Abstract
Hyperandrogenism, insulin resistance, and acanthosis nigricans (HAIR-AN) is a severe subphenotype of polycystic ovary syndrome (PCOS). A 32-year-old woman with HAIR-AN and class 3 obesity presented to an endocrinology clinic after she failed sequential trials of treatment with metformin, estrogen–progestin OCP, spironolactone, leuprolide, and a levonorgestrel intrauterine device. She complained of hirsutism and acanthosis nigricans severely affecting her quality of life and had secondary amenorrhea. Laboratory evaluation showed extremely elevated testosterone and insulin levels and elevated glycated hemoglobin A1c (HbA1c). She underwent laparoscopic sleeve gastrectomy. One year after the surgery, she lost 32% of her body weight and reported normalization of menses, dramatic improvement in hirsutism, and near-resolution of acanthosis nigricans. Her testosterone, insulin, and HbA1c normalized. This case demonstrates the central role of hyperinsulinemia in HAIR-AN and suggests that aggressive measures to normalize insulin resistance and reduce excess weight can effectively treat the reproductive abnormalities in this syndrome. We suggest that bariatric surgery can be an effective cure for HAIR-AN syndrome and that PCOS, including HAIR-AN, should be considered a comorbidity of obesity during evaluation of bariatric surgery candidates.
Keywords: HAIR-AN, hyperandrogenism, insulin resistance, acanthosis nigricans, bariatric surgery, sleeve gastrectomy
Androgen excess disorders, mainly polycystic ovary syndrome (PCOS), affect approximately 10% of women of childbearing age and increase the risks of many comorbidities including type 2 diabetes, cardiovascular disease, obstructive sleep apnea, and others. Hyperandrogenism, insulin resistance, and acanthosis nigricans (HAIR-AN) syndrome is a severe subphenotype of PCOS. Patients with HAIR-AN present with severe insulin resistance, in addition to features of PCOS (oligomenorrhea or amenorrhea secondary to oligo-ovulation or anovulation, clinical and/or biochemical hyperandrogenism, and polycystic ovarian morphology). While some degree of insulin resistance occurs in most cases of PCOS [1], the extreme severity of insulin resistance in HAIR-AN, believed to be due to genetic defects in the insulin signaling pathway [2, 3], leads to manifestations of acanthosis nigricans and central obesity.
HAIR-AN is both a metabolic and reproductive disease. It manifests as an insulin paradox in which ovarian and adrenal tissues remain sensitive to the stimulatory and mitogenic effects of insulin, while major metabolic tissues including skeletal muscle, liver, and adipose tissue are markedly insulin resistant [3]. The resulting hyperinsulinemia stimulates ovarian growth and androgen production and suppresses sex hormone–binding globulin, resulting in increased circulating free androgens. Insulin actions in keratinocytes lead to the development of acanthosis nigricans, which, along with hirsutism, is often the chief complaint of patients presenting with HAIR-AN. Hyperandrogenemia stimulates high-frequency pulses of gonadotropin-releasing hormone (GnRH), which favors luteinizing hormone release relative to follicle-stimulating hormone, perpetuating ovarian hormone imbalance and leading to follicle arrest, anovulation, and cystic follicles.
Diagnosis of HAIR-AN is primarily clinical and is supported by laboratory measurements, ovarian imaging, and exclusion of other endocrinopathies. Medical management of HAIR-AN typically includes targeting androgen excess and hyperinsulinemia through a multipharmaceutical strategy, in combination with lifestyle modifications to reduce excess weight. Treatment with estrogen-progestin oral contraceptives, androgen receptor antagonist spironolactone, and 5-alpha reductase inhibitor flutamide has been reported [4] to alleviate hirsutism. Metabolic derangements in HAIR-AN are often targeted with the insulin sensitizer metformin. Use of the glucagon-like peptide 1 (GLP-1) receptor agonist liraglutide was reported in a recent case series, resulting in improved metabolic profiles and menstrual regularity [5]. Use of the GnRH analogue leuprolide acetate has been reported as well. Most of these treatments resulted in only partial symptomatic and aesthetic relief or required long-term therapy to maintain therapeutic effect. Recently, sleeve gastrectomy was reported to result in weight loss and normalization of insulin sensitivity, but persistently elevated testosterone, in an adolescent with HAIR-AN [6].
