Quantitative sensory testing in chronic pelvic pain shows changes to sensation, including deep tissue and cutaneous inputs, suggesting central and peripheral mechanisms may be important.
Keywords: Quantitative sensory testing, Chronic pelvic pain, Interstitial cystitis, Bladder pain syndrome, Endometriosis
Abstract
Chronic pelvic pain (CPP), despite its high prevalence, is still relatively poorly understood mechanistically. This study, as part of the Translational Research in Pelvic Pain (TRiPP) project, has used a full quantitative sensory testing (QST) paradigm to profile n = 85 women with and without CPP (endometriosis or bladder pain specifically). We used the foot as a control site and abdomen as the test site. Across 5 diagnostically determined subgroups, we found features which are common across different aetiologies, eg, gain of function in pressure pain threshold (PPT) when assessing responses from the lower abdomen or pelvis (referred pain site). However, disease-specific phenotypes were also identified, eg, greater mechanical allodynia in endometriosis, despite there being large heterogeneities within diagnostic groups. The most common QST sensory phenotype was mechanical hyperalgesia (>50% across all the groups). A “healthy’ sensory phenotype was seen in <7% of CPP participants. Specific QST measures correlated with sensory symptoms assessed by the painDETECT questionnaire (pressure-evoked pain [painDETECT] and PPT [QST] [r = 0.47, P < 0.001]; mechanical hyperalgesia (painDETECT) and mechanical pain sensitivity [MPS from QST] [r = 0.38, P = 0.009]). The data suggest that participants with CPP are sensitive to both deep tissue and cutaneous inputs, suggesting that central mechanisms may be important in this cohort. We also see phenotypes such as thermal hyperalgesia, which may be the result of peripheral mechanisms, such as irritable nociceptors. This highlights the importance of stratifying patients into clinically meaningful phenotypes, which may have implications for the development of better therapeutic strategies for CPP.
1. Introduction
Chronic pelvic pain (CPP) affects between 5% and 26.6% of women worldwide.1,19,77,78 Despite the high prevalence of CPP, there is still little understanding of the underlying mechanisms giving rise to and maintaining pain in these women. The Translational Research in Pelvic Pain (TRiPP) study is a collaboration across sites in the United Kingdom, Europe, and United States which focusses specifically on endometriosis and interstitial cystitis or bladder pain syndrome (IC/BPS),15 with an overall aim of better understanding the mechanisms underlying CPP in women.16 Given the increasing evidence of similarities between CPP and other chronic pain conditions, we have focused on both local pelvic and systemic or central mechanisms.
Quantitative sensory testing (QST) is a psychophysical method of testing the function of the somatosensory nervous system.13 Individual somatosensory profiles including “sensory loss” and “sensory gain” have been shown to vary in different aetiologies and on an individual basis for the same diseases.6,13,41 To date, detailed QST profiling has not been performed in women with CPP, although many studies have used single sensory modalities.3,28,30,31,56,75 We used the QST paradigm developed by the German Research Network on Neuropathic Pain (DFNS),40,54 comprising a series of standardised tests to assess the detection and pain thresholds for different types of stimuli. These relate to specific neuroanatomical pathways with separate nerve fibre populations. In addition to characterising the sensory profiles associated with specific pathologies, it has also been shown that, across aetiologies, distinct clusters of sensory profiles can be found.8,70 Four subgroups can be defined using a published algorithm70 representing (1) “sensory loss” (mechanical and thermal sensory loss), (2) “thermal hyperalgesia” (preserved sensory function with heat or cold hyperalgesia), (3) “mechanical hyperalgesia” (loss of thermal sensation with mechanical hyperalgesia or allodynia), and (4) “healthy” (sensation similar to the pain-free population).8 By profiling of human surrogate models, these profiles can be tentatively taken as evidence supporting denervation and peripheral and central sensitisation.69
The aim of this study was to apply the DFNS QST profiling tool to assess sensory phenotypes in women with CPP. We hypothesise that many women with CPP have altered sensory profiles similar to other chronic pain conditions, whereas some features may be specific to their underlying diagnosis and aetiology. Given that the DFNS QST protocol is time consuming, we also aim to determine whether some of the QST measures correlate with scores from the painDETECT questionnaire,23,24 a brief self-report tool that could easily be integrated into clinical practice if shown to be of value in assessing underlying pain mechanisms.
2. Methods
2.1. Participant recruitment
Three sites participated in the study: University of Oxford, UK (OX), Boston's Children's Hospital, USA (BCH) & Instituto de Biologia Molecular e Celular, Portugal (IBMC). Participants from OX and BCH were selected based on criteria from an existing database of participants from parent studies. Participants from IBMC were recruited through urology clinics.15 All appropriate ethical approvals were secured before recruitment into TRiPP (ethics reference 19/YH/0030).
Recruitment was restricted to females aged 18 to 50 who were neither pregnant nor lactating. Participants were recruited into 1 of 5 groups: endometriosis-associated pain (EAP) who have previously received a surgical diagnosis of endometriosis and at least one type of pelvic pain >4/10; endometriosis-associated pain with comorbid bladder pain (EABP) who meet EAP criteria with additional pain experienced in the bladder and urinary symptoms (urinary frequency and/or urgency symptoms)45; bladder pain syndrome (BPS) who fit the clinical presentation of pain perceived to arise from the bladder >4/10 and urinary symptoms (urinary frequency and/or urgency), with no previous surgical diagnosis of endometriosis45; pelvic pain without bladder pain or urinary symptoms and no previous surgical diagnosis of endometriosis (PP), and pain-free controls (CON). In summary, participants were either newly contacted and recruited (IBMC) or contacted after participation in a “parent” study (EndOX: A study to identify possible biomarkers in women with endometriosis, Oxford REC ref:09/H0604/58; Boston Center for Endometriosis [BCE]: A Cross-Institutional Biorepository and Database, IRB-P00004267) and then recruited into the current study (OX and BCH), more detailed information on participant criteria and study protocol can be seen in Demetriou et al.15 Participants had undergone surgery where this was a component of the standard of care. This includes all participants with an endometriosis diagnosis (EAP and EABP) and also all other participants from OX where recruitment was only from clinics. Most participants recruited from the community at BCH and from Urology clinics in IBMC had not had surgery; however, any potential participant in the BPS or PP groups who had had endometriosis identified previously was excluded. More details of the surgical history and where relevant endometriosis staging are given in Table 2. There were no recruitment criteria based on the duration of chronic pain or number of days participants experienced pain per month. Table 2 shows the duration of pain in years for each of the study groups. Pain was not exclusive to the menstrual cycle, with participants reporting noncyclical pelvic pain, dyspareunia, dyschezia, and dysuria (as can be seen in another TRiPP manuscript illustrating the cohort).16
Table 2.
Participant characteristics.
