Abstract

SARS-CoV-2 strains have made an appearance across the globe, causing over 757 million cases and over 6.85 million deaths at the time of writing. The emergence of these variants shows the amplitude of genetic variation to which the wild-type strains have been subjected. The rise of the different SARS-CoV-2 variants resulting from such genetic modification has significantly affected COVD-19’s major impact on proliferation, virulence, and clinics. With the emergence of the variants of concern, the spike protein has been identified as a possible therapeutic target due to its critical role in binding to human cells and pathogenesis. These mutations could be linked to functional heterogeneity and use a different infection strategy. For example, the Omicron variant’s multiple mutations should be carefully examined, as they represent one of the most widely spread strains and hint to us that there may be more genetic changes in the virus. As a result, we applied a common protocol where we reconstructed SARS-CoV-2 variants of concern and performed molecular dynamics simulations to study the stability of the ACE2–RBD complex in each variant. We also carried out free energy calculations to compare the binding and biophysical properties of the different SARS-CoV-2 variants when they interact with ACE2. Therefore, we were able to obtain consistent results and uncover new crucial residues that were essential for preserving a balance between maintaining a high affinity for ACE2 and the capacity to evade RBD-targeted antibodies. Our detailed structural analysis showed that SARS-CoV-2 variants of concern show a higher affinity for ACE2 compared to the Wuhan strain. Additionally, residues K417N and E484K/A might play a crucial role in antibody evasion, whereas Q498R and N501Y are specifically mutated to strengthen RBD affinity to ACE2 and, thereby, increase the viral effect of the COVID-19 virus.
1. Introduction
SARS-CoV-2’s high transmissibility and mutation rates, combined with a lack of robust preexisting immunity in hosts and a slow rate of immunization through vaccinations, have caused COVID-19 cases to surge to over 572 million worldwide by July 2022, as reported by the WHO.1 Although numerous antibodies have been shown to neutralize the wild-type (WT) virus or the Wuhan strain, their efficacy against developing variations, particularly those that have been shown to avoid the host immune response and develop an antibody escape mechanism, should be closely studied.2−5
The spike or S protein of the SARS-CoV-2 virus facilitates viral entrance into the cell.6−8 Viral entrance occurs when the spike protein binds to receptors on the host cell, causing the cell membrane to fuse. The S protein is a structural polypeptide with two subunits, S1 and S2, that engage with the ACE2 receptors and fuse the viral and host cell membranes.9 Moreover, the homo-trimeric spike glycoprotein (residues 1–1273) (Figure 1) has two structural states: active (up) and inactive (down), and it is highly conserved across all human coronaviruses.10 The S protein has been the main focus of several studies since it is the key protein for the virus’s attachment to the cell host.11 More precisely, most of the studies have focused on the receptor binding domain (RBD; residues 331–524) of the SARS-CoV-2 S protein because of its function in binding the angiotensin-converting enzyme 2 (ACE2) receptor (Figure 1) and immune system recognition.12−16 Moreover, the RBD (Figure 1) contains the receptor binding motif (RBM) region (residues 437–508), which is responsible for maintaining contact with the ACE2 protein.8 It is also important to note that treatments against the SARS-CoV-2 virus are developing around the RBD since it is the most crucial protein in the viral process.17−21 The RBD region is also the most affected part of the virus, where several emerging mutations occurred in different lineages.17−21 Due to the RBD’s variability and its fast mutation rate, most of the treatments are focused on targeting the RBD with numerous antibodies issued from COVID-19 patients or other antibody therapies under investigation.22−24 With all this information, we focused on analyzing the RBD to decipher the mutation mechanism and also give more insight into future mutation effects.
Figure 1.
Representation of the interaction between the ACE2 human receptor and the SARS-CoV-2 spike protein. The left panel shows a surface representation of the ACE2 human receptor (upper panel) in a transversal view and the trimeric structure of the SARS-CoV-2 spike protein in a top view (middle panel) and transversal view (lower panel). The middle panel offers a more detailed cartoon representation of the complex formed by the RBD (in blue) and the distal region of ACE2 (in red). The right panel shows the most recurrent mutations in the RBM region. The most recurrent mutations in the five variants of concern are labeled with a red star.
Many COVID-19 variants and sub-variants of concern are currently under investigation. However, five variants of concern (Alpha, Beta, Gamma, Delta, and Omicron) have been classified as dangerous and are evolving rapidly all over the world by the WHO.1 Among these, the Alpha variant was first discovered in the United Kingdom in December 2020 and was declared a variant of concern in December 2020.25,26 It differs from the original Wuhan strain due to many significant alterations in the spike protein.27,28 For instance, one is the N501Y mutation located in the RBD region, which increases the virus’s contagiousness by improving the spike protein binding to cellular receptors.28−31 It also has a D614G mutation, which is likely to aid viral replication,32−37 and a P681H mutation,38 whose function is unknown, but which has appeared many times spontaneously. Moreover, the Alpha variant is predicted to be roughly 50% more transmissible than the original Wuhan strain.25 It is also assumed to be linked to worsening illness severity, but this is not clear.39 However, COVID-19 vaccinations and monoclonal antibody therapies have been reported to be still highly effective against it.40−48
The Beta variant was discovered for the first time in South Africa in December 2020.1 In addition to three of the alterations seen in the Alpha variant,24,27,28 The Beta variant has a K417N mutation located in the RBD region, which may enable the virus resistance to neutralize antibodies produced by vaccination or previous infection.49−51 Although it is expected to be 50% more transmissible than prior variants, there is minimal evidence that Beta is linked to more severe diseases including severe gastrointestinal problems, hearing loss, blood clots that cause tissue death, and gangrene.52,53 Reduced neutralization by antibodies generated by vaccination or as a result of a previous infection is the main source of concern. Despite this, recent vaccines appear to provide effective protection against the Beta variant.54,55
The Gamma variant was first discovered in Brazil in January 2021.1 It contains the E484K, N501Y, and D614G mutations, as do some other variants of concern.56−58 It also carries a K417T/N mutation, which is linked to greater binding to human cells, potentially making the virus easier to transmit,59 and a H655Y mutation, whose function is uncertain.60,61 Moreover, according to a recent study published by Faria and co-workers,58 the Gamma variant is 1.7–2.4 times more transmissible than non-variants of concern. However, existing COVID-19 vaccinations appear to be effective at preventing the Gamma variant.54,62
The Delta variant was first reported in India in May 2021. It has since been confirmed in multiple locations throughout the globe,1 quickly displacing other variants to become the dominant variant in several countries. Delta has the D614G mutation as well as a few other mutations not reported in other variants of concern.63 These include a L452R mutation on the RBD, which is expected to enhance infectivity and may help the virus escape immune cell destruction.64−68 The Delta variant contains the T478K mutation also located on the RBD, which is thought to assist the virus avoid immune detection.69−72 Finally, this variant of concern has a P681R mutation, which is linked to an increased ability to cause serious illnesses.73,74 The Delta variant is thought to be 40–60% more transmissible than the Alpha variant and nearly twice as transmissible as the original Wuhan strain.68,75 Although data suggest that vaccines are slightly less efficient in avoiding infection with the Delta form,44,63 they are still quite effective in preventing serious disease.76,77
The first verified Omicron infection was discovered in a sample collected on November 9, 2021.1 The Omicron variant has been authenticated in several areas throughout the world, including sections of North and South America, Europe, Africa, Asia, and Australia.1 Omicron has a lot of mutations, some of which were identified as dangerous.78 Fifteen mutations can be already found on the RBD: G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, and Y505H.79−83 It is unclear whether Omicron is easier to spread from person to person than other variants or if infection with it causes more severe disease. There is currently no evidence that the symptoms associated with Omicron are distinct from those associated with other variations.84,85 The emergence of the BA.2 sub-lineage, on the other hand, has raised concerns because it looks to be more transmissible.86 Being currently the most transmissible variant, three other sub-lineages (BA.3, BA.4, and BA.5) of Omicron have emerged and are suspected to become the most dominant variants.86 The capacity of BA.4 and BA.5 to avoid immune protection brought on by earlier infection and/or vaccination is presumably the cause of their current observed growth advantage, especially if this immunity has weakened over time.87−89 Currently, there is no evidence that the severity of BA.4 and BA.5 will vary in comparison to earlier Omicron lineage infections.
It is also important to note that many of these variants can contain additional mutations not yet confirmed or only detected in a minority of samples. For instance, the WHO is also monitoring the spread of an Alpha variant with an extra E484K mutation, which could enable the virus to get past the body’s immune defenses by evading neutralizing antibodies produced by vaccination or previous infection.90−92 Another example is the finding of the ’Delta plus’ variant, which was first found in Nepal and carries an extra K417N mutation.77,93−95 These observations show how versatile and adaptable the virus is.
Crystallography96−102 and cryo-EM6,103−111 techniques, as well as computational analyses112−117 and antibody-binding assays106,118−127 have been used to study the SARS-CoV-2 variants RBD–ACE2 complexes. Deep scanning mutagenesis128−131 and in vitro evolution5,132−134 have also been used to investigate SARS-CoV-2 variants effects. Several research papers attempted to map out different classes of antibodies and link them to mutation studies.135,136 To our knowledge, a detailed comparative investigation regrouping all variants of concern utilizing structure-based simulations and free energy approaches is still lacking to understand and have a general view on the SARS-CoV-2 mechanism and particularly of its variants of concern.
In this paper, we used the same protocol to reproduce reliable results for a more accurate comparison between all systems (WT, Alpha, Beta, Gamma, Delta, and Omicron). We present results from structure-based in silico modeling and a full-atomic molecular dynamics (MD) simulation protocol of the RBD–ACE2 complex. These analyses were performed to evaluate the binding free energies of the five variants of concern on the RBD (Table 1). Our results also showed the differences in interactions formed with the ACE2 protein to give more insight on the variants’ mechanisms.
Table 1. Variants of Concerns’ Lineages, Date of Emergence, and the Location of Their Mutations on the RBD.
| variants | lineage | date of emergence | mutations on the RBD |
|---|---|---|---|
| Alpha | B.1.1.7 | September 2020 | N501Y |
| Beta | B.1.351 | August 2020 | K417N, E484K, N501Y |
| Gamma | P.1 | December 2020 | K417T/N, E484K, N501Y |
| Delta | B.1.617.2 | October 2020 | L452R, T478K |
| Omicron | B.1.1.529 | November 2021 | G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H |
2. Material and Methods
2.1. Structural Preparation of the Spike Protein Variants
Our study only focuses on WT SARS-CoV-2 RBD with the human ACE2 receptor and its comparison with five different COVID-19 variants of concern (Alpha, Beta, Gamma, Delta, and Omicron) (RBD residues 331–524). The crystal structure of the ACE2–RBD (PDB: 6M0J(97)) was then retrieved from the RCSB Protein Data Bank in PDB format.137 In fact, here, we focused on the ACE2–RBD complex rather than the glycosylated states of the proteins since we are looking to compare their binding affinities and identify the RBM hotspots. We decided to model Alpha, Beta, Gamma, and Delta mutations since no PDB structures were available at the time the analyses were made. As for the Omicron variant, the respective 7T9L cryo-EM structure was taken from the PDB database.138 The Omicron PDB was used in this study since several crystal structures of this variant of concern were available at the time our analyses were conducted. Another reason is that the majority of papers have mostly focused on this variant of concern due to its many mutations located on the RBD. Additionally, to validate our approach and verify the consistency of our results, we also constructed an Omicron variant model containing all of the RBD mutations, which we will compare with the experimental 7T9L Omicron variant structure.
Therefore, using the 6M0J structure, the Alpha, Beta, Gamma, Delta, and Omicron mutations were introduced using the “mutations wizard” in the PyMOL molecular modeling package (Schrodinger LLC). All residue rotamers were chosen according to the most probable side chain orientation suggested by the program and displaying the least amount of steric clashes within the structure. As our study only focused on the RBD–ACE2 interaction, all glycans were removed from the complexes. Afterward, each complex was protonated according to a physiological pH (pH = 7.4) using PROPKA.139
2.2. MD Simulations
MD simulations were performed on six systems (WT, Alpha, Beta, Gamma, Delta, and Omicron) using the GROMACS tool with the 2020 version.140 The CHARMM36m force field141 was selected along with an explicit solvent TIP3P model.142 A dodecahedron shaped box with an adjusted 12 Å distance between the protein complexes, and the box was used to fit the solvated systems. Then, NA+ ions were introduced to neutralize the whole system. Once the solvated and electroneutral systems were assembled, a full 50,000-step energy minimization was performed to avoid steric clashes using the “steepest descent” algorithm. Each complex was equilibrated with an NVT (number of particles, volume, and temperature) ensemble for 1 ns at a temperature of 300 K and a coupling constant of 0.1 ps. Subsequently, an NPT (number of particles, pressure, and temperature) ensemble was running by setting the temperature at 300 K, and the pressure at 1 bar for 1 ns. As for the electrostatic interactions, they were calculated using the particle-mesh Ewald method.143 Upon the completion of the two equilibration phases, the production phase for each system was performed for 100 ns in triplicate (3 × 100 ns). Originally, 3 runs were performed for each system (WT, Alpha, Beta, Gamma, Delta, and Omicron) for reproducibility purposes (Supporting Information Figures S1–S3). Accordingly, the average C α rmsd and RMSF values for each system were calculated to have an overall view of the protein’s stability. The VMD program144 was used to calculate all hydrogen-bond (HB) and salt bridge (SB) occupancy rates with the angle and distance between the donor and acceptor set to 30° and 3.5 Å, respectively.
