specific | PDQ-8 | Short version of the PDQ-39, contains 8 items representing each of the 8 different domains in the PDQ-39, each of them asking about the frequency a PD-related issue on daily life, with 5 possible answers for each of them | Patient | In-person or remote | 5 | BriefPD-specificGood clinimetric propertiesSensitive to change and responsive to interventionsMinimal important difference availableCan be mapped to utility scores from EQ-5D-3L, so if collected at repeated timepoints then allows approximate calculation of QALYs that can be used in cost-utility analysis, which is commonly used in health technology assessment | Requires a licenseLower reliability and validity than PDQ-39 |
| Resource use | Study-specific combination of CSRI and EHR | Resources used in the treatment and care pathways can be captured from participants/carers using the CSRI questionnaire, and/or from electronic health records, according to the specific study context | Patient, carer, site staff | In-person or remote | 5– 20 | CSRI can be tailored to meet specific study requirements and capture varied types of relevant resource informationEHR can reduce bias and missing data, and patient burden, and allow data collection outside the trial follow-up periodThe combination of CSRI and EHR to capture resource use allows advantages of each to be maximized and disadvantages minimized | Requires extensive input from trial team and other stakeholders during design of data collection plansCSRI can be burdensome for patients/carers to completeEHR can miss important information as they are not generally designed with research in mindEHR can be expensive to obtain |
*LEDD is recommended as Core in trials included PD patients taking symptomatic medication (i.e., not drug-naïve). **A question enquiring about falls is recommended as Core, and as an example, the ProFaNE falls definition is described under this section. CALYs, Capability-Adjusted Life-Years; CGI-I, Clinician Global Impression scale – Improvement; CSRI, Client Service Receipt Inventory; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; HER, Electronic Health Records; HR-QoL, Health-Related Quality of Life; ICECAP, ICEpop CAPability measures; LEDD, Levodopa-Equivalent Daily Dose; MDS-UPDRS, Movement Disorders Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; NMS, non-motor symptoms; OM, outcome measure; PD: Parkinson’s disease; PDD, Parkinson’s disease dementia; PD-MCI, Parkinson’s Disease with mild cognitive impairment; PDQ-39, 39-item Parkinson’s Disease Questionnaire; PDQ-8, 8-item Parkinson’s Disease Questionnaire; PHQ-9, 9-item Patient Health Questionnaire; PPIE, Patient and Public Involvement and Engagement; PRIME-MD, PRIMary care Evaluation of Mental Disorders; QALYs, Quality-Adjusted Life-Years; S&E ADL, Schwab and England Activities of Daily Living Scale; VAS, Visual Analogue Scale.
For overall assessment of health, two global status scales were included: the Clinical Global Impression Scale-Improvement (CGI-I) and the Clinical Global Impression Scale-Severity (CGI-S) [7]. We also included the change in Levodopa-Equivalent Daily Dose (LEDD) [8] for trials including PD patients on antiparkinsonian medications.
For assessment of motor features of PD, we included: the Hoehn and Yahr scale [9], the Movement Disorders Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [10], the remote versions of both the Unified Parkinson’s Disease Rating Scale (UPDRS) and the MDS-UPDRS [11–13], the UPDRS Gait-axial score [14], and the Unified Dyskinesia Rating Scale (UDysRS) [15]. We also included scales on motor symptoms flagged as most bothersome by PPIE representatives, namely gait and balance problems (question about falls (e.g., Prevention of Falls Network Earth (ProFaNE) definition of a fall [16]), Mini-Balance Evaluation Systems Test (Mini-BESTest) [17], Berg Balance Scale [18], Falls Efficacy Scale International (FES-I) [19], and Activities-Specific Balance Confidence Scale (ABC Scale) [20]), and speech and swallowing issues (Generic Scale for Dysphagia-Related Outcomes (Quality of Life) (SWAL-QOL) [21–24], Swallowing Disturbance Questionnaire (SDQ) [25], and Radboud Oral Motor Inventory for Parkinson’s Disease (ROMP) [26]). Amongst the “Diaries and other fluctuation questionnaires”, the Hauser Diary [27–29], the Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD) On/Off Diary [30], and the 9- and 19-item Wearing Off Questionnaires (WOQ-9 [31] and WOQ-19 [32]) were included.
The following global non-motor scales and questionnaires were selected: the Non-Motor Symptoms Questionnaire (NMSQ) [33], the Non-motor Symptoms Scale (NMSS) [34], and the Movement Disorder Society-sponsored Non-motor Rating Scale (MDS-NMS) [35]. Similar to the motor features, specific measures were included for the PPIE-reported most bothersome non-motor symptoms: apathy (Apathy Scale (AS) [36], Apathy Evaluation Scale (AES) [37], and Lille Apathy Rating Scale (LARS) [38]), depression (Geriatric Depression Scale-30 (GDS-30) and GDS-15 [39–41]), 9-item Patient Health Questionnaire (PHQ-9) [42], Columbia Suicide Severity Rating Scale (C-SSRS) [43, 44]), fatigue (Fatigue Severity Scale (FSS) [45]), pain (King’s Parkinson’s Disease Pain Scale (KPPS) [46]), psychosis (Scale for the Assessment of Positive Symptoms in Parkinson’s Disease (SAPS-PD) [47] and its enhanced version (eSAPS-PD) [48]), and sleep (Epworth Sleepiness Scale (ESS) [49], Parkinson’s Disease Sleep Scale-2 (PDSS-2) [50]). Given its relevance and relationship with medication, an autonomic OM was also included (SCales for Outcomes in PArkinson’s disease- AUTonomic symptoms (SCOPA-AUT) [51]).
For global cognitive measures we included the Montreal Cognitive Assessment (MoCA) [52–55], the Mattis Dementia Rating Scale Second Edition (DRS-2) [56], the Parkinson’s Disease-Cognitive Rating Scale (PD-CRS) [57–59], the Addenbrooke’s Cognitive Examination (ACE-III) [60–63], the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-COG) [64], the Mini-Mental State Examination (MMSE) [65], the Mini-Mental Parkinson (MMP) [66, 67], and the SCales for Outcomes in PArkinson’s disease- COGnitive symptoms (SCOPA-COG) [68].
For overall progression of features of PD, Milestone-based outcome measures [69] were included.
Disability measures selected for this review were the Schwab and England Activities of Daily Living (S&E ADL) Scale [70, 71], the Functional Status Questionnaire (FSQ) [72, 73], and part II of the MDS-UPDRS [74].
The ICEpop CAPability measures (ICECAP) [75, 76] was included as a capability measure. Carer measures taken into consideration were the carers quality-of-life questionnaire for parkinsonism (PQoL Carers) [77], the 29-item Parkinson Disease Questionnaire for Carers (PDQ-Carer) [78], and the Zarit Burden Interview (ZBI) [79, 80].
Health-related quality of life (HR-QoL) measures were divided into generic (EQ-5D-5L [81, 82], 36-Item Short Form Survey (SF-36) [83, 84], 12-Item Short Form Survey (SF-12) [85, 86], Patient-Reported Outcomes Measurement Information System/Quality of Life in Neurological Disorders (PROMIS/NeuroQoL) [87–90], Health Utility Index (HUI) [91, 92]) and PD-specific (39- and 8- item versions of the Parkinson’s Disease Questionnaire (PDQ-39 [93], PDQ-8 [94])).
Resource use data collection methods were also considered, specifically the Client Service Receipt Inventory (CSRI) [95–97] and electronic health records. A brief discussion of requirements for using HR-QoL and resource use information in health economics analysis is also included in the Supplementary Material.
Digital measures were divided into active (Oxford Parkinson’s Disease Centre (OPDC) smartphone app [98], CloudUPDRS [99], APDM [100, 101], and mPower smartphone-derived composite score [102]), passive (Parkinson’s Personal KinetiGraph® (formerly Parkinson’s KinetiGraph®) (PKG)-based proxy measures [103], Motor fluctuations Monitor for Parkinson’s Disease (MM4D)-based proxy measures [104], Axivity gait accelerometer [105–107]), and combined active and passive tools (Roche smartphone app [108, 109]).
Furthermore, the following quantitative motor measures were considered: Timed-Up and Go (TUG) 3 metre [110], Purdue Pegboard test [111, 112], Alternate tap test [113], BRadykinesia-Akinesia INcoordination (BRAIN) tap test [114, 115], and 9-hole peg test (9hpt) [112, 116–118]. The OPDC composite clinical score [119] was considered under the composite quantitative motor measures section.
Molecular neuroimaging techniques include dopaminergic single-photon emission computerized tomography (SPECT) [122–129], dopaminergic positron emission tomography (PET) [130–133], non-dopaminergic SPECT [134–140], non-dopaminergic PET [141–155,], and magnetic resonance spectroscopy (MRS) [156, 157].
Considered structural neuroimaging techniques [120] were magnetic resonance imaging (MRI) T1 structural sequence [158–162], diffusion imaging [163–166], multiple parametric mapping protocol, neuromelanin [167, 168] and iron-sensitive sequences [169–172].
The following wet biomarkers were selected for review [173–178]: plasma/serum neurofilament light chain (NfL) [179–189,], plasma tau [190–195], plasma alpha-synuclein (α-syn) [196–205], cerebrospinal fluid (CSF) neurofilament light chain (NfL) [206–210], CSF tau [211, 212], CSF α-syn [213–215], CSF α-syn aggregation [216–221], CSF beta-amyloid (Aβ) [222–229], and salivary markers, such as salivary α-syn [230, 231].
PPIE input revealed that the mean maximum time per study visit varied depending on the frequency of assessments: for 6-monthly visits, it varied between roughly 2 and 3 hours (longer visits more acceptable when remote), and for yearly visits, between 3 and 3.5 hours. Tables 3 and 4 detail the maximum acceptable length of visits for the PPIE WG and for the PPIE broader engagement group.
Table 3.
PPIE WG input on maximal acceptable duration of visits (n = 10)
| Maximum minutes/visit – Remote assessments | Maximum minutes/visit – Clinic assessments | |||
| Mode | Mean | Mode | Mean | |
| Monthly | 60 | 72 | 60 | 72 |
| Every 3 months | 120 | 102 | 120 | 120 |
| Every 6 months | 180 | 138 | 180 | 162 |
| Once a year | 180 | 174 | >180 | 198 |
| Less than once a year | 180 | 162 | >180 | 192 |
Table 4.
PPIE broader engagement group input on maximal acceptable duration of visits (n = 12)
| Maximum minutes/visit – Remote assessments | Maximum minutes/visit – Clinic assessments | |||
| Mode | Mean | Mode | Mean | |
| Monthly | 60 | 66 | 60 | 90 |
| Every 3 months | 120 | 120 | 180 | 150 |
| Every 6 months | 180 | 156 | 180 | 180 |
| Once a year | 180 | 180 | 240 | 210 |
| Less than once a year | 240 | 198 | 240 / Not acceptable frequency | 216 |
240/Not acceptable frequency: the 2 most common answers were either 240 minutes or “this assessment frequency is not acceptable”.
The set of Core outcome measures proposed in Table 2 would take 70 to 90 minutes (i.e., 1 to 1.5 hours) to complete, making it acceptable to patients according to the above.
