Abstract
Background
Side-effect concerns are a major barrier to vaccination against COVID-19 and other diseases. Identifying cost- and time-efficient interventions to improve vaccine experience and reduce vaccine hesitancy—without withholding information about side effects—is critical.
Purpose
Determine whether a brief symptom as positive signals mindset intervention can improve vaccine experience and reduce vaccine hesitancy after the COVID-19 vaccination.
Methods
English-speaking adults (18+) were recruited during the 15-min wait period after receiving their second dose of the Pfizer COVID-19 vaccination and were randomly allocated to the symptom as positive signals mindset condition or the treatment as usual control. Participants in the mindset intervention viewed a 3:43-min video explaining how the body responds to vaccinations and how common side effects such as fatigue, sore arm, and fever are signs that the vaccination is helping the body boost immunity. The control group received standard vaccination center information.
Results
Mindset participants (N = 260) versus controls (N = 268) reported significantly less worry about symptoms at day 3 [t(506)=2.60, p=.01, d=0.23], fewer symptoms immediately following the vaccine [t(484)=2.75, p=.006, d=0.24], and increased intentions to vaccinate against viruses like COVID-19 in the future [t(514)=−2.57, p=.01, d=0.22]. No significant differences for side-effect frequency at day 3, coping, or impact.
Conclusions
This study supports the use of a brief video aimed at reframing symptoms as positive signals to reduce worry and increase future vaccine intentions.
Clinical Trial information
Australian New Zealand Clinical Trials Registry: ACTRN12621000722897p.
Keywords: Vaccination, Mindset, COVID-19, Health Communication, Symptoms
A 4-minute video aimed at reframing symptoms as positive signals lessens worry, reduces symptoms, and increase future vaccine intentions for patients receiving the covid-19 vaccine.
Introduction
The majority of side effects experienced after a vaccination are normal and can indicate the start of establishing immunity [1, 2]. Side effects, such as body aches and mild fever, reveal that the vaccination is working to stimulate the immune system to form antibodies against the infection [3]. Yet, vaccine side effects are not always regarded in this positive light [4]. At best, people may experience side effects as bothersome and unfortunate byproducts of vaccination. Others worry that side effects indicate that their body is particularly sensitive to the vaccine. At worst, people may misinterpret side effects as a sign that the vaccine has caused them to become sick with the infection that the vaccine is designed to protect against or with an idiopathic illness. Such worries can influence how people experience side effects, whether they will take medications and how they respond to them. In a recent international report, concerns about side effects emerged as the primary barrier to COVID-19 vaccine uptake [5].
The process of informed consent requires informing individuals of possible side effects prior to receiving vaccination. Research has shown that expectations of and anxieties about symptoms can increase the awareness and reporting of adverse effects, a process known as the nocebo effect [6]. Studies have found that negative expectations generated by warnings of side effects, or even general awareness of potential adverse reactions to a treatment, can heighten the chance of their occurrence [7]. This issue is complex, as a number of patients do not report any side effects following COVID-19 vaccination and there is no definitive linear relationship between the number and strength of reported side effects and larger immune response [2, 8].
Previous studies suggest that the method and manner of framing and delivering medical information can impact patients’ side-effect reporting [9, 10]. This puts healthcare providers in the difficult position of needing to fully inform patients without causing unnecessary harm or discouraging a treatment that may be beneficial for them [4]. An emerging approach attempts to achieve both goals: It involves truthfully describing minor side effects as a possible indication that a treatment is active and working in the body (see Leibowitz et al. for review) [4, 11–13]. This nuanced approach focuses on instilling adaptive mindsets about the meaning of side effects while still informing patients about them [4].
Research on this approach shows that such interventions can be helpful in improving symptom experiences and outcomes in treatments for pain, hypertension, and allergy. Compared with control groups given a standard, empathetic message about side effects, patients who were informed that side effects may be a sign that treatment is working, were less anxious about side effects and rated them as less threatening and intense [4]. A longitudinal, randomized controlled trial of this approach in patients receiving oral immunotherapy for food allergies found that describing side effects as possible indications that the treatment was working reduced anxiety and lowered the rate at which patients contacted providers with concerns about side effects [13]. Furthermore, evidence suggests this approach does not negatively affect patients who do not experience side effects [10].
The current study is a parallel two-arm randomized controlled trial investigating whether instilling the mindset that side effects of the COVID-19 vaccination can be positive signals of the vaccination working, via a brief (<4-min) animated video, affects the experience of those side effects, as well as future vaccination intentions for COVID-like viruses.
Methods
Setting and Participants
Individuals were recruited from two large community COVID-19 vaccination sites in Auckland, New Zealand from October 2, 2021 to October 24, 2021. Individuals were recruited for the study during their standard 15-min wait time after receiving their vaccination.