2.3. MM-PBSA Calculations
The MM-PBSA (molecular mechanics-Poisson Boltzmann surface area) methodology and the g_mmpbsa program145 were used to compute the binding free energies of each RBD–ACE2 complex as well as their residue decomposition energies. The g_mmpbsa program is a tool that integrates functions from GROMACS140 and APBS146 in order to calculate the binding free energies of protein–protein or protein-ligand complexes.
In this approach, the binding free energy Gbind between protein and ligand/protein includes different energy terms and could be calculated as
The gas-phase interaction energy EMM is equal to the sum of van der Waals energy Evdw and electrostatic energy Eelec. The polar solvation energy GPB and the non-polar solvation energy GSA are added together to form Gsol. The Poisson–Boltzmann (PB) approximation model is used to determine the polar solvation energy, whereas the solvent accessible surface area (SASA) is used to estimate the non-polar solvation energy. Owing to the high computational cost and the fact that considering the entropy, even if it improves the agreement with the experimental values of the binding free energy, it does not change the global profile of the energy,147 the entropy contribution (−TS) is omitted in this work. The binding free energies were decomposed to each residue after computation. It is worth noting that the more negative the energy, the more beneficial the contribution. Positive energy values, on the other hand, indicate unfavorable interactions and a low contribution to the complex. For each binding complex, MM-PBSA calculations were carried out on a total of 90 different conformations for each MD simulation, as in the calculations started from 10 ns simulation time and skipped every 1 ns.
3. Results and Discussion
3.1. Construction of Variant Models
We had to verify that our in silico ACE2–RBD variant models show the root-mean-square deviation (rmsd) values for less than 1 Å (Supporting Information Table S1), which are representative of good models. RBD’s Alpha, Beta, Gamma, Delta, and Omicron variants showed a backbone rmsd of only 0.2, 0.1, 0.2, 0.5, and 0.5 Å from the most recently resolved crystallographic and cryo-EM structures (PDB IDs: 7EKF,977EKG,977EKC,977WBQ,99 and 7T9L(14)) and aligned well against them. Thus, all models are valid to use for further steps.
3.2. Structure Flexibility and Stability of the Simulation Systems
3.2.1. SARS-CoV-2 WT and Variant Systems
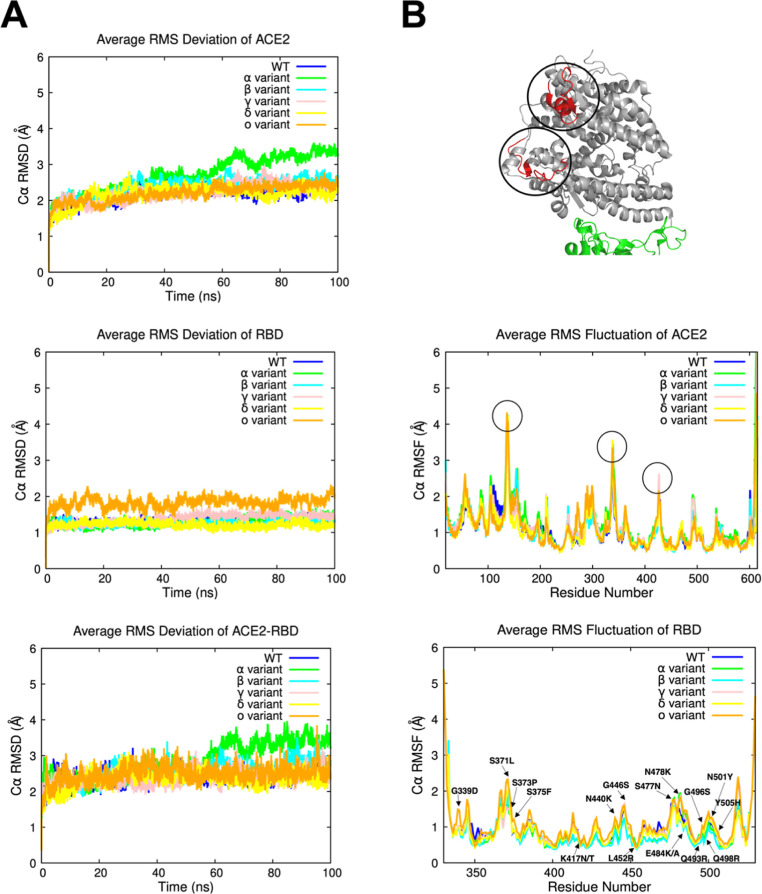
In this section, we report a thorough analysis to evaluate the stability of the ACE2 protein and the RBD of SARS-CoV-2 in the WT and variant systems. Only the Omicron variant is based on experimental data, but the variant systems comprise all modeled structures for the Alpha, Beta, Gamma, Delta, and Omicron variants. Throughout the 100 ns-simulation runs, the stability profiles of SARS-CoV-2 RBD of each system in complex with human ACE2 were analyzed using GROMACS (Figure 2). C α rmsd values of ACE2 and RBD were also studied separately to have detailed information of each of the proteins’ stability (Figure 2A).
Figure 2.
(A) Average C α rmsd values of the ACE2, RBD, and ACE2–RBD complex over 100 ns simulation time for each system. (B) Average C α RMSF values of the ACE2 and the RBD versus the residue number for each system. Highly flexible regions on the ACE2 protein are highlighted with circles and colored in red to easily locate them on the protein. These regions correspond to the circled peaks on the RMSF graph as well.
ACE2 average C α rmsd values are steady and stable, ranging between 2 and 2.5 Å from the initial structure. The Alpha variant shows more fluctuations, with values increasing progressively until reaching a plateau at 3.5 Å (Figure 2). On the other hand, SARS-CoV-2 RBD showed steady C α rmsd values overall (1–2 Å) with no obvious fluctuations (2–2.2 Å) within the Omicron variant (Figure 2). Similarly, ACE2–RBD complexes have proven to be stable during the MD simulations (2–2.5 Å) apart from the Alpha variant, which displayed the same variations as the ACE2 protein with C α rmsd values reaching a maximum of 3.9 Å, stabilizing at the end of the MD simulation (Figure 2). Therefore, the Alpha variant behavior can be explained by the high flexibility of the ACE2 protein and its constant shifting around the RBD toward the end of the MD simulation trajectory (Supporting Information Figures S1A and S3A). As shown in Supporting Information Figures S4 and S5, there were no significant differences in the rmsd values of the resolved and the constructed Omicron variant structures for the ACE2, RBD, and ACE2–RBD complex, supporting the reliability of our protocol.
Then, we computed the average root-mean-square fluctuation (RMSF) of the C α atoms versus the systems’ residue numbers to explore the detailed residual atomic fluctuations (Figure 2). It is also helpful to compare this further with the experimentally obtained B-factors from crystallography, which exhibit similar tendencies but with significantly less overall flexibility because of the cryogenic temperatures and lattice conditions that a protein is subjected to in a crystal. Thus, C α RMSF calculations of the 3 MD runs belonging to each of the variants were added in the Supporting Information Figures S6 and S7 for more details. Three major peaks regions can be observed on the ACE2 protein, all corresponding to the highly flexible loops. We discovered a slightly high fluctuation area specific to the Alpha variant, which is a random coil (P490–P500) reaching maximum C α RMSF values of ∼2 Å. Otherwise, no significant changes in terms of structural flexibility were observed in the Alpha variant as both ACE2 and RBD remained stable and showed similar C α RMSF values to the other variants. Therefore, the increased fluctuations of the ACE2 protein belonging to the Alpha variant (Figure 2B) might be modulated by conformational changes within the protein in order to stabilize itself. As for the RBD, we noticed a slight increase in the RMSF values in the Omicron variant, which corresponds to coiled structured regions of the protein. Similar results were observed for the studied cryo-EM structure as well as the constructed Omicron variant model, with RMSF values displaying the same tendency (Supporting Information Figures S8 and S9). The RBM region, which is the binding region of the RBD, remained stable for all variants, and no significant changes were observed. In order to understand potential changes in the conformations, the 7T9L structure was further compared to the MD-averaged Omicron model and cryo-EM resolved structural complexes. The MD-averaged conformations were superimposed with the 7T9L structure, as seen on Figure 3. To properly analyze protein structures, accurate protein side-chain modeling is required. Hence, when comparing the MD-averaged Omicron conformations to the experimental Omicron structure, we computed the rmsd on a basis of all side chain atoms, excluding hydrogen atoms (Table S2). Lower side chain rmsd values produce ensure more precise results.148 One of the few differences that can be observed is the flexibility of the side chains of the mutated residues located on the RBD surface (G339D, S371L, S373P, S375F, and N440K). The side chain rmsd values of the residues on the RBD surface ranged from 0.37 to 4.08 Å. The conformations’ high quality was confirmed by the fact that residues spanning the RBD interaction surface did not exceed side chain rmsd of 2.3 Å. In fact, it’s essential to remember that the side chains of the residues are flexible components, which explains why there are a few minor variations in the structures. The backbone RBD rmsd values for the MD-averaged Omicron model and cryo-EM resolved structure complexes, respectively, showed exceptionally low values of 0.75 and 0.87 Å when overlaying both structures to the experimental complex (Figure 3). Hence, by doing a direct structural analysis, agreement with experimental structure data for each complex was verified. Overall, C α rmsd and RMSF results showed stable systems over time and can be used further for additional analyses.
Figure 3.
(A) Structural overlay of the MD-averaged Omicron model (magenta) and 7T9L structures (green) with the resolved cryo-EM 7T9L structure (cyan). The 15 mutated residues of the RBD in the Omicron variant have been highlighted and represented in boxes. The backbone RBD rmsd has been specified as well.
3.3. Binding Free Energy Analysis
The binding affinity values have been reported using the molecular mechanics-generalized Born surface area (MM-GBSA) and MM-PBSA approaches in earlier investigations using the WT protein and one of the many SARS-CoV-2 variants.80,149−154 In this study, we compared all known variants of concern that have emerged since the beginning of the pandemic using the MM-PBSA method with the aim of studying the effects of the mutations on the binding energy of the ACE2–RBD complex.
The results showed that the binding free energy of the WT system (−51.5 ± 32.0 kcal mol–1) is lower than that in the other systems (Table 2). On the other hand, the Omicron variant displayed the highest free energy with a value of −142.6 ± 31.4 kcal mol–1. This is mostly due to the introduction of several charged residues with favorable interactions making the ΔEElec energy the key driving factor in the binding process of RBD with ACE2. This is the case of the Alpha variant (N501Y), the Beta variant (E484K and N501Y), the Gamma variant (E484K and N501Y), the Delta variant (L452R and T478K), and the Omicron variant (N440K, T478K, Q493R, Q498R, and N501Y). It can be easily proven with the increasing trend of ΔEElec values of each system reaching −78.3 ± 5.2, −80.3 ± 12.2, −96.2 ± 4.4, −143.6 ± 6.1, −121.5 ± 5.4, and −187.4 ± 5.4 kcal mol–1 for the WT, Alpha, Beta, Gamma, Delta, and Omicron variants, respectively. As for the ΔEvdw, ΔEPolar, and ΔEApolar energy terms, they all seem to be very similar for all systems with no significant changes. The binding free energy of the constructed Omicron variant and the Omicron variant’s cryo-EM structure were also examined. Very similar results have been obtained for both systems, as shown in Supporting Information Table S3. For the Omicron variant model and the resolved Omicron variant structures, respectively, the results showed ΔGbind values of −144.8 28.0 and −142.6 30.4 kcal mol–1. Therefore, these results confirm the reliability of our method of analysis.
Table 2. Summary of the Average Energy Terms ΔEvdw, ΔEElec, ΔEPolar, ΔEApolar, and ΔGbind of the Six Different ACE2–RBD Complexes (WT, Alpha, Beta, Gamma, Delta, and Omicron)a.
| binding
energy components (kcal mol–1) |
|||||
|---|---|---|---|---|---|
| ACE2–RBD complex | ΔEvdw | ΔEElec | ΔEPolar | ΔEApolar | ΔGbind |
| WT | –77.3 ± 6.3 | –78.3 ± 5.2 | 114.1 ± 33.0 | –10.0 ± 0.9 | –51.5 ± 32.0 |
| Alpha | –68.7 ± 15.1 | –80.3 ± 12.2 | 85.6 ± 41.7 | –8.5 ± 2.0 | –72.0 ± 32.9 |
| Beta | –77.5 ± 6.0 | –96.2 ± 4.4 | 67.7 ± 27.9 | –9.1 ± 0.9 | –112.3 ± 27.6 |
| Gamma | –75.7 ± 5.6 | –96.2 ± 5.4 | 78.2 ± 31.1 | –9.3 ± 0.9 | –103.1 ± 29.3 |
| Delta | –77.1 ± 5.4 | –121.5 ± 5.4 | 108.6 ± 35.2 | –10.0 ± 0.9 | –100.0 ± 33.8 |
| Omicron | –77.8 ± 5.3 | –187.4 ± 5.4 | 129.3 ± 32.8 | –10.3 ± 0.9 | –142.6 ± 30.4 |
All computed energies are expressed as mean ± standard deviation.