| EAP | BPS | EABP | PP | CON | |
|---|---|---|---|---|---|
| No. of participants | 25 | 13 | 15 | 6 | 26 |
| Age | 34 (22-50) | 46 (27-51) | 31 (20-51) | 31 (25-34) | 29.5 (21-45) |
| Current pain intensity | 1 (0-6) | 3 (0-8) | 2 (0-7) | 0 (0-2) | 0 (0-2) |
| Duration of pain (y) | 18 (7-33) | 19 (0-37) | 21 (6-35) | 17 (11-22) | — |
| painDETECT score | 9.2 (0-18) | 14.6 (2-29) | 14 (7-26) | 9 (3-13) | N/A |
| painDETECT neuropathic, n (% of group) | 0 (0) | 5 (41.7) | 3 (23.1) | 0 (0) | N/A |
| painDETECT mixed n (% of group) |
4 (23.5) | 1 (8.3) | 5 (38.5) | 1 (16.7) | N/A |
| painDETECT nociceptive n (% of group) | 13 (76.5) | 6 (50) | 5 (38.5) | 5 (83.3) | N/A |
| State anxiety score | 32.1 (20-49) | 41 (20-67) | 33 (22-56) | 28.5 (20-43) | 26 (20-41) |
| Menstrual phase n (% of group) |
|||||
| Menstrual | 2 (8) | 0 (0) | 0 (0) | 1 (16.7) | 4 (16) |
| Proliferative | 1 (4) | 0 (0) | 1 (6.7) | 0 (0) | 4 (16) |
| Secretory | 2 (8) | 1 (8.3) | 2 (13.3) | 2 (33.3) | 3 (12) |
| Taking steroid hormones n (% of group) |
20 (80) | 9 (75) | 12 (80) | 3 (50) | 12 (48) |
| Medications n (% of group) |
|||||
| NSAIDS | 1 (4) | 0 (0) | 2 (13.3) | 1 (16.7) | 1 (3.8) |
| Other over-the-counter painkillers (eg, paracetamol) | 3 (12) | 1 (7.7) | 3 (20) | 0 (0) | 0 (0) |
| Antidepressants/Anxiolytics | 8 (32) | 6 (46.2) | 7 (46.7) | 2 (33.3) | 6 (23.1) |
| Medications for other health conditions (eg, allergy medication) | 15 (60) | 6 (46.2) | 9 (60) | 2 (33.3) | 12 (46.2) |
| Vitamins/minerals/probiotics/herbal | 5 (20) | 1 (7.7) | 2 (13.3) | 0 (0) | 7 (26.9) |
| Opioids | 1 (4) | 0 (0) | 1 (6.7) | 0 (0) | 0 (0) |
| Muscle relaxants | 1 (4) | 2 (15.4) | 0 (0) | 0 (0) | 0 (0) |
| Gabapentinoids | 1 (4) | 0 (0) | 1 (6.7) | 0 (0) | 0 (0) |
| Surgery to diagnose/exclude endometriosis n (% of group) |
25 (100) | 0 (0) | 14 (100) | 2 (33.3) | 0 (0) |
| Stage of endometriosis n (% within those who have had surgery to diagnose/exclude endometriosis) |
|||||
| Stage I | 9 (36) | 0 (0) | 7 (46.7) | 0 (0) | — |
| Stage II | 4 (16) | 0 (0) | 3 (20) | 0 (0) | — |
| Stage III | 2 (8) | 0 (0) | 1 (6.7) | 0 (0) | — |
| Stage IV | 6 (24) | 0 (0) | 2 (13.3) | 0 (0) | — |
| Stage unknown | 4 (16) | 0 (0) | 2 (13.3) | 0 (0) | — |
| No endometriosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | — |
Shown are participant demographics for each of the TRiPP subgroups: EAP (endometriosis-associated pain), BPS (bladder pain and urinary symptoms), EABP (comorbid endometriosis and bladder symptoms), PP (pelvic pain without endometriosis or bladder symptoms), and CON (controls without pelvic pain or endometriosis). Age is given in years with mean and range. Pain intensity is given as median score on 0 to 10 NRS scale with range. Duration of pain is given in years as a median score with range; for the CON group, this is not applicable as they are pain free. Current state anxiety score is given as a mean and range based on State-Trait Anxiety Inventory—State Questionnaire,60 scores of 20 to 39, 40 to 59, and 60 to 80 indicate low, moderate, and high anxiety, respectively. painDETECT25 score is given as mean and range. Medications taken in the 24 hours before the study visit, as broken down to medication groups are shown as counts. Surgery to diagnose or exclude endometriosis is given as counts and percentages per group. The stage of endometriosis shown as count and percentage of those who had received surgery to diagnose or exclude endometriosis.
All participants gave informed consent. Data were collected between January 2020 and August 2021. All participants from IBMC had study visits and questionnaires completed in Portuguese. Validated Portuguese versions of study material was used where available, otherwise material was forward and backward translated. Unfortunately, all control participants were recruited at Boston; therefore, it is not possible to determine the effect of site in this cohort, although previous studies have shown little or no impact of the site of data collection on QST measures.68
2.2. Study visit
After coordinated training of all experimenters, the DFNS QST protocol was performed on the dorsum of the right foot (control site) and the lower abdomen or pelvis (test site).
All QST sessions were performed in a temperature-controlled room at approximately 20°C. Participants were also asked to complete a battery of questionnaires, which included painDETECT23,24 as detailed in our protocol.15 Before the session, participants were also asked to complete a “How are you today?” questionnaire assessing factors that may impact on psychophysical measures: current pain intensity (Numerical Rating Scale [NRS] 0 to 10), state anxiety,58 pain catastrophizing,61 medication and caffeine use that day, and day of menstrual cycle.
The QST script was translated into Portuguese for participants at IBMC. The QST script was forward and back translated before use in this study as no published script was available. A previous study has addressed issues of translating QST scripts into other European languages (not Portuguese, though) and found that it seems possible to produce highly reliable DFNS QST results across different research units, and, more importantly, also across countries and languages.68
Quantitative sensory testing was performed by trained researchers who had undergone training with DFNS in Mannheim (September 2019) or attended a virtual training refresher session before recommencing data collection after the peak of the COVID-19 pandemic halted clinical research. As a measure of outcome quality of this alignment, we averaged DFNS-standardized z-scores across all control subjects: the resulting mean was −0.03 (DFNS 95% CI 0.01 ± 0.25) and SD was 1.26 (DFNS 95% CI 0.99 ± 0.10). Thus, Boston (where CON were collected) had no systematic bias towards overestimating or underestimating QST parameters, but a larger variance than the original 10 sites of DFNS.40
Although researchers tried to stay naïve to the participant group, many participants disclosed information about their pain during the study visit.
Quantitative sensory testing was applied to the lower abdomen or pelvis, below the umbilicus. Specifically, thermal, pinprick, and von Frey stimuli were delivered to the skin of the lower abdomen in varied locations to assess general sensation in the area and to avoid surgical scars, pressure pain threshold was measured on the muscles of the lower abdominal wall, and vibration detection was measured on the symphysis pubis (see Table 1 for more information on QST measures).
Table 1.
Quantitative sensory testing abbreviations and methods.
| Abbreviation | Full name | Method |
|---|---|---|
| CDT | Cold detection threshold | Cooled from baseline until participant senses “cooling.” Three repetitions are performed at each site. Arithmetic mean of change in temperature is used in analysis |
| WDT | Warm detection threshold | Warmed from baseline until participant senses “warming.” Three repetitions are performed at each site. Arithmetic mean of change in temperature is used in analysis |
| TSL | Thermal sensory limen | Warmed and cooled from baseline asking participant to indicate when they feel a change. Calculated by subtracting the arithmetic mean of the cool detections from the arithmetic mean of the warm detections during the alternations |
| PHS | Paradoxical heat sensation | During TSL, if participants report “warm” or “hot” sensations during cooling, this is a paradoxical heat sensation. These are counted, and there is a maximum of 3 |
| CPT | Cold pain threshold | Cooled from baseline until participant feels pain sensation. Three repetitions are performed at each site. Arithmetic mean of absolute temperatures is used in analysis |
| HPT | Heat pain threshold | Heated from baseline until participant feels pain sensation. Three repetitions are performed at each site. Arithmetic mean of absolute temperatures is used in analysis |
| MDT | Mechanical detection threshold or tactile detection threshold | Using von Frey hairs the tactile detection threshold is determined by performing a modified method of limits. Five threshold determinations are made, each with a series of ascending and descending stimulus intensities. The final threshold is the geometric mean of these 5 series of suprathreshold and subthreshold stimuli intensities |
| MPT | Mechanical pain threshold | This test uses weighted pinprick stimuli. Five threshold determinations are made, each with a series of ascending and descending stimulus intensities. The final threshold is the geometric mean of the 5 suprathreshold and subthreshold readings (modified method of limits) |
| MPS | Mechanical pain sensitivity | To test for mechanical pain sensitivity, weighted pinprick stimuli of different stimulus intensities are used so that a stimulus–response function is obtained for pinprick-evoked pain (Numerical Rating Scale; range 0-100). Seven stimuli intensities are applied 5 times each at both test sites in a randomized order, during which the subject is asked to give a numerical pain rating immediately after each stimulus. The degree of pain sensitivity is calculated by the geometrical mean of the pain ratings given for pinprick stimuli (MPS) |
| DMA | Dynamic mechanical allodynia | Dynamic mechanical allodynia is tested by using the same test pattern as described for the MPS. Dynamic innocuous stimuli (Q-tip, cotton wisp, and soft brush) are applied in between the pinprick stimuli in a randomized order. Each of the 3 innocuous stimuli is tested 5 times on each test site. The degree of pain sensitivity is calculated by the geometrical mean of the pain ratings innocuous stimuli (DMA) |
| WUR | Wind-up ratio | The numerical pain rating (NRS; range 0-100) given for an applied series of repetitive pinprick stimuli of the same intensity (10 stimuli with a repetition rate of 1/s, 256 mN) is compared with the numerical pain rating of a single stimulus again of the same intensity. This procedure is repeated 5 times. A “wind-up” ratio is calculated by the arithmetic mean of the pain intensity rating for the series of stimuli divided by the arithmetic mean of the pain intensity rating for the single stimulus |
| VDT | Vibration detection threshold | This test is performed with a standardized tuning fork (64 Hz) that is placed over a bony prominence. The vibration detection threshold is determined by 3 series of descending stimulus intensities determined from the “wandering” tip of a triangle moved by means of the vibration and indicated on the tuning fork29 using the arithmetic mean of the values when the participant just stopped perceiving vibration (in x/8) |
| PPT | Pressure pain threshold | Using a pressure algometer (contact area 1 cm2), the threshold for pressure induced pain is measured above a muscle in 3 series of slowly increasing stimulus intensities (0.5 kg/s, corresponding to ca. 50 kPa/s). The threshold is then determined as the arithmetic mean of the 3 series (in kPa). |
2.3. Data analysis
All data were collected using the official QST form and manually uploaded to a secure database. Data inputting was independently verified. Data were analysed as per protocol,40,54 using MATLAB (R2021a) for data analysis and Prism9 to create figures. Published reference data were used to Z transform the data for the foot.54 Published reference data are not available for the abdomen or pelvis, and therefore, after discussion with the DFNS (R.-D.T. and J.V.), published reference data for the back were used.51 A Z-score greater than 0 shows a gain of function, and a Z-score less than 0 shows a loss of function.