Eligible participants were adults over the age of 18 who had a current and active email address, had just received their second Pfizer COVID-19 vaccination, and were English speaking. Completed participants were 50.7% male, 47.8% female, and 1.5% gender diverse, and reported the following ethnicities: NZ European = 53.8%; Maori = 4.5%; Chinese = 4.5%; Indian = 4.4%; Samoan = 0.9%; Cook Island Maori = 0.8%; Tongan = 0.6%; Niuean = 0.2%; or other = 30.3%. See Table 1 for additional details.
Table 1.
Sample Characteristics of Completed Participants in Intent to Treat Analysis
| Control (n = 268) | Intervention (n = 260) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age | 35.17 | 10.90 | 35.51 | 12.50 |
| Vaccine History (0–5) | 2.47 | 1.70 | 2.47 | 1.68 |
| Vaccine Sensitivity (1–6) | 1.88 | 0.90 | 1.94 | 0.85 |
| Antivax Attitudes (1–6) | 2.28 | 0.80 | 2.40 | 0.85 |
| Gender | ||||
| Male | n = 138 | n = 129 | ||
| Female | n = 125 | n = 127 | ||
| Gender diverse | n = 4 | n = 4 | ||
| Ethnicity | ||||
| NZ European | n = 144 | n = 140 | ||
| Other | n = 124 | n = 120 | ||
Note: Vaccine History is the number of flu shots in the past 5 years (0–5). Vaccine Sensitivity measured with four items of the Brief Perceived Sensitivity to Medication Scale with “medicines” replaced by “vaccinations” [14] (1 = strongly disagree to 6 = strongly agree). Anti-Vaccine Attitudes measured with four items of the Vaccination Attitudes Examination Scale [15] (1 = strongly disagree to 6 = strongly agree). Dropout rates did not significantly differ by condition (15% in the treatment condition and 17% in the control condition) (F = 0.076, p < .783, η2 = 0.000) and there were no differences between participants in the control and mindset intervention conditions with respect to age, gender, vaccine history, or antivax attitudes (all ps > .11) suggesting that missingness was not selective to randomized condition [16].
Ethical approval was obtained from the Health and Disability Ethics Committee (21/CEN/143) and locality approval from the Auckland District Health Board. The study was registered on the Australian New Zealand Clinical Trials Registry (ACTRN12621000722897p). Detailed hypotheses and analytical plans were preregistered on Open Science Framework (OSF) (https://osf.io/2c6a3). Deidentified individual participant data that underlie the results reported in this article in addition to study materials and code are also available on OSF.
Intervention
If randomized to the symptom as positive signals mindset group, participants were shown a short (3:43 min) interventional video that explains how the body responds to vaccinations and how these side effects are signs that the vaccination is working. This information was based on mechanistic evidence implying a link between the development of symptoms as part of the vaccination process and also experimental research linking vaccines with antibody published in JAMA Internal Medicine (e.g., [1]) and information that was already prominently displayed on leading authority websites including the CDC, HHS, and WHO [17–19]. Specifically, the voiceover in our intervention stated:
Understanding how vaccines really work can help us know why some people experience symptoms after receiving the vaccine. It’s common for some to have symptoms such as fever, fatigue, headache, muscle ache, and joint pain in the first few days after getting the vaccine. While it may seem natural to worry about these symptoms, these are actually signs that your body is building immunity and the vaccine is doing what it was designed to do. If you have a particularly hard workout at the gym, your muscles might feel sore, but that’s a sign that your body is getting stronger. Similarly, this vaccine is giving your immune system a workout. This might cause some side effects, but these side effects are a sign that your body is getting stronger and is prepared in case it encounters the virus.
Though the above evidence suggests that side effects are associated with antibody response, research also documented that nearly everyone, regardless of whether or not they experience symptoms, indicates an antibody response to the vaccine which can confer protections (see both Ankuda et al. [1] and Hermann et al. [2] for example). For these reasons, we also included the following information in the intervention condition:
Some people may not experience any symptoms at all. This is okay too. The vaccine is still working and your body is still building immunity. Everyone is different, and we all respond differently to the immune system training that is going on in the body. It’s important to know that your body is working hard to recognize and defeat the covid virus and is growing stronger to prevent you from getting sick if you are exposed to it. Knowing that these symptoms are positive signals that the vaccine is working and the body is building immunity can make these side effects easier to handle. Of course, if your symptoms are more than you can handle or if they last more than a few days, you should reach out to your doctor.
We developed two videos matched to participant gender based on previous studies showing a stronger response to gender consistent models [20–23]. Both the female and male patient videos can be viewed at https://mbl.stanford.edu/. Gender diverse (N = 4) participants were matched to the male patient video.
Figure 1 shows screenshots of the video alongside a summary of key content. This video was made in collaboration with the Stanford Center for Health Education and reviewed by several immunology and infectious disease specialists at the University of Auckland.
Fig. 1.