Then, we carried out our study with a per-residue decomposition energy comparison analysis (Figure 4). To further ensure the accuracy of our findings, we examined the two systems and applied the same protocol for the 7T9L Omicron variant and the Omicron model variant structures (Supporting Information Figure S11). First, we verified that only RBM residues that are within 6 Å of the ACE2 binding region are examined because these are the essential amino acids that play a role in the RBD–ACE2 interaction. The same analysis was also applied in our previously published paper,155 where 8 hot spots were identified (K417, L455, F456, F486, Y489, T500, N501, and Y505) on the WT RBM. Thus, we extended this analysis on the mutated complexes we built for the five variants of the RBD to determine whether the WT RBM residues are remaining hot spots on the five variants under study as well as to detect new hot spots brought on by the insertion of the mutations.
Figure 4.
Bar plots of the free energy component of the RBM hot spots in kcal mol–1. Mutated residues are colored according to the variant color (dark blue for the WT system, green for the Alpha variant, cyan for the Beta variant, pink for the Gamma variant, yellow for the Delta variant, and orange for the Omicron variant). Hot spot residues are considered when their decomposition energy is equal to or greater than −1 kcal mol–1. All computed free energy components are displayed with error bars.
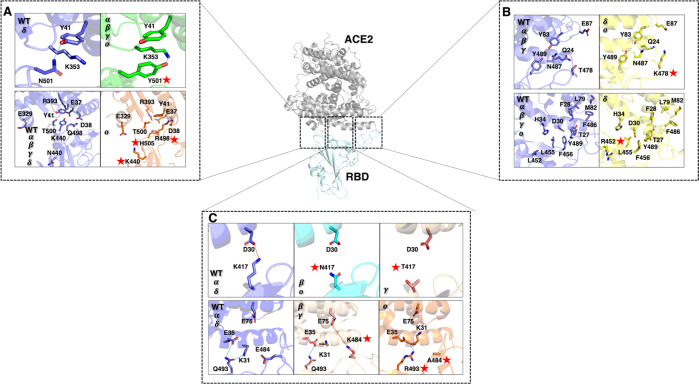
Among the residues (K417, L455, F456, F486, Y489, T500, N501, and Y505) that have been detected as hot spots on the WT RBD, five of them (L455, F456, F486, Y489, and T500) were observed to remain key hot spots on the five variants (Alpha, Beta, Gamma, Delta, and Omicron) (Supporting Information Figure S10). Evidently, the same analysis was performed on the cryo-EM Omicron variant and the constructed Omicron variant structures, where both systems displayed identical results (Supporting Information Figure S12). Moreover, these residues are conserved in all variants of concern. Residue L455 forms intermolecular van der Waals interactions and polar contacts with ACE2 residues D30 and H34, respectively. On the other hand, residues F456, F486 and Y489 are inserted in a hydrophobic pocket on the right edge of the RBM region, which is formed by residues T27, F28, L79, and M82. Thus, the presence of aromatic residues in the pocket may provide additional binding force via π-stacking interactions. For instance, F456–T27, F486–L79, and Y498–F28 interact through hydrophobic interactions. Residue F486 forms an additional interaction through a π–π stacking with residue Y83, while Y489 forms a HB with Y83. Thus, L455, F456, F486, Y489, and T500 can be considered crucial amino acids that have been conserved in all RBD variants, with the key role of maintaining tight binding with ACE2.
As shown in Figure 5, the Delta mutations T452R and T478K were also represented as they are located close to the key unchanged hot spots of the RBM. However, very weak to no interaction was made between these residues and the ACE2 binding region. L452R and T478K are located at a distance above 6 Å of the ACE2 binding surface. Moreover, L452R is facing the opposite side of the RBM region, while T478K is mainly exposed to the solvent and too far from ACE2 residue E87 (∼15 Å), which makes it difficult to maintain steady interactions with ACE2. Both T478K and L452R roles are still uncertain. We can speculate that both types of mutations can improve RBM contacts with the negatively charged and hydrophilic ACE2 interface by making the RBD more positively charged and hydrophilic. However, as stated previously, T478K cannot interact with any ACE2 residues due to its constant exposure to the solvent and great distance from the ACE2 binding surface. On the other hand, T478K can play an important role in the trimeric conformation of the spike protein, where the introduction of a positive mutated residue, a lysine (K), might tighten the RBD interactions to reinforce the closed/inactive state. The L452R mutation is still debated in several studies66,156 since it does not play a crucial role in the interaction with ACE2. However, a paper by Forest-Nault and co-workers157 suggests that the L452R mutation abrogates a hydrophobic patch formed by residues L452, L492, and F490. The loss of this patch could impact the stability of the RBD and possibly its complexation with ACE2, where faster association and dissociation of the RBD have been observed.157
Figure 5.
(A) Representation of the conserved key hot spots of the RBM (L455, F456, F486, and Y489) and their interaction with the ACE2 residues within a 6 Å distance. Mutated residues L452R and T478K were also represented to show their absence of interaction with the ACE2 binding region. (B) Representation of the new key hot spots of the RBM (N501Y and Q498R), the conserved T500 hot spot, and their interaction with the ACE2 residues within a 6 Å distance. Mutated residues N440K and Y505H were also represented to show their weak or absent interaction with the ACE2 binding region. (C) Representation of the new positive and negative key hot spots of the RBM (E484K, E484A and K417N/T), the mutated Q493R residue, and their interaction with the ACE2 residues within a 6 Å distance. Mutated residues are specified with a red star.
New hot spots have also been detected on the RBM surface due to the introduction of several mutations in each variant. The N501Y mutation is common to the Alpha, Beta, Gamma, and Omicron variants, indicating this mutation confers strong advantages to the SARS-CoV-2 virus. As shown in Figure 4, N501 residue has its binding free energy increased from −1.41 kcal mol–1 in the WT system or from −1.72 kcal mol–1 in the Delta variant to −3.37, −3.88, −3.58, and −3.97 kcal mol–1 in the Alpha, Beta, Gamma, and Omicron variants, respectively. In the crystallographic structure of WT RBD–ACE2 complex, N501 residue is known to interact with residue Y41 through a HB. It is also surrounded by the K353 hydrophobic alkyl chain and the Y41 benzene ring (Figure 5). The phenol group on the Y501 side chain in the N501Y mutant can interact through a cation−π interaction with the amine group of the K353 side chain and form a π–π stacking interaction with residue Y41. The higher binding affinity of the N501Y mutant with ACE2 is attributed to these additional stable intermolecular π-interactions with K353 and Y41 (Figure 5). Our results are also in agreement with other studies.30,31 Thus, we can suggest that the N501Y mutation is responsible for improving the binding affinity of the RBD–ACE2 complex, which is directly linked to the enhanced transmissibility of the virus.158 On the other hand, N501Y has been proven to have little effect on the neutralization of antibodies.159
Among the Omicron mutations, residue Q498R showed a high contributing energy (−8.49 kcal mol–1),160 which may be due to its interaction with residue D38 through the formation of an SB (Figure 5). Y505H also had its contribution energy decreased (−1.26 kcal mol–1).160 The Omicron Y505H mutation causes the hydrogen bonding connections between WT RBD Y505 and ACE2 E37 and R393 to be disrupted, which might explain its lower decomposition energy (Figure 4). As for N440K, the location of the mutated residue is too far (∼15–19 Å) from ACE2 residue E329 to form an SB. It is also often exposed to the solvent and cannot interact correctly with the ACE2 interface region. However, like T478K, it might play a role in making the trimeric form of the spike protein stronger by enhancing the interaction of the neighboring RBDs.
E484 is also a shared mutation between several variants (Beta, Gamma, and Omicron) and a hallmark of numerous SARS-CoV-2 lineages. E484 is drastically mutated to a positively charged residue in the Beta and Gamma variants as a lysine (K). However, E484 is altered to an alanine (A) in the Omicron variant. These findings demonstrate E484’s intricate influence and variety of behaviors. As a result, it is not surprising that variants with this mutation have high transmissibility as well as a high rate of antibody escape.161 E484 is positioned on a highly flexible loop of the RBM region. In prior research155 as well on other published papers,150,162−164 we discovered that E484 had extremely positive decomposition energy, as shown in Figure 4 (15.74 kcal mol–1) and was classified as an unfavorable hot spot for the interaction with ACE2. The Alpha (14.22 kcal mol–1) and Delta (14.69 kcal mol–1) variants still show the unfavorable tendency of E484 (Figure 4). This is primarily due to E484’s proximity to negatively charged ACE2 residues like E35 and E75 (Figure 5). The E484K mutation, on the other hand, had a completely different effect, as it not only changed the nature of the residue, but also rendered E484K (−12.96 and −12.98 kcal mol–1 for the Beta and Gamma variants, respectively) a major positive and contributing hot spot for ACE2 binding. As shown in Table 1, the Beta and Gamma variants free energies increased significantly compared to the WT strain. This is consequently due to the important increase of the ΔEElec energies. Thus, E484K is able to bind E75 by forming a SB and using the flexible loop to create a more suitable environment (Figure 5). Both the Beta and Gamma versions carry the same E484K mutation, and particular attention has been given to these specific residue alterations. In fact, the E484K mutation may have a stronger transmissibility than the original strain.161 Additionally, it revealed a reduction in the neutralization activity of some tested antibodies, which could affect how effective the existing vaccines are.5,161,165 As a result, our findings indicated that the E484K mutation may enhance the RBD–ACE2 complex’s binding affinity through more favorable electrostatic forces and tighter interactions on the RBM surface. These findings suggest that the E484K mutation-carrying variants are more transmissible. With several antibodies, other investigations have shown decreased binding affinities, which can result in an immune response escape. This may possibly be because the E484K mutation alters the electrostatic interaction on the RBD surface, decreasing the potency of antibodies. Alternatively, the Omicron E484A mutation did not have any impact on the decomposition energy but rather nullified it (−0.05 kcal mol–1). Thus, the Omicron binding free energy is primarily due to a mixture of additional mutations that function as a compensation for the E484K positive effect.
Similarly, residue K417 is an important hot spot contributing favorably to the ACE2–RBD in the WT SARS-CoV-2 through its SB formation with residue D30 of ACE2 (Figure 5). K417 has been changed to an asparagine (N) in the Beta and Omicron variants, while it was altered to either an asparagine (N) or a threonine (T) in the Gamma variant. As shown in Figure 4, the decomposition energies of K417 are highly favorable compared to the other variants. Moreover, K417 of the Alpha variant shows a higher (−5.76 kcal mol–1) energy than the one observed in the WT strain (−4.26 kcal mol–1) and the Delta variant (−3.27 kcal mol–1). This might be explained by the presence of surrounding mutations that impact the conformation of the RBD and the chemical environment of the residues. It is also clearly shown in Figure 4 that the alteration of the lysine (K) residue considerably reduces the energy of the amino acid. The decomposition energies of K417 have dropped to −0.33, −0.25, and −0.23 kcal mol–1, for the Beta, Gamma, and Omicron variants, respectively. Overall, with the use of other favorable mutations, variants with an altered K417 were able to maintain high binding affinities with ACE2 (Table 2).151
In comparison with the WT, the mutated residue, residue Q493R did show a slight increase in its decomposition energy when comparing the WT with Omicron. However, no significant changes were observed in terms of structure. As shown in Figure 5, in both WT and Omicron systems, Q493R forms a HB with ACE2 residue E35. The non-significant decomposition energy might be due to the non-steady bond formed between those pairs of residues or the constant intramolecular SB interaction of E35 and K31, making Q493R–E35 HB less present.
In summary, out of all mutations that define the Alpha, Beta, and Gamma variants, N501Y promotes association with ACE2 through π-stacking and hydrogen bonding interactions, while E484K contributes through electrostatic attractions, resulting in a higher affinity for the double Gamma variant and the triple Beta variant. The third main mutation in the Beta and Omicron variants, K417N, acts as an unfavorable residue decreasing the ACE2-binding affinity. On the other hand, K417N might help control the infectivity of the different affected variants rather than focusing on tightening the ACE2 interaction. T478K and L452K mutations might be involved in boosting the Delta variant RBD’s affinity for ACE2 by inducing conformational changes on the RBM binding regions, triggering an antibody escape mechanism. As for the rest of the mutations, mostly in the Omicron variant, it is crucial to keep an eye on their impacts because they could be incredibly important for antibody affinities and epitope key site determination. For instance, a combination of these hot spots might increase the strong affinity of the complex formed with ACE2 and RBD. The Delta variant might acquire new mutations like the N501Y or the Q498R, rendering the virus to bind more tightly to human cells. It is, of course, important to also consider other mutations that might be important in the immune escape strategies of SARS-CoV-2. Hence, it remains necessary to monitor the existing mutations and study their effects to predict new possible “gain-of-function” mutations or variants that might gain additional antibody resistance and drastic viral transmission, leading to more aggravating diseases.
3.4. HB and SB Analysis
To analyze the interactions between the ACE2 and the RBD and to highlight the various bonds that were formed and abolished within each system, HB and SB occupancy calculations were performed (Table 3). We chose to illustrate the most significant changes in each system by measuring HB and SB occupancy exceeding 5%.