DISCUSSION
We here present an up-to-date inventory of outcome measures for disease-modifying trials in PD based on expert and PPIE consensus. This inventory and framework will be used to guide the decision to select the outcome measures of the EJS ACT-PD MAMS platform trial, based on their fulfilment of desired criteria for an endpoint (e.g., validated, reliable, sensitive to change, acceptable) and their relevance to the intervention based on its mechanism of action and previously known effects (e.g., wet biomarkers as surrogate or direct markers of target engagement). When selecting and classifying the above measures as Core, Supplemental and Exploratory, a compromise had to be made between measures with the best clinimetric properties, acceptability to patients, feasibility, previous experience of use in PD trials, and regulatory considerations, which potentially might have led to prioritizing measures which appear less “promising” from a purely theoretical point of view (i.e., original validation study results) over others, to achieve an adequate balance and provide a realistic and practical tool. This work could also inform other trial initiatives aiming to identify disease-modifying treatments for PD and the framework used will allow updates with new emerging evidence in the future. However, this inventory of outcome measures was created as part of the development of a MAMS trial for progression of PD and as such, presents some particularities which might have influenced the final list of included outcomes. This type of trial requires large participant numbers across a variety of centers, and has a much longer duration than usual randomized controlled trials (RCTs) (i.e., several years) [232, 233]. Therefore, MAMS trials require endpoints which can be measured in different research settings (ideally remotely), and which are sensitive to changes and capture relevant events in disease progression in the longer term. Alternative trial designs, studies focused on particular PD subpopulations, or those looking into changes in a particular aspect of the disease (e.g., cognition, gait) might require an adaptation of this inventory, although it could provide a basis for such adaptations. All of these caveats emphasize the need for a common core set of outcome measures applicable across trial designs and PD populations, to ensure translatability of results regardless of differences between individual trials. Furthermore, it is important to note that this classification does not intend to dictate the choice of primary endpoint, which should be based on the individual trial characteristics (aim, intervention, population, design), and prioritize, among others, sensitivity to change (i.e., detection of disease-modifying effects), relevance, patient acceptability, and feasibility. We refer the readers to regulatory guidance on this subject [234, 235].
Despite being included as exploratory due to the lack of formal validation in this setting, novel outcome measures, and especially digital endpoints, are a promising alternative to complement the currently available instruments. Their potentially increased sensitivity and the possibility of continuous monitoring in real-life conditions (i.e., at home) is likely to be a valuable addition to the administration of scales in the clinical setting. In line with this, a number of initiatives are looking into the clinical validity of these endpoints and their implementation in clinical research [236, 237]. This group selected some of the digital outcomes with more information on PD populations to be included in the inventory. Nevertheless, this is a fast-moving field and recommendations here could require more frequent revision than for other types of endpoints.
The main strength of our approach was strong expert and PPIE consensus, embedding the patient’s voice into the development and recommendation of outcome measures, as well as evidence from literature reviews, information from other initiatives, and input from regulatory bodies.
Conclusions
With the above methodology, we have identified a broad range of outcome measures which can be potentially included in disease-modifying PD trials, and make recommendations for their inclusion as core, supplementary (for specific arms) and exploratory measures in the EJS ACT-PD MAMS initiative. For other MAMS initiatives, this review aims to serve as a resource from which to select the desired outcome measures according to the requirements of the study (e.g., population, mechanism of action of the intervention, etc.). We also provide a framework for future update of the evidence on outcome measures in disease-modifying PD trials.
EJS ACT-PD CONSORTIUM MEMBERS
Additional EJS ACT-PD consortium members (further details are provided in the Supplementary material): Roger Barker, James Carpenter, Yoav Ben Shlomo, Mark Edwards, Alan Whone, Carl Counsell, Dorothy Salathiel, Sue Whipps, Anna Jewell, Priti Gros, Tom Barber, Shlomi Haar Millo, K Ray Chaudhuri, Anthony HV Schapira, Oliver Bandmann, Simon Stott, George Tofaris, Esther Sammler, Heather Mortiboys, Li Wei, Alan Wong, Susan Duty, David Dexter, Paula Scurfield, Keith Martin, Edwin Jabbari, Stephen Mullin, Huw Morris, David Breen, Christian Lambert, Prasad Korlipara, Monty Silverdale, Kailash Bhatia, Alison Yarnall, Raj Khengar, Helen Collins, Fleur Hudson, Gareth Baxendale, Rebecca Croucher, Sandra Bartolomeu-Pires, Jennifer Allison, Jodie Forbes, Alex Edwards, Sheila Wonnacott, Dilan Athauda, Joy Duffen, Sonia Gandhi, Emily Henderson, Maryanne Graham, Shona Clegg, Karen Matthews, Vince Greaves, Eric Deeson, Laurel Miller, Joel Handley, David Dexter, Helen Matthews, Kevin McFarthing, Amit Batla, Nikul Bashi, Emma Lane, Miriam Parry, Natasha Ratcliffe, Romy Ellis-Doyle, Sally L Collins, Rebecca Chapman, Jesse Cedarbaum, Anthony Lang, Brian Fiske, Richard Wyse, Mahesh Parmar, Adam Boxer, Denise Wilson, Jean Christophe Corvol, Jennifer Harris.
Supplementary Material
ACKNOWLEDGMENTS
This work was done as part of the Edmond J Safra Accelerating Clinical Trials in Parkinson’s Disease (EJS ACT-PD) Initiative, which is funded by the Edmond J Safra Foundation.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JPD-230051.
Contributor Information
on behalf of the EJS ACT-PD Consortium:
Roger Barker, James Carpenter, Yoav Ben Shlomo, Mark Edwards, Alan Whone, Carl Counsell, Dorothy Salathiel, Sue Whipps, Anna Jewell, Priti Gros, Tom Barber, Shlomi Haar Millo, K Ray Chaudhuri, Anthony HV Schapira, Oliver Bandmann, Simon Stott, George Tofaris, Esther Sammler, Heather Mortiboys, Li Wei, Alan Wong, Susan Duty, David Dexter, Paula Scurfield, Keith Martin, Edwin Jabbari, Stephen Mullin, Huw Morris, David Breen, Christian Lambert, Prasad Korlipara, Monty Silverdale, Kailash Bhatia, Alison Yarnall, Raj Khengar, Helen Collins, Fleur Hudson, Gareth Baxendale, Rebecca Croucher, Sandra Bartolomeu-Pires, Jennifer Allison, Jodie Forbes, Alex Edwards, Sheila Wonnacott, Dilan Athauda, Joy Duffen, Sonia Gandhi, Emily Henderson, Maryanne Graham, Shona Clegg, Karen Matthews, Vince Greaves, Eric Deeson, Laurel Miller, Joel Handley, David Dexter, Helen Matthews, Kevin McFarthing, Amit Batla, Nikul Bashi, Emma Lane, Miriam Parry, Natasha Ratcliffe, Romy Ellis-Doyle, Sally L Collins, Rebecca Chapman, Jesse Cedarbaum, Anthony Lang, Brian Fiske, Richard Wyse, Mahesh Parmar, Adam Boxer, Denise Wilson, Jean Christophe Corvol, and Jennifer Harris
CONFLICT OF INTEREST
RSW has received speaking honoraria from GE Healthcare and a writing honorarium from Britannia.
DVW reports consultancy and speaker fees from Bial and Britannia.
MTH received payment for Advisory Board attendance/consultancy for Lundbeck, ESCAPE Bio, Evidera, Manus Neurodynamica, Biogen MA, CuraSen Therapeutics, Roche Products Ltd. MTH is an advisory founder of NeuHealth Digital Ltd (company number: 14492037), a digital biomarker platform to remotely manage condition progression for Parkinson’s.
AJ has been involved in the development and clinical assessment of a smartphone-based tool for Parkinson’s disease (cloudUPDRS).
ML received fees for advising on a secondary analysis of a Parkinson’s RCT (GDNF) sponsored by North Bristol NHS trust.
AN has been involved in the development of the Bradykinesia-Akinesia Incoordination (BRAIN) test. AN is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work).
CBC has received personal fees from AbbVie, Bial, Scient, Orkyn, Abidetex, UCB, Pfizer, EverPharma, Lundbeck, Global Kinetics, Kyowa Kirin, Britannia, and MedScape; and appointments as a Cure Parkinson’s Linked Clinical Trials (LCT) committee member, a Cure Parkinson’s Research Committee member, and a Parkinson’s UK College of Experts Panel member. CBC is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
TF has served on Advisory Boards for Peptron, Voyager Therapeutics, Handl therapeutics, Gain therapeutics, Living Cell Technologies, Abbvie, Bluerock, Bayer & Bial. TF has received honoraria for talks sponsored by Bial, Profile Pharma, Boston Scientific & Novo Nordisk. TF is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review. AS is a member of the MDS-UPDRS Development Group, the MDS-NMS Development Group, the NINDS CDE QoL Group, the MDS Rating Scales Review Committee, and the MDS COA Early and Prodromal PD Working Group. AS has been involved in the development of the MDS-UPDRS, the MDS-NMS, and the PQoL.
AS reports consultancy fees from Biogen, Abbvie, Roche, Bial, and GE Healthcare; license fees from University College London; and royalties from Oxford University Press. CGR, MB, MBu, CSC, BH, CL, GM, PP, KP, LR, CS, CWG and MLZ have no conflict of interest to report.
DATA AVAILABILITY
Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.
ADDITIONAL STATEMENT
For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising from this submission.
REFERENCES
- [1]. Bouça-Machado R, Fernandes A, Ranzato C, Beneby D, Nzwalo H, Ferreira JJ (2022) Measurement tools to assess activities of daily living in patients with Parkinson’s disease: A systematic review. Front Neurosci 16, 945398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Berardi A, Regoli E, Tofani M, Valente D, Fabbrini G, Fabbrini A, Ruggieri M, Panuccio F, Galeoto G (2021) Tools to assess the quality of life in patients with Parkinson’s disease: A systematic review. Expert Rev Pharmacoeconomics Outcomes Res 21, 55–68. [DOI] [PubMed] [Google Scholar]
- [3]. Grinnon ST, Miller K, Marler JR, Lu Y, Stout A, Odenkirchen J, Kunitz S (2012) National Institute of Neurological Disorders and Stroke Common Data Element Project - approach and methods. Clin Trials 9, 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. National Institute of Neurological Disorders and Stroke (2022) Parkinson’s Disease NINDS Common Data Elements. Retrieved 12/01/2023 from: https://www.commondataelements.ninds.nih.gov/Parkinson’s%20Disease
- [5]. Port RJ, Rumsby M, Brown G, Harrison IF, Amjad A, Bale CJ (2021) People with Parkinson’s disease: What symptoms do they most want to improve and how does this change with disease duration? J Parkinsons Dis 11, 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. An EPDA-UCB survey to better understand the challenges of living with Parkinson’s, https://www.parkinsonseurope.org/media/2301/an-epda-ucb-survey-to-better-understand-the-challenges-of-living-with-parkinsons.pdf [Google Scholar]
- [7]. Guy W (1976) ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration, Rockville, MD. [Google Scholar]
- [8]. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25, 2649–2653. [DOI] [PubMed] [Google Scholar]
- [9]. Hoehn MM, Yahr MD (1967) Parkinsonism: Onset, progression, and mortality. Neurology 17, 427–442. [DOI] [PubMed] [Google Scholar]
- [10]. Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, La Pelle N; Movement Disorder Society UPDRS Revision Task Force (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord 23, 2129–2170. [DOI] [PubMed] [Google Scholar]
- [11]. Abdolahi A, Scoglio N, Killoran A, Dorsey ER, Biglan KM (2013) Potential reliability and validity of a modified version of the Unified Parkinson’s Disease Rating Scale that could be administered remotely. Parkinsonism Relat Disord 19, 218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Stillerova T, Liddle J, Gustafsson L, Lamont R, Silburn P (2016) Remotely assessing symptoms of Parkinson’s disease using videoconferencing: A feasibility study. Neurol Res Int 2016, 4802570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Morinan G, Hauser RA, Schrag A, Tang J, O’Keeffe J, MDS-NMS Scale Development Study Group (2022) Abbreviated MDS-UPDRS for remote monitoring in PD identified using exhaustive computational search. Parkinsons Dis 2022, 2920255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Evans JR, Mason SL, Williams-Gray CH, Foltynie T, Trotter M, Barker RA (2011) The factor structure of the UPDRS as an index of disease progression in Parkinson’s disease. J Parkinsons Dis 1, 75–82. [DOI] [PubMed] [Google Scholar]
- [15]. Goetz CG, Nutt JG, Stebbins GT (2008) The unified dyskinesia rating scale: Presentation and clinimetric profile. Mov Disord 23, 2398–2403. [DOI] [PubMed] [Google Scholar]
- [16]. ProFaNE Prevention of Falls Network Earth – Definition of a Fall, http://profane.co/2012/02/22/definition-of-a-fall/.
