Abstract
This article summarizes the status of environmental surveillance (ES) used by the Global Polio Eradication Initiative, provides the rationale for ES, gives examples of ES methods and findings, and summarizes how these data are used to achieve poliovirus eradication. ES complements clinical acute flaccid paralysis (AFP) surveillance for possible polio cases. ES detects poliovirus circulation in environmental sewage and is used to monitor transmission in communities. If detected, the genetic sequences of polioviruses isolated from ES are compared with those of isolates from clinical cases to evaluate the relationships among viruses. To evaluate poliovirus transmission, ES programs must be developed in a manner that is sensitive, with sufficiently frequent sampling, appropriate isolation methods, and specifically targeted sampling sites in locations at highest risk for poliovirus transmission. After poliovirus ceased to be detected in human cases, ES documented the absence of endemic WPV transmission and detected imported WPV. ES provides valuable information, particularly in high-density populations where AFP surveillance is of poor quality, persistent virus circulation is suspected, or frequent virus reintroduction is perceived. Given the benefits of ES, GPEI plans to continue and expand ES as part of its strategic plan and as a supplement to AFP surveillance.
Keywords: Polioviruses, surveillance, environmental sewage, environmental monitoring, disease eradication
Since the launch of the Global Polio Eradication Initiative (GPEI) in 1988, remarkable progress has been made: the number of annual polio cases has decreased by >99%, and only 3 countries remain reservoirs of endemic wild poliovirus (WPV) in 2014. One of the cornerstones of polio eradication efforts is sensitive clinical surveillance for suspected polio cases, or acute flaccid paralysis (AFP) cases, with clinical confirmation of WPV infection by analysis of stool specimens. The main objective of environmental surveillance (ES) for polioviruses is to complement disease-based AFP surveillance for suspected polio cases [1]. Sampling of environmental sewage or wastewater for programmatically relevant polioviruses has been used for many years to supplement AFP surveillance in many countries [2]. ES has played a key role in documenting the elimination of WPV from Egypt and India [3, 4]. ES continues to play an important role in the eradication of WPV from the remaining polio-endemic countries of Pakistan, Afghanistan, and Nigeria. ES can assist in identifying residual WPV transmission, such as where WPV circulates among those who are asymptomatically infected and not showing clinical signs of paralysis. WPV transmission has been documented in Israel, where the ES system has been used since 1988 as an early warning system to detect imported WPV [5–7]. Similarly, ES can also assist in the detection of vaccine-derived polioviruses (VDPVs); such strains, although rare, arise from multiple genetic reversions and sustained transmission over time of the attenuated polioviruses found in oral poliovirus vaccine (OPV), especially in areas with suboptimal vaccine coverage [8].
This article reviews the current uses of ES in the GPEI, providing examples from the polio-endemic countries of Pakistan and Nigeria, in which ES played an important role in polio eradication efforts during 2012–2013. In India, sampling of sewage helped to characterize the epidemiology of poliovirus circulation, as previously described [2,4].In addition, we outline the strengths and limitations of ES and describe how the GPEI plans to continue using ES as a part of its strategic plan (2013–2018) [9].
RATIONALE FOR ES
The examination of stool samples from patients identified through AFP surveillance and subsequent isolation of poliovirus from the stool permits a focused investigation of polio cases and forms the basis for further investigations of the community-level risk of poliovirus transmission [10]. AFP surveillance is the gold standard for the GPEI [11]. In contrast, the examination of composite human fecal samples through ES links poliovirus isolates from unknown individuals to populations served by the sewage or wastewater system. The rationale for ES is based on the characteristic poliovirus excretion pattern. Infected individuals excrete poliovirus in feces for periods of up to several weeks, whether or not they are symptomatic. Large numbers of excreted poliovirus particles remain infectious in the environment for varying durations, depending on the immediate conditions of the wastewater. Accordingly, ES can provide valuable information, particularly in high-density urban populations where AFP surveillance is absent or of poor quality, persistent virus circulation is suspected, or frequent virus reintroduction is perceived.