Table 3. Average RBD–ACE2 Non-covalent Interaction Occupancy (%) During MD Simulations (3 Trajectories for Each System of 100 ns Each)a.
| HB and
SB occupancy (%) |
|||||||
|---|---|---|---|---|---|---|---|
| RBD | ACE2 | WT | Alpha | Beta | Gamma | Delta | Omicron |
| 417 | D30 | SB:94% | SB:80.0% | NI (K417N) | NI (K417T) | SB:98.0% | NI (K417N) |
| 484 | K31 | SB:58.7% | SB:58.3% | NI (E484K) | NI (E484K) | SB:59.4% | NI (E484A) |
| 484 | E75 | NI | NI | SB:19.7% (E484K) | SB:33.7% (E484K) | NI | NI (E484A) |
| 498 | D38 | NI | NI | NI | NI | NI | SB:60.34% (Q498R) |
| 498 | Q42 | HB:32.1% | HB:32.8% | HB:48.3% | HB:41.07% | HB:23.7% | NI (Q498R) |
| 498 | K353 | HB:6.1% | NI | NI | NI | HB:11.9% | NI (Q498R) |
| 505 | E37 | HB:71.2% | HB:47.1% | HB:45.9% | HB:34.04% | HB:56.4% | NI (Y505H) |
| 505 | K353 | NI | NI | NI | NI | NI | HB:24.6% (Y505H) |
All SB bonds were set to a cutoff distance of 10 Å. NI (no interactions) indicates no interaction was formed between residues or occupancies were lower than 5%. For each pair of residues, the type of interaction is specified (SB or HB). The mutated amino acid of each pair of residues is added between brackets under the occupancy rate.
The first SB formed between ACE2 D30 and RBD K417 is significantly present in the WT (94.0%), Alpha (80.0%), and Delta (98.0%) systems as no mutations were introduced to the 417 residue (Figure 5 and Table 3). However, because K417 has been altered to an asparagine (N) or a threonine (T) in the remaining systems, the SB with D30 is no longer present (occupancy < 5%). The average atomic distance between the two residues was also calculated over the simulation time (3 × 100 ns), and the results were compared with those of the WT and Beta systems. Figure 6 illustrates a clear distinction between the two systems, with the distance between D30 and K417 remaining constant with an average distance value of 4 Å, while the distance in Beta is closer to 8–10 Å. As a result, we can assume that the K417N/T mutation, which causes the loss of one of the strongest interactions at the RBD interface, might constitute a convergent strategy employed to avoid being neutralized by antibodies.
Figure 6.
(A) Bar plots of the atomic distance within the formed SB between ACE2 D30 and WT, Beta, and Gamma K417N/T. (B) Histogram of the average SB occupancy (%) of D30-K417N/T in each system (WT, Alpha, Beta, Gamma, Delta, and Omicron). Mutations are marked with a red star. All SB occupancies are displayed with error bars.
Two other SBs are formed between residues E484–K31 and K484–E75 (Table 3). When E484 is non-mutated, a stable SB can be produced with ACE2 residue K31 because the two residues are close to one another. HB occupancy rates of the E484-K31 in the WT, Alpha, and Delta variants are 58.7, 58.3, and 59.4%, respectively. Also, for the majority of the simulation, another stable HB is produced between residues Q493 and K31, which explains why the SB only occurs 50% of the time over the trajectories. On the other hand, a newly formed SB can be observed with ACE2 residue E75 when E484 is changed to a lysine (K). This SB can be seen in both Beta (19.7%) and Gamma (33.7%) systems. This finding supports the dynamic RBD movements that allow for the formation of new bonds to enhance the molecular system’s affinity. As for the Omicron variant, all bonds are abolished when residue E484 is mutated to an alanine (A). E484A, being a small and hydrophobic residue and mostly present in a negatively charged environment, has little to no interaction with the other surrounding residues of the binding interface (Figure 5C and Supporting Information Figure S13A,B).
The ACE2 residues D38, Q42, and K353 can form three distinct HB bonds with Q498, as illustrated in Table 3. Interestingly, Q498 interacts with residue Q42 through an HB throughout all systems (WT 32.1%, Alpha 32.8%, 48.3% Beta, 41.0% Gamma, and 23.7% Delta) with the exception of Omicron. Another important observation is that Q498 is able to form another HB with residue K353 in the WT and Delta systems (Table 3 and Supporting Information Figure S35E). These findings highlight how closely WT and Delta interact, as well as how similar their structural conformations are. On the other hand, to compensate for the loss of the HB originally formed with residues K353 and Q42, the Omicron Q498R mutation allowed the addition of a new SB interaction with residue D38 with a steady SB occupancy rate of 60.3% (Supporting Information Figure S13C).
Y505 did not experience any changes, apart from the Omicron variant, where it is mutated to an histidine (H) residue. Y505-E37 appears to be the most common HB, with occupancy rates of 71.2% in WT, 47.1% in Alpha, 45.9% in Beta, 34.0% in Gamma, and 56.4% in Delta (Table 3 and Supporting Information Figure S13F). To overcome the loss of the Y505–E37 HB in the Omicron system, the Y505H mutation interacts with the ACE2 K353 residue with a stable HB occupancy rate of 24.6% (Supporting Information Figure S13G).
A variety of computational studies have been conducted and continue to be performed, comparing the affinities of SARS-CoV-2 variants of concern with the ACE2 receptor. Several methods, such as MM-PBSA or MM-GBSA,80,149−154 FEP,166 and neural network models,167 were applied in this research. These computational methods have made it possible to compare various variants of concern and predict future mutations, as seen in several neural network models.167
Nevertheless, despite the advances in this research, none have examined the precise pathogenic circulating variants of concern (WT, Alpha, Beta, Gamma, Delta, and Omicron) with the same structural details using the same approach for compatibility and reproducibility purposes, which makes our study distinctive. In addition, our findings are consistent with experimental data, providing further validation of our computational approach and increasing confidence in our results.4,98,99,103 Furthermore, it is important to note that while some studies have compared some sets of variants, most of these did not undertake a comprehensive investigation of all variants of concern. Our study, on the other hand, seeks to close the gap by analyzing all the current variants of concern to have a better understanding of the interaction between ACE2 and RBD. When compared to other studies, our study revealed a comparable trend in affinity and residue decomposition energies. Our research could help in the development of new inhibitors by identifying the binding sites for antibodies that can assist in the prevention of the interaction between ACE2 and RBD. As a result, our study provides a critical understanding of the relationship between SARS-CoV-2 variants of concern and the ACE2 receptor, which may aid in the development of strategies to prevent COVID-19.
In summary, our results demonstrate the newly formed HB and SB interactions resulting from various mutations and being introduced at critical hot spots of the RBD interface. Most of the variations were seen in the RBD binding surface residues K417N/T, E484K/A, Q498R, N501Y, and Y505H. It also provides information on the selection of mutations for which SARS-CoV-2 inquired, primarily to increase its affinity for the ACE2 protein and avoid being eliminated by RBD-targeted antibodies.
4. Conclusions
In this work, we studied the impact of the various mutations of the SARS-CoV-2 RBD WT, Alpha, Beta, Gamma, Delta, and Omicron systems and their affinities with the ACE2 protein. This is the first analysis to overlook all variants and their effects with an identical combination of different computational methods. Molecular modeling enabled the construction of highly similar molecular models of the different RBD–ACE2 systems that fully comply with crystallographic structures. By maintaining the same protocol throughout this study, we were able to ensure the clarity of our work and the reliability of our findings. As a result, even though we were aware that the Omicron model structure would be difficult to accurately model, it contains most of the known mutations in the SARS-CoV-2 virus. Yet, by constructing an Omicron model structure, we were able to confirm the consistency of our approach. In fact, it has been demonstrated that the Omicron model and the resolved cryo-EM Omicron variant structures give results that are very consistent across all the different analyses that we performed. MD simulation results showed the stability of all systems and pointed out flexible regions on both proteins through rmsd and RMSF analyses. The results from the MM-PBSA analysis indicated new hot spots playing crucial roles in enhancing RBD affinity with ACE2. It also showed the impact of the different mutated regions and their ways of increasing the spike protein affinity for ACE2 and mainly escaping RBD-targeted neutralizing antibodies. K417N/T SB has been observed to be abolished when mutated to an asparagine (N) or a threonine (T) residue. The L452R residue is not involved in any interaction; however, it is believed that its mutation is involved in the complex association and dissociation rates. E484K/A mutations are also involved in the formation of a steadier SB with residue E75 when mutated to a lysine (K) in the Beta and Gamma variants and show no interaction when mutated to an alanine (A) in the Omicron variant. The Q498R mutation is involved in the creation of a new SB with residue D38, one of the numerous newly formed bonds in the Omicron variant. The N501Y mutation, which is present in nearly all variants, interacts quite strongly with ACE2 residues through hydrophobic interactions, which increase almost more than twice the decomposition energy of the originally unchanged residue. Therefore, it acts as a crucial hot spot in the mutated ACE2–RBD complex. The Y505H mutation in the Omicron variant shows the abolishment of Y505–R393 and Y505–E37 HB in the rest of the systems and its compensation with the formation of a more dominant interaction with ACE2 residue K353.
Overall, our results demonstrate a strong connection between the computed binding free energies and decomposition energy trends of each variant and its viral effect. It also gives a general view of all variants of concern and their effects at a molecular level, focusing on a variety of key mutations that becoming the new hot spots of the RBD and might be responsible for additional effects in new variants if combined together.
Acknowledgments
This work was supported by the Agence Nationale de la Recherche (PIF21 project, no. ANR-19-CE18-0023). The authors gratefully acknowledge the financial support of the Université de Paris, the CNRS Institute, and the INSERM Institute.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpcb.3c01467.
Protein rmsd on experimental structures and during the MD simulations; RMSF during the MD simulations; MM-PBSA energy terms calculated during the MD simulations; atomic distances extracted during the MD simulations (PDF)
Author Contributions
This manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Notes
The PyMOL software is available to noncommercial users under a distribution-specific license on https://pymol.org/2/. The GROMACS software is free of charge and available on https://www.gromacs.org. The VMD software is available to noncommercial users under a distribution-specific license on http://www.ks.uiuc.edu/Research/vmd/. The g_mmpbsa software is free of charge and available on https://rashmikumari.github.io/g_mmpbsa/.
Supplementary Material
References
- https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed April 10, 2023).