Screenshots from the Symptoms as Positive Signals Mindset video. Note: The first half of the video (A) informs participants about how the COVID-19 vaccine works in their bodies, including that (B) it triggers the body’s natural immune response (C) because it contains instructions that tell the body’s cells how to make an imitation of the spike protein, which (D) calls the immune system to prepare to fight, as if it is facing the real virus; it explains that (E) this preparation of the immune system is like training soldiers to prepare for battle and (F) the immune system is then prepared to fight the real virus. The second half of the video explains why one experiences symptoms following the vaccine, stating that (G) it is common to have symptoms such as fever, fatigue, headache, muscle ache, and joint pain in the first few days after getting the vaccine; (H) it is natural to worry about these symptoms, but they are signs that the body is building immunity and the vaccine is doing what it was designed to do; (I) these symptoms are akin to the body being sore after a gym workout, which is a sign that the body is getting stronger; (J) side effects are a sign that the body is getting stronger and is prepared in case it encounters the virus; (K) experiencing no symptoms is also okay, and the vaccine will still work; (L) if symptoms are more than can be handled or last more than a few days, one should seek medical support. Animated films were produced as a collaboration with the Stanford Center for Health Education Digital Medic and Creative Frontiers https://www.cfrontiers.com/. Both the female and male patient videos can be viewed at https://mbl.stanford.edu/.
The treatment as usual control group only received standard care. For the New Zealand COVID-19 vaccination clinics, this included verbal information from the vaccination center nurse administering the vaccine, which covered common side effects of the Pfizer COVID-19 vaccination.
Data Collection Procedure
Potential participants were invited to take part in a study evaluating the effect of receiving different types of information about the COVID-19 vaccination. All eligible and willing participants provided informed written consent. Participants were provided an iPad and were instructed to read the instructions carefully before answering each set of questions. They began the questionnaire by answering demographic questions (age, gender, ethnicity), followed by questions about previous vaccinations, attitudes toward vaccinations [15], and perceived sensitivity to vaccinations [14], before being randomized to either the mindset intervention or treatment as usual control.
Immediately following the video, participants were asked to rate the information received about the vaccine and side effects in terms of how easy it was to understand, how interesting it was, and how reassuring it was [24]. These questions were presented on a 10-point Likert scale ranging from (1) “not at all” to (10) “extremely.” They were also asked a series of questions to assess their mindsets about the symptoms and their experience of symptoms.
Three days after the vaccination, both groups were emailed a link to Qualtrics to complete the follow-up questionnaire. This questionnaire assessed primary outcomes of side-effect frequency, worry, impact, coping, and intentions (described in more detail below). All 3-day follow-up questionnaires returned were automatically entered into a drawing to win a $500 shopping voucher prize. Participants who did not respond to the follow-up email within 48 hr were sent a reminder email.
Measures
Mindsets about symptoms
The intervention was developed with the intention of instilling the mindset that symptoms can be signs of treatment efficacy. To measure this mindset, participants were asked how much they agreed (1 = strongly disagree; 6 = strongly agree) with the following statement: “Side effects are a sign that the covid-19 vaccine is working.”
Side-effect information evaluation
Participants were asked to rate the information received about the vaccine and side effects in term of how easy it was to understand, how interesting it was and how reassuring it was (1 = not at all; 10 = extremely) a mean of three items based on a measure developed by Crichton and Petrie [24].
Side-effect experiences
At the 3-day follow-up, participants were asked how worried they were about the side effects on a 10-point Likert-style scale (1 = not at all; 10 = extremely), and how bothered they were by the side effects in general and by pain at the injection site.
Side-effect frequency
Immediately following the vaccination and 3 days later participants were asked to report the number of symptoms they experienced. A list of 15 side effects taken from the Side Effect Attribution Scale (SEAS) was presented to participants at the end of the wait period following their first vaccination [25]. These (mostly local) side effects are based on Center for Disease Control and Prevention data as those most frequently reported immediately following the COVID-19 vaccination (e.g., redness at injection site, muscle ache or pain around injection site) [26]. Participants were asked if they had experienced each symptom, with a choice between “yes” or “no.” Three days after vaccination, participants completed the same 15-item measure and 13 additional general symptom items (e.g., cough, vomiting, breathing problems).
Side-effect coping behaviors
At the 3-day follow-up, participants were asked to indicate the degree to which the side effects interfered with their work and other activities on a 10-point Likert-style scale (1 = not at all; 10 = extremely) including the number of days they took off of work as a result of side effects. They were asked whether they contacted a healthcare provider because of side effects (Yes or No) and had taken medication to manage side effects (Yes or No).