ES PROGRAMS
While the goal of AFP surveillance is to detect and investigate all AFP cases in children <15 years of age, using stool sample collection and processing in an accredited polio laboratory for virus isolation and characterization by molecular methods, the goal of ES is to detect poliovirus circulation by means of sewage sampling. Polio laboratories have implemented ES programs in several key countries, namely Egypt, Israel, Pakistan, India, and Nigeria. In all countries that use ES, both WPV and VDPV isolates are detected from environmental sewage sampling after concentration of so-called grabbed samples, using either the 2-phase method (in Pakistan, Egypt, and Nigeria) or the precipitation method (in India), and virus isolation on permissive cells [1]. These polioviruses are sequenced, similar to poliovirus isolates from AFP cases, to characterize their genetic relationship to polioviruses isolated from other environmental samples and from AFP cases. The combination of AFP and ES data are then used to identify areas of increased risk and to specifically target vaccination strategies to better reach susceptible populations. Both Pakistan and Nigeria, 2 of 3 remaining polio-endemic countries, but also Israel, Egypt, and India, which are now polio free, have used ES as a tool to detect WPV transmission and complement AFP surveillance. In these countries, ES is undertaken in large population centers and other strategic locations where the risk of poliovirus circulation is highest.
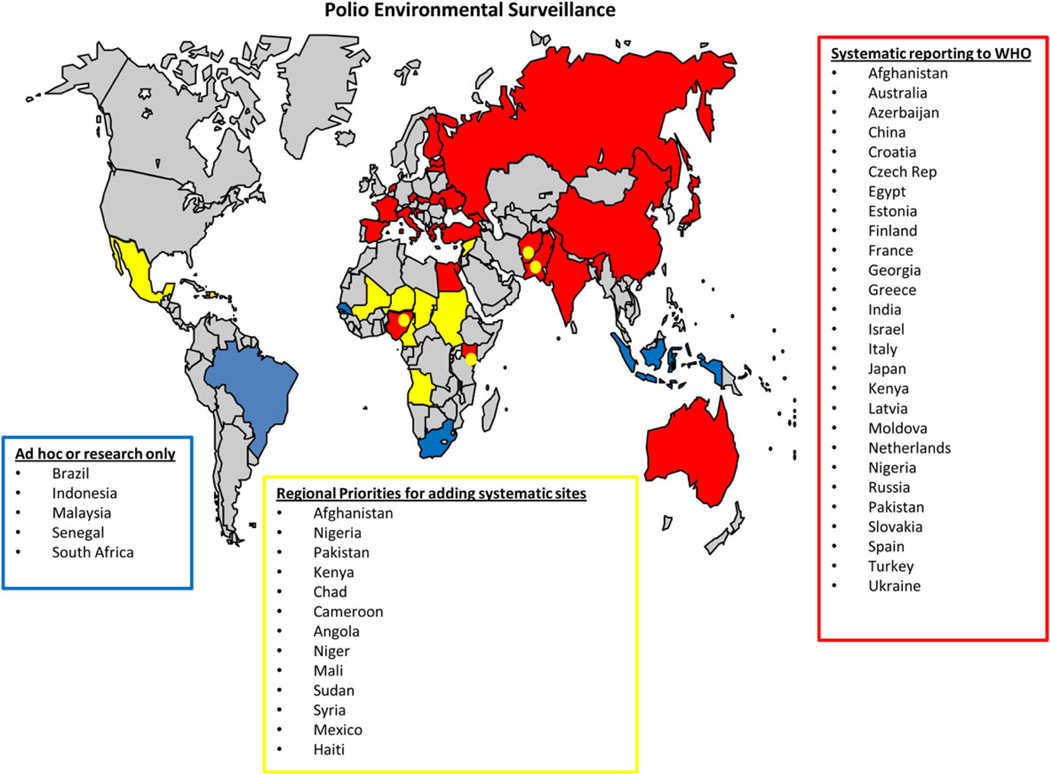
Numerous other non–polio-endemic countries have performed ES for enteroviruses, including WPV and VDPVs, or have conducted research studies, including environmental sampling, for detection of polioviruses (Figure 1). Details on the scope and regularity of ES programs vary by country, and these are reported separately to each World Health Organization regional office for their consideration in monitoring the risk of WPVs.
Figure 1.
Countries that have undertaken environmental surveillance or sampling projects for polioviruses. Data are from the Global Polio Eradication Initiative.