- [17]. Franchignoni F, Horak F, Godi M, Nardone A, Giordano A (2010) Using psychometric techniques to improve the balance evaluation systems test: The mini-bestest. J Rehabil Med 42, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Miranda-Cantellops N, Tiu TK (2023) Berg Balance Testing. StatPearls [Internet], StatPearls Publishing, Treasure Island (FL). [PubMed] [Google Scholar]
- [19]. Tinetti ME, Richman D, Powell L (1990) Falls efficacy as a measure of fear of falling. J Gerontol 45, 239–243. [DOI] [PubMed] [Google Scholar]
- [20]. Powell LE, Myers AM (1995) The Activities-specific Balance Confidence (ABC) scale, J Gerontol A Biol Sci Med Sci 50A, M28–M34. [DOI] [PubMed] [Google Scholar]
- [21]. McHorney CA, Bricker DE, Kramer AE, Rosenbek JC, Robbins JA, Chignell KA, Logemann JA, Clarke C (2000) The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: I. Conceptual foundation and item development. Dysphagia 15, 115–121. [DOI] [PubMed] [Google Scholar]
- [22]. McHorney CA, Earl Bricker D, Robbins J, Kramer AE, Rosenbek JC, Chignell KA (2000) The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. Item reduction and preliminary scaling. Dysphagia 15, 122–133. [DOI] [PubMed] [Google Scholar]
- [23]. McHorney CA, Robbins JA, Lomax K, Rosenbek JC, Chignell K, Kramer AE, Bricker DE (2002) The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia 17, 97–114. [DOI] [PubMed] [Google Scholar]
- [24]. McHorney CA, Martin-Harris B, Robbins JA, Rosenbek J (2006) Clinical validity of the SWAL-QOL and SWAL-CARE outcome tools with respect to bolus flow measures. Dysphagia 21, 141–148. [DOI] [PubMed] [Google Scholar]
- [25]. Lam K, Lam FKY, Kwok KL, Yiu KC, Kan EYL, Woo J, Fat KW, Ko A (2007) Simple clinical tests may predict severe oropharyngeal dysphagia in Parkinson’s disease. Mov Disord 22, 640–644. [DOI] [PubMed] [Google Scholar]
- [26]. Kalf JG, Borm GF, De Swart BJ, Bloem BR, Zwarts MJ, Munneke M (2011) Reproducibility and validity of patient-rated assessment of speech, swallowing, and saliva control in parkinson’s disease. Arch Phys Med Rehabil 92, 1152–1158. [DOI] [PubMed] [Google Scholar]
- [27]. Hauser RA, Friedlander J, Zesiewicz TA, Adler CH, Seeberger LC, O’Brien CF, Molho ES, Factor SA (2000) A home diary to assess functional status in patients with Parkinson’s disease with motor fluctuations and dyskinesia. Clin Neuropharmacol 23, 75–81. [DOI] [PubMed] [Google Scholar]
- [28]. Hauser RA, Deckers F, Lehert P (2004) Parkinson’s disease home diary: Further validation amd implications for clinical trials. Mov Disord 19, 1409–1413. [DOI] [PubMed] [Google Scholar]
- [29]. Hauser RA, Russ H, Haeger DA, Bruguiere-Fontenille M, Müller T, Wenning GK (2006) Patient evaluation of a home diary to assess duration and severity of dyskinesia in parkinson disease. Clin Neuropharmacol 29, 322–330. [DOI] [PubMed] [Google Scholar]
- [30]. Reimer J, Grabowski M, Lindvall O, Hagell P (2004) Use and interpretation of on/off diaries in Parkinson’s disease. J Neurol Neurosurg Psychiatry 75, 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Stacy M, Hauser R, Oertel W, Schapira A, Sethi K, Stocchi F, Tolosa E (2006) End-of-dose wearing off in parkinson disease: A 9-question survey assessment. Clin Neuropharmacol 29, 312–321. [DOI] [PubMed] [Google Scholar]
- [32]. Stacy M, Hauser R (2007) Development of a patient questionnaire to facilitate recognition of motor and non-motor wearing-off in Parkinson’s disease. J Neural Transm 114, 211–217. [DOI] [PubMed] [Google Scholar]
- [33]. Chaudhuri KR, Martinez-Martin P, Schapira AHV, Stocchi F, Sethi K, Odin P, Brown RG, Koller W, Barone P, MacPhee G, Kelly L, Rabey M, MacMahon D, Thomas S, Ondo W, Rye D, Forbes A, Tluk S, Dhawan V, Bowron A, Williams AJ, Olanow CW (2006) International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: The NMSQuest study. Mov Disord 21, 916–923. [DOI] [PubMed] [Google Scholar]
- [34]. Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, Ondo W, Abe K, MacPhee G, MacMahon D, Barone P, Rabey M, Forbes A, Breen K, Tluk S, Naidu Y, Olanow W, Williams AJ, Thomas S, Rye D, Tsuboi Y, Hand A, Schapira AH (2007) The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot study. Mov Disord 22, 1901–1911. [DOI] [PubMed] [Google Scholar]
- [35]. Martinez-Martin P, Schrag A, Weintraub D, Rizos A, Rodriguez-Blazquez C, Chaudhuri KR (2019) Pilot study of the international Parkinson and Movement Disorder Society-sponsored Non-motor Rating Scale (MDS-NMS). Mov Disord Clin Pract 6, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG (1992) Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 4, 134–139. [DOI] [PubMed] [Google Scholar]
- [37]. Marin RS, Biedrzycki RC, Firinciogullari S (1991) Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 38, 143–162. [DOI] [PubMed] [Google Scholar]
- [38]. Sockeel P, Dujardin K, Devos D, Denève C, Destée A, Defebvre L (2006) The Lille apathy rating scale (LARS), a new instrument for detecting and quantifying apathy: Validation in Parkinson’s disease. J Neurol Neurosurg Psychiatry 77, 579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982) Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17, 37–49. [DOI] [PubMed] [Google Scholar]
- [40]. Ertan FS, Ertan T, Kiziltan G, Uyguçgil H (2005) Reliability and validity of the Geriatric Depression Scale in depression in Parkinson’s disease. J Neurol Neurosurg Psychiatry 76, 1445–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. McDonald WM, Holtzheimer PE, Haber M, Vitek JL, McWhorter K, DeLong M (2006) Validity of the 30-item geriatric depression scale in patients with Parkinson’s disease. Mov Disord 21, 1618–1622. [DOI] [PubMed] [Google Scholar]
- [42]. Kroenke K, Spitzer RL, Williams JBW (2001) The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 16, 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Columbia-Suicide Severity Rating Scale (C-SSRS). The Research Foundation for Mental Hygiene, New York, NY. [Google Scholar]
- [44]. Posner K, Brown GK, Stanley B, Brent DA, Yershova K V., Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ (2011) The Columbia-suicide severity rating scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168, 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Krupp LB, Larocca NG, Muir Nash J, Steinberg AD (1989) The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46, 1121–1123. [DOI] [PubMed] [Google Scholar]
- [46]. Chaudhuri KR, Rizos A, Trenkwalder C, Rascol O, Pal S, Martino D, Carroll C, Paviour D, Falup-Pecurariu C, Kessel B, Silverdale M, Todorova A, Sauerbier A, Odin P, Antonini A, Martinez-Martin P (2015) King’s Parkinson’s disease pain scale, the first scale for pain in PD: An international validation. Mov Disord 30, 1623–1631. [DOI] [PubMed] [Google Scholar]
- [47]. Voss TS, Brocht AFD, Ravina B (2010) Performance of the Scale for Assessment of Positive Symptoms in Parkinson’s disease psychosis. Mov Disord 25, 124–125. [DOI] [PubMed] [Google Scholar]
- [48]. Kulick CV, Montgomery KM, Nirenberg MJ (2018) Comprehensive identification of delusions and olfactory, tactile, gustatory, and minor hallucinations in Parkinson’s disease psychosis. Parkinsonism Relat Disord 54, 40–45. [DOI] [PubMed] [Google Scholar]
- [49]. Johns MW (1991) A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 14, 540–545. [DOI] [PubMed] [Google Scholar]
- [50]. Trenkwalder C, Kohnen R, Högl B, Metta V, Sixel-Döring F, Frauscher B, Hülsmann J, Martinez-Martin P, Chaudhuri KR (2011) Parkinson’s disease sleep scale-validation of the revised version PDSS-2. Mov Disord 26, 644–652. [DOI] [PubMed] [Google Scholar]
- [51]. Visser M, Marinus J, Stiggelbout AM, van Hilten JJ (2004) Assessment of autonomic dysfunction in Parkinson’s disease: The SCOPA-AUT. Mov Disord 19, 1306–1312. [DOI] [PubMed] [Google Scholar]
- [52]. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- [53]. Gill DJ, Freshman A, Blender JA, Ravina B (2008) The Montreal Cognitive Assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov Disord 23, 1043–1046. [DOI] [PubMed] [Google Scholar]
- [54]. Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, Weintraub D (2009) Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 73, 1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, Melzer TR, Kirwan J, Keenan R, Wells S, Porter RJ, Watts R, Anderson TJ (2010) The MoCA: Well-suited screen for cognitive impairment in parkinson disease. Neurology 75, 1717–1725. [DOI] [PubMed] [Google Scholar]
- [56]. Matteau E, Dupré N, Langlois M, Provencher P, Simard M (2012) Clinical validity of the Mattis dementia rating scale-2 in Parkinson disease with MCI and dementia. J Geriatr Psychiatry Neurol 25, 100–106. [DOI] [PubMed] [Google Scholar]