In addition, statistical comparisons were made between the pain groups (EAP, EABP, BPS, and PP) and our control group (CON). These comparisons were performed with Student t tests using the Z-transformed data. Multiple comparison correction was performed using Bonferroni correction, when looking at individual QST measures corrections were applied to QST “blocks” of thermal detection, thermal pain thresholds, mechanical pain thresholds, and mechanical detection.
When comparing painDETECT23,24 scores for individual questions, correlations with the relevant QST block were performed (ie, painDETECT question “is cold or heat (bath water) in this area occasionally painful?” correlated with thermal pain QST measures). Normality tests were performed on each painDETECT sensation variable (ie, those scored out of 5, excluding question on time course and spatial properties of the pain) and appropriate tests were used. Paraesthesia symptoms “burning” and “tingling” from painDETECT do not have comparators within QST so have been excluded from this analysis. In addition, the proportion of those reporting clinically significant painDETECT scores for sensations are reported (ie, are any responses >3 of 5, representing strongly or very strongly). To account for interindividual differences in pain sensitivity, painDETECT scores for each of these symptoms were recalculated by subtracting the mean across all 7 responses from each individual response9 when comparing scores between groups. Scores larger than zero thereby indicate a sensation that is more intense than the average individual symptom score.
Other variable scores such as pain intensity (Numerical Rating Scale [NRS] 0 to 10) and state anxiety (State-Trait Anxiety Inventory STAI-S58) were normality tested and are reported appropriately.
For menstrual cycle stage, participants were asked to self-report whether they were taking any hormonal contraceptives, the day of their last menstrual period, and typical length of their menstrual cycle. Those who were not currently taking any form of hormonal contraception, who indicated that they still had menstrual cycles, were categorised by menstrual phase according to the following protocol: based on a 28-day cycle, day 1 to 7 were classified as a menstrual phase, day 8 to 14 were classified as follicular or proliferative and day 15+ was the luteal or secretory phase. For participants whose cycle length deviated from 28 days, 14 days were subtracted from their reported cycle length, the secretory phase being held constant, and the remaining duration was assigned to the proliferative phase. For women who reported a variable cycle length, the min, mean, or max cycle length was determined, and stage was allocated accordingly. The menstrual phase was cross-checked by 2 researchers (L.C. and D.P.) to ensure consistency.
To determine whether individuals with CPP (EAP, BPS, EABP, and PP combined) could be categorised into the clusters previously described,8 a recently developed algorithm64 was used. The clusters are as follows: ‘healthy,’ ‘sensory loss,’ ‘thermal hyperalgesia’ and ‘mechanical hyperalgesia.’ We used a deterministic approach such that participants were sorted into the cluster which they had the greatest score for, based on their abdominal sensory profiles. To assess differences between these clusters in questionnaire measures, analysis of variance or Kruskall–Wallis test was used according to normalcy.
3. Results
3.1. Demographics
Eighty-five women were recruited and underwent the full QST profiling (EAP n = 25, BPS n = 13, EABP n = 15, PP n = 6, and CON n = 26). Overall, most of the pain groups were well matched for age, menstrual cycle phase, and state anxiety scores (Table 2); however, the BPS group were significantly older than the CON and PP groups (P = 0.010 and P = 0.090, respectively) and significantly more anxious than the CON group (P = 0.009). The EAP, BPS, and EABP had significantly higher current pain intensity NRS scores than CON (all P < 0.01), see Table 2. 66.7% of participants were taking hormones, with the remainder being spread across menstrual cycle phases (Table 2) (for n = 1 no data on hormones or cycle were available and for n = 5 participants they were not on hormones, but it was not possible to determine the menstrual stage).
Table 3 shows the proportion of participants that reported clinically significant sensory symptoms on the painDETECT measure. For the endometriosis groups (EAP and EABP), pain attacks was the most common symptom, whereas for the BPS groups ongoing burning pain was the most common and this was also common in the comorbid group EABP. Patients with bladder pain (BPS and EABP) had more frequent pressure-evoked pain than EAP or PP.
Table 3.
Reported symptoms from painDETECT.
| Sensory symptom | EAP (n = 24) | EABP (n = 15) | BPS (n = 13) | PP (n = 6) |
|---|---|---|---|---|
| Burning | 8.3% | 33.3% | 69.2% | 0% |
| Prickling | 0% | 6.7% | 30.8% | 16.7% |
| Mechanical allodynia | 4.2% | 0% | 7.7% | 0% |
| Painful attacks | 37.5% | 53.3% | 23.1% | 16.7% |
| Thermal hyperalgesia | 4.2% | 0% | 0% | 0% |
| Numbness | 4.2% | 6.7% | 0% | 0% |
| Pressure-evoked pain | 12.5% | 33.3% | 23.1% | 0% |
Proportion of participants in each pain group reporting clinically significant symptoms (ie, a score >3, strongly or very strongly) in the painDETECT questionnaire.25
BPS, bladder pain syndrome; EABP, endometriosis-associated pain with comorbid bladder pain; EAP, endometriosis-associated pain; PP, pelvic pain.
One participant in the EAP group reported experiencing pain on the right foot on the day of testing; therefore, their control site data are excluded from all analysis.
3.2. Quantitative sensory testing profiles
Figure 1 shows the sensory profiles at the foot control site (Fig. 1A) and the lower abdomen test site (Fig. 1B) for the CON compared with those with CPP. There are significant differences between the CPP and CON groups for: thermal sensory limen at both the foot and abdomen (t= −2.8, P = 0.032 and t= −3.5, P = 0.004, respectively), suggesting loss of small fibre function in CPP, vibration detection at the foot (t = −3.0, P = 0.017) indicating large fibre defect in CPP, and pressure pain threshold at the abdomen (t = 3.0, P = 0.012) showing large gain of function in CPP suggestive of central sensitization to deep tissue input.
Figure 1.
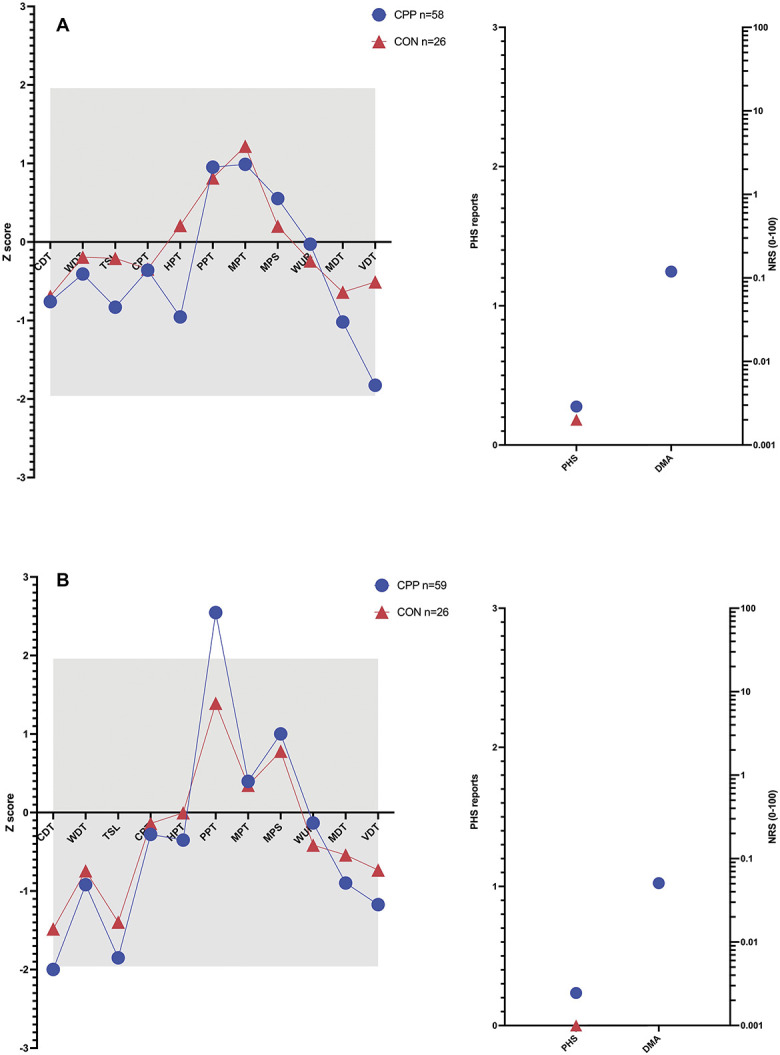
(A) Sensory profiles at the foot control site and (B) sensory profiles at the abdomen. CDT, cold detection threshold; CON group, controls without pain; CPP, participants with chronic pelvic pain; CPT, cold pain threshold; DMA, dynamic mechanical allodynia (DMA ratings do not occur in healthy people (published reference data) or in our CON group); HPT, hot pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; PHS, paradoxical heat sensation; PPT, pressure pain threshold; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold; WUR, wind up ratio.