Pakistan
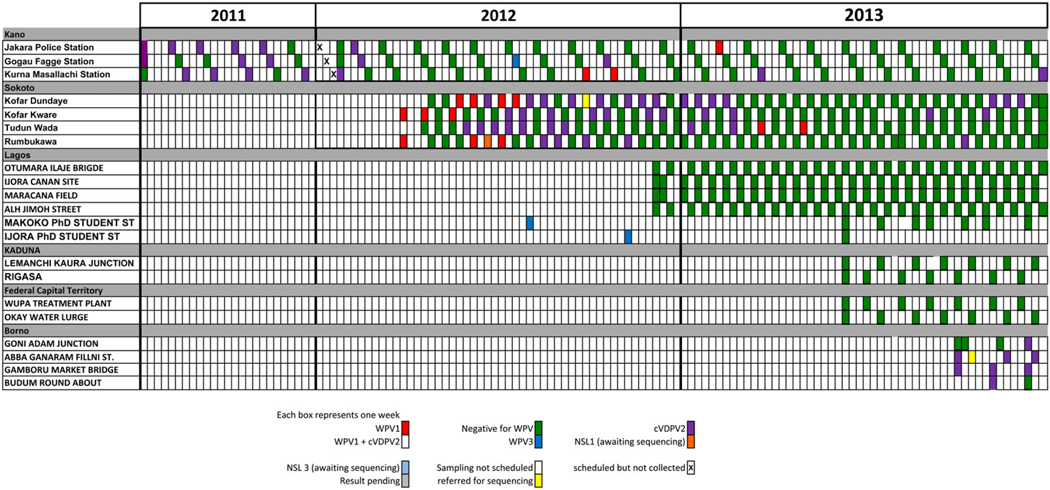
In Pakistan, WPV transmission during 2009–2012 has occurred in conflict-affected and inaccessible areas along the border with Afghanistan. The number of districts affected by WPV has remained largely unchanged from 2009 (17 districts) to 2012 (19 districts), and these districts are located in the northern transmission zone (northwestern Khyber Pakhtunkhwa province and the Federally Administered Tribal Areas, bordering eastern Afghanistan), and in the southern transmission zone (Balochistan, bordering southern Afghanistan). In Pakistan, ES started in Karachi (Sindh province) and Lahore (Punjab) during 2009 and was extended to the other major cities in all 4 provinces (Quetta, in Balochistan; Multan, Rawalpindi, and Faisalabad, in Punjab; and Peshawar, in Khyber Pakhtunkhwa), thereby increasing the number of ES sampling sites from 17 in 2011 to 23 in 2012. The additional sites were in locations where there were concerns regarding gaps in AFP surveillance. The criteria that were considered in selection of the potential sampling sites were (1) sustained confirmation over multiple years of polio cases in a district; (2) high risk for sustained transmission of poliovirus, based on population characteristics, density, immunization coverage, and sanitation; and (3) access for suitable collection of sewage samples. An example of the documented results of ES, by year, city, and sampling site, is shown in Figure 2.
Figure 2.
Weekly results of environmental surveillance in Pakistan, by city and sampling site, 2011–2013. Abbreviations: VDPV, Vaccine-derived poliovirus; WPV, wild poliovirus; WPV-1, wild poliovirus type 1.
Since implementation of ES in Pakistan in 2009, WPV has been isolated frequently from sewage samples collected from all major sites in Pakistan, even in the absence of confirmed WPV cases detected through AFP surveillance. WPV type 1 (WPV1) has been found in all except one sampling site of Karachi, while WPV3 was last detected in mid 2010. Samples from sites in some areas (eg, Sindh and Punjab provinces) have consistently yielded WPV isolates in the absence of WPV-positive AFP cases from the same area, thereby enabling a focus on preventive action in these areas because of the detected circulation in locations where either no AFP cases have yet occurred or are cases have been underreported. Over time, WPV detection at ES sites has shifted to demonstrate widespread circulation to a more focal distribution of positive sites. Genetic sequencing of isolates from both AFP cases and sewage samples has been used to continually characterize epidemiological transmission links and focus response efforts and resources appropriately, such as through supplementary immunization campaigns and/or by enhancing active AFP surveillance. ES has given an enhanced picture of virus circulation and transmission within the provinces and borders of Pakistan and Afghanistan. ES has yielded important information about the circulation of WPV in Pakistan and is allowing the targeting of polio eradication efforts on the reservoir communities within specific locations in Karachi, Lahore, Multan, Peshawar, and Quetta. For example, in Karachi, detection of WPV in environmental samples linked to AFP cases in Khyber Pakhtunkhwa helped document the transmission routes among Pashtun populations who travel between and reside in both locations and the importance of increased vaccination efforts in those mobile communities. In some instances, the early detection of WPV prior to AFP detection has demonstrated its significance and usefulness. For example, WPV was found in Lahore in the absence of AFP cases, which allowed the program to continue the inclusion of vaccination efforts to stop silent WPV transmission within Lahore and prevent spread throughout the province.