Future vaccine intentions
At the 3-day follow-up, participants were asked a series of questions regarding their vaccination intentions: “After getting the COVID-19 vaccine I am less likely to get other vaccinations”; “I will get vaccinated for other viruses like COVID-19 in the future”; “I will get a booster vaccine for COVID-19 if someone recommended it to me”; and “I will recommend the COVID-19 vaccine to family and friends.” Participants rated their agreement with these questions on a six-point Likert-style scale (1 = strongly disagree to 6 = strongly agree).
Vaccine history and attitudes (baseline)
Before randomization to condition participants were asked to complete a series of questions regarding their vaccination history and attitudes. Vaccine history was measured by a single-item measure asking participants how many influenza vaccines they received in five past years. Vaccine Sensitivity was measured with four items of the Brief Perceived Sensitivity to Medication Scale with “medication” replaced by “vaccination” (1 = strongly disagree to 6 = strongly agree) [14]. Anti-Vaccine Attitudes measured with four items of the Vaccination Attitudes Examination Scale (1 = strongly disagree to 6 = strongly agree) [15].
Data, measures, and code can be found on the OSF website.
Sample Size and Power Analysis
A power analysis was conducted with G*Power to calculate the required sample size [27]. Based on Howe et al. [13] and other mindset intervention studies [28, 29], we planned to recruit enough participants to detect a small- to medium-effect size (d = 0.3). With 95% power and 0.05 alpha level, a sample of approximately 290 participants per condition was desired.
Randomization and Blinding
Eligible participants were randomly allocated using block randomization into one of the two conditions: the symptoms as positive signals intervention condition and the control condition. This was done automatically on Qualtrics, a secure online survey software. Qualtrics incorporates a computerized random order generator that randomly presents an interventional video to the symptoms as positive signals condition and no video to the control condition. Qualtrics uses the simple randomization method and was programmed to present videos with an approximate 1:1 ratio. Participants in the intervention condition were gender matched to a video presenting their chosen gender. See Fig. 2 for recruitment flow.
Fig. 2.

Consort diagram showing recruitment flow.
The recruitment and consent process was completed before randomization occurred (see procedure for details). As such, the researcher was blind to participants’ condition throughout recruitment and consent. Similarly, participants were blind to their group allocation and study hypotheses for the duration of the study.
Data Analysis
Primary and secondary outcomes were analyzed using independent samples t-tests and chi-square tests for continuous and categorical outcomes, respectively. Across all items, the skew was <3.0; thus, no data transformations were performed. SPSS software (version 27) was used for all analyses. A two-sided p value of <.05 was used to indicate significance.
Results
Randomization and Dropout
Participants who did not complete follow-up were more likely to be male (t(174) = −2.57, p = .01, d = 0.26), younger (t(541) = −3.83, p < .001, d = 0.40) and have higher antivax attitudes (t(154) = 3.42, p = .002, d = 0.35) but were not different with respect to ethnicity, vaccine sensitivity, or mindset (all ps > .05) [16]. Dropout rates did not significantly differ by condition (15% in the treatment condition and 17% in the control condition) (t(641) = −0.275, p = .783, d = 0.02) and there were no significant differences between participants in the control and mindset intervention conditions with respect to age, gender, ethnicity, vaccine history, or antivax attitudes (all ps > .05) (see Table 1 for baseline characteristics of completed participants) suggesting that missingness was not selective to randomized condition [16]. The final sample thus included 528 adults (260 intervention; 268 control; Mage = 35.3, SD = 11.7).
Manipulation Check: Change in Mindset
Individuals who received the Symptoms as Positive Signals (MINDSET) intervention endorsed the mindset of symptoms as positive signals to a greater extent (M = 4.92, SD = 0.91) than individuals who did not receive the intervention (M = 4.29, SD = 1.19) (t(500) = −6.9, p < .001, d = 0.60). They also reported information that they received about the vaccine more positively (M = 8.41, SD = 1.38) than did individuals who did not receive the intervention (M = 7.82, SD = 1.53) (t(523) = −4.69, p < .001, d = 0.41) (Fig. 3).
Fig. 3.
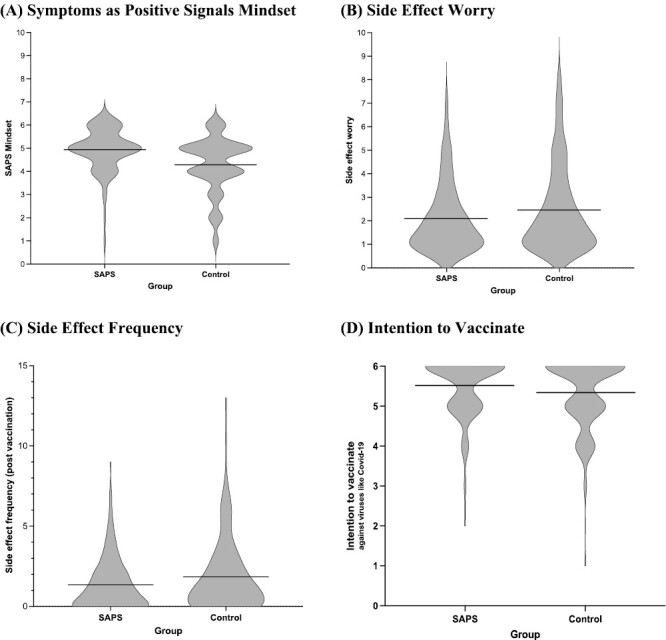
Effects of the mindset intervention versus control on (A) symptoms as positive signals mindset, (B) side-effect worry, (C) side-effect frequency (postvaccination), and (D) intention to vaccinate against viruses like COVID-19.