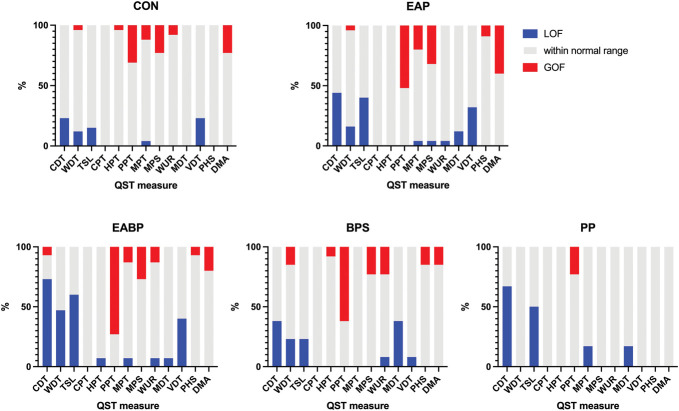
Figure 2 shows the heterogeneity within subgroups showing the proportion with “normal” function, gain of function, and loss of function for each test variable, as well as illustrating differences within the CPP cohort divided into the TRiPP subgroups. At the test site (low abdomen), there was loss of function in thermal detection (indicating small fibre loss), as well as gain of function for pressure pain thresholds, mechanical pain thresholds, mechanical pain sensitivity, or increased dynamic mechanical allodynia across groups.
Figure 2.
Heterogeneity within and between TRiPP subgroups as shown by the distribution of loss of function and gain of function to QST measures at the abdomen test site. Shown in red are the proportion of the group which have gain of function relative to the normal range from reference data. Shown in grey are those within the normal range. Shown in blue is the proportion showing loss of function. BPS, bladder pain; CDT, cold detection threshold; CON, pain-free controls; CPT, cold pain threshold; DMA, dynamic mechanical allodynia; EABP, comorbid endometriosis and bladder pain; EAP, endometriosis-associated pain; HPT, heat pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; PHS, paradoxical heat sensation; PP, pelvic pain without endometriosis or bladder symptoms; PPT, pressure pain threshold; QST, quantitative sensory testing; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold; WUR, wind up ratio.
At the test site, compared with our CON group, those with bladder pain (EABP and BPS combined) showed significant gain of function for pressure pain threshold (t = 3.7, P = 0.003), suggesting central sensitization to deep tissue input as well as loss of function for thermal sensory limen (TSL) (t = −3.0, P = 0.02), suggesting loss of small fibre function. However, those with endometriosis (EAP and EABP combined) had significant loss of function for TSL at both the test (t = −3.5, P = 0.003) and control site (t = −3.2, P = 0.008) (ie, small fibre loss). In the PP compared with CON groups, there was greater dynamic mechanical allodynia (t = −10.7, P < 0.001). However, it should be remembered that this was a small group (n = 6). All other measures were within the 95% confidence intervals from the reference data (shown by grey box).
3.3. Quantitative sensory testing clusters
All 4 previously identified QST clusters were present in the cohort of women with CPP. Profiles were consistent with those of healthy subjects in only a small number (6.8%) of women with CPP; thus, more than 93% had QST sensory profiles suggesting some altered somatosensory processing. Cluster allocation for the TRiPP subgroups (EAP, BPS, EABP, and PP) are shown in Figure 3. For all TRiPP subgroups, the most common cluster allocation was “mechanical hyperalgesia” with at least 50% of participants in each group fitting this cluster, which suggests some central sensitization.
Figure 3.
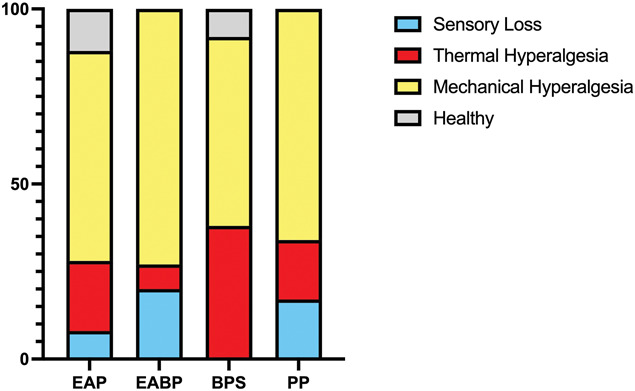
Cluster allocation by group. Sensory phenotypes of sensory loss, thermal hyperalgesia, mechanical hyperalgesia, and healthy determined by published algorithm70 based on individual QST profiles. BPS, bladder pain group; EABP, comorbid endometriosis and bladder pain; EAP, endometriosis-associated pain group; PP, pelvic pain without endometriosis or bladder symptoms; QST, quantitative sensory testing.
There was no significant difference between the clusters for age (P = 0.52), pain intensity rating (NRS 0-10 at the time of testing) (P = 0.72), state anxiety (P = 0.40), or painDETECT score (P = 0.23).
3.4. Comparison with painDETECT scores
Correlations between sensory descriptors from painDETECT and relevant QST measures are shown in Table 4. Significant correlations were found between the descriptor “does slight pressure in this area, eg, with a finger, trigger pain?” (pressure-evoked pain) and the pressure pain threshold (r = 0.47, P < 0.001) and “is cold or heat (bath water) in this area occasionally painful?” (thermal hyperalgesia) and the heat pain threshold (r = 0.32, P = 0.032). The item “is light touching (clothing, a blanket) in this area painful?” (mechanical allodynia) was strongly correlated with mechanical pain sensitivity (r = 0.38, P = 0.009) but not with dynamic mechanical allodynia (r = 0.12, P = 0.378 uncorrected); this item may reflect skin hypersensitivity but not necessarily to light touch.
Table 4.
Correlations between relevant painDETECT measures and quantitative sensory testing measures.
| QST measure | painDETECT question | r | P |
|---|---|---|---|
| CPT | Is cold or heat (bath water) in this area occasionally painful? | 0.25 | 0.12 |
| HPT | Is cold or heat (bath water) in this area occasionally painful? | 0.32* | 0.032* |
| VDT | Do you suffer from a sensation of numbness in the area? | −0.28 | 0.036 (uncorrected) |
| MDT | Do you suffer from a sensation of numbness in the area? | −0.20 | 0.28 |
| PPT | Does slight pressure in this area, eg, with a finger, trigger pain? | 0.47* | <0.001* |
| MPT | Is light touching (clothing, a blanket) in this area painful? | 0.03 | 0.85 (uncorrected) |
| MPS | Is light touching (clothing, a blanket) in this area painful? | 0.38* | 0.009* |
| DMA | Is light touching (clothing, a blanket) in this area painful? | 0.12 | 0.38 (uncorrected) |
Shown are r values from Pearson correlation and P values which are corrected for multiple comparisons (using Bonferroni correction) unless otherwise stated. painDETECT questions relate to clinical symptoms: “Is cold or heat (bath water) in this area occasionally painful?” relates to thermal hyperalgesia; “do you suffer from a sensation of numbness in the area?” relates to numbness; “does slight pressure in this area, eg, with a finger, trigger pain?” relates to pressure evoked pain; “is light touching (clothing, a blanket) in this area painful?” relates to mechanical allodynia.
Significance P < 0.05.
CPT, cold pain threshold; DMA, dynamic mechanical allodynia; HPT, hot pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; PPT, pressure pain threshold; VDT, vibration detection threshold.
In addition, there was a significant difference between the QST clusters described above and individually mean adjusted scores for painDETECT variables: numbness (F = 3.71, P 0.017) and mechanical allodynia (F = 3.15, P = 0.032). Post hoc tests showed for numbness a significant difference between sensory loss and thermal hyperalgesia clusters (t = 2.93, P = 0.018, uncorrected) as well as between thermal hyperalgesia and healthy (t = −2.255, P = 0.041, uncorrected), although these did not withstand multiple comparison correction. For mechanical allodynia, there were significant differences between sensory loss and thermal hyperalgesia clusters (t = −4.10, P = 0.006), sensory loss and mechanical hyperalgesia (t = −5.88, P = 0.0006), and sensory loss and healthy (t = −2.50, P = 0.032, uncorrected).