In Pakistan, future plans for ES include expansion of the program to include samples from insecure and/or inaccessible areas and areas that may harbor highly mobile populations (eg, Bajour, Khyber agency). In addition, in 2012, 2 type 2 VDPVs were isolated in Karachi through ES, which were subsequently genetically linked to circulating VDPV isolates from AFP cases in Balochistan; this linkage and tracing of VDPV pathways demonstrate the importance of ES for activities during and following WPV eradication, as the risk of VDPV is reduced following the switch from trivalent OPV (tOPV) to bivalent OPV (bOPV), tentatively planned for 2016.
Nigeria
From 2010 through 2013, WPV transmission has remained endemic in northern Nigerian states, with Kano state in northern Nigeria considered an epicenter of polio in Nigeria [12]. Transmission of WPV in 2012 in Nigeria has predominantly involved WPV1, and no WPV3 has been detected in 2013. Besides Kano, the states of Sokoto, Borno, Yobe, Kaduna, and Bauchi have been at higher risk for continued WPV transmission than other northern states. In March 2010, the Nigerian Expert Review Committee on Polio Eradication and Routine Immunization recommended the comprehensive assessment of the potential for ES to supplement AFP surveillance in key areas. In July 2011, ES was implemented at 3 sites in Kano. In March 2012, the expert review committee recommended expansion of ES to a second high-risk state; accordingly, ES began in 4 sites in Sokoto state in northwest Nigeria. In November 2012, an academic research project reported the isolation of WPV3 in ES samples in the Makoko area of Lagos. After this identification, ES was also formalized at 4 sites in Lagos.
In Kano State, 4 criteria were used by a technical assessment team to select the sampling sites in relation to the local governmental areas (LGAs) in Kano state: (1) whether the LGAwas classified as high risk for poliovirus transmission, based on existing data (ie, population density, high-risk population, sanitation, living conditions, routine immunization, and SIA coverage); (2) the presence of a sewage disposal channel that receives waste from a substantial proportion of the population in the catchment area, with a minimum amount of waste coming from other areas; (3) the absence of industrial waste in the proposed site or canal; and (4) access to this sewage to take a sample. The technical assessment team determined that 8 high-risk LGAs in Kano state were suitable for ES. Prior to this decision, information on overall sanitation for Kano state was obtained from the State Ministry of Environment and from the Ministry of Health.
During this feasibility assessment, it was documented that there were no organized/functioning sewage wastewater disposal/treatment systems in Kano state; however, there were remnants of 5 nonfunctional sewage-pumping stations. The Kano state population uses various methods of sewage drainage, namely (1) a soak away system, in which sewage is directly absorbed by the soil; (2) drainage of waste through a pipe into the main open sewage channel; (3) direct disposal of waste into the open sewage channel; and (4) open disposal of fecal material in general public landfills. Despite the various methods of waste disposal identified, it was observed that around 60% of population waste is through the open sewage channel, thus making it possible to collect multiple good-quality samples for the purpose of environmental sampling.
There was one major sewage channel, referred to as Jakara, that passes through the city and is the sole drainage system for the identified high-risk LGAs. The drainage is mainly through open drains or through pipes into this collective water channel. However, it does not have a smooth flow because of waste material (eg, shopping bags, dead animals, and cloth) thrown into it, and, at places, it is totally or partially blocked and wastewater flows below or overflows from the side of blockade. The channel has no concrete walls and varies in width, and it narrows while passing through densely populated areas. A member of the technical team was tasked to observe the early morning flow of sewage in the main channel and noted that maximum flow of sewage water occurred between 6:00 AM and 7:00 AM. For the purpose of planning, it was concluded that the best time for collection of samples needed to be between 6:00 AM and 7:00 AM. The technical team inspected the main sewage channel in Kano state. The Jakara channel receives sewage via smaller and medium-sized sewage channels that drain residential areas of the various LGAs in the state. Potential sampling sites were identified at the junctions between the Jakara channel and the smaller/medium-sized channels, based on the established criteria.