Differences in Outcome Measures
Side-effect experiences (primary outcome)
Three days after vaccination, individuals who received the mindset intervention reported being less worried about their side effects (M = 2.10, SD = 1.44) than individuals who did not receive the mindset intervention (M = 2.47, SD = 1.82) (t(506)= 2.60, p = .01, d = 0.23). There were no significant differences between the two groups in how bothered they were by their side effects (t(526) = −1.54, p = .12, d = 0.13) or bothersomeness of pain at the site of injection (t(526) = −0.12, p = .91, d = 0.01).
Frequency of side effects
Individuals who received the mindset intervention reported significantly fewer side effects in the waiting period after receiving their vaccine (M = 1.35, SD = 1.63) compared with those who did not receive the mindset intervention (M = 1.83, SD = 2.29; t(484)= 2.75, p = .006, d = 0.24). There was no statistically significant difference in the experience of side effects at the 3-day follow-up (t(524) = −1.52, p = .13, d = 0.13).
Future vaccine intentions
Individuals who received the mindset intervention reported that they would be more likely to get vaccinated against viruses like COVID-19 in the future (M = 5.52, SD = 0.74) than individuals who did not receive the mindset intervention (M = 5.34, SD = 0.90) (t(514) = −2.57, p = .01, d = 0.22) and also were more likely to disagree with the statement that they would be less likely to get vaccinated against anything in the future (M = 1.41, SD = 0.69) compared with individuals who did not receive the mindset intervention (M = 1.63, SD = 1.05) (t(466) = 2.94, p = .003, d = 0.26). There were no significant differences between groups in intentions to get a booster vaccine for COVID-19 (t(525) = −0.29, p = .78, d = 0.03) or to recommend the COVID-19 vaccine to friends and family (t(524 = 0.17, p = .87, d = 0.01).
Side-effect coping behaviors
There were no significant differences between groups in the extent to which individuals reported taking medication to manage their side effects (χ2 = 0.52, p = .47). Only 3% of the sample endorsed contacting a healthcare professional as a result of their side effects; thus, this variable was not analyzed.
Negative impact of side effects
There were no significant differences between the two groups in how side effects impacted their day-to-day work and activities (t(526) = −0.86, p = .39, d = 0.08). There was also no significant difference between groups in the number of days taken off work (t(452) = −1.27, p = .20, d = 0.12) or whether or not individuals took time off work because of their side effects (χ2 = 1.08, p = .30).
Mediation Model
A 5,000-sample bootstrapping mediation analysis was used to explore whether changes in the symptoms as positive signals mindset could help explain the changes in vaccine intentions [30]. This model determined whether the association between intervention condition (X) and intentions vaccinated against viruses like COVID-19 in the future (Y) was mediated by differences the symptoms as positive signals mindset (M). Results revealed that differences in vaccine intentions were fully mediated by mindset (indirect effect = 0.10, 95% CI [0.048, 0.162]) (Fig. 4).
Fig. 4.

Change in symptoms as positive signals mindset mediates effect of condition on the intentions to vaccinate against future viruses. *p < .05, ***p < .001.
Discussion
To our knowledge, this is the first trial to investigate the effects of a brief video administered to encourage patients to have a more adaptive mindset toward side effects of the COVID-19 vaccine and improve the treatment experience. We found that participants who were randomized to the mindset intervention were more likely to endorse the mindset that side effects could be positive signs that the vaccine was working than were those in the control group. The mindset group, in comparison with the control group, reported fewer side effects in the waiting period following the vaccination and were less worried about their side effects at 3 days. The brief intervention video also had an impact on future vaccine intentions, with participants who were randomized to the mindset group reporting that they were more likely to get vaccinated against viruses like COVID-19 in the future. We did not find that the intervention had an effect on coping, time off work, or use of medical services, possibly because of the low levels of these behaviors in our sample.