- [57]. Pagonabarraga J, Kulisevsky J, Llebaria G, García-Sánchez C, Pascual-Sedano B, Gironell A (2008) Parkinson’s disease-cognitive rating scale: A new cognitive scale specific for Parkinson’s disease. Mov Disord 23, 998–1005. [DOI] [PubMed] [Google Scholar]
- [58]. Fernández de Bobadilla R, Pagonabarraga J, Martínez-Horta S, Pascual-Sedano B, Campolongo A, Kulisevsky J (2013) Parkinson’s disease-cognitive rating scale: Psychometrics for mild cognitive impairment. Mov Disord 28, 1376–1383. [DOI] [PubMed] [Google Scholar]
- [59]. Serrano-Dueñas M, Serrano M, Villena D, Granda D (2017) Validation of the Parkinson’s Disease-Cognitive Rating Scale applying the Movement Disorder Society Task Force Criteria for Dementia associated with Parkinson’s disease. Mov Disord Clin Pract 4, 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR (2006) The Addenbrooke’s Cognitive Examination revised (ACE-R): A brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 21, 1078–1085. [DOI] [PubMed] [Google Scholar]
- [61]. Reyes MA, Lloret SP, Gerscovich ER, Martin ME, Leiguarda R, Merello M (2009) Addenbrooke’s Cognitive Examination validation in Parkinson’s disease. Eur J Neurol 16, 142–147. [DOI] [PubMed] [Google Scholar]
- [62]. McColgan P, Evans JR, Breen DP, Mason SL, Barker RA, Williams-Gray CH (2012) Addenbrooke’s Cognitive Examination-revised for mild cognitive impairment in Parkinson’s disease. Mov Disord 27, 1173–1177. [DOI] [PubMed] [Google Scholar]
- [63]. Rittman T, Ghosh BC, McColgan P, Breen DP, Evans J, Williams-Gray CH, Barker RA, Rowe JB (2013) The Addenbrooke’s Cognitive Examination for the differential diagnosis and longitudinal assessment of patients with parkinsonian disorders. J Neurol Neurosurg Psychiatry 84, 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Rosen WG, Mohs RC, Davis KL (1984) A new rating scale for Alzheimer’s disease. Am J Psychiatry 141, 1356–1364. [DOI] [PubMed] [Google Scholar]
- [65]. Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [66]. Mahieux F, Michelet D, Manifacier MJ, Boller F, Fermanian J, Guillard A (1995) Mini-Mental Parkinson: First validation study of a new bedside test constructed for Parkinson’s disease. Behav Neurol 8, 15–22. [DOI] [PubMed] [Google Scholar]
- [67]. Serrano-Dueñas M, Calero B, Serrano S, Serrano M, Coronel P (2010) Metric properties of the mini-mental Parkinson and SCOPA-COG scales for rating cognitive deterioration in Parkinson’s disease. Mov Disord 25, 2555–2562. [DOI] [PubMed] [Google Scholar]
- [68]. Marinus J, Visser M, Na V, Fr V, Ha M, Am S, Hilten V (2003) Assessment of cognition in Parkinson’s disease. Neurology 61, 1222–1228. [DOI] [PubMed] [Google Scholar]
- [69]. Gonzalez MC, Dalen I, Maple-Grødem J, Tysnes OB, Alves G (2022) Parkinson’s disease clinical milestones and mortality. NPJ Parkinsons Dis 8, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Schwab J, England A (1969) Projection technique for evaluating surgery in Parkinson’s disease. In Third Symposium on Parkinson’s Diseases Disease. Vol. 232, Gillingham F, Donaldson M, eds. Churchill Livingstone, Edinburgh, Scotland, pp. 152–157. [Google Scholar]
- [71]. McRae C, Diem G, Vo A, O’Brien C, Seeberger L (2000) Schwab & England- standardization of administration, Mov Disord 15, 335–336. [DOI] [PubMed] [Google Scholar]
- [72]. Jette AM, Davies AR, Cleary PD, Calkins DR, Rubenstein L V., Fink A, Kosecoff J, Young RT, Brook RH, Delbanco TL (1986) The functional status questionnaire: Reliability and validity when used in primary care. J Gen Intern Med 1, 427–427. [DOI] [PubMed] [Google Scholar]
- [73]. Rubenstein LM, Voelker MD, Chrischilles EA, Glenn DC, Wallace RB, Rodnitzky RL (1998) The usefulness of the Functional Status Questionnaire and Medical Outcomes Study Short Form in Parkinson’s disease research. Qual Life Res 7, 279–290. [DOI] [PubMed] [Google Scholar]
- [74]. Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, Stern MB, Tilley BC, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, Van Hilten JJ, LaPelle N (2007) Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord 22, 41–47. [DOI] [PubMed] [Google Scholar]
- [75]. Al-Janabi H, Flynn TN, Coast J (2012) Development of a self-report measure of capability wellbeing for adults: The ICECAP-A. Qual Life Res 21, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Coast J, Flynn TN, Natarajan L, Sproston K, Lewis J, Louviere JJ, Peters TJ (2008) Valuing the ICECAP capability index for older people. Soc Sci Med 67, 874–882. [DOI] [PubMed] [Google Scholar]
- [77]. Pillas M, Selai C, Quinn NP, Lees A, Litvan I, Lang A, Bower J, Burn D, Low P, Schrag A (2016) Development and validation of a carers quality-of-life questionnaire for parkinsonism (PQoL Carers). Qual Life Res 25, 81–88. [DOI] [PubMed] [Google Scholar]
- [78]. Jenkinson C, Dummett S, Kelly L, Peters M, Dawson J, Morley D, Fitzpatrick R (2012) The development and validation of a quality of life measure for the carers of people with Parkinson’s disease (the PDQ-Carer). Parkinsonism Relat Disord 18, 483–487. [DOI] [PubMed] [Google Scholar]
- [79]. Zarit SH, Reever KE, Bach-Peterson J (1980) Relatives of the impaired elderly: Correlates of feelings of burden. Gerontologist 20, 649–655. [DOI] [PubMed] [Google Scholar]
- [80]. Hagell P, Whalley D, McKenna SP, Lindvall O (2003) Health status measurement in Parkinson’s disease: Validity of the PDQ-39 and Nottingham Health Profile. Mov Disord 18, 773–783. [DOI] [PubMed] [Google Scholar]
- [81]. The EuroQoL Group (1990) EuroQol - a new facility for the measurement of health-related quality of life. Health Policy 16, 199–208. [DOI] [PubMed] [Google Scholar]
- [82]. EuroQol Research Foundation (2019) EQ-5D-5L User Guide v3.0, Computer, Long Beach, CA, pp. 169–232.
- [83]. Ware JE, Sherbourne CD (1992) The MOS 36-item short-form health survey (Sf-36): I. conceptual framework and item selection. Med Care 30, 473–483. [PubMed] [Google Scholar]
- [84]. Brown CA, Cheng EM, Hays RD, Vassar SD, Vickrey BG (2009) SF-36 includes less Parkinson Disease (PD)-targeted content but is more responsive to change than two PD-targeted health-related quality of life measures. Qual Life Res 18, 1219–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. Ware JE, Kosinski M, Keller SD (1996) A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34, 220–233. [DOI] [PubMed] [Google Scholar]
- [86]. Brazier JE, Roberts J (2004) The estimation of a preference-based index from the SF-12. Med Care 42, 851–859. [DOI] [PubMed] [Google Scholar]
- [87]. Gershon RC, Lai JS, Bode R, Choi S, Moy C, Bleck T, Miller D, Peterman A, Cella D (2012) Neuro-QOL: Quality of life item banks for adults with neurological disorders: Item development and calibrations based upon clinical and general population testing. Qual Life Res 21, 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. Rose M, Bjorner JB, Becker J, Fries JF, Ware JE (2008) Evaluation of a preliminary physical function item bank supported the expected advantages of the Patient-Reported Outcomes Measurement Information System (PROMIS). J Clin Epidemiol 61, 17–33. [DOI] [PubMed] [Google Scholar]
- [89]. Nowinski CJ, Siderowf A, Simuni T, Wortman C, Moy C, Cella D (2016) Neuro-QoL health-related quality of life measurement system: Validation in Parkinson’s disease. Mov Disord 31, 725–733. [DOI] [PubMed] [Google Scholar]
- [90]. Shin JY, Pohlig RT, Habermann B (2018) Feasibility of using PROMIS® in individuals with advanced Parkinson’s disease and their caregivers. Res Gerontol Nurs 11, 129–136. [DOI] [PubMed] [Google Scholar]
- [91]. Furlong WJ, Feeny DH, Torrance GW, Barr RD (2001) The Health Utilities Index (HUI®) system for assessing health-related quality of life in clinical studies. Ann Med 33, 375–384. [DOI] [PubMed] [Google Scholar]
- [92]. Horsman J, Furlong W, Feeny D, Torrance G (2003) The Health Utilities Index (HUI®): Concepts, measurement properties and applications. Health Qual Life Outcomes 13, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93]. Peto V, Jenkinson C, Fitzpatrick R, Greenhall R (1995) The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res 4, 241–248. [DOI] [PubMed] [Google Scholar]
- [94]. Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N (1997) The PDQ-8: Development and validation of a short-form Parkinson’s disease questionnaire. Psychol Health 12, 805–814. [Google Scholar]
- [95]. Beecham J, Knapp M (2001) Costing psychiatric interventions. In Measuring Mental Health Needs, Thornicroft G, ed. Royal College of Psychiatrists, London, pp. 200–224. [Google Scholar]
- [96]. McCrone P (2009) Capturing the costs of end-of-life care: Comparisons of multiple sclerosis, Parkinson’s disease, and dementia. J Pain Symptom Manage 38, 62–67. [DOI] [PubMed] [Google Scholar]
- [97]. Personal Social Services Research Unit. Client Service Receipt Inventory (CSRI), https://www.pssru.ac.uk/csri/client-service-receipt-inventory/.