4. Discussion
This is the first study using the comprehensive DFNS QST protocol for profiling and phenotyping women with CPP.40,54 When looking at the abdominal site, we see gain of function in pressure pain thresholds suggesting changes in pain processing pathways to deep tissue input. We also see loss of function in thermal detection thresholds, suggesting loss of small fibre function. However, when divided into clinically defined subgroups (ie, by the presence of endometriosis or bladder pain), more nuanced differences are seen, specifically a gain of function for pressure pain thresholds and a loss of function for heat pain thresholds for those with bladder pain and a gain of function for dynamic mechanical allodynia for those with endometriosis. Both those with endometriosis or bladder pain showed loss of function for thermal sensory limen (detecting changes from warm to cool). There is, however, marked heterogeneity in the sensory profiles seen even within clinically defined subgroups, and this is illustrated by the observation that all 4 sensory phenotypes are represented. Mechanical hyperalgesia seems to be the most common sensory phenotype across all clinical subgroups. Notably, only 6.8% of CPP have “healthy” sensory function.
4.1. Mechanisms of pain
Traditionally, pelvic pain has been considered predominantly a visceral pain condition5 although increasing evidence suggests that there is frequently a central component.33 Given alterations in sensory function found at the abdomen or pelvis, our data suggest that some women with CPP (regardless of underlying pathology) have changes to peripheral nerve function. These changes could be the result of neuropathic-like pain, which the painDETECT scores suggest could be present in this cohort.12,23,24 Alternatively, these could be due to nociplastic or central mechanisms.4,21,36,37,71
The most striking observation is gain of function in PPT. Although there was only a statistically significant difference between CON and those in EABP and BPS groups, Figure 2 illustrates that across all the CPP groups there is a large proportion demonstrating a gain of function in PPT. Although it is not possible to determine from our data how this change is generated, the association with those with bladder pain and urinary symptoms particularly suggests that there may be a potential role for referred hyperalgesia secondary to viscero–somatic communication.74,76
The data also showed significant gain of function in DMA in 22.5% of the participants with endometriosis (EAP and EABP) (as seen in Fig. 2) which is consistent with studies describing allodynia on the abdomen.48,60 We can only speculate about factors generating this observation; however, it is important to remember the role of laparoscopy in both the diagnosis and treatment of endometriosis and the investigation of CPP more broadly.17,34,49 All participants in EAP and EABP groups, and some of the BPS group, were initially recruited into parent studies at the time of surgery, and many will have had multiple surgeries both for their pelvic pain and for other indications (eg, acute appendicitis or caesarean sections). These surgeries carry the risk of postoperative pain, localised hyperexcitability, and numbness.22,52,57,62
The gain of function in pressure pain thresholds suggest peripheral or central sensitization to deep input, whereas mechanical pain measures (MPT, MPS, and DMA) suggest central sensitization to cutaneous input.
Importantly, we also see loss of function in response to stimuli across all subgroups of CPP. Although hyperalgesia and allodynia have been reported in CPP,3,28,30,31,56 such loss of function markers may suggest different mechanisms which could play an important role in understanding CPP. This loss of function could be due to “deafferentation” as has been seen in other conditions.8,29,65 Alternatively, it could be due to “descending defunctionalization” of nonnociceptive somatosensory processing, as has been suggested in neuropathic pain conditions where sensory loss is seen on ipsilateral and contralateral areas.18
The sensory manifestations are not consistent across women, either when considering the cohort as a whole or dividing into clinically determined subgroups. This is, however, consistent with the known heterogeneity of CPP2,11,77 and may contribute to the variation seen in the efficacy of standard treatments.25,77 Strategies to subgroup women based on their somatosensory profiles (and possible underlying mechanisms) could be of enormous benefit in this context.
We explored whether mechanistically relevant clusters could be identified in the present cohort as in other patient groups.8,64,70 The strategy applied70 identifies 4 subgroups in patients with neuropathic pain, believed to represent (1) those with irritable nociceptors14,20 (thermal hyperalgesia), (2) deafferentation10,20,65 (sensory loss), (3) central sensitization10,20 (mechanical hyperalgesia), and (4) those with normal peripheral nerve function. Although it remains to be seen if these clusters respond differently to treatment,8,69,70 there is preliminary evidence suggesting they will7 and differences in pain interference between these clusters have been shown.27 We were able to identify women fitting each of these clusters, suggesting that the 3 mechanistically different sensory abnormal groups are relevant to CPP.
The commonest sensory phenotype allocation was “mechanical hyperalgesia.” Human surrogate models of known central sensitization in the spinal cord had this pattern, in particular mechanical pain measures (MPT, MPS, and DMA); some here may, therefore, have spinal long-term potentiation.55 On the other hand, gain of function for pressure pain threshold has been seen in fibromyalgia,43,46 complex regional pain syndrome,42,66 and after sleep deprivation59 or other stressors44,67; although still central sensitization, the neuronal populations involved may be different. The “thermal hyperalgesia” phenotype seen in the BPS group particularly may represent the concept of irritable nociceptors.8
4.2. Clinical relevance
Chronic pelvic pain is challenging to treat; therefore, it is important to better understand the underlying pain mechanisms and to identify strategies to determine who might respond to specific treatments. The present findings highlight that dysfunction in somatosensory processing pathways is present for many women with CPP no matter the underlying cause. Importantly, we found that >93% of our cohort are classified as having altered somatosensory nervous system function, yet clinically medications targeting peripheral or central somatosensory signalling are not routinely used. This disconnect needs to be addressed to improve patient care.
The sensory phenotypes from QST used in this study represent one potential stratifier; however, the utility of QST clinically is currently limited due to time, equipment and/or training requirements. Although there are ongoing efforts to create simple, clinically accessible, bedside QST tools,38,53,72 the use of patient-completed questionnaires would be even cheaper and more efficient. Although studies have had mixed results when trying to find tight parallels between QST and painDETECT previously,26,32,47,63,73 this study shows correlations between factors assessing particular sensory phenomena. Notably PPT assessed using QST, which clearly showed a gain of function in many participants with CPP, showed strong correlation with the response to the relevant painDETECT question (“does slight pressure in this area, eg, with a finger, trigger pain?”). Interestingly, however, numbness was not correlated with loss of function measures, and this may be an important limitation of this approach. Further work is needed to determine both the utility and acceptability of using painDETECT as a component of clinical and research assessments of CPP in situations where QST is not feasible.
4.3. Limitations
Attempts were made to ensure consistency of data by using the same equipment at all 3 sites and training all personnel together in data collection methods. Although the overall sample size is comparable with other QST studies,35,39,50 when divided into clinical subgroups particularly the PP group is too small to meaningfully interpret results. Where appropriate, comparisons were made between those with and without endometriosis and those with and without bladder pain.
In addition, we cannot exclude that some of the differences we found were based in site differences of this multisite study. This is based in the respective centre's expertise and clinical focus. A future study should aim to include recruiting at centres covering all groups equally.
The lower abdomen or pelvis test site was selected as the most clinically meaningful area as this is often the referred pain site in CPP patients. However, there is no published reference QST data from this site; we, therefore, used published QST data from the trunk area.51 The study compared patient data with a control group (CON), and data were z-transformed using the trunk reference data. We did not consider it appropriate to use the control group as reference data for z-transformation as the sample size is not large enough and the spread of ages is relatively limited.
5. Conclusions
The present multicentre study showed significant changes of somatosensory function in women with CPP, with 6.8% showing a “healthy” sensory profile. Specific sensory alterations are present across different underlying pathologies, whereas others are disease specific. Our findings suggest that there is central sensitization to deep and cutaneous inputs in women with CPP, in addition to a variety of alterations in peripheral nerve function. The data showed a moderate correlation between QST measures and relevant descriptors from painDETECT, suggesting painDETECT may have utility for phenotyping CPP patients in specific settings. Understanding somatosensory processing may be helpful for phenotyping CPP patients. Stratification methods such as these may in future guide personalised pain management and thus should be considered when designing clinical trials.