Here we report a case of nearly complete symptom resolution, biochemical normalization of reproductive and metabolic parameters, and resumption of menses in a 32-year-old woman with HAIR-AN syndrome following sleeve gastrectomy. This case supports the role of insulin resistance as the primary driver of HAIR-AN and suggests that bariatric surgery should be considered as a potentially curative treatment for this syndrome.
Case Presentation
A 32-year-old nulliparous African American woman with a past medical history of HAIR-AN syndrome and obstructive sleep apnea was referred to the endocrinology clinic with chief complaints of acanthosis nigricans and hirsutism. She reported that her quality of life was negatively affected by distress due to worsening patches of thick, hyperpigmented skin on her neck, chest, and underarms, and terminal facial hair requiring daily shaving. She was also concerned about weight gain and irregular menses. She had gained approximately 40 kg in the previous 7 years despite adhering to a healthy diet as counseled by a registered dietitian and exercising regularly with a trainer. Her menstrual cycles had been irregular and infrequent since menarche at age 14. Family history was notable for diabetes, hypertension, hyperlipidemia, and stroke in her close relatives. Past surgical and social histories were noncontributory.
Current medications included metformin 500 mg twice daily and a levonorgestrel 20 mcg intrauterine device. Prior medical treatments had included sequential trials of metformin, spironolactone, combined desogestrel–ethinyl estradiol oral contraceptive (alone and in combination with a levonorgestrel intrauterine device), and leuprolide in combination with norethindrone. She was unable to increase the dose of metformin because of gastrointestinal side effects. Spironolactone was ineffective for reducing hirsutism after 3 years of treatment. Leuprolide treatment had resulted in normalization of her testosterone, but she discontinued it because of intolerable hot flashes and depression. She had been evaluated for bariatric surgery 4 years prior, but was deemed not a candidate because of an insufficiently high body mass index (BMI) of 39 and lack of obesity-related comorbidities.
Diagnostic Assessment
The patient was well appearing, with blood pressure of 129/85 mm Hg and pulse of 88 beats/min. Her weight was 136 kg (299 lb 12.8 oz), height was 1.753 m (5 feet 9 inches), and BMI was 44.27. She had predominantly abdominal adiposity. Terminal hair was present on the chin and cheeks. Patches of acanthosis nigricans were present on the dorsal neck, underarms, and folds of skin on the abdomen and under the breasts. The remainder of the exam was normal.