On the basis of the feasibility assessment and the conclusion of the technical team, as well as available data/field visits, risk factors, and epidemiology, 3 potential sites encompassing different catchment areas were identified for the initial implementation of ES in Kano state: (1) Jakara Police Station, (2) Kurna Masallachi, and (3) Gogau Fagge. Each of these sites are linked to the Jakara channel; however, each serves a different area and/or population as the Jakara channel does not have continuous flow throughout it. In Sokoto state, similar methods and protocols were used to identify 4 sample collection sites in the Sokoto metropolis. The selected sites were (1) Kofar Dundaye, (2) Kofar Kware, (3) Tudun Wada, and (4) Rumbukawa. Three of these sites (Kofar Dundaye, Kofar Kware, and Rumbukawa) are located in Sokoto North LGA, and 1 site (Tudun Wada) is in Sokoto South LGA. Both of these LGAs are known high-risk areas for poliovirus circulation. The results of ES at sampling sites in each city during 2011–2013 are summarized in Figure 3 and in the supplementary data.
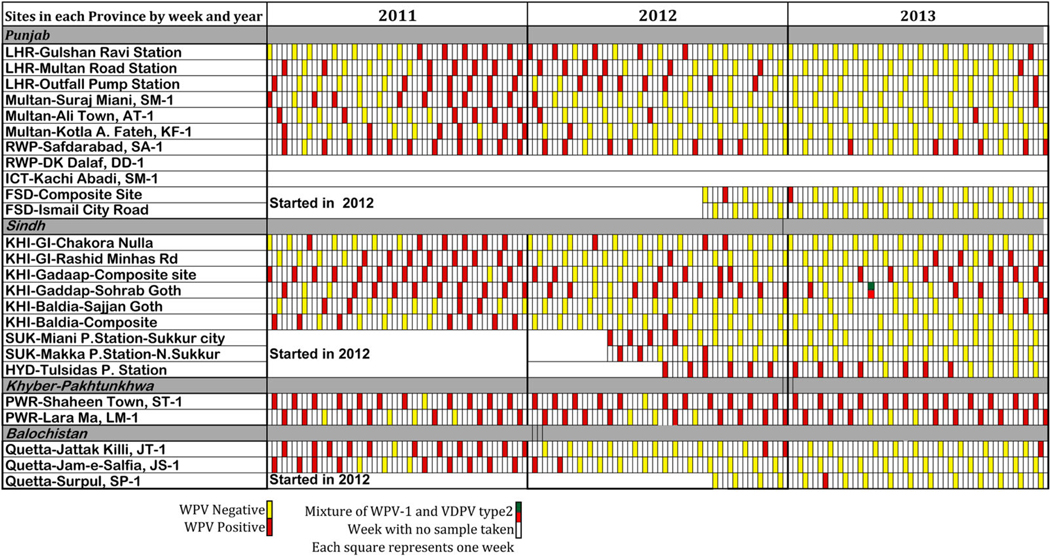
Figure 3.
Weekly results of environmental surveillance in Nigeria, by city and sampling site, 2011–2013. Abbreviations: cVDPV2, circulating vaccine-derived poliovirus type 2; NSL, non-Sabin-like virus; WPV1, wild poliovirus type 1; WPV3, wild poliovirus type 3.
In Nigeria, although AFP surveillance targets continue to be met in all states, virological data have indicated that AFP surveillance alone has not been sufficiently sensitive to detect all chains of WPV transmission in a timely manner. The detection of so-called orphan viruses (defined as polioviruses in which nucleotides at ≥1.5% of positions differ from those of known poliovirus) in ES indicates undetected poliovirus transmission, thus supporting the need to improve surveillance sensitivity. Although ES in Nigeria is not geographically representative, results are consistent with the serotype distribution in AFP cases, as WPV1 has been more frequently detected than WPV3. The ES findings during 2012 have reemphasized that the focus on high-risk areas, as described in the Nigeria National Polio Emergency Plan, must be coupled with continued focus on migrant and mobile communities to reduce the risk of poliovirus movement at the same time as immunity gaps are being closed in reservoir communities.