Conflict of interest statement
L. Coxon: no competing interests. J. Vollert received consulting fees from Vertex Pharmacauticals, Embody Orthopaedic, and Casquar. D. Perro receives financial support from the Canadian Institute of Health Research Doctoral Foreign Study Award. C. E. Lunde: no competing interests. J. Ferreira-Gomes: no competing interests. A. Charrua: no competing interests. P. Abreu-Mendes: no competing interests. J. Birch: no competing interests. J. Meijlink: member of scientific advisory board for Glycologix. L. Hummelshoj: no competing interests. A. Hoffmann: employee of Bayer AG, Germany. Q. Aziz: no competing interests. L. Arendt-Nielsen: no competing interests. E. Pogatzki-Zahn received financial support from Grunenthal and Mundipharma for research activities and advisory and lecture fees from Grünenthal, Novartis, and Mundipharma. In addition, she receives scientific support from the German Research Foundation (DFG), the Federal Ministry of Education and Research (BMBF), the German Federal Joint Committee (G-BA), and the Innovative Medicines Initiative (IMI) 2 Joint Undertaking under grant agreement No 777500. This joint undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA. All money went to the institution E. Pogatzki-Zahn is working for. M. Krassowski: no competing interests. E. Evans: no competing interests. L. Demetriou: no competing interests. S. A. Missmer has been an advisory board member for AbbVie and Roche and receives research funding from the National Institutes of Health, the US Department of Defense, the J. Willard and Alice S. Marriott Foundation, and AbbVie; none are related to the presented work. The J. Willard and Alice S. Marriott Foundation supported enrollment of and data collection from the A2A cohort in Boston from which TRiPP data were sampled. C. M. Becker: Research Grants from Bayer Healthcare, MDNA Life Sciences, Roche Diagnostics, European Commission, and NIH. His employer has received consultancy fees from Myovant and ObsEva for work outside of this project. K. T. Zondervan: reports grant funding from EU Horizon 2020, NIH US, Wellbeing of Women, Bayer AG, Roche Diagnostics, Evotec-Lab282, and MDNA Life Sciences, outside the submitted work. A. W. Horne reports grant funding from the MRC, NIHR, CSO, Wellbeing of Women, Roche Diagnostics, Astra Zeneca, Ferring, Charles Wolfson Charitable Trust, and Standard Life. His employer has received consultancy fees from Roche Diagnostics, AbbVie, Nordic Pharma, and Ferring, outside the submitted work. In addition, A. W. Horne has a patent for a serum biomarker for endometriosis pending. C. B. Sieberg: no competing interests. F. Cruz: consultant, speaker, or investigator for Allergan (Abbvie), Astellas, Bayer, Ipsen, and Recordati. R.-D. Treede: Ad board for BAYER, IASP task force on chronic pain classification. J. Nagel: employee and shareholder of Bayer AG, Germany. K. Vincent declares research funding from Bayer Healthcare and honoraria for consultancy and talks and associated travel expenses from Bayer Healthcare, Grunenthal GmBH, AbbVie, and Eli Lilly.
Acknowledgements
Authors thank Natalie Cuccia (for help collecting data), Miriam Szabo (for data entry), Emily Tan (for data entry), and Lewis Carney (for data entry). This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 777500. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA Companies. This project was preregistered on clinicaltrials.gov: NCT04001244.
Center for Neuroplasticity and Pain (CNAP) is supported by the Danish National Research Foundation (DNRF121). Financial support was provided by the J. Willard and Alice S. Marriott Foundation for establishment of and baseline data collection within the A2A cohort—from which the Boston-based TRiPP population was sampled.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Jan Vollert, Email: j.vollert@imperial.ac.uk.
Danielle Perro, Email: danielle.perro@wrh.ox.ac.uk.
Claire E. Lunde, Email: claire.lunde@wrh.ox.ac.uk.
Joana Ferreira-Gomes, Email: jogomes@med.up.pt.
Ana Charrua, Email: anacharr@gmail.com.
Pedro Abreu-Mendes, Email: pedromendes.uc@gmail.com.
Michal Krassowski, Email: michal.krassowski@wrh.ox.ac.uk.
Judy Birch, Email: judy_b@dsl.pipex.com.
Jane Meijlink, Email: jane@meijlink.eu.
Lone Hummelshoj, Email: lone@endometriosis.org.
Anja Hoffmann, Email: anja.hoffman@bayer.com.
Qasim Aziz, Email: q.aziz@qmul.ac.uk.
Lars Arendt-Nielsen, Email: lan@hst.aau.dk.
Esther Pogatzki-Zahn, Email: esther.pogatzki-zahn@ukmuenster.de.
Emma Evans, Email: emma.evans@wrh.ox.ac.uk.
Lysia Demetriou, Email: lysimachi.demetriou@wrh.ox.ac.uk.
Stephen B. McMahon, Email: stephen.mcmahon@kcl.ac.uk.
Stacey A. Missmer, Email: nhsam@channing.harvard.edu.
Christian M. Becker, Email: christian.becker@wrh.ox.ac.uk.
Krina T. Zondervan, Email: krina.zondervan@wrh.ox.ac.uk.
Andrew W. Horne, Email: andrew.horne@ed.ac.uk.
Francisco Cruz, Email: cruzfjmr@med.up.pt.
Christine B. Sieberg, Email: christine.sieberg@childrens.harvard.edu.
Rolf-Detlef Treede, Email: rolf-detlef.treede@medma.uni-hiedelberg.de.
Jens Nagel, Email: jens.nagel@bayer.com.
Katy Vincent, Email: katy.vincent@wrh.ox.ac.uk.
References
- [1].Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Phys 2014;17:E141–7. [PubMed] [Google Scholar]
- [2].Anothaisintawee T, Attia J, Nickel JC, Thammakraisorn S, Numthavaj P, McEvoy M, Thakkinstian A. Management of chronic prostatitis/chronic pelvic pain syndrome: a systematic review and network meta-analysis. JAMA 2011;305:78–86. [DOI] [PubMed] [Google Scholar]
- [3].As-Sanie S, Harris RE, Harte SE, Tu FF, Neshewat G, Clauw DJ. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet Gynecol 2013;122:1047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aydede M, Shriver A. Recently introduced IASP definition of ‘nociplastic pain’ needs better formulation. PAIN 2018;159:1176–7. [DOI] [PubMed] [Google Scholar]
- [5].Aziz Q, Giamberardino MA, Barke A, Korwisi B, Baranowski AP, Wesselmann U, Rief W, Treede RD. The IASP classification of chronic pain for ICD-11: chronic secondary visceral pain. PAIN 2019;160:69–76. [DOI] [PubMed] [Google Scholar]
- [6].Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807–19. [DOI] [PubMed] [Google Scholar]
- [7].Baron R, Dickenson AH, Calvo M, Dib-Hajj SD, Bennett DL. Maximizing treatment efficacy through patient stratification in neuropathic pain trials. Nat Rev Neurol 2022;19:53–64. [DOI] [PubMed] [Google Scholar]
- [8].Baron R, Maier C, Attal N, Binder A, Bouhassira Di, Cruccu G, Finnerup NB, Haanpaä M, Hansson P, Hüllemann P, Jensen TS, Freynhagen R, Kennedy JD, Magerl W, Mainka T, Reimer M, Rice ASC, Segerdahl M, Serra J, Sindrup S, Sommer C, Tölle T, Vollert J, Treede RD. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. PAIN 2017;158:261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Baron R, Tölle TR, Gockel U, Brosz M, Freynhagen R. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: differences in demographic data and sensory symptoms. PAIN 2009;146:34–40. [DOI] [PubMed] [Google Scholar]
- [10].Baumgärtner U, Magerl W, Klein T, Hopf HC, Treede RD. Neurogenic hyperalgesia versus painful hypoalgesia: two distinct mechanisms of neuropathic pain. PAIN 2022;96:141–51. [DOI] [PubMed] [Google Scholar]
- [11].Clemens JQ, Mullins C, Ackerman AL, Bavendam T, van Bokhoven A, Ellingson BM, Harte SE, Kutch JJ, Lai HH, Martucci KT, Moldwin R, Naliboff BD, Pontari MA, Sutcliffe S, Landis JR. Urologic chronic pelvic pain syndrome: insights from the MAPP Research Network. Nat Rev Urol 2019;16:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Coxon L, Wiech K, Vincent K. Is there a neuropathic-like component to endometriosis-associated pain? Results from a large cohort questionnaire study. Front Pain Res 2021;2:743812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med 2014;15:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, Jensen TS, Sindrup SH. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: A randomised, double-blind, placebo-controlled phenotype-stratified study. PAIN 2014;155:2263–73. [DOI] [PubMed] [Google Scholar]