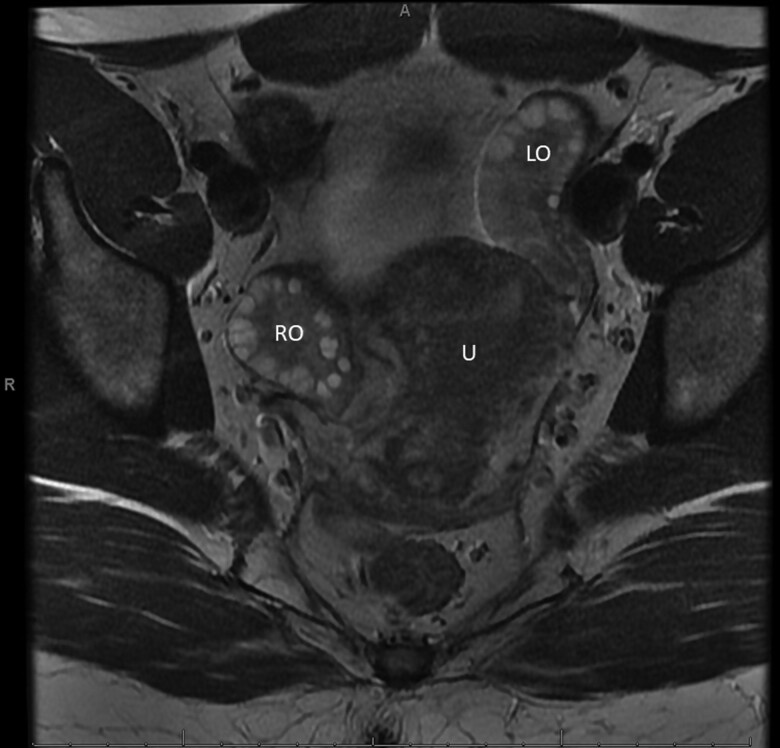
Prior diagnostic assessment was notable for elevated testosterone, insulin, glucose, and HbA1c (Table 1). An endometrial biopsy showed disordered proliferative endometrium. Pelvic magnetic resonance imaging did not find an ovarian tumor, but showed enlarged ovaries (right, 4.7 × 3.2 × 4.1 cm and left, 4.4 × 2.4 × 4.9 cm) with abnormally large numbers of mid-size follicles (approximately 20 follicles per ovary in a single plane, Fig. 1). Transvaginal ultrasound confirmed polycystic ovarian morphology and increased stroma and volume. Screening for other endocrinopathies included normal 17-hydroxyprogesterone, thyroid function tests, prolactin, and cortisol level drawn at 10:50 Am (see Table 1).
Table 1.
Laboratory values before and after surgery
| Reference range | 6 y before surgery | 6 mo before surgery | 14 mo after surgery | |
|---|---|---|---|---|
| Total testosterone | 9-55 ng/dL (0.31-1.91 nmol/L) | 182 (6.32) | 191 (6.63) | 30 (1.04) |
| Free testosterone | 0.06-1.06 ng/dL (0.002-0.037 nmol/L) | 2.76 (0.096) | 5.35 (0.186) | 0.69 (0.024) |
| Bioavailable testosterone | 2.2-20.6 ng/dL (0.076-0.715 nmol/L) | 81.0 (2.811) | ||
| Sex hormone–binding globulin | 1.9-12.35 ug/mL (20-130 nmol/L) | 3.14 (33) | 4.18 (44) | |
| Estradiol | 13-166 pg/mL (47-609 pmol/L) | 47 (173) | 29 (106) | |
| Luteinizing hormone | 2.4-12.6 mIU/mL | 9.1 | 8.5 | |
| Follicle-stimulating hormone | 3.5-12.5 mIU/mL | 5.6 | 7.3 | |
| Prolactin | 4.8-23.3 ng/mL (209-1013 pmol/L) | 14.5 (630) | ||
| Insulin | 3-25 μIU/mL (21-174 pmol/L) | > 1000 (> 6945) | 70 (486) | 17 (118) |
| C-peptide | 1.1-4.4 ng/mL (0.37-1.47 nmol/L) | 5.5 (1.83) | ||
| Glucose | 60-99 mg/dL (3.3-5.5 mmol/L) | 102 (5.7) | 86 (4.8) | |
| Hemoglobin A1c | ≤ 5.6% | 5.8 | 6.6 | 5.2 |
| 17-Hydroxyprogesterone | ≤ 206 ng/dL (≤ 6.23 nmol/L) | 137 (4.15) | ||
| TSH | 0.27-4.20 μIU/mL | 2.16 | ||
| Free T4 | 0.9-1.7 ng/dL (11.6-21.9 pmol/L) | 1.1 (14.1) | ||
| Cortisol | N/A, μg/dL (nmol/L) | 9.4 (259) |
Laboratory values are shown in conventional units, with SI units in parentheses where applicable. Total testosterone was measured by liquid chromatography–tandem mass spectrometry; free testosterone was measured by equilibrium dialysis; all other tests were performed with standard laboratory methods. For estradiol, luteinizing hormone, and follicle-stimulating hormone, reference ranges for the follicular phase are reported. Empty cells represent missing values. Values outside of the reference range are shown in bold.