- Harvey W. T.; Carabelli A. M.; Jackson B.; Gupta R. K.; Thomson E. C.; Harrison E. M.; Ludden C.; Reeve R.; Rambaut A.; Peacock S. J.; Robertson D. L.; Peacock S. J.; Robertson D. L. SARS-CoV-2 Variants, Spike Mutations and Immune Escape. Nat. Rev. Microbiol. 2021, 19, 409–424. 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.; Peng P.; Cao X.; Wu K.; Chen J.; Wang K.; Tang N.; Huang A. Increased Immune Escape of the New SARS-CoV-2 Variant of Concern Omicron. Cell. Mol. Immunol. 2022, 19, 293–295. 10.1038/s41423-021-00836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum M.; Walls A. C.; Sprouse K. R.; Bowen J. E.; Rosen L. E.; Dang H. V.; De Marco A.; Franko N.; Tilles S. W.; Logue J.; et al. Molecular Basis of Immune Evasion by the Delta and Kappa SARS-CoV-2 Variants. Science 2021, 374, 1621–1626. 10.1126/science.abl8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum Y.; Schmidt F.; Zhang F.; DaSilva J.; Poston D.; Lorenzi J. C.; Muecksch F.; Rutkowska M.; Hoffmann H.-H.; Michailidis E.; et al. Escape from Neutralizing Antibodies by SARS-CoV-2 Spike Protein Variants. eLife 2020, 9, e61312 10.7554/elife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Zhang Y.; Wu L.; Niu S.; Song C.; Zhang Z.; Lu G.; Qiao C.; Hu Y.; Yuen K.-Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e9. 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J.; Ye G.; Shi K.; Wan Y.; Luo C.; Aihara H.; Geng Q.; Auerbach A.; Li F. Structural Basis of Receptor Recognition by SARS-CoV-2. Nature 2020, 581, 221–224. 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J.; Ge J.; Yu J.; Shan S.; Zhou H.; Fan S.; Zhang Q.; Shi X.; Wang Q.; Zhang L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Xiao T.; Cai Y.; Chen B. Structure of SARS-CoV-2 Spike Protein. Curr. Opin. Virol. 2021, 50, 173–182. 10.1016/j.coviro.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y.; Zhang J.; Xiao T.; Peng H.; Sterling S. M.; Walsh R. M.; Rawson S.; Rits-Volloch S.; Chen B. Distinct Conformational States of SARS-CoV-2 Spike Protein. Science 2020, 369, 1586–1592. 10.1126/science.abd4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R.; Zhang Y.; Li Y.; Xia L.; Guo Y.; Zhou Q. Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE2. Science 2020, 367, 1444–1448. 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Receptor Recognition Mechanisms of Coronaviruses: A Decade of Structural Studies. J. Virol. 2015, 89, 1954–1964. 10.1128/jvi.02615-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y.; Shang J.; Graham R.; Baric R. S.; Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Zhang C.; Sui J.; Kuhn J. H.; Moore M. J.; Luo S.; Wong S.-K.; Huang I.-C.; Xu K.; Vasilieva N.; et al. Receptor and Viral Determinants of SARS-Coronavirus Adaptation to Human ACE2. EMBO J. 2005, 24, 1634–1643. 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Penninger J. M.; Li Y.; Zhong N.; Slutsky A. S. Angiotensin-Converting Enzyme 2 (ACE2) as a SARS-CoV-2 Receptor: Molecular Mechanisms and Potential Therapeutic Target. Intensive Care Med. 2020, 46, 586–590. 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialo F.; Daniele A.; Amato F.; Pastore L.; Matera M. G.; Cazzola M.; Castaldo G.; Bianco A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2020, 198, 867–877. 10.1007/s00408-020-00408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q.; Hughes T. A.; Kelkar A.; Yu X.; Cheng K.; Park S.; Huang W.-C.; Lovell J. F.; Neelamegham S. Inhibition of SARS-CoV-2 Viral Entry upon Blocking N- and O-Glycan Elaboration. eLife 2020, 9, e61552 10.7554/elife.61552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo J.; Le Bas A.; Ruza R. R.; Duyvesteyn H. M. E.; Mikolajek H.; Malinauskas T.; Tan T. K.; Rijal P.; Dumoux M.; Ward P. N.; et al. Neutralizing Nanobodies Bind SARS-CoV-2 Spike RBD and Block Interaction with ACE2. Nat. Struct. Mol. Biol. 2020, 27, 846–854. 10.1038/s41594-020-0469-6. [DOI] [PubMed] [Google Scholar]
- Linsky T. W.; Vergara R.; Codina N.; Nelson J. W.; Walker M. J.; Su W.; Barnes C. O.; Hsiang T.-Y.; Esser-Nobis K.; Yu K.; et al. De Novo Design of Potent and Resilient HACE2 Decoys to Neutralize SARS-CoV-2. Science 2020, 370, 1208–1214. 10.1126/science.abe0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L.; Goreshnik I.; Coventry B.; Case J. B.; Miller L.; Kozodoy L.; Chen R. E.; Carter L.; Walls A. C.; Park Y.-J.; et al. De Novo Design of Picomolar SARS-CoV-2 Miniprotein Inhibitors. Science 2020, 370, 426–431. 10.1126/science.abd9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J.; Xiang Y.; Huang Z.; Liu X.; Wang M.; Ge G.; Chen H.; Xu J.; Zheng M.; Chen L. Structure-Based Virtual Screening and Identification of Potential Inhibitors of SARS-CoV-2 S-RBD and ACE2 Interaction. Front. Chem. 2021, 9, 740702. 10.3389/fchem.2021.740702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M. I.; MacGowan S. A.; Kutuzov M. A.; Dushek O.; Barton G. J.; van der Merwe P. A. Effects of Common Mutations in the SARS-CoV-2 Spike RBD and Its Ligand, the Human ACE2 Receptor on Binding Affinity and Kinetics. eLife 2021, 10, e70658 10.7554/elife.70658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruprasad L. Human SARS CoV -2 Spike Protein Mutations. Proteins 2021, 89, 569–576. 10.1002/prot.26042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M.; Shayestehpour M.; Mirzaei H. The Impact of Spike Mutated Variants of SARS-CoV2 [Alpha, Beta, Gamma, Delta, and Lambda] on the Efficacy of Subunit Recombinant Vaccines. Braz. J. Infect. Dis. 2021, 25, 101606. 10.1016/j.bjid.2021.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N. G.; Abbott S.; Barnard R. C.; Jarvis C. I.; Kucharski A. J.; Munday J. D.; Pearson C. A. B.; Russell T. W.; Tully D. C.; Washburne A. D.; et al. Estimated Transmissibility and Impact of SARS-CoV-2 Lineage B.1.1.7 in England. Science 2021, 372, eabg3055 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courjon J.; Contenti J.; Demonchy E.; Levraut J.; Barbry P.; Rios G.; Dellamonica J.; Chirio D.; Bonnefoy C.; Giordanengo V.; et al. COVID-19 Patients Age, Comorbidity Profiles and Clinical Presentation Related to the SARS-CoV-2 UK-Variant Spread in the Southeast of France. Sci. Rep. 2021, 11, 18456. 10.1038/s41598-021-95067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi S. S.; Schein C. H.; Braun W. Regional and Temporal Coordinated Mutation Patterns in SARS-CoV-2 Spike Protein Revealed by a Clustering and Network Analysis. Sci. Rep. 2022, 12, 1128. 10.1038/s41598-022-04950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrov D. A. Structural Consequences of Variation in SARS-CoV-2 B.1.1.7. J. Cell. Immunol 2021, 3, 103. 10.33696/immunology.3.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayarri-Olmos R.; Johnsen L. B.; Idorn M.; Reinert L. S.; Rosbjerg A.; Vang S.; Hansen C. B.; Helgstrand C.; Bjelke J. R.; Bak-Thomsen T.; et al. The Alpha/B.1.1.7 SARS-CoV-2 Variant Exhibits Significantly Higher Affinity for ACE-2 and Requires Lower Inoculation Doses to Cause Disease in K18-HACE2 Mice. eLife 2021, 10, e70002 10.7554/elife.70002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socher E.; Conrad M.; Heger L.; Paulsen F.; Sticht H.; Zunke F.; Arnold P. Computational Decomposition Reveals Reshaping of the SARS-CoV-2–ACE2 Interface among Viral Variants Expressing the N501Y Mutation. J. Cell. Biochem. 2021, 122, 1863–1872. 10.1002/jcb.30142. [DOI] [PubMed] [Google Scholar]
- Tian F.; Tong B.; Sun L.; Shi S.; Zheng B.; Wang Z.; Dong X.; Zheng P. N501Y Mutation of Spike Protein in SARS-CoV-2 Strengthens Its Binding to Receptor ACE2. eLife 2021, 10, e69091 10.7554/elife.69091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Jackson C. B.; Mou H.; Ojha A.; Rangarajan E. S.; Izard T.; Farzan M.; Choe H.. The D614G Mutation in the SARS-CoV-2 Spike Protein Reduces S1 Shedding and Increases Infectivity. 2020, Preprint, bioRxiv: 2020.06.12.148726 [Google Scholar]
- Plante J. A.; Liu Y.; Liu J.; Xia H.; Johnson B. A.; Lokugamage K. G.; Zhang X.; Muruato A. E.; Zou J.; Fontes-Garfias C. R.; et al. Spike Mutation D614G Alters SARS-CoV-2 Fitness. Nature 2021, 592, 116–121. 10.1038/s41586-020-2895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Jackson C. B.; Mou H.; Ojha A.; Peng H.; Quinlan B. D.; Rangarajan E. S.; Pan A.; Vanderheiden A.; Suthar M. S.; et al. SARS-CoV-2 Spike-Protein D614G Mutation Increases Virion Spike Density and Infectivity. Nat. Commun. 2020, 11, 6013. 10.1038/s41467-020-19808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y. J.; Chiba S.; Halfmann P.; Ehre C.; Kuroda M.; Dinnon K. H.; Leist S. R.; Schäfer A.; Nakajima N.; Takahashi K.; et al. SARS-CoV-2 D614G Variant Exhibits Efficient Replication Ex Vivo and Transmission in Vivo. Science 2020, 370, 1464–1468. 10.1126/science.abe8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniloski Z.; Jordan T. X.; Ilmain J. K.; Guo X.; Bhabha G.; tenOever B. R.; Sanjana N. E. The Spike D614G Mutation Increases SARS-CoV-2 Infection of Multiple Human Cell Types. eLife 2021, 10, e65365 10.7554/elife.65365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber B.; Fischer W. M.; Gnanakaran S.; Yoon H.; Theiler J.; Abfalterer W.; Hengartner N.; Giorgi E. E.; Bhattacharya T.; Foley B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence That D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827.e19. 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubinski B.; Fernandes M. H. V.; Frazier L.; Tang T.; Daniel S.; Diel D. G.; Jaimes J. A.; Whittaker G. R. Functional Evaluation of the P681H Mutation on the Proteolytic Activation of the SARS-CoV-2 Variant B.1.1.7 (Alpha) Spike. iScience 2022, 25, 103589. 10.1016/j.isci.2021.103589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascall D. J.; Vink E.; Blacow R.; Bulteel N.; Campbell A.; Campbell R.; Clifford S.; Davis C.; Da Silva Filipe A.; Sakka N. E.; Fjodorova L.. et al. The SARS-CoV-2 Alpha Variant Is Associated with Increased Clinical Severity of COVID-19 in Scotland: A Genomics-Based Retrospective Cohort Analysis. 2021, Preprint; Medrxiv, 2021.08.17.21260128 (accessed April 10, 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekliz M.; Adea K.; Vetter P.; Eberhardt C. S.; Hosszu-Fellous K.; Vu D.-L.; Puhach O.; Essaidi-Laziosi M.; Waldvogel-Abramowski S.; Stephan C.; et al. Neutralization Capacity of Antibodies Elicited through Homologous or Heterologous Infection or Vaccination against SARS-CoV-2 VOCs. Nat. Commun. 2022, 13, 3840. 10.1038/s41467-022-31556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonura F.; Genovese D.; Amodio E.; Calamusa G.; Sanfilippo G. L.; Cacioppo F.; Giammanco G. M.; De Grazia S.; Ferraro D. Neutralizing Antibodies Response against SARS-CoV-2 Variants of Concern Elicited by Prior Infection or MRNA BNT162b2 Vaccination. Vaccines 2022, 10, 874. 10.3390/vaccines10060874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. P.; Zeng C.; Carlin C.; Lozanski G.; Saif L. J.; Oltz E. M.; Gumina R. J.; Liu S.-L. Neutralizing Antibody Responses Elicited by SARS-CoV-2 MRNA Vaccination Wane over Time and Are Boosted by Breakthrough Infection. Sci. Transl. Med. 2022, 14, eabn8057 10.1126/scitranslmed.abn8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch K. L.; Zhou S.; Kaul R.; Walker R.; Wu A. H. Evaluation of Neutralizing Antibodies against SARS-CoV-2 Variants after Infection and Vaccination Using a Multiplexed Surrogate Virus Neutralization Test. Clin. Chem. 2022, 68, 702–712. 10.1093/clinchem/hvab283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Luis P.; Aguilar R.; Pelegrin-Pérez J.; Ruiz-Olalla G.; García-Basteiro A. L.; Tortajada M.; Moncunill G.; Dobaño C.; Angulo A.; Engel P. Decreased and Heterogeneous Neutralizing Antibody Responses Against RBD of SARS-CoV-2 Variants After MRNA Vaccination. Front. Immunol. 2022, 13, 816389. 10.3389/fimmu.2022.816389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.; Zhang P.; Matsuoka Y.; Tsybovsky Y.; West K.; Santos C.; Boyd L. F.; Nguyen H.; Pomerenke A.; Stephens T.. et al. Extremely Potent Monoclonal Antibodies Neutralize Omicron and Other SARS-CoV-2 Variants. 