- [15].Demetriou L, Coxon L, Krassowski M, Rahmioglu N, Arendt-Nielsen L, Aziz Q, Becker CM, Birch J, Cruz F, Hoffman A, Horne AW, Hummelshoj L, McMahon S, Meijlink J, Pogatzki-Zahn E, Sieberg CB, Tracey I, Treede R-D, Missmer SA, Zondervan KT, Nagel J, Vincent K. Deep phenotyping of women with endometriosis-associated pain and bladder pain syndrome: the TRiPP (Translational Research in Pelvic Pain) study protocol. medRxiv 2022. doi: 10.1101/2022.05.16.22274828. [DOI] [Google Scholar]
- [16].Demetriou L, Krassowski M, Mendes PA, Garbutt K, Vitonis AF, Wilkins E, Coxon L, Arendt-Nielsen L, Aziz Q, Birch J, Horne AW, Hoffman A, Lunde CE, Meijlink J, Perro D, Rahmioglu N, Terry KL, Pogatzki-Zahn E, Sieberg CB, Treede R-D, Becker CM, Cruz F, Missmer SA, Zondervan KT, Nagel J, Vincent K. Clinical profiling of subgroups of women with chronic pelvic pain. Frontiers in Reproductive Health 2023;5. Available at: https://www.frontiersin.org/articles/10.3389/frph.2023.1140857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dunselman GAJ, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, de Bie B, Heikinheimo O, Horne AW, Kiesel L, Nap A, Prentice A, Saridogan E, Soriano D, Nelen W. ESHRE guideline: management of women with endometriosis. Hum Reprod 2014;29:400–12. [DOI] [PubMed] [Google Scholar]
- [18].Enax-Krumova E, Attal N, Bouhassira D, Freynhagen R, Gierthmühlen J, Hansson P, Kuehler BM, Maier C, Sachau J, Segerdahl M, Tölle T, Treede RD, Ventzel L, Baron R, Vollert J. Contralateral sensory and pain perception changes in patients with unilateral neuropathy. Neurology 2021;97:e389–402. [DOI] [PubMed] [Google Scholar]
- [19].Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am 1997;24:235–58. [DOI] [PubMed] [Google Scholar]
- [20].Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis 1998;5:209–27. [DOI] [PubMed] [Google Scholar]
- [21].Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet 2021;397:2098–110. [DOI] [PubMed] [Google Scholar]
- [22].Fletcher D, Stamer UM, Pogatzki-Zahn E, Zaslansky R, Tanase NV, Perruchoud C, Kranke P, Komann M, Lehman T, Lavand homme P, Vercauteren M, Meissner W, Iohom G, Cinnella G, Aurilio C, Belii A, Filipescu D, Rehberg-Klug B, Decostered I, Suter MR, Blumenthal S, Puig M, Garcia-Filoso A, Brandner B, Varvinskiy AM, Lisnyy I, Kuchyn I. Chronic postsurgical pain in Europe: an observational study. Eur J Anaesthesiol 2015;32:725–34. [DOI] [PubMed] [Google Scholar]
- [23].Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–20. [DOI] [PubMed] [Google Scholar]
- [24].Freynhagen R, Tölle TR, Gockel U, Baron R. The painDETECT project—far more than a screening tool on neuropathic pain. Curr Med Res Opin 2016;32:1033–57. [DOI] [PubMed] [Google Scholar]
- [25].Garzon S, Laganà AS, Casarin J, Raffaelli R, Cromi A, Sturla D, Franchi M, Ghezzi F. An update on treatment options for interstitial cystitis. Przeglad Menopauzalny 2020;19:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gierthmühlen J, Binder A, Förster M, Baron R. Do we measure what patients feel?: an analysis of correspondence between somatosensory modalities upon quantitative sensory testing and self-reported pain experience. Clin J Pain 2018;34:610–17. [DOI] [PubMed] [Google Scholar]
- [27].Gierthmühlen J, Böhmer J, Attal N, Bouhassira D, Freynhagen R, Haanpää M, Hansson P, Jensen TS, Kennedy J, Maier C, Rice ASC, Sachau J, Segerdahl M, Sindrup S, Tölle T, Treede R-D, Ventzel L, Vollert J, Baron R. Association of sensory phenotype with quality of life, functionality, and emotional well-being in patients suffering from neuropathic pain. PAIN 2022;163:1378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Grundström H, Gerdle B, Alehagen S, Berterö C, Arendt-Nielsen L, Kjølhede P. Reduced pain thresholds and signs of sensitization in women with persistent pelvic pain and suspected endometriosis. Acta Obstet Gynecol Scand 2019;98:327–36. [DOI] [PubMed] [Google Scholar]
- [29].Haanpää M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, Cruccu G, Hansson P, Haythornthwaite JA, Iannetti GD, Jensen TS, Kauppila T, Nurmikko TJ, Rice ASC, Rowbotham M, Serra J, Sommer C, Smith BH, Treede RD. NeuPSIG guidelines on neuropathic pain assessment. PAIN 2011;152:14–27. [DOI] [PubMed] [Google Scholar]
- [30].Harte SE, Schrepf A, Gallop R, Kruger GH, Lai HHH, Sutcliffe S, Halvorson M, Ichesco E, Naliboff BD, Afari N, Harris RE, Farrar JT, Tu F, Landis JR, Clauw DJ. Quantitative assessment of nonpelvic pressure pain sensitivity in urologic chronic pelvic pain syndrome: a MAPP Research Network study. PAIN 2019;160:1270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hellman KM, Roth GE, Dillane KE, Garrison EF, Oladosu FA, Clauw DJ, Tu FF. Dysmenorrhea subtypes exhibit differential quantitative sensory assessment profiles. PAIN 2020;161:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hochman JR, Davis AM, Elkayam J, Gagliese L, Hawker GA. Neuropathic pain symptoms on the modified painDETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthritis Cartilage 2013;21:1236–42. [DOI] [PubMed] [Google Scholar]
- [33].Kaya S, Hermans L, Willems T, Roussel N, Meeus M. Central sensitization in urogynecological chronic pelvic pain: a systematic literature review. Pain Physician 2013;16:291–308. [PubMed] [Google Scholar]
- [34].Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A, Saridogan E, Koninckx P, Matorras R, Mueller M, Garcia-Velasco J. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod 2005;20:2698–704. [DOI] [PubMed] [Google Scholar]
- [35].Kinfe T, von Willebrand N, Stadlbauer A, Buchfelder M, Yearwood TL, Muhammad S, Chaudhry SR, Gravius S, Randau T, Winder K, Maihöfner C, Gravius N, Magerl W. Quantitative sensory phenotyping in chronic neuropathic pain patients treated with unilateral L4-dorsal root ganglion stimulation. J Transl Med 2020;18:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kosek E, Clauw D, Nijs J, Baron R, Gilron I, Harris RE, Mico JA, Rice ASC, Sterling M. Chronic nociplastic pain affecting the musculoskeletal system: clinical criteria and grading system. PAIN 2021;162:2629–34. [DOI] [PubMed] [Google Scholar]
- [37].Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice ASC, Rief W, Sluka AK. Do we need a third mechanistic descriptor for chronic pain states? PAIN 2016;157:1382–6. [DOI] [PubMed] [Google Scholar]
- [38].Koulouris AE, Edwards RR, Dorado K, Schreiber KL, Lazaridou A, Rajan S, White J, Garcia J, Gibbons C, Freeman R. Reliability and validity of the Boston bedside quantitative sensory testing battery for neuropathic pain. Pain Med 2020;21:2336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kramer S, Baeumler P, Geber C, Fleckenstein J, Simang M, Haas L, Schober G, Pfab F, Treede RD, Irnich D. Somatosensory profiles in acute herpes zoster and predictors of postherpetic neuralgia. PAIN 2019;160:882–94. [DOI] [PubMed] [Google Scholar]
- [40].Magerl W, Krumova EK, Baron R, Tölle T, Treede RD, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. PAIN 2010;151:598–605. [DOI] [PubMed] [Google Scholar]
- [41].Maier C, Baron R, Tölle TR, Binder A, Birbaumer N, Birklein F, Gierthmühlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihöfner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Üeyler N, Valet M, Wasner G, Treede RD. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. PAIN 2010;150:439–50. [DOI] [PubMed] [Google Scholar]
- [42].Mainka T, Bischoff FS, Baron R, Krumova EK, Nicolas V, Pennekamp W, Treede RD, Vollert J, Westermann A, Maier C. Comparison of muscle and joint pressure-pain thresholds in patients with complex regional pain syndrome and upper limb pain of other origin. PAIN 2014;155:591–7. [DOI] [PubMed] [Google Scholar]
- [43].Maquet D, Croisier JL, Demoulin C, Crielaard JM. Pressure pain thresholds of tender point sites in patients with fibromyalgia and in healthy controls. Eur J Pain 2004;8:111–7 [DOI] [PubMed] [Google Scholar]
- [44].Mertens M, Hermans L, van Oosterwijck J, Meert L, Crombez G, Struyf F, Meeus M. The result of acute induced psychosocial stress on pain sensitivity and modulation in healthy people. Pain Physician 2020;23:E703–12. [PubMed] [Google Scholar]