Abbreviations: N/A, not available; T4, thyroxine; TSH, thyrotropin.
Figure 1.
Axial plane view of pelvic magnetic resonance imaging. LO, left ovary; RO, right ovary; U, uterus.
Treatment
The patient was referred to bariatric surgery for reevaluation. She then underwent laparoscopic sleeve gastrectomy without complications. Briefly, a 36-French tube was inserted along the lesser curvature of the stomach and used as a guide to staple and remove the stomach tissue along the greater curvature. Intraoperative findings included fatty liver. Peak weight loss at 1 year post operation was 47.5 kg.
Outcome and Follow-up
The patient presented to the endocrine clinic 14 months post operation with total weight loss of 43.3 kg (32% of her preoperative body mass). She was not taking any medications. She reported regular monthly menses, dramatic improvement in hirsutism, and near-resolution of acanthosis nigricans. The symptoms of HAIR-AN were no longer distressing to her. Additionally, her obstructive sleep apnea had resolved. Biochemical evaluation was notable for normal testosterone, insulin, and HbA1c (see Table 1). Pelvic ultrasound 7 months post operation showed reduced ovarian volume compared to the preoperative magnetic resonance imaging (right ovary, 17.5 vs 32.3 cc; left ovary, 12.8 vs 27.1 cc).
Discussion
HAIR-AN syndrome is a severe subphenotype of PCOS characterized by a vicious cycle of hyperandrogenism and hyperinsulinemia. Medical management is challenging and often insufficient for symptom resolution. Our patient failed almost all available medical treatments including metformin, spironolactone, and combined oral contraceptive, and was unable to tolerate leuprolide. Treatment with GLP-1 receptor agonist was not attempted, though literature suggests it can be effective for symptoms of HAIR-AN [5]. However, she experienced dramatic resolution of her symptoms alongside above-average weight loss after sleeve gastrectomy. One year after the surgery, there was no clinical or biochemical evidence of hyperandrogenism or insulin resistance, and eumenorrhea was restored, suggesting that the bariatric procedure was curative for this patient. While bariatric surgery has been studied extensively and shown to be effective in PCOS [7], this is the first report to our knowledge of such a procedure to normalize testosterone in a patient with HAIR-AN syndrome.
Many experts note that insulin sensitivity improves significantly within hours after sleeve gastrectomy, and this improvement becomes more dramatic over the following weeks. This case suggests that normalization of insulin resistance and weight loss are sufficient to normalize the reproductive defects, including hormone imbalance and menstrual irregularity, in HAIR-AN. While ample evidence points to interconnections between androgen and insulin signaling pathways in PCOS, this case demonstrates that the vicious cycle of HAIR-AN can be interrupted by targeting hyperinsulinemia and excess weight. It highlights the central role of insulin resistance in this complex syndrome and supports a model of this disease in which insulin acts directly on the ovary to stimulate androgen production. This effect can be highly clinically significant: In a recent case report, a patient with severe insulin resistance was treated with a GnRH analogue resulting in gonadotropin suppression, but experienced persistent multifollicular ovarian growth [8], demonstrating the mitogenic actions of insulin on the ovary.
Contrary to the standard-of-care approach, which focuses primarily on reduction of hyperandrogenism, we suggest that aggressive measures to improve systemic insulin sensitivity should be considered when managing HAIR-AN syndrome. If there is no improvement after at least 1 year of medical management, surgical approaches may be considered. This may include a reconsideration of the current criteria for bariatric surgery, which led to a delay in surgical treatment for our patient until she developed class 3 obesity (BMI > 40). PCOS is associated with cardiovascular disease and poor cardiovascular outcomes [9], while bariatric surgery reduces cardiovascular mortality [10]. We therefore suggest that PCOS and HAIR-AN should be considered indications for bariatric surgery in women with class 2 obesity (BMI 35-40).
Limitations in the evaluation and management of this patient included the lack of conclusive testing to rule out Cushing syndrome and a missed opportunity to try treatment with a GLP receptor agonist before proceeding with bariatric surgery.