- [98]. Arora S, Baig F, Lo C, Barber TR, Lawton MA, Zhan A, Rolinski M, Ruffmann C, Klein JC, Rumbold J, Louvel A, Zaiwalla Z, Lennox G, Quinnell T, Dennis G, Wade-Martins R, Ben-Shlomo Y, Little MA, Hu MT (2018) Smartphone motor testing to distinguish idiopathic REM sleep behavior disorder, controls, and PD, Neurology 91, E1528–E1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99]. Jha A, Menozzi E, Oyekan R, Latorre A, Mulroy E, Schreglmann SR, Stamate C, Daskalopoulos I, Kueppers S, Luchini M, Rothwell JC, Roussos G, Bhatia KP (2020) The CloudUPDRS smartphone software in Parkinson’s study: Cross-validation against blinded human raters. NPJ Parkinsons Dis 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100]. Hasegawa N, Shah VV, Carlson-Kuhta P, Nutt JG, Horak FB, Mancini M (2019) How to select balance measures sensitive to parkinson’s disease from body-worn inertial sensors— separating the trees from the forest. Sensors (Switzerland) 19, 3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101]. Shah VV, McNames J, Mancini M, Carlson-Kuhta P, Spain RI, Nutt JG, El-Gohary M, Curtze C, Horak FB (2020) Quantity and quality of gait and turning in people with multiple sclerosis, Parkinson’s disease and matched controls during daily living. J Neurol 267, 1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102]. Zhan A, Mohan S, Tarolli C, Schneider RB, Adams JL, Sharma S, Elson MJ, Spear KL, Glidden AM, Little MA, Terzis A, Ray Dorsey E, Saria S (2018) Using smartphones and machine learning to quantify Parkinson disease severity the mobile Parkinson disease score. JAMA Neurol 75, 876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103]. Griffiths RI, Kotschet K, Arfon S, Xu ZM, Johnson W, Drago J, Evans A, Kempster P, Raghav S, Horne MK (2012) Automated assessment of bradykinesia and dyskinesia in Parkinson’s disease. J Parkinsons Dis 2, 47–55. [DOI] [PubMed] [Google Scholar]
- [104]. Powers R, Etezadi-Amoli M, Arnold EM, Kianian S, Mance I, Gibiansky M, Trietsch D, Alvarado AS, Kretlow JD, Herrington TM, Brillman S, Huang N, Lin PT, Pham HA, Ullal AV (2021) Smartwatch inertial sensors continuously monitor real-world motor fluctuations in Parkinson’s disease, Sci Transl Med 13, eabd7865. [DOI] [PubMed] [Google Scholar]
- [105]. Rehman RZU, Zhou Y, Din S Del, Alcock L, Hansen C, Guan Y, Hortobágyi T, Maetzler W, Rochester L, Lamoth CJC (2020) Gait analysis with wearables can accurately classify fallers from non-fallers: A step toward better management of neurological disorders. Sensors (Basel) 20, 6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106]. Polhemus A, Ortiz LD, Brittain G, Chynkiamis N, Salis F, Gaßner H, Gross M, Kirk C, Rossanigo R, Taraldsen K, Balta D, Breuls S, Buttery S, Cardenas G, Endress C, Gugenhan J, Keogh A, Kluge F, Koch S, Micó-Amigo ME, Nerz C, Sieber C, Williams P, Bergquist R, de Basea MB, Buckley E, Hansen C, Mikolaizak AS, Schwickert L, Scott K, Stallforth S, van Uem J, Vereijken B, Cereatti A, Demeyer H, Hopkinson N, Maetzler W, Troosters T, Vogiatzis I, Yarnall A, Becker C, Garcia-Aymerich J, Leocani L, Mazzà C, Rochester L, Sharrack B, Frei A, Puhan M; Mobilise-D (2021) Walking on common ground: A cross-disciplinary scoping review on the clinical utility of digital mobility outcomes. NPJ Digit Med 4, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107]. Del Din S, Kirk C, Yarnall AJ, Rochester L, Hausdorff JM (2021) Body-worn sensors for remote monitoring of Parkinson’s disease motor symptoms: Vision, state of the art, and challenges ahead, J Parkinsons Dis 11, S35–S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108]. Pagano G, Boess FG, Taylor KI, Ricci B, Mollenhauer B, Poewe W, Boulay A, Anzures-Cabrera J, Vogt A, Marchesi M, Post A, Nikolcheva T, Kinney GG, Zago WM, Ness DK, Svoboda H, Britschgi M, Ostrowitzki S, Simuni T, Marek K, Koller M, Sevigny J, Doody R, Fontoura P, Umbricht D, Bonni A; PASADENA Investigators; Prasinezumab Study Group (2021) A Phase II Study to Evaluate the Safety and Efficacy of Prasinezumab in Early Parkinson’s Disease (PASADENA): Rationale, design, and baseline data. Front Neurol 12, 705407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109]. Lipsmeier F, Taylor KI, Postuma RB, Kilchenmann T, Wolf D, Zhang YP, Cheng WY, Volkova-Volkmar E, Scotland A, Schjodt-Eriksen J, Boess F, Ness D, Gossens C, Post A, Lindemann M (2019) Preliminary validation of a novel, comprehensive digital biomarker smartphone application to assess motor symptoms in recently diagnosed Parkinson patients, Neurology 92 (15 Supp), 4.7-005. [Google Scholar]
- [110]. Podsiadlo D, Richardson S (1991) The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39, 142–148. [DOI] [PubMed] [Google Scholar]
- [111]. Tiffin J, Asher EJ (1948) The Purdue Pegboard: Norms and studies of reliability and validity. J Appl Psychol 32, 234–247. [DOI] [PubMed] [Google Scholar]
- [112]. Proud EL, Miller KJ, Bilney B, Morris ME, McGinley JL (2020) Construct validity of the 9-Hole Peg Test and Purdue Pegboard Test in people with mild to moderately severe Parkinson’s disease. Physiotherapy 107, 202–208. [DOI] [PubMed] [Google Scholar]
- [113]. Burns BD, Dejong JD (1960) A preliminary report on the measurement of parkinson’s disease. Neurology 10, 1096–1102. [DOI] [PubMed] [Google Scholar]
- [114]. Noyce AJ, Nagy A, Acharya S, Hadavi S, Bestwick JP, Fearnley J, Lees AJ, Giovannoni G (2014) Bradykinesia-akinesia incoordination test: Validating an online keyboard test of upper limb function, PLoS One 9, e96260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115]. Hasan H, Burrows M, Athauda DS, Hellman B, James B, Warner T, Foltynie T, Giovannoni G, Lees AJ, Noyce AJ (2019) The BRadykinesia Akinesia INcoordination (BRAIN) Tap Test: Capturing the sequence effect. Mov Disord Clin Pract 6, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116]. Mathiowetz V, Weber K, Kashman N, Volland G (1985) Adult norms for the nine-hole peg test of finger dexterity. Occup Ther J Res 5, 24–38. [DOI] [PubMed] [Google Scholar]
- [117]. Earhart GM, Cavanaugh JT, Ellis T, Ford MP, Foreman KB, Dibble L (2011) The 9-hole peg test of upper extremity function: Average values, test-retest reliability, and factors contributing to performance in people with Parkinson disease. J Neurol Phys Ther 35, 157–163. [DOI] [PubMed] [Google Scholar]
- [118]. Proud E, Morris ME, Bilney B, Miller KJ, Nijkrake MJ, Munneke M, McGinley JL (2021) Hand dexterity assessment in Parkinson’s disease: Construct validity of the 9-Hole peg test for the more affected hand. Disabil Rehabil 43, 3834–3838. [DOI] [PubMed] [Google Scholar]
- [119]. Lo C, Arora S, Lawton M, Barber T, Quinnell T, Dennis GJ, Shlomo YB, Tao M (2022) A composite clinical motor score as a comprehensive and sensitive outcome measure for Parkinson’s disease. J Neurol Neurosurg Psychiatry 93, 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120]. Saeed U, Lang AE, Masellis M (2020) Neuroimaging advances in Parkinson’s disease and atypical Parkinsonian syndromes. Front Neurol 11, 572976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121]. Nicastro N, Nencha U, Burkhard PR, Garibotto V (2021) Dopaminergic imaging in degenerative parkinsonisms, an established clinical diagnostic tool. J Neurochem 164, 346–363. [DOI] [PubMed] [Google Scholar]
- [122]. Wang L, Zhang Q, Li H, Zhang H (2012) SPECT molecular imaging in Parkinson’s disease. J Biomed Biotechnol 2012, 412486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123]. Palermo G, Giannoni S, Bellini G, Siciliano G, Ceravolo R (2021) Dopamine transporter imaging, current status of a potential biomarker: A comprehensive review. Int J Mol Sci 22, 11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124]. Porter E, Roussakis AA, Lao-Kaim NP, Piccini P (2020) Multimodal dopamine transporter (DAT) imaging and magnetic resonance imaging (MRI) to characterise early Parkinson’s disease. Parkinsonism Relat Disord 79, 26–33. [DOI] [PubMed] [Google Scholar]
- [125]. Simuni T, Siderowf A, Lasch S, Coffey CS, Caspell-Garcia C, Jennings D, Tanner CM, Trojanowski JQ, Shaw LM, Seibyl J, Schuff N, Singleton A, Kieburtz K, Toga AW, Mollenhauer B, Galasko D, Chahine LM, Weintraub D, Foroud T, Tosun D, Poston K, Arnedo V, Frasier M, Sherer T, Chowdhury S, Marek K; Parkinson’s Progression Marker Initiative (2018) Longitudinal change of clinical and biological measures in early Parkinson’s disease: Parkinson’s Progression Markers Initiative Cohort. Mov Disord 33, 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126]. Asenbaum S, Brücke T, Pirker W, Podreka I, Angelberger P, Wenger S, Wöber C, Müller C, Deecke L (1997) Imaging of dopamine transporters with iodine-123-β-CIT and SPECT in Parkinson’s disease. J Nucl Med 38, 1–6. [PubMed] [Google Scholar]
- [127]. Vermeulen RJ, Wolters EC, Tissingh G, Booij J, Janssen AGM, Habraken J, Sokole-Busemann E, Stoof JC, Van Royen EA (1995) Evaluation of [123I]β-CIT binding with SPECT in controls, early and late Parkinson’s disease. Nucl Med Biol 22, 985–991. [DOI] [PubMed] [Google Scholar]
- [128]. Antonini A, Schwarz J, Oertel WH, Pogarell O, Leenders KL (1997) Long-term changes of striatal dopamine D2 receptors in patients with Parkinson’s disease: A study with positron emission tomography and [11C]raclopride. Mov Disord 12, 33–38. [DOI] [PubMed] [Google Scholar]
- [129]. Kim YJ, Ichise M, Ballinger JR, Vines D, Erami SS, Tatschida T, Lang AE (2002) Combination of dopamine transporter and D2 receptor SPECT in the diagnostic evaluation of PD, MSA, and PSP. Mov Disord 17, 303–312. [DOI] [PubMed] [Google Scholar]
- [130]. Snow BJ, Tooyama I, McGeer EG, Yamada T, Calne DB, Takahashi H, Kimura H (1993) Human positron emission tomographic [18F]Fluorodopa studies correlate with dopamine cell counts and levels. Ann Neurol 34, 324–330. [DOI] [PubMed] [Google Scholar]
- [131]. Li W, Lao-Kaim NP, Roussakis AA, Martín-Bastida A, Valle-Guzman N, Paul G, Loane C, Widner H, Politis M, Foltynie T, Barker RA, Piccini P (2018) 11 C-PE2I and 18 F-Dopa PET for assessing progression rate in Parkinson’s: A longitudinal study. Mov Disord 33, 117–127. [DOI] [PubMed] [Google Scholar]
- [132]. Chou KL, Kotagal V, Bohnen NI (2016) Neuroimaging and clinical predictors of fatigue in Parkinson disease. Parkinsonism Relat Disord 23, 45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133]. Brück A, Aalto S, Rauhala E, Bergman J, Marttila R, Rinne JO (2009) A follow-up study on 6-[18F]Fluoro-L-dopa uptake in early Parkinson’s disease shows nonlinear progressionin the putamen. Mov Disord 24, 1009–1015. [DOI] [PubMed] [Google Scholar]