- [45].van de Merwe JP, Nordling J, Bouchelouche P, Bouchelouche K, Cervigni M, Daha LK, Elneil S, Fall M, Hohlbrugger G, Irwin P, Mortensen S, van Ophoven A, Osborne JL, Peeker R, Richter B, Riedl C, Sairanen J, Tinzl M, Wyndaele JJ. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol 2008;53:60–7. [DOI] [PubMed] [Google Scholar]
- [46].Mikkelsson M, Latikka P, Kautiainen H, Isomeri R, Isomäki H. Muscle and bone pressure pain threshold and pain tolerance in fibromyalgia patients and controls. Arch Phys Med Rehabil 1992;73:814–8. [PubMed] [Google Scholar]
- [47].Moore R, Clifford AM, Moloney N, Doody C, Smart KM, O'Leary H. The relationship between clinical and quantitative measures of pain sensitization in knee osteoarthritis. Clin J Pain 2020;36:336–43. [DOI] [PubMed] [Google Scholar]
- [48].Morotti M, Vincent K, Becker CM. Mechanisms of pain in endometriosis. Eur J Obstet Gynecol Reprod Biol 2017;209:8–13. [DOI] [PubMed] [Google Scholar]
- [49].National Institute for Health and Care Excellence. Guideline scope endometriosis: diagnosis and management. Guideline scope, 2015. Available at: https://www.nice.org.uk/guidance/ng73/history [Google Scholar]
- [50].Ng Wing Tin S, Ciampi de Andrade D, Goujon C, Planté-Bordeneuve V, Créange A, Lefaucheur JP. Sensory correlates of pain in peripheral neuropathies. Clin Neurophysiol 2014;125:1048–58. [DOI] [PubMed] [Google Scholar]
- [51].Pfau DB, Krumova EK, Treede RD, Baron R, Toelle T, Birklein F, Eich W, Geber C, Gerhardt A, Weiss T, Magerl W, Maier C. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Reference data for the trunk and application in patients with chronic postherpetic neuralgia. PAIN 2014;155:1002–15. [DOI] [PubMed] [Google Scholar]
- [52].Pogatzki-Zahn EM, Segelcke D, Schug SA. Postoperative pain—from mechanisms to treatment. Pain Rep 2017;2:e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Reimer M, Forstenpointner J, Hartmann A, Otto JC, Vollert J, Gierthmühlen J, Klein T, Hüllemann P, Baron R. Sensory bedside testing: a simple stratification approach for sensory phenotyping. Pain Rep 2020;5:e820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rolke R, Baron R, Maier C, Tölle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihöfner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German research network on neuropathic pain (DFNS): standardized protocol and reference values. PAIN 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- [55].Ruscheweyh R, Wilder-Smith O, Drdla R, Liu XG, Sandkühler J. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol Pain 2011;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Shafrir AL, Martel E, Missmer SA, Clauw DJ, Harte SE, As-Sanie S, Sieberg CB. Pelvic floor, abdominal and uterine tenderness in relation to pressure pain sensitivity among women with endometriosis and chronic pelvic pain. Eur J Obstet Gynecol Reprod Biol 2021;264:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Simanski CJP, Althaus A, Hoederath S, Kreutz KW, Hoederath P, Lefering R, Pape-Köhler C, Neugebauer EAM. Incidence of chronic postsurgical pain (CPSP) after general surgery. Pain Med 2014;15:1222–9. [DOI] [PubMed] [Google Scholar]
- [58].Spielberger R, Lushere RC. State-trait anxiety inventory. Prof Psychol 1971. [Google Scholar]
- [59].Staffe AT, Bech MW, Clemmensen SLK, Nielsen HT, Larsen DB, Petersen KK. Total sleep deprivation increases pain sensitivity, impairs conditioned pain modulation and facilitates temporal summation of pain in healthy participants. PLoS One 2019;14:e0225849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Stratton P, Khachikyan I, Sinaii N, Ortiz R, Shah J. Association of chronic pelvic pain and endometriosis with signs of sensitization and myofascial pain. Obstet Gynecol 2015;125:719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sullivan M, Bishop S, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- [62].Talbot RM, McCrory CR. Mechanisms of postoperative pain-neuropathic. In: Postoperative Pain Management: An Evidence-Based Guide to Practice. Shorten G, Carr DB, Harmon D, Ouig MM, Browne J. (Eds). Elsevier, 2006. pp. 40–62. [Google Scholar]
- [63].Tampin B, Briffa NK, Slater H. Self-reported sensory descriptors are associated with quantitative sensory testing parameters in patients with cervical radiculopathy, but not in patients with fibromyalgia. Eur J Pain 2013;17:621–33. [DOI] [PubMed] [Google Scholar]
- [64].Tampin B, Vollert J, Schmid AB. Sensory profiles are comparable in patients with distal and proximal entrapment neuropathies, while the pain experience differs. Curr Med Res Opin 2018;34:1899–906. [DOI] [PubMed] [Google Scholar]
- [65].Truini A, Padua L, Biasiotta A, Caliandro P, Pazzaglia C, Galeotti F, Inghilleri M, Cruccu G. Differential involvement of A-delta and A-beta fibres in neuropathic pain related to carpal tunnel syndrome. PAIN 2009;145:105–9. [DOI] [PubMed] [Google Scholar]
- [66].Vatine JJ, Tsenter J, Nirel R. Experimental pressure pain in patients with complex regional pain syndrome, type I (Reflex sympathetic dystrophy). Am J Phys Med Rehabil 1998;77:382–7. [DOI] [PubMed] [Google Scholar]
- [67].Vedolin GM, Lobato Vv, Conti PCR, Lauris JRP. The impact of stress and anxiety on the pressure pain threshold of myofascial pain patients. J Oral Rehabil 2009;36:313–21. [DOI] [PubMed] [Google Scholar]
- [68].Vollert J, Attal N, Baron R, Freynhagen R, Haanpää M, Hansson P, Jensen TS, Rice ASC, Segerdahl M, Serra J, Sindrup SH, Tölle TR, Treede RD, Maier C. Quantitative sensory testing using DFNS protocol in Europe: an evaluation of heterogeneity across multiple centers in patients with peripheral neuropathic pain and healthy subjects. PAIN 2016;157:750–8. [DOI] [PubMed] [Google Scholar]
- [69].Vollert J, Magerl W, Baron R, Binder A, Enax-Krumova EK, Geisslingere G, Gierthmuhlen J, Henrich F, Hullemann P, Klein T, Lotsch J, Maier C, Oertel B, Schuh-Hofer S, Tolle TR, Treede RD. Pathophysiological mechanisms of neuropathic pain: Comparison of sensory phenotypes in patients and human surrogate pain models. PAIN 2018;159:1090–102. [DOI] [PubMed] [Google Scholar]
- [70].Vollert J, Maier C, Attal N, Bennett DLH, Bouhassira D, Enax-Krumova EK, Finnerup NB, Freynhagen R, Gierthmühlen J, Haanpää M, Hansson P, Hüllemann P, Jensen TS, Magerl W, Ramirez JD, Rice ASC, Schuh-Hofer S, Segerdahl M, Serra J, Shillo PR, Sindrup S, Tesfaye S, Themistocleous AC, Tölle TR, Treede RD, Baron R. Stratifying patients with peripheral neuropathic pain based on sensory profiles: algorithm and sample size recommendations. PAIN 2017;158:1446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Walsh DA. Nociplastic pain: helping to explain disconnect between pain and pathology. PAIN 2021;162:2627–8. [DOI] [PubMed] [Google Scholar]
- [72].Wasan AD, Alter BJ, Edwards RR, Argoff CE, Sehgal N, Walk D, Moeller-Bertram T, Wallace MS, Backonja M. Test-retest and inter-examiner reliability of a novel bedside quantitative sensory testing battery in postherpetic neuralgia patients. J Pain 2020;21:858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Welte-Jzyk C, Pfau DB, Hartmann A, Daubländer M. Somatosensory profiles of patients with chronic myogenic temporomandibular disorders in relation to their painDETECT score. BMC Oral Health 2018;18:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wesselmann U, Czakanski PP. Pelvic pain: a chronic visceral pain syndrome. Curr Pain Headache Rep 2001;5:13–9. [DOI] [PubMed] [Google Scholar]
- [75].Whitaker LHR, Reid J, Choa A, McFee S, Seretny M, Wilson J, Elton RA, Vincent K, Horne AW. An exploratory study into objective and reported characteristics of neuropathic pain in women with chronic pelvic pain. PLoS One 2016;11:e0151950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yuan T, Greenwood-Van Meerveld B. Abdominal and pelvic pain: current challenges and future opportunities. Front Pain Res (Lausanne) 2021;2:634804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers 2018;4:9. [DOI] [PubMed] [Google Scholar]
- [78].Zondervan KT, Yudkin PL, Vessey MP, Jenkinson CP, Dawes MG, Barlow DH, Kennedy SH. The community prevalence of chronic pelvic pain in women and associated illness behaviour. Br J Gen Pract 2001;51:541–7. [PMC free article] [PubMed] [Google Scholar]