Limitations
This study has several limitations that suggest directions for future research. First, while we intended to recruit participants following their first dose of the COVID-19 vaccine, New Zealand COVID lockdown restrictions prevented us from doing so. It is unknown what impact the video intervention would have had at this first dose, when participants were more naive about side effects. Second, patients watched the videos after receiving the vaccine. While this was intentional, the effects of delivering the intervention before the vaccine, including on vaccine behaviors, is unknown. Third, the control group was not matched to the intervention group on attention or time. The fact that the symptoms as possibly positive signals mindset was a significant mediator of future vaccine intentions supports our theorized mechanisms that content framing symptoms as potentially positive signals was the primary driver of change. However, other interpretations of the mechanism (e.g., merely watching any film or other content within the film not related to the symptoms framing) are possible. Future research is needed to experimentally probe and parse apart the potential mechanisms potentially driving these effects to understand both what is driving the effects herein and also how best to convey this particular mindset in the future (e.g., Could the message be as effectively conveyed in a sentence or a non-animated video? Would the effects be stronger if the intervention was even longer, more elaborate or delivered in person?). Fourth, while the brevity of the intervention is a strength, in terms of accessibility and implementation, a larger dose of intervention (e.g., longer or multiple videos with more information or accompanying learning exercises) may have influenced other outcomes, such as side-effect impact. Fifth, the sample comprised individuals who were already attending a vaccine clinic within a country where attitudes toward the vaccine were overarchingly positive. The effects of the video intervention approach on a sample of a population with greater vaccine hesitancy are unknown. In general the effects of this intervention were small (d’s ranging from 0.2 to 0.4), yet considering the intervention constituted of a <4-min video they is notable. A stronger dose of the intervention may generate larger effects.
Implications
Vaccine hesitancy is a major problem [31], and concerns about side effects are a major barrier to COVID-19 vaccination and other vaccines [5]. Research shows many of the side effects are driven by patients’ negative expectations, with high rates of side effects reported in the placebo condition of the COVID-19 trials [32], and 76% of reported systemic side effects determined to be nocebo responses after the first dose and 51% after second dose. These findings suggest that psychological interventions have potential to reduce the large number of reported side effects as a result of the nocebo effect and thus influence patients subsequent hesitancy to get future vaccines. This method could readily be applied in many areas of medicine to reduce side effects and improve the patient’s experience of treatment. The study design tested a video intervention in a naturalistic setting compared with treatment as usual, meaning it could be easily scaled and implemented as a regular part of COVID-19 vaccination.
Conclusions
The study results add to a growing literature showing that paying attention to the social and psychological context in which a treatment is delivered can meaningfully influence treatment decisions, adherence, experience, and outcomes [13, 25, 33, 34].The study further suggests that “psychologically wise interventions” such as those targeting mindsets may be particularly useful because they do not need to be lengthy or delivered in person but can make use of tablet or digital technology and still be perceived as credible and rated positively by patients [35, 36]. This enables such interventions to be easily adapted for ordinary healthcare settings.
The COVID-19 vaccine is critical in reducing the spread and mortality of the SARS-CoV-1 virus, yet uptake has been polarized and problematic. This study demonstrated that watching a brief (<4-min) video after vaccination explaining how vaccines work and how the experience of symptoms can be positive signals of treatment efficacy led to improved mindsets, reduced worry, reduced frequency of symptoms immediately following vaccination, and increased intentions to vaccinate in the future.
Acknowledgments
The authors are grateful for the collaboration with Stanford Center for Health Education Digital Medic and Creative Fronteirs for the production of the animated films. We also thank Krystal Wright, Sasha Finlay, Emma Lund, and Emerson Bartholomew for help in data collection, Dawson Ward for logistic support at vaccination centers and and Denise Snoad for assistance with the videos.
Contributor Information
Alia J Crum, Department of Psychology, Stanford University, Stanford, CA, USA.
Lauren C Heathcote, Health Psychology Section, Department of Psychology, Institute of Psychiatry Psychology and Neuroscience, King’s College London, London, UK.
Zara Morrison, Department of Psychological Medicine, University of Auckland, Auckland, New Zealand.
Rachael Yielder, Department of Psychological Medicine, University of Auckland, Auckland, New Zealand.
Kari Leibowitz, Department of Psychology, Stanford University, Stanford, CA, USA.
Helen Petousis-Harris, Department of General Practice and Primary Care, University of Auckland, Auckland, New Zealand.
Mark G Thomas, Department of Molecular Medicine and Pathology, University of Auckland, Auckland, New Zealand.
Charles G Prober, Professor of Pediatrics, Microbiology, & Immunology, Stanford Center for Health Education, Stanford University, Stanford, CA, USA.
Jonathan S Berek, Stanford Women’s Cancer Center, Stanford Center for Health Education, Stanford Medicine, Stanford, CA, USA.
Keith J Petrie, Department of Psychological Medicine, University of Auckland, Auckland, New Zealand.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Authors Alia J. Crum, Lauren C. Heathcote, Zara Morrison, Rachael Yielder, Kari Leibowitz, Helen Petousis-Harris, Mark G. Thomas, Charles Prober, Jonathan S. Berek, and Keith J. Petrie declare that they have no conflict of interest.