Finally, in Nigeria, AFP surveillance data have shown that circulating VDPV2 transmission was very low during 2012. However, the findings of ES identified type 2 VDPVs, indicating the existence of population groups with profound immunity gaps to type 2 poliovirus who are excreting the virus in the environment. Therefore, the data from ES in Nigeria demonstrate that in addition to use of tOPV in national polio campaigns, an aggressive strategy needs to be adopted to mop up circulating VDPVs in areas where these strains have been detected in cases, contacts, or the environment.
India
In India, sampling of sewage helped to characterize the epidemiology of poliovirus circulation in settings in which vaccination coverage was high yet paralytic polio cases continued to occur. Much of this development has been previously described [2, 4]. ES of sewage was initiated in Mumbai in 2001. Sampling sites were considered on the basis of the number of paralytic poliomyelitis cases reported annually for the past 10 years, poor environmental sanitation, high population density, and availability of sewage sampling sites representing large populations. Accordingly, 3 sewage sampling sites in municipal wards (F, G, and M) of Mumbai representing large urban slum populations were selected. In the Wadala pumping station, in ward F, samples were collected from a closed drainage system, and samples from the other 2 sites (the Dharava slum in ward G and the Shivaji Nagar slum in ward M) were collected from large open drains (ie, nullah). A 1 liter (L) grab sample was collected between 9:30 and 11:30 AM every week from the 3 sites. Virus from 1 L of sewage samples was concentrated by the PEG6000 precipitation and centrifugation method, which yielded a 200-fold concentration [4].The sampling sites and the sewage sample processing methods have remained unchanged over the past 13 years. WPV1 and WPV3 were isolated from sewage samples from 2001 until 2009. The frequency of each poliovirus serotype isolated in sewage samples in Mumbai was correlated with the endemicity of the serotypes isolated from AFP cases in Uttar Pradesh and Bihar, the 2 states in India where poliovirus eradication was delayed and the most challenging. Phylogenetic analysis of complete VP1 sequences of viruses isolated from sewage samples in Mumbai and AFP cases in the country showed that viruses are exchanged between the commercial capital of India (Mumbai) and the other states very rapidly, within 2 weeks. On at least a few occasions, WPVs of the same genetic lineage were isolated from sewage samples before the detection of a paralytic case through AFP surveillance. A gradual decline in isolation frequency and, finally, complete absence of WPV in sewage samples was observed from 2008 onward. In Mumbai, the last WPV1 strain was detected in sewage samples in November 2010, and the last WPV3 strain was recovered in December 2009 (Figure 4).
Figure 4.
Weekly results of environmental surveillance in India, by city and sampling site, 2010–2011.
ES of sewage was initiated in Delhi in 2010 (Figure 4). Seven sites were identified by use of criteria similar to those used in Mumbai. Samples are collected at 2-week intervals and processed at the National Centre for Disease Control (NCDC) in Delhi exactly as done in Mumbai. WPV1 and WPV3 were detected in sewage samples in Delhi in 2010. The last detection of WPV1 and WPV3 strains in sewage samples in Delhi occurred in August 2010 and July 2010, respectively (Figure 4). The last WPV1 strain detected In West Bengal, India, was genetically linked with virus detected in a sewage sample in Delhi. ES showed that large metropolitan areas are constantly at risk of reintroduction of WPVs from all poliovirus-endemic sites in the country and may also serve to redistribute the viruses, as large numbers of people from all over the country travel to these cities regularly for various purposes, including employment and tourism. Environmental surveillance was thus initiated in Kolkata, West Bengal (in 2010), and in Patna, Bihar (in 2011). No WPV transmission was detected in ES samples from these 2 sites; however, VDPVs were isolated. Absence of WPV in environmental samples in 4 high-risk cities since 2011 has tremendously increased the confidence of the polio eradication program regarding the absence of transmission of indigenous WPV in India. Early detection of WPV importation, if any, will be the key to stopping the spread of WPV importation should it occur. Therefore, in 2013 ES was started in the State of Punjab, which borders Pakistan. Further expansion of ES in 2014 will cover cities in the State of Gujarat, which also borders Pakistan. Samples from Kolkata and Patna are processed at the Institute of Serology, Kolkata, and Patna Medical College, Patna, respectively, until virus is concentrated, and virus isolation and characterization are performed at the Enterovirus Research Centre (ERC)–Mumbai. ES samples from Delhi are processed for virus isolation at NCDC-Delhi, and sequencing is performed at ERC-Mumbai.