2022, Preprint; Medrxiv, 2022.01.12.22269023 (accessed April 10, 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont L.; Snell L. B.; Graham C.; Seow J.; Merrick B.; Lechmere T.; Maguire T. J. A.; Hallett S. R.; Pickering S.; Charalampous T.; et al. Neutralizing Antibody Activity in Convalescent Sera from Infection in Humans with SARS-CoV-2 and Variants of Concern. Nat. Microbiol. 2021, 6, 1433–1442. 10.1038/s41564-021-00974-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narowski T. M.; Raphel K.; Adams L. E.; Huang J.; Vielot N. A.; Jadi R.; de Silva A. M.; Baric R. S.; Lafleur J. E.; Premkumar L. SARS-CoV-2 MRNA Vaccine Induces Robust Specific and Cross-Reactive IgG and Unequal Neutralizing Antibodies in Naive and Previously Infected People. Cell Rep. 2022, 38, 110336. 10.1016/j.celrep.2022.110336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha L. B.; Tedla N.; Bull R. A. Broadly-Neutralizing Antibodies Against Emerging SARS-CoV-2 Variants. Front. Immunol. 2021, 12, 752003. 10.3389/fimmu.2021.752003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. L.; Rappaport J. SARS-CoV-2 Beta Variant Substitutions Alter Spike Glycoprotein Receptor Binding Domain Structure and Stability. J. Biol. Chem. 2021, 297, 101371. 10.1016/j.jbc.2021.101371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Schmidt F.; Weisblum Y.; Muecksch F.; Barnes C. O.; Finkin S.; Schaefer-Babajew D.; Cipolla M.; Gaebler C.; Lieberman J. A.; et al. MRNA Vaccine-Elicited Antibodies to SARS-CoV-2 and Circulating Variants. Nature 2021, 592, 616–622. 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reincke S. M.; Yuan M.; Kornau H.-C.; Corman V. M.; van Hoof S.; Sánchez-Sendin E.; Ramberger M.; Yu W.; Hua Y.; Tien H.; et al. SARS-CoV-2 Beta Variant Infection Elicits Potent Lineage-Specific and Cross-Reactive Antibodies. Science 2022, 375, 782–787. 10.1126/science.abm5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Raddad L. J.; Chemaitelly H.; Ayoub H. H.; Yassine H. M.; Benslimane F. M.; Al Khatib H. A.; Tang P.; Hasan M. R.; Coyle P.; AlMukdad S.; Al Kanaani Z.; et al. Severity, Criticality, and Fatality of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Beta Variant. Clin. Infect. Dis. 2021, 75, e1188–e1191. 10.1093/cid/ciab909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radvak P.; Kwon H.-J.; Kosikova M.; Ortega-Rodriguez U.; Xiang R.; Phue J.-N.; Shen R.-F.; Rozzelle J.; Kapoor N.; Rabara T.; et al. SARS-CoV-2 B.1.1.7 (Alpha) and B.1.351 (Beta) Variants Induce Pathogenic Patterns in K18-HACE2 Transgenic Mice Distinct from Early Strains. Nat. Commun. 2021, 12, 6559. 10.1038/s41467-021-26803-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. R.; Angulo F. J.; Swerdlow D. L.; McLaughlin J. M.; Hazan I.; Ginish N.; Anis E.; Mendelson E.; Mor O.; Zuckerman N. S.; Erster O.; et al. Effectiveness of BNT162b2 MRNA COVID-19 Vaccine against SARS-CoV-2 Variant Beta (B.1.351) among Persons Identified through Contact Tracing in Israel: A Prospective Cohort Study. eClinicalMedicine 2021, 42, 101190. 10.1016/j.eclinm.2021.101190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett K. S.; Gagne M.; Wagner D. A.; O’ Connell S.; Narpala S. R.; Flebbe D. R.; Andrew S. F.; Davis R. L.; Flynn B.; Johnston T. S.; et al. Protection against SARS-CoV-2 Beta Variant in MRNA-1273 Vaccine–Boosted Nonhuman Primates. Science 2021, 374, 1343–1353. 10.1126/science.abl8912. [DOI] [PubMed] [Google Scholar]
- Souza P. F. N.; Mesquita F. P.; Amaral J. L.; Landim P. G. C.; Lima K. R. P.; Costa M. B.; Farias I. R.; Belém M. O.; Pinto Y. O.; Moreira H. H. T.; et al. The Spike Glycoprotein of SARS-CoV-2: A Review of How Mutations of Spike Glycoproteins Have Driven the Emergence of Variants with High Transmissibility and Immune Escape. Int. J. Biol. Macromol. 2022, 208, 105–125. 10.1016/j.ijbiomac.2022.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y.; Omata M. Discovery of a SARS-CoV-2 Variant from the P.1 Lineage Harboring K417T/E484K/N501Y Mutations in Kofu, Japan. J. Infect. 2021, 82, 276–316. 10.1016/j.jinf.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria N. R.; Mellan T. A.; Whittaker C.; Claro I. M.; Candido D. d. S.; Mishra S.; Crispim M. A. E.; Sales F. C. S.; Hawryluk I.; McCrone J. T.; et al. Genomics and Epidemiology of the P.1 SARS-CoV-2 Lineage in Manaus, Brazil. Science 2021, 372, 815–821. 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Wei P.; Kappler J. W.; Marrack P.; Zhang G. SARS-CoV-2 Variants of Concern and Variants of Interest Receptor Binding Domain Mutations and Virus Infectivity. Front. Immunol. 2022, 13, 825256. 10.3389/fimmu.2022.825256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalera A.; Gonzalez-Reiche A. S.; Aslam S.; Mena I.; Laporte M.; Pearl R. L.; Fossati A.; Rathnasinghe R.; Alshammary H.; van de Guchte A.; et al. Mutations in SARS-CoV-2 Variants of Concern Link to Increased Spike Cleavage and Virus Transmission. Cell Host Microbe 2022, 30, 373. 10.1016/j.chom.2022.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A.; Fulton B. O.; Wloga E.; Copin R.; Pascal K. E.; Russo V.; Giordano S.; Lanza K.; Negron N.; Ni M.; et al. Antibody Cocktail to SARS-CoV-2 Spike Protein Prevents Rapid Mutational Escape Seen with Individual Antibodies. Science 2020, 369, 1014–1018. 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández J.; Bruneau N.; Fasce R.; Martín H. S.; Balanda M.; Bustos P.; Ulloa S.; Mora J.; Ramírez E. Neutralization of Alpha, Gamma, and D614G SARS-CoV-2 Variants by CoronaVac Vaccine-induced Antibodies. J. Med. Virol. 2022, 94, 399–403. 10.1002/jmv.27310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas D.; Veyer D.; Baidaliuk A.; Staropoli I.; Guivel-Benhassine F.; Rajah M. M.; Planchais C.; Porrot F.; Robillard N.; Puech J.; et al. Reduced Sensitivity of SARS-CoV-2 Variant Delta to Antibody Neutralization. Nature 2021, 596, 276–280. 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- Motozono C.; Toyoda M.; Zahradnik J.; Saito A.; Nasser H.; Tan T. S.; Ngare I.; Kimura I.; Uriu K.; Kosugi Y.; et al. SARS-CoV-2 Spike L452R Variant Evades Cellular Immunity and Increases Infectivity. Cell Host Microbe 2021, 29, 1124–1136.e11. 10.1016/j.chom.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C.; Sun X.; Ling Z.; Sun B. Jigsaw Puzzle of SARS-CoV-2 RBD Evolution and Immune Escape. Cell. Mol. Immunol. 2022, 19, 848–851. 10.1038/s41423-022-00884-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchesnokova V.; Kulasekara H.; Larson L.; Bowers V.; Rechkina E.; Kisiela D.; Sledneva Y.; Choudhury D.; Maslova I.; Deng K.; et al. Acquisition of the L452R Mutation in the ACE2-Binding Interface of Spike Protein Triggers Recent Massive Expansion of SARS- CoV-2 Variants. J. Clin. Microbiol. 2021, 59, 10. 10.1128/jcm.00921-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmina A.; Wattad S.; Khalaila Y.; Ottolenghi A.; Rosental B.; Engel S.; Rosenberg E.; Taube R. SARS CoV-2 Delta Variant Exhibits Enhanced Infectivity and a Minor Decrease in Neutralization Sensitivity to Convalescent or Post-Vaccination Sera. iScience 2021, 24, 103467. 10.1016/j.isci.2021.103467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D.; Sun Y.; Zhou J.; Ye Q. The Global Epidemic of the SARS-CoV-2 Delta Variant, Key Spike Mutations and Immune Escape. Front. Immunol. 2021, 12, 751778. 10.3389/fimmu.2021.751778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhun H.; Park H.-Y.; Hisham Y.; Song C.-S.; Kim S. SARS-CoV-2 Delta (B.1.617.2) Variant: A Unique T478K Mutation in Receptor Binding Motif (RBM) of Spike Gene. Immune Netw. 2021, 21, e32 10.4110/in.2021.21.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian S.; Potdar V.; Jadhav S.; Yadav P.; Gupta N.; Das M.; Rakshit P.; Singh S.; Abraham P.; Panda S.; Team N. SARS-CoV-2 Spike Mutations, L452R, T478K, E484Q and P681R, in the Second Wave of COVID-19 in Maharashtra, India. Microorganisms 2021, 9, 1542. 10.3390/microorganisms9071542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan M.; Sharma A.; Priyanka; Thakur N.; Rajkhowa T. K.; Choudhary O. P. Delta Variant (B.1.617.2) of SARS-CoV-2: Mutations, Impact, Challenges and Possible Solutions. Hum. Vaccines Immunother. 2022, 18, 2068883. 10.1080/21645515.2022.2068883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goher S. S.; Ali F.; Amin M. The Delta Variant Mutations in the Receptor Binding Domain of SARS-CoV-2 Show Enhanced Electrostatic Interactions with the ACE2. Med. Drug Discovery 2022, 13, 100114. 10.1016/j.medidd.2021.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Liu J.; Johnson B. A.; Xia H.; Ku Z.; Schindewolf C.; Widen S. G.; An Z.; Weaver S. C.; Menachery V. D.; et al. Delta Spike P681R Mutation Enhances SARS-CoV-2 Fitness over Alpha Variant. Cell Rep. 2022, 39, 110829. 10.1016/j.celrep.2022.110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A.; Irie T.; Suzuki R.; Maemura T.; Nasser H.; Uriu K.; Kosugi Y.; Shirakawa K.; Sadamasu K.; Kimura I.; et al. Enhanced Fusogenicity and Pathogenicity of SARS-CoV-2 Delta P681R Mutation. Nature 2022, 602, 300–306. 10.1038/s41586-021-04266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiehzadegan S.; Alaghemand N.; Fox M.; Venketaraman V. Analysis of the Delta Variant B.1.617.2 COVID-19. Clin. Pract. 2021, 11, 778–784. 10.3390/clinpract11040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y.; Yin H.; Yin J.-Y. B. B.1.617.2 (Delta) Variant of SARS-CoV-2: features, transmission and potential strategies. Int. J. Biol. Sci. 2022, 18, 1844–1851. 10.7150/ijbs.66881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L.; Gao Q.; Gao F.; Wang Q.; He Q.; Wu X.; Mao Q.; Xu M.; Liang Z. Impact of the Delta Variant on Vaccine Efficacy and Response Strategies. Expert Rev. Vaccines 2021, 20, 1201–1209. 10.1080/14760584.2021.1976153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E. Heavily Mutated Omicron Variant Puts Scientists on Alert. Nature 2021, 600, 21. 10.1038/d41586-021-03552-w. [DOI] [PubMed] [Google Scholar]
- Lupala C. S.; Ye Y.; Chen H.; Su X.-D.; Liu H. Mutations on RBD of SARS-CoV-2 Omicron Variant Result in Stronger Binding to Human ACE2 Receptor. Biochem. Biophys. Res. Commun. 2022, 590, 34–41. 10.1016/j.bbrc.2021.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa C. H. S.; de Freitas C. A. B.; Alves C. N.; Lameira J. Assessment of Mutations on RBD in the Spike Protein of SARS-CoV-2 Alpha, Delta and Omicron Variants. Sci. Rep. 2022, 12, 8540. 10.1038/s41598-022-12479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Zhou L.; Mo M.; Liu T.; Wu C.; Gong C.; Lu K.; Gong L.; Zhu W.; Xu Z. SARS-CoV-2 Omicron RBD Shows Weaker Binding Affinity than the Currently Dominant Delta Variant to Human ACE2. Signal Transduction Targeted Ther. 2022, 7, 8. 10.1038/s41392-021-00863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed A. M.; Ciling A.; Taha T. Y.; Chen I. P.; Khalid M. M.; Sreekumar B.; Chen P.-Y.; Kumar G. R.; Suryawanshi R.; Silva I.; et al. Omicron Mutations Enhance Infectivity and Reduce Antibody Neutralization of SARS-CoV-2 Virus-like Particles. Proc. Natl. Acad. Sci. U.S.A. 2022, 119, e2200592119 10.1073/pnas.2200592119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N. L.; Clark T.; Raman R.; Sasisekharan R. Insights on the Mutational Landscape of the SARS-CoV-2 Omicron Variant Receptor-Binding Domain. Cell Rep. Med. 2022, 3, 100527. 10.1016/j.xcrm.2022.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.; Li X.; Zhang L.; Wan S.; Zhang L.; Zhou F. SARS-CoV-2 Omicron Variant: Recent Progress and Future Perspectives. Signal Transduction Targeted Ther. 2022, 7, 141. 10.1038/s41392-022-00997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni C.; Valdes A. M.; Polidori L.; Antonelli M.; Penamakuri S.; Nogal A.; Louca P.; May A.; Figueiredo J. C.; Hu C.; et al. Symptom Prevalence, Duration, and Risk of Hospital Admission in Individuals Infected with SARS-CoV-2 during Periods of Omicron and Delta Variant Dominance: A Prospective Observational Study from the ZOE COVID Study. Lancet 2022, 399, 1618–1624. 