- [134]. Kapucu ÖL, Nobili F, Varrone A, Booij J, Vander Borght T, Någren K, Darcourt J, Tatsch K, Van Laere KJ (2009) EANM procedure guideline for brain perfusion SPECT using 99mTc-labelled radiopharmaceuticals, version 2. Eur J Nucl Med Mol Imaging 36, 2093–2102. [DOI] [PubMed] [Google Scholar]
- [135]. Pavel DG, Henderson TA, DeBruin S, Cohen PF (2022) The Legacy of the TTASAAN Report – premature conclusions and forgotten promises about SPECT neuroimaging: A review of policy and practice part II. Front Neurol 13, 851609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136]. Pavel DG, Henderson TA, DeBruin S (2022) The Legacy of the TTASAAN Report— premature conclusions and forgotten promises: A review of policy and practice part I. Front Neurol 12, 749579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137]. Kramberger MG, Štukovnik V, Čuš A, Repovš G, Tomše P, Meglič c NP, Garašević Z, Jensterle J, Pirtošek Z (2010) Parkinson’s disease dementia: Clinical correlates of brain SPECT perfusion and treatment. Psychiatr Danub 22, 446–449. [PubMed] [Google Scholar]
- [138]. Zhu L, Zhao W, Chen J, Li G, Qu J (2022) Systematic review and meta-analysis of diagnostic test accuracy (DTA) studies: The role of cerebral perfusion imaging in prognosis evaluation of mild cognitive impairment. Ann Palliat Med 11, 673–683. [DOI] [PubMed] [Google Scholar]
- [139]. Feigin A, Antonini A, Fukuda M, De Notaris R, Benti R, Pezzoli G, Mentis MJ, Moeller JR, Eidelberg D (2002) Tc-99m ethylene cysteinate dimer SPECT in the differential diagnosis of parkinsonism. Mov Disord 17, 1265–1270. [DOI] [PubMed] [Google Scholar]
- [140]. Juni JE, Waxman AD, Devous MD, Tikofsky RS, Ichise M, Van Heertum RL, Carretta RF, Chen CC (2009) Procedure guideline for brain perfusion SPECT using 99mTc radiopharmaceuticals 3.0. J Nucl Med Technol 37, 191–195. [DOI] [PubMed] [Google Scholar]
- [141]. Kotagal V, Spino C, Bohnen NI, Koeppe R, Albin RL (2018) Serotonin, β-amyloid, and cognition in Parkinson disease. Ann Neurol 83, 994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142]. van der Zee S, Müller MLTM, Kanel P, van Laar T, Bohnen NI (2021) Cholinergic denervation patterns across cognitive domains in Parkinson’s disease. Mov Disord 36, 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143]. Boertien JM, Van Der Zee S, Chrysou A, Gerritsen MJJ, Jansonius NM, Spikman JM, Van Laar T, Verwey NA, Van Harten B, Portman AT, Langedijk MJH, Oomes PG, Jansen BJAM, Van Wieren T, Van Den Bogaard SJA, Van Steenbergen W, Duyff R, Van Amerongen JP, Fransen PSS, Polman SKL, Zwartbol RT, Van Kesteren ME, Braakhekke JP, Trip J, Koops L, De Langen CJ, De Jong G, Hartono JES, Ybema H, Bartels AL, Reesink FE, Postma AG, Vonk GJH, Oen JMTH, Brinkman MJ, Mondria T, Holscher RS, Van Der Meulen AAE, Rutgers AWF, Boekestein WA, Teune LK, Orsel PJL, Hoogendijk JE, Van Laar T; PPNN Study Group (2020) Study protocol of the DUtch PARkinson Cohort (DUPARC): A prospective, observational study of de novo Parkinson’s disease patients for the identification and validation of biomarkers for Parkinson’s disease subtypes, progression and pathophysiology. BMC Neurol 20, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144]. Melzer TR, Stark MR, Keenan RJ, Myall DJ, MacAskill MR, Pitcher TL, Livingston L, Grenfell S, Horne KL, Young BN, Pascoe MJ, Almuqbel MM, Wang J, Marsh SH, Miller DH, Dalrymple-Alford JC, Anderson TJ (2019) Beta amyloid deposition is not associated with cognitive impairment in Parkinson’s disease. Front Neurol 10, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145]. Sabri O, Sabbagh MN, Seibyl J, Barthel H, Akatsu H, Ouchi Y, Senda K, Murayama S, Ishii K, Takao M, Beach TG, Rowe CC, Leverenz JB, Ghetti B, Ironside JW, Catafau AM, Stephens AW, Mueller A, Koglin N, Hoffmann A, Roth K, Reininger C, Schulz-Schaeffer WJ (2015) Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: Phase 3 study. Alzheimers Dement 11, 964–974. [DOI] [PubMed] [Google Scholar]
- [146]. Mashima K, Ito D, Kameyama M, Osada T, Tabuchi H, Nihei Y, Yoshizaki T, Noguchi E, Tanikawa M, Iizuka T, Date Y, Ogata Y, Nakahara T, Iwabuchi Y, Jinzaki M, Murakami K, Suzuki N (2017) Extremely low prevalence of amyloid positron emission tomography positivity in Parkinson’s disease without dementia. Eur Neurol 77, 231–237. [DOI] [PubMed] [Google Scholar]
- [147]. Na S, Jeong H, Park J, Chung Y, Song I (2020) The impact of amyloid-beta positivity with 18F-Florbetaben PET on neuropsychological aspects in Parkinson’s disease dementia. Metabolites 10, 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148]. Chou KL, Dayalu P, Koeppe RA, Gilman S, Spears CC, Albin RL, Kotagal V (2022) Serotonin transporter imaging in multiple system atrophy and Parkinson’s disease, Mov Disord 37, 2301, 2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149]. Fazio P, Ferreira D, Svenningsson P, Halldin C, Farde L, Westman E, Varrone A (2020) High-resolution PET imaging reveals subtle impairment of the serotonin transporter in an early non-depressed Parkinson’s disease cohort. Eur J Nucl Med Mol Imaging 47, 2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150]. Gomperts SN, Locascio JJ, Johnson KA, Growdon H (2016) PET radioligands reveal the basis of dementia in Parkinson’s disease and dementia with Lewy bodies. Neurodegener Dis 2016, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151]. Waggan I, Rissanen E, Tuisku J, Joutsa J, Helin S, Parkkola R, Rinne JO, Airas L (2022) Adenosine A2A receptor availability in patients with early- and moderate-stage Parkinson’s disease. J Neurol 270, 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152]. Ishibashi K, Miura Y, Wagatsuma K, Toyohara J, Ishiwata K, Ishii K (2018) Occupancy of adenosine A2A receptors by istradefylline in patients with Parkinson’s disease using 11C-preladenant PET. Neuropharmacology 143, 106–112. [DOI] [PubMed] [Google Scholar]
- [153]. Guedj E, Varrone A, Boellaard R, Albert NL, Barthel H, van Berckel B, Brendel M, Cecchin D, Ekmekcioglu O, Garibotto V, Lammertsma AA, Law I, Peñuelas I, Semah F, Traub-Weidinger T, van de Giessen E, Van Weehaeghe D, Morbelli S (2022) EANM procedure guidelines for brain PET imaging using [18F]FDG, version 3. Eur J Nucl Med Mol Imaging 49, 632–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154]. Waggan I, Rissanen E, Tuisku J, Matilainen M, Helin S, Parkkola R, Rinne JO, Airas L (2021) Effect of dopaminergic medication on adenosine 2A receptor availability in patients with Parkinson’s disease. Parkinsonism Relat Disord 86, 40–44. [DOI] [PubMed] [Google Scholar]
- [155]. Minoshima S, Drzezga AE, Barthel H, Bohnen N, Djekidel M, Lewis DH, Mathis CA, McConathy J, Nordberg A, Sabri O, Seibyl JP, Stokes MK, Van Laere K (2016) SNMMI Procedure Standard / EANM Practice Guideline for amyloid PET imaging of the brain 1.0. J Nucl Med 57, 1316–1322. [DOI] [PubMed] [Google Scholar]
- [156]. Ciurleo R, Di Lorenzo G, Bramanti P, Marino S (2014) Magnetic resonance spectroscopy: An molecular imaging biomarker for Parkinson’s disease? Biomed Res Int 2014, 519816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157]. Payne T, Sassani M, Buckley E, Moll S, Anton A, Appleby M, Maru S, Taylor R, McNeill A, Hoggard N, Mazza C, Wilkinson ID, Jenkins T, Foltynie T, Bandmann O (2020) Ursodeoxycholic acid as a novel disease-modifying treatment for Parkinson’s disease: Protocol for a two-centre, randomised, double-blind, placebo-controlled trial, the “UP” study, BMJ Open 10, e038911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158]. Li X, Xing Y, Martin-Bastida A, Piccini P, Auer DP (2018) Patterns of grey matter loss associated with motor subscores in early Parkinson’s disease. Neuroimage Clin 17, 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159]. Compta Y, Pereira JB, Ríos J, Ibarretxe-Bilbao N, Junqué C, Bargalló N, Cámara A, Buongiorno M, Fernández M, Pont-Sunyer C, Martí MJ (2013) Combined dementia-risk biomarkers in Parkinson’s disease: A prospective longitudinal study. Parkinsonism Relat Disord 19, 717–724. [DOI] [PubMed] [Google Scholar]
- [160]. Agosta F, Kostic VS, Davidovic K, Kresojević N, Sarro L, Svetel M, Stanković I, Comi G, Klein C, Filippi M (2013) White matter abnormalities in Parkinson’s disease patients with glucocerebrosidase gene mutations. Mov Disord 28, 772–778. [DOI] [PubMed] [Google Scholar]
- [161]. Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT (2004) Cerebral atrophy in Parkinson’s disease with and without dementia: A comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 127, 791–800. [DOI] [PubMed] [Google Scholar]
- [162]. Lanskey JH, McColgan P, Schrag AE, Acosta-Cabronero J, Rees G, Morris HR, Weil RS (2018) Can neuroimaging predict dementia in Parkinson’s disease? Brain 141, 2545–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163]. Rolheiser TM, Fulton HG, Good KP, Fisk JD, McKelvey JR, Scherfler C, Khan NM, Leslie RA, Robertson HA (2011) Diffusion tensor imaging and olfactory identification testing in early-stage Parkinson’s disease. J Neurol 258, 1254–1260. [DOI] [PubMed] [Google Scholar]
- [164]. Seider NA, Adeyemo B, Miller R, Newbold DJ, Hampton JM, Scheidter KM, Rutlin J, Laumann TO, Roland JL, Montez DF, Van AN, Zheng A, Marek S, Kay BP, Bretthorst GL, Schlaggar BL, Greene DJ, Wang Y, Petersen SE, Barch DM, Gordon EM, Snyder AZ, Shimony JS, Dosenbach NUF (2022) Accuracy and reliability of diffusion imaging models. Neuroimage 254, 119138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165]. Archer DB, Bricker JT, Chu WT, Burciu RG, McCracken JL, Lai S, Coombes SA, Fang R, Barmpoutis A, Corcos DM, Kurani AS, Mitchell T, Black ML, Herschel E, Simuni T, Parrish TB, Comella C, Xie T, Seppi K, Bohnen NI, Müller ML, Albin RL, Krismer F, Du G, Lewis MM, Huang X, Li H, Pasternak O, McFarland NR, Okun MS, Vaillancourt DE (2019) Development and validation of the automated imaging differentiation in parkinsonism (AID-P): A multicentre machine learning study, Lancet Digit Health 1, e222–e231. [DOI] [PubMed] [Google Scholar]
- [166]. Andica C, Kamagata K, Hatano T, Saito A, Uchida W, Ogawa T, Takeshige-Amano H, Zalesky A, Wada A, Suzuki M, Hagiwara A, Irie R, Hori M, Kumamaru KK, Oyama G, Shimo Y, Umemura A, Pantelis C, Hattori N, Aoki S (2019) Free-water imaging in white and gray matter in Parkinson’s disease. Cells 8, 839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167]. Biondetti E, Gaurav R, Yahia-Cherif L, Mangone G, Pyatigorskaya N, Valabrègue R, Ewenczyk C, Hutchison M, François C, Corvol JC, Vidailhet M, Lehéricy S (2020) Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson’s disease. Brain 143, 2757–2770. [DOI] [PubMed] [Google Scholar]
- [168]. Matsuura K, Maeda M, Tabei K ichi, Umino M, Kajikawa H, Satoh M, Kida H, Tomimoto H (2016) A longitudinal study of neuromelanin-sensitive magnetic resonance imaging in Parkinson’s disease. Neurosci Lett 633, 112–117. [DOI] [PubMed] [Google Scholar]