In summary, ES helped to detect poliovirus in the final stages of eradication, with poliovirus no longer considered to be endemic in India in 2012, and continues to provide important information documenting the absence of WPVs in sewage, in conjunction with the absence of ongoing WPV cases detected through AFP surveillance.
WPV Transmission in Egypt and Israel
Systematic ES for poliovirus circulation has been conducted in Egypt since 2000, during which surveillance has revealed (1) WPVs in sewage, 1 year after cessation of detection in AFP cases in 2004; (2) 4 independent importations of WPV; and (3) several VDPVs, which have been detected in various locations.
In Egypt in December 2012, WPV1 was isolated from 2 environmental sites in Cairo, although no WPV-confirmed AFP cases were detected, and no additional samples collected subsequently were found to be positive following enhanced sampling and immunization response activities. The WPV1 strain in Cairo was genetically linked to WPV circulating in Sindh province, Pakistan, during 2012. Response vaccination campaigns were implemented in high-risk areas in Cairo, and ES at existing sites was doubled in frequency.
In 2013 in Israel and the West Bank, a related strain of WPV was detected that triggered vaccination responses. Similarly to Egypt, no WPV-confirmed AFP cases were detected [5–7]. However, in late 2013 through early 2014, WPV related to the virus circulating in Israel, the West Bank, and Egypt was detected in AFP cases in Syria (36 cases) and subsequently in Iraq (1 case), thereby leading to further outbreak response efforts throughout these and neighboring Middle Eastern countries.
GPEI 2013–2018 STRATEGIC PLAN
ES plays a role in the GPEI 2013–2018 strategic plan. ES will be geographically expanded to help identify any residual transmission in poliovirus-endemic areas, to provide early indication of new importations into recurrently reinfected areas, and to document the elimination of Sabin viruses following the tOPV-bOPV switch and eventual cessation of tOPV use. Whereas outbreak response activities have historically been driven by isolation of a poliovirus from a paralyzed child, environmental data will also be used more systematically to guide outbreak response planning and implementation. For poliovirus-endemic and other high-risk areas, the detection of a positive environmental sample will help to guide the geographic extent and duration of a response. In previously polio-free areas, the detection of a positive environmental sample will trigger both a virologic and epidemiologic investigation to guide heightened surveillance and, if appropriate, an immunization response. As they become available, new technologies or methods that simplify the time-consuming and labor-intensive nature of collection, processing, and testing of samples could be introduced.
ES EXPANSION AND ASSESSMENT OF SITES
Afghanistan is the only remaining polio-endemic country without established ES, so plans were initiated to begin ES in southern Afghanistan, in Kandahar and Helmand provinces. In 2013, initial site assessments began, with proposals for ES samples to be collected by the Ministry of Health and Ministry of Environment and tested at the Polio Regional Reference Laboratory in Islamabad, Pakistan. Elsewhere, further evaluation of potential sites in countries at highest risk of importation and outbreaks and to supplement AFP surveillance where gaps may exist is of interest to the GPEI. For example, some countries at high risk of importation and recent outbreaks include Kenya, Somalia, and those bordering Nigeria in West and Central Africa.
SUMMARY
As described here, the ES complements AFP surveillance, allowing linkages to characterize continued circulation and identify remaining WPV reservoirs. However, ES has potential limitations for which further research may be needed to overcome. One concern is the potential inability to differentiate between an environmental sample that tests negative for polioviruses, indicating the lack of circulation, and an environmental sample that tests negative for other reasons, such as improper collection site or methods, inadequate storage, low sensitivity of laboratory methods used for concentration and identification, or inaccurate characterization of the target population, either in terms of accurately targeting infected individuals or characterizing the size of the population at risk within the catchment area. In addition, unless closely related viruses (ie, nonpoliovirus enteroviruses) are continually detected in a given area, ES can only confirm the presence of poliovirus at a point in time, not necessarily continuous circulation (ie, transient presence vs actual circulation in the catchment area).
Although genetic sequencing may allow the ability to trace the origin of polioviruses to other circulating polioviruses with similar sequences, the inability to link environmental samples with infected persons may limit the ability to elucidate epidemiological linkages and to appropriately target vaccination strategies. Resource requirements for sample collection and laboratory isolation may require a balanced investment in ES versus case-based AFP surveillance. As the goal of global eradication approaches, the potential expansion of ES may increase the sensitivity of overall poliovirus detection in the final reservoirs of WPV and help document eradication [6]. For certification of polio eradication, the continuation of high-quality ES in existing sampling sites will also be an important consideration for global certification of a polio-free world [6].