10.1016/s0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.; Yisimayi A.; Jian F.; Song W.; Xiao T.; Wang L.; Du S.; Wang J.; Li Q.; Chen X.; et al. BA.2.12.1, BA.4 and BA.5 Escape Antibodies Elicited by Omicron Infection. Nature 2022, 608, 593–602. 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P.; Zhang L.; Krüger N.; Rocha C.; Sidarovich A.; Schulz S.; Kempf A.; Graichen L.; Moldenhauer A.-S.; Cossmann A.; et al. SARS-CoV-2 Omicron Sublineages Show Comparable Cell Entry but Differential Neutralization by Therapeutic Antibodies. Cell Host Microbe 2022, 30, 1103–1111.e6. 10.1016/j.chom.2022.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iketani S.; Liu L.; Guo Y.; Liu L.; Chan J. F.-W.; Huang Y.; Wang M.; Luo Y.; Yu J.; Chu H.; et al. Antibody Evasion Properties of SARS-CoV-2 Omicron Sublineages. Nature 2022, 604, 553–556. 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurhade C.; Zou J.; Xia H.; Liu M.; Yang Q.; Cutler M.; Cooper D.; Muik A.; Sahin U.; Jansen K. U.; et al. Neutralization of Omicron Sublineages and Deltacron SARS-CoV-2 by Three Doses of BNT162b2 Vaccine or BA.1 Infection. Emerg. Microb. Infect. 2022, 11, 1828–1832. 10.1080/22221751.2022.2099305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer-Smadja N.; Bridier-Nahmias A.; Ferré V. M.; Charpentier C.; Garé M.; Rioux C.; Allemand A.; Lavallée P.; Ghosn J.; Kramer L.; et al. Emergence of E484K Mutation Following Bamlanivimab Monotherapy among High-Risk Patients Infected with the Alpha Variant of SARS-CoV-2. Viruses 2021, 13, 1642. 10.3390/v13081642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. K.; Knabl L.; Knabl L.; Wieser M.; Mur A.; Zabernigg A.; Schumacher J.; Kapferer S.; Kaiser N.; Furth P. A.; et al. Immune Transcriptome Analysis of COVID-19 Patients Infected with SARS-CoV-2 Variants Carrying the E484K Escape Mutation Identifies a Distinct Gene Module. Sci. Rep. 2022, 12, 2784. 10.1038/s41598-022-06752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.-T.; Huang W.-H.; Liao T.-L.; Hsiao T.-H.; Chuang H.-N.; Liu P.-Y. SARS-CoV-2 E484K Mutation Narrative Review: Epidemiology, Immune Escape, Clinical Implications, and Future Considerations. Infect. Drug Resist. 2022, 15, 373–385. 10.2147/idr.s344099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S. R.; Spratt A. N.; Cohen A. R.; Naqvi S. H.; Chand H. S.; Quinn T. P.; Lorson C. L.; Byrareddy S. N.; Singh K. Evolutionary Analysis of the Delta and Delta Plus Variants of the SARS-CoV-2 Viruses. J. Autoimmun. 2021, 124, 102715. 10.1016/j.jaut.2021.102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda V. P.; Apostolopoulos V. Global impact of delta plus variant and vaccination. Expert Rev. Vaccines 2022, 21, 597–600. 10.1080/14760584.2022.2044800. [DOI] [PubMed] [Google Scholar]
- Rahman F. I.; Ether S. A.; Islam M. R. The “Delta Plus” COVID-19 Variant Has Evolved to Become the next Potential Variant of Concern: Mutation History and Measures of Prevention. J. Basic Clin. Physiol. Pharmacol. 2022, 33, 109–112. 10.1515/jbcpp-2021-0251. [DOI] [PubMed] [Google Scholar]
- Lan J.; Ge J.; Yu J.; Shan S.; Zhou H.; Fan S.; Zhang Q.; Shi X.; Wang Q.; Zhang L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Han P.; Su C.; Zhang Y.; Bai C.; Zheng A.; Qiao C.; Wang Q.; Niu S.; Chen Q.; Zhang Y.; et al. Molecular Insights into Receptor Binding of Recent Emerging SARS-CoV-2 Variants. Nat. Commun. 2021, 12, 6103. 10.1038/s41467-021-26401-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Zhang Y.; Wu L.; Niu S.; Song C.; Zhang Z.; Lu G.; Qiao C.; Hu Y.; Yuen K.-Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e9. 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P.; Li L.; Liu S.; Wang Q.; Zhang D.; Xu Z.; Han P.; Li X.; Peng Q.; Su C.; et al. Receptor Binding and Complex Structures of Human ACE2 to Spike RBD from Omicron and Delta SARS-CoV-2. Cell 2022, 185, 630–640.e10. 10.1016/j.cell.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W.; Zhou D.; Supasa P.; Liu C.; Mentzer A. J.; Ginn H. M.; Zhao Y.; Duyvesteyn H. M. E.; Tuekprakhon A.; Nutalai R.; et al. Antibody Evasion by the P.1 Strain of SARS-CoV-2. Cell 2021, 184, 2939–2954.e9. 10.1016/j.cell.2021.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutalai R.; Zhou D.; Tuekprakhon A.; Ginn H. M.; Supasa P.; Liu C.; Huo J.; Mentzer A. J.; Duyvesteyn H. M. E.; Dijokaite-Guraliuc A.; et al. Potent Cross-Reactive Antibodies Following Omicron Breakthrough in Vaccinees. Cell 2022, 185, 2116–2131.e18. 10.1016/j.cell.2022.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Liao H.; Meng Y.; Li W.; Han P.; Liu K.; Wang Q.; Li D.; Zhang Y.; Wang L.; et al. Structural Basis of Human ACE2 Higher Binding Affinity to Currently Circulating Omicron SARS-CoV-2 Sub-Variants BA.2 and BA.1.1. Cell 2022, 185, 2952–2960.e10. 10.1016/j.cell.2022.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W.; Zhou D.; Ginn H. M.; Duyvesteyn H. M. E.; Supasa P.; Case J. B.; Zhao Y.; Walter T. S.; Mentzer A. J.; Liu C.; et al. The Antigenic Anatomy of SARS-CoV-2 Receptor Binding Domain. Cell 2021, 184, 2183. 10.1016/j.cell.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S.; Liu P.; Zhang Z.; Xiao T.; Yasimayi A.; Huang W.; Wang Y.; Cao Y.; Xie X. S.; Xiao J. Structures of SARS-CoV-2 B.1.351 Neutralizing Antibodies Provide Insights into Cocktail Design against Concerning Variants. Cell Res. 2021, 31, 1130–1133. 10.1038/s41422-021-00555-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeil S. M.-C.; Janowska K.; McDowell S.; Mansouri K.; Parks R.; Stalls V.; Kopp M. F.; Manne K.; Li D.; Wiehe K.; et al. Effect of Natural Mutations of SARS-CoV-2 on Spike Structure, Conformation, and Antigenicity. Science 2021, 373, eabi6226 10.1126/science.abi6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.; Wang P.; Nair M. S.; Yu J.; Rapp M.; Wang Q.; Luo Y.; Chan J. F.-W.; Sahi V.; Figueroa A.; Guo X. V.; et al. Potent Neutralizing Antibodies against Multiple Epitopes on SARS-CoV-2 Spike. Nature 2020, 584, 450–456. 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- McCallum M.; Bassi J.; De Marco A.; Chen A.; Walls A. C.; Di Iulio J.; Tortorici M. A.; Navarro M.-J.; Silacci-Fregni C.; Saliba C.; et al. SARS-CoV-2 Immune Evasion by the B.1.427/B.1.429 Variant of Concern. Science 2021, 373, 648–654. 10.1126/science.abi7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pymm P.; Adair A.; Chan L.-J.; Cooney J. P.; Mordant F. L.; Allison C. C.; Lopez E.; Haycroft E. R.; O’Neill M. T.; Tan L. L.; et al. Nanobody Cocktails Potently Neutralize SARS-CoV-2 D614G N501Y Variant and Protect Mice. Proc. Natl. Acad. Sci. U.S.A. 2021, 118, e2101918118 10.1073/pnas.2101918118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supasa P.; Zhou D.; Dejnirattisai W.; Liu C.; Mentzer A. J.; Ginn H. M.; Zhao Y.; Duyvesteyn H. M. E.; Nutalai R.; Tuekprakhon A.; et al. Reduced Neutralization of SARS-CoV-2 B.1.1.7 Variant by Convalescent and Vaccine Sera. Cell 2021, 184, 2201–2211.e7. 10.1016/j.cell.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Xiao T.; Cai Y.; Lavine C. L.; Peng H.; Zhu H.; Anand K.; Tong P.; Gautam A.; Mayer M. L.; et al. Membrane Fusion and Immune Evasion by the Spike Protein of SARS-CoV-2 Delta Variant. Science 2021, 374, 1353–1360. 10.1126/science.abl9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.; Mannar D.; Srivastava S. S.; Berezuk A. M.; Demers J.-P.; Saville J. W.; Leopold K.; Li W.; Dimitrov D. S.; Tuttle K. S.; et al. Cryo-Electron Microscopy Structures of the N501Y SARS-CoV-2 Spike Protein in Complex with ACE2 and 2 Potent Neutralizing Antibodies. PLoS Biol. 2021, 19, e3001237 10.1371/journal.pbio.3001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. E. F.; Rezaei R.; Jamieson T. R.; Dave J.; Martin N. T.; Singaravelu R.; Crupi M. J. F.; Boulton S.; Tucker S.; Duong J.; et al. Characterization of Critical Determinants of ACE2–SARS CoV-2 RBD Interaction. Int. J. Mol. Sci. 2021, 22, 2268. 10.3390/ijms22052268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M. H.; Porritt R. A.; Rivas M. N.; Krieger J. M.; Ozdemir A. B.; Garcia G.; Arumugaswami V.; Fries B. C.; Arditi M.; Bahar I. A Monoclonal Antibody against Staphylococcal Enterotoxin B Superantigen Inhibits SARS-CoV-2 Entry in Vitro. Structure 2021, 29, 951–962.e3. 10.1016/j.str.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.; Zia T.; Suleman M.; Khan T.; Ali S. S.; Abbasi A. A.; Mohammad A.; Wei D. Higher Infectivity of the SARS-CoV-2 New Variants Is Associated with K417N/T, E484K, and N501Y Mutants: An Insight from Structural Data. J. Cell. Physiol. 2021, 236, 7045–7057. 10.1002/jcp.30367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik I.; Khan A.; Dwivany F. M.; Fatimawali; Wei D.-Q.; Tallei T. E. Computational Prediction of the Effect of Mutations in the Receptor-Binding Domain on the Interaction between SARS-CoV-2 and Human ACE2. Mol. Divers. 2022, 26, 3309–3324. 10.1007/s11030-022-10392-x. [DOI] [PubMed] [Google Scholar]
- Rath S. L.; Kumar K. Investigation of the Effect of Temperature on the Structure of SARS-CoV-2 Spike Protein by Molecular Dynamics Simulations. Front. Mol. Biosci. 2020, 7, 583523. 10.3389/fmolb.2020.583523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deganutti G.; Prischi F.; Reynolds C. A. Supervised Molecular Dynamics for Exploring the Druggability of the SARS-CoV-2 Spike Protein. J. Comput. Aided Mol. Des. 2021, 35, 195–207. 10.1007/s10822-020-00356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.; Choi Y. K.; Frank M.; Woo H.; Park S.-J.; Yeom M. S.; Seok C.; Im W. Dynamic Interactions of Fully Glycosylated SARS-CoV-2 Spike Protein with Various Antibodies. J. Chem. Theory Comput. 2021, 17, 6559–6569. 10.1021/acs.jctc.1c00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M. H.; Krieger J. M.; Banerjee A.; Xiang Y.; Kaynak B.; Shi Y.; Arditi M.; Bahar I. Impact of New Variants on SARS-CoV-2 Infectivity and Neutralization: A Molecular Assessment of the Alterations in the Spike-Host Protein Interactions. iScience 2022, 25, 103939. 10.1016/j.isci.2022.103939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C. O.; Jette C. A.; Abernathy M. E.; Dam K.-M. A.; Esswein S. R.; Gristick H. B.; Malyutin A. G.; Sharaf N. G.; Huey-Tubman K. E.; Lee Y. E.; et al. SARS-CoV-2 Neutralizing Antibody Structures Inform Therapeutic Strategies. Nature 2020, 588, 682–687. 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti G.; Rapp M.; Guo Y.; Bahna F.; Bimela J.; Reddem E. R.; Yu J.; Wang P.; Liu L.; Huang Y.; et al. Structural Basis for Accommodation of Emerging B.1.351 and B.1.1.7 Variants by Two Potent SARS-CoV-2 Neutralizing Antibodies. Structure 2021, 29, 655–663.e4. 10.1016/j.str.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney A. J.; Starr T. N.; Barnes C. O.; Weisblum Y.; Schmidt F.; Caskey M.; Gaebler C.; Cho A.; Agudelo M.; Finkin S.; et al. Mapping Mutations to the SARS-CoV-2 RBD That Escape Binding by Different Classes of Antibodies. Nat. Commun. 2021, 12, 4196. 10.1038/s41467-021-24435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. F.; Zhao F.; Huang D.; Beutler N.; Burns A.; He W.; Limbo O.; Smith C.; Song G.; Woehl J.; et al. Isolation of Potent SARS-CoV-2 Neutralizing Antibodies and Protection from Disease in a Small Animal Model. Science 2020, 369, 956–963. 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley A. K.; Pymm P.; Esterbauer R.; Dietrich M. H.; Lee W. S.; Drew D.; Kelly H. G.; Chan L.-J.; Mordant F. L.; Black K. A.; et al. Landscape of Human Antibody Recognition of the SARS-CoV-2 Receptor Binding Domain. Cell Rep. 2021, 37, 109822. 10.1016/j.celrep.2021.109822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y.; Nambulli S.; Xiao Z.; Liu H.; Sang Z.; Duprex W. P.; Schneidman-Duhovny D.; Zhang C.; Shi Y. Versatile and Multivalent Nanobodies Efficiently Neutralize SARS-CoV-2. Science 2020, 370, 1479–1484. 