- [169]. Thomas GEC, Leyland LA, Schrag AE, Lees AJ, Acosta-Cabronero J, Weil RS (2020) Brain iron deposition is linked with cognitive severity in Parkinson’s disease. J Neurol Neurosurg Psychiatry 91, 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170]. Uchida Y, Kan H, Sakurai K, Arai N, Kato D, Kawashima S, Ueki Y, Matsukawa N (2019) Voxel-based quantitative susceptibility mapping in Parkinson’s disease with mild cognitive impairment. Mov Disord 34, 1164–1173. [DOI] [PubMed] [Google Scholar]
- [171]. Lee JH, Lee MS (2019) Brain iron accumulation in atypical parkinsonian syndromes: MRI evidences for distinctive patterns. Front Neurol 10, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172]. Cho SJ, Bae YJ, Kim JM, Kim HJ, Baik SH, Sunwoo L, Choi BS, Jung C, Kim JH (2021) Iron-sensitive magnetic resonance imaging in Parkinson’s disease: A systematic review and meta-analysis. J Neurol 268, 4721–4736. [DOI] [PubMed] [Google Scholar]
- [173]. Parnetti L, Gaetani L, Eusebi P, Paciotti S, Hansson O, El-Agnaf O, Mollenhauer B, Blennow K, Calabresi P (2019) CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol 18, 573–586. [DOI] [PubMed] [Google Scholar]
- [174]. Compta Y, Revesz T (2021) Neuropathological and biomarker findings in Parkinson’s disease and Alzheimer’s disease: From protein aggregates to synaptic dysfunction. J Parkinsons Dis 11, 107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175]. Katayama T, Sawada J, Takahashi K, Yahara O (2020) Cerebrospinal fluid biomarkers in Parkinson’s disease: A critical overview of the literature and meta-analyses. Brain Sci 10, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176]. Tönges L, Buhmann C, Klebe S, Klucken J, Kwon EH, Müller T, Pedrosa DJ, Schröter N, Riederer P, Lingor P (2022) Blood-based biomarker in Parkinson’s disease: Potential for future applications in clinical research and practice. J Neural Transm 129, 1201–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177]. Morris HR (2022) Blood based biomarkers for movement disorders. Acta Neurol Scand 146, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178]. Pawlik P, Błochowiak K (2021) The role of salivary biomarkers in the early diagnosis of Alzheimer’s disease and Parkinson’s disease. Diagnostics 11, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179]. Altmann P, Leutmezer F, Zach H, Wurm R, Stattmann M, Ponleitner M, Petzold A, Zetterberg H, Berger T, Rommer P, Bsteh G (2020) Serum neurofilament light chain withstands delayed freezing and repeated thawing. Sci Rep 10, 19982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180]. Altmann P, Ponleitner M, Rommer PS, Haslacher H, Mucher P, Leutmezer F, Petzold A, Wotawa C, Lanzenberger R, Berger T, Zetterberg H, Bsteh G (2021) Seven day pre-analytical stability of serum and plasma neurofilament light chain. Sci Rep 11, 11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181]. Sejbaek T, Nielsen HH, Penner N, Plavina T, Mendoza JP, Martin NA, Elkjaer ML, Ravnborg MH, Illes Z (2019) Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naïve relapsing MS patients. J Neurol Neurosurg Psychiatry 90, 1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182]. Ashton NJ, Janelidze S, Al Khleifat A, Leuzy A, van der Ende EL, Karikari TK, Benedet AL, Pascoal TA, Lleó A, Parnetti L, Galimberti D, Bonanni L, Pilotto A, Padovani A, Lycke J, Novakova L, Axelsson M, Velayudhan L, Rabinovici GD, Miller B, Pariante C, Nikkheslat N, Resnick SM, Thambisetty M, Schöll M, Fernández-Eulate G, Gil-Bea FJ, López de Munain A, Al-Chalabi A, Rosa-Neto P, Strydom A, Svenningsson P, Stomrud E, Santillo A, Aarsland D, van Swieten JC, Palmqvist S, Zetterberg H, Blennow K, Hye A, Hansson O (2021) A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun 12, 3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183]. Mollenhauer B, Dakna M, Kruse N, Galasko D, Foroud T, Zetterberg H, Schade S, Gera RG, Wang W, Gao F, Frasier M, Chahine LM, Coffey CS, Singleton AB, Simuni T, Weintraub D, Seibyl J, Toga AW, Tanner CM, Kieburtz K, Marek K, Siderowf A, Cedarbaum JM, Hutten SJ, Trenkwalder C, Graham D (2020) Validation of serum neurofilament light chain as a biomarker of Parkinson’s disease progression. Mov Disord 35, 1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184]. Oosterveld LP, Verberk IMW, Majbour NK, El-Agnaf OM, Weinstein HC, Berendse HW, Teunissen CE, van de Berg WDJ (2020) CSF or serum neurofilament light added to α-Synuclein panel discriminates Parkinson’s from controls. Mov Disord 35, 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185]. Huang Y, Huang C, Zhang Q, Shen T, Sun J (2022) Serum NFL discriminates Parkinson disease from essential tremor and reflect motor and cognition severity. BMC Neurol 22, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186]. Ygland Rödström E, Mattsson-Carlgren N, Janelidze S, Hansson O, Puschmann A (2021) Serum neurofilament light chain as a marker of progression in Parkinson’s disease: Long-term observation and implications of clinical subtypes. J Parkinsons Dis 12, 571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187]. Ng ASL, Tan YJ, Yong ACW, Saffari SE, Lu Z, Ng EY, Ng SYE, Chia NSY, Choi X, Heng D, Neo S, Xu Z, Keong NCH, Tay KY, Au WL, Tan LCS, Tan EK (2020) Utility of plasma Neurofilament light as a diagnostic and prognostic biomarker of the postural instability gait disorder motor subtype in early Parkinson’s disease. Mol Neurodegener 15, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188]. Sampedro F, Pérez-González R, Martínez-Horta S, Marín-Lahoz J, Pagonabarraga J, Kulisevsky J (2020) Serum neurofilament light chain levels reflect cortical neurodegeneration in de novo Parkinson’s disease. Parkinsonism Relat Disord 74, 43–49. [DOI] [PubMed] [Google Scholar]
- [189]. Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H (2019) Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 90, 870–881. [DOI] [PubMed] [Google Scholar]
- [190]. Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, Chamoun M, Savard M, Kang MS, Therriault J, Schöll M, Massarweh G, Soucy JP, Höglund K, Brinkmalm G, Mattsson N, Palmqvist S, Gauthier S, Stomrud E, Zetterberg H, Hansson O, Rosa-Neto P, Blennow K (2020) Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 19, 422–433. [DOI] [PubMed] [Google Scholar]
- [191]. Gonzalez MC, Ashton NJ, Gomes BF, Tovar-Rios DA, Blanc F, Karikari TK, Mollenhauer B, Pilotto A, Lemstra A, Paquet C, Abdelnour C, Kramberger MG, Bonanni L, Vandenberghe R, Hye A, Blennow K, Zetterberg H, Aarsland D (2022) Association of plasma p-tau181 and p-tau231 concentrations with cognitive decline in patients with probable dementia with Lewy bodies. JAMA Neurol 79, 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192]. Ding XL, Tuo QZ, Lei P (2021) An introduction to ultrasensitive assays for plasma tau detection. J Alzheimers Dis 80, 1353–1362. [DOI] [PubMed] [Google Scholar]
- [193]. Lin CH, Yang SY, Horng HE, Yang CC, Chieh JJ, Chen HH, Liu BH, Chiu MJ (2018) Plasma biomarkers differentiate Parkinson’s disease from atypical parkinsonism syndromes. Front Aging Neurosci 10, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194]. Chen NC, Chen HL, Li SH, Chang YH, Chen MH, Tsai NW, Yu CC, Yang SY, Lu CH, Lin WC (2020) Plasma levels of α-Synuclein, Aβ-40 and T-tau as biomarkers to predict cognitive impairment in Parkinson’s disease. Front Aging Neurosci 12, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195]. Ren J, Pan C, Wang Y, Xue C, Lin H, Xu J, Wang H, Zhang W, Xu P, Chen Y, Liu W (2022) Plasma α-synuclein and phosphorylated tau 181 as a diagnostic biomarker panel for de novo Parkinson’s disease. J Neurochem 161, 506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196]. Youssef P, Kim WS, Halliday GM, Lewis SJG, Dzamko N (2021) Comparison of different platform immunoassays for the measurement of plasma alpha-synuclein in Parkinson’s disease patients. J Parkinsons Dis 11, 1761–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197]. Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Fox M, Goldstein JM, Soriano F, Seubert P, Chilcote TJ (2008) Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis 5, 55–59. [DOI] [PubMed] [Google Scholar]
- [198]. Zubelzu M, Morera-Herreras T, Irastorza G, Gómez-Esteban JC, Murueta-Goyena A (2022) Plasma and serum alpha-synuclein as a biomarker in Parkinson’s disease: A meta-analysis. Parkinsonism Relat Disord 99, 107–115. [DOI] [PubMed] [Google Scholar]
- [199]. Williams SM, Schulz P, Sierks MR (2016) Oligomeric α-synuclein and β-amyloid variants as potential biomarkers for Parkinson’s and Alzheimer’s diseases. Eur J Neurosci 43, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200]. Li XY, Li W, Li X, Li XR, Sun L, Yang W, Cai Y, Chen Z, Wu J, Wang C, Yu S (2021) Alterations of erythrocytic phosphorylated alpha-synuclein in different subtypes and stages of Parkinson’s disease. Front Aging Neurosci 13, 623977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201]. Niu M, Li Y, Li G, Zhou L, Luo N, Yao M, Kang W, Liu J (2020) A longitudinal study on α-synuclein in plasma neuronal exosomes as a biomarker for Parkinson’s disease development and progression. Eur J Neurol 27, 967–974. [DOI] [PubMed] [Google Scholar]
- [202]. Kluge A, Bunk J, Schaeffer E, Drobny A, Xiang W, Knacke H, Bub S, Lückstädt W, Arnold P, Lucius R, Berg D, Zunke F (2022) Detection of neuron-derived pathological α-synuclein in blood. Brain 145, 3058–3071. [DOI] [PubMed] [Google Scholar]
- [203]. Foulds PG, Diggle P, Mitchell JD, Parker A, Hasegawa M, Masuda-Suzukake M, Mann DM, Allsop D (2013) A longitudinal study on a-synuclein in blood plasma as a biomarker for Parkinson’s disease. Sci Rep 3, 2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204]. Malec-Litwinowicz M, Plewka A, Plewka D, Bogunia E, Morek M, Szczudlik A, Szubiga M, Rudzińska-Bar M (2018) The relation between plasma α-synuclein level and clinical symptoms or signs of Parkinson’s disease. Neurol Neurochir Pol 52, 243–251. [DOI] [PubMed] [Google Scholar]
- [205]. Chung CC, Chan L, Chen JH, Hung YC, Hong CT (2021) Plasma extracellular vesicle α-synuclein level in patients with parkinson’s disease. Biomolecules 11, 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206]. Bäckström D, Granåsen G, Mo SJ, Riklund K, Trupp M, Zetterberg H, Blennow K, Forsgren L, Domellöf ME (2022) Prediction and early biomarkers of cognitive decline in Parkinson disease and atypical parkinsonism: A population-based study, Brain Commun 15, fcac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207]. Lerche S, Wurster I, Röben B, Zimmermann M, Machetanz G, Wiethoff S, Dehnert M, Rietschel L, Riebenbauer B, Deuschle C, Stransky E, Lieplt-Scarfone I, Gasser T, Brockmann K (2020) CSF NFL in a longitudinally assessed PD cohort: Age effects and cognitive trajectories. Mov Disord 35, 1138–1144. [DOI] [PubMed] [Google Scholar]