Learning Points
HAIR-AN is a severe manifestation of PCOS caused by insulin signaling defects.
Hyperinsulinemia can stimulate ovarian androgen production.
Both reproductive and metabolic symptoms of HAIR-AN can be effectively treated with bariatric surgery.
Abbreviations
- BMI
body mass index
- GLP-1
glucagon-like peptide 1
- GnRH
gonadotropin-releasing hormone
- HAIR-AN
hyperandrogenism, insulin resistance, and acanthosis nigricans
- HbA1c
glycated hemoglobin A1c
- PCOS
polycystic ovary syndrome
Contributor Information
Zoe Lewin, Department of Medicine, University of Rochester School of Medicine and Dentistry, Rochester, New York 14642, USA.
Wendy S Vitek, Department of Obstetrics and Gynecology, University of Rochester School of Medicine and Dentistry, Rochester, New York 14642, USA.
William O’Malley, Department of Surgery, University of Rochester School of Medicine and Dentistry, Rochester, New York 14642, USA.
Olga Astapova, Department of Medicine, University of Rochester School of Medicine and Dentistry, Rochester, New York 14642, USA.
Contributors
All authors made individual contributions to authorship. W.V. and O.A. were involved in the endocrine diagnosis and medical management of this patient. W.O.M. performed the patient's surgery. Z.L. and O.A. prepared the manuscript. All authors reviewed and approved the final draft.
Funding
This work was supported by the University of Rochester Department of Medicine Pilot Award Program.
Disclosures
The authors have nothing to disclose.
Informed Patient Consent for Publication
Signed informed consent could not be obtained from the patient or a proxy but has been approved by the treating institution.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the present study.
References
- 1. Barber TM, Dimitriadis GK, Andreou A, Franks S. Polycystic ovary syndrome: insight into pathogenesis and a common association with insulin resistance. Clin Med (Lond). 2016;16(3):262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kahn CR, Flier JS, Bar RS, et al. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976;294(14):739–745. [DOI] [PubMed] [Google Scholar]
- 3. Venkatesan AM, Dunaif A, Corbould A. Insulin resistance in polycystic ovary syndrome: progress and paradoxes. Recent Prog Horm Res. 2001;56(1):295–308. [DOI] [PubMed] [Google Scholar]
- 4. Zemtsov A, Wilson L. Successful treatment of hirsutism in HAIR-AN syndrome using flutamide, spironolactone, and birth control therapy. Arch Dermatol. 1997;133(4):431–433. [PubMed] [Google Scholar]
- 5. Livadas S, Androulakis I, Angelopoulos N, Lytras A, Papagiannopoulos F, Kassi G. Liraglutide administration improves hormonal/metabolic profile and reproductive features in women with HAIR-AN syndrome. Endocrinol Diabetes Metab Case Rep. 2020;2020(1):19-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Brien B, Dahiya R, Kimble R. Hyperandrogenism, insulin resistance and acanthosis nigricans (HAIR-AN syndrome): an extreme subphenotype of polycystic ovary syndrome. BMJ Case Rep. 2020;13(4):e231749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li YJ, Han Y, He B. Effects of bariatric surgery on obese polycystic ovary syndrome: a systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15(6):942–950. [DOI] [PubMed] [Google Scholar]
- 8. Singh P, Agress A, Madrigal VK, et al. Massive ovarian growth in a woman with severe insulin-resistant polycystic ovary syndrome receiving GnRH analogue. J Clin Endocrinol Metab. 2019;104(7):2796–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berni TR, Morgan CL, Rees DA. Women with polycystic ovary syndrome have an increased risk of major cardiovascular events: a population study. J Clin Endocrinol Metab. 2021;106(9):e3369–e3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doumouras AG, Lee Y, Paterson JM, et al. Association between bariatric surgery and major adverse diabetes outcomes in patients with diabetes and obesity. JAMA Netw Open. 2021;4(4):e216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the present study.