In the future, after certification, ES data may give insights on the elimination of Sabin viruses following OPV cessation [6]. ES can be used to detect ongoing circulation of type-specific vaccine-related polioviruses to monitor these changes. Surveillance of VDPV emergence and monitoring of cessation of vaccine virus circulation is of high importance during the eradication endgame. In this context, appropriate environmental sites should be established in areas that switch from tOPV to bOPV and after the cessation of all OPV use.
In conclusion, ES has been a valuable tool within polio-endemic countries during current eradication efforts, as well as a method to potentially provide early detection of new importations or VDPV transmission. Both ES and AFP case-based surveillance have proven to be useful and informative tools to guide the final stage of polio eradication efforts.
Acknowledgments.
We thank the following for their efforts in undertaking ES: ministries of health and ministries of the environment in Nigeria, Pakistan, Egypt, and India; and polio eradication partners and implementers, including the National Polio Laboratory (Ibadan, Nigeria), VACSERA (Cairo, Egypt), WHO program offices (in Nigeria, Pakistan, Egypt, and India), the National Institute of Health (Islamabad, Pakistan), and the Enterovirus Research Center (India).
Financial support.
This work was supported by the Global Polio Eradication Initiative.
Footnotes
Supplement sponsorship. This article is part of a supplement entitled “The Final Phase of Polio Eradication and Endgame Strategies for the Post-Eradication Era,” which was sponsored by the Centers for Disease Control and Prevention.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.WHO. Guidelines for environmental surveillance of poliovirus circulation: World Health Organization. Geneva: Department of Vaccines and Biologicals, WHO, 2003. [Google Scholar]
- 2.Hovi T, Shulman LM, Van Der Avoort H, Deshpande J, Roivainen M, De Gourville EM. Role of environmental poliovirus surveillance in global polio eradication and beyond. Epid Infect 2012; 140:1–13. [DOI] [PubMed] [Google Scholar]
- 3.El Bassioni L, Barakat I, Nasr E, et al. Prolonged detection of indigenous wild polioviruses in Sewage from communities in egypt. Am J Epid 2003; 158:807–15. [DOI] [PubMed] [Google Scholar]
- 4.Deshpand JM, Shett SJ, Siddiqu ZA Environmental surveillance system to track wild poliovirus transmission. Appl Environ Microbiol 2003; 69:2919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anis E, Kopel E, Singer SR, et al. Insidious reintroduction of wild poliovirus into Israel, 2013. Euro Surveill 2013; 18:pii=20586. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20586. Accessed 21 August 2014. [DOI] [PubMed]
- 6.Manor Y, Shulman LM, Kaliner E, et al. Intensified environmental surveillance supporting the response to wild poliovirus type 1 silent circulation in Israel, 2013. Euro Surveill 2014; 19:pii=20708. http://www.urosurveillance.org/ViewArticle.aspx?ArticleId=20708. [DOI] [PubMed] [Google Scholar]
- 7.Shulman LM, Gavrilin E, Jorba J, et al. for the Genotype–Phenotype Identification (GPI) Group. Molecular epidemiology of silent introduction and sustained transmission of wild poliovirus type 1, Israel, 2013. Euro Surveill 2014; 19:pii=20709. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20709. Accessed 21 August 2014. [DOI] [PubMed] [Google Scholar]
- 8.Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy of global polio eradication. Ann Rev Microbiol 2005; 59:587–635. [DOI] [PubMed] [Google Scholar]
- 9.Global Polio Eradication Initiative. Polio Eradication and Endgame Strategic Plan (2013–2018). http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/EndGameStratPlan_WHAversion.pdf. Accessed 21 August 2014.
- 10.WHO. Polio laboratory manual. WHO/IVB/0410. Geneva: WHO, 2004. [Google Scholar]
- 11.WHO. Acute flaccid paralysis (AFP) surveillance: the surveillance strategy for poliomyelitis eradication. Wkly Epidemiol Rec 1998; 73:113–4. [Google Scholar]
- 12.CDC. Progress toward poliomyelitis eradication—Nigeria, January 2012–September 2013. MMWR Morb Mortal Wkly Rep 2013; 62:1009–13. [PMC free article] [PubMed] [Google Scholar]