- [208]. Kwon EH, Tennagels S, Gold R, Gerwert K, Beyer L, Tönges L (2022) Update on CSF Biomarkers in Parkinson’s disease. Biomolecules 12, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209]. Bäckström D, Linder J, Jakobson Mo S, Riklund K, Zetterberg H, Blennow K, Forsgren L, Lenfeldt N (2020) NfL as a biomarker for neurodegeneration and survival in Parkinson disease, Neurology 95, e827–e838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210]. Bäckström DC, Domellöf ME, Linder J, Olsson B, Öhrfelt A, Trupp M, Zetterberg H, Blennow K, Forsgren L (2015) Cerebrospinal fluid patterns and the risk of future dementia in early, incident Parkinson disease. JAMA Neurol 72, 1175–1182. [DOI] [PubMed] [Google Scholar]
- [211]. Lifke V, Kollmorgen G, Manuilova E, Oelschlaegel T, Hillringhaus L, Widmann M, von Arnim CAF, Otto M, Christenson RH, Powers JL, Shaw LM, Hansson O, Doecke JD, Li QX, Teunissen C, Tumani H, Blennow K (2019) Elecsys® total-Tau and Phospho-Tau (181P) CSF assays: Analytical performance of the novel, fully automated immunoassays for quantification of tau proteins in human cerebrospinal fluid. Clin Biochem 72, 30–38. [DOI] [PubMed] [Google Scholar]
- [212]. Liu C, Cholerton B, Shi M, Ginghina C, Cain KC, Auinger P, Zhang J (2015) CSF tau and tau/Aβ42 predict cognitive decline in Parkinson’s disease. Parkinsonism Relat Disord 21, 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213]. Mollenhauer B, Bowman FDB, Drake D, Duong J, Blennow K, El-Agnaf O, Shaw LM, Masucci J, Taylor P, Umek RM, Dunty JM, Smith CL, Stoops E, Vanderstichele H, Schmid AW, Moniatte M, Zhang J, Kruse N, Lashuel HA, Teunissen C, Schubert T, Dave KD, Hutten SJ, Zetterberg H (2019) Antibody-based methods for the measurement of α-synuclein concentration in human cerebrospinal fluid – method comparison and round robin study. J Neurochem 149, 126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214]. Constantinides VC, Majbour NK, Paraskevas GP, Abdi I, Safieh-Garabedian B, Stefanis L, El-Agnaf OM, Kapaki E (2021) Cerebrospinal fluid α-synuclein species in cognitive and movements disorders. Brain Sci 11, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215]. Majbour NK, Vaikath NN, Eusebi P, Chiasserini D, Ardah M, Varghese S, Haque ME, Tokuda T, Auinger P, Calabresi P, Parnetti L, El-Agnaf OMA (2016) Longitudinal changes in CSF alpha-synuclein species reflect Parkinson’s disease progression. Mov Disord 31, 1535–1542. [DOI] [PubMed] [Google Scholar]
- [216]. Bargar C, Wang W, Gunzler SA, LeFevre A, Wang Z, Lerner AJ, Singh N, Tatsuoka C, Appleby B, Zhu X, Xu R, Haroutunian V, Zou WQ, Ma J, Chen SG (2021) Streamlined alpha-synuclein RT-QuIC assay for various biospecimens in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol Commun 9, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217]. Kang UJ, Boehme AK, Fairfoul G, Shahnawaz M, Ma TC, Hutten SJ, Green A, Soto C (2019) Comparative study of cerebrospinal fluid α-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov Disord 34, 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218]. Poggiolini I, Gupta V, Lawton M, Lee S, El-Turabi A, Querejeta-Coma A, Trenkwalder C, Sixel-Döring F, Foubert-Samier A, Pavy-Le Traon A, Plazzi G, Biscarini F, Montplaisir J, Gagnon JF, Postuma RB, Antelmi E, Meissner WG, Mollenhauer B, Ben-Shlomo Y, Hu MT, Parkkinen L (2021) Diagnostic value of cerebrospinal fluid alpha-synuclein seed quantification in synucleinopathies. Brain 145, 584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219]. Orrù CD, Ma TC, Hughson AG, Groveman BR, Srivastava A, Galasko D, Angers R, Downey P, Crawford K, Hutten SJ, Kang UJ, Caughey B (2021) A rapid α-synuclein seed assay of Parkinson’s disease CSF panel shows high diagnostic accuracy. Ann Clin Transl Neurol 8, 374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220]. Iranzo A, Fairfoul G, Ayudhaya ACN, Serradell M, Gelpi E, Vilaseca I, Sanchez-Valle R, Gaig C, Santamaria J, Tolosa E, Riha RL, Green AJE (2021) Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: A longitudinal observational study. Lancet Neurol 20, 203–212. [DOI] [PubMed] [Google Scholar]
- [221]. Shahnawaz M, Tokuda T, Waraga M, Mendez N, Ishii R, Trenkwalder C, Mollenhauer B, Soto C (2017) Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol 74, 163–172. [DOI] [PubMed] [Google Scholar]
- [222]. V-PLEX Aβ Peptide Panel 1 (6E10) Kit, https://www.mesoscale.com/en/products/v-plex-abeta-peptide-panel-1-6e10-kit-k15200e/.
- [223]. NEUROLOGY 3-PLEX A (TAU, Aβ42, Aβ40), https://www.quanterix.com/simoa-assay-kits/neurology-3-plex-tau-ab42-ab40/. [Google Scholar]
- [224]. Boulo S, Kuhlmann J, Andreasson U, Brix B, Venkataraman I, Herbst V, Rutz S, Manuilova E, Vandijck M, Dekeyser F, Bjerke M, Pannee J, Charoud-Got J, Auclair G, Mazoua S, Pinski G, Trapmann S, Schimmel H, Emons H, Quaglia M, Portelius E, Korecka M, Shaw LM, Lame M, Chambers E, Vanderstichele H, Stoops E, Leinenbach A, Bittner T, Jenkins RG, Kostanjevecki V, Lewczuk P, Gobom J, Zetterberg H, Zegers I, Blennow K (2020) First amyloid β1-42 certified reference material for re-calibrating commercial immunoassays. Alzheimers Dement 16, 1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225]. Janelidze S, Stomrud E, Brix B, Hansson O (2019) Towards a unified protocol for handling of CSF before β-amyloid measurements. Alzheimers Res Ther 11, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226]. Lewczuk P, Riederer P, O’Bryant SE, Verbeek MM, Dubois B, Visser PJ, Jellinger KA, Engelborghs S, Ramirez A, Parnetti L, Jack CR Jr, Teunissen CE, Hampel H, Lleó A, Jessen F, Glodzik L, de Leon MJ, Fagan AM, Molinuevo JL, Jansen WJ, Winblad B, Shaw LM, Andreasson U, Otto M, Mollenhauer B, Wiltfang J, Turner MR, Zerr I, Handels R, Thompson AG, Johansson G, Ermann N, Trojanowski JQ, Karaca I, Wagner H, Oeckl P, van Waalwijk van Doorn L, Bjerke M, Kapogiannis D, Kuiperij HB, Farotti L, Li Y, Gordon BA, Epelbaum S, Vos SJB, Klijn CJM, Van Nostrand WE, Minguillon C, Schmitz M, Gallo C, Lopez Mato A, Thibaut F, Lista S, Alcolea D, Zetterberg H, Blennow K, Kornhuber J; Members of the WFSBP Task Force Working on this Topic: Peter Riederer, Carla Gallo, Dimitrios Kapogiannis, Andrea Lopez Mato, Florence Thibaut (2018) Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: An update of the Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World J Biol Psychiatry 19, 244–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227]. Lim EW, Aarsland D, Ffytche D, Taddei RN, van Wamelen DJ, Wan YM, Tan EK, Ray Chaudhuri K (2019) Amyloid-β and Parkinson’s disease. J Neurol 266, 2605–2619. [DOI] [PubMed] [Google Scholar]
- [228]. Alves G, Brønnick K, Aarsland D, Blennow K, Zetterberg H, Ballard C, Kurz MW, Andreasson U, Tysnes OB, Larsen JP, Mulugeta E (2010) CSF amyloid-β and tau proteins, and cognitive performance, in early and untreated Parkinson’s Disease: The Norwegian ParkWest study. J Neurol Neurosurg Psychiatry 81, 1080–1086. [DOI] [PubMed] [Google Scholar]
- [229]. Hall S, Surova Y, Öhrfelt A, Zetterberg H, Lindqvist D, Hansson O (2015) CSF biomarkers and clinical progression of Parkinson disease. Neurology 84, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230]. Kharel S, Ojha R, Bist A, Joshi SP, Rauniyar R, Yadav JK (2022) Salivary alpha-synuclein as a potential fluid biomarker in Parkinson’s disease: A systematic review and meta-analysis. Aging Med 5, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231]. Vivacqua G, Mason M, De Bartolo MI, Węgrzynowicz M, Calò L, Belvisi D, Suppa A, Fabbrini G, Berardelli A, Spillantini MG (2022) Salivary α-Synuclein RT-QuIC correlates with disease severity in de novo Parkinson’s disease. Mov Disord 38, 153–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232]. Foltynie T, Gandhi S, Gonzalez-Robles C, Zeissler ML, Mills G, Barker R, Carpenter J, Schrag A, Schapira A, Bandmann O, Mullin S, Duffen J, McFarthing K, Chataway J, Parmar M, Carroll C; EJS ACT-PD Consortium (2023) Towards a multi-arm multi-stage platform trial of disease modifying approaches in Parkinson’s disease. Brain 146, 2717–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233]. James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, Ritchie AW, Parker CC, Russell JM, Attard G, de Bono J, Cross W, Jones RJ, Thalmann G, Amos C, Matheson D, Millman R, Alzouebi M, Beesley S, Birtle AJ, Brock S, Cathomas R, Chakraborti P, Chowdhury S, Cook A, Elliott T, Gale J, Gibbs S, Graham JD, Hetherington J, Hughes R, Laing R, McKinna F, McLaren DB, O’Sullivan JM, Parikh O, Peedell C, Protheroe A, Robinson AJ, Srihari N, Srinivasan R, Staffurth J, Sundar S, Tolan S, Tsang D, Wagstaff J, Parmar MK; STAMPEDE investigators (2016) Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387, 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234]. U.S. Department of Health and Human Services Food and Drug Administration (2022) Patient-Focused Drug Development: Selecting, Developing, or Modifying Fit-for-Purpose Clinical Outcome Assessments.
- [235]. European Medicines Agency, Guideline on clinical investigation of medicinal products in the treatment of Parkinson’s disease (Revision 2), last updated July 6, 2012.
- [236]. Mikolaizak AS, Rochester L, Maetzler W, Sharrack B, Demeyer H, Mazzà C, Caulfield B, Garcia-Aymerich J, Vereijken B, Arnera V, Miller R, Piraino P, Ammour N, Gordon MF, Troosters T, Yarnall AJ, Alcock L, Gaßner H, Winkler J, Klucken J, Schlenstedt C, Watz H, Kirsten AM, Vogiatzis I, Chynkiamis N, Hume E, Megaritis D, Nieuwboer A, Ginis P, Buckley E, Brittain G, Comi G, Leocani L, Helbostad JL, Johnsen LG, Taraldsen K, Blain H, Driss V, Frei A, Puhan MA, Polhemus A, Bosch de Basea M, Gimeno E, Hopkinson NS, Buttery SC, Hausdorff JM, Mirelman A, Evers J, Neatrour I, Singleton D, Schwickert L, Becker C, Jansen CP; clinical validation study (WP4) on behalf of Mobilise-D consortium (2022) Connecting real-world digital mobility assessment to clinical outcomes for regulatory and clinical endorsement – the Mobilise-D study protocol, PLoS One 17, e0269615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237]. O’Hanlon CE, Farmer CM, Ryan J, Ernecoff N (2023) Clinical Outcome Assessments and Digital Health Technologies Supporting Clinical Trial Endpoints in Early Parkinson’s Disease: Roundtable Proceedings and Roadmap for Research RAND Corporation, Santa Monica, CA. https://www.rand.org/pubs/conf_proceedings/CFA2550-1.html [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.