10.1126/science.abe4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M.; Liu H.; Wu N. C.; Wilson I. A. Recognition of the SARS-CoV-2 Receptor Binding Domain by Neutralizing Antibodies. Biochem. Biophys. Res. Commun. 2021, 538, 192–203. 10.1016/j.bbrc.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J.; Zost S. J.; Greaney A. J.; Starr T. N.; Dingens A. S.; Chen E. C.; Chen R. E.; Case J. B.; Sutton R. E.; Gilchuk P.; et al. Genetic and Structural Basis for SARS-CoV-2 Variant Neutralization by a Two-Antibody Cocktail. Nat. Microbiol. 2021, 6, 1233–1244. 10.1038/s41564-021-00972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney A. J.; Loes A. N.; Crawford K. H. D.; Starr T. N.; Malone K. D.; Chu H. Y.; Bloom J. D. Comprehensive Mapping of Mutations in the SARS-CoV-2 Receptor-Binding Domain That Affect Recognition by Polyclonal Human Plasma Antibodies. Cell Host Microbe 2021, 29, 463–476.e6. 10.1016/j.chom.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T. N.; Greaney A. J.; Hilton S. K.; Ellis D.; Crawford K. H. D.; Dingens A. S.; Navarro M. J.; Bowen J. E.; Tortorici M. A.; Walls A. C.; et al. Deep Mutational Scanning of SARS-CoV-2 Receptor Binding Domain Reveals Constraints on Folding and ACE2 Binding. Cell 2020, 182, 1295. 10.1016/j.cell.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T. N.; Greaney A. J.; Addetia A.; Hannon W. W.; Choudhary M. C.; Dingens A. S.; Li J. Z.; Bloom J. D. Prospective Mapping of Viral Mutations That Escape Antibodies Used to Treat COVID-19. Science 2021, 371, 850–854. 10.1126/science.abf9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T. N.; Greaney A. J.; Dingens A. S.; Bloom J. D. Complete Map of SARS-CoV-2 RBD Mutations That Escape the Monoclonal Antibody LY-CoV555 and Its Cocktail with LY-CoV016. Cell Rep. Med. 2021, 2, 100255. 10.1016/j.xcrm.2021.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; VanBlargan L. A.; Bloyet L.-M.; Rothlauf P. W.; Chen R. E.; Stumpf S.; Zhao H.; Errico J. M.; Theel E. S.; Liebeskind M. J.; et al. Identification of SARS-CoV-2 Spike Mutations That Attenuate Monoclonal and Serum Antibody Neutralization. Cell Host Microbe 2021, 29, 477–488.e4. 10.1016/j.chom.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muecksch F.; Weisblum Y.; Barnes C. O.; Schmidt F.; Schaefer-Babajew D.; Wang Z.; C Lorenzi J. C.; Flyak A. I.; DeLaitsch A. T.; Huey-Tubman K. E.; Huey-Tubman K. E.; et al. Affinity Maturation of SARS-CoV-2 Neutralizing Antibodies Confers Potency, Breadth, and Resilience to Viral Escape Mutations. Immunity 2021, 54, 1853–1868.e7. 10.1016/j.immuni.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradník J.; Marciano S.; Shemesh M.; Zoler E.; Harari D.; Chiaravalli J.; Meyer B.; Rudich Y.; Li C.; Marton I.; et al. SARS-CoV-2 Variant Prediction and Antiviral Drug Design Are Enabled by RBD in Vitro Evolution. Nat. Microbiol. 2021, 6, 1188–1198. 10.1038/s41564-021-00954-4. [DOI] [PubMed] [Google Scholar]
- Sánchez-Sendra B.; Albert E.; Zulaica J.; Torres I.; Giménez E.; Botija P.; Beltrán M. J.; Rodado C.; Geller R.; Navarro D. Neutralizing Antibodies against SARS-CoV-2 Variants of Concern Elicited by the Comirnaty COVID-19 Vaccine in Nursing Home Residents. Sci. Rep. 2022, 12, 3788. 10.1038/s41598-022-07849-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L.; Czartoski J.; Wan Y.-H.; Homad L. J.; Rubin V.; Glantz H.; Neradilek M.; Seydoux E.; Jennewein M. F.; MacCamy A. J.; et al. MRNA Vaccination Boosts Cross-Variant Neutralizing Antibodies Elicited by SARS-CoV-2 Infection. Science 2021, 372, 1413–1418. 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman H. M.; Westbrook J.; Feng Z.; Gilliland G.; Bhat T. N.; Weissig H.; Shindyalov I. N.; Bourne P. E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannar D.; Saville J. W.; Zhu X.; Srivastava S. S.; Berezuk A. M.; Tuttle K. S.; Marquez A. C.; Sekirov I.; Subramaniam S. SARS-CoV-2 Omicron Variant: Antibody Evasion and Cryo-EM Structure of Spike Protein–ACE2 Complex. Science 2022, 375, 760–764. 10.1126/science.abn7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søndergaard C. R.; Olsson M. H. M.; Rostkowski M.; Jensen J. H. Improved Treatment of Ligands and Coupling Effects in Empirical Calculation and Rationalization of pKa Values. J. Chem. Theory Comput. 2011, 7, 2284–2295. 10.1021/ct200133y. [DOI] [PubMed] [Google Scholar]
- Berendsen H. J. C.; van der Spoel D.; van Drunen R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. 10.1016/0010-4655(95)00042-e. [DOI] [Google Scholar]
- Huang J.; Rauscher S.; Nawrocki G.; Ran T.; Feig M.; de Groot B. L.; Grubmüller H.; MacKerell A. D. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Nat. Methods 2017, 14, 71–73. 10.1038/nmeth.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark P.; Nilsson L. Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. 10.1021/jp003020w. [DOI] [Google Scholar]
- Darden T.; York D.; Pedersen L. Particle Mesh Ewald: An N log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1993, 98, 10089–10092. 10.1063/1.464397. [DOI] [Google Scholar]
- Humphrey W.; Dalke A.; Schulten K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Kumari R.; Kumar R.; Lynn A. Open Source Drug Discovery Consortium; Lynn, A. G_mmpbsa —A GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. 10.1021/ci500020m. [DOI] [PubMed] [Google Scholar]
- Jurrus E.; Engel D.; Star K.; Monson K.; Brandi J.; Felberg L. E.; Brookes D. H.; Wilson L.; Chen J.; Liles K.; et al. Improvements to the APBS Biomolecular Solvation Software Suite. Protein Sci. 2018, 27, 112–128. 10.1002/pro.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoete V.; Michielin O. Comparison between Computational Alanine Scanning and Per-Residue Binding Free Energy Decomposition for Protein-Protein Association Using MM-GBSA: Application to the TCR-p-MHC Complex. Proteins 2007, 67, 1026–1047. 10.1002/prot.21395. [DOI] [PubMed] [Google Scholar]
- Kimura S. R.; Brower R. C.; Vajda S.; Camacho C. J. Dynamical View of the Positions of Key Side Chains in Protein-Protein Recognition. Biophys. J. 2001, 80, 635–642. 10.1016/s0006-3495(01)76044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P.; Su C.; Zhang Y.; Bai C.; Zheng A.; Qiao C.; Wang Q.; Niu S.; Chen Q.; Zhang Y.; et al. Molecular Insights into Receptor Binding of Recent Emerging SARS-CoV-2 Variants. Nat. Commun. 2021, 12, 6103. 10.1038/s41467-021-26401-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socher E.; Conrad M.; Heger L.; Paulsen F.; Sticht H.; Zunke F.; Arnold P. Computational Decomposition Reveals Reshaping of the SARS-CoV-2–ACE2 Interface among Viral Variants Expressing the N501Y Mutation. J. Cell. Biochem. 2021, 122, 1863–1872. 10.1002/jcb.30142. [DOI] [PubMed] [Google Scholar]
- Jawad B.; Adhikari P.; Podgornik R.; Ching W.-Y. Key Interacting Residues between RBD of SARS-CoV-2 and ACE2 Receptor: Combination of Molecular Dynamics Simulation and Density Functional Calculation. J. Chem. Inf. Model. 2021, 61, 4425–4441. 10.1021/acs.jcim.1c00560. [DOI] [PubMed] [Google Scholar]
- Rath S. L.; Padhi A. K.; Mandal N. Scanning the RBD-ACE2 molecular interactions in Omicron variant. Biochem. Biophys. Res. Commun. 2022, 592, 18–23. 10.1016/j.bbrc.2022.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafary F.; Jafari S.; Ganjalikhany M. R. In silico investigation of critical binding pattern in SARS-CoV-2 spike protein with angiotensin-converting enzyme 2. Sci. Rep. 2021, 11, 6927. 10.1038/s41598-021-86380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal N.; Padhi A. K.; Rath S. L. Molecular Insights into the Differential Dynamics of SARS-CoV-2 Variants of Concern. J. Mol. Graph. Model. 2022, 114, 108194. 10.1016/j.jmgm.2022.108194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoula M.; Naceri S.; Sitruk S.; Flatters D.; Moroy G.; Camproux A. C. Identifying Promising Druggable Binding Sites and Their Flexibility to Target the Receptor-Binding Domain of SARS-CoV-2 Spike Protein. Comput. Struct. Biotechnol. J. 2023, 21, 2339–2351. 10.1016/j.csbj.2023.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan H. H.; Twaddle A.; Marchand B.; Gunsalus K. C. Structural Modeling of the SARS-CoV-2 Spike/Human ACE2 Complex Interface Can Identify High-Affinity Variants Associated with Increased Transmissibility. J. Mol. Biol. 2021, 433, 167051. 10.1016/j.jmb.2021.167051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forest-Nault C.; Koyuturk I.; Gaudreault J.; Pelletier A.; L’Abbé D.; Cass B.; Bisson L.; Burlacu A.; Delafosse L.; Stuible M.; et al. Impact of the Temperature on the Interactions between Common Variants of the SARS-CoV-2 Receptor Binding Domain and the Human ACE2. Sci. Rep. 2022, 12, 11520. 10.1038/s41598-022-15215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H.; Chen Q.; Yang G.; He L.; Fan H.; Deng Y.-Q.; Wang Y.; Teng Y.; Zhao Z.; Cui Y.; et al. Adaptation of SARS-CoV-2 in BALB/c Mice for Testing Vaccine Efficacy. Science 2020, 369, 1603–1607. 10.1126/science.abc4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P.-Y.; Xie X.; Zou J.; Fontes-Garfias C.; Xia H.; Swanson K.; Cutler M.; Cooper D.; Menachery V.; Weaver S.; Dormitzer P.. Neutralization of N501Y Mutant SARS-CoV-2 by BNT162b2 Vaccine-Elicited Sera, 2021. Preprint (In Review), 10.21203/rs.3.rs-143532/v1. [DOI] [PubMed] [Google Scholar]
- Sang P.; Chen Y.-Q.; Liu M.-T.; Wang Y.-T.; Yue T.; Li Y.; Yin Y.-R.; Yang L.-Q. Electrostatic Interactions Are the Primary Determinant of the Binding Affinity of SARS-CoV-2 Spike RBD to ACE2: A Computational Case Study of Omicron Variants. Int. J. Mol. Sci. 2022, 23, 14796. 10.3390/ijms232314796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focosi D.; Maggi F. Neutralising Antibody Escape of SARS-CoV-2 Spike Protein: Risk Assessment for Antibody-based Covid-19 Therapeutics and Vaccines. Rev. Med. Virol. 2021, 31, e2231 10.1002/rmv.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani M.; Brooks B. R.; Klauda J. B. Critical Sequence Hotspots for Binding of Novel Coronavirus to Angiotensin Converter Enzyme as Evaluated by Molecular Simulations. J. Phys. Chem. B 2020, 124, 10034–10047. 10.1021/acs.jpcb.0c05994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavani M.; Riofrío W. A.; Arciniega M. Molecular Dynamics and MM-PBSA Analysis of the SARS-CoV-2 Gamma Variant in Complex with the HACE-2 Receptor. Molecules 2022, 27, 2370. 10.3390/molecules27072370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. B.; Liang Y.; Jin Y. Q.; Zhang J.; Su J. G.; Li Q. M. E484K Mutation in SARS-CoV-2 RBD Enhances Binding Affinity with HACE2 but Reduces Interactions with Neutralizing Antibodies and Nanobodies: Binding Free Energy Calculation Studies. J. Mol. Graph. Model. 2021, 109, 108035. 10.1016/j.jmgm.2021.108035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano E.; Piccini G.; Licastro D.; Casalino L.; Johnson N. V.; Paciello I.; Dal Monego S.; Pantano E.; Manganaro N.; Manenti A.; et al. SARS-CoV-2 Escape from a Highly Neutralizing COVID-19 Convalescent Plasma. Proc. Natl. Acad. Sci. U.S.A. 2021, 118, e2103154118 10.1073/pnas.2103154118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeeva A. P.; Katsamba P. S.; Sampson J. M.; Bahna F.; Mannepalli S.; Morano N. C.; Shapiro L.; Friesner R. A.; Honig B.. Free Energy Perturbation Calculations of Mutation Effects on SARS-CoV-2 RBD::ACE2 Binding Affinity. 2022, Preprint. 10.1101/2022.08.01.502301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Boorla V. S.; Banerjee D.; Chowdhury R.; Cavener V. S.; Nissly R. H.; Gontu A.; Boyle N. R.; Vandegrift K.; Nair M. S.; et al. Computational Prediction of the Effect of Amino Acid Changes on the Binding Affinity between SARS-CoV-2 Spike RBD and Human ACE2. Proc. Natl. Acad. Sci. U.S.A. 2021, 118, e2106480118 10.1073/pnas.2106480118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.