Authors’ Contributions Alia Crum (Conceptualization: Lead; Formal analysis: Lead; Funding acquisition: Lead; Methodology: Lead; Project administration: Lead; Supervision: Lead; Visualization: Lead; Writing – original draft: Lead; Writing – review & editing: Lead), Lauren C. Heathcote (Conceptualization: Supporting; Formal analysis: Equal; Visualization: Equal; Writing – original draft: Equal; Writing – review & editing: Supporting), Zara Morrison (Data curation: Supporting; Formal analysis: Supporting; Methodology: Supporting; Project administration: Supporting; Writing – review & editing: Supporting), Rachael Yielder (Conceptualization: Supporting; Data curation: Supporting; Formal analysis: Supporting; Investigation: Supporting; Methodology: Supporting; Project administration: Supporting; Writing – review & editing: Supporting), Kari Leibowitz (Conceptualization: Supporting; Methodology: Supporting; Writing – review & editing: Supporting), Helen Petousis-Harris (Conceptualization: Supporting; Methodology: Supporting; Writing – review & editing: Supporting), Mark G. Thomas (Conceptualization: Supporting; Methodology: Supporting; Writing – review & editing: Supporting), Charles Prober (Conceptualization: Supporting; Funding acquisition: Supporting; Methodology: Supporting; Writing – review & editing: Supporting), Jonathan S. Berek (Conceptualization: Supporting; Funding acquisition: Supporting; Methodology: Supporting; Writing – review & editing: Supporting), and Keith J. Petrie (Conceptualization: Lead; Data curation: Lead; Formal analysis: Supporting; Funding acquisition: Supporting; Investigation: Lead; Methodology: Lead; Project administration: Lead; Supervision: Lead; Visualization: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Ethical Approval Ethical approval was obtained from the Health and Disability Ethics Committee (21/CEN/143) and locality approval from the Auckland District Health Board.
Informed Consent All participants provided informed consent prior to data collection.
Transparency Statements
1) The study was preregistered in the Australian New Zealand Clinical Trials Registry: ACTRN12621000722897p.
2) The analysis plan was registered prior to beginning data collection on the Open Science Framework (OSF): https://osf.io/2c6a3.
3) Deidentified data from this study are available in a public archive: https://osf.io/2c6a3.
4) Analytic code used to conduct the analyses presented in this study is available in a public archive: https://osf.io/2c6a3.
5) All materials used to conduct the study are available in a public archive: https://osf.io/2c6a3.
References
- 1. Ankuda CK, Leff B, Ritchie CS, Siu AL, Ornstein KA.. Association of the COVID-19 pandemic with the prevalence of homebound older adults in the United States, 2011–2020. JAMA Intern Med. 2021;181(12):1658–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hermann EA, Lee B, Balte PP, et al. Association of symptoms after COVID-19 vaccination with anti-SARS-CoV-2 antibody response in the Framingham Heart Study. JAMA Netw Open. 2022;5:e2237908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ehreth J. The global value of vaccination. Vaccine. 2003;21:596–600. [DOI] [PubMed] [Google Scholar]
- 4. Leibowitz KA, Howe LC, Crum AJ.. Changing mindsets about side effects. BMJ Open. 2021;11:e040134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Global attitudes towards a covid-19 vaccine. Imperial College London. May 2021. https://www.imperial.ac.uk/media/imperial-college/institute-of-global-healthinnovation/GlobalVaccineInsights_ICL-YouGov-Covid-19-Behaviour-Tracker_20210520_v2.pdf.
- 6. Petrie KJ, Rief W.. Psychobiological mechanisms of placebo and nocebo effects: pathways to improve treatments and reduce side effects. Annu Rev Psychol. 2019;70:599–625. [DOI] [PubMed] [Google Scholar]
- 7. Myers MG, Cairns JA, Singer J.. The consent form as a possible cause of side effects. Clin Pharmacol Ther. 1987;42:250–253. [DOI] [PubMed] [Google Scholar]
- 8. Bauernfeind S, Salzberger B, Hitzenbichler F, et al. Association between reactogenicity and immunogenicity after vaccination with BNT162b2. Vaccines. 2021;9:1089–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cormier O’Connor AM, Boyd NF, Tritchler DL, Kriukov Y, Sutherland H, Till JE.. Eliciting preferences for alternative cancer drug treatments: the influence of framing, medium, and rater variables. Med Decis Making. 1985;5:453–463. [DOI] [PubMed] [Google Scholar]
- 10. Webster RK, Weinman J, Rubin GJ.. Medicine-related beliefs predict attribution of symptoms to a sham medicine: a prospective study. Br J Health Psychol. 2018;23:436–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilhelm M, Rief W, Doering BK.. Decreasing the burden of side effects through positive message framing: an experimental proof-of-concept study. Int J Behav Med. 2018;25:381–389. [DOI] [PubMed] [Google Scholar]
- 12. Fernandez A, Kirsch I, Noël L, et al. A test of positive suggestions about side effects as a way of enhancing the analgesic response to NSAIDs. PLoS One. 2019;14:e0209851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Howe LC, Leibowitz KA, Perry MA, et al. Changing patient mindsets about non-life-threatening symptoms during oral immunotherapy: a randomized clinical trial. J Allergy Clin Immunol Pract. 2019;7:1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horne R, Faasse K, Cooper V, et al. The perceived sensitivity to medicines (PSM) scale: an evaluation of validity and reliability. Br J Health Psychol. 2013;18:18–30. [DOI] [PubMed] [Google Scholar]
- 15. Martin LR, Petrie KJ.. Understanding the dimensions of anti-vaccination attitudes: the Vaccination Attitudes Examination (VAX) Scale. Ann Behav Med. 2017;51:652–660. [DOI] [PubMed] [Google Scholar]
- 16. Groenwold RHH, Moons KGM, Vandenbroucke JP.. Randomized trials with missing outcome data: how to analyze and what to report. CMAJ. 2014;186:1153–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. CDC. Understanding How COVID-19 Vaccines Work. Atlanta, GA: Cent Dis Control Prev; 2022. [Google Scholar]
- 18. Health and Human Services. Vaccine Side Effects. Washington, DC: HHSGov; 2021. [Google Scholar]
- 19. WHO. Side Effects of COVID-19 Vaccines. Geneva, Switzerland; 2021. [Google Scholar]
- 20. Bussey K, Bandura A.. Influence of gender constancy and social power on sex-linked modeling. J Pers Soc Psychol. 1984;47:1292–1302. [DOI] [PubMed] [Google Scholar]
- 21. Bandura A, Ross D, Ross SA.. Transmission of aggression through imitation of aggressive models. J Abnorm Soc Psychol. 1961;63:575–582. [DOI] [PubMed] [Google Scholar]
- 22. Mazzoni G, Foan L, Hyland ME, Kirsch I.. The effects of observation and gender on psychogenic symptoms. Health Psychol. 2010;29:181–185. [DOI] [PubMed] [Google Scholar]
- 23. Lorber W, Mazzoni G, Kirsch I.. Illness by suggestion: expectancy, modeling, and gender in the production of psychosomatic symptoms. Ann Behav Med. 2007;33:112–116. [DOI] [PubMed] [Google Scholar]
- 24. Crichton F, Petrie KJ.. Health complaints and wind turbines: the efficacy of explaining the nocebo response to reduce symptom reporting. Environ Res. 2015;140:449–455. [DOI] [PubMed] [Google Scholar]
- 25. MacKrill K, Morrison Z, Petrie KJ.. Increasing and dampening the nocebo response following medicine-taking: a randomised controlled trial. J Psychosom Res. 2021;150:110630. [DOI] [PubMed] [Google Scholar]
- 26. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. Morb Mortal Wkly Rep. 2021;70:283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Faul F, Erdfelder E, Buchner A, Lang A-G.. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. [DOI] [PubMed] [Google Scholar]
- 28. Crum AJ, Salovey P, Achor S.. Rethinking stress: the role of mindsets in determining the stress response. J Pers Soc Psychol. 2013;104:716–733. [DOI] [PubMed] [Google Scholar]
- 29. Yeager DS, Hanselman P, Walton GM, et al. A national experiment reveals where a growth mindset improves achievement. Nature. 2019;573:364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Preacher KJ, Hayes AF.. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. [DOI] [PubMed] [Google Scholar]
- 31. Fridman A, Gershon R, Gneezy A.. COVID-19 and vaccine hesitancy: a longitudinal study. PLoS One. 2021;16:e0250123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haas JW, Bender FL, Ballou S, et al. Frequency of adverse events in the placebo arms of COVID-19 vaccine trials: a systematic review and meta-analysis. JAMA Netw Open. 2022;5:e2143955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rief W, Shedden-Mora MC, Laferton JAC, et al. Preoperative optimization of patient expectations improves long-term outcome in heart surgery patients: results of the randomized controlled PSY-HEART trial. BMC Med. 2017;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Akroyd A, Gunn KN, Rankin S, et al. Optimizing patient expectations to improve therapeutic response to medical treatment: a randomized controlled trial of iron infusion therapy. Br J Health Psychol. 2020;25:639–651. [DOI] [PubMed] [Google Scholar]
- 35. Walton GM, Wilson TD.. Wise interventions: psychological remedies for social and personal problems. Psychol Rev. 2018;125:617–655. [DOI] [PubMed] [Google Scholar]
- 36. Walton GM, Crum AJ.. Handbook of Wise Interventions: How Social Psychology Can Help People Change. New York, USA: Guilford Press; 2020. [Google Scholar]


